Highlights
-
•
We established a CHIKV neutralization assay in a 384-well format.
-
•
We used CHIKV pseudotyped lentiviral vectors encoding luciferase.
-
•
We showed specific neutralization activity of patient sera.
-
•
We developed a new multiplex neutralization assay for CHIKV.
Keywords: Chikungunya virus, Neutralization, Lentiviral vectors
Abstract
Chikungunya virus (CHIKV) is a mosquito-transmitted Alphavirus that causes chikungunya fever and has infected millions of people mainly in developing countries. The associated disease is characterized by rash, high fever and severe arthritis that can persist for years. Since the epidemic on La Réunion in 2006, CHIKV has adapted to Aedes albopictus, which also inhabits temperate regions of the eastern and western hemispheres, including Europe and the United States. A. albopictus might continue migrating north with continuing climate change and CHIKV would then no longer be confined to the developing nations. No treatment or licensed CHIKV vaccine exists. A CHIKV neutralization assay in a 384-well format by using CHIKV-pseudotyped lentiviral vectors was established. This assay system can be used for entry inhibitor screening under a reduced safety level (S2). Production of CHIKV-pseudotyped lentiviral vectors and the reaction volume are optimized. A dose dependent, specific neutralization of CHIKV-pseudotyped vectors with sera of CHIKV-infected individuals could be measured in a 384-well format. A safe and simple multiplex assay for the analysis of CHIKV neutralizing activities was developed and will be able to improve drug and vaccine development as well as it would improve the understanding of CHIKV epidemics regarding antibody responses.
1. Introduction
Chikungunya virus (CHIKV) is a mosquito-transmitted Alphavirus that causes chikungunya fever in humans. Most CHIKV infections are symptomatic, with an incubation period of 2–4 days. The disease is characterized by a sudden onset of fever, headache, malaise, arthralgias or arthritis, myalgias, and lower back pain. The term ‘chikungunya’ means ‘that which bends up’ in the Kimakonde language of Mozambique and describes the disease phenotype. After the acute phase, polyarthritis can be recurrent and may persist for several years after infection. These factors raise a serious public health concern and contribute to a significant economic cost during large outbreaks.
CHIKV was first isolated from the blood of a febrile patient in Tanzania in 1953, and has since been identified as the cause of numerous human epidemics in many areas of Africa, Southeast Asia and on the Indian subcontinent (Thiboutot et al., 2010, Schwartz and Albert, 2010). Aedes aegypti has been the primary CHIKV vector in Asia, but in La Réunion, Aedes albopictus (the Asian tiger mosquito) was the primary vector (Solignat et al., 2009). During this epidemic, CHIKV adapted effectively to A. albopictus, which also inhabits temperate and even cold temperate regions of the eastern and western hemispheres, including Europe and the United States (Medlock et al., 2012, Rochlin et al., 2013). A. albopictus might continue migrating north with continuing climate change and CHIKV would then no longer be confined to the developing nations. A further risk of introducing CHIKV into previously non-endemic areas exists from travelers with viremia, leading to local transmission of the virus, especially in tropical or subtropical areas of the United States and also in southern Europe (Rezza et al., 2007). As a result, the NIAID has designated CHIKV as a Category C pathogen alongside the influenza and SARS-CoV viruses. There is no specific treatment for chikungunya fever and care is only supportive, based on the symptoms. No licensed CHIKV vaccine exists.
CHIKV is a (+) single stranded (ss)RNA virus and belongs to the Alphavirus genus in the Togaviridae family. Alphaviruses enter cells by receptor mediated endocytosis and a subsequent pH-dependent fusion step. CHIKV has three surface proteins: the two transmembrane glycoproteins E2 and E1, and the mainly secreted E3 protein, which presumably facilitates secretion of the E2 and E1 glycoproteins (Mayne et al., 1984, Snyder and Mukhopadhyay, 2012). E1 is a class II viral fusion protein and E2 most likely mediates cell attachment, however the cellular receptor is still unknown. E2 and E1 associate as trimers of heterodimers (E2–E1) on the particle surface (Voss et al., 2010).
The early steps of infection, such as receptor binding and membrane fusion, are carried out solely by the viral glycoproteins. Additionally, the glycoproteins are the major target of neutralizing antibody responses. A tool used to investigate the glycoproteins of viruses is the pseudotyping of vectors with desired glycoproteins. With this strategy, the vectors incorporate a heterologous viral glycoprotein and thereby acquire the host range of the virus the glycoprotein is derived of. They enable studies without the need of using the native virus, which usually requires a higher safety level. Pseudotyping of retro- or lentiviral vectors is frequently used to study viral entry and to evaluate entry inhibitors (Siegert et al., 2005), or to harvest neutralizing antibodies at reduced safety levels (Schnierle et al., 1997, Seaman et al., 2010, Dervillez et al., 2010). It has been previously described that lentiviral vectors can be pseudotyped with CHIKV E1/E2 and transfer the CHIKV host range to these vectors (Salvador et al., 2009, Akahata et al., 2010, Kishishita et al., 2013). This vector system was used to create a reduced safety level assay to analyze sera of CHIKV-infected individuals for their neutralizing activity, and thereby established a multiplexed assay in 384-well format for the analysis of compounds able to inhibit CHIKV entry.
2. Materials and methods
2.1. Cell culture
All cells used in this study were cultured at 37 °C under 5% CO2. HeLa (ATCC: CCL-2), BSC-1 (CCL-26), HEK 293T (CRL-1573), NIH 3T3 (CCL-92), HT 1080 (CCL-121), Huh7 (CCL-185), HepG2 (HB-8065) and A549 (CCL-185) cells were grown in Dulbecco's modified Eagle medium (DMEM; Lonza, Verviers, Belgium). Renca (CRL-2947), HaCat (Boukamp et al., 1988), MCF7 (HTB-22), BHK 21 (CCL-10), Jurkat (TIB-152), HuT78 (TIB-161), Bjab (Kruger et al., 1996) and PM-1 (derived from HuT78 by Lusso et al. (1995)) cells were incubated in Roswell Park Memorial Institute medium (RPMI; Biowest, Nuaille, France). RK13 (CCL-121) and MRC5 (CCL-171) cells were cultured in Eagle's minimal essential medium (EMEM; Biochrom, Berlin, Germany). All media were supplemented with 10% FBS (v/v; PAA, Pasching, Austria) and 5% l-glutamine (200 mM; Lonza, Verviers, Belgium).
2.2. Human sera
Human sera CHIKV 1–3 and DENV 1–3 were obtained from the Bernhard Nocht Institute for Tropical Medicine in Hamburg, Germany. The serum CHIKV 4 was obtained from the Robert-Koch-Institute, Berlin, Germany. The human naïve serum (Nr. 032) was obtained from a healthy volunteer. All human sera used were taken with consent of the patient for diagnostic purposes according to ethical regulations in Germany.
2.3. Plasmids and DNA
The gene for the CHIKV E3-E1 envelope polyprotein was synthesized by GeneArt (Life Technologies, Darmstadt, Germany) on the basis of the “S27-African prototype” sequence (codon-optimized for the expression in mammalian cells). The gene was cloned via blunted ends (PacI and AscI) into the plasmid pIRES2-eGFP (digested with SmaI; Clontech/Takara, 78100 Saint-Germain-en-Laye, France). Furthermore, the plasmids pMDLg/pRRE, pRSVrev, pRRLsinCMV-GFPpre (Dull et al., 1998), pCSII-Luc (Agarwal et al., 2006) (kind gift of N. Somia to R. König), pHIT-G (encoding VSV-G; Soneoka et al., 1995) and pGaLV TM (encoding a modified GaLV Env; Stitz et al., 2000) were used for the production of vector particles.
2.4. Polyethylenimine solution (PEI)
For preparation of PEI transfection solution, 5 g of polyethylenimine were mixed with 5 ml purified water. Afterwards, another 10 ml of purified water were added. Following shaking and total dissolving of the PEI, 0.69 ml of this mixture were added to 39.5 ml of purified water. Then, 9.5 ml of this solution were mixed with another, 35 ml purified water. The pH was adjusted to 7.0 with 1 N HCl. Subsequently, the PEI solution was filled with purified water up to a total weight of 50 g, mixed well, sterile filtered (0.22 μm filters, Sartorius), aliquoted and stored at −20 °C (Boussif et al., 1995).
2.5. Lentiviral vector particle production
HEK 293T cells were seeded in 10 cm dishes in 10 ml DMEM. After 16 h, the subconfluent cells (∼80% density) were cotransfected with the plasmids pRRLsinCMV-GFPpre or pCSII-Luc (5.5 μg), pMDLg/pRRE (2.4 μg), pRSVrev (1.2 μg), and pHIT-G or pIRES2-eGFP-CHIKV E3-E1 (1.9 and 3.0 μg, respectively) using 25 μl PEI solution. PEI was mixed with plasmid DNA in a volume of 1.4 ml serum-free DMEM, incubated for 30 min at room temperature and subsequently added to the cells. For transfection with Lipofectamine® 2000 (according to the manufacturer's protocol; Life Technologies), the following amounts of plasmid DNA were used: pRRLsinCMV-GFPpre or pCSII-Luc (10.0 μg), pMDLg/pRRE (6.5 μg), pRSVrev (2.5 μg), pHIT-G or pIRES2-eGFP-CHIKV E3-E1 (3.5 and 5.3 μg, respectively). After 24 h of incubation, the medium was replaced by 5 ml fresh DMEM per dish. Another 24 h later, the vector particle containing supernatant was harvested, sterile filtered with 0.45 μm filters (Sartorius, Göttingen, Germany), ultracentrifuged (1 h at 50,000 rpm, rotor TLA 100.3; Optima TLX Ultracentrifuge, Beckman Coulter, Krefeld, Germany) and the particles were resuspended in DMEM and/or frozen at −80 °C.
2.6. Transduction of cells with lentiviral vector particles
For the transduction of cells with vector particles produced by the GFP encoding plasmid, 1.25 × 105 cells of the respective cell line per well were seeded in a 1 ml volume in 24-well plates one day prior to transduction. For the human hematopoietic cells, transduction was carried out directly after counting the cells. For all adherent cell lines, 16 h after seeding, the medium was removed and replaced by DMEM containing lentiviral vector particles (eitherVSV-G, GaLV TM or CHIKV Env pseudotyped) up to a volume of 500 μl. After 6 h of incubation at 37 °C, a medium change was performed, substituting the supernatant with 1 ml fresh DMEM. Cells were incubated another 72 h at 37 °C and analyzed by flow cytometry. For this, cells were trypsinated (Trypsin EDTA, Lonza), washed twice with 1 ml PBS + 2% FBS and fixed in 100 μl PBS + 2% paraformaldehyde. Subsequently, the GFP signal was detected with a LSR II SORP flow cytometer (Becton, Dickinson and Company, Heidelberg, Germany).
Transduction of cells with luciferase encoding lentiviral vectors for luciferase assays was performed by seeding 6000 HEK 293T cells per well in a white CELLSTAR 384-well microtiter plate (Greiner Bio-One, Frickenhausen, Germany) in a volume of 20 μl DMEM using the MultiFlo Microplate Dispenser (BioTek, Bad Friedrichshall, Germany). After 24 h of incubation at 37 °C, 10 μl of vector particles (either VSV-G or CHIKV Env pseudotyped, produced with pCSII-Luc) plus 10 μl diluted human serum was added to the cells by using the Matrix Multichannel Equalizer Electronic Pipette (Thermo Scientific). Serum dilutions ranged from 1:30 to 1:2340 (4 times 1:3 dilutions), and were mixed with the vector particles 1:1 resulting in a final dilution of 1:60 to 1:4680. Sera were diluted with DMEM. The vector particle–sera mixtures were incubated in 96-U-well plates (Thermo Scientific, Rockford, IL, USA) at 4 °C for 1 h and subsequently added to the 384-well plates. From every dilution/well in the 96-well plate, 20 μl was transferred to three wells each of the 384-well plate (triplicate assay). After 16 h of incubation, 20 μl of BriteLite (PerkinElmer, Rodgau, Germany) substrate was added to each well using the MultiFlo Microplate Dispenser (BioTek, Bad Friedrichshall, Germany). Following an incubation of 5 min at room temperature, the Luciferase signal was detected with the PHERAstar FS (BMG LABTECH, Ortenberg, Germany).
2.7. Western blot analysis
The Western blot was performed with a BIO-Rad semi-dry blotter. Proteins separated by SDS-PAGE were blotted onto PVDF membranes with 50 mM sodium borate pH 9.0, 20% methanol, and 0.1% SDS at 100 mA per membrane for 75 min. Afterwards, membranes were blocked with Roti-Block™ and proteins were detected with a custom made α-E2 polyclonal antibody (Eurogentec, Cologne, Germany), an anti-rabbit HRP coupled secondary antibody and the ECL detection system (Amersham, Freiburg).
3. Results
3.1. Characterization of CHIKV pseudotyped vector particles
Lentiviral vector particles pseudotyped with the CHIKV glycoproteins were generated by transfection of 293T cells with the following plasmids: a lentiviral vector genome encoding the green fluorescent protein (GFP), the lentiviral packaging vectors and a plasmid encoding the codon-optimized CHIKV glycoproteins E3, E2 and E1. Two days after the transfection, cell supernatants were concentrated by ultracentrifugation and the pellets as well as the cell lysates were subjected to Western blot analysis. In cell lysates, a clear signal for E2 could be observed with an antibody directed against E2 (Fig. 1 A). The Western blot analysis of the pelleted cell supernatants revealed that high levels of CHIKV E2 were incorporated into lentiviral vector particles (Fig. 1B), as detected by the presence of the HIV-1 capsid protein p24 (Fig. 1B, lower panel).
Fig. 1.
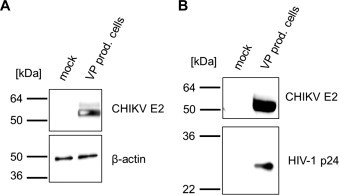
Western blot analysis. HEK 293T cells were cotransfected with the plasmids pRRLsinCMV-GFPpre, pMDLg/pRRE, pRSVrev, and pIRES2-eGFP-CHIKV E3-E1 or remained without additional DNA. After 48 h, cell lysates and concentrated cell supernatants were analyzed by Western blot using an E2-specific antibody and the ECL detection system (Amersham, Freiburg). (A) Cell lysates. Detection of β-actin was used as the loading control. (B) Supernatants. Formation of lentiviral vector particles was controlled by analysis of the HIV-1 p24 protein.
Next, it was proven that the pseudotyped lentiviral vectors are infectious and display the cellular tropism of CHIKV. A series of tissue culture cell lines were transduced either with CHIKV- or VSV-G-pseudotyped vectors encoding GFP. Transduction efficiency was determined by flow cytometry. Lentiviral vectors pseudotyped with the vesicular stomatitis virus G protein (VSV-G) were used as positive control, since they are well known for their very broad cellular tropism. CHIKV-pseudotyped vectors were used to transduce a wide variety of mouse, rabbit, hamster, monkey and human cell lines, which is an extension and in agreement with published data (Salvador et al., 2009) (Fig. 2 ). In contrast, hematopoietic cell lines were unaffected by CHIKV-pseudotyped vectors although VSV-G pseudotyped vectors were able to transduce them (Fig. 2) (Salvador et al., 2009). In general, titers of CHIKV-pseudotyped vectors were lower than VSV-G-pseudotyped vectors. Blocking of the pH-dependent entry pathway by addition of chloroquine during the transduction process occurred as expected for both VSV-G and CHIKV-pseudotyped vectors. Gibon ape leukemia virus (GaLV)-pseudotyped vectors were not affected by chloroquine, because their entry is not pH-dependent (Fig. 3 ).
Fig. 2.
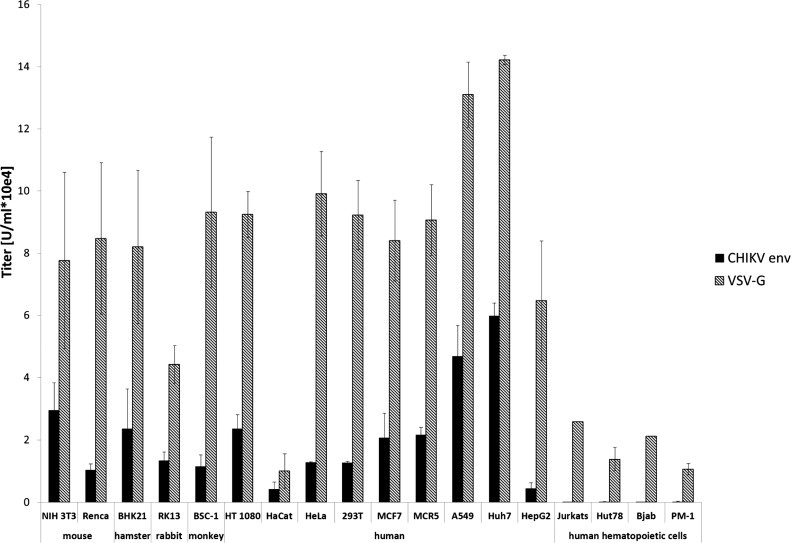
Transduction of tissue culture cells. Different tissue culture cells were transduced with VSV-G (gray) or CHIKV Env (black) pseudotyped vectors encoding GFP. Transduction was analyzed by flow cytometry.
Fig. 3.
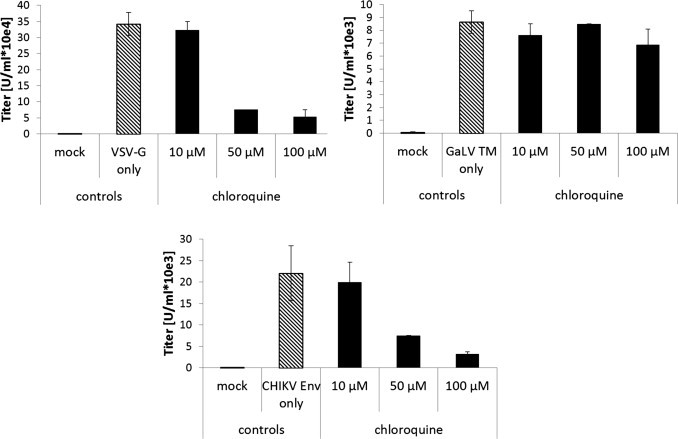
The effect of chloroquine on vector transduction rate. Huh7 cells were transduced with VSV-G, GaLV TM or CHIKV Env pseudotyped vectors encoding GFP in the presence of the indicated amounts of chloroquine. Transduction was analyzed by flow cytometry.
3.2. Development of an assay for neutralizing activity against CHIKV
Screening large numbers of serum samples for diagnostics or compound libraries for drug development requires an optimized assay system in a large-scale format. Advantages of partially automated approaches include substantial increases in throughput, data quality and reproducibility. Automatization of a process requires an easily quantifiable readout. Therefore, the CHIKV-pseudotyped vector system was adjusted. The vector construct (initially a GFP-encoding vector) was replaced by a vector encoding firefly luciferase (pCSII-Luc). This improved the data analysis, since a whole well readout of luminescence can be performed, which is superior over fluorescent readout in medium-throughput to high-throughput approaches. Furthermore, the luminescence signal is stable (depending on the substrate, the half-life can be >5 h), the assay can be used flexibly in various multi-well formats and it is very sensitive with low signal-to-noise ratios, outperforming standard fluorometric or colorimetric assays. In addition, CHIKV-pseudotyped vector production was optimized by changing the transfection reagents. The use of lipofectamine increased the number of infectious particles released by the producer cells by 2.8-fold compared to the transfection reagent polyethylenimine (PEI). The assay was also optimized for a 384-well plate format by changing the reaction volume to 40 μl.
The 293T target cells (6000 cells) were first seeded into the wells using an automated liquid dispenser. Subsequently, the CHIKV-pseudotyped vector particles and serially diluted patient sera were added to the wells. Transduction of target cells by CHIKV-pseudotyped vector particles could be inhibited with the serum of CHIKV-infected patients in a dose dependent manner as measured by luciferase activity. The lowest dilution of 1:60 reduced the luciferase activity 5-fold, whereas the highest dilution of 1:4860 had almost no effect on luciferase activity (Fig. 4A). This phenomenon could not be observed for VSV-G pseudotyped vector particles (Fig. 4B), as the addition of human serum did only slightly change the transduction rate as measured by luciferase activity. There was no effect on luciferase activity after incubation of CHIKV-pseudotyped vector particles with sera of dengue virus infected patients or normal healthy control (Fig. 4A). Once again VSV-G pseudotyped vector particles were not considerably affected by these treatments.
Fig. 4.
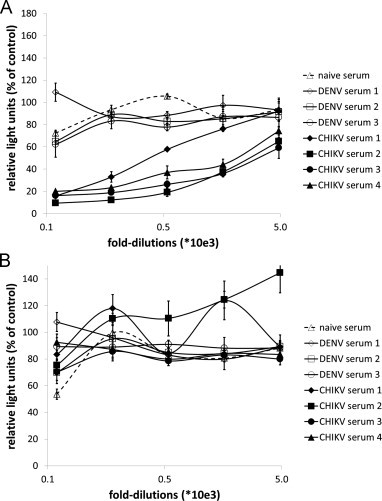
High-throughput analysis of patient serum. (A) Serum of CHIKV infected patients, dengue virus infected patients or healthy controls was serially diluted, incubated with CHIKV Env pseudotyped vector particles and analyzed for its neutralizing activity by detection of relative luciferase activities. The luciferase activity is shown in percentage, relative to untreated control. (B) Serum of CHIKV infected patients, dengue virus infected patients or healthy controls was serially diluted, incubated with VSV-G pseudotyped vector particles and analyzed for its neutralizing activity by detection of relative luciferase activities. The luciferase activity is shown in percentage, relative to untreated control. The values represent mean values of triplicate measurements.
The sera from CHIKV-infected patients (sera 1–3) were furthermore analyzed for their reactivity toward CHIKV (strain S27) by immunofluorescence. Serum number one showed the lowest reactivity (1:1280), whereas sera two and three had titers of either 1:2560 or 1:5120, respectively (Fig. 5 ). The difference in the amount of CHIKV-specific IgG was also reflected in the neutralization assay. Serum number 1 inhibited the transduction of target cells less efficiently then sera two and three. A difference among the two highly reactive sera was not detectable, indicating that the amount of antibodies does not directly correlate with their neutralizing activity. Serum number two contained in addition CHIKV-specific IgM (1:40) indicating that the sample was obtained early after infection, which might explain its high neutralizing activity.
Fig. 5.
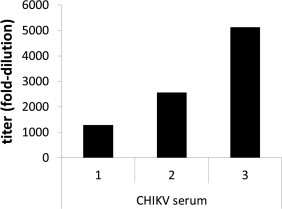
CHIKV-specific reactivity of patient sera. Serum of CHIKV infected patients was serially diluted and analyzed by immunofluorescence. Titers are given in fold dilution.
4. Discussion
Many vaccines rely on the generation of neutralizing antibodies that effectively block the initial viral infection. This result would also be desired for a CHIKV vaccine, since after the acute CHIKV infection phase, persistent polyarthritis frequently occurs in patients. Laboratory diagnosis of viral infection is usually confined to the detection of virus-specific antibodies in the blood, detection of virus antigens or detection of viral genetic material. However, these assays are limited and to provide functional data unable. For instance, nucleic acid amplification can only be used in the viremic phase during CHIKV infections. To determine the neutralizing activity of antibodies or drugs, conventional neutralization assays, like a plaque reduction assay or the inhibition of cytopathogenic effects, are time consuming and rely on the use of fully infectious human pathogens and a reliable reference stock of the virus. Efforts to improve these methods have been described recently. A neutralization assay based on CHIKV replicons expressing secreted Gaussia luciferase (Gluc) as read-out was recently described. The authors showed convincing data with neutralizing antisera of CHIKV infected patients in a 24-well and 96-well format (Glasker et al., 2013). However the production of replicons requires an in vitro transcription and electroporation steps that may resemble technical hurdles. In addition, replicons still bear some residual danger of recombination and the generation of fully replicating CHIKV, although the readout of luciferase in the supernatant of cells is a benefit and omits cell lysis. A second novel approach uses pseudotyped lentiviral vector particles (Kishishita et al., 2013). Here the vector particles needed to be purified by sucrose cushion centrifugation and the assay format was 24-well based. The assay was performed manually and in total took 5 days. Therefore, the method described here, to detect neutralizing activities using pseudotyped lentiviral vector particles in a high-throughput format is a further improvement. This semi-automated neutralization assay for CHIKV was established by using pseudotyped vector particles and the quantitation of luciferase activity. This assay has several advantages to other neutralization assays. It can be performed at a lower biosafety level (S2), thus does not require handling of a human S3 pathogen. Finally, an automated performance of the assay, including the seeding of target cells, makes it fast (3 days) and convenient for the development of entry inhibitors of CHIKV by high throughput screening by using pin tools with specific liquid handling high throughput screening equipment. In addition, the assay will also be helpful for CHIKV vaccine development to screen large numbers of serum samples during clinical trials, in an easy to perform, functional assay. Fast detection of neutralizing or cross-reactive neutralizing antibodies will speed up CHIKV and possibly other Alphavirus vaccine developments and might be useful for basic research to study Alphavirus-specific neutralizing immune responses during large outbreaks.
5. Conclusion
A safe, fast, semi-automated, high throughput assay to detect CHIKV entry inhibitors was developed.
Acknowledgement
We thank Kaitlyn Oliphant for critically reading the manuscript.
References
- Agarwal S., Nikolai B., Yamaguchi T., Lech P., Somia N.V. Construction and use of retroviral vectors encoding the toxic gene barnase. Mol. Ther. 2006;14:555–563. doi: 10.1016/j.ymthe.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Akahata W., Yang Z.Y., Andersen H., Sun S., Holdaway H.A., Kong W.P., Lewis M.G., Higgs S., Rossmann M.G., Rao S., Nabel G.J. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat. Med. 2010;16:334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R.T., Breitkreutz D., Hornung J., Markham A., Fusenig N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussif O., Lezoualc’h F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B., Behr J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervillez X., Klaukien V., Durr R., Koch J., Kreutz A., Haarmann T., Stoll M., Lee D., Carlomagno T., Schnierle B., Mobius K., Konigs C., Griesinger C., Dietrich U. Peptide ligands selected with CD4-induced epitopes on native dualtropic HIV-1 envelope proteins mimic extracellular coreceptor domains and bind to HIV-1 gp120 independently of coreceptor usage. J. Virol. 2010;84:10131–10138. doi: 10.1128/JVI.00165-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T., Zufferey R., Kelly M., Mandel R.J., Nguyen M., Trono D., Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasker S., Lulla A., Lulla V., Couderc T., Drexler J.F., Liljestrom P., Lecuit M., Drosten C., Merits A., Kummerer B.M. Virus replicon particle based chikungunya virus neutralization assay using Gaussia luciferase as readout. Virol. J. 2013;10:235. doi: 10.1186/1743-422X-10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishishita N., Takeda N., Anuegoonpipat A., Anantapreecha S. Development of a pseudotyped-lentiviral-vector-based neutralization assay for chikungunya virus infection. J. Clin. Microbiol. 2013;51:1389–1395. doi: 10.1128/JCM.03109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger U., Pfeiffer T., Bosch V. Generation of lymphocyte cell lines coexpressing CD4 and wild-type or mutant HIV type 1 glycoproteins: implications for HIV type 1 Env-induced cell lysis. AIDS Res. Hum. Retroviruses. 1996;12:783–792. doi: 10.1089/aid.1996.12.783. [DOI] [PubMed] [Google Scholar]
- Lusso P., Cocchi F., Balotta C., Markham P.D., Louie A., Farci P., Pal R., Gallo R.C., Reitz M.S., Jr. Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J. Virol. 1995;69:3712–3720. doi: 10.1128/jvi.69.6.3712-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne J.T., Rice C.M., Strauss E.G., Hunkapiller M.W., Strauss J.H. Biochemical studies of the maturation of the small Sindbis virus glycoprotein E3. Virology. 1984;134:338–357. doi: 10.1016/0042-6822(84)90302-7. [DOI] [PubMed] [Google Scholar]
- Medlock J.M., Hansford K.M., Schaffner F., Versteirt V., Hendrickx G., Zeller H., Van B.W. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12:435–447. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza G., Nicoletti L., Angelini R., Romi R., Finarelli A.C., Panning M., Cordioli P., Fortuna C., Boros S., Magurano F., Silvi G., Angelini P., Dottori M., Ciufolini M.G., Majori G.C., Cassone A. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- Rochlin I., Ninivaggi D.V., Hutchinson M.L., Farajollahi A. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in Northeastern USA: implications for public health practitioners. PLoS ONE. 2013;8:e60874. doi: 10.1371/journal.pone.0060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador B., Zhou Y., Michault A., Muench M.O., Simmons G. Characterization of chikungunya pseudotyped viruses: Identification of refractory cell lines and demonstration of cellular tropism differences mediated by mutations in E1 glycoprotein. Virology. 2009;393:33–41. doi: 10.1016/j.virol.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnierle B.S., Stitz J., Bosch V., Nocken F., Merget-Millitzer H., Engelstadter M., Kurth R., Groner B., Cichutek K. Pseudotyping of murine leukemia virus with the envelope glycoproteins of HIV generates a retroviral vector with specificity of infection for CD4-expressing cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8640–8645. doi: 10.1073/pnas.94.16.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O., Albert M.L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Microbiol. 2010;8:491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- Seaman M.S., Janes H., Hawkins N., Grandpre L.E., Devoy C., Giri A., Coffey R.T., Harris L., Wood B., Daniels M.G., Bhattacharya T., Lapedes A., Polonis V.R., McCutchan F.E., Gilbert P.B., Self S.G., Korber B.T., Montefiori D.C., Mascola J.R. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S., Thaler S., Wagner R., Schnierle B.S. Assessment of HIV-1 entry inhibitors by MLV/HIV-1 pseudotyped vectors. AIDS Res. Ther. 2005;2:7. doi: 10.1186/1742-6405-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A.J., Mukhopadhyay S. The alphavirus E3 glycoprotein functions in a clade-specific manner. J. Virol. 2012;86:13609–13620. doi: 10.1128/JVI.01805-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solignat M., Gay B., Higgs S., Briant L., Devaux C. Replication cycle of chikungunya: a re-emerging arbovirus. Virology. 2009;393:183–197. doi: 10.1016/j.virol.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneoka Y., Cannon P.M., Ramsdale E.E., Griffiths J.C., Romano G., Kingsman S.M., Kingsman A.J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz J., Buchholz C.J., Engelstadter M., Uckert W., Bloemer U., Schmitt I., Cichutek K. Lentiviral vectors pseudotyped with envelope glycoproteins derived from gibbon ape leukemia virus and murine leukemia virus 10A1. Virology. 2000;273:16–20. doi: 10.1006/viro.2000.0394. [DOI] [PubMed] [Google Scholar]
- Thiboutot M.M., Kannan S., Kawalekar O.U., Shedlock D.J., Khan A.S., Sarangan G., Srikanth P., Weiner D.B., Muthumani K. Chikungunya: a potentially emerging epidemic? PLoS Negl. Trop. Dis. 2010;4:e623. doi: 10.1371/journal.pntd.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J.E., Vaney M.C., Duquerroy S., Vonrhein C., Girard-Blanc C., Crublet E., Thompson A., Bricogne G., Rey F.A. Glycoprotein organization of chikungunya virus particles revealed by X-ray crystallography. Nature. 2010;468:709–712. doi: 10.1038/nature09555. [DOI] [PubMed] [Google Scholar]


