Short abstract
Objective
In this cross-sectional study, we aimed to determine the prevalence of asthma and other allergic diseases among a homogenous group of students attending the health colleges of a Saudi university and to investigate the relationship between their atopy profile and associated clinical symptoms of allergic diseases.
Methods
A total of 222 students completed the International Study of Asthma and Allergies in Childhood questionnaire and underwent skin prick testing (SPT) using a standardized panel of allergenic extracts.
Results
Overall prevalence of physician-diagnosed allergic diseases was 27% for bronchial asthma (BA), 13.1% for atopic dermatitis (AD), and 5% for allergic rhinitis (AR). Atopy was present in 90 (40.5%) students. Students with atopic disease were more likely to have physician-diagnosed BA, AR, and AD. Atopy and polysensitization was more frequent among male than female students. SPT results were positive in 20.8% of participants for Bermuda grass, 18.9% for cat fur, and 12.7% for Dermatophagoides pteronyssinus.
Conclusion
The prevalence of atopy and allergic diseases in Saudi young adults is high and worrisome. Comorbid allergic diseases were more prevalent among our participants with atopic disease. Determination of allergen sensitization patterns in patients with atopic disease is crucial for selecting proper preventive and therapeutic strategies.
Keywords: Atopy, asthma, rhinitis, dermatitis, adults, Saudi Arabia
Introduction
Allergic diseases are still considered a serious global public health problem affecting all age groups. These diseases include allergic rhinitis (AR), bronchial asthma (BA), and atopic dermatitis (AD). According to recent international estimates, an average of 300 and 400 million people currently have BA and AR, respectively, with an annual mortality rate of 250,000 deaths. Additionally, the number of people with asthma may rise to 400 million by 2025.1,2
Allergic diseases have adverse effects not only on the quality of life of patients with atopic disease but also on their socioeconomic status. Moreover, allergic diseases occur together in some individuals with atopy. This necessitates a multi-stage approach for diagnosis and treatment, with an increased burden on healthcare systems and substantial financial costs.2–4 Allergic sensitization—the tendency to produce immunoglobulin E (IgE) antibodies in response to antigens—has been reported as a well-known risk factor for the development of allergic diseases.5
Previous epidemiological studies have shown variations in the prevalence of different allergic diseases within and between countries; this indicates the importance of environmental factors in disease pathogenesis, including indoor and outdoor allergens, in addition to genetic and immunological factors and factors such as smoking, obesity, and hormonal changes.6–9 Therefore, the patterns of indoor and outdoor allergens by which individuals with atopic disease are sensitized appear to be crucial in allergic disease epidemiology, diagnosis, and prognosis.2
Allergic disorders are usually diagnosed by taking detailed clinical history, performing physical examination, and conducting laboratory investigations to detect specific antigen (IgE). The skin prick test (SPT) continues to be the most appropriate in vitro diagnostic test to identify patients with atopic disease.10 A standardized method to compare the prevalence and severity of BA and atopic diseases has been established by the International Study of Asthma and Allergic Diseases in Childhood (ISAAC). This methodology explores risk factors among individuals with allergic diseases.11
The prevalence of allergic diseases among children and adolescents has been studied extensively worldwide.4–6,12–14 However, data are scarce regarding the burden of allergic diseases among adults and their risk factors. Moreover, the few epidemiological studies investigating the prevalence of allergic diseases among adults have lacked homogeneity in their adult cohorts.8,15–17 Therefore, in the present study, we selected a group of young adults from among students attending the health colleges of a Saudi university. As a more homogenous group of individuals, our participants shared a possible accumulation of risk factors for allergic diseases such as higher education level and socioeconomic status and an urban lifestyle.7,13 In this study, we aimed to determine the prevalence of allergic diseases, such as asthma and others, among a homogenous group of young adults in the Kingdom of Saudi Arabia, and to investigate the relationship between the atopy profile (monosensitization or polysensitization pattern) and associated clinical symptoms of allergic diseases.
Methods
We conducted this research as a cross-sectional study. The sample was representative of students in the health colleges of Najran University, in southwestern Saudi Arabia, during the period January to May 2018. The study was conducted according to the international guidelines Strengthening the Reporting for Observational Studies in Epidemiology (STROBE).18 Using the World Health Organization (WHO) Manual for Sample Size Determination in Health Studies,19 the minimum sample size calculated for the study was 185 students, based on a conservative estimate of the anticipated population proportion of 4.1%,16 with an absolute precision of 2% and 95% confidence interval. To avoid loss of cases, a total sample was 230 participants (female and male students) was included in the present study. We randomized the sample using a stratified proportional allocation method. Stratification factors were the relative number of students in each health college, the student’s grade level, and sex. This study was conducted according to the principles of the Declaration of Helsinki. The study was revised and approved by the Research Ethical Committee of Najran University. We obtained written consent from all students enrolled in the study.
Interview via questionnaire
We constructed a standardized questionnaire, which was then completed by all participants. The questionnaire was completed during a one-to-one interview with each participant, following the protocol described in the ISAAC Manual and ISAAC Coding and Data Transfer Manual.11 Each interview began with a general discussion regarding the respondents’ understanding of the allergic diseases questions in the three modules. All interviews were conducted by the same physician together with the author of this paper, to ensure consistency. The interviews were transcribed and reviewed by the respondents. Our questionnaire was a modified version of the ISAAC Phase III questionnaire,11 translated into Arabic language. In keeping with the ISAAC recommendations protocol for questionnaire translation, a team of physicians and health educators in the community, including an internist, an otolaryngologist, and an immunologist, revised the translated questionnaire. An epidemiologist and a statistician added their comments to the final version of the questionnaire. After translation, the accuracy of the questionnaire was checked by back translation into English. We performed a pilot test of the questionnaire with a sample of 10 male and 8 female students, to determine its acceptability and the clarity of the questions. The questionnaire was then modified accordingly, taking into consideration any comments with respect to the validity of the content and comprehensiveness.
On the questionnaire, we queried demographic data such as sex, age, grade, and health college. Information on residence as well as parental educational levels was also collected. We composed three modules in the questionnaire, to address different allergic diseases. The module of asthma included questions to identify physician-diagnosed asthma, current or past wheeze at any time, exercise-induced wheezing, and nocturnal cough. The questionnaire module of AR included questions to identify physician-diagnosed AR, current or past rhinitis, and allergic rhinoconjunctivitis. The questionnaire module of AD included questions to identify current or recurrent rash and physician-diagnosed AD.
Skin prick tests (SPTs)
We performed SPTs using the Stallerpoint device l (Stallergenes, Paris, France), with standardized allergenic extracts per the manufacturer’s instructions. Allergens were selected according to the findings of previous studies from different Saudi regions.6,13,20 The allergen panel included Dermatophagoides farina and D. pteronyssinus (house dust mites); ragweed and mugwort (weed pollens); Bermuda grass (grass pollens); olive (tree pollens); Penicillium (molds); cat, horse, and dog hair (animal dander); and cockroach (Blattella germanica) allergens. We used normal saline and histamine hypochloride (10 mg/mL) as negative and positive controls, respectively. SPT results were recorded as positive with a wheal diameter >3 mm to at least one of the allergens or with a wheal diameter 3 mm larger than the negative control. We used the definition of atopy as sensitization to any of the tested allergens, including sensitization to only one allergen (monosensitization) or to two or more allergens (polysensitization).9,10
Data analysis
We coded, validated, and analyzed the data using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). The data were presented using frequency and percent or mean ± standard deviation. We used the chi square test and t-test, with statistical significance set to the 5% level.
Results
In total, 222 students completed the questionnaire, yielding a response rate of 96.5%. A total of 116 male students and 106 female students were included (age range: 19 to 23 years; average age: 21.5 ±1.5 years). Most students were from the college of medicine (36%), followed by nursing (16.2%) and radiology sciences (14.4%). Table 1 shows the prevalence rates of wheezing, asthma, AR, and AD symptoms among Saudi young adults. The overall prevalence of physician-diagnosed allergic diseases was 27% for asthma, 13.1% for AD, and 5% for AR. There was no significant difference in diagnosed AR and AD between male and female students. However, male students were more likely to have physician-diagnosed BA (p = 0.01). We found significant differences between male and female students with respect to symptoms of wheezing over the past 12 months (p = 0.03), exercise-induced wheeze (p = 0.01), wheezing at any time in the past (p = 0.02), nighttime cough (p = 0.001), rhinitis symptoms over the past 12 months (p = 0.01), and rhinitis symptoms at any time in the past (p = 0.005).
Table 1.
Prevalence of associated clinical symptoms of allergic diseases among male and female students.
| Variable | Males (N = 116) | Females (N = 106) | Total | p value |
|---|---|---|---|---|
| Age (y), mean ± SD | 21.3 ± 1.4 | 21 ± 1.8 | 21.2 ± 1.5 | 0.139 |
| Health college, n (%) | 0.24 | |||
| Medicine | 46 (39.7) | 34 (32.1) | 80 (36) | |
| Dentistry | 10 (8.6) | 0 | 10 (4.5) | |
| Pharmacy | 10 (8.6) | 0 | 10 (4.5) | |
| Medical laboratory sciences | 30 (26.8) | 0 | 30 (13.5) | |
| Radiology sciences | 0 (0) | 32 (30.2) | 32 (14.4) | |
| Physiotherapy | 14 (12.1) | 0 | 14 (6.3) | |
| Nursing | 0 (0) | 36 (34) | 36 (16.2) | |
| Asthma, n (%) | ||||
| Wheeze “ever” | 42 (36.2) | 23 (21.7) | 65 (29.3) | 0.018 |
| Current wheeze | 30 (25.9) | 15 (14.2) | 45 (20.3) | 0.031 |
| Physician-diagnosed BA | 40 (34.5) | 20 (18.9) | 60 (27) | 0.009 |
| Exercise-induced asthma | 34 (29.3) | 16 (15.1) | 50 (22.5) | 0.011 |
| Nocturnal cough | 48 (41.4) | 22 (20.8) | 70 (31.5) | 0.001 |
| Rhinitis, n (%) | ||||
| Rhinitis “ever” | 72 (62.1) | 46 (43.4) | 118 (53.2) | 0.005 |
| Current rhinitis | 61 (52.9) | 38 (35.8) | 99 (44.6) | 0.011 |
| Rhinoconjunctivitis | 42 (36.2) | 22 (20.8) | 64 (28.8) | 0.011 |
| Physician-diagnosed AR | 8 (6.9) | 3 (2.8) | 11 (5) | 0.161 |
| Dermatitis, n (%) | ||||
| Recurrent rash “ever” | 28 (24.1) | 24 (22.6) | 52 (23.4) | 0.792 |
| Recurrent rash in past 12 months | 27 (23.3) | 20 (18.9) | 47 (21.2) | 0.424 |
| Recurrent rash typical eczema distribution | 18 (15.5) | 11 (10.4) | 29 (13.1) | 0.261 |
| Recover in past 12 months | 21 (18.1) | 14 (13.2) | 35 (15.8) | 0.318 |
| Physician-diagnosed AD | 18 (15.5) | 11 (10.4) | 29 (13.1) | 0.261 |
BA, bronchial asthma; AR, allergic rhinitis; AD, atopic dermatitis.
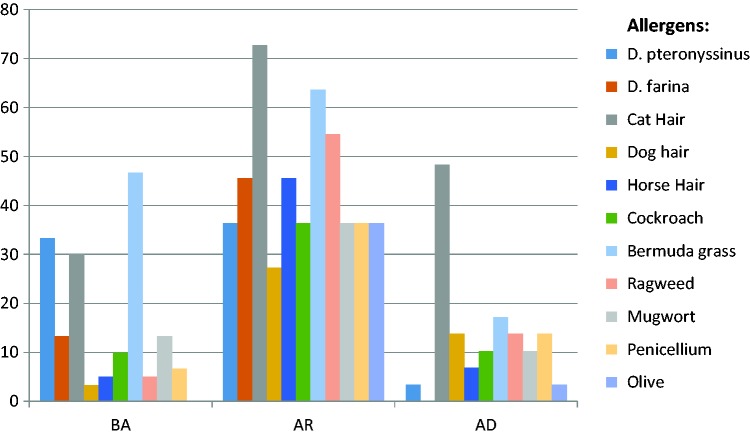
Atopy was present in 90 (40.5%) students. We identified positive SPT reactivity for Bermuda grass (20.8%), cat fur (18.9%), D. pteronyssinus (12.7%), ragweed (9%), mugwort (8%), and D. farina (7.1%). Figure 1 shows the distribution of SPT sensitization to various allergens among patients with physician-diagnosed allergic diseases. Participants with BA had increased positive SPT reactivity to different allergens, including Bermuda grass, D. pteronyssinus, and cat fur. Students with AR had increased sensitization to cat fur, ragweed, and Bermuda grass. Sensitization to cat fur, dog hair, Penicillium, and Bermuda grass was more frequent among patients with AD.
Figure 1.
Distribution of sensitization patterns to various allergens among students with different allergic diseases.
BA: Bronchial asthma; AR: Allergic rhinitis; AD: Atopic dermatitis.
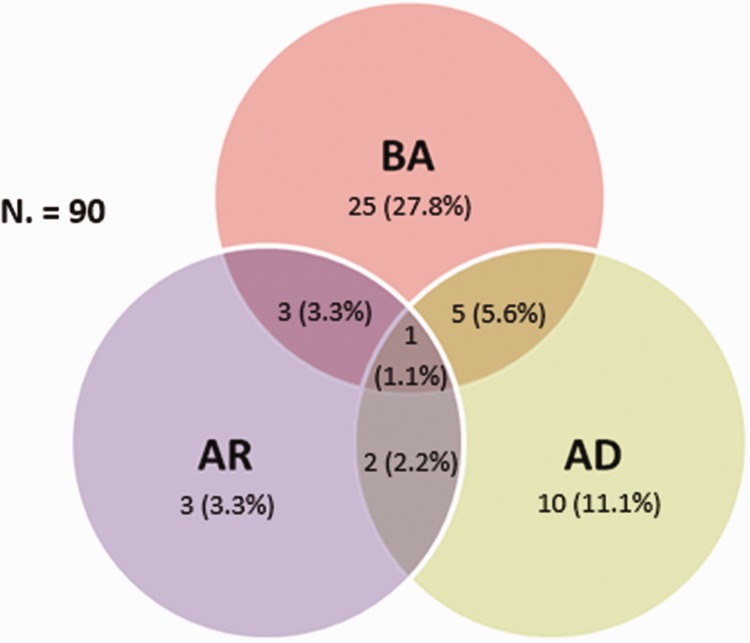
Among the 90 students with atopy, 54.4% were symptomatic for one or more allergic diseases. The prevalence of BA among students with atopic disease was 27.8%, concomitant with AD, was 5.6%, with AR 3.3%, and with both disorders 1.1% (Figure 2). Students who had atopic disease were more likely to have physician-diagnosed BA (p = 0.009), diagnosed AR (p = 0.008), and diagnosed AD (p = 0.04). Students with atopic disease reported more wheezing symptoms over the past 12 months (p < 0.001), past wheezing (p < 0.001), rhinitis symptoms over the past 12 months (p = 0.02), and rhinitis symptoms at any time in the past (p = 0.03), as compared with students who did not have atopy (Table 2).
Figure 2.
Venn diagram of comorbid allergic diseases among Saudi young adults with atopic disease.
BA, bronchial asthma; AR, allergic rhinitis; AD, atopic dermatitis.
Table 2.
Distribution of clinical symptoms of allergic diseases among students with and without atopy.
| Variable, n (%) | Participants without atopy(N = 122) | Participants with atopy(N = 90) | p value |
|---|---|---|---|
| Asthma | |||
| Wheeze “ever” | 25 (20.4) | 40 (44.4) | <0.001 |
| Current wheeze | 13 (10.7) | 32 (35.6) | <0.001 |
| Physician-diagnosed BA | 26 (21.3) | 34 (37.8) | 0.009 |
| Exercise-induced asthma | 28 (25) | 22 (24.4) | 0.921 |
| Nocturnal cough | 42 (38.2) | 28 (31.1) | 0.286 |
| Rhinitis | |||
| Rhinitis “ever” | 58 (51.8) | 60 (66.7) | 0.031 |
| Current rhinitis | 47 (42) | 52 (57.8) | 0.023 |
| Rhinoconjunctivitis | 26 (23.2) | 38 (42.2) | 0.003 |
| Physician-diagnosed AR | 2 (1.8) | 9 (10) | 0.008 |
| Dermatitis | |||
| Recurrent rash “ever” | 28 (25) | 24 (26.7) | 0.781 |
| Recurrent rash in past 12 months | 27 (24.1) | 20 (22.2) | 0.747 |
| Recurrent rash typical eczema distribution | 18 (16.1) | 11 (12.2) | 0.426 |
| Recover in past 12 months | 23 (20.5) | 12 (13.2) | 0.167 |
| Physician-diagnosed AD | 11 (9.8) | 18 (20) | 0.04 |
BA, bronchial asthma; AR, allergic rhinitis; AD, atopic dermatitis.
In this study, more male than female students were atopic (p = 0.003). There was no significant difference in monosensitization and polysensitization between male and female students. However, female students were significantly more sensitized against dog hair and Bermuda grass allergens whereas males were significantly more sensitized to cat hair and ragweed allergens (Table 3).
Table 3.
Distribution of atopy and sensitization patterns to different allergens among male and female students.
| Variable, n (%) | Males (N=116) | Females (N=106) | p value |
|---|---|---|---|
| Atopy | 58 (50) | 32 (30.2) | 0.003 |
| Monosensitization | 26 (22.4) | 16 (15.1) | 0.166 |
| Polysensitization | 0.08 | ||
| 2 allergens | 12 (10.7) | 8 (7.5) | |
| 3 allergens | 8 (7.1) | 6 (5.7) | |
| ≥4 allergens | 10 (8.6) | 4 (3.8) | |
| D. pteronyssinus | 17 (14.7) | 10 (9.4) | 0.229 |
| D. farina | 10 (8.6) | 5 (4.7) | 0.248 |
| Cat hair | 28 (24.1) | 12 (11.3) | 0.013 |
| Dog hair | 3 (2.6) | 9 (8.5) | 0.05 |
| Horse hair | 8 (6.9) | 5 (4.7) | 0.486 |
| Cockroach | 9 (7.8) | 6 (5.7) | 0.536 |
| Bermuda grass | 12 (10.3) | 32 (30.2) | 0.0002 |
| Ragweed | 14 (12.1) | 5 (4.7) | 0.049 |
| Mugwort | 11 (9.5) | 6 (5.7) | 0.289 |
| Penicillium | 7 (6) | 6 (5.7) | 0.924 |
| Olive | 2 (1.7) | 4 (3.8) | 0.336 |
Discussion
The findings of this study further demonstrate a continuous surge in the prevalence of asthma and other allergic diseases among Saudi young adults. Previous studies have found that the prevalence rates of allergic diseases among adults in different regions of Saudi Arabia ranged from 4.1% to 23% for BA, from 5.3% to 25% for AR, and from 6.1% to 13% for AD.15–17,21–24
Because Saudi Arabia covers a vast territory with marked variations in topographical, meteorological, and climatic conditions, important regional variations in allergic disease prevalence can be observed. Previous epidemiological studies have reported the highest prevalence of asthma and other allergic diseases in Hofuf, Madinah, and Najran, and the lowest rates have been reported in Qassim, Dammam, and Taif.25 Since 2010, some studies have noted plateauing or even declining trends of allergic diseases among children and adolescents in Saudi Arabia.16,26 However, recent studies report a growing prevalence rate of allergic diseases.6,24,25 These increasing trends have been expected by the WHO1 and have been well documented in many recent studies worldwide.12,27 Although the results of our study could be owing to the selection of a homogenous group of young adults with possible accumulation of risk factors for allergic diseases,7,13 a real increase in the prevalence over time, in comparison with previous Saudi reports, cannot be ruled out. It is noteworthy that similar high prevalence rates of allergic diseases have been reported among schoolchildren in the same region as our study site.6
In this study, the prevalence of atopy among young adults was 41%, as detected by SPTs. Previous Saudi studies have showed sensitization rates ranging from 18% to 75% among Saudi populations of different age groups.6,20,28–30 A Global Allergy and Asthma European Network (GALEN) report investigating the geographical variation in SPT positivity among 3451 participants aged 18 to 75 years from 13 European countries found that the sensitization rate varied from 31.4% in Denmark to 52.9% in Germany, with higher rates in younger than older adults.8
There have been few studies focusing on determination of the clinical features of allergic diseases in adults. Consistent with results from previous studies, the symptoms of BA, AR, and AD in our study were significantly associated with adults who had atopic disease, as compared with those who did not.9,31–33 Recent evidence suggests that asthma, rhinitis, and eczema are coexisting disorders that are becoming more prevalent among people with atopic disease; these disorders also occur together in individuals without atopic disease and are strongly associated with specific IgE antibodies.9,34 In this study, 12% of participants with atopy had more than one allergic disease, and 1% had “atopic march”. Atopic march refers to the natural history or typical progression of allergic diseases that often begins early in life; these include atopic dermatitis, food allergy, allergic rhinitis, and asthma. In previous clinical studies, a relatively low number of patients were found to be following the classic course of atopic march. This seems to exist simultaneously with atopic disorders and may be associated with greater functional impairment, which can be reflected in decreased activity and lower quality of life in comparison with participants who have a single allergic disease.4,35 Therefore, the identification of participants with atopic disease and comorbid allergic disorders is essential for accurate monitoring and prompt treatment.
In this study, the sex distribution showed that male students had more frequent atopy and were polysensitized more often than female students; this corresponds with the findings of many previous studies. This sex susceptibility to developing more chronic atopic diseases could be owing to hormonal changes and sex-specific differences in environmental exposures.12,25 The rate of polysensitization in this study (21.6%) was similar to that of many previous reports with a 10% to 22% polysensitization rate among adults.8,9,33 It has been reported that severe allergic diseases are more likely to occur with polysensitization. This could be owing to the increased duration of exposure, recurrent exposure, and extent of inflammation in polysensitized individuals.4
Sensitization to indoor and outdoor allergens is crucial for the development of atopic disorders. The distribution of allergens varies with different environmental factors, local climates, geographic areas, and lifestyles of individuals. Moreover, the prevalence of sensitization to allergens may differ across age groups.8 These facts suggest the paramount importance of local epidemiological data on allergen sensitization, to support evidence-based prevention and management of allergic diseases in different countries and regions. In our study in the Najran region of Saudi Arabia, we found that grass pollens, cat fur, and house dust mites were the most common allergens affecting young adults. Similar patterns of sensitization were reported in a recent study among schoolchildren in the same region.6 However, house dust mites are the most frequent indoor allergen associated with atopic diseases, according to previous epidemiological studies conducted in Saudi Arabia and worldwide.8,28,30 Our finding can be explained in that the Najran region is an agricultural area located in southern Saudi Arabia, with high altitude. These factors promote a prolonged pollination period of grasses and a higher exposure rate, leading to polysensitization against different types of grass pollens.35
Sensitization varies for each specific allergic disease.14 We found that grass pollens and cat fur were the predominant allergens associated with all allergic diseases. Contrary to these findings, many studies have reported a close association of indoor allergens with BA and of outdoor allergens with AR.4,31
Limitations
This study had some limitations. First, self-reporting of symptoms associated with allergic diseases using a questionnaire may involve reporting bias and misclassification. However, we can reasonably expect that the health college students enrolled in our study had sufficient medical background to differentiate whether they had symptoms of asthma, rhinitis, or dermatitis. Second, our study was limited to the Najran region of Saudi Arabia. Therefore, our results may not reflect the remaining areas of Saudi Arabia. Finally, the absence of detailed information regarding participants’ place of birth, the presence of pets or smokers in the family, family history of atopic disorders, and other social and environmental risk factors are a potential limitation of this study. Therefore, further large-scale studies among adults of different age groups from various Saudi regions are important, to explore possible risk and protective factors of allergic diseases in adults.
Conclusion
Our study revealed a high prevalence of atopy and allergic diseases among Saudi young adults. Comorbid allergic diseases were more prevalent among our participants with atopic disease. Male participants had clinical symptoms of allergic disorders and were polysensitized more frequently than female participants. Determining allergen sensitization patterns in patients with atopic disease is crucial to selecting proper preventive and therapeutic strategies.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by a grant from Prince Meshaal bin Abdulaziz Chair for Endemic Diseases, Najran University.
ORCID iD
Jobran Miree Alqahtani https://orcid.org/0000-0003-3198-7576
References
- 1.Global Asthma Network. The Global Asthma Report 2014. http://www.globalasthmareport.org/ (accessed 24 February 2018).
- 2.Global Initiative for Asthma. The 2018 GINA report, Global strategy for asthma management and prevention. https://ginasthma.org/gina-reports/ (accessed 28 February 2018).
- 3.Zuberbier T, Lotvall J, Simoens Set al. Economic burden of inadequate management of allergic diseases in the European Union: a GA2LEN review. Allergy 2014; 69: 1275–1279. [DOI] [PubMed] [Google Scholar]
- 4.Ha EK, Baek JH, Lee Set al. Association of polysensitization, allergic multimorbidity, and allergy severity: a cross-sectional study of school children. Internat Arch Allergy Immunol 2016; 171: 251–260. [DOI] [PubMed] [Google Scholar]
- 5.Sly PD, Boner AL, Bjorksten Bet al. Early identification of atopy in the prediction of persistent asthma in children. Lancet 2008; 372: 1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alqahtani JM. Asthma and other allergic diseases among Saudi schoolchildren in Najran: the need for a comprehensive intervention program. Ann Saud Med 2016; 36: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Amato G, Cecchi L, D’Amato Met al. Urban air pollution and climate change as environmental risk factors of respiratory allergy: an update. J Invest Allergol Clin Immunol 2010; 20: 95–102. [PubMed] [Google Scholar]
- 8.Newson RB, van Ree R, Forsberg Bet al. Geographical variation in the prevalence of sensitization to common aeroallergens in adults: the GA2LEN survey. Allergy 2014; 69: 643–651. [DOI] [PubMed] [Google Scholar]
- 9.Steiner UC, Bachmann LM, Soyka MBet al. Relationship between rhinitis, asthma, and eczema and the presence of sensitization in young Swiss adults. Allergy Rhinol 2018; 9: 2152656718773606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanno LK, Calderon MA, Smith HEet al. Dissemination of definitions and concepts of allergic and hypersensitivity conditions. World Allergy Org J 2016; 9: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The International Study of Asthma and Allergic Diseases (ISAAC). http://isaac.auckland.ac.nz/story/index.html (accessed 26 February 2018).
- 12.Garcia-Larsen V, Potts JF, Del Giacco Set al. Changes in symptoms of asthma and rhinitis by sensitization status over ten years in a cohort of young Chilean adults. BMC Pulm Med 2016; 16: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alqahtani JM, Asaad AM, Awadalla NJet al. Environmental determinants of bronchial asthma among Saudi school children in Southwestern Saudi Arabia. Internat J Environ Res Pub Health 2017; 14: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wickman M, Asarnoj A, Tillander Het al. Childhood to-adolescence evolution of IgE antibodies to pollens and plant foods in the BAMSE cohort. J Allergy Clin Immunol 2014; 133: 580–582. [DOI] [PubMed] [Google Scholar]
- 15.Al Ghobain M, Al-Hajjaj M, Al Moamary M. . Asthma prevalence among 16- to 18-year-old adolescents in Saudi Arabia using the ISAAC questionnaire. BMC Pub Health 2012; 12: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moradi-Lakeh M, El Bcheraoui C, Daoud Fet al. Prevalence of asthma in Saudi adults: findings from a national household survey, 2013. BMC Pulm Med 2015; 15: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarraf H, Aydin O, Mungan Det al. Prevalence of asthma among the adult general population of five Middle Eastern countries: results of the SNAPSHOT program. BMC Pulm Med 2018; 18: 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.STROBE Statement, Version 4 (published Oct/Nov 2007). http://www.strobe-statement.org/index.php?id = available-checklists/ (accessed 28 February 2018).
- 19.Lwanga SK, Lemeshow S. Sample size determination in health studies. Geneva: World Health Organization, 1990. [Google Scholar]
- 20.Koshak EA. Skin test reactivity to indoor allergens correlates with asthma severity in Jeddah, Saudi Arabia. Allergy Asthma Clin Immunol 2006; 2: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabry EY. Prevalence of allergic diseases in a sample of Taif citizens assessed by an original Arabic questionnaire (phase I): a pioneer study in Saudi Arabia. Allergol Immunopathol 2011; 39: 96–105. [DOI] [PubMed] [Google Scholar]
- 22.Fatani MI, Bahashwan E, Alfif KAet al. The prevalence of urticaria and its clinical patterns in Makkah, Saudi Arabia. J Health Sci 2015; 5: 6–9. [Google Scholar]
- 23.Alzeer I, andSuliteen I.. Asthma and allergies among adolescents: comparison of symptoms and prevalence between urban and rural settings. Internat J Med Sci Pub Health 2016; 5: 1–6. [Google Scholar]
- 24.Hasnain SM, Alqassim A, Hasnain Set al. Emerging status of asthma, allergic rhinitis and eczema in the Middle East. J Dis Glob Health 2016; 7: 128–136. [Google Scholar]
- 25.Hussain SM, Farhana SA, Alnasser SM. Time trends and regional variation in prevalence of asthma and associated factors in Saudi Arabia: a systematic review and meta-analysis. Biomed Res Internat 2018; 2018: ID 8102527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harfi H, Al Abbad K, Alsaeed H. Decreased prevalence of allergic rhinitis, asthma and eczema in Riyadh city, Saudi Arabia. Trends Med Res 2010; 5: 57–62. [Google Scholar]
- 27.Backman H, Raisanen P, Hedman Let al. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016 – results from three population surveys. Clin Exper Allergy 2017; 47: 1426–1435. [DOI] [PubMed] [Google Scholar]
- 28.Al-Frayh A. IgE mediated sensitization to weeds pollen in Saudi Arabia. J Allergy Clin Immunol 2007; 119: Abstract 232. [Google Scholar]
- 29.Hasanain SM, Al-Frayh AR, Subiza JLet al. Sensitization to indigenous pollen and molds and other outdoor and indoor allergens in allergic patients from Saudi Arabia, United Arab Emirates, and Sudan. World Allergy Org J 2012; 5: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badran HS, Hussein A, Salah Met al. Identification and prevalence of allergic, non-allergic and local allergic rhinitis patients in Western area, Saudi Arabia. Ann Oto Rhinol Laryngol 2016; 125: 634–643. [DOI] [PubMed] [Google Scholar]
- 31.Toppila-Salmi S, Huhtala H, Karjalainen Jet al. Sensitization pattern affects the asthma risk in Finnish adult population. Allergy 2015; 70: 1112–1120. [DOI] [PubMed] [Google Scholar]
- 32.Steinegger L, Regenass S, Bachmann LMet al. Atopy and related clinical symptoms among Swiss medical students from 2007 to 2015. Allergy Asthma Clin Immunol 2018; 14: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguilar D, Pinart M, Koppelman GHet al. Computational analysis of multimorbidity between asthma, eczema and rhinitis. PLoS One 2017; 12: e0179125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bousquet J, Schunemann HJ, Samolinski Bet al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol 2012; 130: 1049–1062. [DOI] [PubMed] [Google Scholar]
- 35.Katotomichelakis M, Nikolaidis C, Makris Met al. The clinical significance of the pollen calendar of the Western Thrace/northeast Greece region in allergic rhinitis. Internat Forum Allergy Rhinol 2015; 5: 1156–1163. [DOI] [PubMed] [Google Scholar]