Abstract
Giardia duodenalis, the most prevalent human intestinal parasite causes the disease, giardiasis. On an annual basis G. duodenalis infects ~1 billion people, of which ~280 million develop symptomatic disease. Giardiasis can be severe and chronic, causing malnutrition, stunted growth and poor cognitive development in children. Current treatment options rely on drugs with declining efficacy and side-effects. To improve the health and well-being of millions of people world-wide, new anti-Giardia drugs with different modes of action to currently used drugs are required. The Medicines for Malaria Venture's Pathogen Box, a collection of bio-active compounds specifically chosen to stimulate infectious disease drug discovery, represents an opportunity for the discovery of new anti-Giardia agents. While the anti-Giardia activity of Pathogen Box compounds has been reported, this work failed to identify known anti-Giardia controls within the compound set. It also reported the activity of compounds previously screened and shown to be inactive by others, suggesting data may be inaccurate. Given these concerns the anti-Giardia activity of Pathogen Box compounds was re-assessed in the current study. Data from this work identified thirteen compounds with anti-Giardia IC50 values ≤2 μM. Five of these compounds were reference compounds (marketed drugs with known anti-microbial activity), or analogues of compounds with previously described anti-Giardia activity. However, eight, including MMV676358 and MMV028694, which demonstrated potent sub-μM IC50s against assemblage A, B and metronidazole resistant parasites (0.3 μM and 0.9 μM respectively), may represent new leads for future drug development. Interestingly, only four of these compounds were identified in the previously reported Pathogen Box screen highlighting the importance of assay selection and design when assessing compounds for activity against infectious agents.
Keywords: Pathogen box, Giardia duodenalis, Drug discovery
Graphical abstract
Highlights
-
•
13 compounds with anti-Giardia IC50 values < 2 μM were identified.
-
•
8 compounds represent new leads for drug development.
-
•
MMV676358 and MMV028694 demonstrated the most promising acting.
-
•
Data highlight the importance of assay selection and design in drug discovery.
1. Introduction
Giardia duodenalis (aka G. lamblia or G. intestinalis) infects a wide range of mammalian hosts and is the most prevalent human intestinal parasite (Thompson and Monis, 2004; Ankarklev et al., 2010). Each year Giardia parasites infect ~1 billion people, causing approximately 280 million cases of giardiasis (Einarsson et al., 2016). While these figures are likely to be underestimates (Li et al., 2017), infection rates are clearly higher in developing countries (20–30%) as compared to industrialized countries (2–7%) (Thompson et al., 1993; Kappus et al., 1994; Savioli et al., 2006; Escobedo and Cimerman, 2007; Yoder et al., 2012; Gibney et al., 2014). However, they do vary considerably within regions and are often higher in children. In Australia, for example, Giardia parasites are the most common intestinal parasite identified in Indigenous Australian communities with carriage rates of 25–67% in children and ~12% in adults (Gracey et al., 1983; Gill and Jones, 1985; Meloni et al., 1993; Asher et al., 2014).
Giardiasis, is a neglected parasitic disease (Savioli et al., 2006) characterized by watery diarrhoea, nausea, vomiting, epigastric pain, and weight loss (Farthing, 1997; Ankarklev et al., 2010). It is usually self-limiting, however, infections can become severe and chronic leading to failure to thrive and growth retardation in children (Al-Mekhlafi et al., 2005; Botero-Garces et al., 2009; Abou-Shady et al., 2011). There is also increasing evidence demonstrating that G. duodenalis infections are associated with post-infectious disorders including irritable bowel syndrome, chronic fatigue and food allergies (Halliez and Buret, 2013; Hanevik et al., 2014; Bartelt and Sartor, 2015; Litleskare et al., 2018).
Despite growing evidence to suggest that Giardia parasites result in significant morbidity, there is no human vaccine for this pathogen and treatment depends on an arsenal of chemotherapeutics that have limitations including declining efficacy. The most commonly used drugs include the 5-nitroimidazoles and the benzimidazoles, with the 5-nitroimidazole, metronidazole, being widely acknowledged as the “gold-standard” therapy. However, treatment with metronidazole has been associated with clinical failure rates as high as 45–70% (reviewed in Lalle and Hanevik, 2018). Metronidazole is also very distasteful, requires a long treatment regimen (500 mg, 3 times/day for 5–7 days or 2g once/day for 3–5 days (Lalle and Hanevik, 2018)) and can cause side-effects including headache, vomiting, anorexia and nausea (Escobedo and Cimerman, 2007; Lalle, 2010). While the benzimidazoles, such as albendazole, are associated with comparably fewer side effects (Meloni et al., 1990) the efficacy of these agents can be very variable (25–90%) (Gardner and Hill, 2001). To improve these treatment options and to ensure the health and well-being of millions world-wide, particularly young children and those in marginalized communities, new anti-Giardia drugs with different modes of action to current agents need to be identified and developed.
To stimulate the discovery of new anti-infective compounds, the Medicines for Malaria Venture (MMV) developed the Pathogen Box, a collection of 400 molecules, with known activity against one or more key disease-causing pathogens. As all compounds in the Pathogen Box have known biological activity, including cytotoxicity, they represent excellent starting points for drug discovery efforts. To facilitate anti-Giardia drug discovery, the Pathogen Box was recently assessed for compounds displaying activity against Giardia trophozoites (Hennessey et al., 2018). In this study, a transgenic G. duodenalis parasite line expressing red-shifted firefly luciferase under the control of the β-tubulin promoter was used to quantify growth inhibition. Data identified 15 compounds that were able to inhibit growth by at least 95% following 24h exposure at a concentration of 16 μM. The activity of these compounds and an additional three compounds demonstrating >75% inhibition of G. duodenalis and Cryptosporidium parvum at 16 μM were further assessed in follow-up dose response assays. These data identified ten compounds with 24h IC50 values <2 μM (Hennessey et al., 2018). However, the previously published anti-Giardia compounds, mebendazole (IC50 value of <1 μM) (Morgan et al., 1993; Katiyar et al., 1994; Chen et al., 2011) and benznidazole (IC50 values of <1 μM) (Chen et al., 2011) were not identified as active compounds in this study. In addition, clofazimine and iodoquinol were identified as active compounds (24h IC50 values of 1.8 μM and 2.5 μM respectively), whereas previous studies had reported these drugs to be inactive (Bonilla-Santiago et al., 2008; Chen et al., 2011). As these discrepancies raised concerns around previously published data, the aim of the current study was to reassess the anti-Giardia activity of the Pathogen Box compounds using wild-type parasites and a previously validated image-based live cell assay (Hart et al., 2017). The activity of selected compounds was also assessed using 3H-thymidine incorporation as a comparator.
2. Materials and methods
2.1. Compounds
Pathogen Box compounds were obtained from the Medicines for Malaria Venture (MMV; https://www.mmv.org/mmv-open/pathogen-box/about-pathogen-box) as frozen 10 mM stocks prepared in DMSO (10 μL). Albendazole, mebendazole and metronidazole (Sigma-Aldrich, USA) were prepared in 100% dimethyl sulfoxide (DMSO) to stock concentrations of 20 mM and stored at −20 °C until required.
2.2. Parasite culture
Metronidazole-sensitive G. duodenalis assemblage B parasites (BRIS/91/HEPU/1279; AB MTZS), metronidazole-sensitive assemblage A parasites (BRIS/87/HEPU/713; AA MTZS) and laboratory generated metronidazole-resistant assemblage B parasites (BRIS/91/HEPU/1279m1; AB MTZR), were obtained from the QIMR Berghofer (Townson et al., 1992; Upcroft et al., 1995; Nolan et al., 2011). Trophozoites were grown axenically at 37 °C in Kiester's modified TYI-S-33 medium supplemented with 10% foetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin, in sealed 8 mL screw-capped borosilicate vials (Keister, 1983; Meloni and Thompson, 1987). Metronidazole resistant parasites (BRIS/91/HEPU/1279m1) were routinely cultured in the presence of 15 μM metrondazole. However parasites were grown without drug for at least two days prior to experimental use.
2.3. Primary screen of Pathogen Box compounds
The activity of Pathogen Box compounds was assessed in 96-well flat bottom micro-titre plates (Corning Costar, USA) against G. duodenalis assemblage B parasites BRIS/91/HEPU/1279 at 5 μM in singlicate on two separate occasions. To avoid evaporation in test wells, all outer wells were filled with PBS (200 μL). In addition to test wells (100 μL of 10 μM test compound; 0.5% DMSO) all assay plates included medium only (200 μL medium), negative (100 μL medium), positive (100 μL of 10 μM albendazole; 0.5% DMSO) and vehicle controls (100 μL medium containing 0.5% DMSO) in triplicate wells. All test and control wells, except medium controls, were inoculated with parasites (1.5 × 104 BRIS/91/HEPU/1279 trophozoites in 100 μL; final volume 200 μL 0.25% DMSO and final concentration of test compounds 5 μM). Plates were then incubated in sealed chambers filled with 3% O2, 5% CO2 in N2 for 48h as previously described (Gut et al., 2011; Hart et al., 2017). Plates were imaged using brightfield and digital phase-contrast microscopy using the Operetta (PerkinElmer, USA) and automatically enumerated using Harmony and PhenoLogic™ software as previously described (Hart et al., 2017). Inhibition of parasite growth was calculated as a percentage relative to the vehicle controls minus any background and Z′ values were calculated as previously described (Zhang et al., 1999).
2.4. Dose response activity assessments
The dose response activity of selected compounds was assessed in 96-well micro-titre plates by preparing 1:2 serial dilutions of test or control compounds in triplicate (0.2% DMSO; 100 μL). All experiments were performed on at least three occasions, with albendazole control plates included in each assay. Metronidazole control plates were also included when the sensitivity of parasites resistant to this compound was assessed. To avoid evaporation in test wells, all outer wells were filled with PBS (200 μL). In addition to triplicate test wells all assay plates also included medium only (200 μL medium), negative (100 μL medium) and vehicle controls (100 μL medium containing 0.2% DMSO) in triplicate wells. All test and control wells, with the exception of medium only controls, were inoculated with parasites (1.5 × 104 trophozoites in 100 μL; final volume 200 μL 0.1% DMSO). Plates were incubated in sealed culture chambers filled with 3% O2, 5% CO2 in N2 until growth assessment as previously described (Hart et al., 2017). Inhibition of parasite growth was calculated as a percentage relative to the vehicle control minus background and IC50 values were calculated using log-linear interpolation as previously described (Huber and Koella, 1993). Student's t-tests were used to compare the activity of compounds against the different Giardia parasite lines.
2.5. 3H-thymidine incorporation assay
The dose response activity of selected compounds was also assessed using a 3H-thymidine incorporation assay. Plates were prepared as described for imaging assays (Section 2.4) and all assays were performed in triplicate on at least three occasions. However, following the addition of parasites to assay plates were labelled with 3H-thymidine (1.0 μCi/well). Plates were then incubated at 37 °C in air-tight chambers filled in 3% O2, 5% CO2, in N2 for 48 h before being frozen. Parasite growth was indirectly assessed via the assessment of 3H-thymidine incorporation. In brief, assay plates were thawed, harvested onto glass-fibre filter-mats, air-dried and sealed in sample bags with 5 mL of beta-plate scintillant (PerkinElmer, USA), and counted (Microbeta2 2450, PerkinElmer, USA) (Bell et al., 1991; Downey et al., 2009). Inhibition of parasite growth was calculated as a percentage relative to the vehicle controls minus any background, and further analysed as previously described (section 2.4).
2.6. Minimum lethal concentration assay
Minimum lethal concentration assays were performed using G. duodenalis assemblage B parasites (BRIS/91/HEPU/1279; AB MTZS). In brief, duplicate dose response assay plates were prepared in 96-well micro-titre plates as previously described (section 2.4). Following 48 h incubation at 37 °C in sealed chambers (3% O2, 5% CO2 in N2), the growth of parasites was assessed by light microscopy. Plates were then incubated on ice for 30 min and the contents of each well transferred to individual 8 mL borosilicate vials filled with culture medium. The minimum lethal concentration or the lowest concentration of each compound that prevented all parasite growth was estimated by light microscopy following a further four days incubation at 37 °C. Each assay was performed on three separate occasions with albendazole as the positive control.
3. Results
3.1. Primary screen of Pathogen Box compounds
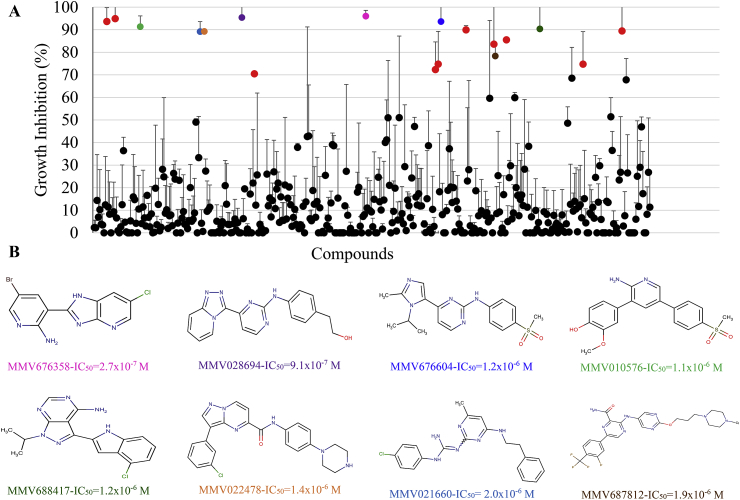
Our screen of the Pathogen Box (average Z’ factor 0.73 ± 0.17; values indicative of an excellent assay) identified 25 compounds able to inhibit trophozoite growth by >50% at 5 μM, 18 compounds able to inhibit trophozoite growth by >70% at 5 μM and seven compounds able to inhibit trophozoite growth by >90% at 5 μM (Fig. 1 and Supplementary Table 1). Among the 26 reference compounds (marketed drugs with known anti-microbial activity), six inhibited trophozoite growth by >50%. These were MMV003152 (mebendazole; 94.5%); MMV001499 (nifurtimox; 93.6%), MMV688978 (auranofin; 89.4%), MMV688773 (benznidazole; 74.7%), MMV688991 (nitazoxanide; 67.8%) and MMV687800 (clofazimine; 59.6%) (Supplementary Table 1).
Fig. 1.
Anti-Giardia activity of Pathogen Box compounds and the structures of compounds demonstrating anti-Giardia IC50values ≤2 μM. The anti-Giardia activity of Pathogen Box compounds was assessed at 5 μM against BRIS/91/HEPU/1279 trophozoites for 48 h. Two separate singlicate assays were performed with data presented as mean % growth ± SD. Compounds that inhibited trophozoite growth by >70% at 5 μM are coloured (A). The structures and 48 h IC50 values of compounds demonstrating anti-Giardia IC50 values <2 μM against BRIS/91/HEPU/1279 trophozoites, as determined via imaging and automated counting, are displayed as mean ± SD of three independent experiments performed in triplicate (B).
3.2. Follow-up dose response activity
The dose response activity of 18 compounds demonstrating >70% activity at 5 μM was determined against BRIS/91/HEPU/1279 trophozoites (Table 1). The inhibitory activity of albendazole in these assays, (IC50 0.12 μM and 0.09 μM at 24h and 48h respectively; Table 1) was consistent with previously published data (Cruz et al., 2003; Chen et al., 2011; Hart et al., 2017). Data derived from these experiments identified thirteen compounds with anti-Giardia 48h IC50 values ≤2 μM. While five of these compounds were reference compounds, or analogues of compounds with previously described anti-Giardia activity (MMV003152 (mebendazole) 48 h IC50 0.46 μM; MMV001499 (nifurtimox) 48 h IC50 1.34 μM; MMV688978 (auranofin) 48 h IC50 0.48 μM; MMV688773 (benznidazole) 48 h IC50 1.30 μM and MMV688262 (delamanid) 48 h IC50 0.36 μM), eight, MMV676358, MMV028694, MMV676604, MMV010576, MMV688417, MMV022478, MMV021660 and MMV687812 were non-reference compounds (Table 1; Fig. 1B). Of these eight compounds, MMV676358 demonstrated the most potent activity with IC50 values of 0.34 μM and 0.27 μM for 24 and 48 h respectively (Table 1). MMV028694 also demonstrated sub-μM anti-Giardia activity with a 48 h IC50 values of 0.91 μM (Table 1). The activity of these compounds against BRIS/91/HEPU/1279 trophozoites was further supported by 3H-thymidine incorporation dose response assays (IC50 values of 0.45 μM and 1.9 μM respectively; Table 2) and images of treated parasites (Fig. 2).
Table 1.
Anti-Giardia activity of Pathogen Box compounds demonstrating >70% growth inhibition at 5 μM.
| Compound | Activity in current study |
Previously described activity |
Previously described cytotoxicity (CC50 M) |
|||||
|---|---|---|---|---|---|---|---|---|
| 5 μM 48h Inhibition (%±SD) | 24 h IC50 (M±SD) | 48 h IC50 (M±SD) | 16 μM 24h Inhibition (%±SD)a | 24 h IC50 (M±SD) | 48 h IC50 (M±SD) | HepG2b | HEK293c | |
| MMV676358 | 96 ± 3 | 3.4 × 10−7±7.0 × 10−8 | 2.7 × 10−7±8.1 × 10−8 | 90 ± 2 | Not assessed | Not assessed | >10 × 10−6 | >20 × 10−6 |
| MMV028694 | 95 ± 8 | 1.6 × 10−6±2.8 × 10−7 | 9.1 × 10−7±2.3 × 10−7 | 99 ± 0 | 3.9 × 10−6±1.3 × 10−6a | Not assessed | 8.1-15.9 × 10−6a,b | >20 × 10−6 |
| MMV003152 mebendazole | 95 ± 6 | 5.9 × 10−7±3.6 × 10−7 | 4.6 × 10−7±3.3 × 10−7 | 26 ± 0 | Not assessed | 0.6-1.2 × 10−7d,e | Not assessed | Not assessed |
| MMV001499 nifurtimox | 94 ± 6 | 1.5 × 10−6±0.5 × 10−7 | 1.3 × 10−6±1.0 × 10−6 | 97 ± 1 | 6.4 × 10−7±1.2 × 10−7a | 3.7 × 10−7c | Not assessed | Not assessed |
| MMV676604 | 94 ± 11 | 1.7 × 10−6±6.4 × 10−7 | 1.2 × 10−6±5.2 × 10−7 | 92 ± 2 | Not assessed | Not assessed | 2.0 × 10−6 (CC20) | 2.7 × 10−6 |
| MMV010576 | 91 ± 5 | 1.9 × 10−6±7.0 × 10−7 | 1.1 × 10−6±5.6 × 10−7 | 94 ± 0 | 1.9 × 10−6±2.8 × 10−7 | Not assessed | >10 × 10−6 | >20 × 10−6 |
| MMV688417 | 90 ± 14 | 2.0 × 10−6±7.9 × 10−7 | 1.2 × 10−6±3.4 × 10−7 | 77 ± 0 | Not assessed | Not assessed | Not assessed | <20 × 10−6 |
| MMV687273 | 90 ± 2 | 9.2 × 10−6±0.0 × 10−6 | 2.3 × 10−6±1.2 × 10−6 | 89 ± 0 | Not assessed | Not assessed | 7.1 × 10−6 | >20 × 10−6 |
| MMV688978 Auranofin |
89 ± 11 | 1.1 × 10−6±2.8 × 10−7 | 4.8 × 10−7±7.8 × 10−8 | 98 ± 0 | 3.7 × 10−6±4.6 × 10−7a | 4.0-6.0 × 10−6f | Not assessed | Not assessed |
| MMV022478 | 89 ± 1 | 2.4 × 10−6±1.2 × 10−6 | 1.4 × 10−6±0.6 × 10-x | 98 ± 0 | 2.4 × 10−6±1.2 × 10−7a | Not assessed | >10 × 10−6 | 8.1 × 10−6 |
| MMV021660 | 89 ± 4 | 2.3 × 10−6±2.2 × 10−7 | 2.0 × 10−6±3.8 × 10−7 | 90 ± 4 | Not assessed | Not assessed | 6.5 × 10−6 | <20 × 10−6 |
| MMV688262 delamanid | 86 ± 0 | 1.1 × 10−6±7.6 × 10−7 | 3.6 × 10−7±7.2 × 10−8 | 97 ± 0 | 5.5 × 10−7±4.0 × 10−8a | Not assessed | 7.2 × 10−5 | Not assessed |
| MMV687807 | 84 ± 21 | 5.6 × 10−6±1.6 × 10−6 | 5.2 × 10−6±2.1 × 10−6 | 97 ± 0 | 5.1 × 10−7±6.0 × 10−8a | Not assessed | 7.0 × 10−7 | 2.0 × 10−6 |
| MMV687812 | 78 ± 5 | 2.6 × 10−6±5.1 × 10−7 | 1.9 × 10−6±3.4 × 10−7 | 98 ± 0 | 1.2 × 10−6±1.2 × 10−7a | Not assessed | 3.9 × 10−6 | <20 × 10−6 |
| MMV676602 | 75 ± 15 | 7.4 × 10−6±2.4 × 10−6 | 4.3 × 10−6±1.1 × 10−6 | 94 ± 1 | Not assessed | Not assessed | <3.0 × 10−7(CC20) | 1.3 × 10−6 |
| MMV688773 Benznidazole |
75 ± 15 | 2.0 × 10−6±9.8 × 10−7 | 1.3 × 10−6±9.1 × 10−7 | 57 ± 10 | Not assessed | 4.0 × 10−7d | Not assessed | Not assessed |
| MMV676599 | 72 ± 12 | >10 × 10−6 | 8.8 × 10−6±1.4 × 10−6 | 21 ± 3 | Not assessed | Not assessed | 1.9 × 10−5(CC20) | Not assessed |
| MMV062221 |
70 ± 0 |
>10 × 10−6 |
>10 × 10−6 |
35 ± 2 |
Not assessed |
Not assessed |
>10 × 10−6 |
>20 × 10−6 |
| Albendazole | 92 ± 8 | 1.2 × 10−7±4.0 × 10−8 | 9.0 × 10−8±2.0 × 10−8 | NA | 2.7 × 10−8-2.3 × 10−7g,h | 4.0 × 10−8i-4.0 × 10−7j | Not assessed | Not assessed |
| Metronidazole | Not assessed | 4.8 × 10−6±1.4 × 10−6 | 2.0 × 10−6±5.0 × 10−7 | Not assessed | 2.2 × 10−6g,h | 1.0–9.0 × 10−6k | Not assessed | Not assessed |
Pathogen_Box_Activity_Biological_Data_Structures.xlsx (https://www.mmv.org/mmv-open/pathogen-box/about-pathogen-box).
Table 2.
Anti-Giardia activity of selected Pathogen Box compounds.
| Compound | Inhibition (%) |
Current study 48h IC50±SD (M) |
Previous study; 24h IC50±SD (M) | ||
|---|---|---|---|---|---|
| Current study (5 μM, 48h) | Previous study (16 μM, 24h) | Imaging | 3H-Thymidine incorporation | ||
| MMV676358 | 96.0 ± 2.6 | 90 ± 0 | 2.7 × 10−7±8.1 × 10−8 | 4.5 × 10−7±1.4 × 10−7 | Not assessed |
| MMV028694 |
95.4 ± 8.0 |
99 ± 0 |
9.1 × 10−7±2.3 × 10−7 |
1.9 × 10−6±4.0 × 10−8 |
3.9 × 10−6±1.3 × 10−6 |
| MMV688844 | 24.6 ± 9.7 | 98 ± 0 | >10 × 10−6 | >10 × 10−6 | 2.3 × 10−6±4.3 × 10−7 |
| MMV676395 | 13.8 ± 19.5 | 98 ± 0 | >10 × 10−6 | >10 × 10−6 | 1.6 × 10−6±2.0 × 10−7 |
| MMV495543 | 0.0 ± 0.0 | 97 ± 2 | >10 × 10−6 | Not assessed | 2.8 × 10−6±2.1 × 10−7 |
| MMV687800 clofazimine |
59.1 ± 34.5 |
97 ± 0 |
6.6 × 10−6±7.0 × 10−7 |
Not assessed |
1.8 × 10−6±2.3 × 10−7 |
| mebendazole | 94.9 ± 5.8 | 26 ± 0 | 5.0 × 10−8±7.0 × 10−9 | 1.6 × 10−7±9.0 × 10−8 | Not assessed; >1.6 × 10−5 |
| albendazole | 92.3 ± 8.1 | Not assessed | 9.0 × 10−8±2.0 × 10−8 | 1.7 × 10−7±4.0 × 10−8 | 4.1 × 10−7± |
Fig. 2.
The effect of selected compounds on Giardia trophozoite growth.Giardia trophozoites were treated with vehicle control or test compounds to a final concentration of 5 μM for 48 h. Brightfield images were then taken using a PerkinElmer Operetta® using a 20× Olympus objective. Scale bar indicates 50 μm.
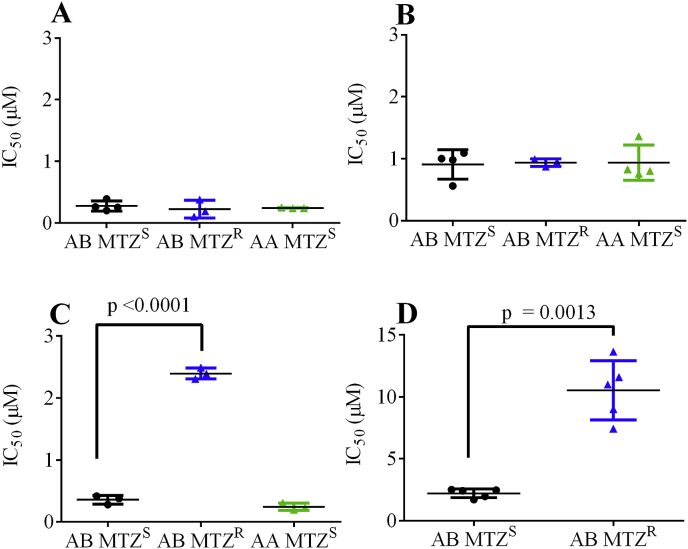
Given the promising 48 h IC50 values of MMV676358 and MMV028694 against metronidazole-sensitive G. duodenalis assemblage B parasites (BRIS/91/HEPU/1279; AB MTZS) further studies were performed to determine the activity of these compounds against assemblage A (BRIS/87/HEPU/713; AA MTZS) and metronidazole resistant parasites ((BRIS/91/HEPU/1279m1; AB MTZR; Fig. 3). The minimum lethal concentration of these compounds against BRIS/91/HEPU/1279; AB MTZS was also assessed. Data from these studies demonstrated that MMV676358 and MMV028694 were cidal, with minimum lethal concentrations of 0.62 μM and 6.25 μM respectively and that these compounds were active against all parasite lines, with similar 48 h IC50 values (Fig. 3A and B). The nitroimidazoles, MMV688262 (delamanid) and metronidazole were used as controls in studies with metronidazole resistant parasites, with data demonstrating both compounds to have reduced activity against these parasites (7-fold higher IC50 for MMV688262 p < 0.0001 and 4-fold higher IC50 for metronidazole p = 0.0013 compared to sensitive parasites; Fig. 3C and D). The minimum lethal concentration of albendazole was 0.25 μM (>0.12 μM), as previously described (Cedillo-Rivera and Munoz, 1992).
Fig. 3.
Anti-Giardia activity of MMV676358, MMV028694, delamanid and metronidazole against assemblage A, B and metronidazole resistant parasites. Dose response assays were performed to determine the 48 h IC50 values of MMV676358 (A), MMV028694 (B), delamanid (C) and metronidazole (D) against G. duodenalis. All experiments were performed at least three times in triplicate and data are shown as IC50 values (mean ± SD).
An additional four compounds (MMV688844, MMV676395, MMV495543 and MMV687800) that were identified as anti-Giardia hits previously (Hennessey et al., 2018), but did not reach 70% growth inhibition in the current study (<70% growth inhibition at 5 μM) were also assessed in dose response assays. The IC50 values of these compounds and representive images obtained during assays (Table 2; Fig. 2) supported initial screening data with all IC50 values determined to be > 5 μM. As compound was available, and data did not reflect the activity previously reported (Hennessey et al., 2018), the activity of MMV688844 and MMV676395 was also determined using 3H-thymidine incorporation. Data from these assays further supported these compounds to have IC50 values of >5 μM (Table 2). Importantly, mebendazole and albendazole controls were active at concentrations previously reported in the literature in these assays (Table 2).
4. Discussion
Thirteen Pathogen Box compounds demonstrated promising in vitro activity against Giardia trophozoites in the current study (IC50 < 2 μM). While five of these compounds were reference compounds (MMV003152, mebendazole (Katiyar et al., 1994; Chen et al., 2011); MMV001499, nifurtimox (Chen et al., 2011); MMV688978, Auranofin (Tejman-Yarden et al., 2013) and MMV688773, benznidazole (Chen et al., 2011)), or analogues of compounds with previously described anti-Giardia activity (MMV688262, delamanid), eight may represent new leads for anti-Giardia drug development (Table 1; Fig. 1B). The most potent of these compounds were MMV676358 and MMV028694, each displaying sub-μM IC50 values against multiple Giardia parasite lines, including the metronidazole-resistant assemblage B parasites, BRIS/91/HEPU/1279m1 (Fig. 3) and minimum lethal concentrations of 0.62 μM and 6.25 μM respectively.
With demonstrated activity against Plasmodium falciparum and P. berghei (https://www.mmv.org/mmv-open/pathogen-box/about-pathogen-box), MMV676358 forms part of the malaria disease set within the Pathogen Box. However, the mode of action of this compound against malaria parasites is not known. While our studies investigating the activity of MMV676358 against multiple G. duodenalis assemblages and metronidazole resistant parasites suggests that the mode of action of this compound against Giardia is cidal at sub-μM concentrations (Fig. 2 and minimum lethal concentration 0.62 μM) and different to the 5-nitroimidazoles, additional studies will be required to determine the target of this compound in these parasites. However, compounds sharing the 3-(1H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine core of MMV676358 have been shown to modulate the activity of kinases (patent document WO2011006567 and WO2007056155). Further studies investigating the anti-parasitic activity of this compound together with additional analogues appear warranted given that MMV676358 has also demonstrated good selectivity for parasites over human cells (SI > 37; Table 1) and has no obvious structural liabilities (Fig. 1B). In addition, although the previous screen of Pathogen Box compounds against Giardia parasites did not identify MMV676358 as a potent inhibitor of parasites (IC50 not determined) (Hennessey et al., 2018), likely due to the high screening concentration chosen, this work did demonstrate good inhibition (90 ± 2%) at the screening concentration of 16 μM (Table 1).
MMV028694, the second compound with sub-μM activity against Giardia parasites, also forms part of the malaria disease set, but has demonstrated activity against multiple parasites including Trypanosoma (Duffy et al., 2017). While the mode of action of MMV028694 has not been reported, structurally similar compounds have been identified as inhibitors of human MAP kinase enzymes (Palmer et al., 2013), so the anti-parasitic activity of MMV028694 may be associated with kinase inhibition (reviewed in (Veale, 2019). MAP kinase enzymes have been identified in G. duodenalis and could represent promising new therapeutic targets (Manning et al., 2011). Importantly studies with metronidazole resistant parasites suggest that the activity of MMV028694 is different to this currently used 5-nitroimidazole (Fig. 3). This observation taken together with the cidal (minimum lethal concentrations 6.25 μM) anti-Giardia activity and selectivity of this compound (IC50 < 1 μM; SI >10; Table 1), supports further mode of action and structure activity relationship studies. In addition, MMV028694 has no obvious structural liabilities and its anti-Giardia activity, while lower than reported in the current study, was supported by Hennessey et al. (2018) (IC50 3.9 μM; Table 1).
Four of the remaining six compounds, (MMV676604, MMV010576, MMV688417 and MMV022478) that demonstrated promising anti-Giardia activity in the current study have also been shown to inhibit protein kinases. MMV676604 (aka AZD5438; IC50 1.2 μM; SI > 8; Table 1; structural homology to MMV028694), with previously reported activity against Trypanosoma and Leishmania parasites, is a human cyclin-dependent kinase inhibitor (Byth et al., 2009). Whereas the 2-aminopyridine MMV010576, the indole MMV688417 and the pyrazolopyrimidine MMV022478, have been linked to the inhibition of P. falciparum phosphatidylinositol 4-kinase (Pf3D7_0509800; Paquet et al., 2017), Toxoplasma gondii calcium-dependent protein kinase1 (TgCDPK1; TGME49_301440) (Johnson et al., 2012; Veale, 2019) and human protein kinase C (PKCβII) (Gatto et al., 2013) respectively. While the target(s) of these compounds in G. duodenalis remains unknown, the genome of these parasites is predicted to encode multiple protein kinases, including a phosphatidylinositol 4-kinase (21% sequence identity to Pf3D7_0509800). MMV676604, MMV010576 and MMV688417 represent ideal opportunities for anti-Giardia drug development given that a significant amount of investigative work, including structure activity relationship studies has already been performed (Johnson et al., 2012; Veale, 2019), a factor which has the potential to reduce the time and cost associated with drug discovery efforts. MMV010576, for example, has undergone medicinal chemical optimisation for malaria, resulting in the development of the extremely promising clinical candidate MMV390048 (Younis et al., 2012; Paquet et al., 2017). Protein kinases are also an attractive but unexploited class of drug targets in Giardia parasites with evidence suggesting that compounds with selectivity for parasite kinases can be developed (an average 40% sequence identity has been described between human and Giardia protein kinases) (Manning et al., 2011). While less research has been performed with MMV022478, this compound has also demonstrated promising activity against multiple parasites including Toxoplasma (Spalenka et al., 2018) and Schistosoma species (Pasche et al., 2019). However, the activity of this compound against human protein kinase C (PKCβII) suggests that structure activity relationship activity optimisation studies would be required to further develop the activity of this compound (SI ~6 to >7; Table 1).
The remaining two Pathogen Box compounds with anti-Giardia IC50 values of ≤2 μM, MMV021660 and MMV687812, belong to the tuberculosis compound set. While MMV687812 is part of a group of compounds designed to inhibit Mycobacterium DNA gyrase (Shirude et al., 2013), the mode of action of MMV021660 against M. tuberculosis is less clear (reviewed in (Veale, 2019). While further studies would be required to define and optimise the activity of these compounds for Giardia, this work is feasible given the availability of analogues and is highly likely to result in the development of compounds with a different mode of action to those currently used in the clinic. Additional studies against drug resistant parasites should also be pursued given limited compound availability precluded this work in the current study. Importantly, although the dose response activity of MMV021660 was not assessed as part of the previously reported anti-Giardia Pathogen Box screen, both MMV021660 and MMV687812 were reported to inhibit the growth of parasites (90 ± 4% growth inhibition at 16 μM and 24 h IC50 1.2 μM respectively) further supporting additional studies with these compounds.
A comparison of the data generated in the current study with that reported by Hennessey et al. (2018), highlighted the importance of assay choice in phenotypic drug discovery. While both studies identified most known anti-Giardia compounds and the compounds discussed above as active agents, there were several obvious differences in the data generated. One clear difference was the inability of the Hennessey et al. (2018) screen to demonstrate the anti-Giardia activity of mebendazole (26 ± 0% inhibition after 24h exposure to 16 μM compared to a 24 h IC50 0.59 μM in the current study; Table 1). While the authors indicate that this discrepancy may be due to slow-action, we saw no indication of this in the current study (Table 1) and hypothesize that the reduced activity of mebendazole may have been associated with the genetic modification made to the parasites used during this research. The selection of transgenic parasites with puromycin has been shown to alter their gene expression (Su et al., 2007). In addition, the transgenic G. duodenalis parasite line used in this work expressed a red-shifted firefly luciferase under the control of the β-tubulin promoter and the activity of benzimidazoles, including mebendazole, in Giardia has been linked to β-tubulin and the inhibition of cytoskeleton polymerization (Holberton and Ward, 1981; Chavez et al., 1992; MacDonald et al., 2004). This idea is further supported by the relatively high IC50 reported for albendazole in this study (Table 1) and may indicate a limitation of the assay to detect the activity of compounds with this defined mode of action.
The Pathogen Box screen performed by Hennessey et al. (2018), also failed to detect the previously reported anti-Giardia activity of benznidazole (IC50 values of <1 μM) (Chen et al., 2011) (57 ± 10% inhibition at 16 μM). While the reason for this observation is unknown, particularly given that the work was performed with high compound concentrations in an anaerobic environment, it may be also be associated with the altered gene expression of transgenic parasites (Su et al., 2007). Although we have observed the activity of selected nitroimidazole compounds to be time dependent (e.g. Table 1 metronidazole 24 h IC50 4.8 μM vs 48 h IC50 2.0 μM), this idea does not adequately explain the poor activity of benznidazole reported by Hennessey et al. (2018) in their 24 h assay. The current study detected the activity of benznidazole at 24 and 48 h in a microaerophilic environment (3% O2, 5% CO2 in N2), which is known to inhibit the activation of benznidazole and other nitroheterocyclic compounds (Edwards and Shanson, 1980). Indeed, the reduced activity of nitazoxanide (67.8% inhibition at 5 μM) reported in the current study, is likely to be associated with assay environment (3% O2, 5% CO2 in N2). Previous work has shown nitazoxanide activation to be more sensitive to oxygen than metronidazole (Muller et al., 2015). The microaerophilic conditions used in the current study were chosen to better mimic the oxygen available in the mammalian intestine (He et al., 1999; Zheng et al., 2015).
The current study was unable to confirm the anti-Giardia activities of clofazimine (MMV687800; IC50 < 5 μM; Table 2; Fig. 2) and iodoquinol (MMV002817) (Supplemental Table S1). However, the activity of clofazimine was highly variable in our hands, an observation that may be associated with the activity reported by Hennessey et al. (2018). We were also unable to confirm the activity of MMV495543, MMV676395, MMV676501 and MMV688844 (Supplemental Table S1) with additional imaging and 3H-thymidine assays for selected compounds also supporting these findings (Fig. 2; Table 2).
In an era of declining anti-Giardia drug efficacy, the current study has identified several new compounds that warrant further investigation. These studies may be particularly beneficial given that the modes of action of identified compounds appear novel and that many of these compounds have already been studied by multiple groups, making analogues and resources readily available. The current study has also highlighted the impact of phenotypic assay design on data generation, directly demonstrating the potential impact of transgenic parasites and assay screening concentration on study outcomes.
Declaration of competing interest
None.
Acknowledgements
We thank the Medicines for Malaria Venture (MMV) for supplying Pathogen Box compounds. We also thank QIMR Berghofer for providing the G. duodenalis parasites used in these studies. ST, CH and TSA were supported by the Australian NHMRC Project Grant APP1141069.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2020.03.002.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Abou-Shady O., El Raziky M.S., Zaki M.M., Mohamed R.K. Impact of Giardia lamblia on growth, serum levels of zinc, copper, and iron in Egyptian children. Biol. Trace Elem. Res. 2011;140:1–6. doi: 10.1007/s12011-010-8673-6. [DOI] [PubMed] [Google Scholar]
- Al-Mekhlafi M.S., Azlin M., Nor Aini U., Shaik A., Sa'iah A., Fatmah M.S., Ismail M.G., Ahmad Firdaus M.S., Aisah M.Y., Rozlida A.R., Norhayati M. Giardiasis as a predictor of childhood malnutrition in Orang Asli children in Malaysia. Trans. R. Soc. Trop. Med. Hyg. 2005;99:686–691. doi: 10.1016/j.trstmh.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Ankarklev J., Jerlstrom-Hultqvist J., Ringqvist E., Troell K., Svard S.G. Behind the smile: cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 2010;8:413–422. doi: 10.1038/nrmicro2317. [DOI] [PubMed] [Google Scholar]
- Asher A.J., Holt D.C., Andrews R.M., Power M.L. Distribution of Giardia duodenalis assemblages A and B among children living in a remote indigenous community of the Northern Territory, Australia. PloS One. 2014;9 doi: 10.1371/journal.pone.0112058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt L.A., Sartor R.B. Advances in understanding Giardia: determinants and mechanisms of chronic sequelae. F1000Prime Rep. 2015;7:62. doi: 10.12703/P7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C.A., Cory M., Fairley T.A., Hall J.E., Tidwell R.R. Structure-activity relationships of pentamidine analogs against Giardia lamblia and correlation of antigiardial activity with DNA-binding affinity. Antimicrob. Agents Chemother. 1991;35:1099–1107. doi: 10.1128/aac.35.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Santiago R., Wu Z., Zhang L., Widmer G. Identification of growth inhibiting compounds in a Giardia lamblia high-throughput screen. Mol. Biochem. Parasitol. 2008;162:149–154. doi: 10.1016/j.molbiopara.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero-Garces J.H., Garcia-Montoya G.M., Grisales-Patino D., Aguirre-Acevedo D.C., Alvarez-Uribe M.C. Giardia intestinalis and nutritional status in children participating in the complementary nutrition program, Antioquia, Colombia. Rev. Inst. Med. Trop. Sao Paulo. 2009;51:155–162. doi: 10.1590/s0036-46652009000300006. May to October 2006. [DOI] [PubMed] [Google Scholar]
- Byth K.F., Thomas A., Hughes G., Forder C., McGregor A., Geh C., Oakes S., Green C., Walker M., Newcombe N., Green S., Growcott J., Barker A., Wilkinson R.W. AZD5438, a potent oral inhibitor of cyclin-dependent kinases 1, 2, and 9, leads to pharmacodynamic changes and potent antitumor effects in human tumor xenografts. Mol. Canc. Therapeut. 2009;8:1856–1866. doi: 10.1158/1535-7163.MCT-08-0836. [DOI] [PubMed] [Google Scholar]
- Cedillo-Rivera R., Chavez B., Gonzalez-Robles A., Tapia A., Yepez-Mulia L. In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis trophozoites. J. Eukaryot. Microbiol. 2002;49:201–208. doi: 10.1111/j.1550-7408.2002.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Cedillo-Rivera R., Munoz O. In-vitro susceptibility of Giardia lamblia to albendazole, mebendazole and other chemotherapeutic agents. J. Med. Microbiol. 1992;37:221–224. doi: 10.1099/00222615-37-3-221. [DOI] [PubMed] [Google Scholar]
- Chavez B., Cedillo-Rivera R., Martinez-Palomo A. Giardia lamblia: ultrastructural study of the in vitro effect of benzimidazoles. J. Protozool. 1992;39:510–515. doi: 10.1111/j.1550-7408.1992.tb04841.x. [DOI] [PubMed] [Google Scholar]
- Chen C.Z., Kulakova L., Southall N., Marugan J.J., Galkin A., Austin C.P., Herzberg O., Zheng W. High-throughput Giardia lamblia viability assay using bioluminescent ATP content measurements. Antimicrob. Agents Chemother. 2011;55:667–675. doi: 10.1128/AAC.00618-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A., Sousa M.I., Azeredo Z., Leite E., Figueiredo de Sousa J.C., Cabral M. Isolation, excystation and axenization of Giardia lamblia isolates: in vitro susceptibility to metronidazole and albendazole. J. Antimicrob. Chemother. 2003;51:1017–1020. doi: 10.1093/jac/dkg150. [DOI] [PubMed] [Google Scholar]
- Downey A.S., Graczyk T.K., Sullivan D.J. In vitro activity of pyrvinium pamoate against Entamoeba histolytica and Giardia intestinalis using radiolabelled thymidine incorporation and an SYBR Green I-based fluorescence assay. J. Antimicrob. Chemother. 2009;64:751–754. doi: 10.1093/jac/dkp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S., Sykes M.L., Jones A.J., Shelper T.B., Simpson M., Lang R., Poulsen S.A., Sleebs B.E., Avery V.M. Screening the Medicines for malaria venture pathogen Box across multiple pathogens reclassifies starting points for open-source drug discovery. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00379-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlind T.D., Hang T.L., Chakraborty P.R. Activity of the anthelmintic benzimidazoles against Giardia lamblia in vitro. J. Infect. Dis. 1990;162:1408–1411. doi: 10.1093/infdis/162.6.1408. [DOI] [PubMed] [Google Scholar]
- Edwards D.I., Shanson D. Metronidazole inactivation by aerobes. J. Antimicrob. Chemother. 1980;6:402–403. doi: 10.1093/jac/6.3.402. [DOI] [PubMed] [Google Scholar]
- Einarsson E., Ma'ayeh S., Svard S.G. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol. 2016;34:47–52. doi: 10.1016/j.mib.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Escobedo A.A., Cimerman S. Giardiasis: a pharmacotherapy review. Expet Opin. Pharmacother. 2007;8:1885–1902. doi: 10.1517/14656566.8.12.1885. [DOI] [PubMed] [Google Scholar]
- Farthing M.J. The molecular pathogenesis of giardiasis. J. Pediatr. Gastroenterol. Nutr. 1997;24:79–88. doi: 10.1097/00005176-199701000-00018. [DOI] [PubMed] [Google Scholar]
- Gardner T.B., Hill D.R. Treatment of giardiasis. Clin. Microbiol. Rev. 2001;14:114–128. doi: 10.1128/CMR.14.1.114-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto G.J., Jr., Ao Z., Kearse M.G., Zhou M., Morales C.R., Daniels E., Bradley B.T., Goserud M.T., Goodman K.B., Douglas S.A., Harpel M.R., Johns D.G. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J. Enzym. Inhib. Med. Chem. 2013;28:95–104. doi: 10.3109/14756366.2011.636360. [DOI] [PubMed] [Google Scholar]
- Gibney K.B., O'Toole J., Sinclair M., Leder K. Disease burden of selected gastrointestinal pathogens in Australia, 2010. Int. J. Infect. Dis. 2014;28:176–185. doi: 10.1016/j.ijid.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Gill J.S., Jones H.I. Intestinal parasites and bacteria in aboriginal children in south west Australia. Aust. Paediatr. J. 1985;21:45–49. doi: 10.1111/j.1440-1754.1985.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Gracey M., Burke V., Robinson J. Patterns of intestinal infection in Australian Aboriginal children. Ann. Trop. Paediatr. 1983;3:35–39. doi: 10.1080/02724936.1983.11748265. [DOI] [PubMed] [Google Scholar]
- Gut J., Ang K.K., Legac J., Arkin M.R., Rosenthal P.J., McKerrow J.H. An image-based assay for high throughput screening of Giardia lamblia. J. Microbiol. Methods. 2011;84:398–405. doi: 10.1016/j.mimet.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Halliez M.C., Buret A.G. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J. Gastroenterol. 2013;19:8974–8985. doi: 10.3748/wjg.v19.i47.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanevik K., Wensaas K.A., Rortveit G., Eide G.E., Morch K., Langeland N. Irritable bowel syndrome and chronic fatigue 6 years after Giardia infection: a controlled prospective cohort study. Clin. Infect. Dis. 2014;59:1394–1400. doi: 10.1093/cid/ciu629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C.J., Munro T., Andrews K.T., Ryan J.H., Riches A.G., Skinner-Adams T.S. A novel in vitro image-based assay identifies new drug leads for giardiasis. Int. J. Parasitol. Drugs Drug. Resist. 2017;7:83–89. doi: 10.1016/j.ijpddr.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G., Shankar R.A., Chzhan M., Samouilov A., Kuppusamy P., Zweier J.L. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4586–4591. doi: 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey K.M., Rogiers I.C., Shih H.W., Hulverson M.A., Choi R., McCloskey M.C., Whitman G.R., Barrett L.K., Merritt E.A., Paredez A.R., Ojo K.K. Screening of the Pathogen Box for inhibitors with dual efficacy against Giardia lamblia and Cryptosporidium parvum. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holberton D.V., Ward A.P. Isolation of the cytoskeleton from Giardia. Tubulin and a low-molecular-weight protein associated with microribbon structures. J. Cell Sci. 1981;47:139–166. doi: 10.1242/jcs.47.1.139. [DOI] [PubMed] [Google Scholar]
- Huber W., Koella J.C. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993;55:257–261. doi: 10.1016/0001-706x(93)90083-n. [DOI] [PubMed] [Google Scholar]
- Johnson S.M., Murphy R.C., Geiger J.A., DeRocher A.E., Zhang Z., Ojo K.K., Larson E.T., Perera B.G., Dale E.J., He P., Reid M.C., Fox A.M., Mueller N.R., Merritt E.A., Fan E., Parsons M., Van Voorhis W.C., Maly D.J. Development of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitors with potent anti-toxoplasma activity. J. Med. Chem. 2012;55:2416–2426. doi: 10.1021/jm201713h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappus K.D., Lundgren R.G., Juranek D.D., Roberts J.M., Spencer H.C. Intestinal parasitism in the united-states - update on a continuing problem. Am. J. Trop. Med. Hyg. 1994;50:705–713. doi: 10.4269/ajtmh.1994.50.705. [DOI] [PubMed] [Google Scholar]
- Katiyar S.K., Gordon V.R., McLaughlin G.L., Edlind T.D. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob. Agents Chemother. 1994;38:2086–2090. doi: 10.1128/aac.38.9.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D.B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Lalle M. Giardiasis in the post genomic era: treatment, drug resistance and novel therapeutic perspectives. Infect. Disord. - Drug Targets. 2010;10:283–294. doi: 10.2174/187152610791591610. [DOI] [PubMed] [Google Scholar]
- Lalle M., Hanevik K. Treatment-refractory giardiasis: challenges and solutions. Infect. Drug Resist. 2018;11:1921–1933. doi: 10.2147/IDR.S141468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang H., Wang R., Zhang L. Giardia duodenalis infections in humans and other animals in China. Front. Microbiol. 2017;8:2004. doi: 10.3389/fmicb.2017.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litleskare S., Rortveit G., Eide G.E., Hanevik K., Langeland N., Wensaas K.A. Prevalence of irritable bowel syndrome and chronic fatigue 10 Years after Giardia infection. Clin. Gastroenterol. Hepatol. 2018;16:1064–1072. doi: 10.1016/j.cgh.2018.01.022. e1064. [DOI] [PubMed] [Google Scholar]
- MacDonald L.M., Armson A., Thompson A.R., Reynoldson J.A. Characterisation of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol. Biochem. Parasitol. 2004;138:89–96. doi: 10.1016/j.molbiopara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Manning G., Reiner D.S., Lauwaet T., Dacre M., Smith A., Zhai Y., Svard S., Gillin F.D. The minimal kinome of Giardia lamblia illuminates early kinase evolution and unique parasite biology. Genome Biol. 2011;12:R66. doi: 10.1186/gb-2011-12-7-r66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni B.P., Thompson R.C. Comparative studies on the axenic in vitro cultivation of Giardia of human and canine origin: evidence for intraspecific variation. Trans. R. Soc. Trop. Med. Hyg. 1987;81:637–640. doi: 10.1016/0035-9203(87)90438-x. [DOI] [PubMed] [Google Scholar]
- Meloni B.P., Thompson R.C., Hopkins R.M., Reynoldson J.A., Gracey M. The prevalence of Giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley. Med. J. Aust. 1993;158:157–159. doi: 10.5694/j.1326-5377.1993.tb121692.x. [DOI] [PubMed] [Google Scholar]
- Meloni B.P., Thompson R.C., Reynoldson J.A., Seville P. Albendazole: a more effective antigiardial agent in vitro than metronidazole or tinidazole. Trans. R. Soc. Trop. Med. Hyg. 1990;84:375–379. doi: 10.1016/0035-9203(90)90324-8. [DOI] [PubMed] [Google Scholar]
- Morgan U.M., Reynoldson J.A., Thompson R.C. Activities of several benzimidazoles and tubulin inhibitors against Giardia spp. in vitro. Antimicrob. Agents Chemother. 1993;37:328–331. doi: 10.1128/aac.37.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Rout S., Leitsch D., Vaithilingam J., Hehl A., Muller N. Comparative characterisation of two nitroreductases from Giardia lamblia as potential activators of nitro compounds. Int. J. Parasitol. Drugs Drug. Resist. 2015;5:37–43. doi: 10.1016/j.ijpddr.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan M.J., Jex A.R., Upcroft J.A., Upcroft P., Gasser R.B. Barcoding of Giardia duodenalis isolates and derived lines from an established cryobank by a mutation scanning-based approach. Electrophoresis. 2011;32:2075–2090. doi: 10.1002/elps.201100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer W.S., Alam M., Arzeno H.B., Chang K.C., Dunn J.P., Goldstein D.M., Gong L., Goyal B., Hermann J.C., Hogg J.H., Hsieh G., Jahangir A., Janson C., Jin S., Ursula Kammlott R., Kuglstatter A., Lukacs C., Michoud C., Niu L., Reuter D.C., Shao A., Silva T., Trejo-Martin T.A., Stein K., Tan Y.C., Tivitmahaisoon P., Tran P., Wagner P., Weller P., Wu S.Y. Development of amino-pyrimidine inhibitors of c-Jun N-terminal kinase (JNK): kinase profiling guided optimization of a 1,2,3-benzotriazole lead. Bioorg. Med. Chem. Lett. 2013;23:1486–1492. doi: 10.1016/j.bmcl.2012.12.047. [DOI] [PubMed] [Google Scholar]
- Paquet T., Le Manach C., Cabrera D.G., Younis Y., Henrich P.P., Abraham T.S., Lee M.C.S., Basak R., Ghidelli-Disse S., Lafuente-Monasterio M.J., Bantscheff M., Ruecker A., Blagborough A.M., Zakutansky S.E., Zeeman A.M., White K.L., Shackleford D.M., Mannila J., Morizzi J., Scheurer C., Angulo-Barturen I., Martinez M.S., Ferrer S., Sanz L.M., Gamo F.J., Reader J., Botha M., Dechering K.J., Sauerwein R.W., Tungtaeng A., Vanachayangkul P., Lim C.S., Burrows J., Witty M.J., Marsh K.C., Bodenreider C., Rochford R., Solapure S.M., Jimenez-Diaz M.B., Wittlin S., Charman S.A., Donini C., Campo B., Birkholtz L.M., Hanson K.K., Drewes G., Kocken C.H.M., Delves M.J., Leroy D., Fidock D.A., Waterson D., Street L.J., Chibale K. Antimalarial efficacy of MMV390048, an inhibitor of Plasmodiumphosphatidylinositol 4-kinase. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aad9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasche V., Laleu B., Keiser J. Early antischistosomal leads identified from in vitro and in vivo screening of the Medicines for malaria venture pathogen Box. ACS Infect. Dis. 2019;5:102–110. doi: 10.1021/acsinfecdis.8b00220. [DOI] [PubMed] [Google Scholar]
- Savioli L., Smith H., Thompson A. Giardia and Cryptosporidium join the 'neglected diseases initiative. Trends Parasitol. 2006;22:203–208. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Shirude P.S., Madhavapeddi P., Tucker J.A., Murugan K., Patil V., Basavarajappa H., Raichurkar A.V., Humnabadkar V., Hussein S., Sharma S., Ramya V.K., Narayan C.B., Balganesh T.S., Sambandamurthy V.K. Aminopyrazinamides: novel and specific GyrB inhibitors that kill replicating and nonreplicating Mycobacterium tuberculosis. ACS Chem. Biol. 2013;8:519–523. doi: 10.1021/cb300510w. [DOI] [PubMed] [Google Scholar]
- Spalenka J., Escotte-Binet S., Bakiri A., Hubert J., Renault J.H., Velard F., Duchateau S., Aubert D., Huguenin A., Villena I. Discovery of new inhibitors of Toxoplasma gondii via the pathogen Box. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01640-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L.H., Lee G.A., Huang Y.C., Chen Y.H., Sun C.H. Neomycin and puromycin affect gene expression in Giardia lamblia stable transfection. Mol. Biochem. Parasitol. 2007;156:124–135. doi: 10.1016/j.molbiopara.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Tejman-Yarden N., Miyamoto Y., Leitsch D., Santini J., Debnath A., Gut J., McKerrow J.H., Reed S.L., Eckmann L. A reprofiled drug, auranofin, is effective against metronidazole-resistant Giardia lamblia. Antimicrob. Agents Chemother. 2013;57:2029–2035. doi: 10.1128/AAC.01675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.C., Monis P.T. Variation in Giardia: implications for taxonomy and epidemiology. Adv. Parasitol. 2004;58:69–137. doi: 10.1016/S0065-308X(04)58002-8. [DOI] [PubMed] [Google Scholar]
- Thompson R.C., Reynoldson J.A., Mendis A.H. Giardia and giardiasis. Adv. Parasitol. 1993;32:71–160. doi: 10.1016/s0065-308x(08)60207-9. [DOI] [PubMed] [Google Scholar]
- Townson S.M., Laqua H., Upcroft P., Boreham P.F., Upcroft J.A. Induction of metronidazole and furazolidone resistance in Giardia. Trans. R. Soc. Trop. Med. Hyg. 1992;86:521–522. doi: 10.1016/0035-9203(92)90095-t. [DOI] [PubMed] [Google Scholar]
- Upcroft J.A., Boreham P.F., Campbell R.W., Shepherd R.W., Upcroft P. Biological and genetic analysis of a longitudinal collection of Giardia samples derived from humans. Acta Trop. 1995;60:35–46. doi: 10.1016/0001-706x(95)00100-s. [DOI] [PubMed] [Google Scholar]
- Veale C.G.L. Unpacking the pathogen box-an open source tool for fighting neglected tropical disease. ChemMedChem. 2019;14:386–453. doi: 10.1002/cmdc.201800755. [DOI] [PubMed] [Google Scholar]
- Yoder J.S., Gargano J.W., Wallace R.M., Beach M.J., Centers for Disease, C.,Prevention Giardiasis surveillance--United States, 2009-2010. MMWR Surveill. Summ. 2012;61:13–23. [PubMed] [Google Scholar]
- Younis Y., Douelle F., Feng T.S., Gonzalez Cabrera D., Le Manach C., Nchinda A.T., Duffy S., White K.L., Shackleford D.M., Morizzi J., Mannila J., Katneni K., Bhamidipati R., Zabiulla K.M., Joseph J.T., Bashyam S., Waterson D., Witty M.J., Hardick D., Wittlin S., Avery V., Charman S.A., Chibale K. 3,5-Diaryl-2-aminopyridines as a novel class of orally active antimalarials demonstrating single dose cure in mice and clinical candidate potential. J. Med. Chem. 2012;55:3479–3487. doi: 10.1021/jm3001373. [DOI] [PubMed] [Google Scholar]
- Zheng L., Kelly C.J., Colgan S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 2015;309:C350–C360. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.