Graphical abstract
Keywords: Coronaviruses, COVID-19, Origin, Outbreak, Spread
Abstract
The coronavirus disease 19 (COVID-19) is a highly transmittable and pathogenic viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which emerged in Wuhan, China and spread around the world. Genomic analysis revealed that SARS-CoV-2 is phylogenetically related to severe acute respiratory syndrome-like (SARS-like) bat viruses, therefore bats could be the possible primary reservoir. The intermediate source of origin and transfer to humans is not known, however, the rapid human to human transfer has been confirmed widely. There is no clinically approved antiviral drug or vaccine available to be used against COVID-19. However, few broad-spectrum antiviral drugs have been evaluated against COVID-19 in clinical trials, resulted in clinical recovery. In the current review, we summarize and comparatively analyze the emergence and pathogenicity of COVID-19 infection and previous human coronaviruses severe acute respiratory syndrome coronavirus (SARS-CoV) and middle east respiratory syndrome coronavirus (MERS-CoV). We also discuss the approaches for developing effective vaccines and therapeutic combinations to cope with this viral outbreak.
Introduction
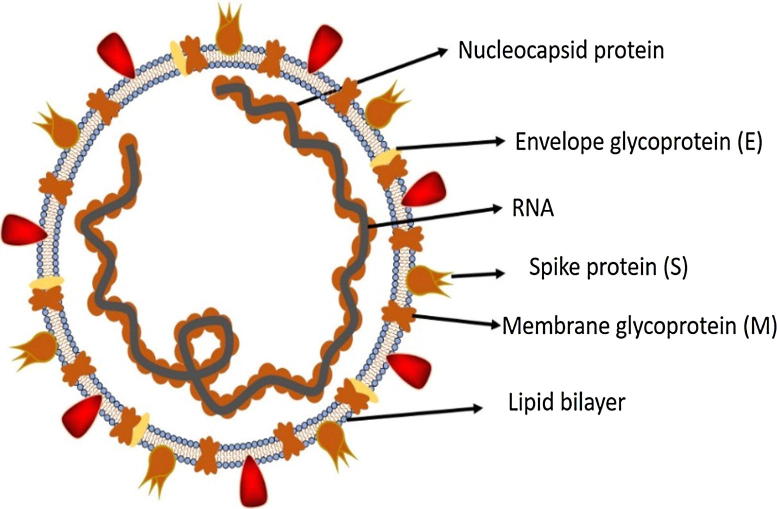
Coronaviruses belong to the Coronaviridae family in the Nidovirales order. Corona represents crown-like spikes on the outer surface of the virus; thus, it was named as a coronavirus. Coronaviruses are minute in size (65–125 nm in diameter) and contain a single-stranded RNA as a nucleic material, size ranging from 26 to 32kbs in length (Fig. 1). The subgroups of coronaviruses family are alpha (α), beta (β), gamma (γ) and delta (δ) coronavirus. The severe acute respiratory syndrome coronavirus (SARS-CoV), H5N1 influenza A, H1N1 2009 and Middle East respiratory syndrome coronavirus (MERS-CoV) cause acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) which leads to pulmonary failure and result in fatality. These viruses were thought to infect only animals until the world witnessed a severe acute respiratory syndrome (SARS) outbreak caused by SARS-CoV, 2002 in Guangdong, China [1]. Only a decade later, another pathogenic coronavirus, known as Middle East respiratory syndrome coronavirus (MERS-CoV) caused an endemic in Middle Eastern countries [2].
Fig. 1.
Structure of respiratory syndrome causing human coronavirus.
Recently at the end of 2019, Wuhan an emerging business hub of China experienced an outbreak of a novel coronavirus that killed more than eighteen hundred and infected over seventy thousand individuals within the first fifty days of the epidemic. This virus was reported to be a member of the β group of coronaviruses. The novel virus was named as Wuhan coronavirus or 2019 novel coronavirus (2019-nCov) by the Chinese researchers. The International Committee on Taxonomy of Viruses (ICTV) named the virus as SARS-CoV-2 and the disease as COVID-19 [3], [4], [5]. In the history, SRAS-CoV (2003) infected 8098 individuals with mortality rate of 9%, across 26 contries in the world, on the other hand, novel corona virus (2019) infected 120,000 induviduals with mortality rate of 2.9%, across 109 countries, till date of this writing. It shows that the transmission rate of SARS-CoV-2 is higher than SRAS-CoV and the reason could be genetic recombination event at S protein in the RBD region of SARS-CoV-2 may have enhanced its transmission ability. In this review article, we discuss the origination of human coronaviruses briefly. We further discuss the associated infectiousness and biological features of SARS and MERS with a special focus on COVID-19.
Comparative analysis of emergence and spreading of coronaviruses
In 2003, the Chinese population was infected with a virus causing Severe Acute Respiratory Syndrome (SARS) in Guangdong province. The virus was confirmed as a member of the Beta-coronavirus subgroup and was named SARS-CoV [6], [7]. The infected patients exhibited pneumonia symptoms with a diffused alveolar injury which lead to acute respiratory distress syndrome (ARDS). SARS initially emerged in Guangdong, China and then spread rapidly around the globe with more than 8000 infected persons and 776 deceases. A decade later in 2012, a couple of Saudi Arabian nationals were diagnosed to be infected with another coronavirus. The detected virus was confirmed as a member of coronaviruses and named as the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). The World health organization reported that MERS-coronavirus infected more than 2428 individuals and 838 deaths [8]. MERS-CoV is a member beta-coronavirus subgroup and phylogenetically diverse from other human-CoV. The infection of MERS-CoV initiates from a mild upper respiratory injury while progression leads to severe respiratory disease. Similar to SARS-coronavirus, patients infected with MERS-coronavirus suffer pneumonia, followed by ARDS and renal failure [9].
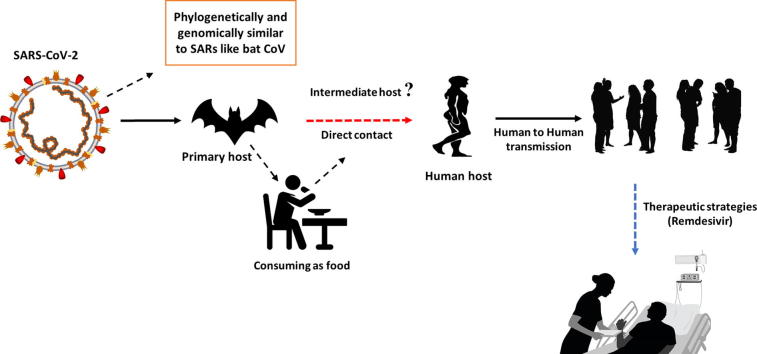
Recently, by the end of 2019, WHO was informed by the Chinese government about several cases of pneumonia with unfamiliar etiology. The outbreak was initiated from the Hunan seafood market in Wuhan city of China and rapidly infected more than 50 peoples. The live animals are frequently sold at the Hunan seafood market such as bats, frogs, snakes, birds, marmots and rabbits [10]. On 12 January 2020, the National Health Commission of China released further details about the epidemic, suggested viral pneumonia [10]. From the sequence-based analysis of isolates from the patients, the virus was identified as a novel coronavirus. Moreover, the genetic sequence was also provided for the diagnosis of viral infection. Initially, it was suggested that the patients infected with Wuhan coronavirus induced pneumonia in China may have visited the seafood market where live animals were sold or may have used infected animals or birds as a source of food. However, further investigations revealed that some individuals contracted the infection even with no record of visiting the seafood market. These observations indicated a human to the human spreading capability of this virus, which was subsequently reported in more than 100 countries in the world. The human to the human spreading of the virus occurs due to close contact with an infected person, exposed to coughing, sneezing, respiratory droplets or aerosols. These aerosols can penetrate the human body (lungs) via inhalation through the nose or mouth (Fig. 2) [11], [12], [13], [14].
Fig. 2.
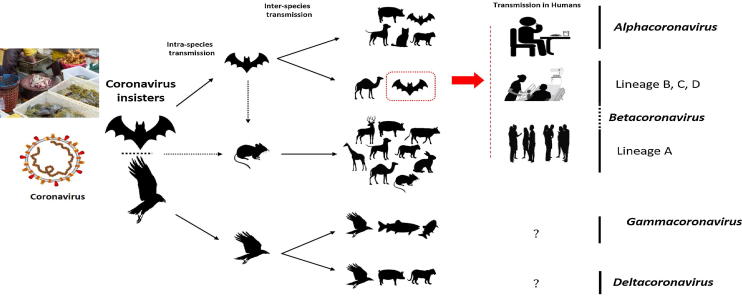
The key reservoirs and mode of transmission of coronaviruses (suspected reservoirs of SARS-CoV-2 are red encircled); only α and β coronaviruses have the ability to infect humans, the consumption of infected animal as a source of food is the major cause of animal to human transmission of the virus and due to close contact with an infected person, the virus is further transmitted to healthy persons. Dotted black arrow shows the possibility of viral transfer from bat whereas the solid black arrow represent the confirmed transfer. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Primary reservoirs and hosts of coronaviruses
The source of origination and transmission are important to be determined in order to develop preventive strategies to contain the infection. In the case of SARS-CoV, the researchers initially focused on raccoon dogs and palm civets as a key reservoir of infection. However, only the samples isolated from the civets at the food market showed positive results for viral RNA detection, suggesting that the civet palm might be secondary hosts [15]. In 2001 the samples were isolated from the healthy persons of Hongkong and the molecular assessment showed 2.5% frequency rate of anti-bodies against SARS-coronavirus. These indications suggested that SARS-coronavirus may be circulating in humans before causing the outbreak in 2003 [16]. Later on, Rhinolophus bats were also found to have anti-SARS-CoV antibodies suggesting the bats as a source of viral replication [17]. The Middle East respiratory syndrome (MERS) coronavirus first emerged in 2012 in Saudi Arabia [9]. MERS-coronavirus also pertains to beta-coronavirus and having camels as a zoonotic source or primary host [18]. In a recent study, MERS-coronavirus was also detected in Pipistrellus and Perimyotis bats [19], proffering that bats are the key host and transmitting medium of the virus [20], [21]. Initially, a group of researchers suggested snakes be the possible host, however, after genomic similarity findings of novel coronavirus with SARS-like bat viruses supported the statement that not snakes but only bats could be the key reservoirs (Table 1) [22], [23]. Further analysis of homologous recombination revealed that receptor binding spike glycoprotein of novel coronavirus is developed from a SARS-CoV (CoVZXC21 or CoVZC45) and a yet unknown Beta-CoV [24]. Nonetheless, to eradicate the virus, more work is required to be done in the aspects of the identification of the intermediate zoonotic source that caused the transmission of the virus to humans.
Table 1.
Comparative analysis of biological features of SARS-CoV and SARS-CoV-2.
| Features | SARS-CoV | SARS-CoV-2 | Reference |
|---|---|---|---|
| Emergence date | November 2002 | December 2019 | [37], [79], [80], [81] |
| Area of emergence | Guangdong, China | Wuhan, China | |
| Date of fully controlled | July 2003 | Not controlled yet | |
| Key hosts | Bat, palm civets and Raccon dogs | Bat | [22], [82], [83] |
| Number of countries infected | 26 | 109 | [84] |
| Entry receptor in humans | ACE2 receptor | ACE2 receptor | [22], [55], [85] |
| Sign and symptoms | fever, malaise, myalgia, headache, diarrhoea, shivering, cough and shortness of breath | Cough, fever and shortness of breath | [12], [23], [85] |
| Disease caused | SARS, ARDS | SARS, COVID-19 | [85], [86] |
| Total infected patients | 8098 | 123882 | [84] |
| Total recovered patients | 7322 | 67051 | |
| Total died patients | 776 (9.6% mortality rate) | 4473 (3.61% mortality rate) |
Key features and entry mechanism of human coronaviruses
All coronaviruses contain specific genes in ORF1 downstream regions that encode proteins for viral replication, nucleocapsid and spikes formation [25]. The glycoprotein spikes on the outer surface of coronaviruses are responsible for the attachment and entry of the virus to host cells (Fig. 1). The receptor-binding domain (RBD) is loosely attached among virus, therefore, the virus may infect multiple hosts [26], [27]. Other coronaviruses mostly recognize aminopeptidases or carbohydrates as a key receptor for entry to human cells while SARS-CoV and MERS-CoV recognize exopeptidases [2]. The entry mechanism of a coronavirus depends upon cellular proteases which include, human airway trypsin-like protease (HAT), cathepsins and transmembrane protease serine 2 (TMPRSS2) that split the spike protein and establish further penetration changes [28], [29]. MERS-coronavirus employs dipeptidyl peptidase 4 (DPP4), while HCoV-NL63 and SARS-coronavirus require angiotensin-converting enzyme 2 (ACE2) as a key receptor [2], [26].
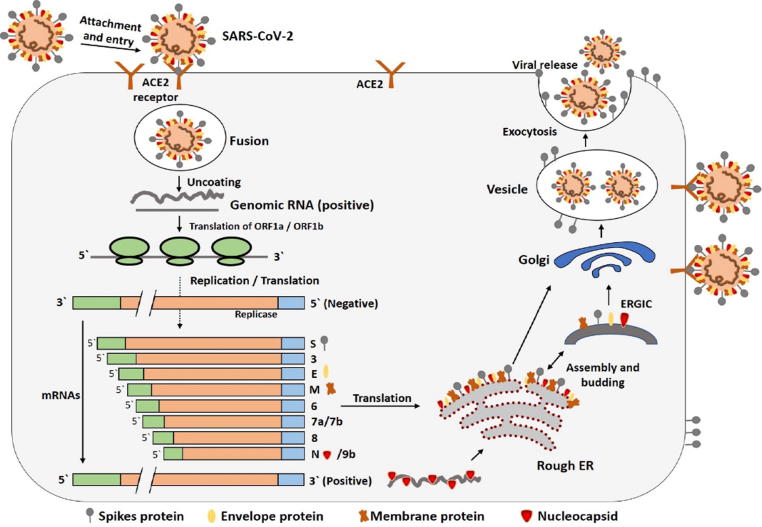
SARS-CoV-2 possesses the typical coronavirus structure with spike protein and also expressed other polyproteins, nucleoproteins, and membrane proteins, such as RNA polymerase, 3-chymotrypsin-like protease, papain-like protease, helicase, glycoprotein, and accessory proteins [30], [31]. The spike protein of SARS-CoV-2 contains a 3-D structure in the RBD region to maintain the van der Waals forces [32]. The 394 glutamine residue in the RBD region of SARS-CoV-2 is recognized by the critical lysine 31 residue on the human ACE2 receptor [33]. The entire mechanism of pathogenicity of SARS-CoV-2, from attachment to replication is well mentioned in Fig. 3.
Fig. 3.
The life cycle of SARS-CoV-2 in host cells; begins its life cycle when S protein binds to the cellular receptor ACE2. After receptor binding, the conformation change in the S protein facilitates viral envelope fusion with the cell membrane through the endosomal pathway. Then SARS-CoV-2 releases RNA into the host cell. Genome RNA is translated into viral replicase polyproteins pp1a and 1ab, which are then cleaved into small products by viral proteinases. The polymerase produces a series of subgenomic mRNAs by discontinuous transcription and finally translated into relevant viral proteins. Viral proteins and genome RNA are subsequently assembled into virions in the ER and Golgi and then transported via vesicles and released out of the cell. ACE2, angiotensin-converting enzyme 2; ER, endoplasmic reticulum; ERGIC, ER–Golgi intermediate compartment.
Genomic variations in SARS-CoV-2
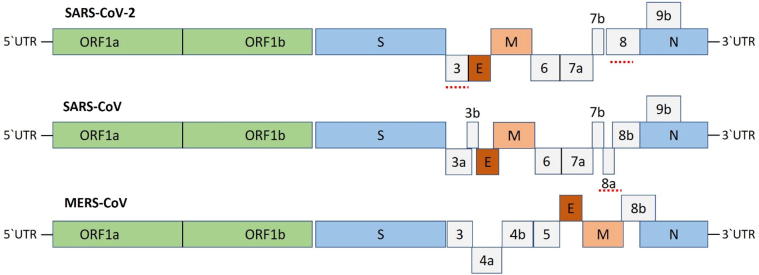
The genome of the SARS-CoV-2 has been reported over 80% identical to the previous human coronavirus (SARS-like bat CoV) [34]. The Structural proteins are encoded by the four structural genes, including spike (S), envelope (E), membrane (M) and nucleocapsid (N) genes. The orf1ab is the largest gene in SARS-CoV-2 which encodes the pp1ab protein and 15 nsps. The orf1a gene encodes for pp1a protein which also contains 10 nsps [34], [35], [36]. According to the evolutionary tree, SARS-CoV-2 lies close to the group of SARS-coronaviruses [37], [38] (Fig. 5). Recent studies have indicated notable variations in SARS-CoV and SARS-CoV-2 such as the absence of 8a protein and fluctuation in the number of amino acids in 8b and 3c protein in SARS-CoV-2 [34] (Fig. 4). It is also reported that Spike glycoprotein of the Wuhan coronavirus is modified via homologous recombination. The spike glycoprotein of SARS-CoV-2 is the mixture of bat SARS-CoV and a not known Beta-CoV [38]. In a fluorescent study, it was confirmed that the SARS-CoV-2 also uses the same ACE2 (angiotensin-converting enzyme 2) cell receptor and mechanism for the entry to host cell which is previously used by the SARS-CoV [39], [40]. The single N501T mutation in SARS-CoV-2's Spike protein may have significantly enhanced its binding affinity for ACE2 [33].
Fig. 5.
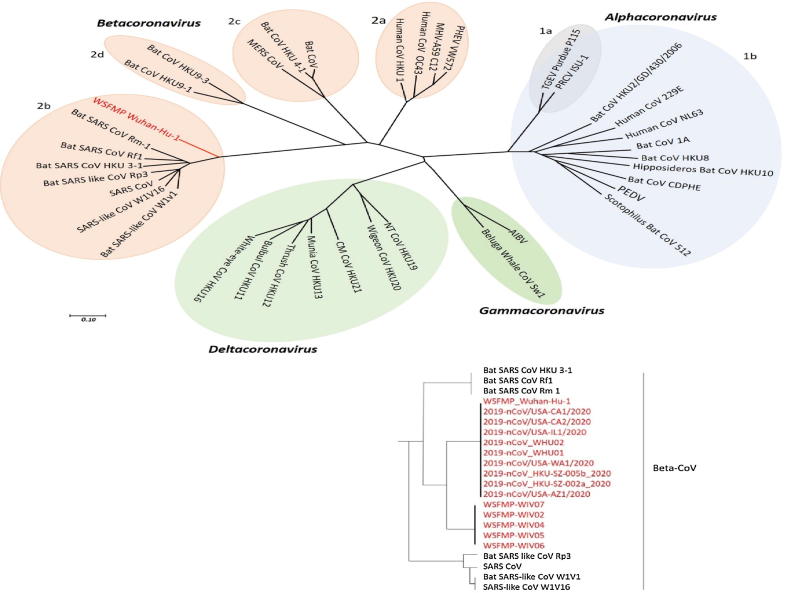
Phylogenetic tree of coronaviruses (content in red is the latest addition of newly emerged SARS-CoV-2 and WSFMP Wuhan-Hu-1 is used as a reference in the tree); The phylogenetic tree showing the relationship of Wuhan-Hu-1 (denoted as red) to selected coronavirus is based on nucleotide sequences of the complete genome. The viruses are grouped into four genera (prototype shown): Alphacoronavirus (sky blue), Betacoronavirus (pink), Gammacoronavirus (green) and Deltacoronavirus (light blue). Subgroup clusters are labeled as 1a and 1b for the Alphacoronavirus and 2a, 2b, 2c, and 2d for the Betacoronavirus. This tree is based on the published trees of Coronavirinae [3], [41] and reconstructed with sequences of the complete RNA- dependent RNA polymerase- coding region of the representative novel coronaviruses (maximum likelihood method using MEGA 7.2 software). severe acute respiratory syndrome coronavirus (SARS- CoV); SARS- related coronavirus (SARSr- CoV); the Middle East respiratory syndrome coronavirus (MERS- CoV); porcine enteric diarrhea virus (PEDV); Wuhan seafood market pneumonia (Wuhan-Hu-1). Bat CoV RaTG13 Showed high sequence identity to SARS-CoV-2 [42]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Betacoronaviruses genome organization; The Betacoronavirus for human (SARS-CoV-2, SARS-CoV and MERS-CoV) genome comprises of the 5′-untranslated region (5′-UTR), open reading frame (orf) 1a/b (green box) encoding non-structural proteins (nsp) for replication, structural proteins including spike (blue box), envelop (maroon box), membrane (pink box), and nucleocapsid (cyan box) proteins, accessory proteins (light gray boxes) such as orf 3, 6, 7a, 7b, 8 and 9b in the SARS-CoV-2 genome, and the 3′-untranslated region (3′-UTR). The doted underlined in red are the protein which shows key variation between SARS-CoV-2 and SARS-CoV. The length of nsps and orfs are not drawn in scale. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The major obstacle in research progress
Animal models play a vital role to uncover the mechanisms of viral pathogenicity from the entrance to the transmission and designing therapeutic strategies. Previously, to examine the replication of SARS-CoV, various animal models were used which showed the symptoms of severe infection [43]. In contrast to SARS-CoV, no MERS-CoV pathogenesis was observed in small animals. Mice are not vulnerable to infection by MERS-coronavirus due to the non-compatibility of the DPP4 receptor [44]. As the entire genome of the 2019-novel coronavirus is more than 80% similar to the previous human SARS-like bat CoV, previously used animal models for SARS-CoV can be utilized to study the infectious pathogenicity of SARS-CoV-2. The human ACE2 cell receptor is recognized by both SARS and Novel coronaviruses. Conclusively, TALEN or CRISPR-mediated genetically modified hamsters or other small animals can be utilized for the study of the pathogenicity of novel coronaviruses. SARS-CoV has been reported to replicate and cause severe disease in Rats (F344), where the sequence analysis revealed a mutation at spike glycoprotein [45]. Thus, it could be another suitable option to develop spike glycoprotein targeting therapeutics against novel coronaviruses. Recently, mice models and clinical isolates were used to develop any therapeutic strategy against SARS-CoV-2 induced COVID-19 [46], [47]. In a similar study, artificial intelligence prediction was used to investigate the inhibitory role of the drug against SARS-CoV-2 [48]. SARS-CoV-2 infected patients were also used to conduct randomized clinical trials [46], [49], [50]. It is now important that the scientists worldwide collaborate the design a suitable model and investigate the in vivo mechanisms associated with pathogenesis of SARS-CoV-2.
Potential therapeutic strategies against COVID-19
Initially, interferons-α nebulization, broad-spectrum antibiotics, and anti-viral drugs were used to reduce the viral load [49], [51], [52], however, only remdesivir has shown promising impact against the virus [53]. Remdesivir only and in combination with chloroquine or interferon beta significantly blocked the SARS-CoV-2 replication and patients were declared as clinically recovered [46], [50], [52]. Various other anti-virals are currently being evaluated against infection. Nafamostat, Nitazoxanide, Ribavirin, Penciclovir, Favipiravir, Ritonavir, AAK1, Baricitinib, and Arbidol exhibited moderate results when tested against infection in patients and in-vitro clinical isolates [46], [48], [50], [52]. Several other combinations, such as combining the antiviral or antibiotics with traditional Chinese medicines were also evaluated against SARS-CoV-2 induced infection in humans and mice [46]. Recently in Shanghai, doctors isolated the blood plasma from clinically recovered patients of COVID-19 and injected it in the infected patients who showed positive results with rapid recovery [54]. In a recent study, it was identified that monoclonal antibody (CR3022) binds with the spike RBD of SARS-CoV-2. This is likely due to the antibody’s epitope not overlapping with the divergent ACE2 receptor-binding motif. CR3022 has the potential to be developed as a therapeutic candidate, alone or in combination with other neutralizing antibodies for the prevention and treatment of COVID-19 infection [55].
Vaccines for SARS-CoV-2
There is no available vaccine against COVID-19, while previous vaccines or strategies used to develop a vaccine against SARS-CoV can be effective. Recombinant protein from the Urbani (AY278741) strain of SARS-CoV was administered to mice and hamsters, resulted in the production of neutralizing antibodies and protection against SARS-CoV [56], [57]. The DNA fragment, inactivated whole virus or live-vectored strain of SARS-CoV (AY278741), significantly reduced the viral infection in various animal models [58], [59], [60], [61], [62], [63]. Different other strains of SARS-CoV were also used to produce inactivated or live-vectored vaccines which efficiently reduced the viral load in animal models. These strains include, Tor2 (AY274119) [64], [65], Utah (AY714217) [66], FRA (AY310120) [59], HKU-39849 (AY278491) [57], [67], BJ01 (AY278488) [68], [69], NS1 (AY508724) [70], ZJ01 (AY297028) [70], GD01 (AY278489) [69] and GZ50 (AY304495) [71]. However, there are few vaccines in the pipeline against SARS-CoV-2. The mRNA based vaccine prepared by the US National Institute of Allergy and Infectious Diseases against SARS-CoV-2 is under phase 1 trial [72]. INO-4800-DNA based vaccine will be soon available for human testing [73]. Chinese Centre for Disease Control and Prevention (CDC) working on the development of an inactivated virus vaccine [74], [75]. Soon mRNA based vaccine’s sample (prepared by Stermirna Therapeutics) will be available [76]. GeoVax-BravoVax is working to develop a Modified Vaccina Ankara (MVA) based vaccine [77]. While Clover Biopharmaceuticals is developing a recombinant 2019-nCoV S protein subunit-trimer based vaccine [78].
Although research teams all over the world are working to investigate the key features, pathogenesis and treatment options, it is deemed necessary to focus on competitive therapeutic options and cross-resistance of other vaccines. For instance, there is a possibility that vaccines for other diseases such as rubella or measles can create cross-resistance for SARS-CoV-2. This statement of cross-resistance is based on the observations that children in china were found less vulnerable to infection as compared to the elder population, while children are being largely vaccinated for measles in China.
Conclusion and perspective
The novel coronavirus originated from the Hunan seafood market at Wuhan, China where bats, snakes, raccoon dogs, palm civets, and other animals are sold, and rapidly spread up to 109 countries. The zoonotic source of SARS-CoV-2 is not confirmed, however, sequence-based analysis suggested bats as the key reservoir. DNA recombination was found to be involved at spike glycoprotein which assorted SARS-CoV (CoVZXC21 or CoVZC45) with the RBD of another Beta CoV, thus could be the reason for cross-species transmission and rapid infection. According to phylogenetic trees, SARS-CoV is closer to SARS-like bat CoVs. Until now, no promising clinical treatments or prevention strategies have been developed against human coronaviruses. However, the researchers are working to develop efficient therapeutic strategies to cope with the novel coronaviruses. Various broad-spectrum antivirals previously used against influenza, SARS and MERS coronaviruses have been evaluated either alone or in combinations to treat COVID-19 patients, mice models, and clinical isolates. Remdesivir, Lopinavir, Ritonavir, and Oseltamivir significantly blocked the COVID-19 infection in infected patients. It can be cocluded that the homologus recombination event at the S protein of RBD region enhanced the transmission ability of the virus. While the decision of bring back the nationals from infected area by various countries and poor screening of passengers, become the leading cause of spreading virus in others countries.
Most importantly, human coronaviruses targeting vaccines and antiviral drugs should be designed that could be used against the current as well as future epidemics. There are many companies working for the development of effective SARS-CoV-2 vaccines, such as Moderna Therapeutics, Inovio Pharmaceuticals, Novavax, Vir Biotechnology, Stermirna Therapeutics, Johnson & Johnson, VIDO-InterVac, GeoVax-BravoVax, Clover Biopharmaceuticals, CureVac, and Codagenix. But there is a need for rapid human and animal-based trails as these vaccines still require 3–10 months for commercialization. There must be a complete ban on utilizing wild animals and birds as a source of food. Beside the development of most efficient drug, a strategy to rapidly diagnose SARS-CoV-2 in suspected patient is also required. The signs and symptoms of SARS-CoV-2 induced COVID-19 are a bit similar to influenza and seasonal allergies (pollen allergies). Person suffering from influenza or seasonal allergy may also exhibit temprature which can be detected by thermo-scanners, hence the person will become suspected. Therefore, an accurate and rapid diagnostic kit or meter for detection of SARS-CoV-2 in suspected patients is required, as the PCR based testing is expensive and time consuming. Different teams of Chinese doctors should immediately sent to Eurpean and other countries, especially spain and Italy to control the over spread of COVID-19, because Chinese doctors have efficiently controlled the outbreak in china and limited the mortality rate to less than 3% only. The therapeutic strategies used by Chinese, should also be followed by other countries.
Acknowledgments
Acknowledgments
The authors acknowledge the Postdoctoral grant from The Second Affiliated Hospital of Zhengzhou University (for S.K).
Declaration of Competing Interest
The authors of this manuscript declare no conflict of interest.
Biographies
Muhammad Adnan Shereen is a PhD researcher at Wuhan University, working on Zika virus and coronavirus in the aspects of pathogenesis, drug screening and molecular mechanisms. He is an author in 8 articles published in journals with impact factor more than 5 including the recently accepted paper in Nature microbiology.
Suliman Khan has completed his PhD degree from Chinese Academy of Sciences and currently working at second affiliated hospital of Zhengzhou university as postdoctoral scientist. He has published more than 25 articles and 5 on SARS-CoV-2 in well reputed journals including Clinical microbiology and infection (CMI) and Journal of clinical microbiology (ASM-JCM) as first and corresponding author.
Abeer Kazmi is a PhD student at Wuhan University.
Nadia Bashir is a PhD student at Wuhan University working on coronaviruses. She is an author in more than 5 papers published or accepted in renowned journals.
Rabeea Siddique is a PhD student at Zhengzhou university. She has published more than 10 papers in well reputed journals as first or coauthor.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Zhong N., Zheng B., Li Y., Poon L., Xie Z., Chan K. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. The Lancet. 2003;362(9393):1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;105924 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization WH. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization, 2020.
- 6.Peiris J., Guan Y., Yuen K. Severe acute respiratory syndrome. Nat Med. 2004;10(12):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyrc K., Berkhout B., Van Der Hoek L. Identification of new human coronaviruses. Expert Review of Anti-infective Therapy. 2007;5(2):245–253. doi: 10.1586/14787210.5.2.245. [DOI] [PubMed] [Google Scholar]
- 8.Rahman A., Sarkar A. Risk factors for fatal middle east respiratory syndrome coronavirus infections in Saudi Arabia: analysis of the WHO Line List, 2013–2018. Am J Public Health. 2019;109(9):1288–1293. doi: 10.2105/AJPH.2019.305186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memish Z.A., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A.A., Stephens G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 10.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020 doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance. 2020;25(4). [DOI] [PMC free article] [PubMed]
- 13.Parry J. China coronavirus: cases surge as official admits human to human transmission. British Medical Journal Publishing Group; 2020. [DOI] [PubMed]
- 14.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kan B., Wang M., Jing H., Xu H., Jiang X., Yan M. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J Virol. 2005;79(18):11892–11900. doi: 10.1128/JVI.79.18.11892-11900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng B.J., Guan Y., Wong K.H., Zhou J., Wong K.L., Young B.W.Y. SARS-related virus predating SARS outbreak, Hong Kong. Emerg Infect Dis. 2004;10(2):176. doi: 10.3201/eid1002.030533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Z., Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008;133(1):74–87. doi: 10.1016/j.virusres.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paden C., Yusof M., Al Hammadi Z., Queen K., Tao Y., Eltahir Y. Zoonotic origin and transmission of Middle East respiratory syndrome coronavirus in the UAE. Zoonoses Public Health. 2018;65(3):322–333. doi: 10.1111/zph.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annan A., Baldwin H.J., Corman V.M., Klose S.M., Owusu M., Nkrumah E.E. Human betacoronavirus 2c EMC/2012–related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013;19(3):456. doi: 10.3201/eid1903.121503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huynh J., Li S., Yount B., Smith A., Sturges L., Olsen J.C. Evidence supporting a zoonotic origin of human coronavirus strain NL63. J Virol. 2012;86(23):12816–12825. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau S.K., Li K.S., Tsang A.K., Lam C.S., Ahmed S., Chen H. Genetic characterization of Betacoronavirus lineage C viruses in bats reveals marked sequence divergence in the spike protein of pipistrellus bat coronavirus HKU5 in Japanese pipistrelle: implications for the origin of the novel Middle East respiratory syndrome coronavirus. J Virol. 2013;87(15):8638–8650. doi: 10.1128/JVI.01055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting. Wuhan. Emerging Microbes & Infections. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3(6):e00473–e512. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glowacka I., Bertram S., Müller M.A., Allen P., Soilleux E., Pfefferle S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertram S., Glowacka I., Müller M.A., Lavender H., Gnirss K., Nehlmeier I. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J Virol. 2011;85(24):13363–13372. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;1–5 doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou P., Yang X., Wang X., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature [Internet]. 2020;Feb:3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Science China Life Sciences. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020 doi: 10.1002/jmv.26234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. International Journal of Infectious Diseases. 2020;91:264–6. [DOI] [PMC free article] [PubMed]
- 38.Li B, Si H-R, Zhu Y, Yang X-L, Anderson DE, Shi Z-L, et al. Discovery of Bat Coronaviruses through Surveillance and Probe Capture-Based Next-Generation Sequencing. mSphere. 2020;5(1). [DOI] [PMC free article] [PubMed]
- 39.Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;1–4 doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corman V.M., Ithete N.L., Richards L.R., Schoeman M.C., Preiser W., Drosten C. Rooting the phylogenetic tree of middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J Virol. 2014;88(19):11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv; 2020.
- 43.Gretebeck L.M., Subbarao K. Animal models for SARS and MERS coronaviruses. Cur Opin Virol. 2015;13:123–129. doi: 10.1016/j.coviro.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cockrell A.S., Peck K.M., Yount B.L., Agnihothram S.S., Scobey T., Curnes N.R. Mouse dipeptidyl peptidase 4 is not a functional receptor for Middle East respiratory syndrome coronavirus infection. J Virol. 2014;88(9):5195–5199. doi: 10.1128/JVI.03764-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagata N., Iwata N., Hasegawa H., Fukushi S., Yokoyama M., Harashima A. Participation of both host and virus factors in induction of severe acute respiratory syndrome (SARS) in F344 rats infected with SARS coronavirus. J Virol. 2007;81(4):1848–1857. doi: 10.1128/JVI.01967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. BioScience Trends. 2020 doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 48.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng C.S., Kasumba D.M., Fujita T., Luo H. Spatio-temporal characterization of the antiviral activity of the XRN1-DCP1/2 aggregation against cytoplasmic RNA viruses to prevent cell death. Cell Death Differ. 2020:1–20. doi: 10.1038/s41418-020-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang B.X., Fish E.N. Elsevier; 2019. editors. Global virus outbreaks: Interferons as 1st responders. Seminars in immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;1–3 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2):e00221–e318. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derebail V.K., Falk R.J. ANCA-associated vasculitis—refining therapy with plasma exchange and glucocorticoids. Mass Medical Soc. 2020 doi: 10.1056/NEJMe1917490. [DOI] [PubMed] [Google Scholar]
- 55.Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. bioRxiv; 2020. [DOI] [PMC free article] [PubMed]
- 56.Bisht H., Roberts A., Vogel L., Subbarao K., Moss B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology. 2005;334(2):160–165. doi: 10.1016/j.virol.2005.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kam Y.W., Kien F., Roberts A., Cheung Y.C., Lamirande E.W., Vogel L. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcγRII-dependent entry into B cells in vitro. Vaccine. 2007;25(4):729–740. doi: 10.1016/j.vaccine.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Z-y, Kong W-p, Huang Y., Roberts A., Murphy B.R., Subbarao K. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stadler K., Roberts A., Becker S., Vogel L., Eickmann M., Kolesnikova L. SARS vaccine protective in mice. Emerg Infect Dis. 2005;11(8):1312. doi: 10.3201/eid1108.041003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kapadia S.U., Rose J.K., Lamirande E., Vogel L., Subbarao K., Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340(2):174–182. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci. 2004;101(17):6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buchholz U.J., Bukreyev A., Yang L., Lamirande E.W., Murphy B.R., Subbarao K. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci. 2004;101(26):9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bukreyev A., Lamirande E.W., Buchholz U.J., Vogel L.N., Elkins W.R., St Claire M. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363(9427):2122–2127. doi: 10.1016/S0140-6736(04)16501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.See R.H., Zakhartchouk A.N., Petric M., Lawrence D.J., Mok C.P., Hogan R.J. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J Gen Virol. 2006;87(3):641–650. doi: 10.1099/vir.0.81579-0. [DOI] [PubMed] [Google Scholar]
- 65.Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004;78(22):12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spruth M., Kistner O., Savidis-Dacho H., Hitter E., Crowe B., Gerencer M. A double-inactivated whole virus candidate SARS coronavirus vaccine stimulates neutralising and protective antibody responses. Vaccine. 2006;24(5):652–661. doi: 10.1016/j.vaccine.2005.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takasuka N., Fujii H., Takahashi Y., Kasai M., Morikawa S., Itamura S. A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice. Int Immunol. 2004;16(10):1423–1430. doi: 10.1093/intimm/dxh143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang L., Zhu Q., Qin E., Yu M., Ding Z., Shi H. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol. 2004;23(6):391–394. doi: 10.1089/104454904323145272. [DOI] [PubMed] [Google Scholar]
- 69.Qin E., Shi H., Tang L., Wang C., Chang G., Ding Z. Immunogenicity and protective efficacy in monkeys of purified inactivated Vero-cell SARS vaccine. Vaccine. 2006;24(7):1028–1034. doi: 10.1016/j.vaccine.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou J., Wang W., Zhong Q., Hou W., Yang Z., Xiao S.-Y. Immunogenicity, safety, and protective efficacy of an inactivated SARS-associated coronavirus vaccine in rhesus monkeys. Vaccine. 2005;23(24):3202–3209. doi: 10.1016/j.vaccine.2004.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qu D., Zheng B., Yao X., Guan Y., Yuan Z.-H., Zhong N.-S. Intranasal immunization with inactivated SARS-CoV (SARS-associated coronavirus) induced local and serum antibodies in mice. Vaccine. 2005;23(7):924–931. doi: 10.1016/j.vaccine.2004.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McKay BLP. Drugmakers rush to develop vaccines against china virus the wall street journal. [cited 2020 28 January]; Available from: <https://www.wsj.com/articles/drugmakers-rush-to-develop-vaccines-against-china-virus-11579813026>.
- 73.Inovio IP. Inovio selected by cepi to develop vaccine against new coronavirus inovio. [cited 2020 29 January]; Available from: <http://ir.inovio.com/news-and-media/news/press-release-details/2020/Inovio-Selectedby-CEPI-to-Develop-Vaccine-Against-NewCoronavirus/default.aspx>.
- 74.J.-H.Z.W. Lee LZ. Chinese scientists race to develop vaccine as coronavirus death toll jumps: South china morning post. [cited 2020 29 January]; Available from: <https://www.scmp.com/news/china/society/article/3047676/numbercoronavirus-cases-china-doubles-spread-rate-accelerates>.
- 75.Cheung E. China coronavirus: Hong kong researchers have already developed vaccine but need time to test it, expert reveals: South china morning post. [cited 2020 29 January]; Available from: https://www.scmp.com/news/hongkong/health-environment/article/3047956/china-coronavirus-hong-kong-researchers-have.
- 76.Xinhua. China fast-tracks novel coronavirus vaccine development xinhua. [cited 202 29 January]; Available from: http://www.xinhuanet.com/english/2020-01/28/c_138739378.htm.
- 77.Geo-Vax. Geovax and bravovax (wuhan, china) to collaborate on development of coronavirus vaccine. [cited 2020 3 March]; Available from: https://www.geovax.com/news/geovax-and-bravovax-wuhan-china-to-collaborate-ondevelopment-of-coronavirus-vaccine.
- 78.Clover B. Clover initiates development of recombinant subunit- trimer vaccine for wuhan coronavirus (2019-ncov). [cited 2020 6 March]; Available from: http://www.cloverbiopharma.com/index.php?m=content&c=index&a=show&catid=11&id=40.
- 79.Huang Y. The SARS epidemic and its aftermath in China: a political perspective. Learning from SARS: Preparing for the next disease outbreak; 2004. p. 116–36.
- 80.Holmes K.V. SARS coronavirus: a new challenge for prevention and therapy. J Clin Investig. 2003;111(11):1605–1609. doi: 10.1172/JCI18819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perlman S. Another decade, another coronavirus. Mass Medical Soc. 2020 [Google Scholar]
- 83.Bolles M., Donaldson E., Baric R. SARS-CoV and emergent coronaviruses: viral determinants of interspecies transmission. Current Opin Virol. 2011;1(6):624–634. doi: 10.1016/j.coviro.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vara V. Coronavirus outbreak: The countries affected. 11 MARCH 2020; Available from: https://www.pharmaceutical-technology.com/features/coronavirus-outbreak-the-countries-affected/.
- 85.Shi Y., Yi Y., Li P., Kuang T., Li L., Dong M. Diagnosis of severe acute respiratory syndrome (SARS) by detection of SARS coronavirus nucleocapsid antibodies in an antigen-capturing enzyme-linked immunosorbent assay. J Clin Microbiol. 2003;41(12):5781–5782. doi: 10.1128/JCM.41.12.5781-5782.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dong N, Yang X, Ye L, Chen K, Chan EW-C, Yang M, et al. Genomic and protein structure modelling analysis depicts the origin and infectivity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. bioRxiv. 2020.