Abstract
Bone remodeling is a strictly regulated dynamic process that cycles between bone formation and resorption, and interleukin‐17 (IL‐17) critically orchestrates the activation and differentiation of both osteoblasts and osteoclasts. Mesenchymal stem cells (MSCs) within their native environment receive biochemical stimuli from surrounding cells that influences their differentiation into bone precursors, while the roles of osteocytes in regulating the osteogenic differentiation of MSCs remain unclear. This study investigated the specific roles of IL‐17 signaling cascades and osteocyte‐specific pathways in the osteogenesis of MSCs. Using a transwell coculture (CC) system, we explored the effects of osteocytes and osteoblasts on the osteogenesis of MSCs with and without IL‐17 supplementation. A polycaprolactone (PCL) three‐dimensional (3D) culture model was used to evaluate their osteogenic potential in the presence of osteocytes and IL‐17. Notably, IL‐17 induced osteogenesis in MSCs, which could be attenuated by blocking IL‐17 receptor A. The osteogenic differentiation of MSCs promoted by IL‐17 was further enhanced by CC with osteocytes. Moreover, proinflammatory cytokines IL‐6 and IL‐1β played an important role in IL‐17‐dependent differentiation, via the phosphorylation of AKT, signal transducer and activator of transcription 3, and extracellular signal‐regulated kinase 1/2 signaling pathways in the MSC niche. The present study confirms a synergistic effect of osteocytes and IL‐17 in the production of biochemical signals to stimulate the osteogenic differentiation of MSCs, which could be further promoted in the PCL 3D‐scaffold. These findings provide important insight into the mechanisms of MSCs activation and osteogenic differentiation within the native stem cell niche, and suggest a possible role of IL‐17 in bone tissue engineering.
Keywords: interleukin‐17, mesenchymal stem cells, osteocytes, osteogenesis
This study investigated the specific roles of interleukin‐17 (IL‐17) signaling cascades and osteocyte‐specific pathways in the osteogenesis of mesenchymal stem cells (MSCs). The results show a synergistic effect of osteocytes and IL‐17 in the osteogenic differentiation of MSCs, which could be further promoted by polycaprolactone three‐dimensional scaffold. Inflammatory factors IL‐6 and IL‐1β play an important role in IL‐17‐dependent differentiation, and AKT, signal transducer and activator of transcription 3 and extracellular signal‐regulated kinase 1/2 signaling pathways in the MSC niche are activated by osteocytes and IL‐17.
1. INTRODUCTION
Bone loss diseases have significant effects on the activities of daily living, are caused by disruptions in the delicate balance between bone formation by osteoblasts and bone resorption by osteoclasts (Ikebuchi et al., 2018). Once bone tissue is subjected to significant damage or deformation, localized self‐repair is challenging, and innovative treatment strategies are needed to regenerate bone and recover the original anatomical structure. Inflammatory cytokines play key pathogenetic roles in diseases characterized by bone loss (Abrahamsen, Bonnevie‐Nielsen, Ebbesen, Gram, & Beck‐Nielsen, 2000; Lubberts, 2015). Yet, at the initial stages of bone repair, inflammation also plays a key role in aiding bone regeneration (Marsell & Einhorn, 2011). Bone repair is a complex process, initiated by the release of various inflammatory substances, and the subsequent remodeling of a callus type tissue, coordinated by the interactions between osteoblasts and osteoclasts (Mountziaris & Mikos, 2008).
Interleukin (IL)‐17 is a proinflammatory cytokine and an important trigger for bone remodeling (Kim et al., 2014; Sebastian, Kannan, Norazmi, & Nurul, 2018). In the previous review, we concluded that IL‐17 regulates the differentiation of various cells involved in bone remodeling, including osteoblasts, osteoclasts, and periodontal ligament cells (Liao, Zhang, & Yang, 2017). In early studies, the role of IL‐17 in promoting osteoclastic differentiation was confirmed: excessive IL‐17 in osteoarthritic diseases exacerbates bone damage (Akitsu et al., 2015). Our previous study also found that IL‐17 can promote osteoclast differentiation with the help of osteocytes (Liao et al., 2017). In recent years, the IL‐17‐promoting osteogenic effect has been reported, that is, IL‐17 promotes osteoblast differentiation and maturation, which is manifested by increased extracellular matrix calcium deposition and alkaline phosphatase activity (Kocic et al., 2012; Zhang et al., 2011).
Mesenchymal stem cells (MSCs) are multipotent progenitor cells and their osteogenic differentiation potential has been used to promote periodontal tissue regeneration by autologous transplantation of MSCs (Chen et al., 2016; Kawaguchi et al., 2004). IL‐17 receptor A (IL‐17RA) is particularly highly expressed on MSCs, in both humans and mice (Miossec & Kolls, 2012; Osta, Lavocat, Eljaafari, & Miossec, 2014). Studies have also reported the potential effects of IL‐17 on the osteogenic differentiation of hMSCs (Croes et al., 2016; Huang et al., 2009). However, the regulation mechanism of IL‐17 on MSCs remains unclear.
MSCs are found within the stem cell niche environment in vivo (Li & Xie, 2005). The stem cell niche in a bone comprises MSCs and their progenies, and a group of supporting cells, including fibroblasts, endothelial cells, adipocytes, osteoblasts, and osteocytes (Kuhn & Tuan, 2010). The supporting cells guide niche function and the activities of these cells (Schofield, 1978). In particular, osteoblasts and osteocytes are crucial regulators of bone formation (Csaki, Matis, Mobasheri, & Shakibaei, 2009; Heino, Hentunen, & Vaananen, 2004). Osteocytes are derived from osteoblasts and become embedded in the bone matrix at the end of osteogenic differentiation. Osteocytes are believed to be responsible for the initiation of bone adaptation (Bellido, 2014). We previously found that both IL‐17 and IL‐17RA were expressed in the osteocyte‐like MLO‐Y4 cell line and induced osteoclastogenesis of macrophage under cocultured (CC) conditions (Liao et al., 2017). However, how osteocytes modulate osteogenesis of MSCs is still unclear.
Mitogen‐activated protein kinases (MAPK) pathways are critical in IL‐17 signaling (Moseley, Haudenschild, Rose, & Reddi, 2003; Song & Qian, 2013). A previous study showed that IL‐17 can induce the osteogenic differentiation of C2C12 immortalized mouse myoblasts via the activation of extracellular signal‐regulated kinase 1/2 (ERK1/2) signaling (Kocic et al., 2012). Additionally, the signal transducer and activator of transcription 3 (STAT3) pathway may be activated by IL‐17‐induced osteoblast differentiation in ankylosing spondylitis (Jo et al., 2018). However, IL‐17 signaling in osteogenesis of MSCs still needs to be explored.
This study aimed to investigate how IL‐17 mediates the osteogenic differentiation of MSCs specifically through the signaling pathways activated by osteocytes. We performed CC experiments using previously established osteoblast and osteocyte cell lines and MSCs, supplementing with IL‐17 or relevant inhibitors. Further, we used a three‐dimensional (3D) polycaprolactone (PCL) scaffold culture to investigate the osteogenic potential of MSCs induced by IL‐17 and osteocytes. Our findings support the potential benefits of IL‐17–induced MSCs in the treatment of bone loss diseases and in the induction of self‐renewal and self‐repair of damaged osseous tissue.
2. MATERIALS AND METHODS
2.1. Cell culture
2.1.1. Isolation and characterization of mouse bone marrow MSCs
Mouse MSCs (mMSCs) were isolated and cultured from mouse compact bone following a published protocol (Zhu et al., 2010). Briefly, freshly isolated long bones from eight male C57BL/6 mice 2–3 weeks old; Laboratory Animal Unit of the Faculty of Medicine, HKU) were crushed and then digested with collagenase at 37°C for 1 hr. Isolated cells and small bone chips were washed thoroughly and cultured in Dulbecco's modified Eagle's medium/F12 with l‐glutamine and 10% fetal bovine serum (FBS; Sigma‐Aldrich, St. Louis, MO) for several days to allow mesenchymal cells to migrate out from the bone fragments. Adherent cells were passaged further and maintained in a humidified incubator with 5% CO2 at 37°C.
At Passage 1, we removed CD11b+ cells from the cell suspension using CD11b MicroBeads, LS columns, and MiniMACS separators, according to the manufacturer's instructions (Miltenyi Biotec, Teterow, Germany). The CD11b antigen is expressed on bone marrow monocytes/macrophages in mice (Fogg et al., 2006). The remaining cells were collected in the flow through buffer and further cultured.
At Passage 3, cells were subjected to flow cytometric analysis and sorting (BD FACSAria III, San Jose, CA). Cells were harvested and stained for CD105 (endoglin), CD29 (β1 integrin), and Sca‐1 antibody (a murine hematopoietic stem cell and MSC marker; R&D systems; Chang et al., 2013). The CD105+/CD29+/Sca‐1+/CD11b− MSCs were collected and cultured for subsequent experiments (Figure S1). Three different mMSC lines were used for the experiments presented in the present work. The three lines independently showed similar results.
2.1.2. Culture of osteoblast lineage cells MC3T3‐E1, MLO‐A5 and MLO‐Y4
Preosteoblast cell line MC3T3‐E1 cells were cultured in alpha‐modified minimum essential media (α‐MEM) containing 10% FBS, 2 mM l‐glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. The MLO‐A5 (postosteoblast cell line) and MLO‐Y4 (osteocyte‐like cell line) were kind gifts from Dr Lynda Bonewald (Indiana University, Indianapolis, IN). MLO‐Y5 cells express high levels of markers of late osteoblasts and have been used to study the differentiation of osteoblasts into osteocytes (Dallas et al., 2009) and the mineralization process (Barragan‐Adjemian et al., 2006). MLO‐Y4 is a murine‐derived cell line that shares features with primary osteocytes, including high levels of osteocalcin (OCN), low levels of alkaline phosphatase (ALP), and abundant dendritic processes (Kato, Windle, Koop, Mundy, & Bonewald, 1997). The MLO‐A5 and MLO‐Y4 cells were cultured in α‐MEM with 5% FBS (Gibco, Grand Island, NY), 5% calf serum (Gibco), and antibiotics (Rosser & Bonewald, 2012).
2.1.3. CC experiment
MSCs were CC with osteoblasts or osteocytes using transwell supports (12 or 24 mm, 0.4 μm pores; Sigma‐Aldrich). These membranes ensured that the osteoblasts or osteocytes were physically separated from the MSCs to prevent direct intercellular contact. MSCs (6,000 cells/cm2) were cultured in the bottom chamber until subconfluence, whereas MC3T3‐E1 (MC3T3‐E1 CC), MLO‐A5 (MLO‐A5 CC), or MLO‐Y4 (MLO‐Y4 CC) cells (6,000 cells/cm2) were cultured on the insert in their own standard medium for 24 hr before CC. When MSCs reached subconfluence, the inserts, seeded with CC cells, were placed on top of the MSCs in the same wells to create a CC system. Cells on each side of the membrane received the same standard medium (α‐MEM containing 10% FBS, 2 mM l‐glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) or osteogenic medium (OM). As a control, MSCs in the bottom chamber were cultured with MSCs on the insert.
2.2. Osteogenic differentiation and staining
OM contains standard medium supplemented with 50 ng/mL L‐thyroxine, 20 mM β‐glycerol phosphate, 100 nM dexamethasone, and 50 μM ascorbic acid (all from Sigma‐Aldrich). The cell culture or CC medium was changed every 3–4 days. After 7–14 day, cells were fixed in 10% formalin, and stained with Alizarin red and ALP staining (Sigma‐Aldrich) to analyze the appearance of mineral nodules and ALP expression, respectively, as described elsewhere. For quantification of Alizarin red, cells were destained overnight in 10% (w/v) cetylpyridinium chloride at room temperature with continuous agitation. The absorbance (562 nm) was read using a SpectraMax M2 microplate reader (Molecular Devices, Orleans Drive Sunnyvale, CA; Daniele et al., 2017). ALP activity was determined by an assay based on the hydrolysis of p‐nitrophenylphosphate to p‐nitrophenol (Gharibi, Abraham, Ham & Evans, 2011). Cells were washed with phosphate‐buffered saline, and 50 ml of phosphatase substrate/alkaline buffer (pH 10.3) was added to each well. After 20 min in the dark, absorbance (405 nm) was read on a SpectraMax M2 microplate reader. ALP activity was normalized to protein concentration in parallel experimental plates. For all staining, there were three independent experiments with at least two repeated wells each time.
2.3. Exogenous IL‐17A induction and IL‐17RA blocking
MSCs, MC3T3‐E1, MLO‐A5, and MLO‐Y4 cells were stimulated with 0.5, 5, or 50 ng/ml IL‐17 (R&D Systems, Minneapolis, MN) diluted in the medium. For the blocking assay, cells were treated with 0.8 μg/ml IL‐17A receptor neutralizing antibody (R&D Systems) for 2 hr in serum‐free medium, and then stimulated with 50 ng/ml IL‐17. The control group was cultured in the standard medium under the same conditions.
2.4. IL‐6 and IL‐1β antibody blocking and MEK1/2, JNK, STAT3 signaling inhibition
For antibody blocking experiments, MSCs were treated with 8 μg/ml purified anti‐mouse IL‐1β antibody or 8 μg/ml purified anti‐mouse IL‐6 antibody (BioLegend, San Diego, CA) in serum‐free medium for 2 hr and then stimulated with 50 ng/ml IL‐17. IgG (BioLegend) was used in the control group under the same conditions.
To study signaling inhibition, when MSCs reached subconfluence, MSCs were treated with 10 μM of the AKT inhibitor Perifosine, the JAK1 and JAK2 inhibitor AZD1480 (which is used to inhibit STAT3 signaling), or the MEK1/2 inhibitor U0126 (all obtained from InvivoGen, San Diego, CA), all solubilized in 0.1% dimethylsulfoxide (DMSO), in serum‐free medium for 2 hr, and then stimulated with 50 ng/ml IL‐17. DMSO alone was used in the control group under the same conditions.
2.5. Real‐time quantitative polymerase chain reaction
The total RNA isolated from each sample was reverse‐transcribed using SuperScript III (Thermo Fisher Scientific) in a Veriti Thermal Cycler (Applied Biosystems, Foster City, CA). The polymerase chain reaction (PCR) amplification was performed using a StepOnePlus Real‐Time PCR System (Applied Biosystems). The target genes were normalized to GAPDH. The messenger RNA (mRNA) expression of runt‐related transcription factor 2 (RUNX2), OCN, COL‐1, IL‐17R, IL‐6, and IL‐1β was evaluated. The primer sequences are shown in Table 1.
Table 1.
Primers used in real‐time polymerase chain reaction gene expression analysis
| Gene | Primer sequences (5′‐3′) |
|---|---|
| GAPDH | |
| Forward | GCATCTCCCTCACAATTTCCA |
| Reverse | TGCAGCGAACTTTATTGATGGT |
| ALP | |
| Forward | AACCCAGACACAAGCATTCC |
| Reverse | GCCTTTGAGGTTTTTGGTCA |
| RUNX2 | |
| Forward | TTCTCCAACCCACGAATGCAC |
| Reverse | CAGGTACGTGTGGTAGTGAGT |
| OCN | |
| Forward | CGCTCTGTCTCTCTGACCTC |
| Reverse | TCACAAGCAGGGTTAAGCTC |
| COL‐1 | |
| Forward | AATGGTGCTCCTGGTATTGC |
| Reverse | GGCACCAGTGTCTCCTTTGT |
| IL‐17RA | |
| Forward | TCAGGGCCAGTGTGAAAACA |
| Reverse | CATGTCGGGTTATCAGGGAAA |
| IL‐6 | |
| Forward | TAGTCCTTCCTACCCCAATTTCC |
| Reverse | TTGGTCCTTAGCCACTCCTTC |
| IL‐1β | |
| Forward | GCAACTGTTCCTGAACTCAACT |
| Reverse | ATCTTTTGGGGTCCGTCAACT |
Abbreviations: ALP, Alkaline phosphatase; COL, collagen; IL, interleukin; OCN, osteocalcin; RA, receptor A; RUNX2, runt‐related transcription factor 2.
2.6. Enzyme‐linked immunosorbent assay
The media were collected and centrifuged to remove cell debris. The expression levels of IL‐6 and IL‐1β in the culture supernatants were measured by enzyme‐linked immunosorbent assay (all kits from R&D Systems) according to the manufacturer's instructions. The absorbance (450 nm) of each well was determined using a SpectraMax M2 microplate reader (Molecular Devices), with 540 nm used for wavelength correction.
2.7. Western blotting
The cell lysates were prepared using a radioimmunoprecipitation cell‐lysis buffer (Thermo Fisher Scientific). The total protein concentration was determined using the bicinchoninic acid reagent system (Thermo Fisher Scientific). The protein extracts (20 μg of total cell protein) were subjected to 7.5–15% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and electrotransferred onto polyvinylidene difluoride membranes. Nonspecific binding was blocked using Tris‐buffered saline with 0.5% Tween‐20 containing 5% skimmed milk powder. Specific antigens were immunodetected using appropriate primary and secondary antibodies and visualized using a western blotting detection kit (Advansta, San Jose, CA). Membranes were stripped using Restore Western Blot Stripping Buffer (Thermo Fisher Scientific) and reprobed for GAPDH to confirm equal loading. The primary antibodies were: rabbit anti‐pSTAT3, rabbit anti‐pERK1/2, rabbit anti‐pSARK/JNK, rabbit anti‐pp38, rabbit anti‐pAKT and rabbit anti‐GAPDH (1:1,000; Cell Signaling Technology). The secondary antibodies were all obtained from Cell Signaling Technology (1:3,000).
2.8. 3D culture with PCL scaffold
PCL is a biodegradable polyester material approved by the FDA for its use in implants, adhesion barriers, and drug delivery devices. More recently, PCL has also been exploited as a biomaterial for bone tissue engineering (Tarafder & Bose, 2014). In our study, mMSCs were cultured on 21‐mm‐diameter 3D PCL disks (1.5‐mm height, 300‐micron fiber diameter, 300‐micron spacing; 3D Biotek, Hillsborough, NJ). The scaffolds with mMSCs were placed at the bottom chamber of 12‐well Transwell plates and MLO‐Y4 osteocytes seeded in the upper chamber were placed over this 3D culture system in osteogenic media, similar to the other transwell assays. After 10 days of culture in OM with IL‐17, Alizarin red staining was used to analyze the appearance of mineralized nodules.
Furthermore, we repeated the assay with human induced pluripotent stem cell‐derived MSCs (hereafter referred to as hMSCs); these cells were a gift from Dr. Chen (Li Ka Shing Medicine School, HKU) and were previously obtained with a clinically compliant protocol and successfully used in regeneration (Lian et al., 2010; Sun et al., 2012).
2.9. Statistical analysis
We used one‐way or two‐way analysis of variance to compare data from more than two groups, with three levels of significance: *p ≤ .05, **p ≤ .01, and ***p < .001. Data are reported as the mean ± SD. Each experiment was repeated at least three times independently.
3. RESULTS
3.1. IL‐17 enhances the osteogenic differentiation of mMSCs
To explore the osteogenic role of IL‐17 at different stages along the osteoblastic lineage, we first examined the effects of IL‐17 on the mineralization of mMSCs, MC3T3‐E1 pre‐osteoblasts, MLO‐A5 postosteoblasts, and MLO‐Y4 osteocytes. We found enhanced mineralized nodule formation in mMSCs in response to IL‐17 under osteogenic induction conditions (14 days) and also in MLO‐Y4, especially at high IL‐17 concentrations (14 days; Figure 1a,b). However, IL‐17 seemingly inhibited osteoblastic differentiation of MC3T3‐E1 cells (14 days) and had no effect on MLO‐A5 cells (10 days). MLO‐A5 cultures were assessed at the earlier timepoint because of their faster mineralization speed as compared with the other three type of cells (Helfrich & Ralston, 2012). In a standard medium (without osteogenic supplements), none of the cells formed nodules (data not shown).
Figure 1.
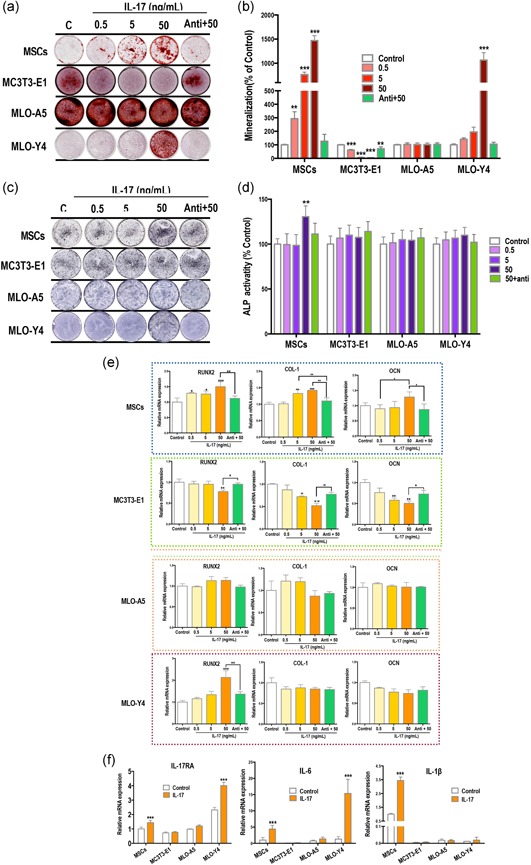
IL‐17 enhances the osteogenic differentiation of mMSCs but not MC3T3‐E1. (a,b) Increased mineralized nodule formation (red) in mesenchymal stem cells (MSCs) and MLO‐Y4 osteocytes after treatment with 0.5–50 ng/ml IL‐17 in osteogenic induction medium for 14 days. Mineralization of MC3T3‐E1 cells was inhibited, and there was no change in mineralization levels in MLO‐A5 cells. (c,d) Alkaline phosphatase (ALP) expression (purple) of MSCs was enhanced by IL‐17 (7 days). (e,f) mRNA expression of factors after IL‐17 treatment for 24 hr. Treatment with IL‐17 increased runt‐related transcription factor 2 (RUNX2), collagen‐1 (COL‐1) and osteocalcin (OCN) in MSCs, and only RUNX2 in MLO‐Y4 cells. All three factors were decreased in MC3T3‐E1 cells and no significant changes were measured in MLO‐A5 cells. (f) IL‐17 receptor A (RA) and IL‐6 were upregulated in MSCs and MLO‐Y4 cells, and IL‐β was significantly upregulated in MSCs. IL, interleukin; mMSC, mouse MSCs; mRNA, messenger RNA
ALP staining was performed on Day 7. We found increased ALP expression in mMSCs with high concentrations of IL‐17, but no evident change in ALP expression in MC3T3‐E1, MLO‐A5 cells or MLO‐Y4 (Figure 1b,c).
We next examined changes in the expression of various osteogenic differentiation factors after IL‐17 stimulation for 24 hr. The relative mRNA expression levels of RUNX2, collagen‐1 (COL‐1) and OCN in MSCs were enhanced by IL‐17 in a concentration‐dependent manner (Figure 1c). Only RUNX2 was increased in MLO‐Y4 cells by IL‐17, and IL‐17 treatment inhibited the expression of RUNX2, COL‐1, and OCN in MC3T3‐E1 cells (Figure 1d). IL‐17 had no significant effect on the expression of osteogenic factors in the MLO‐A5 cells (Figure 1d).
We found that blocking IL‐17RA attenuated the effects of IL‐17 in MSCs, MLO‐Y4, and MC3T3‐E1 cells. Therefore, we next investigated changes in the expression of IL‐17RA and inflammatory factors after IL‐17 stimulation for 24 hr. We found enhanced expression of IL‐17RA mRNA in MSCs and MLO‐Y4 cells after 50 ng/ml IL‐17 stimulation (Figure 1f). IL‐6 expression was higher in MSCs and MLO‐Y4 cells as compared with MC3T3‐E1 and MLO‐Y5 cells, and this expression was further increased after stimulation with IL‐17 (Figure 1f). IL‐1β mRNA expression was also significantly elevated by IL‐17 stimulation in MSCs (Figure 1f).
3.2. MSCs undergo osteogenic differentiation when CC with osteocytes
We used a transwell CC system to investigate the influence of osteoblasts and osteocytes on the osteogenic differentiation of MSCs (Figure 2a). Using Alizarin red staining, we found significant mineralized nodule formation in MSCs CC with MLO‐Y4 cells for 14 days, which was further enhanced following supplementation of 50 ng/ml IL‐17 (Figure 2b,c). There was no change observed in the MC3T3‐E1 and MLO‐A5 cocultures.
Figure 2.
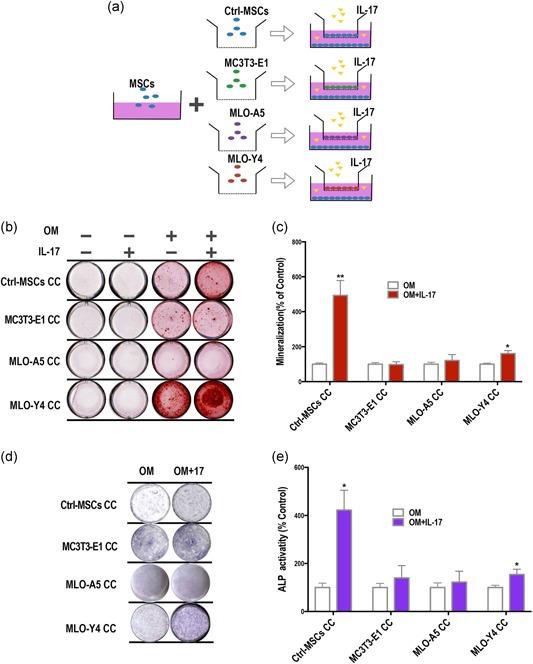
Osteogenic differentiation of mesenchymal stem cells (MSCs) after coculture (CC) with osteocytes is further enhanced by interleukin‐17 (IL‐17). (a) We used a transwell CC system, with MSCs seeded in the lower chamber and various osteogenic cells in the upper chambers. Cells were grown in osteoinductive media (OM) with or without IL‐17. MSCs in both the upper and lower chambers served as a control. (b,c) Alizarin red staining to detect mineralized nodule formation (red) in the lower chambers following CC with or without IL‐17 stimulation (50 ng/ml). IL‐17 enhanced mineralization in control‐MSCs (top row). No obvious change was found for MSCs in MC3T3‐E1 CC and MLO‐A5 CC (second and third rows). An increase in Alizarin red staining was found for MSCs in MLO‐Y4 CC, which was further increased with IL‐17. (d,e) ALP staining and quantification of ALP activity. IL‐17 induced ALP expression (purple) in MSCs under control CC condition (top row). ALP expression was also induced in MSC in CC with MLO‐Y4 cells (bottom row; Day 7). Each experiment was repeated at least three times independently and three different mMSC cell lines were used. ALP, alkaline phosphatase
In the control group, IL‐17 caused an increase in ALP staining in MSCs. Likewise, CC with MLO‐Y4 cells induced ALP expression in MSCs, and this was further enhanced by IL‐17 stimulation(Figure 2d,e). Given the positive effects on MSC osteogenic induction with coculturing, we further investigated the intercellular relationship between MSCs and osteocytes.
3.3. IL‐17 upregulated inflammatory factors IL‐6 and IL‐1β and activated ERK1/2, AKT, and STAT3 signaling in MSC in stem cell niches
We tested for changes in the expression of secreted inflammatory cytokines IL‐6 and IL‐1β after CC of MSCs with MLO‐Y4 osteocytes on days 7, 10, and 14 (Figure 3a). IL‐6 was induced following IL‐17 stimulation and in response to MLO‐Y4 CC, with a significant synergistic effect of MLO‐Y4 CC with IL‐17 treatment. IL‐1β expression followed a similar pattern but at a much lower level. We also noted that both IL‐6 and IL‐1β decreased at Day 14 as compared with Day 7 and Day 10.
Figure 3.
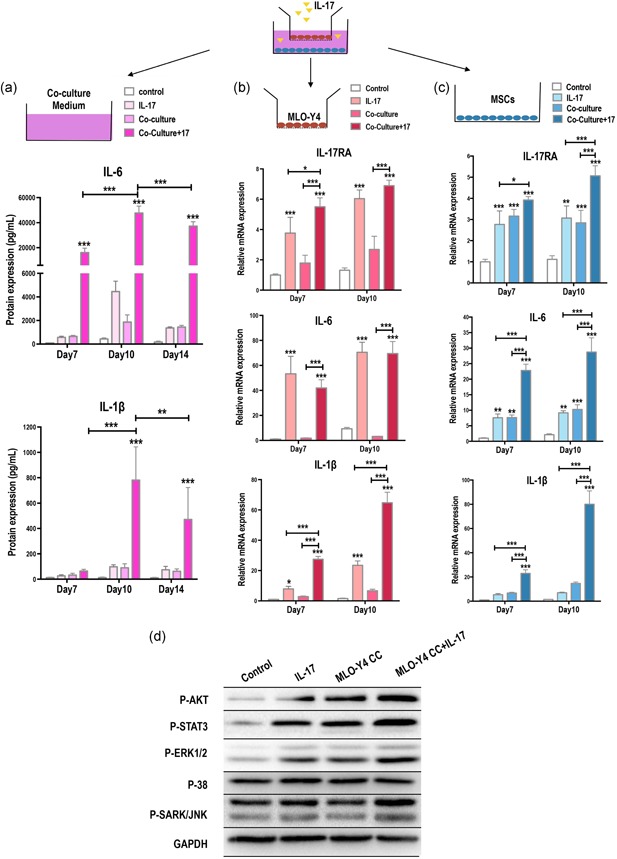
Interleukin (IL)‐17 upregulates inflammatory factors, IL‐6 and IL‐1β, and activates ERK, AKT, and STAT3 signaling in mesenchymal stem cells (MSC)–MLO‐Y4 cocultures (CC). (a) The medium from CCs of MSCs and MLO‐Y4 osteocytes was analyzed by ELISA on days 7, 10, and 14. IL‐6 and IL‐1β were synergistically induced by IL‐17 (50 ng/ml) and osteocyte CC. (b) MLO‐Y4 osteocytes in the upper chamber of the transwell CC were tested for changes in the mRNA expression levels of IL‐17RA, IL‐6, and IL‐1β after IL‐17 treatment. (c) MSCs in the lower chambers of the transwell CC were tested for changes in the mRNA expression levels of IL‐17RA, IL‐6, and IL‐1 β after IL‐17 treatment. (d) Increased phosphorylation of signaling regulators ERK1/2, AKT, and STAT3 after IL‐17 stimulation in MLO‐Y4 CCs. Phosphorylated SARK/JNK and p38 were unchanged (24 hr). ELISA, enzyme‐linked immunosorbent assay; ERK1/2, extracellular signal‐regulated kinase 1/2; mRNA, messenger RNA; RA, receptor A; STAT3, signal transducer and activator of transcription 3. *p < .05, **p < .01, ***p < .001
We next analyzed the expression of inflammatory mediators of MLO‐Y4 osteocytes in the upper chamber and MSCs in the lower chamber separately after CC for 7 and 10 days (Figure 3b). We found that the MLO‐Y4 osteocytes were more sensitive to IL‐17 stimulation than CC treatment, with enriched levels of IL‐17RA, IL‐6 and IL‐1β mRNA (Figure 3c). In the MSCs, IL‐17RA was upregulated by IL‐17 and further enhanced after CC with osteocytes, suggesting that there may be a synergistic activation of downstream signaling induced by IL‐17 and osteocytes. Similarly, osteocyte CC led to a further increase in IL‐17–mediated increase in IL‐6 and IL‐1β. These results highlight a potential synergistic relationship between osteocytes and IL‐17 in the osteoinduction of MSCs. The findings also suggest that IL‐17 activates downstream inflammatory factors, such as IL‐6 and IL‐1β.
To assess the molecular mechanism of osteoblastic differentiation of MSCs, we evaluated the expression of MAPKs, AKT, and STAT3 signaling molecules, which are known to be crucial regulators of osteoblastic differentiation (Baker, Sohn, & Tuan, 2015). We found that as early as 24 hr after stimulation, phosphorylated ERK1/2, AKT, and STAT3 were activated by IL‐17 and further enhanced in MLO‐Y4 CC, whereas there were no changes in the phosphorylation of SARK/JNK or p38 (Figure 3d).
3.4. IL‐17 activates intercellular signaling of osteogenesis via IL‐6 and IL‐1β
Anti‐IL‐6 and anti‐IL‐1β antibodies were used to neutralize IL‐6 and IL‐1β induced by IL‐17 in the CC system. At Day 14, MSC mineralization in OM with IL‐17 was effectively inhibited in the presence of anti‐IL‐6 antibodies both in the control‐MSCs CC and MLO‐Y4 CC. (Figure 4a,b).
Figure 4.
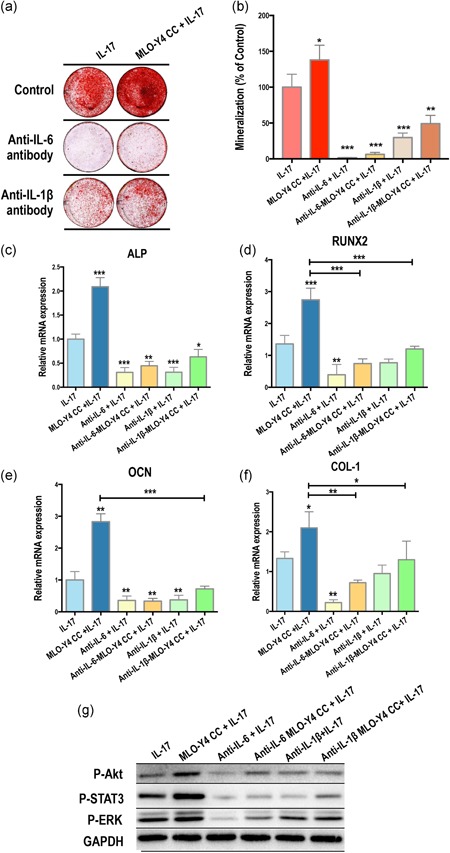
Interleukin (IL)‐17 activates intercellular signaling of osteogenesis via IL‐6 and IL‐1β. Anti‐IL‐6 and anti‐IL‐1β antibodies were used in the IL‐17–induced coculture (CC) system. Both antibodies could effectively inhibit (a,b) the formation of mineral nodules, (c) the mRNA expression of ALP, RUNX2, OCN, and COL‐1 and (d) AKT, STAT3, and ERK1/2 phosphorylation in the control‐MSCs CC and MLO‐Y4 CC at Day 14 after IL‐17 treatment; albeit, the effects of IL‐1β neutralization were weaker than that of IL‐6. ALP, alkaline phosphatase; COL‐1, collagen‐1; ERK1/2, extracellular signal‐regulated kinase 1/2; mRNA, messenger RNA; OCN, osteocalcin; RUNX2, runt‐related transcription factor 2; STAT3, signal transducer and activator of transcription 3
Osteogenic factors, ALP, RUNX2, OCN, and COL‐1, were also analyzed after neutralization with antibodies against IL‐6 and IL‐1β for 24 hr (Figure 4c–f). Consistently, anti‐IL‐6 antibodies significantly downregulated the mRNA expression levels of osteogenic factors in the control‐MSCs CC and MLO‐Y4 CC, with the effects of anti‐IL‐1β much weaker.
Following this, we investigated changes in the activation of AKT, STAT3, and ERK1/2 signaling after antibody blocking for 24 hr (Figure 4e). Activated signaling induced by MLO‐Y4 CC with IL‐17 stimulation was effectively depressed by the IL‐6 antibody, whereas the suppression of anti‐IL‐1β was not as strong as the anti‐IL‐6 antibody.
3.5. AKT, STAT3, and ERK1/2 pathways are activated in osteoblastic differentiation
We inhibited AKT, STAT3, and ERK1/2 pathways in the CC with IL‐17 stimulation to examine their effect in osteogenic differentiation. We first confirmed that the inhibitors could suppress AKT, STAT3, and ERK1/2 expression in MSCs, with a loss of phosphorylation in western blotting (Figure 5a). Next, using Alizarin Red staining, we found a prominent inhibition of mineralization in MSCs grown in the MLO‐Y4 CC system with IL‐17 stimulation on Day 14 (Figure 5b,c) with the weakest inhibition of mineralization seen with the ERK1/2 inhibitor.
Figure 5.
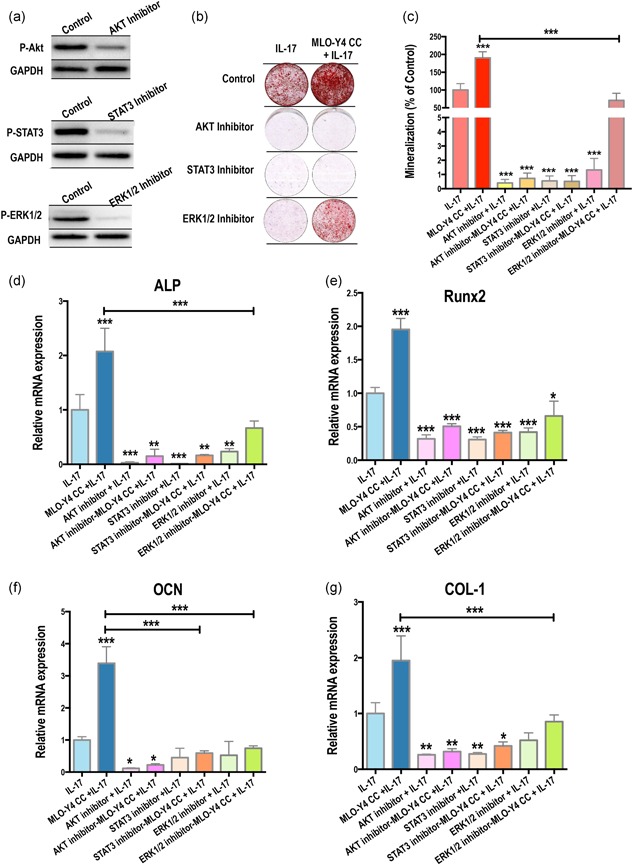
AKT, STAT3, and ERK1/2 pathways are activated in IL‐17–mediated osteoblastic differentiation. (a) Ten micrometer of the AKT inhibitor Perifosine, the JAK1 and JAK2 inhibitor AZD1480 (to inhibit STAT3 signaling), and the MEK1/2 inhibitor U0126, solubilized in 0.1% DMSO in serum‐free medium efficiently suppressed AKT, STAT3, and ERK1/2 expression, respectively, in MSCs. (b,c) IL‐17–induced coculture systems with 10 μM of inhibitors inhibited mineralization (14 days). (d–g) Relative mRNA expression of ALP, RUNX2, OCN, and COL‐1 was significantly decreased by the suppression of AKT, STAT3, and ERK1/2 signaling pathways. ALP, alkaline phosphatase; COL‐1, collagen‐1; ERK1/2, extracellular signal‐regulated kinase 1/2; IL, interleukin; mRNA, messenger RNA; MSC, mesenchymal stem cell; OCN, osteocalcin; RUNX2, runt‐related transcription factor 2; STAT3, signal transducer and activator of transcription 3. (*p < .05, **p < .01, ***p < .001)
We next investigated changes in the relative mRNA expression of osteogenic factors with various kinase inhibitors (Figure 5d–g). We found that suppression of AKT, STAT3, and ERK1/2 pathways led to reductions in the expression of ALP, RUNX2, OCN, and COL‐1, further confirming the role of these pathways in the IL‐17–mediated enhancement of osteoblastic differentiation in MSCs.
3.6. 3D culture on PCL scaffold enhances IL‐17‐stimulated osteogenesis of MSCs when CC with MLO‐Y4 osteocytes
Finally, we employed a PCL scaffold for mMSCs and hMSCs culture and used transwell assay with MLO‐Y4 CC to examine whether the PCL scaffold could further enhance osteogenic differentiation and mineralization. Compared with the control cultures, cultures grown on the PCL scaffold showed an obvious increase in mineralized nodule formation in both mMSCs and hMSCs as early as Day 10 (Figure 6). Additionally, IL‐17 and CC with MLO‐Y4 osteocytes could synergistically enhance osteogenesis in these cultures.
Figure 6.
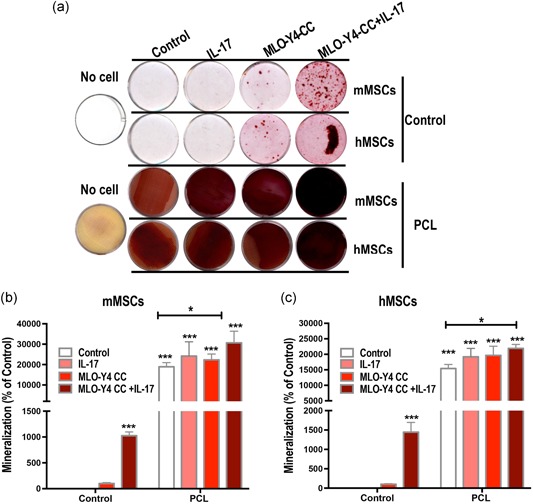
Three‐dimensional culture of mesenchymal stem cells (MSCs) on a polycaprolactone (PCL) scaffold enhances IL‐17‐stimulated osteogenesis when cocultured with MLO‐Y4 osteocytes. (a) Alizarin red staining shows mineralized nodule formation (red) in mouse MSCs (mMSCs) and human‐induced pluripotent stem cell‐derived MSCs (hMSCs) cultures on Day 10. Growth on the PCL scaffold promoted osteogenesis, and this could be further enhanced by coculture with MLO‐Y4 osteocytes and IL‐17 stimulation. (b) Quantification of mMSCs mineralazation. (c) Quantification of hMSCs mineralization. IL, interleukin
4. DISCUSSION
Our study reveals that osteocytes rather than osteoblasts can stimulate the osteogenesis of MSCs. The crucial markers of osteogenic differentiation, calcium deposition, and ALP were notably elevated when MSCs were CC with osteocytes as compared with other cell types or alone (Figure 2b–e). This finding agrees with previous studies suggesting that only osteocyte‐derived factors can stimulate osteogenesis in MSCs (Heino et al., 2004; Hoey, Kelly, & Jacobs, 2011). However, it has been reported previously that early osteogenic markers, such as ALP and bone sialoprotein, can be promoted in MSCs cultured with conditioned media from osteoblasts (Hoey et al., 2011). The discrepancies in the regulatory roles of osteocytes and osteoblasts from previous studies may be attributed to the use of different cell types and species across different work. Furthermore, these variances may reflect the changes to cells that were immortalized at different stages along the osteoblastic lineage. According to our results (Figure 2), MSCs osteogenesis is influenced by the stage at which the cell lines were immortalized (preosteoblast, postosteoblast, and osteocyte).
When both osteocytes and IL‐17 were incorporated in the CC, we found that the biochemical signals enhanced the osteogenic differentiation of MSCs, with increased mineralized nodule formation as compared with the CCs with osteocytes alone or IL‐17–stimulation alone (Figure 2b,c). There are several reasons for the synergistic relationship between osteocytes and IL‐17. The first is the high sensitivity of osteocytes to IL‐17. We found that IL‐17 could induce mineralized nodule formation in osteocytes (Figure 1a,b) high expression levels of IL‐17RA in osteocytes but not in the other cell types under physiological conditions (Figure 1f). Furthermore, IL‐17 stimulated MSCs to produce factors that regulate osteocyte activity and vice versa.
To explain the intercellular interactions, we analyzed the medium from the CC of MSCs and osteocytes and found that the exogenous application of IL‐17 led to higher levels of IL‐6 and IL‐1β (Figure 3a). Exogenous IL‐6 has been found to enhance ALP activity in osteocytes and osteoblasts, and the conditioned medium from IL‐6‐treated osteocytes could stimulate OCN production in osteoblasts at Day 14 (Bakker, Kulkarni, Klein‐Nulend, & Lems, 2014). Moreover, IL‐1β effectively and rapidly induces hMSC mineralization and differentiation into osteoblasts (Sonomoto et al., 2012). In our study, we found that IL‐6 and IL‐1β played essential roles in the osteogenesis of the MSC niche (Figure 4a,b). It confirms that proinflammatory cytokines and osteoinductive factors act synergistically to initiate tissue repair by modulating the differentiation of bone progenitor cells.
To explore the signaling pathways active within these IL‐17–induced events, we analyzed the involvement of the AKT, JAK/STAT, and MAPK pathways, because previous reports have indicated that these molecules are associated with IL‐17 signaling (Hoey et al., 2011; Kocic et al., 2012; Moseley et al., 2003). In our study, we found that IL‐17 activated AKT, STAT3, and ERK1/2 pathways in MSCs and that their activations could be further enhanced in CC with osteocytes (Figure 3d). When IL‐6 and IL‐1β were blocked, the IL‐17–mediated activation of AKT, STAT3, and ERK1/2 was suppressed to varying degrees (Figure 4g), indicating that these signaling molecules depend on IL‐6 and IL‐1β. Individually blocking each of these pathways effectively inhibited IL‐17–induced osteogenesis (Figure 5b,c). This finding confirms that IL‐17 stimulates the osteogenic differentiation of the MSC niche through ERK1/2, STAT3, and AKT activation in an IL‐6/IL‐1β–dependent manner.
In this exploratory process, we found higher levels of IL‐6 in the medium of the stem cell niche than IL‐1β after IL‐17 induction (Figure 3a). Accordingly, blocking IL‐6 was more effective than blocking IL‐1β to inhibit osteogenesis (Figure 4a,b). These findings suggest that IL‐6 plays an important role in the signaling downstream of IL‐17. In the signaling inhibition experiments, we found that the inhibitors of STAT3 and AKT had stronger effects than the inhibitor of ERK1/2 on osteogenesis inhibition, suggesting different transcriptional regulation levels. A balance between ERK1/2 and STAT3 activation has been suggested in many cell types (Pricola, Kuhn, Haleem‐Smith, Song, & Tuan, 2009), and the specific activation of MAP kinases in IL‐17–mediated differentiation independent on the cell type. ERK1/2 activation in response to IL‐17 in MSCs may be attributable to ligand‐specific cell surface receptors.
The use of PCL for tissue engineering has increased significantly over the past decade (Xue et al., 2017). Here, we used a PCL 3D‐scalfold to enhance the osteogenic differentiation potency of MSCs induced by IL‐17 and osteocyte CC. PCL scaffold cell culture is another 3D culture rather than transwell CC, which is the extension of the previous in vitro study. Additionally, we used human MSCs besides mouse MSCs, which further verified the results of mMSCs. Our findings suggest the potential benefits of this combination of agents for bone tissue engineering: an IL‐17–induced MSC niche can promote the self‐renewal and self‐repair of damaged osseous tissue in the context of bone loss. Future studies will further explore the utility of these 3D models for in vivo bone repair.
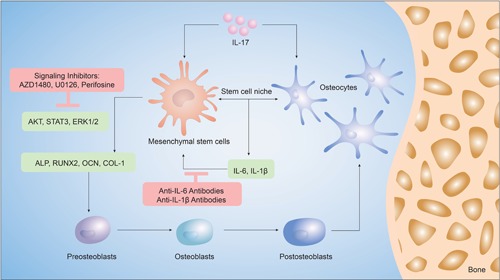
The synergistic effects of IL‐17 and osteocytes in the MSC niche are summarized in Figure 7. We suggest a relationship in the IL‐17–osteocyte network, which provides new insight into the role of osteocytes as regulatory cells that produce the biochemical factors required to drive osteogenesis in the MSC niche.
Figure 7.
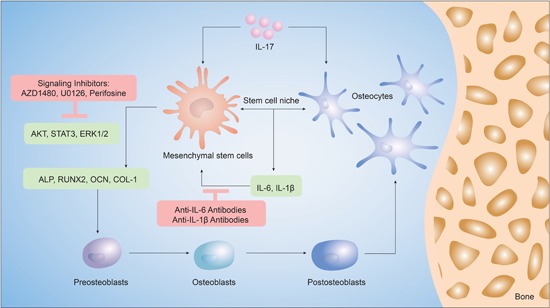
Schematic of the findings of this study. Mesenchymal stem cells (MSCs) exposed to interleukin (IL)‐17 and osteocytes in coculture underwent differentiation along the osteogenic lineage, better than when exposed to either stimulus alone. IL‐17 induces the expression of inflammatory markers, IL‐6 and IL‐1β, which in turn, increase the expression of markers of osteoblastic differentiation in MSCs, such as alkaline phosphatase (ALP), runt‐related transcription factor 2 (RUNX2), osteocalcin (OCN) and collagen‐1 (COL‐1). The activation of extracellular signal‐regulated kinase 1/2 (ERK1/2), AKT, and signal transducer and activator of transcription 3 (STAT3) regulated IL‐17 induced osteogenesis in the MSC niche, which could be inhibited by antibodies of IL‐6 and IL‐1β
In conclusion, osteogenesis of MSCs driven by IL‐17 is enhanced in CC with osteocytes. Inflammatory factors IL‐6 and IL‐1β play important roles in IL‐17‐dependent differentiation, by activating ERK1/2, AKT, and STAT3 signaling pathways in the MSC niche.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Study design: C. L. and Y. Y. Study conduct: C. L. Data collection: C. L. Data analysis: C. L. Data interpretation: C. L, C. Z., L. J. Drafting manuscript: C. L. Revising manuscript content: Y. Y., C. Z., and L. J. Approving final version of manuscript: C. L., C. Z., L. J., and Y. Y. C. L. takes responsibility for the integrity of the data analysis.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was supported by HKU Research Funding (201409176098) and the Fundamental Research Funds for the Central Universities (22120180397).
We thank Rebecca Jackson, PhD, from Liwen Bianji, Edanz Group China, for editing the English text of a draft of this manuscript.
Liao C, Zhang C, Jin L, Yang Y. IL‐17 alters the mesenchymal stem cell niche towards osteogenesis in cooperation with osteocytes. J Cell Physiol. 2020;235:4466–4480. 10.1002/jcp.29323
REFERENCES
- Abrahamsen, B. , Bonnevie‐Nielsen, V. , Ebbesen, E. N. , Gram, J. , & Beck‐Nielsen, H. (2000). Cytokines and bone loss in a 5‐year longitudinal study—hormone replacement therapy suppresses serum soluble interleukin‐6 receptor and increases interleukin‐1‐receptor antagonist: The Danish osteoporosis prevention study. Journal of Bone and Mineral Research, 15(8), 1545–1554. 10.1359/jbmr.2000.15.8.1545 [DOI] [PubMed] [Google Scholar]
- Akitsu, A. , Ishigame, H. , Kakuta, S. , Chung, S. H. , Ikeda, S. , Shimizu, K. , … Iwakura, Y. (2015). IL‐1 receptor antagonist‐deficient mice develop autoimmune arthritis due to intrinsic activation of IL‐17‐producing CCR2(+)Vgamma6(+)gammadelta T cells. Nature Communications, 6, 7464 10.1038/ncomms8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, N. , Sohn, J. , & Tuan, R. S. (2015). Promotion of human mesenchymal stem cell osteogenesis by PI3‐kinase/Akt signaling, and the influence of caveolin‐1/cholesterol homeostasis. Stem Cell Research & Therapy, 6, 238 10.1186/s13287-015-0225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker, A. D. , Kulkarni, R. N. , Klein‐Nulend, J. , & Lems, W. F. (2014). IL‐6 Alters osteocyte signaling toward osteoblasts but not osteoclasts. Journal of Dental Research, 93(4), 394–399. 10.1177/0022034514522485 [DOI] [PubMed] [Google Scholar]
- Barragan‐Adjemian, C. , Nicolella, D. , Dusevich, V. , Dallas, M. R. , Eick, J. D. , & Bonewald, L. F. (2006). Mechanism by which MLO‐A5 late osteoblasts/early osteocytes mineralize in culture: Similarities with mineralization of lamellar bone. Calcified Tissue International, 79(5), 340–353. 10.1007/s00223-006-0107-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido, T. (2014). Osteocyte‐driven bone remodeling. Calcified Tissue International, 94(1), 25–34. 10.1007/s00223-013-9774-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. , Liu, F. , Lee, M. , Wu, B. , Ting, K. , Zara, J. N. , … Wang, C. Y. (2013). NF‐κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta‐catenin degradation. Proceedings of the National Academy of Sciences of the United States of America, 110(23), 9469–9474. 10.1073/pnas.1300532110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Shou, P. , Zheng, C. , Jiang, M. , Cao, G. , Yang, Q. , Shi, Y. , … Shi, Y. (2016). Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death and Differentiation, 23(7), 1128–1139. 10.1038/cdd.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croes, M. , Oner, F. C. , van Neerven, D. , Sabir, E. , Kruyt, M. C. , Blokhuis, T. J. , … Alblas, J. (2016). Proinflammatory T cells and IL‐17 stimulate osteoblast differentiation. Bone, 84, 262–270. 10.1016/j.bone.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Csaki, C. , Matis, U. , Mobasheri, A. , & Shakibaei, M. (2009). Co‐culture of canine mesenchymal stem cells with primary bone‐derived osteoblasts promotes osteogenic differentiation. Histochemistry and Cell Biology, 131(2), 251–266. 10.1007/s00418-008-0524-6 [DOI] [PubMed] [Google Scholar]
- Dallas, S. L. , Veno, P. A. , Rosser, J. L. , Barragan‐Adjemian, C. , Rowe, D. W. , Kalajzic, I. , & Bonewald, L. F. (2009). Time lapse imaging techniques for comparison of mineralization dynamics in primary murine osteoblasts and the late osteoblast/early osteocyte‐like cell line MLO‐A5. Cells Tissues Organs, 189(1‐4), 6–11. 10.1159/000151745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele, S. , Natali, L. , Giacomelli, C. , Campiglia, P. , Novellino, E. , Martini, C. , & Trincavelli, M. L. (2017). Osteogenesis Is improved by low tumor necrosis factor alpha concentration through the modulation of Gs‐coupled receptor signals. Molecular and Cellular Biology, 37(8), e00442–e00516. 10.1128/MCB.00442-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg, D. K. , Sibon, C. , Miled, C. , Jung, S. , Aucouturier, P. , Littman, D. R. , … Geissmann, F. (2006). A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science, 311(5757), 83–87. 10.1126/science.1117729 [DOI] [PubMed] [Google Scholar]
- Gharibi, B. , Abraham, A. A. , Ham, J. , & Evans, B. A. J. (2011). Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. Journal of Bone and Mineral Research, 26(9), 2112–2124. 10.1002/jbmr.424 [DOI] [PubMed] [Google Scholar]
- Heino, T. J. , Hentunen, T. A. , & Vaananen, H. K. (2004). Conditioned medium from osteocytes stimulates the proliferation of bone marrow mesenchymal stem cells and their differentiation into osteoblasts. Experimental Cell Research, 294(2), 458–468. 10.1016/j.yexcr.2003.11.016 [DOI] [PubMed] [Google Scholar]
- Helfrich, M. H. , & Ralston, S. H. (2012). Bone research protocol (Sencond Edition). New York City, USA: Humama Press. [Google Scholar]
- Hoey, D. A. , Kelly, D. J. , & Jacobs, C. R. (2011). A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochemical and Biophysical Research Communications, 412(1), 182–187. 10.1016/j.bbrc.2011.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Kim, H. J. , Chang, E. J. , Lee, Z. H. , Hwang, S. J. , Kim, H. M. , … Kim, H. H. (2009). IL‐17 stimulates the proliferation and differentiation of human mesenchymal stem cells: Implications for bone remodeling. Cell Death and Differentiation, 16(10), 1332–1343. 10.1038/cdd.2009.74 [DOI] [PubMed] [Google Scholar]
- Ikebuchi, Y. , Aoki, S. , Honma, M. , Hayashi, M. , Sugamori, Y. , Khan, M. , … Suzuki, H. (2018). Coupling of bone resorption and formation by RANKL reverse signalling. Nature, 561(7722), 195–200. 10.1038/s41586-018-0482-7 [DOI] [PubMed] [Google Scholar]
- Jo, S. , Wang, S. E. , Lee, Y. L. , Kang, S. , Lee, B. , Han, J. , … Kim, T. H. (2018). IL‐17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Research & Therapy, 20, 115 10.1186/s13075-018-1582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, Y. , Windle, J. J. , Koop, B. A. , Mundy, G. R. , & Bonewald, L. F. (1997). Establishment of an osteocyte‐like cell line, MLO‐Y4. Journal of Bone and Mineral Research, 12(12), 2014–2023. 10.1359/jbmr.1997.12.12.2014 [DOI] [PubMed] [Google Scholar]
- Kawaguchi, H. , Hirachi, A. , Hasegawa, N. , Iwata, T. , Hamaguchi, H. , Shiba, H. , … Kurihara, H. (2004). Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. Journal of Periodontology, 75(9), 1281–1287. 10.1902/jop.2004.75.9.1281 [DOI] [PubMed] [Google Scholar]
- Kim, Y. G. , Park, J. W. , Lee, J. M. , Suh, J. K. , Chang, B. S. , Um, H. S. , … Lee, Y. (2014). IL‐17 inhibits osteoblast differentiation and bone regeneration in rat. Archives Oral Biology, 59(9), 897–905. 10.1016/j.archoralbio.2014.05.009 [DOI] [PubMed] [Google Scholar]
- Kocic, J. , Santibanez, J. F. , Krstic, A. , Mojsilovic, S. , Dordevic, I. O. , Trivanovic, D. , … Bugarski, D. (2012). Interleukin 17 inhibits myogenic and promotes osteogenic differentiation of C2C12 myoblasts by activating ERK1,2. Biochimica Et Biophysica Acta‐Molecular Cell Research, 1823(4), 838–849. 10.1016/j.bbamcr.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Kuhn, N. Z. , & Tuan, R. S. (2010). Regulation of stemness and stem cell niche of mesenchymal stem cells: Implications in tumorigenesis and metastasis. Journal of Cellular Physiology, 222(2), 268–277. 10.1002/jcp.21940 [DOI] [PubMed] [Google Scholar]
- Li, L. , & Xie, T. (2005). Stem cell niche: Structure and function. Annual Review of Cell and Developmental Biology, 21, 605–631. 10.1146/annurev.cellbio.21.012704.131525 [DOI] [PubMed] [Google Scholar]
- Lian, Q. Z. , Zhang, Y. L. , Zhang, J. Q. , Zhang, H. K. , Wu, X. G. , Zhang, Y. , … Tse, H. F. (2010). Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation, 121(9), 1113–U1191. 10.1161/Circulationaha.109.898312 [DOI] [PubMed] [Google Scholar]
- Liao, C. , Zhang, C. , & Yang, Y. (2017). Pivotal roles of interleukin‐17 as the epicenter of bone loss diseases. Current Pharmaceutical Design, 23(41), 6302–6309. 10.2174/1381612823666170519120040 [DOI] [PubMed] [Google Scholar]
- Liao, C. S. , Cheng, T. F. , Wang, S. , Zhang, C. F. , Jin, L. J. , & Yang, Y. Q. (2017). Shear stress inhibits IL‐17A‐mediated induction of osteoclastogenesis via osteocyte pathways. Bone, 101, 10–20. 10.1016/j.bone.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Lubberts, E. (2015). The IL‐23‐IL‐17 axis in inflammatory arthritis. Nature Reviews Rheumatology, 11(7), 415–429. 10.1038/nrrheum.2015.53 [DOI] [PubMed] [Google Scholar]
- Marsell, R. , & Einhorn, T. A. (2011). The biology of fracture healing. Injury, 42(6), 551–555. 10.1016/j.injury.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec, P. , & Kolls, J. K. (2012). Targeting IL‐17 and Th17 cells in chronic inflammation. Nature Reviews Drug Discovery, 11(10), 763–776. 10.1038/nrd3794 [DOI] [PubMed] [Google Scholar]
- Moseley, T. A. , Haudenschild, D. R. , Rose, L. , & Reddi, A. H. (2003). Interleukin‐17 family and IL‐17 receptors. Cytokine & Growth Factor Reviews, 14(2), 155–174. 10.1016/S1359-6101(03)00002-9 [DOI] [PubMed] [Google Scholar]
- Mountziaris, P. M. , & Mikos, A. G. (2008). Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Engineering Part B‐Reviews, 14(2), 179–186. 10.1089/ten.teb.2008.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osta, B. , Lavocat, F. , Eljaafari, A. , & Miossec, P. (2014). Effects of interleukin‐17A on osteogenic differentiation of isolated human mesenchymal stem cells. Frontiers in Immunology, 5, 425 10.3389/fimmu.2014.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pricola, K. L. , Kuhn, N. Z. , Haleem‐Smith, H. , Song, Y. J. , & Tuan, R. S. (2009). Interleukin‐6 maintains bone marrow‐derived mesenchymal stem cell stemness by an ERK1/2‐dependent mechanism. Journal of Cellular Biochemistry, 108(3), 577–588. 10.1002/jcb.22289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser, J. , & Bonewald, L. F. (2012). Studying osteocyte function using the cell lines MLO‐Y4 and MLO‐A5. Methods in Molecular Biology, 816, 67–81. 10.1007/978-1-61779-415-5_6 [DOI] [PubMed] [Google Scholar]
- Schofield, R. (1978). The relationship between the spleen colony‐forming cell and the stem cell. Blood Cells, 4(1‐2), 8–25. [PubMed] [Google Scholar]
- Sebastian, A. A. , Kannan, T. P. , Norazmi, M. N. , & Nurul, A. A. (2018). Interleukin‐17A promotes osteogenic differentiation by increasing OPG/RANKL ratio in stem cells from human exfoliated deciduous teeth (SHED). Journal of Tissue Engineering and Regenerative Medicine, 12(8), 1856–1866. 10.1002/term.2706 [DOI] [PubMed] [Google Scholar]
- Song, X. Y. , & Qian, Y. C. (2013). The activation and regulation of IL‐17 receptor mediated signaling. Cytokine, 62(2), 175–182. 10.1016/j.cyto.2013.03.014 [DOI] [PubMed] [Google Scholar]
- Sonomoto, K. , Yamaoka, K. , Oshita, K. , Fukuyo, S. , Zhang, X. M. , Nakano, K. , … Tanaka, Y. (2012). Interleukin‐1 beta induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt‐5a/receptor tyrosine kinase‐like orphan receptor 2 pathway. Arthritis and Rheumatism, 64(10), 3355–3363. 10.1002/art.34555 [DOI] [PubMed] [Google Scholar]
- Sun, Y. Q. , Deng, M. X. , He, J. , Zeng, Q. X. , Wen, W. P. , Wong, D. S. H. , … Fu, Q. L. (2012). Human pluripotent stem cell‐derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells, 30(12), 2692–2699. 10.1002/stem.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarafder, S. , & Bose, S. (2014). Polycaprolactone‐coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: In vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. Acs Applied Materials & Interfaces, 6(13), 9955–9965. 10.1021/am501048n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, R. Y. , Qian, Y. N. , Li, L. H. , Yao, G. D. , Yang, L. , & Sun, Y. P. (2017). Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue‐derived mesenchymal stem cells. Stem Cell Research & Therapy, 8, 148 10.1186/s13287-017-0588-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F. , Tanaka, H. , Kawato, T. , Kitami, S. , Nakai, K. , Motohashi, M. , … Maeno, M. (2011). Interleukin‐17A induces cathepsin K and MMP‐9 expression in osteoclasts via celecoxib‐blocked prostaglandin E2 in osteoblasts. Biochimie, 93(2), 296–305. 10.1016/j.biochi.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Zhu, H. , Guo, Z. K. , Jiang, X. X. , Li, H. , Wang, X. Y. , Yao, H. Y. , … Mao, N. (2010). A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nature Protocols, 5(3), 550–560. 10.1038/nprot.2009.238 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


