Highlights
-
•
We review the outbreak of severe acute respiratory syndrome (SARS) in 2002–2003 and antiviral treatment of patients.
-
•
We review efforts towards the rational design of anti-SARS therapeutics.
-
•
We present a comprehensive list of all available 3-dimensional structures of coronavirus proteins.
-
•
We discuss the emerging MERS coronavirus and review the few antivirals available for treatment.
-
•
We critically discuss which lessons have been learned from SARS and which are yet to be learned.
Keywords: Severe acute respiratory syndrome, Coronavirus, Antiviral therapy, Vaccine, Middle East respiratory syndrome
Abstract
This article introduces a series of invited papers in Antiviral Research marking the 10th anniversary of the outbreak of severe acute respiratory syndrome (SARS), caused by a novel coronavirus that emerged in southern China in late 2002. Until that time, coronaviruses had not been recognized as agents causing severe disease in humans, hence, the emergence of the SARS-CoV came as a complete surprise. Research during the past ten years has revealed the existence of a diverse pool of coronaviruses circulating among various bat species and other animals, suggesting that further introductions of highly pathogenic coronaviruses into the human population are not merely probable, but inevitable. The recent emergence of another coronavirus causing severe disease, Middle East respiratory syndrome (MERS), in humans, has made it clear that coronaviruses pose a major threat to human health, and that more research is urgently needed to elucidate their replication mechanisms, identify potential drug targets, and develop effective countermeasures. In this series, experts in many different aspects of coronavirus replication and disease will provide authoritative, up-to-date reviews of the following topics:
– clinical management and infection control of SARS;
– reservoir hosts of coronaviruses;
– receptor recognition and cross-species transmission of SARS-CoV;
– SARS-CoV evasion of innate immune responses;
– structures and functions of individual coronaviral proteins;
– anti-coronavirus drug discovery and development; and
– the public health legacy of the SARS outbreak.
Each article will be identified in the last line of its abstract as belonging to the series “From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses.”
1. Introduction
“Those who cannot remember the past are condemned to repeat it” – George Santayana.
Ten years ago, a novel coronavirus causing pneumonia in humans emerged in Guangdong, China. The first known patient was a 45-year old man in the city of Foshan, who developed fever and respiratory symptoms on November 16, 2002, transmitting infection to his wife and three other family members. The second index case was a restaurant chef in Shenzhen who became ill on 10 December, returned to his home in Heyuan and transmitted infection to health care workers (HCWs) in the local hospital, including the physician who accompanied him in an ambulance to Guangzhou provincial hospital. This scenario of the emergence of clusters of cases of severe respiratory disease among family members and hospital workers, each cluster apparently going extinct after a few rounds of secondary or tertiary transmission, was played out repeatedly in subsequent weeks in a number of municipalities in Guangdong province. The index cases of many of these early case clusters were food handlers or chefs working in restaurants where a variety of exotic and game animals were slaughtered on the premises (Xu et al., 2004a). During subsequent weeks, the outbreak became self-sustaining, with large clusters of transmission in hospitals spilling back into the community (Table 1 ).
Table 1.
SARS and its aftermath: a chronology of events over the past ten years. Partly based on WHO Western Pacific Region (2006).
| Date | Key events |
|---|---|
| 16 November 2002 | A 45-year-old man in Foshan city, Guangdong province, China develops an atypical pneumonia and infects four relatives. This is the first identified case of SARS from epidemiological investigations. |
| 10 December 2002 | A 35-year-old restaurant worker in Shenzhen develops pneumonia and 8 health care workers in contact with him become ill. |
| 8 January 2003 | A 26-year-old man working in the game animal trade in Guangxi Province (adjacent province to Guangdong) develops pneumonia and infects family members. |
| January 2003 | Pneumonia outbreaks in Guangzhou (capital city of Guangdong Province). |
| 23 January 2003 | Guangdong Health Bureau circulates document giving case definition and control measures to health bureaus and hospitals in the province. |
| 30 January 2003 | A patient hospitalized in Guangzhou transmits infection to more than 50 hospital staff and 19 relatives, the first of many “super-spreading” events. |
| 11 February 2003 | WHO receives reports of an outbreak of respiratory disease in Guangdong, 305 cases and 5 deaths. One-third of cases are health care workers infected while caring for patients with similar illness. |
| 21 February 2003 | A doctor from Guangdong caring for patients with atypical pneumonia checks in at Hotel M in Hong Kong to attend a wedding. He had been ill since 15 February, but now deteriorates further and is hospitalized on 22 February. He infects 16 other guests and one visitor at this hotel, some of whom travel on to Vietnam, Singapore and Toronto where they initiate local clusters of transmission. |
| 26 February 2003 | A Hotel M contact is admitted to a private hospital in Hanoi and is the source of an outbreak there. Seven health care workers ill by 5 March. |
| 4 March 2003 | A Hotel M contact admitted to Prince of Wales Hospital, Hong Kong. He had been ill since 24 February, but his illness is not severe and not recognized as a possible case of the new “atypical pneumonia”. By 7 March, health care workers at this hospital report a respiratory illness. Overall, he infects 50 health care workers, 17 medical students, 30 other patients and 42 visitors to the ward and 4 family members. |
| 5 March 2003 | A Hotel M contact dies in Toronto. Five family members affected. |
| 12 March 2003 | WHO issues global alert. |
| 14 March 2003 | Singapore and Toronto report clusters of atypical pneumonia. In retrospect, both groups have an epidemiological link to Hotel M. One of the doctors who had treated patients in Singapore has gone to New York and develops symptoms while traveling. He is quarantined as his flight lands in transit in Frankfurt, Germany. He has infected two family members travelling with him and one crew member. |
| 15 March 2003 | The WHO has received reports of over 150 cases of this new disease, now named Severe Acute Respiratory Syndrome (SARS). Travel advisory issued. |
| 17 March 2003 | A WHO multi-center laboratory network is established for the study of SARS causation and diagnosis. |
| 21– 27 March 2003 | A novel coronavirus is identified in patients with SARS. |
| 14 April 2003 | Mapping of the full genome of SARS-CoV is completed. |
| 16 April 2003 | WHO announces that SARS-CoV is the causative agent of SARS. |
| 23 May 2003 | A virus related to SARS-CoV is detected in animals in Guangdong. |
| 5 July 2003 | Absence of further transmission in Taiwan signals the end of the SARS outbreak in humans. |
| September 2003 – February 2004 | Laboratory-acquired SARS cases reported in Singapore, Taiwan and Beijing. The case in Beijing leads to limited community transmission in Beijing and Anhui. |
| December 2003 – January 2004 | Transient re-emergence of SARS infecting humans from animal markets. |
| 23 May 2005 | The International Health Regulations are adopted by the Fifty-eighth World Health Assembly on 23 May 2005. They enter into force on 15 June 2007. |
| September 2012 | A novel coronavirus causing respiratory disease is isolated in Saudi Arabia. Earlier cases in Jordan (April 2012) were retrospectively diagnosed. The aetiological agent is a novel human β-coronavirus, subsequently named the Middle East respiratory syndrome (MERS) coronavirus. |
| 1 April – 23 May 2013 | Outbreak of over 20 cases of MERS reported in hospitals in Al-Ahsa, in eastern Saudi Arabia. |
| As of 7 September 2013 | 114 confirmed cases of MERS have been reported to WHO, leading to 54 deaths. Index cases have occurred in Jordan, Saudi Arabia, Qatar and the United Arab Emirates. Imported cases, sometimes with limited secondary transmission, have been reported from France, Germany, Italy, Tunesia and the United Kingdom. |
The first “super-spreading” event, which became a hallmark of the epidemiology of this disease, occurred with the hospitalization of a 44-year old man in Guangzhou on 30 January, 2003. He was to transmit infection to 19 relatives and more than 50 hospital staff. On 21 February, one of the doctors infected as part of this extended hospital outbreak traveled to Hong Kong, where he stayed one night in a hotel and was hospitalised the next day. During his stay, he transmitted infection to 16 other hotel guests and one visitor, who traveled on to their destinations, seeding outbreaks of this disease in Vietnam, Singapore, Toronto and in Hong Kong. On 12 March, following the outbreaks in mainland China, Hong Kong and Vietnam, the World Health Organization (WHO) issued a global alert about an unusual pneumonia which appeared to cause outbreaks of disease in hospitals. This led to the recognition and reporting of additional case clusters in Toronto and Singapore, prompting the WHO to issue an Emergency Travel Advisory on 15 March, providing an early case definition and naming the disease Severe Acute Respiratory Syndrome (WHO Western Pacific Region, 2006). A chronology of events during and after the SARS outbreak is provided in Table 1.
At this stage, a number of possible aetiological agents were being proposed, including Chlamydia, paramyxoviruses, human metapneumovirus, and coronavirus, among others. The WHO coordinated the sharing of information among laboratories, resulting in the consensus that the etiological agent was a novel coronavirus, to be named SARS coronavirus (SARS-CoV) (Peiris et al., 2003, Ksiazek et al., 2003, Drosten et al., 2003, Kuiken et al., 2003). This WHO-mediated information-sharing allowed laboratories, epidemiologists, and clinicians to achieve rapid consensus on clinical virology, patient management and virus transmission (World Health Organization Multicentre Collaborative Network for Severe Acute Respiratory Syndrome Diagnosis, 2003).
SARS spread rapidly along routes of air-travel, affecting 25 countries and territories across five continents and sickening over 8000 people, leading to the death of almost 800. Fortunately, it had an unusual feature that permitted the success of basic public health measures in controlling person-to-person transmission: the “viral load” in upper respiratory tract secretions was low in the first 5 days of illness, then increased progressively, peaking early in the second week (see Cheng et al., in this series). As a result, transmission was less common in the first days of illness, providing an opportunity for case detection and isolation to interrupt transmission. Patients were most infectious when they were hospitalised, contributing to transmission in hospitals, especially those in more developed settings where invasive and potentially aerosol-generating interventions such as bronchoscopy were more likely to be carried out.
The SARS-CoV was also unusually stable in the environment, more so than other coronaviruses or other respiratory viruses, making infection control in hospitals a challenge (see Cheng et al., in this series). It has been speculated that the enhanced stability of the SARS-CoV at lower temperatures and lower humidity, especially in air-conditioned environments, may help explain the explosive outbreaks that occurred in some regions, compared to others (Chan et al., 2011). However, as awareness grew, patients began to be identified and hospitalized earlier in the illness (Leung et al., 2004), and as effective infection control modalities were better implemented, it became possible to interrupt transmission in the community and in hospitals. Thus, on 5 July 2003, it was possible for the WHO to announce that “all known chains of human-to-human transmission of the SARS virus now appear to be broken”. Such an outcome could hardly have been imagined in the dark days of March–April, when, for example, an unprecedented cluster of around 300 cases emerged over a few days in the Amoy Gardens housing estate in Hong Kong.
2. The source of the SARS-coronavirus
Once the outbreak had ended, the zoonotic source of the virus still remained to be identified. Epidemiological investigations had found that the index patients of the initial case clusters in November–December 2002 were food handlers or restaurant workers, especially those exposed to exotic wild-game animals, regarded as a winter delicacy in southern China. The increasing affluence of the past decade had led to this trade becoming highly organised and commercialised, with hundreds of diverse exotic wild-life being housed in large central markets. Investigation of these “wet markets” led to the detection of a virus closely related to SARS-CoV in a range of small mammalian species, such as Himalayan palm civets (Paguma larvata), raccoon dogs (Nyctereutes procyonoides) and others. People working in these markets had a high prevalence of antibodies to SARS-CoV, even though they gave no history of having had SARS, while people working in other areas of the markets, such as vegetable stalls, or people in the community did not (Guan et al., 2003). This identified wild-game animal markets as the interface which facilitated the maintenance and amplification of SARS-CoV precursor viruses, allowing repeated exposure of the human population and leading to inter-species transmission events.
In late 2003 and early 2004, four more patients with a SARS-like illness were diagnosed. Phylogenetically, these re-emergent viruses were more closely related to viruses found at that time in game-animal markets than to those that caused the SARS epidemic the year before (Liang et al., 2004). This confirmed the contention that wet markets were the source of initial human infection and led to their closure, very likely pre-empting a re-emergence of SARS. Further work established that wild-caught palm civets showed no evidence of SARS-CoV infection, suggesting that they were intermediate, amplifying hosts, rather than the true reservoir. Novel coronaviruses, including some closely related to SARS-CoV, have been identified in bats, including insectivorous Rhinolophid bats (Li et al., 2005b, Lau et al., 2005; see Drexler et al., in this series). Subsequent work has identified the virus–receptor interactions and receptor restrictions that permit or restrict interspecies transmission events of SARS-CoV-like viruses (Li et al., 2005a; see the review by F. Li, in this series).
3. Antiviral therapy during the SARS outbreak
In the early phase of the epidemic, physicians had to manage severely ill patients, including some of their own colleagues, without reliable knowledge of the virus and its susceptibility to antiviral drugs. The treatment regimens that were applied will be described in detail by Cheng et al. in this series. Initially, patients were given ribavirin, a broadly active antiviral compound that is effective against some RNA viruses, such as hepatitis C virus and Lassa virus, but in retrospect showed little benefit for SARS patients. The in vitro activity of ribavirin on SARS-CoV replication in cell culture gave contradictory results, depending on the cell type used (Cinatl et al., 2003a; Morgenstern et al., 2005).
Many SARS patients were treated with a combination of ribavirin and corticosteroids, with mixed results (see Cheng et al., in this series). Interferon-α was administered to patients in mainland China and in Toronto, Canada. In the reports from China, a beneficial effect could not be clearly ascribed to interferon-α, as it was always used in combination with immunoglobulins or thymosin (Zhao et al., 2003). A preliminary, uncontrolled study from Toronto suggested that treatment with a combination of interferon-α and corticosteroids was superior to corticosteroids alone (Loutfy et al., 2003). In SARS-CoV-infected cell culture, interferon-β had much superior effects over interferon-α (Cinatl et al., 2003b). Polyethyleneglycol-modified interferon-α was demonstrated to protect macaques from SARS-CoV prophylactically and to reduce viral replication and tissue pathology when administered therapeutically (Haagmans et al., 2004).
Interestingly, the HIV-protease inhibitor lopinavir, often combined with ritonavir, appeared to show some benefit for SARS patients (Chu et al., 2004; see the review by Cheng et al., in this series). The antiviral effect of these compounds was also observed in cell culture. As the coronavirus genome does not code for an aspartic protease related to the HIV protease, Wu et al. (2004) tested the potency of lopinavir against the isolated SARS-CoV main protease (also called the 3C-like protease, 3CLpro), which is a cysteine protease, and found an IC50 of around 50 μM. This low potency is probably not sufficient to explain the beneficial effects of lopinavir/ritonavir in the clinical treatment of SARS patients. While ritonavir is known to boost the immune system, this cannot explain the observed activity in cell culture. An alternative explanation could be the anti-apoptotic activity of these HIV protease inhibitors (Maturrese et al., 2002). More information on the proposed binding mode of lopinavir to the SARS-CoV main protease and on attempts to improve its inhibitory potency will be presented in the review of this molecular target in this series by Zhang et al.
In mainland China, traditional Chinese medicines (TCMs) were employed in addition to one or more of the therapies described above, but it is difficult to assess their effect, as no systematic studies were carried out (see Cheng et al., in this series). The only TCM for which anti-SARS-CoV activity was demonstrated in cell culture (but at relatively high concentrations), was glycyrrhizin, a compound found in liquorice (Cinatl et al., 2003a).
4. Early attempts at rational design of anti-SARS drugs
Within three weeks of the discovery of the SARS-CoV, its complete nucleotide sequence had been determined by Marra et al. (2003) and Rota et al. (2003), making more rational approaches to antiviral drug discovery possible. For example, Luo et al. (2004) noticed some sequence similarities between the SARS-CoV nucleocapsid (N) protein and the capsid protein (CA) of HIV. The latter binds to cyclophilin A (CypA), a peptidyl prolyl cis/trans isomerase of the host cell, which is incorporated into the HIV particle (Gamble et al., 1996). Accordingly, Luo et al. (2004) reasoned that the N protein of SARS-CoV may also bind to CypA, and could indeed determine the Kd value to 60–160 nM. This interaction was blocked by cyclosporin A, an inhibitor of CypA, which however is used as an immunosuppressive drug. Through virtual screening techniques that employed the modelled SARS-CoV N-CypA complex as a target, Luo et al. were able to identify some other inhibitors of this interaction, but most of them showed some degree of cell toxicity. Chen et al. (2005) confirmed the interaction between CypA and the N protein and even provided evidence for an incorporation of CypA into the virion, similar to what had been shown previously for HIV-1.
However, once the SARS epidemic was over, the idea of blocking SARS-CoV replication by inhibiting CypA was not followed further, until Pfefferle et al. (2011) detected a specific interaction between CypA and the SARS-CoV non-structural protein 1 (Nsp1) (and similarly, of the HCoV-NL63 Nsp1) by yeast-two-hybrid and other protein–protein interaction techniques (see the article by von Brunn and colleagues in this series). Consequently, they tested CsA for antiviral effects against a large range of coronaviruses and found it to be a “pan-coronavirus inhibitor” (Pfefferle et al., 2011; see also de Wilde et al., 2011). However, as CsA also displayed antiviral activity against the γ-coronavirus, infectious bronchitis virus (IBV), which lacks Nsp1, it is possible that the mechanism originally proposed by Luo et al. (2004) may contribute to CsA’s anticoronaviral activity (Ma-Lauer et al., 2012). Interestingly, there are anecdotal reports that cyclosporin A was occasionally used to treat SARS patients (e.g., So et al., 2003).
Efforts to design anti-SARS drugs were initiated early during the outbreak, but were hampered by a lack of structural data on molecular targets. By the end of March 2003, when the SARS-CoV was discovered as the etiological agent causing the disease, only one crystal structure of a coronavirus protein was available, that of the main protease (Mpro or 3CLpro) of the porcine coronavirus, transmissible gastroenteritis virus (TGEV) (Anand et al., 2002). However, in mid-May 2003, at the peak of the SARS outbreak in Beijing, the same group published the first structure of a synthetic inhibitor, a peptidyl methyl ketone, bound to the TGEV Mpro, as well as the structure of a second coronavirus Mpro, that of human coronavirus 229E, and a homology model of the SARS-CoV enzyme based on these structures (Anand et al., 2003). Furthermore, these authors suggested that AG7088 (rupintrivir), a Michael-acceptor-type inhibitor of the 3C protease of human rhinovirus, should be a good starting point for anti-SARS drug design. A little later, this compound itself was shown to have little activity against the virus (Shie et al., 2005), but derivatives of rupintrivir turned out to be quite active in virus-infected cell culture (Shie et al., 2005, Yang et al., 2005). These studies were facilitated by the determination of the crystal structure of the SARS-CoV main protease itself in June, 2003 (Yang et al., 2003). Since then, many inhibitors have been designed and synthesized that target the coronavirus Mpro, but few of them have undergone systematic toxicity and other preclinical studies, so that these compounds are not yet available for clinical trials in case of a recurrence of SARS (or for treatment of patients infected with the new human coronavirus, MERS-CoV, see below). The numerous studies aimed at designing inhibitors of coronavirus main proteases will be summarized by Zhang et al. in this series.
5. The SARS-CoV genome and proteome
Coronaviruses are enveloped viruses with a single-stranded RNA genome of positive polarity. This is the largest known RNA genome, with a size of 27–32 kb (27.8 kb in the case of SARS-CoV). The 14 open reading frames (ORFs) of the SARS-CoV genome code for at least 28 proteins (Fig. 1 ). The structural proteins are encoded in the 3′-terminal third of the genome. The spike glycoprotein (S) protrudes from the surface of the viral particle (hence the name “coronavirus”) and is responsible for receptor binding and membrane fusion. In late 2003, angiotensin-converting enzyme 2 (ACE2) was identified as the receptor of SARS-CoV on the surface of human cells (Li et al., 2003, Wang et al., 2004). The structure of the complex between the receptor-binding domain of the SARS-CoV spike protein and ACE2 was determined by Li et al. (2005a), who will review this and subsequent work in this series. Proteolytic processing of the S protein, a prerequisite for membrane fusion, will be reviewed by Simmons, Pöhlmann, and colleagues. Inhibitors of the host-cell proteases involved have also been shown to prevent cell entry of SARS-CoV (see, e.g., Adedeji et al., 2013). Peptides corresponding to the heptad repeats of the trimeric S protein have been demonstrated to inhibit the fusion of the viral envelope with the host-cell membrane (e.g., Sainz et al., 2006, Liu et al., 2009).
Fig. 1.
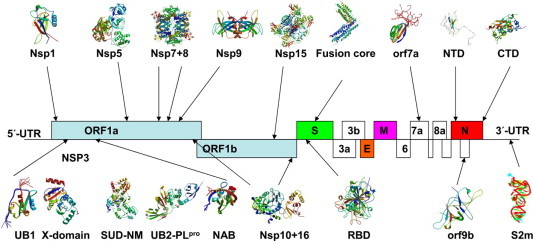
Structure of the RNA genome of SARS-CoV. Three-dimensional structures are depicted for those proteins for which they are available. References to the corresponding publications and PDB codes can be found in Table 2.
Another most important structural protein of coronaviruses is the nucleocapsid (N) protein, which encapsulates the genomic RNA and has roles in its replication and transcription (see, e.g., Tylor et al., 2009, Grossoehme et al., 2009). Current knowledge of this protein will be reviewed by Huang and colleagues in this series. The matrix (M) protein and the envelope (E) glycoprotein complete the structural proteins, although in the case of SARS-CoV, some of the accessory proteins (see below) are also believed to be incorporated into the viral particle.
ORF1 comprises about two-thirds of the SARS-CoV genome and codes for two huge polyproteins, pp1a (about 486 kD) and pp1ab (about 790 kD). Ribosome slippage at a frameshift site near the 3′-terminus of ORF1a leads to translation of the entire ORF1ab (Namy et al., 2006). The polyproteins are processed by two viral cysteine proteases, a papain-like protease (PLpro, a domain in Nsp3) and the main protease (Mpro or 3CLpro, Nsp5) into a total of 15 or 16 non-structural proteins (Nsps). Most of them are components of the viral replication/transcription complex (RTC) but they may also adopt additional functions (for reviews, see Sawicki et al., 2005, Sawicki et al., 2007, Pasternak et al., 2006, Masters, 2006, Perlman and Netland, 2009).
The RTC, consisting of the majority of the coronaviral Nsps and some as yet unidentified host proteins, assembles at virus-induced double-membrane vesicles (DMVs) and other unusual membrane structures, which have been derived from the ER membrane (Knoops et al., 2008). In recent years, significant progress has been made in unravelling the structures of these DMVs, as will be reviewed by Snijder and colleagues in this series. After assembly of the RTC, a nested set of (sub)genomic mRNAs is synthesized and subsequently translated into the structural and accessory proteins (Sawicki et al., 2007, Pasternak et al., 2006). Finally, the structural proteins assemble into progeny virions, along with the newly synthesized genomic RNA. After budding through membranes of the intermediate ER-to-Golgi compartment (Krijnse-Locker et al., 1994), the mature virions egress from the host cell via exocytosis.
Because of their obviously essential role, the coronaviral proteases are the target of intense structural, functional, mechanistic, and inhibitor-discovery studies, and will be dealt with in separate reviews in this series by Mesecar, Baker and colleagues (on PLpro) and Zhang et al. (on Mpro). Significant progress has also been made over the past 10 years in elucidating the structures of other Nsps, in the hope of learning something about their function. Table 2 lists the structures known to date. This effort has been made possible through several structural proteomics projects (Bartlam et al., 2005, Bartlam et al., 2007, Canard et al., 2008, Hilgenfeld et al., 2008). Overall, this approach has been quite successful, although for those proteins (mostly encoded by ORF1a) for which we know the 3D structures, the functions are still not quite clear in many cases (Nsp1, Nsp3 domains other than the PLpro, Nsp9, Nsp10, also partly Nsp7+8). In contrast, for several proteins (mostly encoded by ORF1b) for which we know the functions, little structural information is available. The most painful lack of structural information concerns Nsp12, the RNA-dependent RNA-polymerase (RdRp), and Nsp13, the helicase, both of which are obvious drug targets. Progress in characterizing Nsp3 domains other than the PLpro will be summarized by Lei et al., on Nsp7–Nsp10 by Xiao et al., and on Nsp12–Nsp16 by Canard and colleagues in articles in this series.
Table 2.
Three-dimensional protein structures for SARS-CoV and other coronaviruses.
| Protein (or RNA) | Virus and PDB code | Reference |
|---|---|---|
| Nsp1 | SARS-CoV (2HSX; 2GDT); TGEV (3ZBD) | Almeida et al. (2007) and Jansson (2013) |
| Nsp3 UB1 | SARS-CoV (2GRI) ; MHV (2M0I) | Serrano et al. (2007) and Keane and Giedroc (2013) |
| Nsp3 PL1pro | TGEV (3MP2) | Wojdyla et al. (2010) |
| Nsp3 X-domain | SARS-CoV (2ACF; 2FAV); HCoV 229E (3EWQ; 3EJG); IBV (3EWO; 3EJF; 3EKE); FCoV (3ETI; 3EW5) | Saikatendu et al., 2005, Egloff et al., 2006, Xu et al., 2009a, Piotrowski et al., 2009 and Wojdyla et al. (2009) |
| Nsp3 SUD | SARS-CoV (2W2G; 2WCT; 2KQV; 2KQW; 2JZF; 2RNK) | Tan et al., 2009, Johnson et al., 2010a and Chatterjee et al. (2009) |
| Nsp3 PL2pro | SARS-CoV (2FE8) | Ratia et al. (2006) |
| Nsp3 NAB | SARS-CoV (2K87) | Serrano et al. (2009) |
| Nsp4-C | FCoV (3GZF) ; MHV (3VC8) | Manolaridis et al., 2009, Xu et al., 2009b |
| Nsp5 | TGEV (1LVO); HCoV 229E (1P9S); SARS-CoV (1UJ1; 2BX3; 2BX4); HKU1 (3D23); IBV (2Q6D) | Anand et al., 2002, Anand et al., 2003, Yang et al., 2003, Tan et al., 2005, Zhao et al., 2008 and Xue et al. (2008) |
| Nsp7 | SARS-CoV (1YSY; 2KYS) | Peti et al., 2005, Johnson et al., 2010b |
| Nsp7 + 8 complex | SARS-CoV (2AHM); FCoV (3UB0) | Zhai et al. (2005) and Xiao et al. (2012) |
| Nsp9 | SARS-CoV (1UW7; 1QZ8); HCoV 229E (2J97) | Sutton et al., 2004, Egloff et al., 2004 and Ponnusamy et al. (2008) |
| Nsp10 | SARS-CoV (2FYG; 2G9T; 2GA6) | Joseph et al. (2006) and Su et al. (2006) |
| Nsp15 | SARS-CoV (2H85); MHV (2GTH) | Ricagno et al. (2006) and Xu et al. (2006) |
| Nsp10 + 16 complex | SARS-CoV (2XYQ; 2XYR; 3R24) | Decroly et al. (2011) and Chen et al. (2011) |
| Hemagglutinin-esterase | BCoV (3CL4) | Zeng et al. (2008) |
| orf7a | SARS-CoV (1XAK; 1YO4) | Nelson et al. (2005) and Hänel et al. (2006) |
| orf9b | SARS-CoV (2CME) | Meier et al. (2006) |
| Spike RBD alone and in complex with receptor | SARS-CoV (2GHV; 2AJF); HCoV NL63 (3KBH); PRCV (4F5C); MHV (3R4D); MERS-CoV (4L3N; 4KR0; 4KQZ; 4L72) | Hwang et al., 2006, Li et al., 2005a, Wu et al., 2009, Reguera et al., 2012, Peng et al., 2011, Chen et al., 2013a, Lu et al., 2013 and Wang et al. (2013) |
| Spike fusion core | SARS-CoV (1WYY; 2BEQ; 2BEZ; 1ZV7; 1ZVB; 1ZV8; 1ZVA; 2FXP; 1WNC); MHV (1WDF; 1WDG); HCoV NL63 (2IEQ) | Duquerroy et al., 2005, Supekar et al., 2004, Deng et al., 2006, Hakansson-McReynolds et al., 2006, Xu et al., 2004b and Zheng et al. (2006) |
| Nucleocapsid-NTD | IBV (2C86; 2GEC; 2BXX); HCoV OC43 (4J3K); SARS-CoV (2OFZ; 2OG3; 1SSK); MHV (3HD4) | Jayaram et al., 2006, Fan et al., 2005, Chen et al., 2013b, Saikatendu et al., 2007, Huang et al., 2004 and Grossoehme et al. (2009) |
| Nucleocapsid-CTD | IBV (2CA1; 2GE7; 2GE8); SARS-CoV (2CJR; 2JW8; 2GIB) | Jayaram et al., 2006, Chen et al., 2007, Takeda et al., 2008, Yu et al., 2006 |
| S2m | SARS-CoV (1XJR) | Robertson et al. (2004) |
Structures elucidated by nuclear magnetic resonance (NMR) techniques are indicated by a Protein Data Bank (PDB) code in italics. Abbreviations: NTD, N-terminal domain; CTD, C-terminal domain; HCoV, human coronavirus; FCoV, feline coronavirus; IBV, infectious bronchitis virus; TGEV, transmissible gastroenteritis virus. Only structures of the free proteins are listed here, inhibitor complexes are excluded. The crystal structure of the S2m element of the SARS-CoV genomic RNA is also included. Only those structures have been included for which a PDB entry is available.
Finally, the SARS-CoV genome encodes several accessory proteins, some of which undergo rapid evolution. For example, early in the SARS outbreak ORF8 coded for one protein, but during the evolution of the virus in early 2003 it lost 29 nucleotides, and subsequently coded for two separate accessory proteins, 8a and 8b. It is thought that this event was responsible for the increased efficiency of human-to-human transmission that surfaced about the same time, triggering the epidemic (The Chinese SARS Molecular Epidemiology Consortium, 2004, Oostra et al., 2007). Structural information for SARS-CoV accessory proteins is still very scarce (see Table 2), partly due to the fact that many of these gene products are membrane proteins. In the present series of articles, the accessory proteins of SARS-CoV will be reviewed by D.X. Liu and colleagues, and evolutionary aspects of SARS-CoV will be the subject of an article by Gorbalenya and colleagues.
6. Lessons learned
The SARS epidemic vividly demonstrated that we now live in a “global village,” and that an infectious disease emerging anywhere in the world has the potential to spread globally in a short period of time. One legacy of the outbreak was the formulation of the international Health Regulations (IHR) in 2005 (http://www.who.int/ihr/en/) and their acceptance by the World Health Assembly in 2007. The IHR require countries to report unusual and unexplained outbreaks of infectious disease and to develop the public health capacity to detect and respond to such diseases, when and where they occur (in this series, see the review by P. Gully). SARS and other contemporary zoonotic threats, such as H5N1 avian influenza, have highlighted the need for collaboration among those responsible for human and animal health, and the environment. This led to the formalization of the concept of “One Health”, which fosters collaborative effects of multiple disciplines to attain optimal health for people, animals, and the environment. The relevant international organizations, the Food and Agriculture Organization (FAO), the World Organisation for Animal Health (OIE) and the WHO now have a formal agreement and framework within which they can coordinate activities to assess risks at the animal/human/ecosystem interface (FAO–OIE–WHO Collaboration; see http://www.who.int/influenza/resources/documents/tripartite_concept_note_hanoi_042011_en.pdf (accessed 7th August 2013)). SARS also contributed to the enhanced emphasis now being placed on better understanding of viral diversity in wildlife and the need to understand the ecological and biological bases of inter-species transmission of these pathogens.
7. Ten years after: Middle East respiratory syndrome coronavirus (MERS-CoV)
Ten years after the SARS outbreak, it is worth recounting these events in detail and to summarise subsequent understanding of the SARS-CoV, because we continue to be confronted by novel emerging disease threats. Due to increased research efforts, two additional human coronaviruses, HCoV NL63 and HCoV HKU1, were discovered in 2004/2005 (van der Hoek et al., 2004, Woo et al., 2005). In 2012, we saw another novel coronavirus emerge in the Middle East (Zaki et al., 2012). The novel Middle East respiratory syndrome (MERS) coronavirus is a beta-coronavirus, like the SARS-CoV but it belongs to lineage c rather than b (see Drexler et al. in this series). It causes severe pneumonia as well as renal failure, with a high fatality rate. Index cases have originated in Jordan, Qatar, Saudi Arabia, and the United Arab Emirates, while travel-associated cases have been diagnosed in France, Germany, Italy, Tunisia, and the UK. As of 7 September 2013, 114 cases have been confirmed, with 54 deaths (http://www.who.int/csr/don/2013_09_07/en/index.html).
MERS patients tend to be elderly and have other underlying illnesses. Secondary transmission has been reported in hospitals, but in one such event in France, the disease did not appear to be highly transmissible to healthy HCWs; instead, it targeted patients who were immunocompromised (Mailles et al., 2013). The largest cluster of cases was reported from a health facility in Al-Ahsa in the Eastern Province of Saudi Arabia where transmission to other patients and family members, as well as a few HCWs, has occurred (Assiri et al., 2013). In contrast to SARS, relatively few HCWs have been affected so far. Although viruses closely related to the MERS-CoV have been detected in Pipistrellus bats found in Europe and Africa, a more epidemiologically plausible zoonotic source and phylogenetically proximate virus remain to be identified (see Drexler et al., in this series; Annan et al., 2013). Very recently, high neutralizing-antibody titers to MERS-CoV have been detected in dromedary camels in Oman and in Egypt, suggesting that they may be an intermediate transmitter of the virus (Reusken et al., 2013; Perera et al., 2013). These events are uncannily reminiscent of the emergence of SARS in late 2002. Sero-epidemiological studies are needed to define the full extent of secondary transmission of MERS-CoV and whether the infection is more widespread in the community.
The receptor for MERS-CoV has been identified to be dipeptidyl peptidase IV (DPP4) which is expressed in the human respiratory tract and is conserved across many species, including bats (Raj et al., 2013). Biological understanding gleaned from the viral–host receptor interactions in restriction of interspecies transmission of SARS-CoV (see the review by Li, in this series) will be relevant to MERS-CoV. Two crystal structures of the complex between the receptor-binding domain (RBD) of the MERS-CoV spike protein and DPP4 have been published very recently (Lu et al., 2013, Wang et al., 2013), as has the structure of the RBD alone (Chen et al., 2013a; see the review by F. Li, in this series). A crystal structure for the MERS-CoV main protease has also been communicated (Ren et al., 2013), and an article on the macrodomains of the virus by Lei et al. is in preparation. All of these structures will be included in reviews in this series describing individual proteins of SARS-CoV and MERS-CoV.
MERS-CoV appears to replicate efficiently in human respiratory tissues (Chan et al., 2013), targeting alveolar epithelial cells and the endothelium of blood vessels in the lung, indicating a potential for disseminating beyond the respiratory tract as was seen for SARS-CoV (see Cheng et al., in this series). As with SARS-CoV, the novel coronavirus appears to avoid eliciting host interferon responses, but remain sensitive to the action of interferon (see the review by Frieman, Baric, and colleagues in this series; Chan et al., 2013, Falzarano et al., 2013a, de Wilde et al., 2013). The therapeutic options tried for SARS, including interferon therapy, as summarized by Cheng et al. in this series, may be pertinent to MERS.
8. Lessons yet to be learned: the current status of antiviral therapy and vaccine development for SARS and MERS
Huge progress has been made over the past ten years in the elucidation of the functions and structures of the proteins of the SARS-CoV, and research on vaccine development has also progressed, with a number of strategies being developed and evaluated in experimental animal models. However, it should also be noted that after 2005–2006, it became difficult to obtain funding for research on SARS-CoV in many countries, especially for efforts to discover new antiviral therapies. Similarly, there was no incentive to further develop SARS-CoV vaccines, in the absence of an overt threat to human health. Funding agencies and peer reviewers were probably short-sighted in this respect, but many virologists also failed to take seriously the threat of the re-emergence of SARS or of a SARS-like virus.
Even though many inhibitors of the SARS-CoV main protease have been designed on the basis of crystal structures (see the review by Zhang et al., in this series), few have been tested in SARS-CoV-infected cell culture, let alone in an animal model. For the other prime drug targets among the viral nonstructural proteins, the RdRp (Nsp12) and the helicase (Nsp13), the situation is even more discouraging, because it is difficult to obtain these enzymes in an active form, and numerous attempts to crystallize them have failed. Thus, the frustrating conclusion after ten years of excellent basic research on SARS-CoV is that we are still left with the therapies that showed only uncertain effects in the treatment of patients in 2003, i.e. interferon-α and lopinavir/ritonavir, which seem to be all that can be offered for the specific antiviral treatment of MERS patients. Very recently, it was shown that in MERS-CoV-infected rhesus macaques, the course of disease was much milder upon treatment with a combination of interferon-α2b and ribavirin (Falzarano et al., 2013b). After the largely negative experiences in treating SARS patients, corticosteroids are no longer a treatment option. There may be some hope that non-immunosuppressive derivatives of cyclosporin A, such as DEBIO-025, can be used for therapy, but such compounds remain to be tested in virus-infected cell culture. Also, a peptidic ketoamide designed on the basis of the crystal structure of the Mpro of the bat coronavirus HKU4, which is highly similar to the corresponding enzyme from MERS-CoV, appears to be promising, with good antiviral activity against the MERS-CoV (Lin et al., in preparation), and so do some α,β-unsaturated esters (Ma et al., in preparation). However, these compounds are only now in safety studies in animals, and it will take a long time until they can be used in the clinic. If the current MERS-CoV outbreak is over by then, there is a danger that both funding and enthusiasm for developing these and other compounds will once again wane, and we will be in the same “drug-less” situation when the next zoonotic transmission of a coronavirus into the human population will occur.
In conjunction with producing novel antivirals, there is a need to develop and consolidate global networks that can rapidly respond to emerging infectious disease crises such as MERS, so that novel therapeutic options may be scientifically evaluated in controlled clinical trials. Some examples of such networks are emerging, including ISARIC, the International Severe Acute Respiratory and Emerging Infection Consortium. This global initiative aims to ensure that clinical researchers have open access to the protocols and data-sharing processes needed to facilitate a rapid response to emerging diseases that may turn into epidemics or pandemics (http://isaric.tghn.org/).
As regards vaccine development, the long-lived neutralizing antibody response in those who recovered from SARS provides hope that active and passive immunization strategies are feasible, at least in principle. A number of vaccine strategies were developed and tested in laboratory animals, including recombinant vectored vaccines expressing SARS-CoV S protein, DNA vaccines, inactivated whole-virus vaccines and recombinant-protein vaccines (for a review, see Gillim-Ross and Subbarao, 2006). These studies showed that the S protein is crucial for eliciting effective protective antibody responses, and that there is a good correlation between neutralizing- antibody titers and protection from challenge in animal models. The N protein can induce antigen-specific T-cell-mediated immune responses. An inactivated whole-virus vaccine was tested in phase-1 clinical trials in China. Human monoclonal antibodies that neutralize SARS-CoV were shown to be protective for passive prophylaxis and immunotherapy in laboratory animals (Gillim-Ross and Subbarao, 2006). However, in the absence of a re-emergence of SARS, there was little incentive to pursue these initiatives, and recent years have not seen progress towards a credible SARS vaccine.
In conclusion, the SARS outbreak taught us many lessons, but that of the necessity of developing new antiviral therapies was not learned. The lack of progress we have detailed with regard to antivirals over the past 10 years is equally relevant to the development of coronavirus vaccines, for essentially the same reasons. Together with the emergence of the MERS-CoV, we hope that the articles in this series will help change the attitude of researchers and funding policy makers this time around!
Acknowledgement
The authors thank Yibei Xiao for help with Fig. 1 and Table 2.
References
- Adedeji A.O., Severson W., Jonsson C., Singh K., Weiss S.R., Sarafianos S.G. Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three different mechanisms. J. Virol. 2013;87:8017–8028. doi: 10.1128/JVI.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M.S., Johnson M.A., Herrmann T., Geralt M., Wüthrich K. Novel beta-barrel fold in the nuclear magnetic resonance structure of the replicase nonstructural protein 1 from the severe acute respiratory syndrome coronavirus. J. Virol. 2007;81:3151–3161. doi: 10.1128/JVI.01939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand K., Palm G.J., Mesters J.R., Siddell S.G., Ziebuhr J., Hilgenfeld R. Structure of coronavirus main proteinase reveals combination of a chymotrypsin fold with an extra alpha-helical domain. EMBO J. 2002;21:3213–3224. doi: 10.1093/emboj/cdf327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- Annan A., Baldwin H.J., Corman V.M., Klose S.M., Owusu M., Nkrumah E.E., Badu E.K., Anti P., Agbenyega O., Meyer B., Oppong S., Sarkodie Y.A., Kalko E.K., Lina P.H., Godlevska E.V., Reusken C., Seebens A., Gloza-Rausch F., Vallo P., Tschapka M., Drosten C., Drexler J.F. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg. Infect. Dis. 2013;19:456–459. doi: 10.3201/eid1903.121503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A., Alabdullatif Z.N., Assad M., Almulhim A., Makhdoom H., Madani H., Alhakeem R., Al-Tawfiq J.A., Cotten M., Watson S.J., Kellam P., Zumla A.I., Memish Z.A., The KSA MERS-CoV Investigation Team Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlam M., Yang H., Rao Z. Structural insights into SARS coronavirus proteins. Curr. Opin. Struct. Biol. 2005;15:664–672. doi: 10.1016/j.sbi.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlam M., Xu Y., Rao Z. Structural proteomics of the SARS coronavirus: a model response to emerging infectious diseases. J. Struct. Funct. Genomics. 2007;8:85–97. doi: 10.1007/s10969-007-9024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canard B., Joseph J.S., Kuhn P. International research networks in viral structural proteomics: again, lessons from SARS. Antiviral Res. 2008;78:47–50. doi: 10.1016/j.antiviral.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Peiris J.S., Lam S.Y., Poon L.L., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;2011:734690. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R.W., Chan M.C., Agnihothram S., Chan L.L., Kuok D.I., Fong J.H., Guan Y., Poon L.L., Baric R.S., Nicholls J.M., Peiris J.S. Tropism of and innate immune responses to the novel human betacoronavirus lineage C virus in human ex vivo respiratory organ cultures. J. Virol. 2013;87:6604–6614. doi: 10.1128/JVI.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A., Johnson M.A., Serrano P., Pedrini B., Joseph J.S., Neuman B.W., Saikatendu K., Buchmeier M.J., Kuhn P., Wüthrich K. Nuclear magnetic resonance structure shows that the severe acute respiratory syndrome coronavirus-unique domain contains a macrodomain fold. J. Virol. 2009;83:1823–1836. doi: 10.1128/JVI.01781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Mi L., Xu J., Yu J., Wang X., Jiang J., Xing J., Shang P., Qian A., Li Y., Shaw P.X., Wang J., Duan S., Ding J., Fan C., Zhang Y., Yang Y., Yu X., Feng Q., Li B., Yao X., Zhang Z., Li L., Xue X., Zhu P. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005;191:755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y., Chang C.K., Chang Y.W., Sue S.C., Bai H.I., Riang L., Hsiao C.D., Huang T.H. Structure of the SARS coronavirus nucleocapsid protein RNA-binding dimerization domain suggests a mechanism for helical packaging of viral RNA. J. Mol. Biol. 2007;368:1075–1086. doi: 10.1016/j.jmb.2007.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Su C., Ke M., Jin X., Xu L., Zhang Z., Wu A., Sun Y., Yang Z., Tien P., Ahola T., Liang Y., Liu X., Guo D. Biochemical and structural insights into the mechanisms of SARS coronavirus RNA ribose 2′-O-methylation by nsp16/nsp10 protein complex. PLoS Pathog. 2011;7:e1002294. doi: 10.1371/journal.ppat.1002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Rajashankar K.R., Yang Y., Agnihothram S.S., Liu C., Lin Y.L., Baric R.S., Li F. Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J. Virol. 2013;87:10777–10783. doi: 10.1128/JVI.01756-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I., Yuann J.M.P., Chang Y.M., Lin S.Y., Zhao J., Perlman S., Shen Y.Y., Huang T.H., Hou M.H. Crystal structure-based exploration of the important role of Arg106 in the RNA-binding domain of human coronavirus OC43 nucleocapsid protein. Biochim. Biophys. Acta. 2013;1834:1054–1062. doi: 10.1016/j.bbapap.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., Peiris J.S., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Zevenhoven-Dobbe J.C., van der Meer Y., Thiel V., Narayanan K., Makino S., Snijder E.J., van Hemert M. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011;92:2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Ray V.S., Oudshoorn D., Bestebroer T.M., van Nieuwkoop S., Limpens R.W., Posthuma C.C., van der Meer Y., Bárcena M., Haagmans B.L., Snijder E.J., van den Hoogen B.G. Human coronavirus-EMC replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-alpha treatment. J. Gen. Virol. 2013;94:1749–1760. doi: 10.1099/vir.0.052910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroly E., Debarnot C., Ferron F., Bouvet M., Coutard B., Imbert I., Gluais L., Papageorgiou N., Sharff A., Bricogne G., Ortiz-Lombardia M., Lescar J., Canard B. Crystal structure and functional analysis of the SARS-coronavirus RNA cap 2’-O-methyltransferase nsp10/nsp16 complex. PLoS Pathog. 2011;7:e1002059. doi: 10.1371/journal.ppat.1002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Liu J., Zheng Q., Yong W., Lu M. Structures and polymorphic interactions of two heptad-repeat regions of the SARS virus S2 protein. Structure. 2006;14:889–899. doi: 10.1016/j.str.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguière A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Duquerroy S., Vigouroux A., Rottier P.J., Rey F.A., Bosch B.J. Central ions and lateral asparagine/glutamine zippers stabilize the post-fusion hairpin conformation of the SARS coronavirus spike glycoprotein. Virology. 2005;335:276–285. doi: 10.1016/j.virol.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff M.P., Ferron F., Campanacci V., Longhi S., Rancurel C., Dutartre H., Snijder E.J., Gorbalenya A.E., Cambillau C., Canard B. The severe acute respiratory syndrome-coronavirus replicative protein nsp9 is a single-stranded RNA-binding subunit unique in the RNA virus world. Proc. Natl. Acad. Sci. USA. 2004;101:3792–3796. doi: 10.1073/pnas.0307877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff M.P., Malet H., Putics A., Heinonen M., Dutartre H., Frangeul A., Gruez A., Campanacci V., Cambillau C., Ziebuhr J., Ahola T., Canard B. Structural and functional basis for ADP-ribose and poly(ADP-ribose) binding by viral macro domains. J. Virol. 2006;80:8493–8502. doi: 10.1128/JVI.00713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci. Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzarano, D., de Wit, E., Rasmussen, A.L., Feldmann, F., Okumura, A., Scott, D.P., Brining, D., Bushmaker, T., Martellaro, C., Baseler, L., Benecke, A.G., Katze, M.G., Munster, V.J., Feldmann, H., 2013b. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat. Med., in press (E-published ahead of print, 10.1038/nm.3362). [DOI] [PMC free article] [PubMed]
- Fan H., Ooi A., Tan Y.W., Wang S., Fang S., Liu D.X., Lescar J. The nucleocapsid protein of coronavirus Infectious Bronchitis Virus: crystal structure of its N-terminal domain and multimerization properties. Structure. 2005;13:1859–1868. doi: 10.1016/j.str.2005.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble T.R., Vajdos F.F., Yoo S., Worthylake D.K., Houseweart M., Sundquist W.I., Hill C.P. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87:1285–1294. doi: 10.1016/s0092-8674(00)81823-1. [DOI] [PubMed] [Google Scholar]
- Gillim-Ross L., Subbarao K. Emerging respiratory viruses: challenges and vaccine strategies. Clin. Microbiol. Rev. 2006;19:614–636. doi: 10.1128/CMR.00005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossoehme N.E., Li L., Keane S.C., Liu P., Dann C.E., 3rd, Leibowitz J.L., Giedroc D.P. Coronavirus N protein N-terminal domain (NTD) specifically binds the transcriptional regulatory sequence (TRS) and melts TRS-cTRS RNA duplexes. J. Mol. Biol. 2009;394:544–557. doi: 10.1016/j.jmb.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J., Butt K.M., Wong K.L., Chan K.W., Lim W., Shortridge K.F., Yuen K.Y., Peiris J.S., Poon L.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A.M., Rimmelzwaan G.F., van Amerongen G., van Riel D., de Jong T., Itamura S., Chan K.-H., Tashiro M., Osterhaus A.D.M.E. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson-McReynolds S., Jiang S., Rong L., Caffrey M. Solution structure of the severe acute respiratory syndrome-coronavirus heptad repeat 2 domain in the prefusion state. J. Biol. Chem. 2006;281:11965–11971. doi: 10.1074/jbc.M601174200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänel K., Stangler T., Stoldt M., Willbold D. Solution structure of the X4 protein coded by the SARS related coronavirus reveals an immunoglobulin like fold and suggests a binding activity to integrin I domains. J. Biomed. Sci. 2006;13:281–293. doi: 10.1007/s11373-005-9043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenfeld R., Tan J., Chen S., Shen X., Jiang H. Structural proteomics of emerging viruses: the examples of SARS-CoV and other coronaviruses. In: Sussman J., Silman I., editors. Structural Proteomics. World Scientific; Singapore: 2008. pp. 361–433. [Google Scholar]
- Huang Q., Yu L., Petros A.M., Gunasekera A., Liu Z., Xu N., Hajduk P., Mack J., Fesik S.W., Olejniczak E.T. Structure of the N-terminal RNA-binding domain of the SARS CoV nucleocapsid protein. Biochemistry. 2004;43:6059–6063. doi: 10.1021/bi036155b. [DOI] [PubMed] [Google Scholar]
- Hwang W.C., Lin Y., Santelli E., Sui J., Jaroszewski L., Stec B., Farzan M., Marasco W.A., Liddington R.C. Structural basis of neutralization by a human anti-severe acute respiratory syndrome spike protein antibody, 80R. J. Biol. Chem. 2006;281:34610–34616. doi: 10.1074/jbc.M603275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson A.M. Structure of alphacoronavirus transmissible gastroenteritis virus nsp1 has implications for coronavirus nsp1 function and evolution. J. Virol. 2013;87:2949–2955. doi: 10.1128/JVI.03163-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram H., Fan H., Bowman B.R., Ooi A., Jayaram J., Collisson E.W., Lescar J., Prasad B.V. X-Ray structures of the N- and C-terminal domains of a coronavirus nucleocapsid protein: Implications for nucleocapsid formation. J. Virol. 2006;80:6612–6620. doi: 10.1128/JVI.00157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.A., Chatterjee A., Neuman B.W., Wüthrich K. SARS coronavirus unique domain: three-domain molecular architecture in solution and RNA binding. J Mol Biol. 2010;400:724–742. doi: 10.1016/j.jmb.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.A., Jaudzems K., Wüthrich K. NMR structure of the SARS-CoV nonstructural protein 7 in solution at pH 6.5. J. Mol. Biol. 2010;402:619–628. doi: 10.1016/j.jmb.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J.S., Saikatendu K.S., Subramanian V., Neuman B.W., Brooun A., Griffith M., Moy K., Yadav M.K., Velasquez J., Buchmeier M.J., Stevens R.C., Kuhn P. Crystal structure of nonstructural protein 10 from the severe acute respiratory syndrome coronavirus reveals a novel fold with two zinc-binding motifs. J. Virol. 2006;80:7894–7901. doi: 10.1128/JVI.00467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane S.C., Giedroc D.P. Solution structure of mouse hepatitis virus (MHV) nsp3a and determinants of the interaction with MHV nucleocapsid (N) protein. J. Virol. 2013;87:3502–3515. doi: 10.1128/JVI.03112-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops K., Kikkert M., van den Worm S.H., Zevenhoven-Dobbe J.C., van der Meer Y., Koster A.J., Mommaas A.M., Snijder E.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6:e226. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J., Ericsson M., Rottier P.J., Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J. Cell Biol. 1994;124:55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaa n.G.F, van Amerongen G., van Riel D., Laman J.D., de Jon g T., van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.C., Stöhr K., Peiris J.S., Osterhaus A.D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K.P., Woo P.C., Li K.S., Huang Y., Tsoi H.W., Wong B.H., Wong S.S., Leung S.Y., Chan K.H., Yuen K.Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung G.M., Hedley A.J., Ho L.M., Chau P., Wong I.O., Thach T.Q., Ghani A.C., Donnelly C.A., Fraser C., Riley S., Ferguson N.M., Anderson R.M., Tsang T., Leung P.Y., Wong V., Chan J.C., Tsui E., Lo S.V., Lam T.H. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann. Int. Med. 2004;141:662–673. doi: 10.7326/0003-4819-141-9-200411020-00006. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–678. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Liang G., Chen Q., Xu J., Liu Y., Lim W., Peiris J.S., Anderson L.J., Ruan L., Li H., Kan B., Di B., Cheng P., Chan K.H., Erdman D.D., Gu S., Yan X., Liang W., Zhou D., Haynes L., Duan S., Zhang X., Zheng H., Gao Y., Tong S., Li D., Fang L., Qin P., Xu W., SARS Diagnosis Working Group Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg. Infect. Dis. 2004;10:1774–1781. doi: 10.3201/eid1010.040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu I.J., Kao C.L., Hsieh S.C., Wey M.T., Kan L.S., Wang W.K. Identification of a minimal peptide derived from heptad repeat (HR) 2 of spike protein of SARS-CoV and combination of HR1-derived peptides as fusion inhibitors. Antiviral Res. 2009;81:82–87. doi: 10.1016/j.antiviral.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B. Interferon Alphacon-1 plus corticosteroids in severe acute respiratory syndrome. J. Am. Med. Assoc. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J., Zhang B., Shi Y., Yan J., Gao G.F. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Luo H., Zheng S., Gui C., Yue L., Yu C., Sun T., He P., Chen J., Shen J., Luo X., Li Y., Liu H., Bai D., Shen J., Yang Y., Li F., Zuo J., Hilgenfeld R., Pei G., Chen K., Shen X., Jiang H. Nucleocapsid protein of SARS coronavirus tightly binds to human cyclophilin A. Biochem. Biophys. Res. Commun. 2004;321:557–565. doi: 10.1016/j.bbrc.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailles A., Blanckaert K., Chaud P., Van der Werf S., Lina B., Caro V., Campese C., Guery B., Prouvost H., Lemaire X., Paty M., Haeghebaert S., Antoine D., Ettahar N., Noel H., Behillil S., Hendricx S., Manuguerra J., Enouf V., La Ruche G., Semaille C., Coignard B., Levy-Bruhl D., Weber F., Saura C., Che D., Investigation Team First cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro Surveill. 2013;18 (June 13, 2013) pii: 20502. [PubMed] [Google Scholar]
- Ma-Lauer Y., Lei J., Hilgenfeld R., von Brunn A. Virus–host interactomes – antiviral drug discovery. Curr. Opin. Virol. 2012;2:614–621. doi: 10.1016/j.coviro.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolaridis I., Wojdyla J.A., Panjikar S., Snijder E.J., Gorbalenya A.E., Berglind H., Nordlund P., Coutard B., Tucker P.A. Structure of the C-terminal domain of nsp4 from feline coronavirus. Acta Crystallogr. D Biol. Crystallogr. 2009;65:839–846. doi: 10.1107/S0907444909018253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturrese P., Giammarioli A.M., Cauda R., Malorni W. Antiapoptotic activity by HIV protease inhibitors either alone or boostered. J. Acquir. Immun. Defic. Syndr. 2002;31:545–546. doi: 10.1097/00126334-200212150-00015. [DOI] [PubMed] [Google Scholar]
- Meier C., Aricescu A.R., Assenberg R., Aplin R.T., Gilbert R.J., Grimes J.M., Stuart D.I. The crystal structure of ORF-9b, a lipid binding protein from the SARS coronavirus. Structure. 2006;14:1157–1165. doi: 10.1016/j.str.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern B., Michaelis M., Baer P.C., Doerr H.W., Cinatl J., Jr. Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem. Biophys. Res. Commun. 2005;326:905–908. doi: 10.1016/j.bbrc.2004.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namy O., Moran S.J., Stuart D.I., Gilbert R.J.C., Brierley I. A mechanical explanation of RNA pseudoknot function in programmed ribosomal frameshifting. Nature. 2006;441:244–247. doi: 10.1038/nature04735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Pekosz A., Lee C.A., Diamond M.S., Fremont D.H. Structure and intracellular targeting of the SARS-coronavirus Orf7a accessory protein. Structure. 2005;13:75–85. doi: 10.1016/j.str.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra M., de Haan C.A.M., Rottier P.J.M. The 29-nucleotide deletion present in human but not in animal severe acute respiratory syndrome coronaviruses disrupts the functional expression of open reading frame 8. J. Virol. 2007;81:13876–13888. doi: 10.1128/JVI.01631-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak A.O., Spaan W.J., Snijder E.J. Nidovirus transcription: how to make sense? J. Gen. Virol. 2006;87:1403–1421. doi: 10.1099/vir.0.81611-0. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T., Cheng V.C., Chan K.H., Tsang D.N., Yung R.W., Ng T.K., Yuen K.Y., SARS study group Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G., Sun D., Rajashankar K.R., Qian Z., Holmes K.V., Li F. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl. Acad. Sci. USA. 2011;108:10696–10701. doi: 10.1073/pnas.1104306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, R.A., Wang, P., Gomaa, M.R., El-Shesheny, R., Kandeil, A., Bagato, O., Siu, L.Y., Shehata, M.M., Kayed, A.S., Moatasim, Y., Li, M., Poon, L.L., Guan, Y., Webby, R.J., Ali, M.A., Peiris, J.S.M., Kayali, G., 2013. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 18, pii: 20574. [DOI] [PubMed]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti W., Johnson M.A., Herrmann T., Neuman B.W., Buchmeier M.J., Nelson M., Joseph J., Page R., Stevens R.C., Kuhn P., Wüthrich K. Structural genomics of the severe acute respiratory syndrome coronavirus: nuclear magnetic resonance structure of the protein nsP7. J. Virol. 2005;79:12905–12913. doi: 10.1128/JVI.79.20.12905-12913.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle S., Schöpf J., Kögl M., Friedel C.C., Müller M.A., Carbajo-Lozoya J., Stellberger T., von Dall’Armi E., Herzog P., Kallies S., Niemeyer D., Ditt V., Kuri T., Züst R., Pumpor K., Hilgenfeld R., Schwarz F., Zimmer R., Steffen I., Weber F., Thiel V., Herrler G., Thiel H.-J., Schwegmann-Weßels C., Pöhlmann S., Haas J., Drosten C., von Brunn A. The SARS-coronavirus-host interactome: Identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011;7:e1002331. doi: 10.1371/journal.ppat.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski Y., Hansen G., Boomaars-van der Zanden A.L., Snijder E.J., Gorbalenya A.E., Hilgenfeld R. Crystal structures of the X-domains of a Group-1 and a Group-3 coronavirus reveal that ADP-ribose-binding may not be a conserved property. Protein Sci. 2009;18:6–16. doi: 10.1002/pro.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy R., Moll R., Weimar T., Mesters J.R., Hilgenfeld R. Variable oligomerization modes in coronavirus non-structural protein 9. J. Mol. Biol. 2008;383:1081–1096. doi: 10.1016/j.jmb.2008.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R., Muth D., Demmers J.A., Zaki A., Fouchier R.A., Thiel V., Drosten C., Rottier P.J., Osterhaus A.D., Bosch B.J., Haagmans B.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratia K., Saikatendu K.S., Santarsiero B.D., Barretto N., Baker S.C., Stevens R.C., Mesecar A.D. Severe acute respiratory syndrome coronavirus papain-like protease: structure of a viral deubiquitinating enzyme. Proc. Natl. Acad. Sci. USA. 2006;103:5717–5722. doi: 10.1073/pnas.0510851103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera J., Santiago C., Mudgal G., Ordoño D., Enjuanes L., Casasnovas J.M. Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. Plos Pathog. 2012;8:e1002859. doi: 10.1371/journal.ppat.1002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z., Yan L., Zhang N., Guo Y., Yang C., Lou Z., Rao Z. The newly emerged SARS-Like coronavirus HCoV-EMC also has an “Achilles’ heel”: current effective inhibitor targeting a 3C-like protease. Prot. Cell. 2013;4:248–250. doi: 10.1007/s13238-013-2841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B.E.M., Haagmans B.L., Müller M.A., Gutierrez C., Godeke G.J., Meyer B., Muth D., Raj V.S., Smits-De Vries L., Corman V.M., Drexler J.F., Smits S.L., El Tahir Y.E., de Sousa R., van Beek J., Nowotny N., van Maanen K., Hidalgo-Hermoso E., Bosch B.J., Rottier P., Osterhaus A., Gortázar-Schmidt C., Drosten C., Koopmans M.P.G. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative seroligical study. Lancet Infect. Dis. 2013 doi: 10.1016/S1473-3099(13)70164-6. in press (E-published: August 09, 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricagno S., Egloff M.P., Ulferts R., Coutard B., Nurizzo D., Campanacci V., Cambillau C., Ziebuhr J., Canard B. Crystal structure and mechanistic determinants of SARS coronavirus nonstructural protein 15 define an endoribonuclease family. Proc. Natl. Acad. Sci. USA. 2006;103:11892–11897. doi: 10.1073/pnas.0601708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M.P., Igel H., Baertsch R., Haussler D., Ares M., Jr, Scott W.G. The structure of a rigorously conserved RNA element within the SARS virus genome. PloS Biol. 2004;3:86–94. doi: 10.1371/journal.pbio.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Saikatendu K.S., Joseph J.S., Subramanian V., Clayton T., Griffith M., Moy K., Velasquez J., Neuman B.W., Buchmeier M.J., Stevens R.C., Kuhn P. Structural basis of severe acute respiratory syndrome coronavirus ADP-ribose-1″-phosphate dephosphorylation by a conserved domain of nsP3. Structure. 2005;13:1665–1675. doi: 10.1016/j.str.2005.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikatendu K.S., Joseph J.S., Subramanian V., Neuman B.W., Buchmeier M.J., Stevens R.C., Kuhn P. Ribonucleocapsid formation of severe acute respiratory syndrome coronavirus through molecular action of the N-terminal domain of N protein. J. Virol. 2007;81:3913–3921. doi: 10.1128/JVI.02236-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz B., Jr., Mossel E.C., Gallaher W.R., Wimley W.C., Peters C.J., Wilson R.B., Garry R.F. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) infectivity by peptides analogous to the viral spike protein. Virus Res. 2006;120:146–155. doi: 10.1016/j.virusres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S.G., Sawicki D.L., Younker D., Meyer Y., Thiel V., Stokes H., Siddell S.G. Functional and genetic analysis of coronavirus replicase–transcriptase proteins. PLoS Pathog. 2005;1:e39. doi: 10.1371/journal.ppat.0010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S.G., Sawicki D.L., Siddell S.G. A contemporary view of coronavirus transcription. J. Virol. 2007;81:20–29. doi: 10.1128/JVI.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P., Johnson M.A., Almeida M.S., Horst R., Herrmann T., Joseph J.S., Neuman B.W., Subramanian V., Saikatendu K.S., Buchmeier M.J., Stevens R.C., Kuhn P., Wüthrich K. Nuclear magnetic resonance structure of the N-terminal domain of nonstructural protein 3 from the severe acute respiratory syndrome coronavirus. J. Virol. 2007;81:12049–12060. doi: 10.1128/JVI.00969-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P., Johnson M.A., Chatterjee A., Neuman B.W., Joseph J.S., Buchmeier M.J., Kuhn P., Wüthrich K. Nuclear magnetic resonance structure of the nucleic acid-binding domain of severe acute respiratory syndrome coronavirus nonstructural protein 3. J. Virol. 2009;83:12998–13008. doi: 10.1128/JVI.01253-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie J.J., Fang J.M., Kuo T.H., Kuo C.J., Liang P.H., Huang H.J., Wu Y.T., Jan J.T., Cheng Y.S., Wong C.H. Inhibition of the severe acute respiratory syndrome 3CL protease by peptidomimetic alpha, beta-unsaturated esters. Bioorg. Med. Chem. 2005;13:5240–5252. doi: 10.1016/j.bmc.2005.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So, L.K.Y., Lau, A.C.-W., Yam, L.Y.-C., 2003. SARS Treatment. In: Kamps, B.S., Hoffmann, C. (Eds.), www.SARSreference.com, pp. 144–166 (last accessed on 14.07.13).
- Su D., Lou Z., Sun F., Zhai Y., Yang H., Zhang R., Joachimiak A., Zhang X.C., Bartlam M., Rao Z. Dodecamer structure of severe acute respiratory syndrome coronavirus nonstructural protein nsp10. J. Virol. 2006;80:7902–7908. doi: 10.1128/JVI.00483-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar V.M., Bruckmann C., Ingallinella P., Bianchi E., Pessi A., Carfí A. Structure of a proteolytically resistant core from the severe acute respiratory syndrome coronavirus S2 fusion protein. Proc. Natl. Acad. Sci. USA. 2004;101:17958–19763. doi: 10.1073/pnas.0406128102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton G., Fry E., Carter L., Sainsbury S., Walter T., Nettleship J., Berrow N., Owens R., Gilbert R., Davidson A., Siddell S., Poon L.L., Diprose J., Alderton D., Walsh M., Grimes J.M., Stuart D.I. The nsp9 replicase protein of SARS-coronavirus, structure and functional insights. Structure. 2004;12:341–353. doi: 10.1016/j.str.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M., Chang C.K., Ikeya T., Güntert P., Chang Y.H., Hsu Y.L., Huang T.H., Kainosho M. Solution structure of the C-terminal dimerization domain of SARS coronavirus nucleocapsid protein solved by the SAIL-NMR method. J. Mol. Biol. 2008;380:608–622. doi: 10.1016/j.jmb.2007.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Verschueren K.H., Anand K., Shen J., Yang M., Xu Y., Rao Z., Bigalke J., Heisen B., Mesters J.R., Chen K., Shen X., Jiang H., Hilgenfeld R. PH-dependent conformational flexibility of the SARS-CoV main proteinase (Mpro) dimer: molecular dynamics simulations and multiple X-ray structure analyses. J. Mol. Biol. 2005;354:25–40. doi: 10.1016/j.jmb.2005.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Vonrhein C., Smart O.S., Bricogne G., Bollati M., Kusov Y., Hansen G., Mesters J.R., Schmidt C.L., Hilgenfeld R. The SARS-unique domain (SUD) of SARS coronavirus contains two macrodomains that bind G-quadruplexes. PLoS Pathog. 2009;5:e1000428. doi: 10.1371/journal.ppat.1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Chinese SARS Molecular Epidemiology Consortium Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- Tylor S., Andonov A., Cutts T., Cao J., Grudesky E., van Domselaar G., Li X., He R. The SR-rich motif in SARS-CoV nucleocapsid protein is important for virus replication. Can. J. Microbiol. 2009;55:254–260. doi: 10.1139/w08-139. [DOI] [PubMed] [Google Scholar]
- van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C., Wertheim-van Dillen P.M., Kaandorp J., Spaargaren J., Berkhout B. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Chen J., Zheng A., Nie Y., Shi X., Wang W., Wang G., Luo M., Liu H., Tan L., Song X., Wang Z., Yin X., Qu X., Wang X., Qing T., Ding M., Deng H. Expression cloning of functional receptor used by SARS coronavirus. Biochem. Biophys. Res. Commun. 2004;315:439–444. doi: 10.1016/j.bbrc.2004.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P., Guo D., Fu L., Cui Y., Liu X., Arledge K.C., Chen Y.-H., Zhang L., Wang X. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23:986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Western Pacific Region . WHO Press; 2006. SARS: How a Global Epidemic was Stopped. p. 306. [Google Scholar]
- Wojdyla J.A., Manolaridis I., Snijder E.J., Gorbalenya A.E., Coutard B., Piotrowski Y., Hilgenfeld R., Tucker P.A. Structure of the X (ADRP) domain of nsp3 from feline coronavirus. Acta Crystallogr. D Biol. Crystallogr. 2009;65:1292–1300. doi: 10.1107/S0907444909040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojdyla J.A., Manolaridis I., van Kasteren P.B., Kikkert M., Snijder E.J., Gorbalenya A.E., Tucker P.A. Papain-like protease 1 from transmissible gastroenteritis virus: crystal structure and enzymatic activity toward viral and cellular substrates. J Virol. 2010;84:10063–10073. doi: 10.1128/JVI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y., Wong B.H., Poon R.W., Cai J.J., Luk W.K., Poon L.L., Wong S.S., Guan Y., Peiris J.S., Yuen K.Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Multicentre Collaborative Network for Severe Acute Respiratory Syndrome Diagnosis A multicentre collaboration to investigate the cause of severe acute respiratory syndrome. Lancet. 2003;361:1730–1733. doi: 10.1016/S0140-6736(03)13376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.Y., Jan J.T., Ma S.H., Kuo C.J., Juan H.F., Cheng Y.S.E., Hsu H.H., Huang H.C., Wu D., Brik A., Liang F.S., Liu R.S., Fang J.M., Chen S.T., Liang P.H., Wong C.H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. USA. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Li W., Peng G., Li F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc. Natl. Acad. Sci. USA. 2009;106:19970–19974. doi: 10.1073/pnas.0908837106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Ma Q., Restle T., Shang W., Svergun D.I., Ponnusamy R., Sczakiel G., Hilgenfeld R. Nonstructural proteins 7 and 8 of feline coronavirus form a 2:1 heterotrimer that exhibits primer-independent RNA polymerase activity. J. Virol. 2012;86:4444–4454. doi: 10.1128/JVI.06635-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.H., He J.F., Evans M.R., Peng G.W., Field H.E., Yu D.W., Lee C.K., Luo H.M., Lin W.S., Lin P., Li L.H., Liang W.J., Lin J.Y., Schnur A. Epidemiologic clues to SARS origin in China. Emerg. Infect. Dis. 2004;10:1030–1037. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Liu Y., Lou Z., Qin L., Li X., Bai Z., Pang H., Tien P., Gao G.F., Rao Z. Structural basis for coronavirus-mediated membrane fusion: crystal structure of mouse hepatitis virus spike protein fusion core. J. Biol. Chem. 2004;279:30514–30522. doi: 10.1074/jbc.M403760200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhai Y., Sun F., Lou Z., Su D., Xu Y., Zhang R., Joachimiak A., Zhang X.C., Bartlam M., Rao Z. New antiviral target revealed by the hexameric structure of mouse hepatitis virus nonstructural protein nsp15. J. Virol. 2006;80:7909–7917. doi: 10.1128/JVI.00525-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Cong L., Chen C., Wei L., Zhao Q., Xu X., Ma Y., Bartlam M., Rao Z. Crystal structures of two coronavirus ADP-ribose-1″-monophosphatases and their complexes with ADP-ribose: a systematic structural analysis of the viral ADRP domain. J. Virol. 2009;83:1083–1092. doi: 10.1128/JVI.01862-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Lou Z., Ma Y., Chen X., Yang Z., Tong X., Zhao Q., Xu Y., Deng H., Bartlam M., Rao Z. Crystal structure of the C-terminal cytoplasmic domain of non-structural protein 4 from mouse hepatitis virus A59. PLoS One. 2009;4:e6217. doi: 10.1371/journal.pone.0006217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X., Yu H., Yang H., Xue F., Wu Z., Shen W., Li J., Zhou Z., Ding Y., Zhao Q., Zhang X.C., Liao M., Bartlam M., Rao Z. Structures of two coronavirus main proteases: implications for substrate binding and antiviral drug design. J. Virol. 2008;82:2515–2527. doi: 10.1128/JVI.02114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Yang M., Ding Y., Liu Y., Lou Z., Zhou Z., Sun L., Mo L., Ye S., Pang H., Gao G.F., Anand K., Bartlam M., Hilgenfeld R., Rao Z. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. USA. 2003;100:13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., Zhao Q., Zhou Z., Pei D., Ziebuhr J., Hilgenfeld R., Yuen K.X., Wong L., Gao G., Chen S., Chen Z., Ma D., Bartlam M., Rao Z. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3:1742–1752. doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.M., Oldham M.L., Zhang J., Chen J. Crystal structure of the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein dimerization domain reveals evolutionary linkage between corona- and arteriviridae. J. Biol. Chem. 2006;281:17134–17139. doi: 10.1074/jbc.M602107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zeng Q., Langereis M.A., van Vliet A.L., Huizinga E.G., de Groot R.J. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc. Natl. Acad. Sci. USA. 2008;105:9065–9069. doi: 10.1073/pnas.0800502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y., Sun F., Li X., Pang H., Xu X., Bartlam M., Rao Z. Insights into SARS-CoV transcription and replication from the structure of the nsp7-nsp8 hexadecamer. Nat. Struct. Mol. Biol. 2005;12:980–986. doi: 10.1038/nsmb999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Zhang F., Xu M., Huang K., Zhong W., Cai W., Yin Z., Huang S., Deng Z., Wei M., Xiong J., Hawkey P.M. Description and treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J. Med. Microbiol. 2003;52:715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Li S., Xue F., Zou Y., Chen C., Bartlam M., Rao Z. Structure of the main protease from a global infectious human coronavirus, HCoV-HKU1. J. Virol. 2008;82:8647–8655. doi: 10.1128/JVI.00298-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Deng Y., Liu J., van der Hoek L., Berkhout B., Lu M. Core structure of S2 from the human coronavirus NL63 spike glycoprotein. Biochemistry. 2006;45:15205–15215. doi: 10.1021/bi061686w. [DOI] [PubMed] [Google Scholar]


