Graphical abstract
Keywords: Blood mRNA, Faecal immunochemical test, CEACAM6, LGALS4, TSPAN8, COL1A2
Abstract
Prevention is essential to reduce Colorectal Cancer (CRC) mortality. We previously reported a panel of four genes: CEACAM6, LGALS4, TSPAN8, COL1A2 (CELTiC) able to discriminate patients with CRC. Here, we assessed the CELTiC panel by quantitative polymerase chain reaction, in the blood of 174 healthy subjects, who resulted negative to the faecal immunochemical test (FITN). Using non-parametric statistic and multinomial logistic models, the FITN were compared to previously analysed subjects: 36 false positive FIT (NFIT), who were negative at colonoscopy, 36 patients with low risk lesions (LR) and 92 patients with high risk lesions or CRC (HR/CRC). FITN showed a significantly lower expression of the four genes when compared to HR/CRC. Moreover, FITN showed a significantly lower expression of TSPAN8 and COL1A2 compared to NFIT and LR patients.
The multinomial logistic model confirmed that TSPAN8 alone specifically discriminated FITN from NFIT, LR and HR/CRC, while LGALS4 was able to differentiate FITN from false positive FIT. Finally, ROC curves analysis of the comparisons between FITN and HR/CRC, LR or NFIT reported AUC greater than 0.87, with a sensitivity and specificity of 83% and 76%, respectively. The CELTiC panel was confirmed a useful tool to identify CRC patients and to discriminate false FIT positive subjects.
Introduction
Secondary prevention dealing with the early diagnosis of the biological onset of the disease, before the clinical manifestations, is a powerful weapon to overcome cancer. Therefore, secondary prevention is often implemented through organized screening programs. Already in 1968, for diseases involving large portions of the population, the World Health Organization (WHO) established universal criteria for screening, based on acceptability [1].
Colorectal cancer (CRC) is the third cancer for worldwide incidence, with 1.8 million new cases in 2018, and the second for cancer mortality with 880,000 deaths [2], [3]. Most cases of CRC develop over many years, through multiple steps of systematic selection of genetic alterations that drive the transformation from normal tissue to carcinoma. Thus, secondary prevention with the early detection and screening proves particularly apt for this disease. The main screening test adopted for colorectal cancer is the faecal occult blood test. This test detects haemoglobin by immunochemical antibody-based assay (FIT) to human globin, or by guaiac colorimetry (FOBT) to haem. Nowadays, FIT is the most used colorectal cancer screening test worldwide [4], [5].
In Italy, over the last 10 years, the faecal occult blood test has been used as the screening test. In 2017, over 6 million citizens were invited, 75% of them were aged between 50 and 69, the main target population for the screening. The total participation stands at 42%, ranging from the 53% in Northern Italy to the 24% in the South [6], [7]. When FIT is positive, colonoscopy is performed for the final diagnosis. As predicted, the screening programs have also yielded an increase in survival rates and a decrease in incidence and mortality for CRC [8], [9]. However, incidence and mortality remain high, and the methods used for the screening and CRC diagnosis present some disadvantages. FIT has good sensitivity (79%) and specificity (94%) to detect CRC, but the sensitivity to detect early adenomas is low (30–50%) and it is not able to detect polyps [1], [8], [10]. Moreover, a number of false positive results can affect National Health Care costs and produce anxiety for patients [10], [11], [12]. Colonoscopy presents high sensitivity and specificity also in detecting adenomas and polyps. However, colonoscopy is an expensive and invasive procedure with possible complications for patients. New alternatives and non-invasive techniques are desirable to convince more people to participate in screening programs [8], [10] and to possibly further decrease the mortality for CRC.
Liquid biopsy refers to the analysis of tumour-derived biomarkers detected in biological fluids of cancer patients [13]. Among the possible biological fluids, peripheral blood is one of the most studied. It is generally recognised that a blood sample offers many advantages, in particular, the minimally invasive procedure and the possibility to describe a more comprehensive molecular map of the disease. In our previous study [14], using bioinformatics analysis, we identified a panel of four mRNAs as promising biomarkers of CRC in whole blood samples, namely carcinoembryonic antigen-related cell-adhesion molecule 6 (CEACAM6), lectin galactoside-binding soluble 4 (LGALS4), tetraspanin 8 (TSPAN8) and collagen type I alpha 2 chain (COL1A2). CEACAM6 is a glycosylphosphatidylinositol (GPI)-anchored cell surface glycoprotein with a role in cell adhesion. It is also a tumor marker in serum immunoassay determination of CRC carcinoma [15], [16]. LGALS4 is a β-galactoside binding protein, with a role as microvillar lipid raft stabilizer/organizer [17]. It is expressed specifically in the small intestine, colon and rectum and it is involved in cancer cell invasion [18]. TSPAN8 is a multipass membrane glycoprotein acting as “molecular facilitator”, forming a web of glycolipid-enriched membrane microdomains called TEM (tetraspanin-enriched-microdomains). It is involved in the regulation of cell development, activation and motility, by promoting angiogenesis and it was found as component of the exosomes [19], [20]. Finally, COL1A2 is the most abundant collagen in the human body that interacts with other matrix proteins and anchors cells to the extracellular matrix. It is necessary for angiogenesis and it was reported de-regulated in cancer [21]. The panel, named with the acronym CELTiC, was subsequently tested on 101 FIT-positive subjects scheduled for colonoscopy yielding promising results to distinguish among healthy subjects (N), patients with low risk lesion (polyps) (LR) and patients with high risk lesions (advanced adenomas or CRC) (HR/CRC) [22].
The aim of the present study was to evaluate the expression of the CELTiC panel in blood samples of controlled healthy subjects, who resulted negative to the FIT (FITN). In the same group, we also evaluated the influences of gender and age on the level of expression of the CELTiC panel. In addition, the calculated expression values were compared to the groups of CRC and high-risk subjects (HR), low risk subjects (LR) and false positive FIT subjects (NFIT). These data were available from our previous work [14], [22] and confirmed the power of the CELTiC panel for the early diagnosis of CRC, also when the comparison is performed with the negative FIT subjects (FITN), collected in this study.
Materials and methods
Population
In the present study 174 peripheral blood samples (1 mL) were collected from subjects who resulted negative to FIT evaluation (FITN) at the S. Antonio Laboratory for Clinical Analysis, Bologna (Italy), from April to July 2018. The subjects were healthy asymptomatic people aged from 50 to 70 years old resulting negative at the FIT screening program of the Emilia Romagna region in the last 2 years and recruited to participate in a volunteer campaign of the University of Bologna. Healthy FITN subjects were asked to fill a questionnaire on the presence of any clinical signs related to the digestive tract. People reporting clinical signs were excluded from the healthy FITN group.
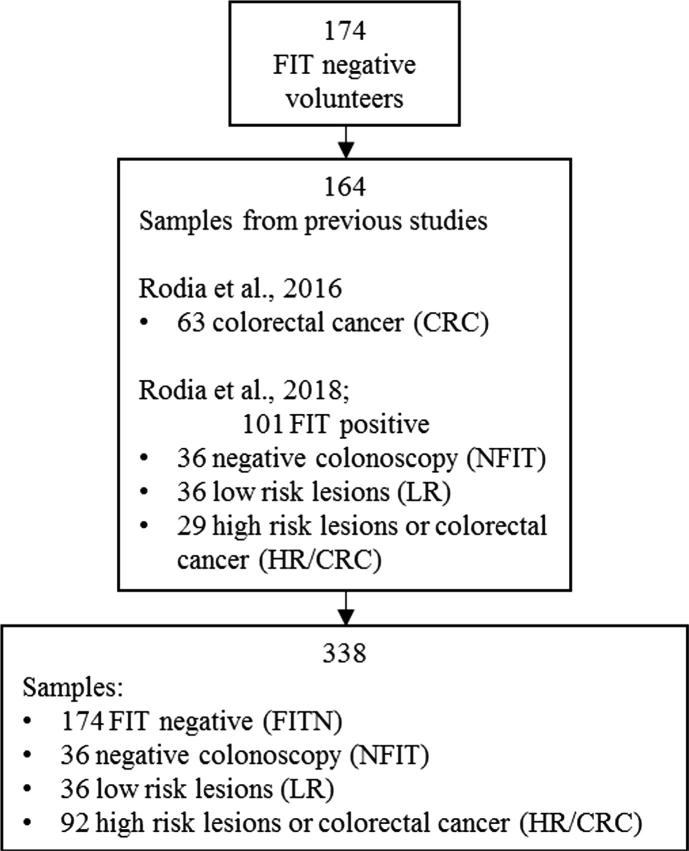
This is a cross-sectional study and different samples of subjects with specific characteristics were evaluated. For the subsequent comparison with patients and statistical analysis, the results obtained from 164 samples collected in our previous studies [14], [22] were included, divided into 3 groups: 36 false positive FIT detected by negative-colonoscopy (NFIT), 36 low risk lesions (LR), 92 high risk lesions or full-blown colorectal cancers (HR/CRC).
The study was conducted after approval by the ethical committee of the Sant’Orsola - Malpighi Hospital, Bologna (155/2007/U/sper approved 22/01/2008; EM 120/2016/U approved 14/06/2016).
All the participants gave written informed consent to the participation in the study.
All the procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the ethical principles for medical research involving human subjects of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
RNA extraction
Whole blood samples were used to extract RNA as previously reported [14], [22]. The blood was collected in EDTA tubes and lysed within 1 h of collection. In brief, 1 mL of whole blood was diluted with diethyl pyrocarbonate (DEPC) water (1:2 ratio), lysed with TRIzol® LS (Liquid Samples) reagent, (cat. 10296010, Invitrogen, Carlsbad, CA), and total RNA was extracted according to the manufacturer’s protocol. Subsequently, standard ethanol precipitation was applied to the total extracted RNA, the pellet dissolved in 20 µL RNase-free water and stored at −20 °C. The quality and the quantity of all RNA samples were checked and quantified by a Nanodrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and the integrity tested by agarose gel electrophoresis. A 260/280 nm ratio greater than 1.8 and the presence on a 1% agarose gel of two clear bands, corresponding to the 28S and 18S subunits, with no sign of smear, were considered acceptable.
Reverse transcription and qRT-PCR assay
For each sample, 300 ng of total RNA was reverse transcribed with the RevertAid RT Reverse Transcription kit (cat. K1691, Thermo Fisher Scientific TM, Waltham, MA, USA) and amplified using the iTaq universal SYBR Green Supermix (cat. 1725122, Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions.
Real-time PCRs were performed with the CFX96 instrument (Bio-Rad) in duplicate, at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min, with melting curve analysis. Each qPCR run included a negative control without the cDNA template and a positive control of cDNA derived from the HT-29 cell line, in which all the tested genes are expressed. Primer sequences and the calibration test have been previously described [14]. Expression values of the four markers of the CELTiC panel were measured as ΔCq (Quantification Cycle) after normalization on the beta 2 microglobulin (B2M; NCBI reference sequence: NM_004048) housekeeping gene expression. We selected B2M as the housekeeping gene upon a comprehensive meta-analysis performed in our previous study and according to the literature [14], [23]
Technical variability (imprecision)
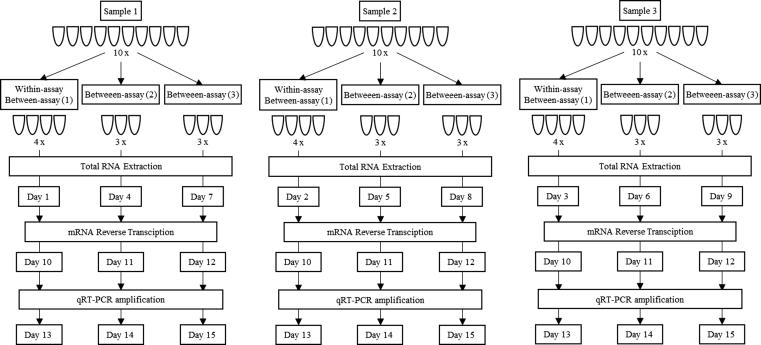
Three samples were selected to calculate the within-assay (repeatability) and between-assay (intermediate precision) variability of the CELTiC panel, each divided into ten technical replicates. For each sample, total RNA from four aliquots (replicates) was extracted in one day to calculate within-assay variability. From the other six aliquots, total RNA was extracted on two different days (three replicates per day). For technical reasons, it was not possible to perform the entire protocol in one day; therefore, each step of the protocol (extraction, reverse-transcription and qPCR) for each sample was performed on different days (Fig. 1). Within-assay and between-assay Coefficients of Variation (CVs) were calculated with the following formula:
Fig. 1.
Graphical representation of the technical variability assay. Three samples were selected. Each sample was divided into 10 aliquots (replicates). For each sample, four aliquots (replicates) were extracted in one day to calculate within-assay variability. The other six aliquots were extracted on two different days (three replicates per day). After extraction of all the replicates of each sample, total RNA was reverse-transcribed and cDNA amplified on different days.
Statistical analysis
The mean, median, standard deviation, range (minimum - maximum), and frequency were reported as descriptive statistics. Within FITN, Kruskal-Wallis rank sum test was performed to evaluate if differences were present for sex (M; F) and/or age (50–59; 60–70) on the expression of each marker of the CELTiC panel.
The Kruskal-Wallis rank sum test was also applied to compare each marker’s expression among all groups (FITN, NFIT, LR, HR/CRC); adjusted p-values were calculated for multiple comparison and p-values lower than 0.05 were considered statistically significant. Pearson correlation coefficients (r) among the four markers were also reported with their p-values.
A multinomial logistic regression model was estimated to study the relationship between the outcomes and a linear combination of the proposed markers adding age and sex to the model; the output for this model was reported using FITN as reference group.
Logistic regression models were estimated and the receiving operating characteristic (ROC) curve analysis was reported to assess the accuracy of these models in discriminating among different combinations of the four groups of subjects. The area under the curve (AUC) is reported together with the relative optimal values of sensitivity and specificity.
STATA, version 14.0, and RStudio, version 1.0.143, were used to perform statistical analyses.
Results
Study population
To assess the efficacy of the CELTiC panel, the data of 174 subjects aged from 50 to 70 years old, negative at the FIT screening program in the last 2 years, were analysed and compared to the results obtained from the samples of our previous studies [14], [22] (Fig. 2). Of note, the ΔCq values inversely correlate with the amount of gene expression. Interestingly, a different expression by gender was unveiled in FITN (Table 1). In fact, FITN subjects display statistically significant different values for CEACAM6 and COL1A2 in male (13.5 ± 1.2; 11.1 ± 1.4) compared to female (14.1 ± 1.1; 11.7 ± 1.5) (p = 0.002; p = 0.04). Notably, CEACAM6 appeared less expressed (higher Cq values) in the older female group (60–70 y.o.) compared to the older male (14.1 ± 0.9 vs 13.3 ± 1.3; p = 0.04).
Fig. 2.
Enrollment and outcomes. Study plan describing admission of 174 subjects with negative fecal immunochemical test (FITN). The distribution of cases of 164 samples collected in our previous studies [14], [22] is also reported.
Table 1.
FIT negative group divided by sex (F, M) and age groups (50–59, 60–70): means and standard deviation of quantification cycles (ΔCq ± SD) of the CELTiC panel.
|
CEACAM6 |
LGALS4 |
TSPAN8 |
COL1A2 |
||
|---|---|---|---|---|---|
| ΔCq ± SD | ΔCq ± SD | ΔCq ± SD | ΔCq ± SD | ||
| FITN | n = 174 | 13.8 ± 1.2 | 15.2 ± 0.7 | 11.6 ± 1.4 | 11.4 ± 1.5 |
| M | n = 82 | 13.5 ± 1.2* | 15.2 ± 0.7 | 11.4 ± 1.3 | 11.1 ± 1.4* |
| F | n = 92 | 14.1 ± 1.1* | 15.2 ± 0.7 | 11.8 ± 1.5 | 11.7 ± 1.5* |
| 50–59 | n = 106 | 13.9 ± 1.2 | 15.1 ± 0.7 | 11.6 ± 1.4 | 11.4 ± 1.6 |
| 60–70 | n = 68 | 13.7 ± 1.2 | 15.2 ± 0.7 | 11.6 ± 1.3 | 11.4 ± 1.4 |
| M_50-59 | n = 50 | 13.7 ± 1.2 | 15.1 ± 0.7 | 11.4 ± 1.4 | 11.3 ± 1.5 |
| F_50-59 | n = 56 | 14.1 ± 1.2 | 15.2 ± 0.7 | 11.8 ± 1.5 | 11.6 ± 1.6 |
| M_60-70 | n = 32 | 13.3 ± 1.3‡ | 15.2 ± 0.8 | 11.3 ± 1.9 | 11.0 ± 1.2 |
| F_60-70 | n = 36 | 14.1 ± 0.9‡ | 15.3 ± 0.7 | 11.9 ± 1.4 | 11.9 ± 1.4 |
CEACAM6, carcinoembryonic antigen-related cell-adhesion molecule 6; LGALS4, lectin galactoside-binding soluble 4; TSPAN8, tetraspanin 8; COL1A2, collagen type I alpha 2 chain; ΔCq, mean quantification cycles after normalisation on reference gene; SD, standard deviation. FITN, faecal immunochemical test negative; F, females, M, males; 50–59, people aged between 50 and 59 years old; 60–70, people aged between 60 and 70 years old. * indicates a significant difference between males and females (p value < 0.05); ‡ indicates a significant difference between older males and older females (p value < 0.05).
Technical variability
To evaluate the technical variability of the protocol and further confirm the robustness of the proposed panel of biomarkers, we determined the within and between coefficients of variations as measures of the repeatability (within-assay variability, replicates of the same sample analysed during the same run and the same day) and intermediate precision (between-assay variability, replicates of the same sample analysed during different runs in different days), respectively, as required for the clinical diagnostic assay [24], [25]. The procedures of the assay are summarized in Fig. 1, while Table 2 shows the data for within and between assays (CV % for Cq and ΔCq). The CELTiC panel showed high repeatability and precision with low CVs for both the within and between-run. Focusing on the ΔCq, in the within-assay analysis, CEACAM6 reported an overall mean CV of 1.6%, LGALS4 of 2.1%, TSPAN8 of 4.8% and COL1A2 of 4.7%. The between-assay evaluation showed 1.2% for CEACAM6, 1.3% for LGALS4, 7.7% for TSPAN8, and 8.9% for COL1A2. Further confirming the precision of the method, the within-assay and between-assay CVs were lower than the overall biologic CVs for FITN, (CEACAM6, 8.5%; LGALS4, 4.6%; TSPAN8, 12.1%; COL1A2, 13.2%) considered as a measure of the biological variability of the group.
Table 2.
Within-assay and between-assay coefficient of variations (CV %) of the four markers of the CELTiC panel for the three samples analysed.
| WITHIN-ASSAY |
BETWEEN-ASSAY |
WITHIN-ASSAY |
BETWEEN-ASSAY |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cq | SD | CV % | Cq | SD | CV % | ΔCq | SD | CV % | ΔCq | SD | CV % | |
| Sample 1 | ||||||||||||
| B2M | 18.2 | 0.1 | 0.3 | 17.8 | 0.3 | 1.5 | ||||||
| CEACAM6 | 33.7 | 0.4 | 1.3 | 33.5 | 0.3 | 0.9 | 15.5 | 0.4 | 2.4 | 15.6 | 0.2 | 1.3 |
| LGALS4 | 33.6 | 0.7 | 2.0 | 33.3 | 0.4 | 1.1 | 15.5 | 0.6 | 4.0 | 15.5 | 0.2 | 1.4 |
| TSPAN8 | 29.0 | 1.4 | 4.8 | 29.0 | 1.4 | 4.8 | 10.0 | 0.2 | 1.6 | 10.9 | 1.4 | 13.2 |
| COL1A2 | 29.4 | 0.4 | 1.5 | 29.2 | 1.3 | 4.6 | 10.3 | 0.3 | 3.4 | 11.0 | 1.3 | 12.2 |
| Sample 2 | ||||||||||||
| B2M | 17.1 | 0.1 | 0.8 | 17.0 | 0.2 | 1.4 | ||||||
| CEACAM6 | 33.1 | 0.1 | 0.3 | 32.9 | 0.5 | 1.4 | 15.9 | 0.1 | 0.8 | 15.9 | 0.2 | 1.5 |
| LGALS4 | 33.1 | 0.2 | 0.7 | 32.7 | 0.4 | 1.2 | 15.9 | 0.3 | 2.1 | 15.7 | 0.1 | 0.9 |
| TSPAN8 | 28.1 | 0.9 | 3.0 | 27.6 | 0.5 | 1.8 | 10.9 | 0.8 | 7.5 | 10.6 | 0.3 | 2.5 |
| COL1A2 | 28.2 | 0.1 | 0.4 | 27.8 | 0.5 | 1.8 | 11.7 | 0.8 | 6.8 | 11.1 | 0.5 | 4.6 |
| Sample 3 | ||||||||||||
| B2M | 17.1 | 0.2 | 1.0 | 17.1 | 0.1 | 0.6 | ||||||
| CEACAM6 | 30.8 | 0.3 | 1.0 | 31.1 | 0.2 | 0.6 | 13.8 | 0.2 | 1.6 | 13.9 | 0.1 | 0.8 |
| LGALS4 | 31.8 | 0.2 | 0.5 | 32.0 | 0.2 | 0.6 | 14.7 | 0.0 | 0.3 | 14.8 | 0.2 | 1.5 |
| TSPAN8 | 28.7 | 0.6 | 2.0 | 29.2 | 0.9 | 2.9 | 11.6 | 0.6 | 5.2 | 12.1 | 0.9 | 7.5 |
| COL1A2 | 28.7 | 0.3 | 1.0 | 29.6 | 1.2 | 4.0 | 11.6 | 0.4 | 3.9 | 12.4 | 1.2 | 9.9 |
| Mean values | ||||||||||||
| B2M | 17.5 | 0.1 | 0.7 | 17.3 | 0.2 | 1.2 | ||||||
| CEACAM6 | 32.5 | 0.3 | 0.8 | 32.5 | 0.3 | 1.0 | 15.1 | 0.2 | 1.6 | 15.2 | 0.2 | 1.2 |
| LGALS4 | 32.8 | 0.3 | 1.0 | 32.7 | 0.3 | 0.9 | 15.4 | 0.3 | 2.1 | 15.4 | 0.2 | 1.3 |
| TSPAN8 | 28.6 | 1.0 | 3.4 | 28.6 | 0.9 | 3.2 | 10.9 | 0.5 | 4.8 | 11.2 | 0.9 | 7.7 |
| COL1A2 | 28.8 | 0.3 | 1.0 | 28.9 | 1.0 | 3.5 | 11.2 | 0.5 | 4.7 | 11.5 | 1.0 | 8.9 |
B2M, beta 2 microglobulin; CEACAM6, carcinoembryonic antigen-related cell-adhesion molecule 6; LGALS4, lectin galactoside-binding soluble 4; TSPAN8, tetraspanin 8; COL1A2, collagen type I alpha 2 chain; ΔCq, mean quantification cycles after normalisation on housekeeping gene B2M; SD, standard deviation.
Descriptive statistics and comparisons among FITN, NFIT, LR and HR/CRC
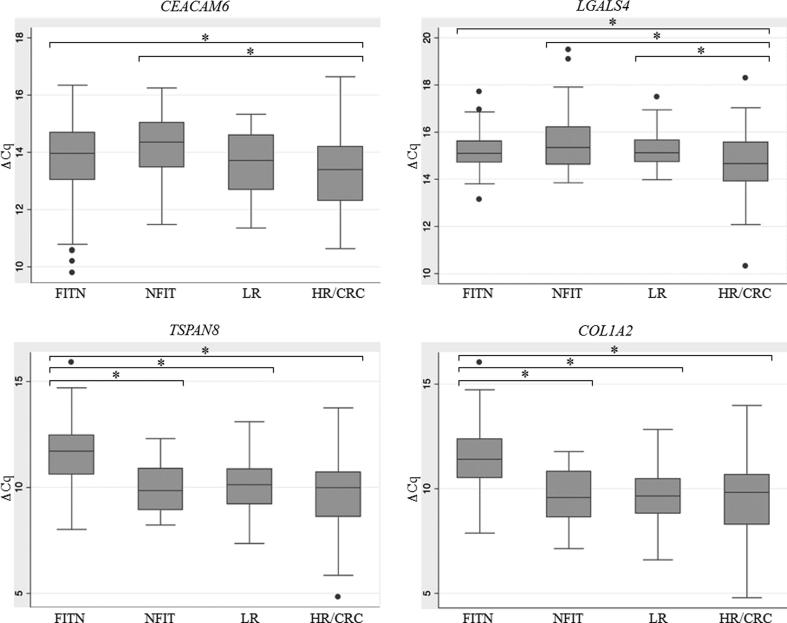
The comparison of the expression values of the CELTiC panel was performed by including the data of the 164 samples collected in our previous studies [14], [22] for a total of 338 subjects. The box-plot distributions of ΔCq for each marker of the CELTiC panel are reported in Fig. 3. Every marker of the CELTiC panel displayed a statistically significant different expression in FIT negative subjects (CEACAM6, 13.8 ± 1.2; LGALS4, 15.2 ± 0.7; TSPAN8, 11.6 ± 1.4; COL1A2, 11.4 ± 1.5) compared to the HR/CRC patients (CEACAM6, 13.3 ± 1.2; LGALS4, 14.7 ± 1.3; TSPAN8, 9.6 ± 1.9; COL1A2, 9.6 ± 2.0) (Table 3 and Fig. 3). Interestingly, TSPAN8 and COL1A2 expression levels were also able to distinguish FIT negative subjects from both false positive FIT (Fig. 3) (TSPAN8, 10.0 ± 1.2; COL1A2, 9.7 ± 1.3, respectively) and low risk patients (Fig. 3) (TSPAN8, 9.9 ± 1.4; COL1A2, 9.7 ± 1.4, respectively) (p < 0.001) (Table 3). Moreover, CEACAM6 showed significant different expression in false positive FIT (14.2 ± 1.1) respect to HR/CRC subjects (Fig. 3) and LGALS4 expression was significantly different in HR/CRC when compared to both false positive FIT (15.7 ± 1.3) and low risk subjects (15.3 ± 0.8) (p < 0.001) (Fig. 3, Table 3).
Fig. 3.
Box-plot of the quantification cycles (ΔCq), normalised on the housekeeping gene, of the CELTiC markers for the four groups analysed. CEACAM6, carcinoembryonic antigen-related cell-adhesion molecule 6; LGALS4, lectin galactoside-binding soluble 4; TSPAN8, tetraspanin 8; COL1A2, collagen type I alpha 2 chain. FITN, healthy FIT negative; NFIT, negative-colonoscopy FIT-positive; LR, low risk; HR/CRC, high risk/colorectal cancer. * indicate significant difference between groups (p < 0.05).
Table 3.
Descriptive statistic and Kruskal-Wallis rank sum test (adjusted p values) between groups. The distribution of age and sex for each group is also reported.
| FITN |
NFIT |
LR |
HR/CRC |
FITN vs NFIT | FITN vs LR | FITN vs HR/CRC | |
|---|---|---|---|---|---|---|---|
| ΔCq ± SD |
ΔCq ± SD |
ΔCq ± SD |
ΔCq ± SD |
||||
| N = 174 | N = 36 | N = 36 | N = 92 | ||||
| CEACAM6 | 13.8 ± 1.2 | 14.2 ± 1.1 | 13.6 ± 1.2 | 13.3 ± 1.2 | 0.365 | 1.000 | 0.008 |
| median | 14.0 | 14.4 | 13.7 | 13.4 | |||
| min | 9.8 | 11.5 | 11.4 | 10.6 | |||
| max | 16.3 | 16.2 | 15.3 | 16.6 | |||
| LGALS4 | 15.2 ± 0.7 | 15.7 ± 1.3 | 15.3 ± 0.8 | 14.7 ± 1.3 | 0.742 | 1.000 | <0.001 |
| median | 15.1 | 15.4 | 15.1 | 14.7 | |||
| min | 13.1 | 13.8 | 14.0 | 10.3 | |||
| max | 17.7 | 19.5 | 17.5 | 18.3 | |||
| TSPAN8 | 11.6 ± 1.4 | 10.0 ± 1.2 | 9.9 ± 1.4 | 9.6 ± 1.9 | <0.001 | <0.001 | <0.001 |
| median | 11.7 | 9.8 | 10.1 | 10.0 | |||
| min | 8.0 | 8.2 | 7.4 | 4.8 | |||
| max | 15.9 | 12.3 | 13.1 | 13.8 | |||
| COL1A2 | 11.4 ± 1.5 | 9.7 ± 1.3 | 9.7 ± 1.4 | 9.6 ± 2.0 | <0.001 | <0.001 | <0.001 |
| median | 11.4 | 9.6 | 9.7 | 9.8 | |||
| min | 7.9 | 7.1 | 6.6 | 4.8 | |||
| max | 16.0 | 11.8 | 12.8 | 14.0 | |||
| Sex n (%) | |||||||
| Male | 82 (47.1) | 10 (27.8) | 19 (52.8) | 48 (52.2) | |||
| Female | 92 (52.9) | 26 (72.2) | 17 (47.2) | 44 (47.8) | |||
| Age n (%) | |||||||
| 50–59 | 106 (60.9) | 19 (52.8) | 10 (27.8) | 23 (25.0) | |||
| 60–70 | 68 (39.1) | 17 (47.2) | 26 (72.2) | 42 (45.7) | |||
| >70 | 27 (29.3) |
CEACAM6, carcinoembryonic antigen-related cell-adhesion molecule 6; LGALS4, lectin galactoside-binding soluble 4; TSPAN8, tetraspanin 8; COL1A2, collagen type I alpha 2 chain; ΔCq, mean quantification cycles after normalisation on reference gene; SD, standard deviation. FITN, faecal immunochemical test negative; NFIT, negative colonoscopy positive faecal immunochemical test; LR, low risk; HR/CRC, high risk - colorectal carcinoma.
Multinomial logit model
Before estimating the Multinomial Logit Model, the pairwise correlation among CELTiC biomarkers was computed (Table 4). TSPAN8 and COL1A2 showed a high correlation (r = 0.96; p < 0.001). We decided to omit COL1A2 from the regression model given that there is a high correlation between this marker and TSPAN8. Thus, in order to prevent collinearity problems, the multinomial logistic regression model considered only CEACAM6, LGALS4, TSPAN8, age and sex as independent variables.
Table 4.
CELTiC markers correlations coefficients (r).
|
CEACAM6 |
LGALS4 |
TSPAN8 |
COL1A2 |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | r | p value | |
| CEACAM6 | 1 | |||||||
| LGALS4 | 0.1799 | 0.0009 | 1 | |||||
| TSPAN8 | 0.1777 | 0.001 | 0.3054 | 0 | 1 | |||
| COL1A2 | 0.1872 | 0.0005 | 0.2663 | 0 | 0.9583 | 0 | 1 | |
CEACAM6, carcinoembryonic antigen-related cell-adhesion molecule 6; LGALS4, lectin galactoside-binding soluble 4; TSPAN8, tetraspanin 8; COL1A2, collagen type I alpha 2 chain
The multinomial logistic regression model is reported in Table 5 with FIT negative subjects as the reference group. Comparing FITN with HR/CRC, TSPAN8 is the only marker that reports a statistically significant result with an odds ratio (OR) of 0.37 (p < 0.001), in accordance to the Kruskal-Wallis analysis reported in Table 3. The same holds when comparing FITN with LR (OR = 0.39 and p < 0.001). Comparing FITN with false positive FIT subjects (NFIT), in addition to TSPAN8 (OR = 0.37 and p < 0.001) also LGALS4 provides an important role with an OR of 2.16 (p = 0.001). No discriminating capabilities were detected for CEACAM6, in any comparison setting. It is interesting to note the effect of sex in the comparison between FITN and NFIT (OR = 0.28 and p = 0.008), while age was significant comparing FITN with LR (OR = 1.11 and p < 0.001) or with HR/CRC patients (OR = 1.18 and p < 0.001). In fact, the OR highlights reduced risk for males to be classified as NFIT respect to females and a greater risk for older people to be affected by LR or HR/CRC lesions.
Table 5.
Multinomial logit models.
| FITN vs NFIT | OR | p value | 95% CI |
|---|---|---|---|
| Age | 1.05 | 0.093 | 0.99 to 1.12 |
| Sex | 0.28* | 0.008 | 0.11 to 0.72 |
| TSPAN8 | 0.37 | 0.000 | 0.26 to 0.51 |
| LGALS4 | 2.16 | 0.001 | 1.38 to 3.36 |
| CEACAM6 | 1.35 | 0.142 | 0.90 to 2.01 |
| FITN vs LR | OR | p value | 95% CI |
| Age | 1.11 | 0.000 | 1.05 to 1.18 |
| Sex | 0.52* | 0.139 | 0.22 to 1.24 |
| TSPAN8 | 0.39 | 0.000 | 0.28 to 0.53 |
| LGALS4 | 1.47 | 0.099 | 0.93 to 2.34 |
| CEACAM6 | 0.93 | 0.666 | 0.65 to 1.31 |
| FITN vs HR/CRC | OR | p value | 95% CI |
| Age | 1.18 | 0.000 | 1.12–1.24 |
| Sex | 0.64* | 0.221 | 0.31 to 1.31 |
| TSPAN8 | 0.37 | 0.000 | 0.28 to 0.49 |
| LGALS4 | 0.87 | 0.502 | 0.58 to 1.30 |
| CEACAM6 | 0.94 | 0.687 | 0.71 to 1.26 |
CEACAM6, carcinoembryonic antigen-related cell-adhesion molecule 6; LGALS4, lectin galactoside-binding soluble 4; TSPAN8, tetraspanin 8; OR, odds ratio; CI confidence intervals. FITN, faecal immunochemical test negative; NFIT, negative colonoscopy positive faecal immunochemical test; LR, low risk; HR/CRC, high risk - colorectal carcinoma.
*The reference group for sex is female.
Diagnostic values of CELTiC
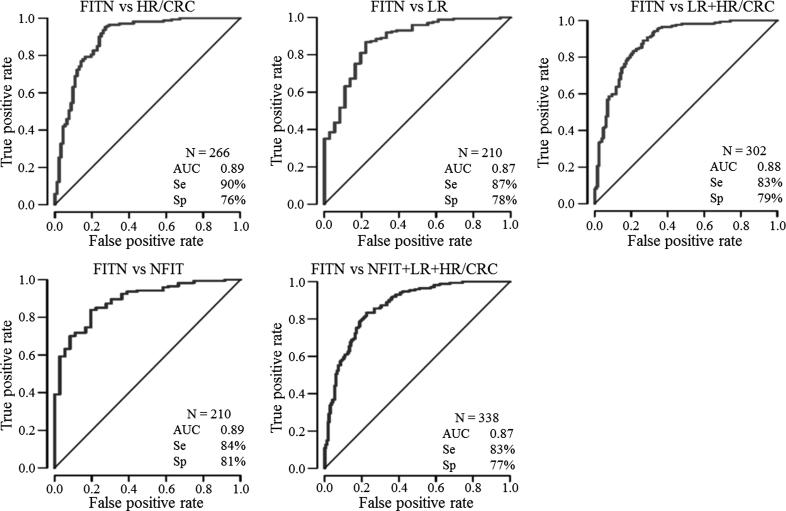
Next, we performed the ROC curve analysis to evaluate the diagnostic accuracy of the CELTiC panel by calculating the sensitivity and specificity for the optimal cut-point (Fig. 4): simple two groups comparisons were considered together with several aggregations of all groups with the exception of FITN. Comparing FIT negative subjects to HR/CRC or LR groups, CELTiC reached AUCs of 0.89 and 0.87 with sensitivities of 90% and 87% and specificities of 76% and 78%, respectively. When the comparison was performed between FITN and the two groups combined (LR with HR/CRC) the performances were still high, with an AUC of 0.88, a sensitivity of 83% and a specificity of 79%. The comparison between FITN and false positive FIT reached an AUC of 0.89 with a sensitivity of 84% and a specificity of 81%. The overall performances of the CELTiC panel comparing FIT negative subject to false positive FIT, LR and HR/CC showed an AUC of 0.87 with a sensitivity of 83% and a specificity of 77%. All these results confirm the diagnostic potential of the studied panel, extending the possibility to discriminate also LR and false positive FIT.
Fig. 4.
ROC curves of the CELTiC panel for the comparisons of FITN vs the other groups. AUC, area under curve; Se, sensitivity; Sp, specificity; FITN, healthy FIT negative; NFIT, negative-colonoscopy FIT-positive; LR, low risk; HR/CRC, high risk/colorectal cancer.
Discussion
The use of blood biomarkers in CRC screening is still limited, probably due to the low level of standardization of the new tests proposed and the limited number of subjects included [13], even though blood-based tumour biomarker analyses are appealing and widely pursued due to their promising role in diagnosis, malignant evaluation and prognosis prediction [13], [26]. Over the years, several markers have been proposed for patients affected by CRC as a possible diagnostic tool, including cell-free and circulating tumour DNA, mRNA, micro- and long-non-coding-RNA, circulating tumour cells, cancer stem cells, as well as extracellular vesicles, proteomes and metabolomes [13], [26], [27], [28]. This has led to the implementation of panels of biomarkers to detect CRC and/or its early stages starting from blood samples.
In the present study, to continue and validate our approach based on the blood CELTiC test, designed to implement colorectal cancer screening, we introduce a standardized healthy reference group that is essential for a correct comparison with pathological cases. Of note, it is not possible to perform colonoscopy on healthy subjects, so we referred to 174 subjects, who had been previously screened as negative at the FIT test. To our knowledge, this is the best modality to establish the group of colon healthy subjects.
We analyzed the 174 negative FIT cases also in respect to the gender and their distribution in age groups (50–59 and 60–70 years old) for the expression of the CELTiC panel.
Nowadays, interest is constantly growing on gender differences in diagnosis and therapy. Interestingly, we observed statistically significant differences for the expression of CEACAM6 and COL1A2 in gender. The expression of CEACAM6 showed statistically significant differences between males and females in the group of 60–70.
Sex was confirmed as a possible factor of influence also in the comparison between FITN and NFIT, indeed we unveiled an increased risk for female subjects to result false FIT positive. These difference might be explained with the higher adhesion of female population to screening programs as previously reported in literature [29]. On the other hand, a significant increasing incidence risk in older people was highlighted when comparing LR to FITN or HR/CRC to FITN. This different age distribution and increasing incidence risk are consistent with the knowledge that sporadic CRC is a disease of the elderly. Our results support current data on the importance of gender and age differences in CRC incidence and mortality [2], [11], and suggest that more studies are needed to better define gender- and age-specific reference intervals for the early diagnosis of CRC. Therefore, to correctly interpret the comparisons among groups we included, in the statistical analysis evaluation, age and sex.
To further optimise the protocol and validate our results, we calculated the within-assay and between-assay coefficient of variations (CV) for each gene of the CELTiC panel as measures of precision and repeatability. All CVs of the tested genes, including the reference B2M, were below 5% for Cq (Table 2), and in line with data previously reported [30]. The CV values for ΔCq were below the 25% suggested for qPCR [24], [31], [32], [33]. In addition, all the SDs and CVs of the replicates (technical variability) were lower than the SDs and CVs of the whole FITN group (biological variability), further confirming the robustness of the CELTiC panel protocol. Therefore, considering that our study focused on relative gene expression and not on the absolute quantification of the mRNA copy numbers, [30], [33] our results can be considered acceptable and reliable.
The expression of the CELTiC panel in FITN repeated the data we previously published [22]. Moreover, the multinomial logit model confirms the prominent role of TSPAN8 to discriminate between FITN and the other tested groups. In addition, ROC curves corroborate the diagnostic value of the CELTiC panel for CRC. The AUC sensitivities and specificities reported here are promising and comparable to the previous work, thus supporting the reliability of our standardization with the new FITN group as reference group and further consolidating the clinical relevance, with an enlargement of the data set from 231 to 338 subjects.
Finally, the proposed test is based on the measurements of four genes using quantitative reverse transcription PCR (qRT-PCR). Our choice to investigate on mRNA markers could have potential advantages related to the high amount of copies of RNA that can be detected in early diagnosis [34]. Moreover, also the measurements of extracellular RNAs in biofluids, protected by microvesicles or RNA-binding proteins, are appealing to monitor the disease development [34]. Nowadays multigene mRNA signature-based assays are being increasingly incorporated into clinical management for systematic evaluation of RNA-seq-based classifiers [34].
This method is fast, sensitive, high-throughput for the number of clinical samples that can be analysed and cost-effective. The results reported here confirm the values of sensitivity and specificity detected in the previous studies. Furthermore, we showed that CELTiC panel also has the potential to discriminate false positive FIT patients, which may reach 15% of the tested cases [35]. Thus, the CELTiC panel associated with FIT analysis may reduce the number of false positive FIT, which leads to costly and superfluous colonoscopies.
The present study has some limitations such as: LR and NFIT groups are based on few samples. We are aware of the need to expand the number of the cases, in order to obtain cut-off values that could distinguish the analyzed groups (FITN, NFIT, LR, HR/CRC).
Conclusion
By testing the CELTiC panel in 174 FIT negative subjects, we confirmed our previous findings on the efficacy of the proposed blood-based biomarkers as potential candidates to implement the non-invasive screening of CRC. TSPAN8 and LGALS4 could be particularly useful to identify patients affected by high-risk lesions or CRC and possibly to exclude false positive FIT subjects. The low technical variability further confirmed the reliability and the robustness of the protocol. Additional studies are needed to increase the number of FIT positive subjects to better discriminate false positive FIT from patients affected by low risk lesions.
Compliance with ethics requirements
The study was conducted after approval by the ethical committee of the Sant’Orsola - Malpighi Hospital, Bologna (155/2007/U/sper approved 22/01/2008; EM 120/2016/U approved 14/06/2016).
All the participants gave written informed consent to the participation in the study. All the procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the ethical principles for medical research involving human subjects of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Acknowledgments
Acknowledgments
The authors wish to acknowledge Dr. Maria Carmen Biffoni and Dr. Daniela Solmi of the Laboratorio Analisi S. Antonio Bologna for the sample collections, the Fondazione Enzo Piccinini for the financial support and all the blood donor volunteers.
Declaration of Competing Interest
RS, GM and LM have intellectual property rights on a patent pending (WO2016/185451 A1: method and kit for diagnosis of colorectal cancer). The remaining authors declared no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Hadjipetrou A., Anyfantakis D., Galanakis C.G., Kastanakis M., Kastanakis S. Colorectal cancer, screening and primary care: a mini literature review. World J Gastroenterol. 2017;23(133):6049–6058. doi: 10.3748/wjg.v23.i33.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Senore C., Basu P., Anttila A., Ponti A., Tomatis M., Vale D.B. Status of implementation and organization of cancer screening in The European Union Member States—Summary results from the second European screening report. Int J Cancer. 2018;142:44–56. doi: 10.1002/ijc.31043. [DOI] [PubMed] [Google Scholar]
- 5.Benson V.S., Atkin W.S., Green J., Nadel M.R., Patnick J., Smith R.A. Toward standardizing and reporting colorectal cancer screening indicators on an international level: the international colorectal cancer screening network. Int J Cancer. 2012;130:2961–2973. doi: 10.1002/ijc.26310. [DOI] [PubMed] [Google Scholar]
- 6.Venturelli F., Sampaolo L., Carrozzi G., Zappa M., Giorgi Rossi P., Rossi P.G. Associations between cervical, breast and colorectal cancer screening uptake, chronic diseases and health-related behaviours: Data from the Italian PASSI nationwide surveillance. Prev Med. 2019;120:60–70. doi: 10.1016/j.ypmed.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Zorzi M., Hassan C., Capodaglio G., Fedato C., Montaguti A., Turrin A. Long-term performance of colorectal cancer screening programmes based on the faecal immunochemical test. Gut. 2018;67:2124–2130. doi: 10.1136/gutjnl-2017-314753. [DOI] [PubMed] [Google Scholar]
- 8.Schreuders E.H., Ruco A., Rabeneck L., Schoen R.E., Sung J.J.Y., Young G.P. Colorectal cancer screening: A global overview of existing programmes. Gut. 2015;64:1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 9.Idigoras Rubio I., Arana-Arri E., Portillo Villares I., Bilbao Iturribarrria I., Martinez-Indart L., Imaz-Ayo N. Participation in a population-based screening for colorectal cancer using the faecal immunochemical test decreases mortality in 5 years. Eur J Gastroenterol Hepatol. 2019;31:197–204. doi: 10.1097/MEG.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 10.Rosso C., Cabianca L., Gili F.M. Non-invasive markers to detect colorectal cancer in asymptomatic population. Minerva Biotecnol. 2019;31(1):23–29. [Google Scholar]
- 11.Botteri E., Crosta C., Bagnardi V., Tamayo D., Sonzogni A.M., De Roberto G. Predictors of advanced colorectal neoplasia at initial and surveillance colonoscopy after positive screening immunochemical faecal occult blood test. Dig Liver Dis. 2016;48:321–326. doi: 10.1016/j.dld.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Tao S., Seiler C.M., Ronellenfitsch U., Brenner H. Comparative evaluation of nine faecal immunochemical tests for the detection of colorectal cancer. Acta Oncol. 2013;52:1667–1675. doi: 10.3109/0284186X.2013.789141. [DOI] [PubMed] [Google Scholar]
- 13.Normanno N., Cervantes A., Ciardiello F., De Luca A, De Luca A., Pinto C. The liquid biopsy in the management of colorectal cancer patients: Current applications and future scenarios. Cancer Treat Rev. 2018;70:1–8. doi: 10.1016/j.ctrv.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Rodia M.T., Ugolini G., Mattei G., Montroni I., Zattoni D., Ghignone F. Systematic large-scale meta-analysis identifies a panel of two mRNAs as blood biomarkers for colorectal cancer detection. Oncotarget. 2016;7:30295–30306. doi: 10.18632/oncotarget.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gemei M., Mirabelli P., Di Noto R., Corbo C., Iaccarino A., Zamboli A. CD66c is a novel marker for colorectal cancer stem cell isolation, and its silencing halts tumor growth in vivo. Cancer. 2013;119(4):729–738. doi: 10.1002/cncr.27794. [DOI] [PubMed] [Google Scholar]
- 16.Kuroki M., Matsushita H., Matsumoto H., Hirose Y., Senba T., Yamamoto T. Nonspecific cross-reacting antigen-50/90 (NCA-50/90) as a new tumor marke. Anticancer Res. 1999;19:5599–5606. [PubMed] [Google Scholar]
- 17.Cao Z.Q., Guo X.L. The role of galectin-4 in physiology and diseases. Protein Cell. 2016;7(5):314–324. doi: 10.1007/s13238-016-0262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makoto W., Ichiro T., Naoki K., Yuhki Y., Matsuo E.I., Susumu I. Clinical significance of circulating galectins as colorectal cancer markers. Oncol Rep. 2011;25(5):1217–1226. doi: 10.3892/or.2011.1198. [DOI] [PubMed] [Google Scholar]
- 19.Richardson M.M., Jennings L.K., Zhang X.A. Tetraspanins and tumor progression. Clin Exp Metastasis. 2011;28(3):261–270. doi: 10.1007/s10585-010-9365-5. [DOI] [PubMed] [Google Scholar]
- 20.Nazarenko I., Rana S., Baumann A., McAlear J., Hellwig A., Trendelenburg M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70(4):1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 21.Greco C., Bralet M.P., Ailane N., Dubart-Kupperschmitt A., Rubinstein E., Le Naour F. E-cadherin/p120-catenin and tetraspanin Co-029 cooperate for cell motility control in human colon carcinoma. Cancer Res. 2010;70(19):7674–7683. doi: 10.1158/0008-5472.CAN-09-4482. [DOI] [PubMed] [Google Scholar]
- 22.Rodia M.T., Solmi R., Pasini F., Nardi E., Mattei G., Ugolini G. LGALS4, CEACAM6, TSPAN8, and COL1A2: blood markers for colorectal cancer—validation in a cohort of subjects with positive fecal immunochemical test result. Clin Colorectal Cancer. 2018;17(2):e217–e228. doi: 10.1016/j.clcc.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Hamm A., Prenen H., Van Delm W, Di Matteo M, Wenes M., Delamarre E. Tumour-educated circulating monocytes are powerful candidate biomarkers for diagnosis and disease follow-up of colorectal cancer. Gut. 2016;65:990–1000. doi: 10.1136/gutjnl-2014-308988. [DOI] [PubMed] [Google Scholar]
- 24.Broeders S., Huber I., Grohmann L., Berben G., Taverniers I., Mazzara M. Guidelines for validation of qualitative real-time PCR methods. Trends Food Sci Technol. 2014;37:115–126. [Google Scholar]
- 25.Mattocks C.J., Morris M.A., Matthijs G., Swinnen E., Corveleyn A., Dequeker E. A standardized framework for the validation and verification of clinical molecular genetic tests. Eur J Hum Genet. 2010;18:1276–1288. doi: 10.1038/ejhg.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganepola G.A., Nizin J., Rutledge J.R., Chang D.H. Use of blood-based biomarkers for early diagnosis and surveillance of colorectal cancer. World J Gastrointest Oncol. 2014;6:83–97. doi: 10.4251/wjgo.v6.i4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada T., Matsuda A., Koizumi M., Shinji S., Takahashi G., Iwai T. Liquid biopsy for the management of patients with colorectal cancer. Digestion. 2019;99:39–45. doi: 10.1159/000494411. [DOI] [PubMed] [Google Scholar]
- 28.Yörüker E.E., Holdenrieder S., Gezer U. Blood-based biomarkers for diagnosis, prognosis and treatment of colorectal cancer. Clin Chim Acta. 2016;455:26–32. doi: 10.1016/j.cca.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Navarro M., Nicolas A., Ferrandez A., Lanas A. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol. 2017;23(20):3632. doi: 10.3748/wjg.v23.i20.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29 doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kralik P., Ricchi M. A basic guide to real time PCR in microbial diagnostics: Definitions, parameters, and everything. Front Microbiol. 2017;8:1–9. doi: 10.3389/fmicb.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 33.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 34.Byron S.A., Van Keuren-Jensen K.R., Engelthaler D.M., Carpten J.D., Craig D.W. Translating RNA sequencing into clinical diagnostics: Opportunities and challenges. Nat Rev Genet. 2016;17(5):257–271. doi: 10.1038/nrg.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amitay E.L., Cuk K., Niedermaier T., Weigl K., Brenner H. Factors associated with false-positive fecal immunochemical tests in a large German colorectal cancer screening study. Int J Cancer. 2019;144:2419–2427. doi: 10.1002/ijc.31972. [DOI] [PubMed] [Google Scholar]