Abstract
Porcine epidemic diarrhea virus (PEDV) invades porcine intestinal epithelial cells (IECs) and causes diarrhea and dehydration in pigs. In the present study, we showed a suppression of PEDV infection in porcine jejunum intestinal epithelial cells (IPEC-J2) by an increase in autophagy. Autophagy was activated by rapamycin at a dose that does not affect cell viability and tight junction permeability. The induction of autophagy was examined by LC3I/LC3II conversion. To confirm the autophagic-flux (entire autophagy pathway), autophagolysosomes were examined by an immunofluorescence assay. Pre-treatment with rapamycin significantly restricted not only a 1 h infection but also a longer infection (24 h) with PEDV, while this effect disappeared when autophagy was blocked. Co-localization of PEDV and autophagosomes suggests that PEDV could be a target of autophagy. Moreover, alleviation of PEDV-induced cell death in IPEC-J2 cells pretreated with rapamycin demonstrates a protective effect of rapamycin against PEDV-induced epithelial cell death. Collectively, the present study suggests an early prevention against PEDV infection in IPEC-J2 cells via autophagy that might be an effective strategy for the restriction of PEDV, and opens up the possibility of the use of rapamycin in vivo as an effective prophylactic and prevention treatment.
Keywords: Porcine epidemic diarrhea virus, Rapamycin, Antiviral effect, Autophagy, IPEC-J2
Highlights
-
•
Rapamycin has an antiviral effect against PEDV infection.
-
•
Rapamycin prevents PEDV-induced cell death.
-
•
Rapamycin-induced autophagy restricted PEDV infection in porcine intestinal epithelial cells.
1. Introduction
Porcine epidemic diarrhea virus (PEDV) is a positive single-strand RNA and a member of the Coronaviridae family that is composed of four genera known as the alpha-, beta-, gamma-, and delta-coronaviruses, where PEDV belongs to the alpha-coronavirus. (Belouzard et al., 2012). The virus invades porcine small intestinal epithelial cells (Kim and Lee, 2013) causing diarrhea and dehydration in pigs. Upon PEDV infection, the mortality of piglets may reach 100% (Alvarez et al., 2015), which can cause huge economic losses in the swine industry. Because of the genetic variation particularly in the S gene (Jung and Saif, 2015, Lee, 2015), the available PEDV vaccines cannot prevent all PEDV infections. Because of this, further development of prophylactic and treatment strategies is necessary to restrict PEDV infection.
To protect against PEDV infection, various prophylactics have been developed using Escherichia coli expressing PEDV neutral single chain variable fragments (Pyo et al., 2009), plant extracts (Choi et al., 2009, Lee et al., 2015, Yang et al., 2015), and chemicals such as antiviral drugs (Kim and Lee, 2013). Most studies have used African monkey kidney epithelial cell lines such as VeroE6 while PEDV targets the porcine cells. This raised the question of whether autophagy could have a protective effect against PEDV in porcine intestinal epithelial cells (IECs).
Autophagy is a destructive mechanism for unnecessary or dysfunctional intracellular components (Reggiori and Klionsky, 2002). These components, such as UNC-51-like kinase (ULK), class III phosphatidylinositol-3-kinase (PI3K) complex and autophagy-related genes (ATG), are assembled into isolated membrane bound compartments from the plasma membrane that is isolation and nucleation (Deretic et al., 2013, Dunn, 1994). The isolated membrane bound compartments mature to complete vesicles known as autophagosomes containing ATG and microtubule-associated protein 1A/1B-light chain 3 (LC3)-II, which is produced as a result of LC3-I lipidation by phosphatidylethanolamine. The autophagosomes fuse with lysosomes containing lysosomal enzymes in a low pH environment (Dunn, 1994). The fused vesicle, called an autophagolysosome, undergoes degradation along with its components. This autophagic pathway is critical to maintain cellular homeostasis during stressful conditions such as cellular starvation, infection, or organelle damage (Levine et al., 2011, Mizushima et al., 2010).
In general, autophagy occurs as an antiviral defense mechanism (Deretic et al., 2013, Kobayashi et al., 2014, Levine et al., 2011, Moy et al., 2014), however when some viruses such as hepatitis C virus (HCV), mouse hepatitis virus (MHV), coxsackievirus, and herpes simplex virus 2 (HSV-2) infect a target cell, they induce autophagy in order to utilize it for their replication (Dreux and Chisari, 2009, Harris et al., 2015, Prentice et al., 2004, Yakoub and Shukla, 2015). MHV uses autophagy to enhance its replication in embryonic stem cells (Prentice et al., 2004), whereas the replication of MHV is not related with autophagy in primary murine embryonic fibroblasts and bone marrow derived macrophages (Zhao et al., 2007), which suggests that some viruses have different autophagy responses in different cell types. Therefore, a non-transformed porcine jejunum intestinal epithelial cells (IPEC-J2) that closely resembles the target cells of PEDV should better be used in order to define the relationship between PEDV infection and autophagy at the cellular and molecular level.
Interestingly, several recent studies have shown that chemical or physiological autophagy inducers such as rapamycin, SMER28, and starvation prevent viral infections including rift valley fever virus (Moy et al., 2014), HSV-1 (Yakoub and Shukla, 2015), and transmissible gastroenteritis virus (Guo et al., 2016). However, the role of autophagy activation in the restriction of PEDV remains unknown. Thus, the objective of the present study was to examine the effect of autophagy induction in porcine IECs infected with PEDV.
2. Materials and methods
2.1. Cells, viruses, and reagents
African green monkey kidney cells (VeroE6) were cultured in Eagle's minimal essential medium (MEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Invivogen, USA) and 1% antibiotics (Invitrogen, USA). IPEC-J2 cells (DSMZ, Germany) were cultured in Advanced Dulbecco's modified Eagle's F12 Ham medium (DMEM-F12; Gibco, USA) supplemented with 5% FBS, 1% insulin-transferrin-selenium-X 100× (ITS-X; Gibco, USA), and 1% antibiotics, as described previously (Gu et al., 2016). Both cells were incubated at 37 °C in a humidified atmosphere with 5% CO2. PEDV, strain SM98, was propagated in VeroE6 cells in MEM containing 2 μg/mL of trypsin. Confluent IPEC-J2 cells were inoculated with PEDV at a multiplicity of infection (MOI) of 0.1 for 1 h or 24 h at 37 °C. Unattached viruses were removed by washing with phosphate-buffered saline (PBS). The infected cells were then incubated in fresh media for various time periods.
2.2. Virus titration
After infection, PEDV-infected IPEC-J2 cells were cultured for the required incubation period followed by 2 freeze/thaw cycles. The supernatant was collected and PEDV titer was determined by tissue culture infectious dose 50 (TCID50) (Chen et al., 2014a, Zhao et al., 2014) or a plaque assay by inoculating the supernatant on a monolayer of VeroE6 cells.
2.3. Transepithelial electrical resistance (TEER) assay
IPEC-J2 cells were seeded on 0.33 cm2 polyethylene terephthalate membrane inserts with 0.4 mm pore size (Corning, USA). When the cells reached confluence and were treated with rapamycin, TEER was measured by an epithelial tissue volt-ohmmeter (EVOM2; World Precision Instruments, USA). All values were expressed as Ω × cm2.
2.4. Permeability measurement
Confluent IPEC-J2 cells, cultured on 0.33 cm2 polyethylene terephthalate membrane inserts with 0.4 mm pore size (Corning), were treated with 100 nM rapamycin for 24 h and then washed. Cell culture medium containing 2.2 mg/mL 4 kDa FITC-dextran was added to the upper compartment of the transwell plate. After 1 h of incubation, the amount of fluorescence in the lower compartment of the transwell plate was measured with a fluorescent microplate reader, Victor 3 (Perkin Elmer, USA). Excitation and emission wavelengths were 490 and 520 nm, respectively.
2.5. MTT assay
Confluent IPEC-J2 cells, cultured in a 96-well culture plate, were treated with 100 nM rapamycin for 24 h. At the end of the incubation, 10 μL of a 5 mg/mL MTT solution was added to each well for 4 h and the media was discarded. Then, 100 μL of DMSO was added to each well and shaken for 5 min to solubilize the formazan formed in the viable cells. Absorbance was measured at 580 nm using a microplate reader, VersaMax (Molecular Devices, USA). Cell viability was calculated as the percent ratio of absorbance of the samples against the non-treated control medium.
2.6. Western blot
Confluent IPEC-J2 cells were treated with or without 100 nM rapamycin, washed with PBS, and lysed in a RIPA lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxychloate, 0.1% SDS) containing a protease inhibitors. Protein quantitation of lysates was performed using the Micro BCA kit (Thermo, USA). The same amount of protein extract was loaded on 12% Tris–glycine polyacrylamide gels and electrophoresed. Then, the proteins were transferred onto a polyvinylidene fluoride (PVDF) microporous membrane for 2 h at 4 °C and blocked with 5% skim milk in TBS-T (1 M Tris-HCl, 5 M NaCl, 10% Tween-20) for 90 min. The PVDF microporous membrane was incubated with rabbit anti-LC3B IgG antibody or mouse anti-β-actin IgG antibody overnight. Subsequently, the membrane was washed and incubated with goat anti-rabbit or anti-mouse IgG-HRP antibody for 1 h. The target protein was visualized with an enhanced chemiluminescence system (GE Healthcare, USA), followed by analysis using ChemiDoc XRS (Bio-Rad, USA). The proportion of the LC3-I and LC3-II was calculated by using Image J software.
2.7. Real-time qRT-PCR
Total RNA was isolated using the TRIzol reagent according to the manufacturer's instructions and reverse-transcribed to generate complementary DNA (cDNA) using oligo-dT primers (Bioneer, Korea). Real-time qRT-PCR was performed using a StepOne Plus real-time PCR system (Applied Biosystems, USA). The PCR reaction was carried out in a 96-well reaction plate with 10 μL SYBR® green PCR master mix, 0.5 μL primers, 1 μL cDNA template, and 8 μL nuclease-free H2O. Thermal cycles consisting of 2 min at 50 °C, 10 min at 95 °C, 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C were repeated 40 times. Relative quantification of target genes was calculated using the 2−ΔΔCt method. The nucleotide sequences of specific primers for PEDV nucleocapsid protein (PEDV N; Forward primer: 5′-GTCTGACAACAGCGGCAAAA-3′, Reverse primer: 5′-TTTCGCCCTTGGGAATTCTC-3′) and PEDV spike protein (PEDV S; Forward primer: 5′-TGTTTATTCTGTCACGCCATGTT-3′, Reverse primer: 5′-CCAGGCAACTCCCTAGTATTGCT-3′) were used. Target gene expression was normalized to the mRNA level of porcine glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Forward primer: 5′-TGGGCGTGAACCATGAGAA-3′, Reverse primer: 5′-CCTCCACGATGCCGAAGT-3′).
2.8. Confocal immunofluorescence microscopy
Confluent IPEC-J2 cells were treated with or without PEDV in the absence or presence of pre-treatment with 100 nM rapamycin. The cells were washed, fixed with 100% methyl-alcohol for 15 min at −20 °C, and blocked with PBS containing 5% FBS and 0.3% Triton-100X for 1 h at room temperature. The cells were incubated with rabbit anti-LC3B IgG antibody and mouse anti-PEDV N IgG2a antibody, followed by staining with goat anti-rabbit IgG conjugated with Alexa Fluor 488, goat anti-mouse IgG2a conjugated with Alex Fluor 594, and DAPI for nuclei. Images were captured using a confocal laser scanning microscope, LSM700 (Carl Zeiss, Germany).
2.9. Transfection
Transfection was performed by using Lipofectamine 3000 according to the manufacturer's protocol. In short, IPEC-J2 cells were seeded onto a cell culture slide (SPL, South Korea). When the cells had reached 70–90% confluence, the cells were transiently transfected with the ptfLC3 plasmid (Addgene plasmid # 21074) gifted from Tamotsu Yoshimori. Forty eight hours after transfection, the cells were treated with or without 100 nM rapamycin for 24 h, then fixed with 100% methyl-alcohol for 15 min at −20 °C. The localization of LC3 puncta was observed by a confocal laser scanning microscope, LSM700 (Carl Zeiss, Germany).
2.10. Flow cytometry
Floating IPEC-J2 cells were collected, and the attached cells were washed with PBS and trypsinized for 5 min. The floating cells and trypsinized cells were added together and stained with propidium iodide at a pre-determined optimal concentration. The cells were incubated for 20 min at 4 °C in the dark. After staining, the cells were washed, and the intensity of the markers was examined by a FACS Canto II flow cytometer (BD Biosciences, USA). All flow cytometric data were analyzed using FlowJo software (Tree Star, USA).
2.11. Transmission electron microscopy (TEM)
For ultrastructural analysis, confluent IPEC-J2 cells were infected with PEDV in the absence or presence of pre-treatment with 100 nM rapamycin. The cells were collected and fixed with Karnovsky's fixative buffer for 2 h at 4 °C, washed with 0.05 M sodium cacodylate buffer (pH 7.2), then with 1% osmium tetroxide in 0.05 M sodium cacodylate buffer (pH 7.2), and washed with distilled water. Subsequently, the cells were subjected to En bloc staining for 30 min at 4 °C by using 0.5% uranyl acetate, and sequentially dehydrated by using 30, 50, 70, 80, 90, and 100% ethanol for 10 min each at room temperature. Next, specimens were incubated in 100% propylene oxide for 10 min twice at room temperature, then a 1:1 mixture of propylene oxide and Spurr's resin for 2h, followed by Spurr's resin alone overnight at room temperature. Then, Spurr's resin was added once more for 2h at room temperature, followed by polymerization at 70 °C for 24 h. Finally, specimens were sectioned using an ultramicrotome (EM UC7, Leica, Germany) and were examined by using a transmission electron microscope (JEM1010, JEOL, Japan).
2.12. Statistical analysis
Statistical analysis (One-way ANOVA with Tukey's post-test) was performed using GraphPad Prism 5 (GraphPad Software, USA). Differences were considered significant if p < 0.05.
3. Results
3.1. Rapamycin induces autophagy in IPEC-J2 cells
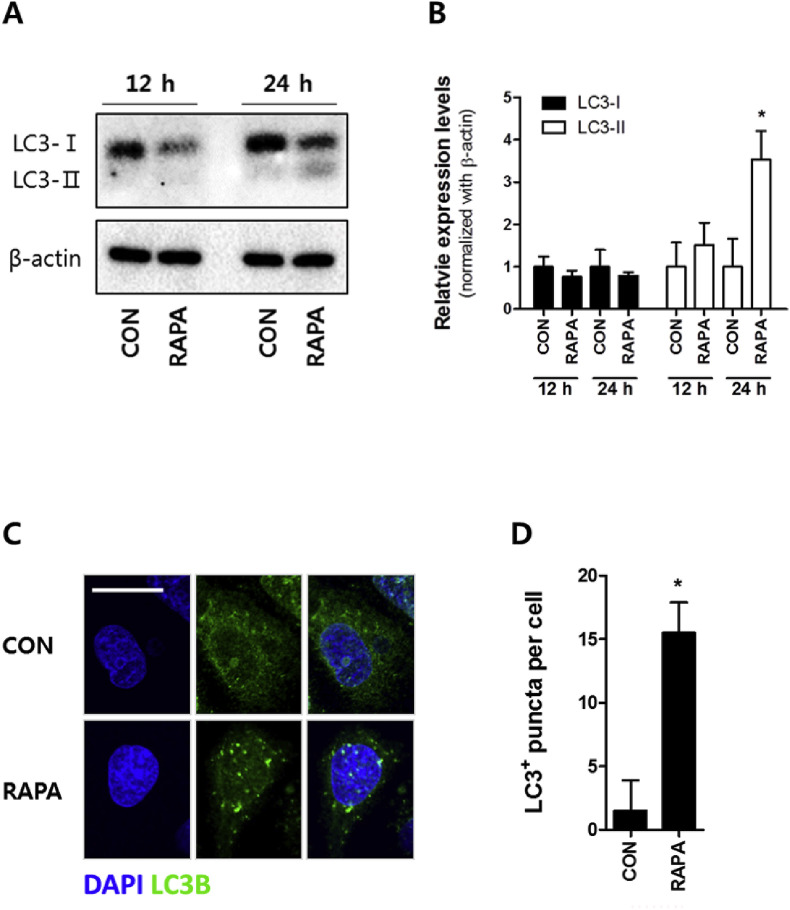
As a preliminary experiment, we have examined antiviral effect of rapamycin at a various concentration using plaque assay in IPEC-J2 cells. As shown in Fig. S3, the rapamycin could restrict PEDV infection in a dose-dependent manner and, therefore, we picked 100 nM as a minimal dose showing a significant antiviral effect. In order to examine whether rapamycin induces autophagy in porcine IECs, LC3 conversion of IPEC-J2 was examined by immunoblot after 100 nM rapamycin treatment for 12 or 24 h. Rapamycin-treatment for 12 h was not sufficient for the activation of autophagy while LC3-I was reduced and, at the same time, LC3-II was increased at 24 h after the treatment compared with control (Fig. 1 A and B). The number of LC3 puncta, indicating autophagosomes, was higher in cells treated with rapamycin for 24 h than untreated cells (Fig. 1C and D). Taken together, these results suggest that 100 nM rapamycin activated autophagy without biological damage to the cell (Fig. S1).
Fig. 1.
Rapamycin activates autophagy in IPEC-J2 cells. Confluent IPEC-J2 cells were treated with 100 nM rapamycin for 24 h. The activation of autophagy was examined by (A) immunoblot for LC3 conversion using rabbit anti-LC3B IgG antibody, (B) relative expression levels of the LC3-I and LC3-II by calculating with Image J, and (C) confocal immunofluorescence microscopy for the formation of autophagosomes. The scale bar indicates 20 μm. (D) The number of puncta per cell was counted, and the values are expressed by mean ± S.D. of at least 5 replicates. The representative data from at least 3 independent experiments are shown. * indicates a significant difference at p < 0.05 compared to its control. CON: control; RAPA: rapamycin.
3.2. The entire process of autophagy pathway is observed in IPEC-J2 cells when treated with rapamycin
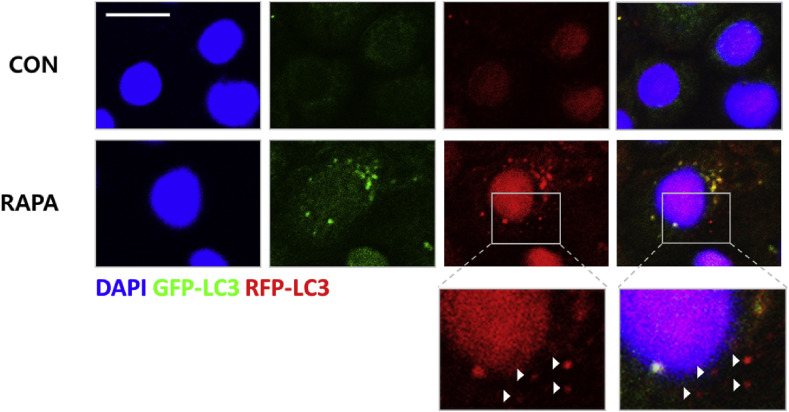
It is well known that the generation of autophagolysosomes is critical for the degradation of unnecessary components including virus particles after infection (Deretic et al., 2013, Levine et al., 2011). However, until now, the formation of autophagolysosomes indicating autophagic-flux in porcine IEC has not been examined. To investigate if rapamycin can induce autophagolysosome formation in porcine IECs, we used transiently transfected IPEC-J2 cells with ptfLC3 plasmids, in which LC3 proteins are conjugated with red fluorescent protein (RFP) and green fluorescent protein (GFP) (Kimura et al., 2007). GFP is unstable and degraded at low pH whereas RFP is relatively stable in an acidic environment. Therefore, GFP disappears while RFP remains alone in acidic conditions of autophagolysosomes after fusion with lysosomes (Kimura et al., 2007). The formation of autophagolysosomes, which appeared as red spots, was observed in the rapamycin-treated group (Fig. 2 ) suggesting that rapamycin treatment induced autophagic-flux in porcine IECs.
Fig. 2.
Rapamycin induces autophagic-flux in IPEC-J2 cells. IPEC-J2 cells at a confluency of 70–90% were transfected with the ptfLC3 plasmid for 2 days. Then, they were treated with100 nM of rapamycin for an additional 24 h. The autophagosomes (yellow spots) and autophagolysosomes (red spots marked by white-arrow heads in the extended images) in the merged image were examined by confocal immunofluorescence microscopy. Magnification is 200× and the scale bar indicates 20 μm. The representative data from at least 3 independent experiments are shown. CON: control, RAPA: rapamycin. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
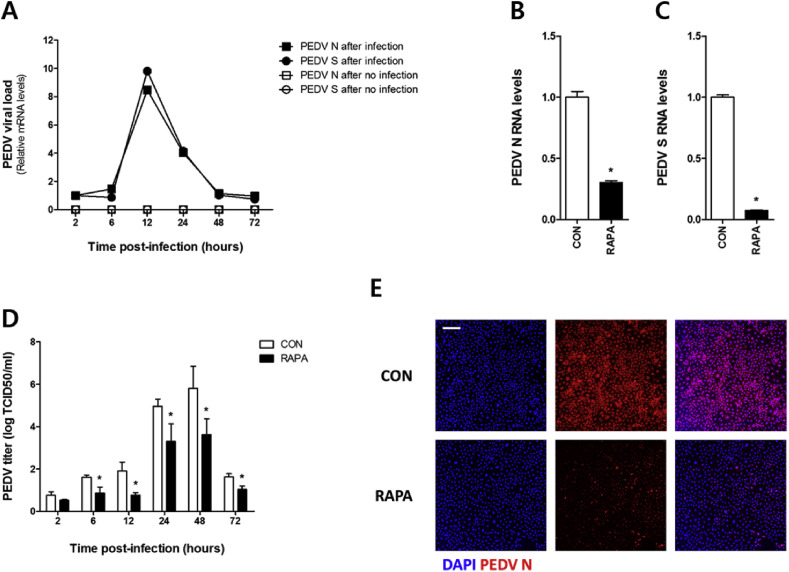
3.3. Rapamycin-treatment down regulates PEDV infection
After PEDV infection, viral replication was examined by PEDV mRNA levels (Fig. 3 A). The rapamycin pretreated group showed reduced PEDV N and S mRNA levels (Fig. 3B, C), a lower PEDV titer (Fig. 3D), and lower PEDV infection in the cells (Fig. 3E) than control cells. These results showed that rapamycin treatment induced autophagy in porcine IECs that may inhibit PEDV infection.
Fig. 3.
Pre-treatment with rapamycin limits PEDV infection in IPEC-J2 cells. IPEC-J2 cells pretreated with or without 100 nM of rapamycin were infected with 0.1 MOI PEDV for 1 h (A) mRNA of PEDV N and S proteins was estimated by real-time qRT-PCR at each time point in IPEC-J2 cells. The mRNA of PEDV (B) N and (C) S was estimated by real-time qRT-PCR at 24 h after the infection. (D) PEDV titer was measured by TCID50 at 2, 6, 12, 24, 48 and 72 h after the infection. (E) PEDV infection was examined by confocal immunofluorescence microscopy at 24 h after the infection. Magnification is 100× and the scale bar indicates 200 μm. All values are expressed as mean ± S. D. of at least 3 replicates. The representative results from at least 3 independent experiments are shown. * indicates a significant difference at p < 0.05. CON: control, RAPA: rapamycin.
3.4. Autophagy restricts PEDV infection in IPEC-J2 cells
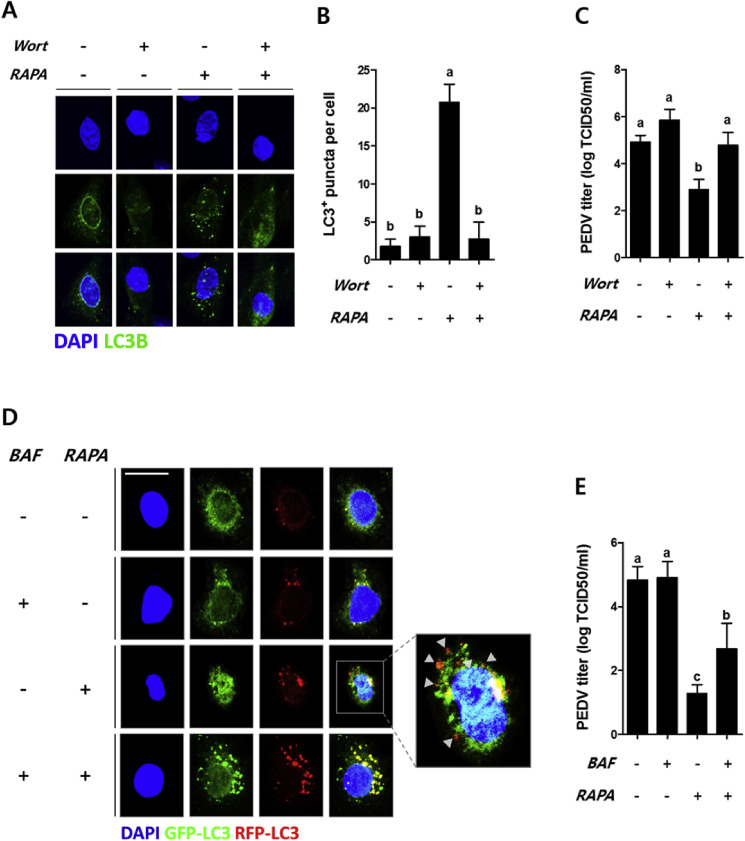
To examine whether autophagy is involved in the restriction of PEDV, 1 μM wortmannin that is non-toxic to IPEC-J2 (Fig. S4B), a PI3K inhibitor (Blommaart et al., 1997), was used to inhibit autophagy. The inhibitor blocked the formation of autophagosomes (Fig. 4 A) and decreased the number of rapamycin-induced LC3 puncta (Fig. 4B) in the wortmannin-treated group when compared with the wortmannin-untreated group. When autophagy was inhibited, a higher PEDV titer was observed compared to the group treated with rapamycin without inhibition (Fig. 4C), indicating that autophagosomes could reduce PEDV infection.
Fig. 4.
Autophagy limits PEDV infection in porcine epithelial cells. Confluent IPEC-J2 cells were treated with or without 1 μM of wortmannin for 1 h prior to 100 nM of rapamycin treatment for 24 h. (A) Autophagy inhibition was observed using a confocal immunofluorescence microscope. (B) The number of puncta per cell was counted, and the values are expressed as mean ± S. D. from at least 5 replicates. (C) After the inhibition of autophagy induced by rapamycin, the cells were infected with 0.1 MOI PEDV for 1 h, and virus titer was estimated using TCID50 at 24 h after infection. (D) IPEC-J2 cells at a confluency of 70–90% were transfected with the ptfLC3 plasmid and treated with 100 nM rapamycin for 24 h, followed by 0.2 μM bafilomycin A1 for 1 h, and the formation of autophagolysosomes were examined using confocal immunofluorescence microscopy. In the merged image, the yellow spots are autophagosomes and white arrow heads indicate autophagolysosomes. (E) After the inhibition of autophagolysosomes, 0.1 MOI PEDV was inoculated for 1 h, and PEDV titer was estimated using TCID50 at 24 h after infection. Magnification of immunofluorescence images is 400× and the scale bar indicates 20 μm. All values other than displaying LC3 puncta are expressed as mean ± S. D. from at least 3 replicates. The figures are representative data from at least 3 independent experiments. Different letters indicate significant differences at p < 0.05 between treatment groups. RAPA: rapamycin, Wort: wortmannin, BAF: bafilomycin A1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To validate the impact of autophagy on restricting PEDV infection further, another inhibitor, 200 nM bafilomycin A1 that is non-toxic to IPEC-J2 (Fig. S4C), was used to block the formation of autophagolysosomes by interfering with the fusion of autophagosomes and lysosomes (Yamamoto et al., 1998). The inhibition of autophagolysosome formation was detected by using IPEC-J2 cells transfected with the ptfLC3 plasmid (Fig. 4D). In these cells, PEDV titer was significantly increased compared with cells only treated with rapamycin (Fig. 4E).
Collectively, these results suggested that autophagy is closely related with restriction of PEDV infection, and especially showed that activation of autophagic-flux such as the formation of autophagolysosomes is important in suppressing PEDV infection.
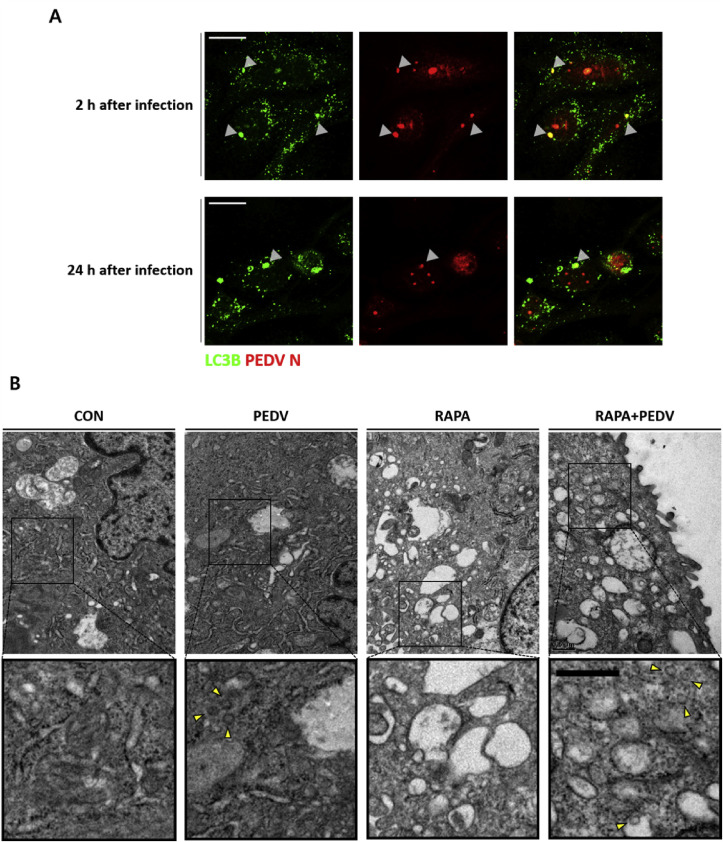
3.5. PEDV is a potential target of autophagy
To further validate the restriction of PEDV by autophagy, co-localization of autophagosomes and PEDV was examined at 2 and 24 h post-infection (Fig. 5 A). Occurrence of co-localization was relatively higher at 2 h post-infection than at 24 h post-infection. Moreover, rapamycin treatment induced increase of the formation of double membrane vesicles (Fig. 5B). After PEDV infection in IPEC-J2 cells, viral particles assumed to be PEDV due to their mostly round shape (Lavazza et al., 2015) and diameter ranging from 95 to 190 nm (Lee, 2015) were found in the cytosol of the cells (Fig. 5B). In particular, cells pretreated with rapamycin had viral particles within vesicles after PEDV infection (Fig. 5B). Therefore, these results showed that PEDV could be positioned within autophagosomes induced by rapamycin, and PEDV might be suppressed by rapamycin-induced autophagy soon after infection.
Fig. 5.
Co-localization of PEDV and autophagosomes. (A) IPEC-J2 cells were treated with 100 nM of rapamycin for 24 h and infected with 0.1 MOI PEDV for 1 h. Co-localization of autophagosomes and PEDV (N protein) was observed (arrow heads) by confocal immunofluorescence microscopy at 2 (upper panel) and 24 h post-infection (lower panel). Magnification is 400× and the scale bar indicates 20 μm. (B) IPEC-J2 cells pretreated with or without 100 nM of rapamycin were infected with or without 0.1 MOI PEDV for 1 h. Each sample was fixed and processed for electron microscopy analysis at 2 h after infection. Yellow arrow heads indicate PEDV in the extended images. Magnification is 40,000× with the scale bar indicating 500 nm. These figures are representative images from at least 3 independent experiments. CON: control, RAPA: rapamycin. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
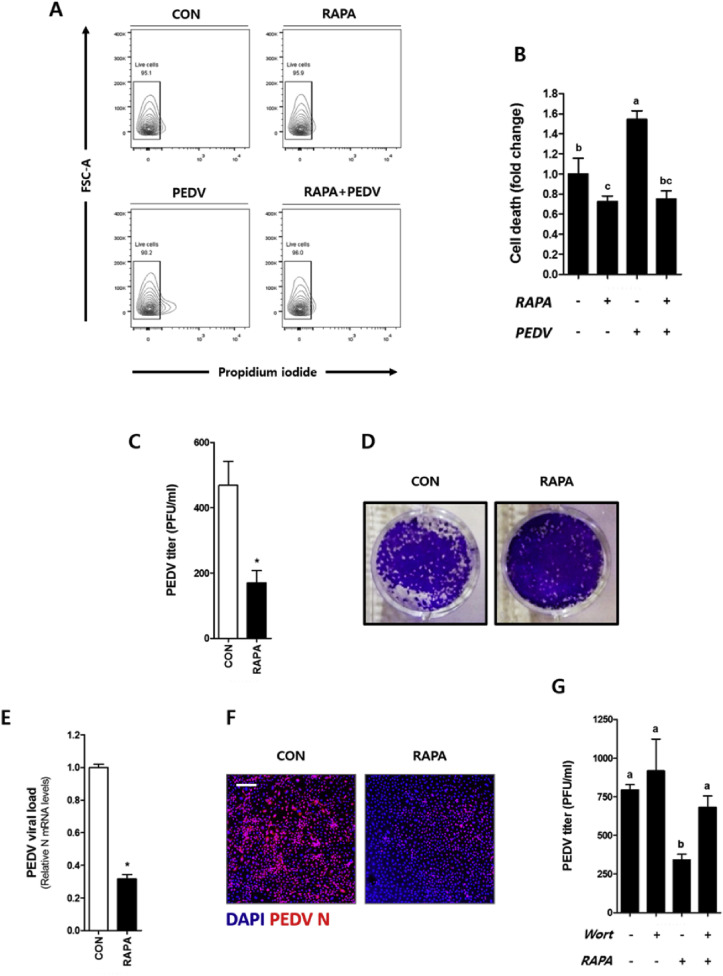
3.6. Rapamycin has a protective effect on cell death induced by longer PEDV infection
In general, most studies on in vitro viral infection use infection times of 60 min (Guo et al., 2016, Shelly et al., 2009, Zhao et al., 2014). For example, Zhao et al. showed a slight change in tight junction protein expression and permeability of porcine IECs, followed by a rapid recovery after 1 h of PEDV or TEGV infection (Zhao et al., 2014). This finding opened up the question of whether PEDV infection for 1 h in vitro appropriately depicts an actual infection, because it is known that PEDV infection induces necrotic death in porcine intestinal epithelial cells in vivo (Jung et al., 2014).
In the present study, PEDV infection for 24 h induced cell death (Fig. 6 A and B), however infection for 12 h did not (Fig. S2). The protective effect of rapamycin pre-treatment in porcine IECs against the longer PEDV infection was also investigated. The results showed a down regulation in the number of plaque forming units (PFU) in the rapamycin pretreated group (Fig. 6C and D). We also confirmed a lower PEDV infection level in the rapamycin-treated group compared to controls as measured by PEDV mRNA level (Fig. 6E) as well as confocal immunofluorescence microscopy (Fig. 6F). Moreover, to examine whether the restriction of longer PEDV infection is due to rapamycin-induced autophagy, we used wortmannin to block autophagy and confirmed a high PEDV titer compared to rapamycin-treated cells without autophagy inhibition (Fig. 6G). These results demonstrated that rapamycin-induced autophagy can restrict a longer PEDV infection for 24 h. In addition, alleviation of PEDV-induced cell death was observed in cells pretreated with rapamycin (Fig. 6A and B).
Fig. 6.
Pre-treatment with rapamycin protects against longer PEDV infection in porcine epithelial cells. IPEC-J2 cells pretreated with or without 100 nM of rapamycin for 24 h were infected with PEDV for 24 h, or not infected. (A) The cells were stained with PI and examined by flow cytometry. (B) The cell death was expressed by fold change. The supernatant was collected after infection and (C) PEDV titer was estimated by a plaque assay and (D) images was taken. (E) mRNA of PEDV N was measured by real-time qRT-PCR. (F) PEDV infection was examined by confocal immunofluorescence microscopy at the magnification of 100×, with the scale bar indicating 200 μm. (G) After the inhibition of autophagy induced by rapamycin, cells were inoculated with PEDV for 24 h, and PEDV titer was estimated using a plaque assay. All values are expressed as mean ± S. D. from at least 3 replicates. The representative data from at least 3 independent experiments are shown. An asterisk (*) indicates a significant difference at p < 0.05. Different letters indicate significant differences at p < 0.05 between treatment groups. CON: control, RAPA: rapamycin, Wort: wortmannin.
4. Discussion
PEDV invasion of porcine intestinal epithelial cells (IECs) causes severe diarrhea, dehydration, and high mortality in suckling pigs. Thus, it is valuable to examine and understand the defense mechanisms against PEDV. In light of the fact that autophagy has been suggested as one of the antiviral mechanisms against a number of viruses (Kudchodkar and Levine, 2009) other than PEDV, the current study discovered that PEDV can be restricted by autophagy in porcine IECs.
When PEDV infects porcine IECs, its entrance is mediated by receptors such as aminopeptidase N protein (Belouzard et al., 2012) or sugars (Deng et al., 2016, Liu et al., 2015) expressed on porcine IECs (Cong et al., 2015, Jung and Saif, 2015). It has been reported that the virus infected epithelium results in barrier dysfunction by downregulation of tight junction proteins (Zhao et al., 2014) and an increase in interleukin (IL)-8 (Xu et al., 2013) and necrotic cell death (Jung and Saif, 2015). However, the mechanism that defends against PEDV infection in porcine IECs is unknown. Since viral translation for replication occurs in the cytoplasm of target cells (Lee, 2015), we postulated that autophagy which removes unnecessary cytoplasmic components (Levine et al., 2011, Reggiori and Klionsky, 2002) might be a potential effective defense mechanism against PEDV. With reference to existing results showing that autophagy can restrict invasion of other viruses including Transmissible gastroenteritis coronavirus (Guo et al., 2016), HSV-1 (Yakoub and Shukla, 2015), and Rift Valley fever virus (Moy et al., 2014), we demonstrated that rapamycin treatment in porcine IECs has a protective function against PEDV through the uptake of the virus into autophagosomes at an early stage of infection. To the best of our knowledge, this study is the first to describe the defensive mechanism of autophagy against PEDV infection in porcine IECs.
Autophagy pathway progresses with the stage of initiation, nucleation, elongation and maturation. Especially, the final step of autophagy pathway is to degrade autophagic cargo in autophagosome after the fusion with lysosome. It has been suggested that Beclin 1 is importantly related with progression of the final step (Glick et al., 2010). Coronavirus has the viral papain-like protease (PLP) 2, which could interrupt the autophagic process. The membrane-associated PLP2 of PEDV, a viral protein encoded by coronaviruses, induced an incomplete autophagy, demonstrated by showing the accumulation of autophagosomes and inhibiting formation of autophagolysosome when Beclin1 was removed (Chen et al., 2014b). On the other hand, rapamycin could up-regulate the expression of beclin1 (Xie et al., 2013). Upregulation of beclin1 expression in cells treated with rapamycin might help formation of autophagolysosome, even if PEDV infects and makes PLP2. Although the antiviral mechanism of rapamycin has not been fully elucidated, we postulated that the rapamycin likely has an antiviral effect in porcine epithelial cells infected with PEDV via increasing beclin1 and/or inducing the formation of autophagolysosome.
It has been reported that PEDV infection in pigs induced an inflammatory response via increased production of pro-inflammatory cytokines including IL-18, IL-12, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ in serum (Annamalai et al., 2015) and monocyte-derived dendritic cells (Gao et al., 2016). Since IECs are in close contact with laminar propria cells, an injured epithelium could lead to the excessive expression of inflammatory cytokines and trigger diarrhea by inducing gut inflammation (Neurath, 2014). Thus, prevention of PEDV infection in porcine IEC before the induction of high levels of inflammation is crucial to reduce diarrhea provoked by PEDV.
PEDV induces acute necrosis in porcine IECs (Jung et al., 2014). However, it is important to mention that viral infection for 1 h, most often used in various PEDV studies, could not generate cell death in vitro and may not accurately depict an actual PEDV infection. To mimic the repeated infection, IPEC-J2 cells were infected with PEDV for 24 h in the present study that resulted in a cell death. Interestingly, PEDV-induced cell death was alleviated by rapamycin pre-treatment. These results highlighted that autophagy induced by pre-treatment of rapamycin was enough to prevent necrosis induced by the longer infection with PEDV.
Findings such as necrosis in porcine IECs (Jung and Saif, 2015) and increased inflammation in the ileum (Annamalai et al., 2015) by PEDV infection may help to recommend the use of rapamycin since it is outstanding for suppressing immune reactions (Saunders et al., 2001) and is an effective antiviral drug that can alleviate induction of inflammation and PEDV infection via autophagy. Additionally, it would be very interesting to determine whether the protective effect of rapamycin on PEDV influences immune cells by using a co-culture system of porcine IECs and porcine immune cells.
In conclusion, rapamycin triggers autophagic-flux and reduces PEDV infection. The reduction of PEDV-induced cell death by pretreatment with rapamycin was also found, suggesting (1) that activation of autophagy might be a good strategy for protecting PEDV and (2) the potency of rapamycin as a powerful prophylactic against PEDV.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Authors' contributions
CH Yun conceived the study, and S Ko designed the study. MJ Gu, YC Kye, CG Kim, Y Lim, JE Lee, and S Ko carried out all experiments and analyzed the samples. S Ko drafted the manuscript. H Chu, BC Park, SH Han, and CH Yun guided other authors and discussed the meaning and direction of the results.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (PJ01112401), Rural Development Administration, Research Institute of Agriculture and Life Sciences, National Research Foundation (NRF-2015R1D1A1A02061577), and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agri-Bioindustry Technology Development Program, which is funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA 115082-2 and 916004-2), Republic of Korea.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.antiviral.2017.08.010.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alvarez J., Sarradell J., Morrison R., Perez A. Impact of porcine epidemic diarrhea on performance of growing pigs. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annamalai T., Saif L.J., Lu Z., Jung K. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Veterinary Immunol. Immunopathol. 2015;168:193–202. doi: 10.1016/j.vetimm.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blommaart E.F., Krause U., Schellens J.P., Vreeling-Sindelárová H., Meijer A.J. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- Chen Q., Li G., Stasko J., Thomas J.T., Stensland W.R., Pillatzki A.E., Gauger P.C., Schwartz K.J., Madson D., Yoon K.-J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014;52:234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wang K., Xing Y., Tu J., Yang X., Zhao Q., Li K., Chen Z. Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein & Cell. 2014;5:912–927. doi: 10.1007/s13238-014-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.-J., Kim J.-H., Lee C.-H., Ahn Y.-J., Song J.-H., Baek S.-H., Kwon D.-H. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antivir. Res. 2009;81:77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y., Li X., Bai Y., Lv X., Herrler G., Enjuanes L., Zhou X., Qu B., Meng F., Cong C. Porcine aminopeptidase N mediated polarized infection by porcine epidemic diarrhea virus in target cells. Virology. 2015;478:1–8. doi: 10.1016/j.virol.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F., Ye G., Liu Q., Navid M.T., Zhong X., Li Y., Wan C., Xiao S., He Q., Fu Z.F. Identification and comparison of receptor binding characteristics of the spike protein of two porcine epidemic diarrhea virus strains. Viruses. 2016;8:55. doi: 10.3390/v8030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Saitoh T., Akira S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux M., Chisari F.V. Autophagy proteins promote hepatitis C virus replication. Autophagy. 2009;5:1224–1225. doi: 10.4161/auto.5.8.10219. [DOI] [PubMed] [Google Scholar]
- Dunn W.A. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–143. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Gao Q., Zhao S., Qin T., Yin Y., Yu Q., Yang Q. Effects of inactivated porcine epidemic diarrhea virus on porcine monocyte-derived dendritic cells and intestinal dendritic cells. Res. veterinary Sci. 2016;106:149–158. doi: 10.1016/j.rvsc.2016.03.023. [DOI] [PubMed] [Google Scholar]
- Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M.J., Song S.K., Lee I.K., Ko S., Han S.E., Bae S., Ji S.Y., Park B.-C., Song K.-D., Lee H.-K. Barrier protection via Toll-like receptor 2 signaling in porcine intestinal epithelial cells damaged by deoxynivalnol. Veterinary Res. 2016;47:1. doi: 10.1186/s13567-016-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Yu H., Gu W., Luo X., Li R., Zhang J., Xu Y., Yang L., Shen N., Feng L. Autophagy negatively regulates transmissible gastroenteritis virus replication. Sci. Rep. 2016;6 doi: 10.1038/srep23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.G., Morosky S.A., Drummond C.G., Patel M., Kim C., Stolz D.B., Bergelson J.M., Cherry S., Coyne C.B. RIP3 regulates autophagy and promotes coxsackievirus B3 infection of intestinal epithelial cells. Cell Host microbe. 2015;18:221–232. doi: 10.1016/j.chom.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Veterinary J. 2015;204:134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Wang Q., Scheuer K.A., Lu Z., Zhang Y., Saif L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014;20 doi: 10.3201/eid2004.131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Lee C. Ribavirin efficiently suppresses porcine nidovirus replication. Virus Res. 2013;171:44. doi: 10.1016/j.virusres.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Noda T., Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Orba Y., Yamaguchi H., Takahashi K., Sasaki M., Hasebe R., Kimura T., Sawa H. Autophagy inhibits viral genome replication and gene expression stages in West Nile virus infection. Virus Res. 2014;191:83–91. doi: 10.1016/j.virusres.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Kudchodkar S.B., Levine B. Viruses and autophagy. Rev. Med. Virol. 2009;19:359–378. doi: 10.1002/rmv.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavazza A., Tittarelli C., Cerioli M. The use of convalescent sera in immune-electron microscopy to detect non-suspected/new viral agents. Viruses. 2015;7:2683–2703. doi: 10.3390/v7052683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Porcine epidemic diarrhea virus: an emerging and re-emerging epizootic swine virus. Virol. J. 2015;12:1. doi: 10.1186/s12985-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Park J.-S., Lee S.-W., Hwang S.-Y., Young B.-E., Choi H.-J. Porcine epidemic diarrhea virus infection: inhibition by polysaccharide from Ginkgo biloba exocarp and mode of its action. Virus Res. 2015;195:148–152. doi: 10.1016/j.virusres.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Levine B., Mizushima N., Virgin H.W. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Tang J., Ma Y., Liang X., Yang Y., Peng G., Qi Q., Jiang S., Li J., Du L. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol. 2015;89:6121–6125. doi: 10.1128/JVI.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Yoshimori T., Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy R.H., Gold B., Molleston J.M., Schad V., Yanger K., Salzano M.-V., Yagi Y., Fitzgerald K.A., Stanger B.Z., Soldan S.S. Antiviral autophagy restricts rift valley Fever virus infection and is conserved from flies to mammals. Immunity. 2014;40:51–65. doi: 10.1016/j.immuni.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- Prentice E., Jerome W.G., Yoshimori T., Mizushima N., Denison M.R. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 2004;279:10136–10141. doi: 10.1074/jbc.M306124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo H.-M., Kim I.-J., Kim S.-H., Kim H.-S., Cho S.-D., Cho I.-S., Hyun B.-H. Escherichia coli expressing single-chain Fv on the cell surface as a potential prophylactic of porcine epidemic diarrhea virus. Vaccine. 2009;27:2030–2036. doi: 10.1016/j.vaccine.2009.01.130. [DOI] [PubMed] [Google Scholar]
- Reggiori F., Klionsky D.J. Autophagy in the eukaryotic cell. Eukaryot. Cell. 2002;1:11–21. doi: 10.1128/EC.01.1.11-21.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders R.N., Metcalfe M.S., Nicholson M.L. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59:3–16. doi: 10.1046/j.1523-1755.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- Shelly S., Lukinova N., Bambina S., Berman A., Cherry S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z.G., Xie Y., Dong Q.R. Inhibition of the mammalian target of rapamycin leads to autophagy activation and cell death of MG63 osteosarcoma cells. Oncol. Lett. 2013;6:1465–1469. doi: 10.3892/ol.2013.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhang H., Zhang Q., Dong J., Liang Y., Huang Y., Liu H.-J., Tong D. Porcine epidemic diarrhea virus E protein causes endoplasmic reticulum stress and up-regulates interleukin-8 expression. Virol. J. 2013;10:1. doi: 10.1186/1743-422X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakoub A.M., Shukla D. Autophagy stimulation abrogates herpes simplex virus-1 infection. Sci. Rep. 2015;5:9730. doi: 10.1038/srep09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Tagawa Y., Yoshimori T., Moriyama Y., Masaki R., Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- Yang J.-L., Ha T.-K.-Q., Dhodary B., Pyo E., Nguyen N.H., Cho H., Kim E., Oh W.K. Oleanane triterpenes from the flowers of camellia japonica inhibit porcine epidemic diarrhea virus (PEDV) replication. J. Med. Chem. 2015;58:1268–1280. doi: 10.1021/jm501567f. [DOI] [PubMed] [Google Scholar]
- Zhao S., Gao J., Zhu L., Yang Q. Transmissible gastroenteritis virus and porcine epidemic diarrhoea virus infection induces dramatic changes in the tight junctions and microfilaments of polarized IPEC-J2 cells. Virus Res. 2014;192:34–45. doi: 10.1016/j.virusres.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Thackray L.B., Miller B.C., Lynn T.M., Becker M.M., Ward E., Mizushima N., Denison M.R., Virgin I., Herbert W. Coronavirus replication does not require the autophagy gene ATG5. Autophagy. 2007;3:581–585. doi: 10.4161/auto.4782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.