Highlights
-
•
Agreement between molecular tests for Rotavirus group A (RVA) detection was 80–92%.
-
•
The agreement between all assays was 81–100% in samples containing high viral loads.
-
•
The sensitivity of RVA RT-iiPCR was 3–4 copies of in vitro transcribed dsRNA.
-
•
The field-deployable RT-iiPCR system holds promise for on-site detection of RVA.
Keywords: Diagnosis, Insulated isothermal PCR, Point of need, Real-time RT-PCR, Rotavirus
Abstract
There is no gold standard for detection of Rotavirus Group A (RVA), one of the main causes of diarrhea in neonatal animals. Sensitive and specific real-time RT-PCR (rtRT-PCR) assays are available for RVA but require submission of the clinical samples to diagnostic laboratories. Patient-side immunoassays for RVA protein detection have shown variable results, particularly with samples from unintended species. A sensitive and specific test for detection of RVA on the farm would facilitate rapid management decisions. The insulated isothermal RT-PCR (RT-iiPCR) assay works in a portable machine to allow sensitive and specific on-site testing. The aim of this investigation was to evaluate a commercially available RT-iiPCR assay for RVA detection in feces from different animal species. This assay was compared to an in-house rtRT-PCR assay and a commercially available rtRT-PCR kit, as well as an ELISA and EM for RVA detection. All three PCR assays targeted the well-conserved NSP5 gene. Clinical fecal samples from 108 diarrheic animals (mainly cattle and horses) were tested. The percentage of positive samples by ELISA, EM, in-house rtRT-PCR, commercial rtRT-PCR, and RT-iiPCR was 29.4%, 31%, 36.7%, 51.4%, 56.9%, respectively. The agreement between different assays was high (81.3–100%) in samples containing high viral loads. The sensitivity of the RT-iiPCR assay appeared to be higher than the commercially available rtRT-PCR assay, with a limit of detection (95% confidence index) of 3–4 copies of in vitro transcribed dsRNA. In conclusion, the user-friendly, field-deployable RT-iiPCR system holds substantial promise for on-site detection of RVA.
1. Introduction
Rotavirus is classified as a member of family Reoviridae, genus Rotavirus. It is non-enveloped, 60–80 nm in diameter, and the genome length is approximately 18.5 Kb. The genome is composed of 11 segments of double-stranded RNA and encodes six structural proteins (VP1–4, 6 and 7) and six non-structural proteins (NSP1-6) (Desselberger, 2014, Zhou et al., 2015). The virus capsid displays icosahedral symmetry and contains three layers, including an outer layer composed of the VP7 protein, with VP4 protein spikes, an inner or middle VP6 glycoprotein layer, and the core shell formed by VP2 (Desselberger, 2014).
Antigenic epitope analysis of the VP6 glycoprotein classifies the genus Rotavirus into 8 groups (A–H) (Chandler-Bostock et al., 2015, Matthijnssens et al., 2012). Rotaviruses are characterized by relatively high antigenic and genetic diversity, as a result of accumulation of point mutations (genetic drift), and/or reassortment of genomic segments (genetic shift) (Matthijnssens et al., 2012). Although host species barriers and host range restriction exist in rotavirus, reassortment can result in interspecies transmission, which also contributes to the diversity and evolution of rotavirus (Martella et al., 2010, Zhou et al., 2015).
RVA is one of the main causative agents of diarrhea in young humans and animals (Cho et al., 2013, Zhou et al., 2015). It is ubiquitous in the environment and relatively resistance to disinfectants. The adult animals are the main source of infection for newborn animals, and serological surveys revealed that 50–100% of adult animals have an immune response against RVA (Schlafer and Scott, 1979). Clinically healthy newborn animals may also shed RVA, but the prevalence is lower compared to animals exhibiting diarrhea (Kaminjolo and Adesiyun, 1994). Studies have shown that detection of rotavirus in the presence of diarrhea is a significant finding in calf diarrhea (Cho et al., 2013). Additionally, rotavirus has been shown to be significantly associated with liquid diarrhea in 9- to 21-day-old dairy calves (Al Mawly et al., 2015) and also in beef calves (Cho et al., 2013).
There are many assays used for diagnosis of RVA. Historically, diagnostic laboratories routinely used electron microscopy (EM) for detection of the virus. However, due to the costs of the microscope and its maintenance, as well as the required technical expertise, this method has lost favor. Additionally, EM lacks sensitivity, requiring ∼106 viral particles/ml for virus detection (Maes et al., 2003). Immunoassays (such as ELISAs) have replaced EM as the diagnostic test of choice for detection of viral antigen because of their ease of use and speed of obtaining a result (Desselberger, 2014). Most of the commercially available ELISA kits are based on monoclonal antibodies against VP6 glycoprotein, which is expressed at a high level during infection. The VP6 glycoprotein is conserved among RVA of different animal species, allowing cross-reactivity and detection of the virus from different hosts. Therefore, the commercially available human RVA ELISA assays have been used for detection of RVA in animals (Bailey et al., 2013). However, as a result of antigenic drift, there are several variants or genotypes of RVA VP6 that have been described (Mino et al., 2015). Differences among these VP6 genotypes influence the performance of various commercially available VP6 based immunoassays. Therefore, diagnostic kit validation for each species is necessary. Not all the assays have been validated for use in all animal species, and this is particularly true for horses (Mino et al., 2015). In fact, one human RVA rapid immunoassay developed for patient-side use was shown not to work in detection of RVA in horses (Slovis et al., 2014).
Recently, molecular techniques such as RT-PCR and real-time rtRT-PCR have replaced other diagnostic tests with the advantage of higher analytical sensitivity and specificity (Slovis et al., 2014). However, these types of assays are run in commercial or diagnostic laboratories and require expensive equipment and advanced technical skills, resulting in increasing costs to the producer, which leads to a reduction in the use of laboratory assays to support field disease investigations (Izzo et al., 2012). Therefore, the aim of our investigation was to evaluate a recently available insulated isothermal RT-PCR (RT-iiPCR) reagent set (POCKIT™ Rotavirus A Reagent Set, GeneReach USA, Lexington, MA, USA) with use of a portable PCR machine, which could potentially be used for point-of-need detection for RVA in the feces of different animal species. The assay was compared to an in-house rtRT-PCR assay, a commercially available rtRT-PCR kit, a commercially available ELISA, and EM.
2. Materials and methods
2.1. Clinical specimens
A total of 108 fecal samples from clinically affected animals, submitted to the University of Tennessee, College of Veterinary Medicine, Clinical Virology Laboratory, were used for comparison between different diagnostic assays. Nucleic acids were extracted with an automated nucleic acid extraction system according to the manufacturer’s instructions (taco™ mini, GeneReach USA) from all 108 samples for molecular testing (in-house real-time RT-PCR, the commercially available real-time RT-PCR, and the RT-iiPCR reagent set). Briefly, 200 mg of fecal sample was added to 1 ml PBS and 200 μl of the supernatant was used for extraction. Nucleic acids were tested immediately following extraction or were stored at −80 °C until tested.
2.2. Electron microscopy
Approximately five grams of fecal material were suspended in 10 ml distilled water and centrifuged at 14,000g for 50 min. The fecal pellet was re-suspended in 2 ml of distilled water and 100 μl of the suspension was mixed with 100 μl of 3% phosphotungstic acid (pH 7.4) in 1 ml of distilled water. The mixture was then nebulized onto a Carbon Type-B, 200-mesh copper grid (TED Pella, Redding, CA, USA). The grids were examined by an electron microscope (Zeiss Auriga, University of Tennessee, Advanced Microscopy and Imaging Center, Knoxville, TN, USA). Eighty four of the fecal samples were available for testing by EM.
2.3. ELISA
A commercially available sandwich ELISA, targeting the VP6 of human RVA (Premier™ Rotaclone ELISA kit, Meridian Bioscience, Cincinnati, OH, USA), was used for testing the fecal samples, according to the manufacturer’s instructions. Eighty-five of the fecal samples were available for testing by ELISA.
2.4. In-house rtRT-PCR assay
The published nucleotide sequences of RVA non-structural protein 5 (NSP5), from different animal species (62 bovine, 12 equine, 4 caprine, 1 ovine and 3 canine), were retrieved from GenBank1 and aligned using MAFFT software.2 Primers and a probe were designed, using the GenScript online software,3 to amplify an area of 141 bp. Primer and probe sequences are shown in Table 1 . One-step real-time RT-PCR assay was performed using the SuperScript® III Platinum® One-Step qRT-PCR Kit (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA, USA) in a StepOne™ real-time PCR system (Applied Biosystems, Thermo Fisher Scientific, Foster City, CA, USA). The proper concentrations of primers, probe and magnesium were optimized. The test was performed in 25-μl total reaction volume containing 5 μl of the extracted nucleic acid, 400 nM of each primer, 200 nM of probe, 3 mM magnesium and 500 nM ROX reference dye. Following optimization, the cycle parameters were: 50 °C for 30 min, 95 °C for 2 min, followed by 45 cycles at 95 °C for 15 s and 60 °C for 1 min. The assay sensitivity was determined and optimized by using standard RNA, which was produced by cloning the PCR product from a positive clinical sample with the TA Cloning® Kit with pCR™2.1 Vector and One Shot® INVαF' Chemically Competent Escherichia coli (Invitrogen, Thermo Fisher Scientific, USA) according to manufacturer’s instructions. The purified plasmids were sequenced to confirm correct orientation, linearized, and used as a template for synthesis of in vitro transcribed RNA using the MEGAscript T7 Transcription Kit (Invitrogen, Thermo Fisher Scientific, USA), according to manufacturer’s instructions. RNA copy numbers were calculated and a standard curve was generated from ten-fold serial dilutions of the RNA standard at a range from 1 × 10−1 to 1 × 10−6. The reproducibility of the assay was evaluated by calculation of intra- and inter-assay coefficient of variation (CV). The assay specificity was evaluated by testing DNA and RNA of different pathogens that are known to cause diarrhea in various animal species, including Neorickettsia risticii (Potomac horse fever), Rhodococcus equi, Clostridium difficile, E. coli, Salmonella enterica Typhimurium and Dublin, Crytosporidium parvum, bovine viral diarrhea virus genotypes I and II, and bovine coronavirus.
Table 1.
Primer/probe sequences for in-house real-time RT-PCR assay.
| Primer/Probe | Sequence | Positiona |
|---|---|---|
| RotaReal-F | 5′ TTCTGCTTCAAACGAYCCACTC 3′ | 21–234 |
| RotaReal-R | 5′ GAGAAATCYACTTGRTCGCA 3′ | 353–334 |
| RotaReal-Probe | 5′ FAM- TCCATAGAYACRCCAGYRTCTGCRTTTGTC- BHQ 3′ | 296–267 |
According to GenBank accession number GU937876.
2.5. RT-iiPCR assay
The RT-iiPCR test (POCKIT™ Rotavirus A Reagent Set) targets the NSP5 gene. All the components of the RT-iiPCR reaction were lyophilized in one tube. The lyophilized Premix was rehydrated before reaction in 50 μl Premix Buffer B and 5 μl of sample RNA were added to the mixture. The mixture was transferred to an R-tube, centrifuged and tested by the POCKIT™ nucleic acid analyzer (GeneReach USA) as previously described(Wilkes et al., 2014). The assay specificity was evaluated as described for the in-house rtRT-PCR assay. Double-stranded rotavirus NS5 RNA was synthesized and used to determine sensitivity of the RT-iiPCR reagent set. Briefly, a plasmid containing a partial sequence of the NSP5 gene of the RVA/horse-wt/ZAF/EqRV-SA1/2006/G14P[12] strain (GenBank accession JQ345499) and the bovine rotavirus strain KJ44 strain (GenBank accession DQ494399) were used to generate positive and negative strand RNA by in vitro transcription using the MAXIscript® T7 kit and MEGAscript® SP6 Kit (Life Technologies, Darmstadt, Germany), respectively. Residual DNA was removed using the Ambion® Turbo DNA-free™ kit (Life Technologies). The two RNA products were annealed to form double-stranded RNA. Residual single strand RNA was removed by RNase A treatment. After phenol-chloroform extraction, integrity of the double-stranded RNA preparation was confirmed by polyacrylamide gel electrophoresis analysis. Concentration of RNA was determined in a NanoDrop 1000 Spectrophotometer (NanoDrop Technologies, Houston, TX, USA). Serial dilutions of double-stranded RNA were made in 40 ng/μl yeast tRNA. Single use aliquots were stored at −80 °C. Additionally, the sensitivity of the rotavirus RT-iiPCR reagent set was evaluated by comparison with the commercially available rtRT-PCR assay using 10-fold serial dilutions of nucleic acid extracted from a positive bovine clinical sample.
2.6. Commercially available rtRT-PCR assay
The nucleic acid samples were tested by the LSI VetMAX™ Triplex Ruminant Rotavirus & Coronavirus Real-Time PCR Kit (Thermo Fisher Scientific, USA), according to manufacturer’s instructions.
2.7. Statistical analysis
The percentage of agreement between the different diagnostic assays and Cohen’s Kappa coefficient were calculated using SPSS Statistics software.4
3. Results
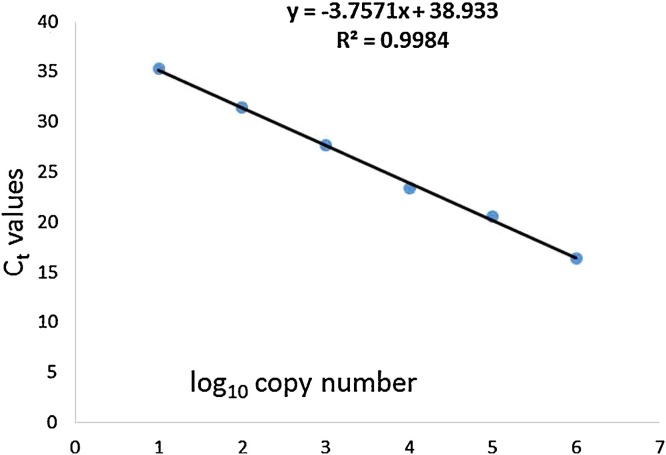
The standard curve for the in-house rtRT-PCR assay generated from serially diluted standard RNA was linear (slope = −3.757) (Fig. 1 ) and the coefficient of linear regression (R2) was 0.998. The assay efficiency was estimated to be 84.6%. The developed assay was sensitive and able to detect 70 genomic equivalents of the target NSP5 gene per reaction. Furthermore, it was specific, with no amplification detected from DNA or RNA from other pathogens known to cause diarrhea in animal species. The assay was reproducible with intra- and inter-assay CVs ranging from 0.009 to 0.022 and 0.004 to 0.033, respectively.
Fig. 1.
Standard curve for in-house real-time RT-PCR assay for rotavirus group A. The assay was linear over 6 orders of magnitude and was able to detect as few as 70 genomic equivalents per reaction.
The limit of detection (95% confidence interval) for the RT-iiPCR reagent set was 4 and 3 copies of dsRNA, based on using log dilutions of in vitro transcribed dsRNA containing target bovine and equine rotavirus sequences, respectively. The comparison between sensitivity of the RT-iiPCR assay and commercially available rtRT-PCR assay using 10-fold serial dilutions of nucleic acid from a positive sample showed that the RT-iiPCR reagent set had a 100-fold increase in sensitivity. Like the in-house assay, this assay was also specific, with no amplification in any of the samples used for specificity testing.
There was variation in the percentage of positive samples detected by each assay. The RT-iiPCR assay detected the most, while EM detected the least. The overall percentages of positive samples by each diagnostic assay are shown in Table 2 . There was a significant difference in the number of positive samples detected with the in-house rtRT-PCR assay versus the other two molecular tests. These differences are evident when comparing the Kappa coefficients (Table 3 ).
Table 2.
Percentage of positive samples by different diagnostics assays.
| Animal species | Assays |
||||
|---|---|---|---|---|---|
| EM | ELISAa | Commercial RT-iiPCR | Commercial real-time rtRT-PCR kitb | In-house rtRT-PCR assay | |
| Bovine | 21/68 (30.9%) | 20/54 (37%) | 54/76 (71%) | 52/76 (68.4%) | 35/76 (46%) |
| Equine | 1/6 (16.7%) | 2/21 (9.5%) | 3/22 (13.6%) | 2/22 (9.1%) | 2/22 (9.1%) |
| Caprine | 1/4 (25%) | 0/4 (0%) | 1/4 (25%) | 0/4 (0%) | 0/4 (0%) |
| Canine | 3/6 (50%) | 3/6 (50%) | 4/6 (66.7%) | 3/6 (50%) | 3/6 (50%) |
| Total | 26/84 (31%) | 25/85 (29.4%) | 62/108 (57.4%) | 57/108 (52.8%) | 40/108 (37%) |
Premier™ Rotaclone ELISA kit, Meridian Bioscience, USA.
LSI VetMAX™ triple ruminant Rotavirus and Coronavirus Real-Time PCR kit, Thermo Fisher Scientific, USA.
Table 3.
Comparison of reliability of different diagnostic tests in detection of Rotavirus group A.
| Tests in comparison | Test by test agreement |
Agreementc | |
|---|---|---|---|
| Percentage of agreement | Kappa | ||
| EM vs ELISAa | 85.5% | 0.68 | Substantial |
| EM vs commercial RT-iiPCR assay | 64.3% | 0.37 | Fair |
| EM vs commercial real-time rtRT-PCR kitb | 67.5% | 0.40 | Fair |
| EM vs in-house rtRT-PCR assay | 78.6% | 0.56 | Moderate |
| ELISA vs commercial RT-iiPCR assay | 74.1% | 0.46 | Moderate |
| ELISA vs commercial rtRT-PCR kit | 81.2% | 0.59 | Moderate |
| ELISA vs in-house rtRT-PCR assay | 89.4% | 0.74 | Substantial |
| Commercial RT-iiPCR vs commercial rtRT-PCR kit | 91.7% | 0.84 | Almost Perfect |
| Commercial RT-iiPCR vs in-house rtRT-PCR assay | 79.6% | 0.62 | Substantial |
| Commercial rtRT-PCR kit vs in-house rtRT-PCR assay | 84.3% | 0.68 | Substantial |
Premier™ Rotaclone ELISA kit, Meridian Bioscience, USA.
LSI VetMAX™ triple ruminant Rotavirus and Coronavirus Real-Time PCR kit, Thermo Fisher Scientific, USA.
The EM assay results showed substantial agreement in comparison to ELISA results. This agreement becomes fair when compared to the RT-iiPCR reagent set and the commercial rtRT-PCR kit, and moderate in comparison to the in-house rtRT-PCR assay. The ELISA showed moderate agreement with the RT-iiPCR reagent set and commercial rtRT-PCR assay but had substantial agreement with the in-house rtRT-PCR assay. A perfect agreement was found between the RT-iiPCR reagent set and the commercial rtRT-PCR assay (Table 3). Comparison between the different assays showed a strong correlation for samples containing high viral loads (Ct values ≤25) (Table 4 ).
Table 4.
Percentage agreement between positive samples by Commercial Real-time rtRT-PCR kita versus other assays, broken down by rtRT-PCR Ct values.
| Ct Range | Assays |
|||
|---|---|---|---|---|
| EM | ELISAb | Commercial RT-iiPCR | In-house rtRT-PCR assay | |
| 5–15 | 100% | 100% | 100% | 100% |
| 16–25 | 81.3% | 100% | 100% | 94% |
| 26–35 | 12% | 40% | 100% | 83.3% |
| 36–45 | 0% | 22.2% | 87% | 9% |
LSI VetMAX™ triple ruminant Rotavirus and Coronavirus Real-Time PCR kit, Thermo Fisher Scientific, USA.
Premier™ Rotaclone ELISA kit, Meridian Bioscience, USA.
4. Discussion
RVA is one of the most prevalent causative agents of diarrhea in farm animals (Athanassious et al., 1994, Izzo et al., 2011, Slovis et al., 2014). It causes significant economic losses as a result of decreased weight gain, treatment costs, and high mortalities. Therefore, development of a highly sensitive and specific test for point-of-need diagnosis would be beneficial to the veterinary practitioner and producer because delays associated with shipping samples to a diagnostic laboratory could be avoided, aiding in rapid control and prevention decisions (Izzo et al., 2012).
There are many commercially available lateral flow immunochromatography assays (LAT) that can be used for on-site detection of RVA. One brand of LAT showed favorable results in comparison to virus isolation and ELISA (Maes et al., 2003). However, when compared to qRT-PCR, the results have been variable. The limitation of antibody-based tests for the detection of enteric pathogens is the requirement of high concentration of free antigen to generate a positive reaction, the free antigen is decreased significantly during the course of disease. Therefore, these tests have lower sensitivity and could miss positive samples collected late in the course of clinical disease, when compared to RT-PCR (Izzo et al., 2012, Maes et al., 2003). Depending on the test used and species tested, these tests can also have low specificity (Izzo et al., 2012) or may not work at all (Slovis et al., 2014).
Commercial ELISA assays and other immunoassays, while easy to use and rapid for detection, are mostly designed for human use and care must be taken when applying these tests for animal use. The ELISA used in this study was able to detect RVA from three different animal species. The agreement between EM and ELISA was 85.5%, which was similar to previously published reports (Athanassious et al., 1994, Benfield et al., 1984, Reynolds et al., 1984), and both of these methods lacked the sensitivity achieved with molecular assays, which has also been previously reported (Izzo et al., 2012). Interestingly, three positive samples by ELISA in this study were negative by EM and all three molecular assays. The ELISA assay absorbance values for these samples ranged from 0.2 to 0.5, which indicates low viral load according to the manufacturer. These samples were considered false positives. False positive results are not uncommon for ELISA assays as a result of the complex sample matrix (i.e. gut microbiota), which can increase the probability of cross-reactions. The type of antibodies (polyclonal versus monoclonal) used strongly affects their detection efficiency. The use of monoclonal antibodies is usually associated with increased specificity of the assay, but this also creates potential problems with regard to amino acid variability among the rotaviruses from different species
Another complication with detection of rotavirus is the fact that it can be detected in both healthy and diseased animals. Establishing a causal relationship may be difficult without demonstration of classic histopathological changes (Izzo et al., 2012), particularly when using highly sensitive molecular detection methods. Considering animals shed up to 108–1012 virions/ml of feces during the acute phase of infection (Izzo et al., 2012), positive results obtained with less sensitive methods (EM and ELISA) are more likely to be associated with causality. In human medicine, ELISA diagnosis is highly correlated with disease in RVA infection (Phillips et al., 2009).
Related to this concept is the idea that magnitude of viral shedding (based on Ct value) can help determine disease etiology (Phillips et al., 2009). Evidence of high viral load in samples tested gives the clinician more confidence that the virus is the cause of the disease process (Izzo et al., 2012). A high correlation between Ct values ≤25 and clinical disease was found in one human study that compared RVA shedding in clinically healthy subjects versus those with diarrhea (Phillips et al., 2009). We found a higher correlation between test methods when we evaluated samples with Ct values ≤25. However, Ct values from different protocols must be interpreted with care because the values may not equate to the same viral load per gram of feces (Phillips et al., 2009). This was actually seen in this study when comparing between the commercial rtRT-PCR assay and the in-house rtRT-PCR assay, particularly with the positive equine samples. The Ct values obtained by the in-house rtRT-PCR assay were 21 and 18.3, compared to 26.18 and 36.01, respectively, by the commercial rtRT-PCR kit. While we did not have additional equine samples to further examine this, these high Ct values from equine samples are consistent with a previous report (Matthijnssens et al., 2015) in which the same commercial molecular assay was also used for diarrheic samples from equine. Ct values for positive samples from that study ranged from 26.18–36.01. The authors attributed the high Ct values to degradation of the viral RNA in the samples or mild infection. While these are certainly possibilities, these results do raise questions about the sensitivity of this commercial rtRT-PCR assay in the diagnosis of equine RVA. This disparity may potentially be attributed to mismatches in primer and probe binding areas. The designed primers and probe of the in-house rtRT-PCR assay contain several degenerate bases, in an attempt to avoid problems with base mismatches. However, as seen with the sensitivity testing and comparison between the assays, the in-house molecular test was less sensitive that the other molecular assays. Based on the findings in this study when comparing Ct values, it is important to note that the possibility of the significance of lower concentrations of RNA may not be excluded. Ct values can be affected by many factors, not just mismatches in primer/probe binding regions or inappropriate handling and storage of the sample, but also stage of the disease and the quality of the sample collected (Izzo et al., 2012).
The commercial RT-iiPCR assay performed in a portable PCR machine was shown to have higher sensitivity than the other molecular methods tested in this study. While it is possible some of the positive samples could have been false positives, we believe it is more likely associated with the increased sensitivity seen with this test, which was demonstrated with side-by-side testing, versus the commercial rtRT-PCR assay, of serial dilutions of nucleic acid from a clinical sample. High sensitivity of RT-iiPCR assays has been consistently demonstrated in previous reports (Ambagala et al., 2015, Balasuriya et al., 2014, Wilkes et al., 2014). The higher sensitivity may be attributed to the performance of the reaction in gradient temperature that results from the thermal convective phenomena associated with this type of PCR (Krishnan et al., 2002). This allows primers and probes to anneal to sequences with mismatches (Ambagala et al., 2015).
The commercial RT-iiPCR assay incorporates a fluorescent hydrolysis probe, which increases the specificity of the assay, functioning more like a real-time PCR than a conventional PCR. However, rather than obtaining a Ct value that requires interpretation, the portable machine detects the fluorescent signal before and after the reaction and automatically converts it into a positive or negative result. The numerical value for these fluorescent signals can be obtained from the machine for some determination about amount of virus present in the sample, but the sensitivity of the signal does not correlate as well as a threshold cycle value does for real-time PCR. The automated interpretation of the iiPCR machine does however make the method easier to use without the need for advanced training. The portable machine is small and light weight and can be operated with a car battery.
5. Conclusions
The three RT-PCR assays evaluated in the study were shown in general to have comparable performance for RVA detection in fecal samples. The real-time PCR assays are excellent tools for diagnostic laboratories, and the RT-iiPCR assay working in a portable PCR machine is highly sensitive and specific and shows promise for on-farm molecular detection of RVA.
Conflict of interest
M.A. Soltan and R. P. Wilkes declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. P.Y. Lee, Y.L. Tsai, C.F. Tsai, H.F. Chang, and H.T. Wang are affiliated with GeneReach USA. However, this work does not alter our adherence to all the Veterinary Journal’s policies on sharing data and materials.
Acknowledgement
We wish to thank all the veterinarians who submitted samples and made this work possible.
Footnotes
References
- Al Mawly J., Grinberg A., Prattley D., Moffat J., Marshall J., French N. Risk factors for neonatal calf diarrhoea and enteropathogen shedding in New Zealand dairy farms. Vet. J. 2015;203:155–160. doi: 10.1016/j.tvjl.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambagala A., Pahari S., Fisher M., Lee P.A., Pasick J., Ostlund E.N., Johnson D.J., Lung O. A rapid field-deployable reverse transcription-insulated isothermal polymerase chain reaction assay for sensitive and specific detection of bluetongue virus. Transbound. Emerg. Dis. 2015 doi: 10.1111/tbed.12388. [DOI] [PubMed] [Google Scholar]
- Athanassious R., Marsolais G., Assaf R., Dea S., Descoteaux J.P., Dulude S., Montpetit C. Detection of bovine coronavirus and type A rotavirus in neonatal calf diarrhea and winter dysentery of cattle in Quebec: evaluation of three diagnostic methods. Can. Vet. J. 1994;35:163–169. [PMC free article] [PubMed] [Google Scholar]
- Bailey K.E., Gilkerson J.R., Browning G.F. Equine rotaviruses-current understanding and continuing challenges. Vet. Microbiol. 2013;167:135–144. doi: 10.1016/j.vetmic.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasuriya U.B., Lee P.Y., Tiwari A., Skillman A., Nam B., Chambers T.M., Tsai Y.L., Ma L.J., Yang P.C., Chang H.F., Wang H.T. Rapid detection of equine influenza virus H3N8 subtype by insulated isothermal RT-PCR (iiRT-PCR) assay using the POCKIT™ nucleic acid analyzer. J. Virol. Methods. 2014;207:66–72. doi: 10.1016/j.jviromet.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Benfield D.A., Stotz I.J., Nelson E.A., Groon K.S. Comparison of a commercial enzyme-linked immunosorbent assay with electron microscopy fluorescent antibody, and virus isolation for the detection of bovine and porcine rotavirus. Am. J. Vet. Res. 1984;45:1998–2002. [PubMed] [Google Scholar]
- Chandler-Bostock R., Hancox L.R., Payne H., Iturriza-Gomara M., Daly J.M., Mellits K.H. Diversity of group A rotavirus on a UK pig farm. Vet. Microbiol. 2015;180:205–211. doi: 10.1016/j.vetmic.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.I., Han J.I., Wang C., Cooper V., Schwartz K., Engelken T., Yoon K.J. Case-control study of microbiological etiology associated with calf diarrhea. Vet. Microbiol. 2013;166:375–385. doi: 10.1016/j.vetmic.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desselberger U. Rotaviruses. Virus Res. 2014;190:75–96. doi: 10.1016/j.virusres.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Izzo M.M., Kirkland P.D., Mohler V.L., Perkins N.R., Gunn A.A., House J.K. Prevalence of major enteric pathogens in Australian dairy calves with diarrhoea. Aust. Vet. J. 2011;89:167–173. doi: 10.1111/j.1751-0813.2011.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo M.M., Kirkland P.D., Gu X., Lele Y., Gunn A.A., House J.K. Comparison of three diagnostic techniques for detection of rotavirus and coronavirus in calf faeces in Australia. Aust. Vet. J. 2012;90:122–129. doi: 10.1111/j.1751-0813.2011.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminjolo J.S., Adesiyun A.A. Rotavirus infection in calves piglets, lambs and goat kids in Trinidad. Br. Vet. J. 1994;150:293–299. doi: 10.1016/S0007-1935(05)80009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan M., Ugaz V.M., Burns M.A. PCR in a Rayleigh-Benard convection cell. Science (New York, N.Y) 2002;298:793. doi: 10.1126/science.298.5594.793. [DOI] [PubMed] [Google Scholar]
- Maes R.K., Grooms D.L., Wise A.G., Han C., Ciesicki V., Hanson L., Vickers M.L., Kanitz C., Holland R. Evaluation of a human group a rotavirus assay for on-site detection of bovine rotavirus. J. Clin. Microbiol. 2003;41:290–294. doi: 10.1128/JCM.41.1.290-294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Banyai K., Matthijnssens J., Buonavoglia C., Ciarlet M. Zoonotic aspects of rotaviruses. Vet. Microbiol. 2010;140:246–255. doi: 10.1016/j.vetmic.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J., Otto P.H., Ciarlet M., Desselberger U., Van Ranst M., Johne R. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 2012;157:1177–1182. doi: 10.1007/s00705-012-1273-3. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J., Ons E., De Coster S., Conceicao-Neto N., Gryspeerdt A., Van Ranst M., Raue R. Molecular characterization of equine rotaviruses isolated in Europe in 2013: implications for vaccination. Vet. Microbiol. 2015;176:179–185. doi: 10.1016/j.vetmic.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino S., Kern A., Barrandeguy M., Parreno V. Comparison of two commercial kits and an in-house ELISA for the detection of equine rotavirus in foal feces. J. Virol. Methods. 2015;222:1–10. doi: 10.1016/j.jviromet.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Phillips G., Lopman B., Tam C.C., Iturriza-Gomara M., Brown D., Gray J. Diagnosing rotavirus A associated IID: using ELISA to identify a cut-off for real time RT-PCR. J. Clin. Virol. 2009;44:242–245. doi: 10.1016/j.jcv.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Reynolds D.J., Chasey D., Scott A.C., Bridger J.C. Evaluation of ELISA and electron microscopy for the detection of coronavirus and rotavirus in bovine faeces. Vet. Rec. 1984;114:397–401. doi: 10.1136/vr.114.16.397. [DOI] [PubMed] [Google Scholar]
- Schlafer D.H., Scott F.W. Prevalence of neutralizing antibody to the calf rotavirus in New York cattle. Cornell Vet. 1979;69:262–271. [PubMed] [Google Scholar]
- Slovis N.M., Elam J., Estrada M., Leutenegger C.M. Infectious agents associated with diarrhoea in neonatal foals in central Kentucky: a comprehensive molecular study. Equine Vet. J. 2014;46:311–316. doi: 10.1111/evj.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viera A.J., Garrett J.M. Understanding interobserver agreement: the kappa statistic. Fam. Med. 2005;37:360–363. [PubMed] [Google Scholar]
- Wilkes R.P., Tsai Y.L., Lee P.Y., Lee F.C., Chang H.F., Wang H.T. Rapid and sensitive detection of canine distemper virus by one-tube reverse transcription-insulated isothermal polymerase chain reaction. BMC Vet. Res. 2014;10:213. doi: 10.1186/s12917-014-0213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Wang Y.H., Ghosh S., Tang W.F., Pang B.B., Liu M.Q., Peng J.S., Zhou D.J., Kobayashi N. Genomic characterization of G3P[6], G4P[6] and G4P[8] human rotaviruses from Wuhan, China: evidence for interspecies transmission and reassortment events. Infect. Genet. Evol. 2015;33:55–71. doi: 10.1016/j.meegid.2015.04.010. [DOI] [PubMed] [Google Scholar]