Highlights
-
•
Rapid real-time LAMP assays with QPrimer for influenza and RS virus were developed.
-
•
The assays demonstrated high sensitivity and specificity in clinical specimens.
-
•
The assays are useful for experimental, hospital and quarantine laboratories.
Keywords: Real-time, RT-LAMP assay, Influenza virus, Respiratory syncytial virus
Abstract
Influenza virus and respiratory syncytial virus cause acute upper and lower respiratory tract infections, especially in children and the elderly. Early treatment for these infections is thought to be important, so simple and sensitive detection methods are needed for use at clinical sites. Therefore, in this study, real-time reverse transcription loop-mediated isothermal amplification assays with quenching primer for influenza virus and respiratory syncytial virus were developed. Evaluation of a total of 113 clinical specimens compared to real-time RT-PCR assays showed that the novel assays could distinguish between the types and subtypes of influenza virus and respiratory syncytial virus and had 100% diagnostic specificity. The diagnostic sensitivity of each assay exceeded 85.0% and the assays showed sufficient clinical accuracy. Furthermore, positive results could be obtained in around 15 min using the novel assays in cases with high concentrations of virus. The developed assays should be useful for identifying influenza virus and respiratory syncytial virus cases not only in experimental laboratories but also in hospital and quarantine laboratories.
1. Introduction
Influenza virus (IV) and respiratory syncytial virus (RSV) infections are common causes of acute upper and lower respiratory tract infections such as pneumonia and bronchiolitis and lead to high rates of hospitalization, especially in children and the elderly (Falsey and Walsh, 2000; Jain et al., 2015; Sugaya et al., 2000; Zhou et al., 2012). Antiviral drugs for IV, such as oseltamivir and zanamivir, reduce the duration, frequency of symptoms, and hospitalization if administered within 48 h of the onset of symptoms (Aoki et al., 2003; Hayden et al., 1997). Moreover, rapid detection of these viruses is important in the clinical management of patients and for the reduction of healthcare costs (Bonner et al., 2003). However, the clinical signs and symptoms of these viruses are sometimes similar, and it can be difficult to distinguish between causative viruses especially in the incipient stage of disease (Zambon et al., 2001).
In addition to the widely used detection methods for these diseases, including viral cultures, serology, real-time reverse transcription PCR (rRT-PCR), and rapid antigen detection tests (RADTs), several new methods that are easy-to-use and sensitive are currently being developed (Guatelli et al., 1990; Ishiguro et al., 2003; Kouguchi et al., 2010). One newly developed test is the loop-mediated isothermal amplification (LAMP) method, a rapid and sensitive nucleic acid amplification method that is performed under isothermal conditions and requires less complicated equipment than PCR (Nagamine et al., 2002; Notomi et al., 2000). This method can yield results in less than 1 h and can be utilized for the detection of many kinds of viral genomes (Kurosaki et al., 2016; Shirato et al., 2014; Yamazaki et al., 2013). A LAMP reaction can be monitored in real time by measuring the progressive increase in sample turbidity due to the precipitation of magnesium pyrophosphate (Mori et al., 2001), but this technology may compromise the specificity of the test because of the exponential amplification of primer dimers (Njiru, 2012) and, in some cases, the detection of non-specific LAMP products made by host-derived DNA. DNA intercalators or fluorescent dyes, such as calcein, can also be used for real-time monitoring of the LAMP reaction (Seyrig et al., 2015; Tomita et al., 2008). Although these methods yield higher analytical sensitivity and shortened reaction times compared with turbidity-based real-time LAMP, the detection principles of the methods are the same.
In this study, quenching primer (QPrimer) was utilized for the detection of LAMP products by targeting an internal sequence of the amplicon. QPrimer has a cytosine labeled with a fluorescent dye such as BODIPY® FL at the 5' end. When QPrimer hybridizes to its target nucleotide sequence, the fluorescence is quenched by photoinduced electron transfer between the fluorescent dye and a guanine residue in the target (Crockett and Wittwer, 2001; Kurata et al., 2001; Torimura et al., 2001). The establishment of a novel real-time reverse transcription LAMP (rRT-LAMP) assay for the detection of IV and RSV using QPrimer was reported here.
2. Material and methods
2.1. Primer design for the rRT-LAMP assay
Primers for detecting influenza A (IAV) and influenza A subtype H1pdm09 (A/H1pdm) viruses were modified for the circulating strains from those originally described by Nakauchi et al. (Nakauchi et al., 2011b). Primers for detecting influenza B virus (IBV), influenza A subtype H3 (A/H3) virus, respiratory syncytial virus type A (RSV A), and respiratory syncytial virus type B (RSV B) were designed using conserved regions of the NS gene of IBV, the HA gene of influenza A/H3 virus, and the N genes of RSVs (Table 1 ). LAMP primers were designed from candidate conserved regions using Primer Explorer V4 software (Eiken Chemical, Tokyo, Japan). All primers were synthesized by Life Technologies Japan (Tokyo, Japan) and cartridge-purified.
Table 1.
rRT-LAMP primers designed for IV and RSV.
| Target | Name of primer | Sequence (5' to 3') | Reference or source |
|---|---|---|---|
| IAV | F3-1 | GACTTGAAGATGTCTTTGC | (Nakauchi et al., 2011b) |
| F3-2 | GACTGGAAAGTGTCTTTGC | ||
| B3-1 | TRTTATTTGGGTCTCCATT | ||
| B3-2 | TRTTGTTTGGGTCCCCATT | ||
| FIP | TTAGTCAGAGGTGACARRATTGCAGATCTTGAGGCTCTC | ||
| BIP | TTGTKTTCACGCTCACCGTGTTTGGACAAAGCGTCTACG | ||
| LF | GTCTTGTCTTTAGCCA | ||
| LB | CMAGTGAGCGAGGACTG | ||
| QPrimer | CMAGTGAGCGAGGACTG | ||
| IBV | F3 | GCAACCAATGCCACCATA | This study |
| B3 | TTCTCTCTTCAAGRGACATC | ||
| FIP | TAGTCAAGGGCYCTTTGCCACTTTGAAGCAGGAATTCTGGA | ||
| BIP | CAAGACCGCCTAAACAGACTAAACTTTTACTTTCAGGCTCACTT | ||
| LF | TGAAAGYCTTTCATAGCAC | ||
| LB | CAAGAATAAAGACTCACAAC | ||
| QPrimer | CAAGAATAAAGACTCACAAC | ||
| A/H1pdm | F3 | AGCTAAGAGAGCAATT | (Nakauchi et al., 2011b) |
| B3 | TTTCCCTTTATCATTAATGTAGGATTTG | ||
| FIP | ACCTTTGTTCGAGTCATGATTGGTCTCAGTGTCATCATTTGAAAGGTTT | ||
| BIP | TAACGGCAGCATGTCCTCAGTATGAATTTCCTTTTTTAACTAGCCA | ||
| LF | CCATGAACTTGTCTTGGGGAATA | ||
| LB | GCTGGAGCAAAAAGCTTCTACA | ||
| QPrimer | CCATGAACTTGTCTTGGGGAATA | ||
| A/H3 | F3-1 | AGCTGGTTCAGARTTCCT | This study |
| F3-2 | AATTGAAGTTACTAATGCTACTG | ||
| B3 | CGGCACATCATARGGGTAAC | ||
| FIP | AGAGCATCTATTAGTGTGCAGTTTCAAYAGGTGAAATATGCRAC | ||
| BIP | GGAGACCCTCAGTGTGATGGTGCTGTRGGCTTTGC | ||
| LF | CCATCAAGGATCTGATGAGGAC | ||
| LB | AGAARTGGGACCTTTTTGTTGAAC | ||
| QPrimer | CCATCAAGGATCTGATGAGGAC | ||
| RSV A | F3 | GAGTTGAAGGGATTTTTGCA | This study |
| B3 | TGGGTTGTTCAATATATGGTAGA | ||
| FIP | TAACTGATTTTGCTAAGACCCCCGGATTGTTTATGAATGCCTATGG | ||
| BIP | CAAGCAGAAATGGAACAAGTTGTGCTGCTTCTCCACCCAATT | ||
| LF | CACCGTAACATCACTTG | ||
| LB | GAGGTGTATGAGTATGCTCAGA | ||
| QPrimer | CACCGTAACATCACTTG | ||
| RSV B | F3 | TGACATCAGAAATACAAGTCAAT | This study |
| B3 | CGTTTTTTAAGACATTGTTTGCC | ||
| FIP | CATCCCACAGTCTGGAGAATCAAGAAAGTCCTACAAAAAAATGC | ||
| BIP | GCTGCCCTTGTAATAACCAAATTAGCTCCTAATTACTGCAGTAAGACC | ||
| LF | GAGCCACTTCTCCCATC | ||
| LB | CAGCAGGAGATAGATCA | ||
| QPrimer | CGAGCCACTTCTCCCATC | ||
2.2. Clinical specimens
From November 2014 through May 2015 and from November 2015 through March 2016, 113 nasal aspirates, secretions, or swabs were collected from patients presenting with influenza-like illnesses at the outpatient department of Showa General Hospital. Participants or the parents of participants provided written informed consent. This study was approved by the institutional medical ethical committees of the National Institute of Infectious Diseases and Showa General Hospital. Nasal aspirates, secretions, or swabs were collected in 1 mL of universal transport medium (UTM; Copan, Brescia, Italy) and frozen at −80 °C until use.
2.3. In vitro-transcribed RNA
In vitro-transcribed RNA was used as a standard for the rRT-LAMP assay. RNA transcripts for the rRT-LAMP assay for IV were prepared from the full-length of M and HA genes of A/Narita/1/2009 (H1N1)pdm09 (GISAID accession nos. EPI180038 and EPI179437), HA gene of A/Texas/50/2012 (H3N2) (EPI391247), and NS gene of B/Massachusetts/02/2012 (EPI439259). The primers Uni12 (5′-AGCAAAAGCAGG-3′) or Uni9 (5′-AGCAGAAGC-3′) (Zou, 1997) were used for reverse transcription using a SuperScript ® III Reverse Transcriptase Kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. The entire coding region of each gene was amplified by PCR using Phusion High-Fidelity DNA Polymerase (New England BioLabs, Ipswich, MA) with paired primers, with the reverse primer containing the T7 promoter sequence. RNA was transcribed using the T7 RiboMAX™ Express Large Scale RNA Production System (Promega, Madison, WI) and treated with TURBO® DNase (Thermo Fisher Scientific) to degrade the template DNA. The dNTPs and NTPs were removed using MicroSpin G-25 Columns (GE Healthcare, Piscataway, NJ) according to the manufacturer’s instructions. The transcribed RNAs were quantified using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific), and the absorbance value was used to calculate the copy numbers of the transcribed RNAs. The integrity of each transcribed RNA was assessed with a 2100 BioAnalyzer (Agilent Technologies, Santa Clara, CA). RNA transcripts for the rRT-LAMP assay for RSV were prepared from the full-length of N gene of RSV/OsakaC.JPN/16.2012 (Genbank accession no. LC415429) and RSV/OsakaC.JPN/38.2011 (LC415430) and produced as described above.
2.4. RNA extraction and rRT-LAMP assay
Viral RNA was extracted by QIAamp® Viral RNA Mini kit (Qiagen, Dusseldorf, Germany) using 140 μL of UTM mixed with clinical specimens according to the manufacturer's instructions. RT-LAMP was performed using a 25 μL volume reaction mix that contained 5 μL template RNA, 1.4 mM of each dNTP, 0.8 M betaine, 20 mM Tris−HCl (pH 8.8), 70 mM KCl, 8 mM MgSO4, 15 mM (NH4)2SO4, 0.1% Tween 20, 8 U Bst 2.0 polymerase (New England Biolabs), and 0.25 U AMV reverse transcriptase (Nippon Gene, Tokyo, Japan). The reaction mixture also contained 0.2 μM each of F3 and B3 primers, 1.6 μM each of FIP and BIP primers, and 0.8 μM each of LF and LB primers for each assay. In the IAV and A/H3 assay, the final concentration of each F3 and B3 primer was 0.2 μM, although more than 2 primers were used as F3 and B3 primers. For the novel rRT-LAMP assay, 5% of the LB or LF primer of each assay was substituted by the QPrimer-5 G (Nippon Steel and Sumikin Eco-Tech, Tokyo, Japan). The rRT-LAMP reaction was performed at 63 °C for 30 min using LightCycler® 480 II (Roche, Basel, Switzerland). Fluorescence was measured at wavelengths of 465 nm (excitation) and 510 nm (emission) after 4 min of reaction and again every 1 min thereafter. The results were determined by observation in real time and considered positive following fluorescence quenching. To compare with a turbidity-based rRT-LAMP assay, the time to positivity was considered as the first time the fluorescence quench rate increased by more than 3% within 3 min. To obtain the relative fluorescence rate at each detection point, the fluorescence intensity measured at each time point was divided by that measured at the beginning. The turbidity-based rRT-LAMP assay was conducted at 63 °C for 30 min using Loopamp Realtime Turbidimeter LA-320C (Eiken Chemical). Turbidity readings of the optimal density at 650 nm (OD650) were obtained every 6 s, and the reaction was considered positive when the turbidity values were over 0.05. To obtain corrected absorbance, the average turbidity from 2 to 5 min after the initiation of the LAMP reaction was used as the correction base line. GraphPad Prism 5.0 software (Graph Pad Software, La Jolla, CA) was used to generate the figures.
2.5. rRT-PCR assay
All viral RNA extracted from the 113 specimens was tested using the one-step rRT-PCR assays for detection of the types and subtypes of IV as the reference test, as described previously (Nakauchi et al., 2014, 2011a). In addition, another 15 viral respiratory pathogens were identified by rRT-PCR assays developed by Do et al. (2010) and Kaida et al. (2014), namely, RSV A and B; human parainfluenza virus type 1, 2, 3, and 4; influenza C virus; human rhinoviruses; human metapneumovirus; human coronavirus OC43, 229E, NL63, and HKU1; human bocavirus; and human adenovirus.
2.6. Validation and evaluation of QPrimer-based rRT-LAMP assay
The sensitivity of the QPrimer-based rRT-LAMP assays was assessed using various concentrations of quantified in vitro-transcribed RNA in triplicate at each concentration. The type/subtype specificity of the QPrimer-based rRT-LAMP assays for IV was validated using 24 representative subtypes of IAV and IBV (Table 2 ). All statistical analyses were performed with the MedCalc free statistical calculator (http://www.medcalc.org, MedCalc Software bvba, Ostend, Belgium).
Table 2.
Panel of 24 IVs used to determine the analytical specificity of the rRT-LAMP.
| Subtype | Virus |
|---|---|
| H1N1 | A/duck/Alberta/35/76 |
| H1N1 | A/Brisbane/59/2007 |
| H1N1pdm09 | A/Narita/1/2009 |
| H2N3 | A/duck/Germany/1215/73 |
| H3N8 | A/duck/Ukraine/1/63 |
| H3N2 | A/Uruguay/716/2007 |
| H4N6 | A/duck/Czechoslovakia/56 |
| H4N6 | A/duck/Hyogo/1/2010 |
| H5N1 | A/whooper swan/Hokkaido/4/2011 |
| H5N1 | A/blow fly/Kyoto/93/2004 |
| H5N2 | A/chicken/Ibaraki/1/2005 |
| H6N2 | A/turkey/Massachusetts/3740/65 |
| H7N1 | A/duck/Hong Kong/301/1978 |
| H7N9 | A/Anhui/1/2013 |
| H8N4 | A/turkey/Ontario/6118/68 |
| H8N4 | A/duck/Shizuoka/45/2011 |
| H9N2 | A/turkey/Wisconsin/1/66 |
| H10N7 | A/chicken/Germany/N/49 |
| H11N6 | A/duck/England/56 |
| H12N5 | A/duck/Alberta/60/76 |
| H13N6 | A/gull/Maryland/704/77 |
| H14N5 | A/mallard/Gurjev/263/82 |
| H15N8 | A/duck/Australia/341/83 |
| TypeB | B/Massachusetts/2/2012 |
3. Results
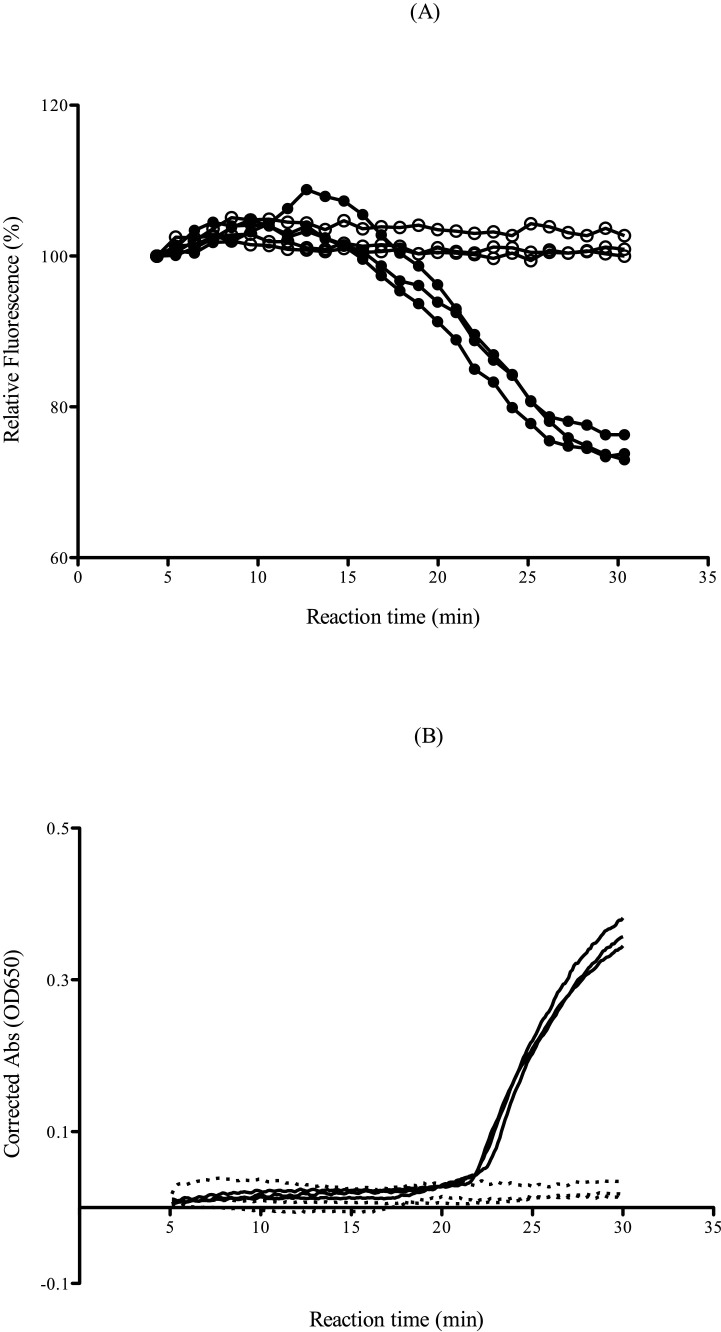
The principle of guanine quenching was used to detect the amplification process in the novel rRT-LAMP. Fluorescence quenching is detectable in real time because QPrimer reduces the fluorescence once it is incorporated within the LAMP product. Thus, the fluorescence signal is at a maximum at the beginning of the amplification reaction and is quenched progressively throughout the amplification process down to a stable plateau, where it remains until the end of the reaction. On the other hand, the precipitation of magnesium pyrophosphate increases as the LAMP reaction proceeds in the turbidity-based rRT-LAMP assay (Fig. 1 ). The reaction times of rRT-LAMP for IAV in the two types of LAMP detection, namely, turbidity and QPrimer, were examined. To compare the reaction times in the two formats, various concentrations of in vitro-transcribed RNA were used. The RNA detection time at each dilution point by QPrimer was earlier than that by turbidity measurements, and the detection rate of target RNAs was more stable in the novel QPrimer-based rRT-LAMP assay compared with the turbidity-based RT-LAMP assay (Table 3 ).
Fig. 1.
Comparative reaction times of the rRT-LAMP assay for IAV. The assay was performed using in vitro-transcribed standard RNA. (A) LAMP products were detected by fluorescence quenching using QPrimer (novel QPrimer-based rRT-LAMP). The results of 5000 copies/reaction (filled circles) and negative control samples (open circles) are indicated. (B) LAMP products were detected by real-time turbidity (conventional turbidity-based rRT-LAMP). The results of 5000 copies/reaction (straight lines) and negative control samples (dotted lines) are indicated.
Table 3.
Reaction times (min) of novel and conventional rRT-LAMP assays for detecting IAV in in vitro-transcribed standard RNA.a
| The way of detection | Concentration of RNA (copies/reaction) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 5000 | 500 | 250 | 50 | 25 | 5 | 0.5 | N.C. | |
| QPrimer (novel rRT-LAMP) | 16.0 | 19.0 | 22.0 | 26.0 | 25.0 | – | – | – |
| 17.0 | 23.0 | 19.0 | – | – | – | – | – | |
| 16.0 | 21.0 | 27.0 | – | – | – | – | – | |
| Turbidity (conventional rRT-LAMP) | 23.2 | 27.2 | 26.8 | – | – | – | – | – |
| 23.3 | – | 25.6 | 29.9 | – | – | – | – | |
| 23.8 | 26.1 | 29.9 | – | – | – | – | – | |
The assays were carried out in triplicate at each concentration.
The analytical sensitivity of the QPrimer-based rRT-LAMP assays was observed using testing various dilutions of quantified in vitro-transcribed RNA of each target gene in triplicate. As shown in Table 4 , the assays enabled the detection of each target gene at 25–250 copies/reaction at the lowest concentration, and no false positive results were observed for any of the negative control samples in either assay. The type/subtype specificity of the assays for IV were validated using 24 representative IAVs and IBV (Table 2). The assay showed positive reactions only for each target type/subtype virus and no cross-reactivity with any other subtypes of IAV and IBV (data not shown).
Table 4.
Number of positive results of the QPrimer-based rRT-LAMP assay in the detection of each in vitro-transcribed standard RNA.a
| Target | Standard RNA | Concentration of RNA (copies/reaction) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 5000 | 500 | 250 | 50 | 25 | 5 | 0.5 | N.C. | ||
| IAV | A/Narita/1/2009 (H1N1)pdm09 M gene | 3 | 3 | 3 | 1 | 1 | 0 | 0 | 0 |
| IBV | B/Massachusetts/2/2012 NS gene | 3 | 3 | 3 | 2 | 1 | 0 | 0 | 0 |
| A/H1pdm | A/Narita/1/2009 (H1N1)pdm09 HA gene | 3 | 3 | 3 | 2 | 1 | 0 | 0 | 0 |
| A/H3 | A/Texas/50/2012 (H3N2) HA gene | 3 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| RSV A | RSV/OsakaC.JPN/16.2012 N gene | 3 | 3 | 2 | 2 | 0 | 0 | 0 | 0 |
| RSV B | RSV/OsakaC.JPN/38.2011 N gene | 3 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
The assays were carried out in triplicate at each concentration.
To assess the utility of the QPrimer-based rRT-LAMP assay for the clinical diagnosis of IV and RSV, an evaluation of 113 clinical specimens collected from patients with influenza-like illness was conducted. Table 5 summarizes the comparison of results between QPrimer-based rRT-LAMP and rRT-PCR. The QPrimer-based rRT-LAMP assays had higher than 85.0% sensitivity and 100% specificity for all targets. The average threshold cycle (Ct) values in the reference rRT-PCR assay for samples, which were positive by both QPrimer-based rRT-LAMP and rRT-PCR, were 24.9 (range, 18.6–32.7) for IAV, 26.1 (range, 18.6–33.9) for IBV, 26.2 (range, 20.9–34.1) for A/H1pdm virus, 24.0 (range, 17.8–29.1) for A/H3 virus, 23.6 (range, 19.5–32.9) for RSV A, and 28.2 (range, 21.4–37.2) for RSV B. Among the samples, 1–3 showed false negative results in the QPrimer-based rRT-LAMP assay, with Ct values between 31.0 and 39.8 in the reference rRT-PCR assay. Four specimens were co-infected with IV and RSV in this evaluation. Of these, IBV and RSV B were not detected by QPrimer-based rRT-LAMP in 1 specimen co-infected with IBV and RSV A or in 1 specimen co-infected with RSV A and RSV B. On the other hand, both pathogens were detected by QPrimer-based rRT-LAMP in the other 2 specimens co-infected with RSV A and RSV B. Among the 113 specimens, 14 (12.4%) that were negative for all pathogens tested by rRT-PCR were also negative via the QPrimer-based rRT-LAMP assays. Furthermore, the QPrimer-based rRT-LAMP did not amplify RNA from specimens that contained other respiratory viruses not targeted by RT-LAMP in this study. That is, none of the samples showed false positive results via QPrimer-based rRT-LAMP assay.
Table 5.
Performance of novel QPrimer-based rRT-LAMP assay for IV and RSV compared with the reference rRT-PCR assay.
| Target | LAMP result | rRT-PCR result |
LAMP sensitivity % (95% CI) | LAMP specificity % (95% CI) | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| IAV | Positive | 26 | 0 | 89.7 (72.7–97.8) | 100 (95.7–100) |
| Negative | 3 | 84 | |||
| IBV | Positive | 10 | 0 | 90.9 (58.7–99.8) | 100 (96.5–100) |
| Negative | 1 | 102 | |||
| A/H1pdm | Positive | 16 | 0 | 88.9 (65.3–98.6) | 100 (96.2–100) |
| Negative | 2 | 95 | |||
| A/H3 | Positive | 10 | 0 | 90.9 (58.7–99.8) | 100 (96.5–100) |
| Negative | 1 | 102 | |||
| RSV A | Positive | 17 | 0 | 85.0 (62.1–96.8) | 100 (96.1–100) |
| Negative | 3 | 93 | |||
| RSV B | Positive | 13 | 0 | 92.9 (66.1–99.8) | 100 (96.3–100) |
| Negative | 1 | 99 | |||
4. Discussion
In this study, a rapid, specific, and sensitive detection assay for IV and RSV using a novel rRT-LAMP method was developed. Currently, RADTs are widely used and popular at clinical sites because it is considered important to provide early treatment for IV and RSV and avoid the unnecessary use of antibiotic therapy. However, the sensitivity and specificity of some RADTs remain relatively poor, especially those for RSV (sensitivity range, 71.15–80.77%) (Bell et al., 2014; Dunn et al., 2014; Kanwar et al., 2015). The novel QPrimer-based rRT-LAMP assays enabled the detection of each target gene at 25–250 copies/reaction at the lowest concentration (Table 4) and showed less sensitive than the reference rRT-PCR assays which limit of detection were 6–9 copies/reaction as determined in previous studies (Nakauchi et al., 2014,). However, the QPrimer-based rRT-LAMP assays showed high sensitivity (≥85.0%) and sufficient clinical accuracy in clinical specimens from patients with influenza-like illness compared with RADTs. Furthermore, the design of QPrimer in the novel method is simple and universally applicable; specifically, part of one of the loop primers with a cytosine at the 5′ end is substituted by the QPrimer, or it can add a cytosine to the 5′ end of the QPrimer if there are no suitable loop primers with a cytosine at the 5′ end, such as with the RSV B primer sets in this study (Table 1). The QPrimer-based rRT-LAMP assays can be conducted using general-purpose real-time PCR instruments and an isothermal nucleic acid amplification system with fluorescence measurements.
The conventional turbidity-based rRT-LAMP detection of a target by turbidity is simple. However, because it cannot exclude nonspecific reactions caused by primer dimers, the time taken to positivity can be delayed. In this study, QPrimer was utilized for detection by targeting an internal sequence of the amplicon. By using QPrimer, reaction time delay by turbidity-based rRT-LAMP assay was overcame (Table 3). In addition, higher diagnostic test specificity was achieved and all assays had 100% specificity (Table 5). These valuable results agree with current reports on QPrimer and quenching probe (QProbe), which are used for amplicon detection in some nucleotide amplification methods (Ayukawa et al., 2017; Hiramatsu et al., 2017; Toyama et al., 2015). An rRT-PCR assay usually takes 1–2 h after sample preparation, whereas the novel QPrimer-based rRT-LAMP assay provides positive results in around 15 min for samples with high concentrations of viral RNA, suggesting that this assay can be characterized by its rapid results. The results suggest that the QPrimer-based rRT-LAMP assay could be used as a fast and sensitive diagnostic test for detecting IV and RSV.
In conclusion, the newly developed rRT-LAMP assay with QPrimer for IV and RSV demonstrated high diagnostic sensitivity (≥85.0%) and high diagnostic specificity (100%) in clinical specimens from patients with influenza-like illness. The QPrimer-based rRT-LAMP assays can be performed without skilled personnel, and positive results can be achieved faster than they are by rRT-PCR assay. The QPrimer-based rRT-LAMP assay can be used to test for other pathogens and is a powerful tool for use not only in experimental laboratories but also in hospital and quarantine laboratories.
Competing interests
The authors declare that they have no competing interests.
Funding
This research was supported by a Grant-in-Aid (Grant Number JP18fk0108030) from Japan Agency for Medical Research and Development (AMED).
References
- Aoki F.Y., Macleod M.D., Paggiaro P., Carewicz O., El Sawy A., Wat C., Griffiths M., Waalberg E., Ward P., Group I.S. Early administration of oral oseltamivir increases the benefits of influenza treatment. J. Antimicrob. Chemother. 2003;51:123–129. doi: 10.1093/jac/dkg007. [DOI] [PubMed] [Google Scholar]
- Ayukawa Y., Hanyuda S., Fujita N., Komatsu K., Arie T. Novel loop-mediated isothermal amplification (LAMP) assay with a universal QProbe can detect SNPs determining races in plant pathogenic fungi. Sci. Rep. 2017;7:4253. doi: 10.1038/s41598-017-04084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J.J., Anderson E.J., Greene W.H., Romero J.R., Merchant M., Selvarangan R. Multicenter clinical performance evaluation of BD veritor system for rapid detection of respiratory syncytial virus. J. Clin. Virol. 2014;61:113–117. doi: 10.1016/j.jcv.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Bonner A.B., Monroe K.W., Talley L.I., Klasner A.E., Kimberlin D.W. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112:363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- Crockett A.O., Wittwer C.T. Fluorescein-labeled oligonucleotides for real-time pcr: using the inherent quenching of deoxyguanosine nucleotides. Anal. Biochem. 2001;290:89–97. doi: 10.1006/abio.2000.4957. [DOI] [PubMed] [Google Scholar]
- Do D.H., Laus S., Leber A., Marcon M.J., Jordan J.A., Martin J.M., Wadowsky R.M. A one-step, real-time PCR assay for rapid detection of rhinovirus. J Mol Diagn. 2010;12:102–108. doi: 10.2353/jmoldx.2010.090071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J., Obuekwe J., Baun T., Rogers J., Patel T., Snow L. Prompt detection of influenza A and B viruses using the BD Veritor System Flu A+B, Quidel(R) Sofia(R) Influenza A+B FIA, and Alere BinaxNOW(R) Influenza A&B compared to real-time reverse transcription-polymerase chain reaction (RT-PCR) Diagn. Microbiol. Infect. Dis. 2014;79:10–13. doi: 10.1016/j.diagmicrobio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Falsey A.R., Walsh E.E. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 2000;13:371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatelli J.C., Whitfield K.M., Kwoh D.Y., Barringer K.J., Richman D.D., Gingeras T.R. Isothermal, in vitro amplification of nucleic acids by a multienzyme reaction modeled after retroviral replication. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7797. doi: 10.1073/pnas.87.19.7797b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F.G., Osterhaus A.D., Treanor J.J., Fleming D.M., Aoki F.Y., Nicholson K.G., Bohnen A.M., Hirst H.M., Keene O., Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. N. Engl. J. Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K., Matsuda H., Nemoto T., Nosaka T., Saito Y., Naito T., Takahashi K., Ofuji K., Ohtani M., Suto H., Yasuda T., Hida Y., Kimura H., Soya Y., Nakamoto Y. Identification of novel variants in HLA class II region related to HLA DPB1 expression and disease progression in patients with chronic hepatitis C. J. Med. Virol. 2017;89:1574–1583. doi: 10.1002/jmv.24814. [DOI] [PubMed] [Google Scholar]
- Ishiguro T., Saitoh J., Horie R., Hayashi T., Ishizuka T., Tsuchiya S., Yasukawa K., Kido T., Nakaguchi Y., Nishibuchi M., Ueda K. Intercalation activating fluorescence DNA probe and its application to homogeneous quantification of a target sequence by isothermal sequence amplification in a closed vessel. Anal. Biochem. 2003;314:77–86. doi: 10.1016/s0003-2697(02)00618-8. [DOI] [PubMed] [Google Scholar]
- Jain S., Williams D.J., Arnold S.R., Ampofo K., Bramley A.M., Reed C., Stockmann C., Anderson E.J., Grijalva C.G., Self W.H., Zhu Y., Patel A., Hymas W., Chappell J.D., Kaufman R.A., Kan J.H., Dansie D., Lenny N., Hillyard D.R., Haynes L.M., Levine M., Lindstrom S., Winchell J.M., Katz J.M., Erdman D., Schneider E., Hicks L.A., Wunderink R.G., Edwards K.M., Pavia A.T., McCullers J.A., Finelli L., Team C.E.S. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida A., Kubo H., Takakura K., Sekiguchi J., Yamamoto S.P., Kohdera U., Togawa M., Amo K., Shiomi M., Ohyama M., Goto K., Hase A., Kageyama T., Iritani N. Associations between co-detected respiratory viruses in children with acute respiratory infections. Jpn. J. Infect. Dis. 2014;67:469–475. doi: 10.7883/yoken.67.469. [DOI] [PubMed] [Google Scholar]
- Kanwar N., Hassan F., Nguyen A., Selvarangan R. Head-to-head comparison of the diagnostic accuracies of BD Veritor System RSV and Quidel(R) Sofia(R) RSV FIA systems for respiratory syncytial virus (RSV) diagnosis. J. Clin. Virol. 2015;65:83–86. doi: 10.1016/j.jcv.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Kouguchi Y., Teramoto M., Kuramoto M. Real-time nucleic acid sequence-based amplification (NASBA) using an adenine-induced quenching probe and an intercalator dye. J. Appl. Microbiol. 2010;109:1724–1732. doi: 10.1111/j.1365-2672.2010.04801.x. [DOI] [PubMed] [Google Scholar]
- Kurata S., Kanagawa T., Yamada K., Torimura M., Yokomaku T., Kamagata Y., Kurane R. Fluorescent quenching-based quantitative detection of specific DNA/RNA using a BODIPY((R)) FL-labeled probe or primer. Nucleic Acids Res. 2001;29:E34. doi: 10.1093/nar/29.6.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki Y., Magassouba N., Oloniniyi O.K., Cherif M.S., Sakabe S., Takada A., Hirayama K., Yasuda J. Development and evaluation of reverse transcription-loop-mediated isothermal amplification (RT-LAMP) assay coupled with a portable device for rapid diagnosis of Ebola virus disease in Guinea. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y., Nagamine K., Tomita N., Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001;289:150–154. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- Nagamine K., Hase T., Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes. 2002;16:223–229. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]
- Nakauchi M., Yasui Y., Miyoshi T., Minagawa H., Tanaka T., Tashiro M., Kageyama T. One-step real-time reverse transcription-PCR assays for detecting and subtyping pandemic influenza A/H1N1 2009, seasonal influenza A/H1N1, and seasonal influenza A/H3N2 viruses. J. Virol. Methods. 2011;171:156–162. doi: 10.1016/j.jviromet.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakauchi M., Yoshikawa T., Nakai H., Sugata K., Yoshikawa A., Asano Y., Ihira M., Tashiro M., Kageyama T. Evaluation of reverse transcription loop-mediated isothermal amplification assays for rapid diagnosis of pandemic influenza A/H1N1 2009 virus. J. Med. Virol. 2011;83:10–15. doi: 10.1002/jmv.21934. [DOI] [PubMed] [Google Scholar]
- Nakauchi M., Takayama I., Takahashi H., Oba K., Kubo H., Kaida A., Tashiro M., Kageyama T. Real-time RT-PCR assays for discriminating influenza B virus Yamagata and Victoria lineages. J. Virol. Methods. 2014;205:110–115. doi: 10.1016/j.jviromet.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njiru Z.K. Loop-mediated isothermal amplification technology: towards point of care diagnostics. PLoS Negl. Trop. Dis. 2012;6:e1572. doi: 10.1371/journal.pntd.0001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyrig G., Stedtfeld R.D., Tourlousse D.M., Ahmad F., Towery K., Cupples A.M., Tiedje J.M., Hashsham S.A. Selection of fluorescent DNA dyes for real-time LAMP with portable and simple optics. J. Microbiol. Methods. 2015;119:223–227. doi: 10.1016/j.mimet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Shirato K., Yano T., Senba S., Akachi S., Kobayashi T., Nishinaka T., Notomi T., Matsuyama S. Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP) Virol. J. 2014;11:139. doi: 10.1186/1743-422X-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya N., Mitamura K., Nirasawa M., Takahashi K. The impact of winter epidemics of influenza and respiratory syncytial virus on paediatric admissions to an urban general hospital. J. Med. Virol. 2000;60:102–106. [PubMed] [Google Scholar]
- Tomita N., Mori Y., Kanda H., Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008;3:877–882. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- Torimura M., Kurata S., Yamada K., Yokomaku T., Kamagata Y., Kanagawa T., Kurane R. Fluorescence-quenching phenomenon by photoinduced electron transfer between a fluorescent dye and a nucleotide base. Anal. Sci. 2001;17:155–160. doi: 10.2116/analsci.17.155. [DOI] [PubMed] [Google Scholar]
- Toyama T., Nishimura Y., Ogata Y., Sei K., Mori K., Ike M. Effects of planting Phragmites australis on nitrogen removal, microbial nitrogen cycling, and abundance of ammonia-oxidizing and denitrifying microorganisms in sediments. Environ. Technol. 2015:1–8. doi: 10.1080/09593330.2015.1074156. [DOI] [PubMed] [Google Scholar]
- Yamazaki W., Mioulet V., Murray L., Madi M., Haga T., Misawa N., Horii Y., King D.P. Development and evaluation of multiplex RT-LAMP assays for rapid and sensitive detection of foot-and-mouth disease virus. J. Virol. Methods. 2013;192:18–24. doi: 10.1016/j.jviromet.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Zambon M.C., Stockton J.D., Clewley J.P., Fleming D.M. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358:1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- Zhou H., Thompson W.W., Viboud C.G., Ringholz C.M., Cheng P.Y., Steiner C., Abedi G.R., Anderson L.J., Brammer L., Shay D.K. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin. Infect. Dis. 2012;54:1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S. A practical approach to genetic screening for influenza virus variants. J. Clin. Microbiol. 1997;35:2623–2627. doi: 10.1128/jcm.35.10.2623-2627.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]