Short abstract
Kasabach–Merritt syndrome (KMS) is a rare complication of hemangioma. KMS mostly occurs in the pediatric population with typical clinical manifestations, including thrombocytopenia, consumptive coagulation, and purpura. However, the pathogenesis of KMS is still unclear and the KMS therapy is controversial. We report here a case of KMS and multiple, giant, hepatic hemangiomas in a 34-year-old female patient who was successfully treated in our hospital. Glucocorticoid along with supportive treatments was administrated immediately to reverse fatal disseminated intravascular coagulation and acute hemolysis. After the acute phase, glucocorticoid was tapered slowly and sirolimus was added to treat the hemangiomas. In conclusion, the risk factors of gestation, interventional treatment, and autoimmune disturbance might contribute to the pathogenesis of KMS. Additionally, treatment with glucocorticoid and sirolimus is effective in KMS and multiple giant hepatic hemangiomas.
Keywords: Kasabach–Merritt syndrome, hemangioma, glucocorticoid, sirolimus, disseminated intravascular coagulation, purpura, thrombocytopenia, anemia
Abbreviations
KMS = Kasabach–Merritt syndrome, DIC = disseminated intravascular coagulation.
Introduction
Kasabach–Merritt syndrome (KMS) is a rare complication of hemangioma that is related to Kaposiform hemangioendothelioma and tufted angioma. KMS mostly occurs in the pediatric population. Typical clinical manifestations of KMS include thrombocytopenia, consumptive coagulation, and purpura. We report a case of KMS and multiple giant hepatic hemangiomas in a patient who was successfully treated with glucocorticoid and sirolimus. We speculate that gestation, interventional treatment, and autoimmune disturbance might be risk factors of KMS.
Case report
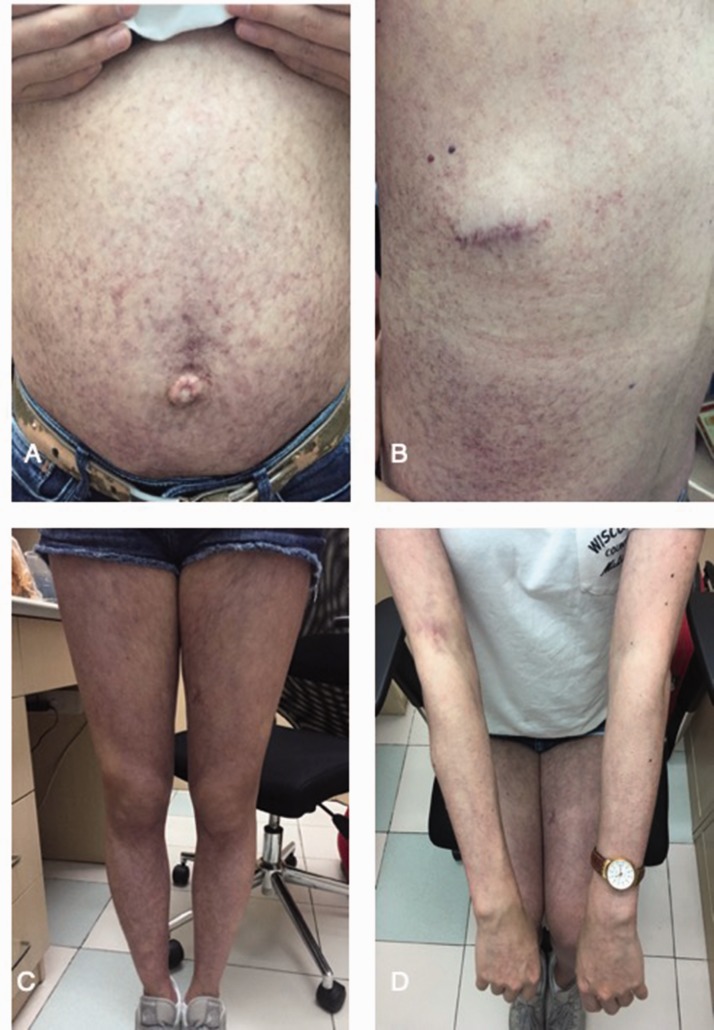
A 34-year-old female patient with a 6-day history of nausea, vomiting, dark urine, and fever was admitted to our hospital on 6 July 2016. She received hepatic hemangioma embolization with bleomycin 8 days before admission. She had a history of recurrent purpura and subcutaneous masses for 20 years. Multiple giant hepatic hemangiomas were found when she was pregnant in 1998. She had received resection and embolization of subcutaneous masses many times since 2000. After admission to our department, a physical examination showed petechiae, purpura, and subcutaneous masses over her limbs and trunk. Her abdomen was distended with palpable hepatomegaly and an umbilical hernia (Figure 1). Subcutaneous masses that were sampled from the breast were biopsied and cavernous hemangioma was found.
Figure 1.
Petechiae, purpura, and umbilical hernia were clearly seen in the patient’s distended abdomen (a), back (b), lower limbs (c) and upper limbs (d).
Routine blood and liver function tests showed a low erythrocyte count (1.31 × 109/L, normal range: 3.8–5.0 ×109/L), hemoglobin level (38 g/L, normal range: 115–150 g/L), and platelet count (43 × 109/L, normal range: 125–350 ×109/L). Furthermore, there were elevated levels of reticulocytes (3.99%, normal range: 0.5% to 1.5%), bilirubin (total bilirubin: 120 µmol/L, normal range: 3–22 µmol/L; conjugated bilirubin: 34 µmol/L, normal range 0–5 µmol/L; unconjugated bilirubin: 86 µmol/L, normal range: 2–19 µmol/L), and lactate dehydrogenase (1612 U/L, normal range: 313–618 U/L). These laboratory findings suggested that the patient had acute hemolytic anemia with thrombocytopenia. Her coagulation tests showed a prolonged prothrombin time (16.3 seconds, normal range: 11–14.5 seconds) and activated partial thromboplastin time (43 seconds, normal range: 28–40 seconds), low fibrinogen level (1.39 g/L, normal range: 2–4.5 g/L), elevated D-dimer level (>20,000 µg/L, normal range: <500 µg/L), and the presence of fibrinogen degradation products (197.7 mg/L, normal range: <5.0 mg/L). These tests confirmed that the patient had signs of consumptive coagulation. Additionally, >4.5% schistocytes were found in a peripheral blood smear, which suggested that the anemia in this patient was due to microangiopathic hemolytic anemia. Furthermore, an immunological test showed antinuclear antibodies of 1 : 1000 and anti-ribosomal antibody was positive with low levels of complement C3 (0.356 g/L, normal range : 0.790–1.520 g/L) and C4 (0.020 g/L, normal range : 0.160–0.380 g/L).
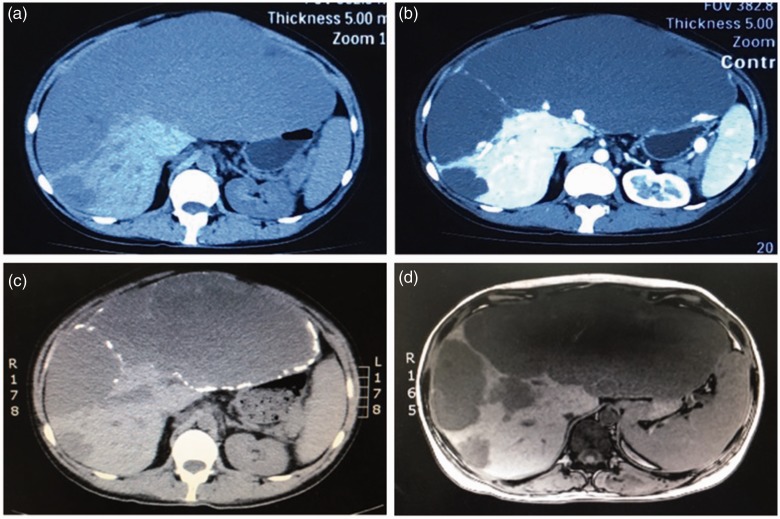
An abdominal non-enhanced and contrast-enhanced computed tomography scan showed multiple giant hepatic hemangiomas. An enhanced magnetic resonance imaging scan, including T1-weighted imaging and T2-weighted imaging, confirmed the findings of the computed tomography scan. The largest hemangioma was approximately 20 × 15×10 cm as shown by computed tomography and magnetic resonance imaging (Figure 2).
Figure 2.
Abdominal non-contrast computed tomography (a) and contrast-enhanced computed tomography (b) show multiple hepatic giant hemangiomas. T1-weighed magnetic resonance imaging (c) and T2-weighed magnetic resonance imaging (d) confirm the findings of the computed tomography scan and show that the largest hemangioma measures approximately 20 × 10 cm.
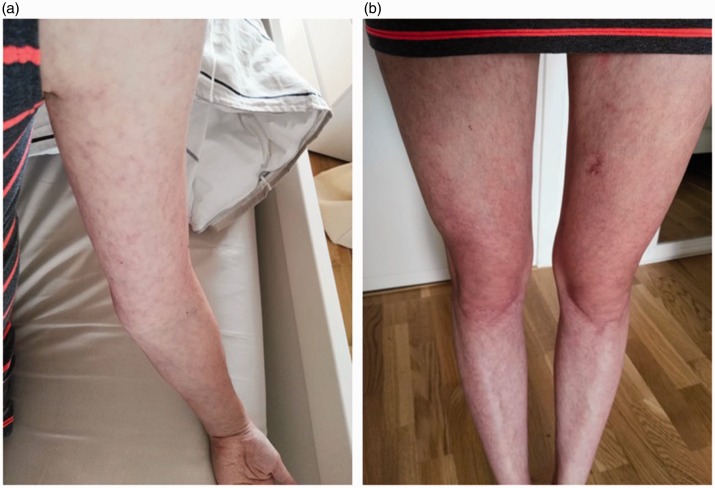
KMS was diagnosed on the presentations of unexplained thrombocytopenia, disseminated intravascular coagulation (DIC), and microangiopathic hemolytic anemia along with skin manifestations and hepatic hemangiomas. Biopsy of the hepatic hemangioma was not performed because of the severe anemia and high risk of bleeding. After diagnosis, methylprednisolone (2 mg/kg daily, with a slow taper) and transfusions of fresh frozen plasma were provided immediately. Within 3 weeks of treatment, the blood hemoglobin level and platelet count were elevated to 116 g/L and 74 × 109/L, respectively. However, the skin masses did not considerably change in size. Accordingly, sirolimus was then added to the therapeutic regimen. The initial dose was 0.8 mg/m2 twice daily, which was then adjusted to maintain a blood concentration of 5 to 15 ng/L. The subcutaneous masses then perceptibly diminished starting from 1 week later. Five months after the completion of glucocorticoid and sirolimus therapy, the hemoglobin level, platelet count, and coagulation test results were normalized. Her subcutaneous masses were remarkably diminished (Figure 3) and the size of the hepatic hemangiomas was also decreased on ultrasound film. According to an ultrasound scan, the size of the hepatic hemangiomas was decreased to 75% of the original size after 5 months of treatment with sirolimus. The patient’s condition remained stable during 2 years of follow-up.
Figure 3.
Manifestations of the skin (a, b) were greatly alleviated after treatment of sirolimus.
We obtained informed consent from the patient involved in this case report for publication. All of the medical procedures were standard. Therefore, approval from the ethics committee was not required.
Discussion
To date, the pathogenesis of KMS remains unclear. However, in the development of KMS, circulatory platelets might become trapped and activated by malformed hemangiomas, which subsequently induce intravascular coagulopathy and secondary hyperfibrinolysis.1,2 Activation of platelets promotes growth of hemangiomas.3,4 In our patient, skin manifestations, including recurrent petechiae, purpura, and subcutaneous masses, were initially found at the age of 14 years. When she was 26, she became pregnant and was diagnosed with hepatic hemangiomas. The hepatic hemangiomas became increasingly larger in the following years. Eight days before the current admission when she first had embolization for the hepatic hemangiomas, fatal DIC and hemolytic anemia occurred. According to the atypical progression of disease, we suggest that hemangiomas that are associated with KMS might be exacerbated by gestation. This view is in accordance with previous reports.5,6 Additionally, in our case, interventional therapy of hepatic hemangiomas appeared to be closely associated with the occurrence of DIC and acute hemolysis anemia. Therefore, clinicians should be cautious when treating a pregnant woman with hepatic hemangioma and performing interventional embolization in a patient with KMS.
Interestingly, positive results of antinuclear antibodies of 1:1000 and anti-ribosomal antibody, and low complement C3 and C4 levels were found in our patient. However, there were no other signs and symptoms of autoimmune diseases. Taking into account these abnormal results, especially the positive finding of anti-ribosomal antibody (known as a highly specific marker of systemic lupus erythematosus), we propose that autoimmune disturbance might contribute to the pathogenesis of KMS. There might be some unrecognized antiplatelet antibodies, which could result in destruction of platelets, and sedimentation of the immune complex, which could aggravate hemangiomas. To some extent, this hypothesis explains the effectiveness of glucocorticoid and sirolimus in the treatment of KMS because of their anti-inflammatory and immunosuppressive effects. However, the role of autoimmune disturbance in the pathogenesis of KMS requires further investigation.
Treatment of KMS is performed mainly to manage the coagulopathy and hemangiomas. Pharmacological therapies include glucocorticoids, vincristine, interferon alpha, sirolimus, and other supportive drugs.7 Surgery, embolization, and radiation therapy are also available options.7 However, there is still no consensus on the treatment of KMS because of its rarity. Findings in our case suggest that glucocorticoid, as well as supportive therapy, should be used as soon as possible to control fatal DIC and acute hemolysis. When the patient’s condition becomes stable, sirolimus, which is a promising drug for treating KMS,8–10 should be used to manage subcutaneous and visceral hemangiomas.
Findings in our case suggest that gestation, interventional treatment, and autoimmune disturbance might be risk factors of KMS. Clinicians should be careful when treating a pregnant woman with hepatic hemangioma and performing interventional embolization on a patient with KMS. Additionally, clinicians should pay more attention to immunological alternations in patients with KMS. With regard to treatment of fatal DIC and hemolytic anemia in KMS, glucocorticoids should be administrated immediately along with supportive treatment. To further manage hemangiomas, sirolimus might be a good choice with rapid and safe pharmacological efficacy.
Acknowledgements
We are grateful for the co-operation of our patient.
Compliance with ethics guidelines
Neither the authors, their immediate families, nor any research foundation with which they are affiliated received any financial payments or other benefits from any commercial entity related to the subject of this article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Yaqun Liu https://orcid.org/0000-0002-5606-4527
References
- 1.Kasabach H, Merritt K. Capillary hemangioma with extensive purpura : report of a case. Arch Pediatr Adolesc Med 1940; 59 : 1063–1070. [Google Scholar]
- 2.Lyons L, North P, Mac Let al. Kaposiform hemangioendothelioma : a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol 2004; 28 : 559–568. [DOI] [PubMed] [Google Scholar]
- 3.Hall G. Kasabach-Merritt syndrome : pathogenesis and management. Br J Haematol 2001; 112 : 851–862. [DOI] [PubMed] [Google Scholar]
- 4.Lopezgutierrez J. Current management of vascular tumors in the neonate. Curr Pediatr Rev 2015; 11 : 226–232. [DOI] [PubMed] [Google Scholar]
- 5.Girjia S, Chakrapani R. Kasabach–Merritt syndrome in two successive pregnancies. Int J Dermatol 1998; 37 : 690–693. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Jr, Kirk RF. Pregnancy associated with giant hemangiomata, thrombocytopenia, and fibrinogenopenia (Kasabach-Merritt syndrome). Report of a case. Obstet Gynecol 1967; 29 : 24–29. [PubMed] [Google Scholar]
- 7.Rafferty C, Ciara M, Alan Det al. Recent advances in the pathobiology and management of Kasabach–Merritt phenomenon. Br J Haematol 2015; 171 : 38–51. [DOI] [PubMed] [Google Scholar]
- 8.Kai L, Wang Z, Yao Wet al. Sirolimus, a promising treatment for refractory Kaposiform hemangioendothelioma. J Cancer Res Clin Oncol 2014; 140 : 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oza VS, Mamlouk MD, Hess CPet al. Role of sirolimus in advanced kaposiform hemangioendothelioma. Pediatr Dermatol 2016; 33 : 88–92. [DOI] [PubMed] [Google Scholar]
- 10.Ji Y, Chen S, Xiang Bet al. Sirolimus for the treatment of progressive kaposiform hemangioendothelioma : a multicenter retrospective study. Int J Cancer 2017; 141 : 848–855. [DOI] [PubMed] [Google Scholar]