Short abstract
Objective
This study compared Chinese herbal injections (CHIs) plus chemotherapy with chemotherapy alone in terms of clinical efficacy and safety for treating patients with esophageal cancer (EC).
Methods
Randomized controlled trials (RCTs) of CHIs combined with chemotherapy for treating EC published in English and Chinese databases were identified. The main outcomes were clinical efficacy, performance status, and adverse reactions. Random-effects models were fitted to calculate the odds ratios and 95% confidence intervals for all pair-wise comparisons.
Results
In total, 29 RCTs of eight CHIs were included in this study. The results of cluster analysis demonstrated that Compound Kushen injection plus chemotherapy was the optimal choice for improving the clinical efficacy rate. Shenfu injection was associated with a relatively high performance status. Compound Kushen injection and Shenfu injection were inferior to other CHIs in terms of preventing leukopenia and gastrointestinal side effects.
Conclusions
The combination of Compound Kushen injection with chemotherapy could improve efficacy and reduce adverse drug reactions versus chemotherapy alone in patients with EC.
Keywords: Chinese herbal injection, esophageal cancer, network meta-analysis, systematic review, chemotherapy, adverse drug reactions, traditional Chinese medicine
Introduction
Globally, esophageal cancer (EC) has emerged as the leading cause of cancer-related death, and it represents a major global health challenge because of its poor prognosis, increasing incidence, and high mortality rates.1–3 EC is more common in developed countries than in developing countries. Furthermore, the characteristics of EC are epidemiologically and biologically distinct, and an estimated 4,292,000 new cases and 2,814,000 deaths were estimated to occur in China in 2015.4,5 Following the approach of surgery alone, locally advanced EC frequently and eventually progresses to metastatic disease despite multimodality therapy, and chemotherapy is recommended as an adjunctive therapy for such patients.6–8 Nevertheless, improvements in the efficacy of chemotherapy have been marginal over the past three decades, and it is widely recognized that chemotherapy is associated with significant hematologic toxicity and modest effects against EC.7,9 As the critical backbone of complementary and alternative medicine, traditional Chinese medicine (TCM) has gradually gained global recognition for treating EC based on its favorable effects on symptoms and the disease, it has become a promising and active area of both fundamental and clinical research in the field of anti-cancer therapy. In addition, it has been elucidated that the anti-tumor mechanisms of TCM involve the regulation of autologous immune function, inhibition of tumor cell growth and proliferation, and induction of tumor cell autophagy and apoptosis.10–12 According to accumulated evidenced-based data, the combination of Chinese herbal injections (CHIs) and chemotherapy or radiotherapy is associated with considerable efficacy in treating patients with digestive system malignancies.13–15 However, a paucity of scientific research exists regarding the combined use of CHIs plus chemotherapy in patients with EC. Thus, the present research used network meta-analysis (NMA) to provide evidence-based hierarchies of the comparative efficacy and safety of CHIs combined with chemotherapy and provide additional insights for identifying optimal CHI-based regimens for treating EC.
Materials and methods
The procedure of the current research was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines “NMA extended version.”16
Literature search
A comprehensive literature search was performed, tested, and directed by a well-trained librarian in consultation with our review team to identify individual studies in multiple databases from their inception to March 5, 2018. The databases searched in this study included Embase, PubMed, Cochrane Library, the China National Knowledge Infrastructure Database (CNKI), and the Wanfang Database. No language restriction was applied. The searching strategies were divided into three parts: EC, CHIs, and randomized controlled trials (RCTs). In addition, the search terms for EC included “Esophageal Neoplasms, Esophageal Neoplasm, Esophagus Neoplasm, Esophagus Neoplasms, Cancer of Esophagus, Cancer of the Esophagus, Esophagus Cancer, Esophagus Cancers, Esophageal Cancer, and Esophageal Cancers.” A combination of MeSH and free-text terms was adopted. In the Chinese databases, using CNKI as an example, the search term for EC was “esophageal cancer” together with a full-text search for “random.” Additionally, we manually searched bibliographies and added related references to ensure that all relevant RCTs were included in the analysis.
Inclusion and exclusion criteria
In the present systematic review, we included RCTs meeting the eligibility criteria as follows: RCTs using CHIs combined with chemotherapy to treat EC; patients meeting the diagnostic criteria regarding the pathology, cytology, or histology of EC, regardless of gender, race, or disease severity; and the intervention of interest in this NMA was the combination of CHIs and chemotherapy. All doses, treatment schedules, and durations of different regimens were eligible. By contrast, the patients in control group only received chemotherapy. In addition, the clinical efficacy rate and performance status were the primary outcomes of interest, and adverse drug reactions (ADRs) including leukopenia and gastrointestinal side effects were considered safety outcomes.
We excluded studies meeting any of the following criteria: diagnosis of other primary tumors; CHIs were applied non-intravenously, and the comparator treatment was not chemotherapy; the studies were not RCTs; and essential information could not be extracted, such as drug names, dosage, duration of treatment, and outcomes.
Data extraction and risk of bias assessment
The screening of citation titles and abstracts among the search records was conducted by two reviewers independently to assess study eligibility according to the inclusion and exclusion criteria. The full text of citations considered to describe potentially eligible articles was independently reviewed. The consensus principle was used to resolve disagreements between the reviewers. The following data were extracted and acquired from eligible studies: study information (e.g., title, author names, publication date), baseline characteristics of the population (e.g., sample size, age, gender, performance status, tumor status), and study characteristics (e.g., study design, items of quality evaluation, dosing regimens, treatment duration, outcomes). The quality assessment of each eligible study was performed by two investigators independently in accordance with the Cochrane Risk of Bias Assessment Tool.17 The quality assessment was divided into seven domains, namely randomization sequence generation, allocation concealment, blinding of both participants and outcome assessors, incomplete outcome data, selective reporting, and other bias. Each domain was rated as low, unclear, or high risk according to the corresponding details in the eligible studies.
Statistical analyses
First, a pair-wise meta-analysis was performed using the random-effect model in consideration of within- and between-study heterogeneity among the included RCTs. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated and calculated to produce a summary effect size for each endpoint.18,19 The data were analyzed using WinBUGS 1.4.3 software. Bayesian inference was performed using Markov Chain Monte Carlo simulation, and 200,000 iterations were used, with the first 10,000 iterations discarded as a burn-in for annealing to eliminate the impact of the initial value in the WinBUGS program.20,21 Subsequently, Stata software (Stata Corp, College Station, TX, USA) was employed to generate network geometry, depict plots, determine the effect size, evaluate the assumptions of the network meta-analysis, and determine the relative rankings of the interventions.22–24 As a valuable graphical tool, Stata software can provide comprehensive and easily understandable methods for displaying statistical analysis results. The network geometry was determined to explore comparative relationships among the different treatments. Node sizes indicated the total sample sizes for treatments, and the line thickness corresponded to the number of trials.25 In addition, the surface under the cumulative ranking curves (SUCRA) was applied to rank the treatments to identify superiority. The interventions with higher SUCRA values were associated with the highest probability of being more effective. SUCRA ranges between 0 and 1, where 1 indicates that the treatment had a 100% probability of being ranked first and a 0% probability of being ranked last.26,27 Conceptually, the comparison-adjusted funnel plot was completed to evaluate the clinical and methodological heterogeneity. Additionally, cluster analysis was conducted to identify the optimal regimens in consideration of two different outcomes simultaneously. The interventions in the upper right region of the cluster analysis plots were more preferable options than the other regimens.28,29
Results
Literature selection and baseline characteristics
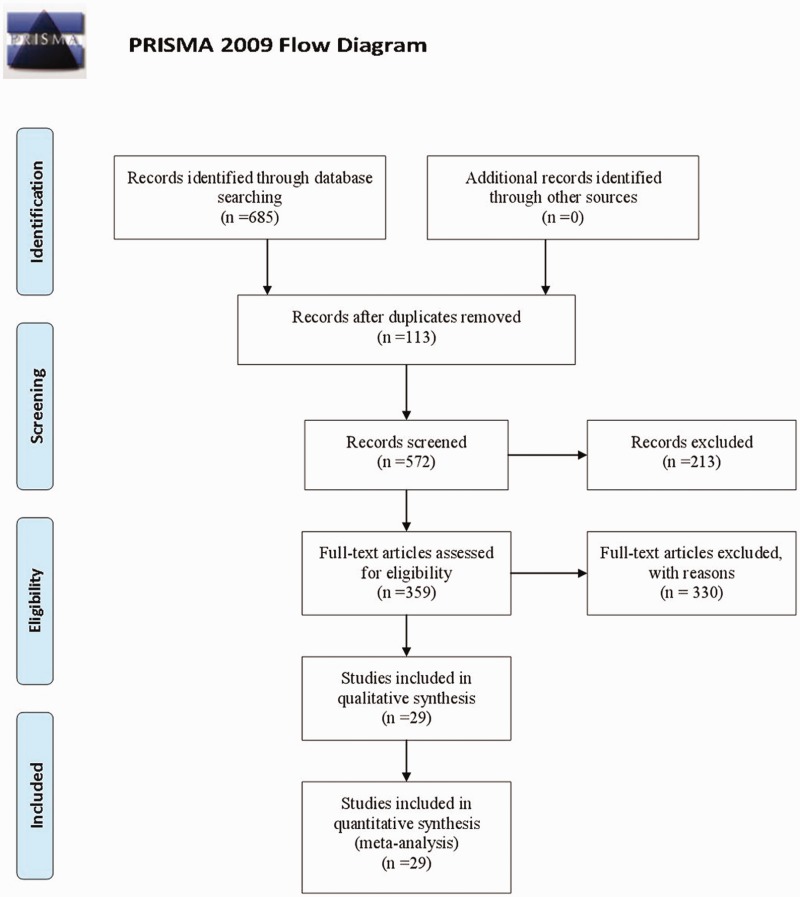
Initially, we identified 685 references after reviewing the titles and abstracts in our comprehensive search, and 359 citations were collected for additional review after excluding duplicate and irrelevant papers through reading titles and abstracts. Then, by reading the full text of potentially eligible articles, 29 RCTs of eight CHIs met our inclusion criteria.30–58 The literature screening process is shown in Figure 1. The number of studies of each CHI was as follows: Compound Kushen injection, seven trials; Aidi injection, six trials; Xiaoaiping injection, five trials; Huachansu injection, five trials; Shenqi Fuzheng injection, two trials; Kanglaite injection, two trials; Shenfu injection, one trial; and elemene injection, one trial.
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses flow diagram.
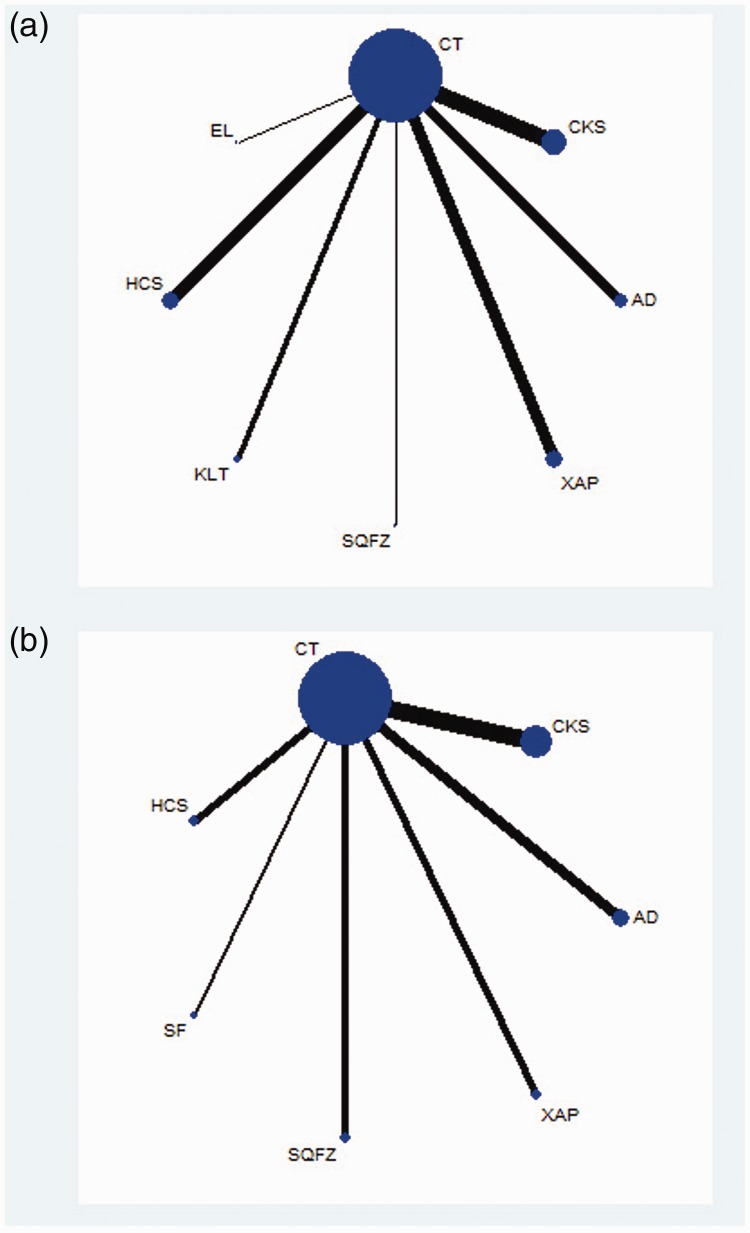
The 29 RCTs included a total of 1967 patients. Among them, 1001 patients were included in the CHI group, and 966 patients were included in the control group. The basic characteristics of the included studies are summarized in Table 1. A network plot of the clinical efficacy rate and performance status in the Bayesian analysis is depicted in Figure 2.
Table 1.
Basic characteristics of the included studies.
| Study ID | Sex(M/F) | Meanage | N(E/C) | TNMstage | EST(m) | KPS score | CHI + chemotherapy regimen | Chemotherapy regimen | Treatment(days) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen X 2006 | 41/19 | 57.4 | 30/30 | NR | NR | >70 | AD 50 mL + 5-FU 500–750 mg/m2+ DDP 50 mg/m2 | 5-FU 500–750 mg/m2 + DDP 50 mg/m2 | 21 days × 2 | ①②③ |
| Sun J 2013 | 59/47 | 63 | 53/53 | NR | ≥3 | ≥60 | AD 60–100 mL + PTX 150 mg/m2 + DDP 20 mg/m2 | PTX 150 mg/m2 + DDP 20 mg/m2 | 14 days × 2 | ③ |
| Yan HX 2013 | 39/22 | 61.9 | 30/31 | II–IV | NR | >70 | AD 100 mL + DDP 20 mg/m2 + 5-FU 750 mg/m2 | DDP 20 mg/m2 + 5-FU 750 mg/m2 | 10 days | ①②③ |
| Huang XY 2014 | 26/20 | 60.5 | 24/22 | III–IV | >3 | ≥60 | AD 50 mL + DDP 30 mg/m2 + 5-FU 500 mg/m2 | DDP 30 mg/m2 + 5-FU 500 mg/m2 | 28 days × 2 | ①②③ |
| Wu J 2013 | 38/5 | 57 | 22/21 | III–IV | ≥3 | ≥70 | AD 10 mL + DOC 75 mg/m2 + DDP 100 mg/m2 | DOC 75 mg/m2 + DDP 100 mg/m2 | 14 days × 3 | ③ |
| Sun NJ 2016 | 45/25 | 46.5 | 35/35 | NR | NR | NR | AD 50 mL + DDP 30 mg/m2 + 5-FU 500 mg/m2 | DDP 30 mg/m2 + 5-FU 500 mg/m2 | 28 days × 2 | ①③ |
| Lu S 2009 | 62/58 | 50.9 | 60/60 | II–III | NR | >60 | SF 50 mL + NVB 25–30 mg/m2 + l-OHP 85 mg/m2 | NVB 25–30 mg/m2+ l-OHP 85 mg/m2 | 8 days | ②③ |
| Zhang SQ 2011 | 54/29 | 55 | 42/41 | NR | >3 | ≥50 | SQFZ 250 mL + DDP 25 mg/m2 + CF 200 mg/m2 + 5-FU 500–750 mg/m2 | DDP 25 mg/m2 + CF 200 mg/m2 + 5-FU 500–750 mg/m2 | 21 days× 2 | ②③ |
| Xu M 2010 | 50/10 | 58 | 30/30 | II–III | >3 | >70 | SQFZ 250 mL + DDP 100 mg/m2 + 5-FU 1000 mg/m2 | DDP 100 mg/m2 + 5-FU 1000 mg/m2 | 10 days × 2 | ①②③ |
| Zheng HY 2012 | 32/28 | 61 | 31/29 | NR | ≥3 | ≥60 | CKS 10–20 mL + DOC 175 mg/m2 + DDP 20 mg/m2 + 5-FU 750 mg/m2 | DOC 175 mg/m2 + DDP 20 mg/m2 + 5-FU 750 mg/m2 | 28 days× 3 | ①②③ |
| Hou JX 2012 | 38/22 | 54.4 | 30/30 | III–IV | NR | ≥60 | CKS 15 mL + DDP 20 mg/m2 + 5-FU 500 mg/m2 | DDP 20 mg/m2 + 5-FU 500 mg/m2 | 21 days × 2 | ①②③ |
| Zhu LN 2011 | 48/30 | 50.5 | 42/36 | NR | >3 | >60 | CKS 30 mL + PTX 150 mg/m2 + NED 75 mg/m2 | PTX 150 mg/m2 + NED 75 mg/m2 | 21 days × 2 | ①②③ |
| Su HT 2010 | 41/29 | 62.8 | 36/34 | NR | NR | NR | CKS 20 mL + DDP 15 mg/m2 + 5-FU 750 mg/m2 | DDP 15 mg/m2 + 5-FU 750 mg/m2 | 21 days × 4 | ① |
| Gao QH 2008 | 24/11 | 62 | 20/15 | I–III | NR | NR | CKS 20 mL + DDP 25 mg/m2 + 5-FU 500 mg/m2 | DDP 25 mg/m2 + 5-FU 500 mg/m2 | 30 days × 2–5 | ①② |
| Li EX 2002 | NR | 60 | 30/27 | II–IV | NR | 50–80 | CKS 20 mL + DDP 20 mg/m2 + 5-FU 500 mg/m2 | DDP 20 mg/m2 + 5-FU 500 mg/m2 | 21 days × 2 | ①② |
| Chen XY 2004 | NR | 35-76 | 37/30 | III–IV | NR | NR | CKS 20 mL + DDP 30 mg/m2 + 5-FU 300 mg/m2 | DDP 30 mg/m2 + 5-FU 300 mg/m2 | 21–28 days × 2 | ①③ |
| Zhang GQ 2006 | 37/21 | 35-71 | 29/29 | NR | NR | ≥60 | HCS 30 mL + CF 100 mg/m2 + 5-FU 750 mg/m2 + DDP 20 mg/m2 | CF 100 mg/m2 + 5-FU 750 mg/m2 + DDP 20 mg/m2 | 21 days × 2 | ①② |
| Liu DG 2007 | NR | 53 | 30/30 | NR | NR | ≥40 | HCS 20 mL + OPT 7 mg/m2 + XF 200 mg/m2 + CTX 600 mg/m2 | OPT 7 mg/m2 + XF 200 mg/m2 + CTX 600 mg/m2 | 15 days | ①④ |
| Liu HM 2011 | 48/22 | 61.1 | 35/35 | III–IV | NR | NR | HCS 20 mL + NVB 50 mg/m2 + DDP 80–100 mg/m2 | NVB 50 mg/m2 + DDP 80–100 mg/m2 | 21 days × 2 | ① |
| Han ZY 2012 | 85/43 | NR | 68/60 | IV | >3 | ≥60 | HCS 20 mL + 5-FU 250 mg/m2 + DDP 60 mg/m2 | 5-FU 250 mg/m2 + DDP 60 mg/m2 | 21 days × 2 | ①②③ |
| Xu JC 2008 | NR | NR | 60/60 | NR | NR | NR | HCS 20–30 mL + DDP 20–30 mg/m2 + CF 200 mg/m2 + 5-FU 0.5–0.75 g/m2 | DDP 20–30 mg/m2 + CF 200 mg/m2 + 5-FU 0.5–0.75 g/m2 | 15 days × 3 | ① |
| Bai XH 2002 | 19/5 | 52 | 15/9. | NR | NR | 72.5 | KLT 200 mL + CF 200 mg/m2 + 5-FU 500 mg/m2 + DDP 20 mg/m2 | CF 200 mg/m2 + 5-FU 500 mg/m2 + DDP 20 mg/m2 | 21 days × 3 | ① |
| Li XY 1999 | 75/20 | 57.1 | 44/51 | NR | >3 | >60 | KLT 200 mL + DDP 40 mg/m2 + 5-FU 500–700 mg/m2 | DDP 40 mg/m2 + 5-FU 500-700 mg/m2 | 28 days | ① |
| Wang XQ 2016 | 32/20 | 55.4 | 26/26 | NR | NR | NR | EL 400–500 mg + PTX 135 mg/m2 + DDP 75–80 mg/m2 | PTX 135 mg/m2 + DDP 75–80 mg/m2 | 21 days × 2 | ①③ |
| Lin Q 2013 | 37/13 | 55.9 | 30/30 | III–IV | >3 | NR | XAP 60 mL + l-OHP 85 mg/m2 + CF 400 mg/m2 + 5-FU 400 mg/m2 | l-OHP 85 mg/m2 + CF 400 mg/m2 + 5-FU 400 mg/m2 | 7 days × 4 | ①②③ |
| Li HF2015 | 65/35 | NR | 50/50 | >3 | NR | XAP 60 mL + DDP 30 mg/m2 + 5-FU 500 mg/m2 | DDP 30 mg/m2 + 5-FU 500 mg/m2 | 21 days × 3 | ① | |
| Wang XJ 2015 | 35/27 | 56.6 | 31/31 | III–IV | >3 | ≥60 | XAP 40 mL + PTX 135 mg/m2 + DDP 25 mg/m2 | PTX 135 mg/m2 + DDP 25 mg/m2 | 21 days × 2 | ① |
| Li K 2007 | 19/11 | 67.2 | 15/15 | NR | ≥3 | ≥50 | XAP 60 mL + 5-FU 500 mg/m2 + DDP 30 mg/m2 | 5-FU 500 mg/m2 + DDP 30 mg/m2 | 21 days × 3 | ①③ |
| Yang J 2013 | 27/5 | 28/72 | 16/16 | NR | NR | 40–90 | XAP 40 mL + 5-FU 0.1 g/m2 + DDP 80–100 mg/m2 + ADM 80 mg/m2/ MMC 12 mg/m2 | 5-FU 0.1 g/m2 + DDP 80–100 mg/m2 + ADM 80 mg/m2/ MMC 12 mg/m2 | 28 days × 2–3 | ①②③ |
NR, not reported. ① Clinical efficacy rate; ② performance status; ③ adverse reaction. AD, Aidi injection; SF, Shenfu injection; SQFZ, Shenqi Fuzheng injection; KLT, Kanglaite injection; EL, elemene injection; XAP, Xiaoaiping injection; CKS, Compound Kushen injection; HCS, Huachansu injection.
Figure 2.
Network graph of the clinical efficacy rate (a) and performance status (b).
NOTE: Node sizes indicate the total sample sizes for treatments, and the line thickness corresponds to the number of trials. AD, Aidi injection; SF, Shenfu injection; SQFZ, Shenqi Fuzheng injection; KLT, Kanglaite injection; EL, elemene injection; XAP, Xiaoaiping injection; CKS, Compound Kushen injection; HCS, Huachansu injection.
Quality assessment of included studies
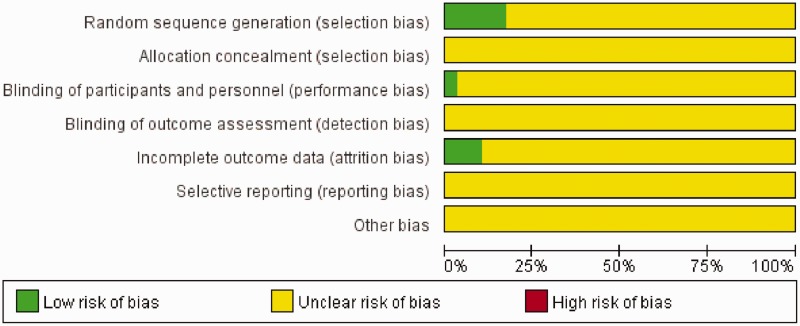
Regarding selection bias, four (13.79%) trials adopted a random number table, and one (3.45%) trial used direct sampling to randomize subjects into groups. These studies were rated as having a low risk of bias. Only one (3.45%) trial was considered to have a low risk of performance bias because it used a blinding method. Regarding attrition bias, three (10.34%) trials were rated as low risk based on their follow-up data. The risk of the remaining RCTs was considered unclear.
In addition, the results of quality evaluation for the included RCTs indicated that 27 (93.10%) trials evaluated adverse events, whereas four (13.79%) trials described medical ethics. All 29 RCTs mentioned randomization and descried the inclusion and exclusion criteria, whereas no study described the estimation of sample size. The bias graph of the included RCTs is shown in Figure 3.
Figure 3.
Bias graph of the included randomized controlled trials.
Clinical efficacy outcomes
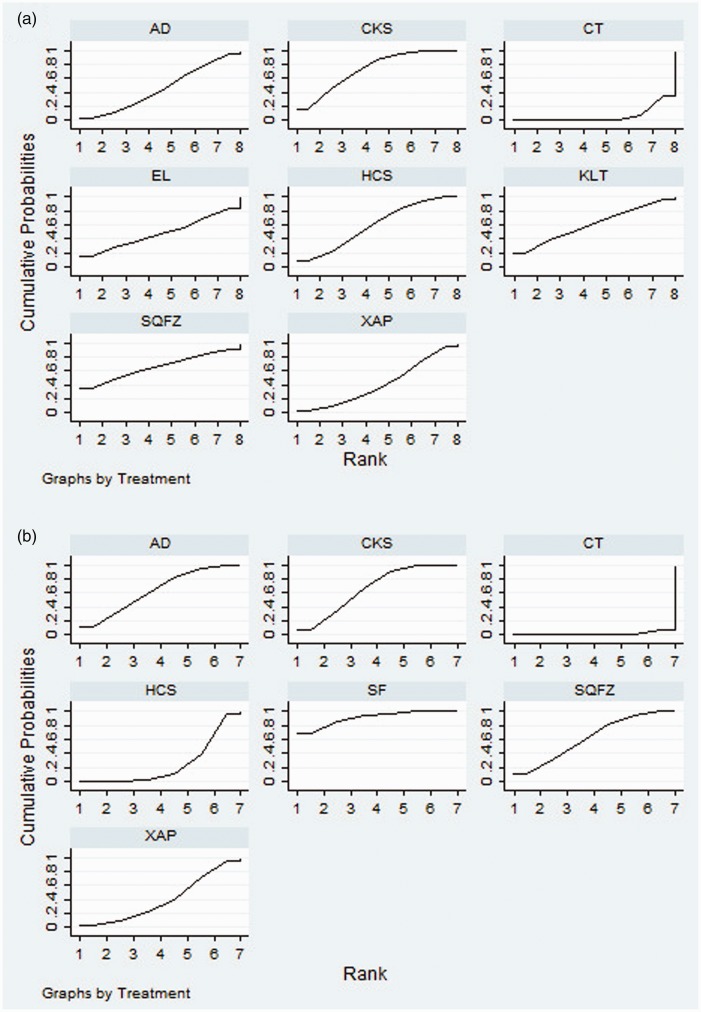
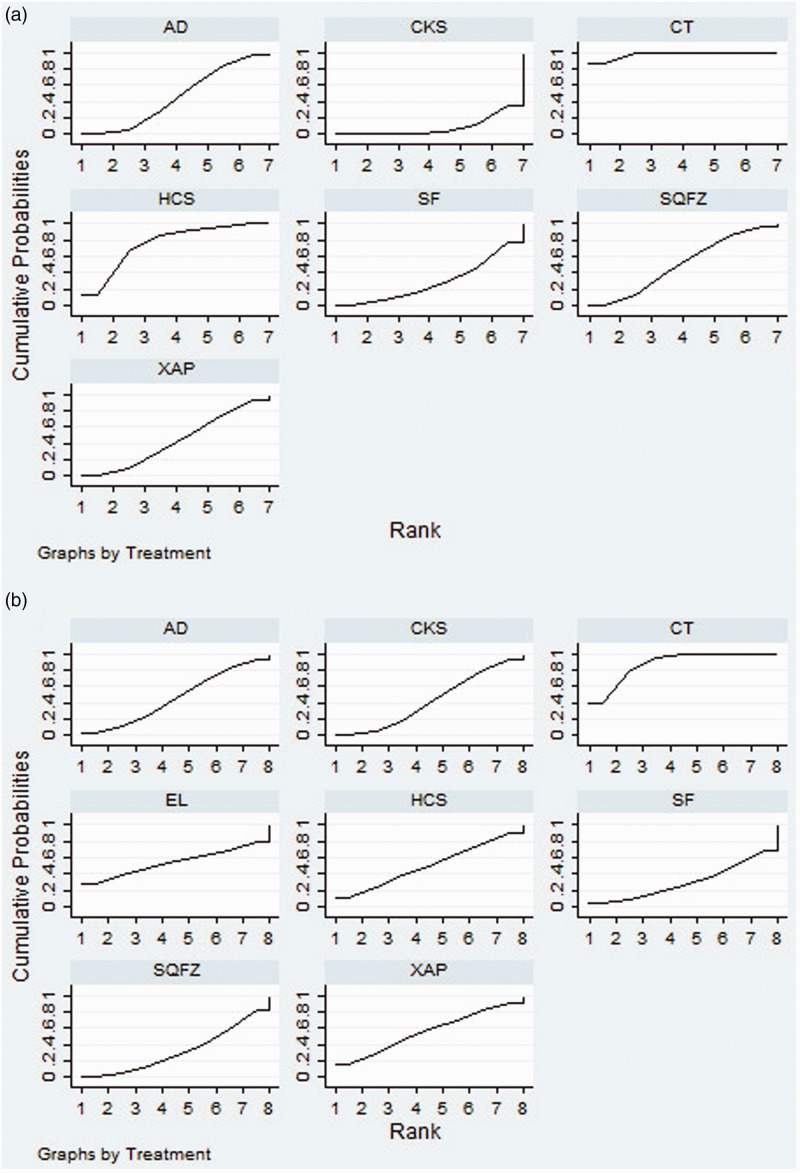
Twenty-five RCTs of seven CHIs presented clinical efficacy rates. The results revealed that compared with the effects of chemotherapy only, Compound Kushen injection + chemotherapy (OR = 2.73, 95% CI = 1.55–4.85) and Huachansu Injection + chemotherapy (OR = 2.26, 95% CI = 1.20–4.17) were associated with significantly improved clinical outcomes (Table 2). According to the SUCRA of clinical efficacy, the seven CHIs were ranked as follows: Compound Kushen (72.61%) > Shenqi Fuzheng (65.83%) > Kanglaite (61.4%) > Huachansu (59.17%) > elemene (48.14%) > Aidi (46.2%) > Xiaoaiping (39.81%) (Figure 4a).
Table 2.
Results of the network meta-analysis of the clinical efficacy rate (upper right quadrant) and performance status (lower left quadrant).
| AD + CT | 0.54 (0.25–1.15) | 1.49 (0.27–8.75) | 1.47 (0.56–3.81) | 1.21 (0.44–3.20) | 1.29 (0.33–5.07) | 1.02 (0.18–5.73) | 0.91 (0.32–2.57) | |
| 0.27 (0.12–0.57) | CT | 2.77 (0.59–13.9) | 2.73 (1.55–4.85) | 2.26 (1.20–4.17) | 2.40 (0.78–7.55) | 1.9 (0.41–9.02) | 1.70 (0.84–3.44) | |
| 0.98 (0.29–3.32) | 3.69 (1.52–9.41) | SQFZ + CT | 0.99 (0.18–5.16) | 0.81 (0.14–4.28) | 0.87 (0.12–5.88) | 0.68 (0.073–6.12) | 0.61 (0.10–3.32) | |
| 1.03 (0.37–2.67) | 3.88 (2.16–6.93) | 1.05 (0.35–3.00) | CKS + CT | 0.83 (0.35–1.90) | 0.88 (0.25–3.16) | 0.70 (0.14–3.63) | 0.62 (0.25–1.54) | |
| 0.49 (0.16–1.49) | 1.84 (0.86–4.14) | 0.50 (0.15–1.66) | 0.48 (0.18–1.29) | HCS + CT | 1.07 (0.30–3.95) | 0.84 (0.16–4.57) | 0.75 (0.29–1.93) | |
| KLT + CT | 0.79 (0.12–5.40) | 0.70 (0.18–2.66) | ||||||
| EL + CT | 0.89 (0.16–4.84) | |||||||
| 0.67 (0.18–2.41) | 2.49 (0.93–7.11) | 0.67 (0.17–2.68) | 0.64 (0.20–2.19) | 1.36 (0.38–4.89) | XAP + CT | |||
| 1.8 (0.44–7.31) | 6.77 (2.19–22.44) | 0.55 (0.12–2.33) | 0.57 (0.15–2.04) | 0.27 (0.066–1.08) | 0.37 (0.079–1.69) | SF + CT |
Values in bold indicate statistically significant results. AD, Aidi injection; SF, Shenfu injection; SQFZ, Shenqi Fuzheng injection; KLT, Kanglaite injection; EL, elemene injection; XAP, Xiaoaiping injection; CKS, Compound Kushen injection; EL, HCS, Huachansu injection; CT, chemotherapy.
Figure 4.
Rank of the cumulative probabilities for the clinical efficacy rate (a) and performance status (b).
NOTE: Higher surface under the cumulative ranking curves (SUCRA) values indicated higher probabilities that the treatments were more effective and superior than other therapies. AD, Aidi injection; SF, Shenfu injection; SQFZ, Shenqi Fuzheng injection; KLT, Kanglaite injection; EL, elemene injection; XAP, Xiaoaiping injection; CKS, Compound Kushen injection; HCS, Huachansu injection; CT, chemotherapy.
Performance status
Fifteen RCTs of six CHIs were included in the performance status analysis. Aidi + chemotherapy (OR = 0.27, 95% CI = 0.12–0.57), Shenqi Fuzheng + chemotherapy (OR = 3.69, 95% CI = 1.52–9.41), Compound Kushen + chemotherapy (OR = 3.88, 95% CI = 2.16–6.93), and Shenfu + chemotherapy (OR = 6.77, 95% CI = 2.19–22.44) were associated with favorable responses in terms of performance status compared with the effects of chemotherapy alone (Table 2). The six CHIs were ranked in terms of performance status as follows: Shenfu (88.29%) > Compound Kushen (65.84%) > Aidi (63.84%) > Shenqi Fuzheng (62.17%) > Xiaoaiping (41.97%) > Huachansu (26.34%) (Figure 4b).
Safety outcomes
In total, 14 RCTs of six CHIs provided data regarding the incidence of leukopenia. The results demonstrated that Aidi + chemotherapy (OR = 3.29, 95% CI = 1.51–7.52), Shenfu + chemotherapy (OR = 0.22, 95% CI = 0.051–0.88), Shenqi Fuzheng + chemotherapy (OR = 0.33, 95% CI = 0.10–0.97) , Compound Kushen + chemotherapy (OR = 0.13, 95% CI = 0.049–0.32), and Xiaoaiping + chemotherapy (OR = 0.28, 95% CI = 0.090–0.78) were linked to lower rates of leukopenia than chemotherapy alone. In addition, no significant difference in clinical efficacy was observed among the CHIs (Table 3). According to the cumulative probabilities for leukopenia, the six CHIs were ranked as follows: Compound Kushen (90.47%) > Shenfu (68.45%) > Xiaoaiping (58.5%) > Aidi (53.59%) > Shenqi Fuzheng (50.28%) > Huachansu (24.85%). The top three CHIs were Compound Kushen, Shenfu, and Xiaoaiping (Figure 5c).
Table 3.
Results of network meta-analysis for leucopenia (upper right quadrant) and gastrointestinal side effects (lower left quadrant).
| AD+CT | 3.29 (1.51,7.52) | 0.72 (0.14,3.64) | 1.08 (0.27,4.17) | 0.42 (0.12,1.44) | 1.98 (0.41,9.73) | 0.91 (0.23,3.47) | |
| 2.73 (0.74,10.7) | CT | 0.22 (0.051,0.88) | 0.33 (0.10,0.97) | 0.13 (0.049,0.32) | 0.60 (0.15,2.31) | 0.28 (0.090,0.78) | |
| 0.59 (0.037,9.20) | 0.22 (0.019,2.37) | SF+CT | 1.50 (0.25,9.08) | 0.59 (0.11,3.22) | 2.73 (0.39,19.88) | 1.26 (0.21,7.27) | |
| 0.72 (0.080,6.45) | 0.26 (0.045,1.47) | 1.22 (0.063,24.56) | SQFZ+CT | 0.39 (0.092,1.67) | 1.83 (0.32,10.84) | 0.84 (0.18,3.96) | |
| 0.93 (0.15,5.50) | 0.34 (0.095,1.08) | 1.59 (0.10,22.88) | 1.3 (0.15,10.31) | CKS+CT | 4.65 (0.92,24.58) | 2.13 (0.50,8.72) | |
| 1.13 (0.075,17.27) | 0.42 (0.038,4.40) | 1.92 (0.066,58.5) | 1.58 (0.083,29.94) | 1.22 (0.086,18.41) | HCS+CT | 0.46 (0.080,2.50) | |
| 1.34 (0.088,21.66) | 0.49 (0.044,5.36) | 2.28 (0.076,70.44) | 1.87 (0.097,36.95) | 1.43 (0.10,22.89) | 1.18 (0.041,35.28) | XAP+CT | |
| 1.05 (0.015,41.99) | 0.39 (0.0066,11.68) | 1.77 (0.017,118.3) | 1.46 (0.018,68.67) | 1.13 (0.017,43.76) | 0.92 (0.0088,59.64) | 1.29 (0.020,138) | EL+CT |
AD, Aidi injection; SF, Shenfu injection; SQFZ, Shenqi Fuzheng injection; KLT, Kanglaite injection; EL, elemene injection; XAP, Xiaoaiping injection; CKS, Compound Kushen injection; HCS, Huachansu injection; CT, chemotherapy.
Figure 5.
Rank of the cumulative probabilities for leukopenia (a) and gastrointestinal side effects (b).
NOTE: Higher surface under the cumulative ranking curves (SUCRA) values indicated higher probabilities that the treatments were more effective and superior than other therapies. AD, Aidi injection; SF, Shenfu injection; SQFZ, Shenqi Fuzheng injection; KLT, Kanglaite injection; EL, elemene injection; XAP, Xiaoaiping injection; CKS, Compound Kushen injection; HCS, Huachansu injection; CT, chemotherapy.
Fourteen eligible RCTs studying seven CHIs reported data regarding the incidence of gastrointestinal side effects. However, no significant differences in the rates of gastrointestinal side effects were observed among the treatments. Based on the occurrence of gastrointestinal side effects, the CHIs were ranked as follows: Shenfu (68.67%) > Shenqi Fuzheng (64.95%) > Compound Kushen (56.55%) > Aidi (53.58%) > elemene (50.78%) > Huachansu (48.57%) > Xiaoaiping (43.32%) (Figure 5d).
Additionally, the SUCRAs of different treatments for outcomes are shown in Table 4.
Table 4.
SUCRA values of different interventions for outcomes.
| Clinical efficacy rate | Performance status | Leukopenia | Gastrointestinal side effects | |
|---|---|---|---|---|
| AD + CT | 46.2% | 63.84% | 53.59% | 53.58% |
| CT | 6.84% | 1.55% | 3.86% | 13.58% |
| CKS + CT | 72.61% | 65.84% | 90.47% | 56.55% |
| EL + CT | 48.14% | NR | NR | 50.78% |
| HCS + CT | 59.17% | 26.34% | 24.85% | 48.57% |
| KLT + CT | 61.4% | NR | NR | NR |
| SF + CT | NR | 88.29% | 68.45% | 68.67% |
| SQFZ + CT | 65.83% | 62.17% | 50.28% | 64.95% |
| XAP + CT | 39.81% | 41.97% | 58.5% | 43.32% |
AD, Aidi injection; SF, Shenfu injection; SQFZ, Shenqi Fuzheng injection; KLT, Kanglaite injection; EL, elemene injection; XAP, Xiaoaiping injection; CKS, Compound Kushen injection; HCS, Huachansu injection; CT, chemotherapy.
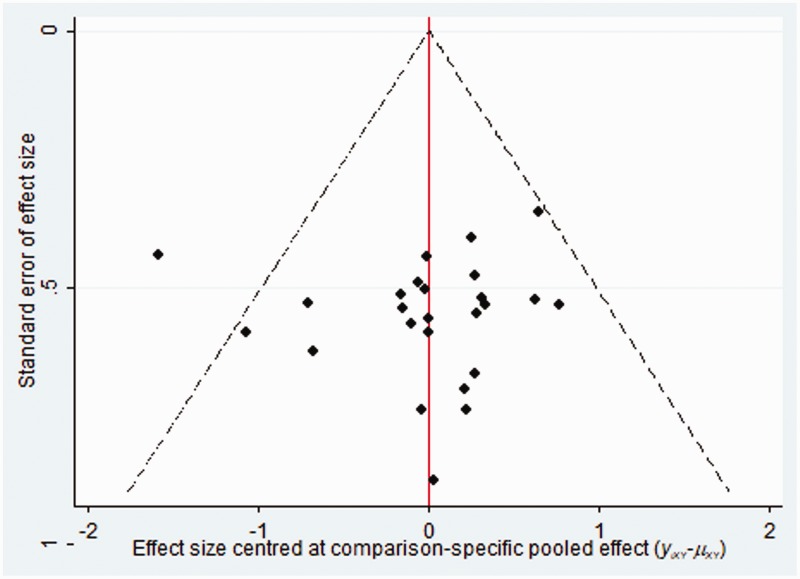
Publication bias
The publication bias of the included RCTs was evaluated using funnel plots. The funnel plots were not visually symmetrical, indicating the existence of bias. The lack of negative results and large-scale clinical controlled trials might have also contributed to the bias (Figure 6).
Figure 6.
Funnel plots of the included randomized controlled trials.
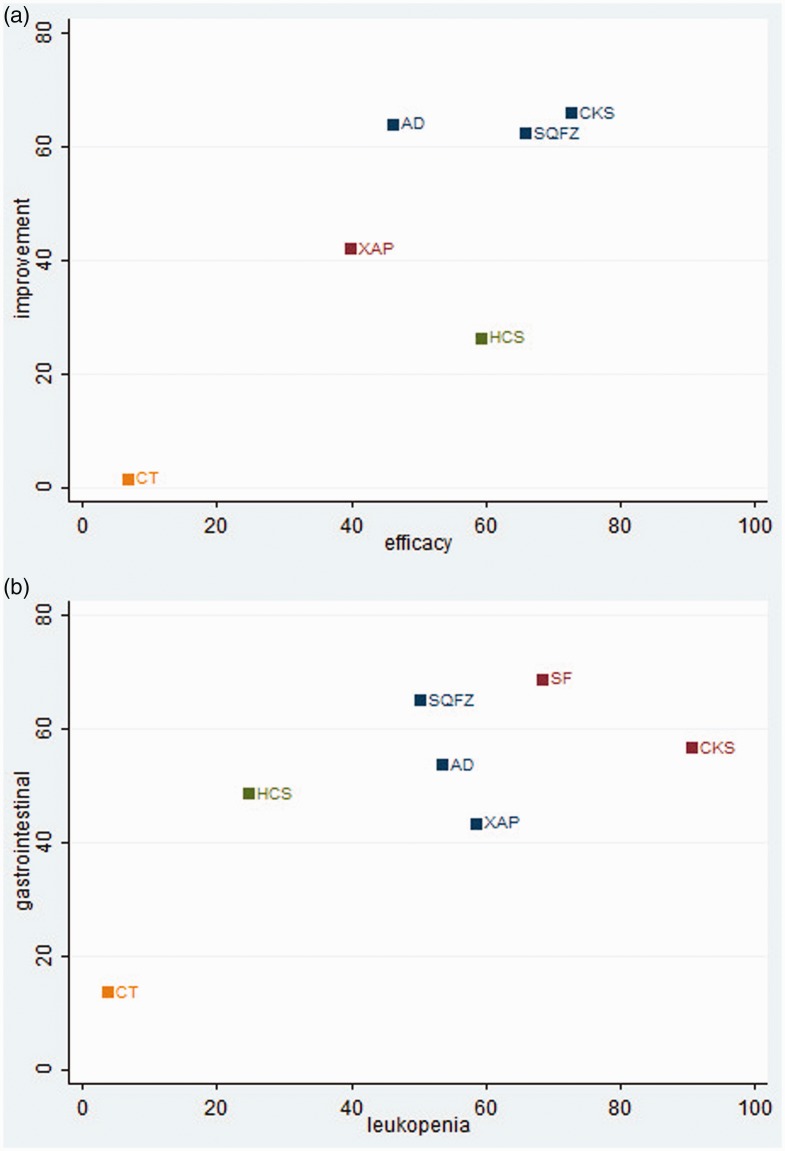
Cluster analysis
Cluster analysis of five CHIs revealed that the combination of Compound Kushen injection and chemotherapy was superior to the other regimens in terms of both clinical efficacy and performance status. Furthermore, the results for six CHIs concerning the incidence of leukopenia and gastrointestinal side effects indicated that Compound Kushen injection combined with chemotherapy was associated with lower rates of ADRs (Figure 7).
Figure 7.
Cluster analysis plots. (a) Clinical efficacy rate (x-axis) and performance status (y-axis). (b) Rates of leukopenia (x-axis) and gastrointestinal effects (y-axis).
NOTE: Interventions in the upper-right section of the cluster analysis plots were more preferable treatment options. AD, Aidi Injection; SF, Shenfu injection; SQFZ, Shenqi Fuzheng injection; XAP, Xiaoaiping injection; CKS, Compound Kushen injection; HCS, Huachansu injection, CT: chemotherapy.
Discussion
We conducted the current NMA to evaluate the comparative clinical efficacy and safety of all reported combinations of CHIs and chemotherapy in the treatment of EC. Overall, the results revealed that Compound Kushen injection combined with chemotherapy was the best option for both improving clinical efficacy and reducing the incidence of leukopenia and gastrointestinal side effects.
Compound Kushen Injection is prepared from the medicinal plants Sophora flavescens and Heterosmilax chinensis. Recently, the results of clinical and pharmacological research indicated that Compound Kushen injection has utility as a selective adjunct treatment for patients with cancer. Systematic reviews have provided moderate evidence that Compound Kushen injection is beneficial for alleviating cancer-related pain, and it appears to have beneficial effects on the rates of side effects in patients receiving chemotherapy.59–61 Additionally, this CHI improved the efficacy and performance status of chemotherapy in patients with advanced colon cancer and reduced ADR rates among postoperative patients with breast cancer.62,63 The candidate anti-cancer molecular mechanisms of Compound Kushen injection include the perturbation of cell cycle progression, downregulation of actin cytoskeletal and focal adhesion genes, inhibition of tumor growth, and increases of the cell energy charge.64–67 Similarly as a variety of anti-tumor compounds, matrine and oxymatrine can inhibit the proliferation and differentiation of cancer cells, prevent metastasis and invasion, induce cell cycle arrest, accelerate apoptosis, and restrain angiogenesis,68 and their underlying mechanisms have been validated and predicted using network pharmacology methods. The findings illustrated that the treatments target important pathways of cancers, including glycometabolism, amino acid metabolism, and PI3K-Akt signaling.69,70 Additionally, when using Compound Kushen injection in combination with chemotherapy in clinical practice, attention must be paid to ADRs to promote the rational use of TCM, improve their acceptance, and increase the recognition of the anti-cancer effects of CHIs. In addition, the selection of treatments in the clinic should also depend on multiple aspects including safety, patient preference, the specific disease situation, costs, local availability, clinician experience, and the duration of chemotherapy.
To our knowledge, this is the first study to compare different CHIs in combination with chemotherapy in the treatment of EC. Our study had several advantages. First, this study searched an extensive period and included a large sample of patients with EC. The searching strategies of our study were also more comprehensive given that both English and Chinese databases were searched and RCTs were identified using the websites of other relevant organizations. In addition, the search terms were divided into three parts, and the search strategy used a combination of MeSH terms and full-text words. Finally, our study comprehensively sorted the various outcome indicators including clinical efficacy, performance status, and rates of leukopenia and gastrointestinal side effects via cluster analysis.
Several limitations of our study should be noted. First, published data rather than individual patient information, which contains a more detailed appraisal of outcomes, were gathered in the present study. Second, the overall survival rate was the crucial indicator in the efficacy evaluation, whereas the follow-up data were insufficient for comparing the survival benefits of different CHIs. In addition, some included studies did not report randomization, blinding methods, and allocation concealment adequately, and these factors might undermine the validity of the overall findings. Finally, there may have been clinical heterogeneity because of the diversity of chemotherapeutic drugs. In this regard, our findings suggested that attention should be paid to the academic, scientific, and systematic components of clinical trials, such as recognizing the important endpoints of efficacy and safety outcomes, focusing on improving the methodological quality of clinical trials as well as the training of specialists and clinicians.
Conclusion
The available evidence in the present NMA demonstrated that the combination of Compound Kushen injection and chemotherapy was the optimal regimen in terms of efficacy and safety for patients with esophageal cancer. However, direct comparisons among different CHs are warranted and required to robustly demonstrate the possible and potential differences among these adjunctive therapies for chemotherapy.
Supplemental Material
Supplemental material, IMR898336 Supplemental Material for Systematic review and network meta-analysis comparing Chinese herbal injections with chemotherapy for treating patients with esophageal cancer by Dan Zhang, Jiarui Wu, Haojia Wang, Wei Zhou, Mengwei Ni, Xinkui Liu and Xiaomeng Zhang in Journal of International Medical Research
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
The design of the study and collection, analysis, and interpretation of data were supported by the Young Scientists Training Program of Beijing University of Chinese Medicine and the National Nature Science Foundation of China (Grant nos. 81473547 and 81673829).
ORCID iD
Jiarui Wu https://orcid.org/0000-0002-1617-6110
Supplemental material
Supplemental material for this article is available online.
References
- 1.Smyth EC, Lagergren J, Fitzgerald RCet al. Oesophageal cancer. Nat Rev Dis Primers 2017; 3: 17048. doi: 10.1038/nrdp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Short MW, Burgers KG, Fry VT. Esophageal cancer. Am Fam Physician 2017; 95: 22–28. [PubMed] [Google Scholar]
- 3.Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg 2018; 41: 210–215. doi: 10.1016/j.asjsur.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PDet al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015; 21: 7933–7943. doi: 10.3748/wjg.v21.i26.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun L, Zhao F, Zeng Yet al. Risks and benefits of multimodal esophageal cancer treatments: a meta-analysis. Med Sci Monit 2017; 23: 889–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohda M, Kuwano H. Current status and future prospects for esophageal cancer treatment. Ann Thorac Cardiovasc Surg 2017; 23: 1–11. doi: 10.5761/atcs.ra.16-00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janmaat VT, Steyerberg EW, van der Gaast Aet al. Palliative chemotherapy and targeted therapies for esophageal and gastroesophageal junction cancer. Cochrane Database Syst Rev 2017; 11: CD004063. doi: 10.1002/14651858.CD004063.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ku GY. Systemic therapy for esophageal cancer: chemotherapy. Chin Clin Oncol 2017; 6: 49. doi: 10.21037/cco.2017.07.06. [DOI] [PubMed] [Google Scholar]
- 10.Zhang YS, Shen Q, Li J. Traditional Chinese medicine targeting apoptotic mechanisms for esophageal cancer therapy. Acta Pharmacol Sin 2016; 37: 295–302. doi: 10.1038/aps.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai YM, Zhu H, Niu JXet al. Identification of herb pairs in esophageal cancer. Complement Med Res 2017; 24: 40–45. doi: 10.1159/000454699. [DOI] [PubMed] [Google Scholar]
- 12.Sheng J, Zou X, Cheng Zet al. Recent advances in herbal medicines for digestive system malignancies. Front Pharmacol 2018; 9: 1249. doi: 10.3389/fphar.2018.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D, Wu J, Liu Set al. Network meta-analysis of Chinese herbal injections combined with the chemotherapy for the treatment of pancreatic cancer. Medicine 2017; 96: e7005. doi: 10.1097/MD.0000000000007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D, Zheng J, Ni Met al. Comparative efficacy and safety of Chinese herbal injections combined with the FOLFOX regimen for treating gastric cancer in China: a network meta-analysis. Oncotarget 2017; 8: 68873–68889. doi: 10.18632/oncotarget.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D, Ni M, Wu Jet al. The optimal Chinese herbal injections for use with radiotherapy to treat esophageal cancer: a systematic review and Bayesian network meta-analysis. Front Pharmacol 2019; 9: 1470. doi: 10.3389/fphar.2018.01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutton B, Salanti G, Caldwell DMet al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gotzsche PCet al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achana FA, Cooper NJ, Bujkiewicz Set al. Network meta-analysis of multiple outcome measures accounting for borrowing of information across outcomes. BMC Med Res Methodol 2014; 14: 92. doi: 10.1186/1471-2288-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sven S, Denny B, Eric-Jan W. Bayesian inference for the information gain model. Behav Res Methods 2011; 43: 297–309. Published online 2011 Feb 8. doi: 10.3758/s13428-010-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christian HH, Pamela W, Allan Wet al. A practical guide to pre‐trial simulations for Bayesian adaptive trials using SAS and BUGS. Pharm Stat 2018; 17: 854–865. doi: 10.1002/pst.1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciprian M, Crainiceanu A, Jeffrey G. Bayesian functional data analysis using WinBUGS. J Stat Softw 2010; 32: i11. [PMC free article] [PubMed] [Google Scholar]
- 22.Shim S, Yoon BH, Shin ISet al. Network meta-analysis: application and practice using Stata. Epidemiol Health 2017; 39: e2017047. doi: 10.4178/epih.e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72: 39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaimani A, Higgins JP, Mavridis Det al. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaimani A, Mavridis D, Salanti G. A hands-on practical tutorial on performing meta-analysis with Stata. Evid Based Ment Health 2014; 17: 111–116. doi: 10.1136/eb-2014-101967. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Carter RE. Ranking of the most effective treatments for cardiovascular disease using SUCRA: is it as sweet as it appears? Eur J Prev Cardiol 2018; 25: 842–843. doi: 10.1177/2047487318767199. [DOI] [PubMed] [Google Scholar]
- 27.Tonin FS, Rotta I, Mendes AMet al. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract 2017; 15: 943. doi: 10.18549/PharmPract.2017.01.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hans-Peter P. Network-meta analysis made easy: detection of inconsistency using factorial analysis-of-variance models. BMC Med Res Methodol 2014; 14: 61. doi: 10.1186/1471-2288-14-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ 2013; 346: f2914. doi: 10.1136/bmj.f2914. [DOI] [PubMed] [Google Scholar]
- 30.Chen XB, Xiao YJ, Luo SXet al. Clinical observation of Aidi injection combined with DF regimen in the treatment of advanced esophageal cancer. Basic Clin Oncol 2006; 19: 211–212. [Google Scholar]
- 31.Sun J, Sheng ZJ. Clinical observation of Aidi injection combined with PT regimen in the treatment of advanced esophageal cancer. Cancer Res Clin 2013; 25: 547–549. [Google Scholar]
- 32.Yan HX, Yang GW, Wang ZHet al. Effects of Aidi injection combined with chemotherapy on immune function in patients with esophageal cancer. Chin Trad Pat Med 2013; 35: 33–36. [Google Scholar]
- 33.Huang XY. Clinical observation of Aidi injection combined with chemotherapy in the treatment of advanced esophageal cancer. Med Aesthetics Beauty 2014; 11: 70–71. [Google Scholar]
- 34.Wu J, Long GX. Clinical observation of 22 cases of advanced esophageal cancer treated with Chinese medicine Aidi injection combined with chemotherapy. J Intern Med Crit Care 2013; 19: 100–101. [Google Scholar]
- 35.Sun NJ. On the clinical value of Aidi injection combined with chemotherapy in the treatment of advanced esophageal cancer. Pharm Front 2016; 6: 212–213. [Google Scholar]
- 36.Lu S, Xiong GZ, Yang Yet al. Clinical observation of attenuating effect of Shenfu injection in adjuvant chemotherapy after esophageal cancer surgery. Cancer Prev Treat 2009; 22: 305–306. [Google Scholar]
- 37.Zhang SQ, Lin LF, Qiu XHet al. Clinical observation of Shenqi Fuzheng injection combined with chemotherapy in the treatment of advanced esophageal cancer. Chin Med Herald 2011; 8: 74–76. [Google Scholar]
- 38.Xu M. Clinical study of Shenqi Fuzheng injection in adjuvant chemotherapy after esophageal cancer surgery. China: Liaoning University of Traditional Chinese Medicine, 2010. [Google Scholar]
- 39.Zheng HY, Hou XS. Therapeutic effect of compound Kushen injection combined with chemotherapy on postoperative recurrence of esophageal cancer. Chin J Info Tradit Chin Med 2012; 9: 85. [Google Scholar]
- 40.Hou JX. Clinical study of compound Kushen injection combined with chemotherapy in the treatment of advanced esophageal cancer. J Tradit Chin Med 2012; 27: 1085–1086. [Google Scholar]
- 41.Zhu LN, Fan QX. The role of compound Kushen injection in chemotherapy of advanced esophageal squamous cell carcinoma. Chin J Clin Oncol Rehabil 2011; 3: 278–279. [Google Scholar]
- 42.Su TH. Clinical observation of 36 cases of advanced esophageal carcinoma treated by compound Kushen injection. Chin Med Guide 2010; 8: 75–76. [Google Scholar]
- 43.Gao QH, Yang L, Yue Zet al. Clinical observation of Yanshu in the treatment of esophageal cancer. J Liaoning Coll Tradit Chin Med 2008; 10: 102–103. [Google Scholar]
- 44.Li EX, Shang JT, Li Yet al. Clinical observation of Yanshu injection combined with PF regimen in the treatment of esophageal cancer with cancerous perforation signs. Chin J Oncol 2002; 24: 408. [Google Scholar]
- 45.Chen XY, Qu ZY. Therapeutic effect of Yanshu injection on advanced esophageal squamous cell carcinoma. Chin J Misdiagn 2004; 4: 871–872. [Google Scholar]
- 46.Zhang GQ. Observation on the short-term efficacy of Huachansu combined with chemotherapy in the treatment of advanced esophageal cancer. Jilin J Tradit Chin Med 2006; 26: 25. [Google Scholar]
- 47.Liu DG, Liu Y, Ma YG. Clinical observation of Huachansu combined with chemotherapy in the treatment of advanced esophageal cancer. Chin J Pract Intern Med 2007; S1: 333. [Google Scholar]
- 48.Liu HM, Zheng YL, Liu XLet al. Treatment of advanced esophageal cancer with Huachansu combined with chemotherapy. Chin J Exp Tradit Med Form 2011; 17: 235–237. [Google Scholar]
- 49.Han ZY, Zhou YQ, Zhang JWet al. Therapeutic effect of Huachansu combined with low-dose chemotherapy on advanced esophageal cancer. The first National TCM Cancer Summit Forum Proceedings. 2012; 1: 78–80. [Google Scholar]
- 50.Xu JC. Therapeutic effect of Huachansu injection on patients with advanced esophageal cancer. J Clin Psychosom Dis 2008; 14: 364–364. [Google Scholar]
- 51.Bai XH, Yao YM, Hong XT. Clinical observation of 24 cases of esophageal cancer treated with Kanglaite combined with chemotherapy. Shaanxi J Oncol 2002; 10: 59–59. [Google Scholar]
- 52.Li XY, Shen SJ, Fan QX. Clinical observation of Kanglaite injection combined with DF regimen in the treatment of esophageal cancer. Chin J Clini Oncol 1999; 26: 296. [Google Scholar]
- 53.Wang XQ. Clinical value of cisplatin combined with paclitaxel and elemene injection in the treatment of advanced esophageal cancer. North Med 2016; 13: 90–91. [Google Scholar]
- 54.Lin Q, Rong JY. Efficacy of mFOLFOX6 regimen combined with Xiaoaiping in the treatment of advanced esophageal cancer. Guangxi Med J 2013; 7: 912–913. [Google Scholar]
- 55.Li HF, Sun ZQ, Li YJet al. Clinical observation of chemotherapy combined with Xiaoaiping injection in the treatment of advanced esophageal cancer. North Med 2015; 5: 110. [Google Scholar]
- 56.Wang XJ, Zhao MX, Yang Xet al. Therapeutic effect of Xiaoaiping combined with TP regimen in the treatment of advanced esophageal cancer. Mod J Integr Tradit Chin West Med 2015; 33: 3699–3701. [Google Scholar]
- 57.Li K, Zou HW. Clinical observation of Xiaoaiping combined with chemotherapy in the treatment of advanced esophageal cancer. Chin J Cancer Prev Treat 2007; 14: 1272–1273. [Google Scholar]
- 58.Yang J. Xiaocanping injection in the treatment of advanced liver cancer in 16 cases. Shanghai Med J 2013; 9: 27–28. [Google Scholar]
- 59.Guo YM, Huang YX, Shen HHet al. Efficacy of compound Kushen injection in relieving cancer-related pain: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2015; 2015: 840742. doi: 10.1155/2015/840742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bao Y, Kong X, Yang Let al. Complementary and alternative medicine for cancer pain: an overview of systematic reviews. Evid Based Complement Alternat Med 2014; 2014: 170396. doi: 10.1155/2014/170396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yanju B, Yang L, Hua Bet al. A systematic review and meta-analysis on the use of traditional Chinese medicine compound kushen injection for bone cancer pain. Support Care Cancer 2014; 22: 825–836. doi: 10.1007/s00520-013-2063-5. [DOI] [PubMed] [Google Scholar]
- 62.Yu L, Zhou Y, Yang Yet al. Efficacy and safety of compound Kushen injection on patients with advanced colon cancer: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2017; 2017: 7102514. doi: 10.1155/2017/7102514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ao M, Xiao X, Li Q. Efficacy and safety of compound Kushen injection combined with chemotherapy on postoperative Patients with breast cancer: a meta-analysis of randomized controlled trials. Medicine 2019; 98: e14024. doi: 10.1097/MD.0000000000014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qu Z, Cui J, Harata-Lee Yet al. Identification of candidate anti-cancer molecular mechanisms of Compound Kushen Injection using functional genomics. Oncotarget 2016; 7: 66003–66019. doi: 10.18632/oncotarget.11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nourmohammadi S, Aung TN, Cui Jet al. Effect of compound Kushen injection, a natural compound mixture, and its identified chemical components on migration and invasion of colon, brain, and breast cancer cell lines. Front Oncol 2019; 9: 314. doi: 10.3389/fonc.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H, Hu H, Rong Het al. Effects of compound Kushen injection on pathology and angiogenesis of tumor tissues. Oncol Lett 2019; 17: 2278–2282. doi: 10.3892/ol.2018.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui J, Qu Z, Harata-Lee Yet al. Cell cycle, energy metabolism and DNA repair pathways in cancer cells are suppressed by Compound Kushen Injection. BMC Cancer 2019; 19: 103. doi: 10.1186/s12885-018-5230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W, You RL, Qin WJet al. Anti-tumor activities of active ingredients in Compound Kushen Injection. Acta Pharmacol Sin 2015; 36: 676–679. doi: 10.1038/aps.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meng Z, Liu X, Wu Jet al. Mechanisms of compound Kushen injection for the treatment of lung cancer based on network pharmacology. Evid Based Complement Alternat Med 2019; 2019: 4637839. doi: 10.1155/2019/4637839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao L, Wang KX, Zhou YZet al. Uncovering the anticancer mechanism of Compound Kushen Injection against HCC by integrating quantitative analysis, network analysis and experimental validation. Sci Rep 2018; 8: 624. doi: 10.1038/s41598-017-18325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, IMR898336 Supplemental Material for Systematic review and network meta-analysis comparing Chinese herbal injections with chemotherapy for treating patients with esophageal cancer by Dan Zhang, Jiarui Wu, Haojia Wang, Wei Zhou, Mengwei Ni, Xinkui Liu and Xiaomeng Zhang in Journal of International Medical Research