Highlights
-
•
Nitazoxanide is a broad-spectrum antiviral in Phase 3 trials for treating influenza.
-
•
In a Phase 2/3 trial, oral nitazoxanide reduced the duration of influenza symptoms.
-
•
Treatment with oral nitazoxanide also reduced shedding of influenza virus.
-
•
Data suggest a role in treating a broader spectrum of viral respiratory infections.
-
•
Other development opportunities include viral gastroenteritis and dengue fever.
Keywords: Nitazoxanide, Antiviral therapy, Broad-spectrum, Influenza, Thiazolides
Abstract
Originally developed and commercialized as an antiprotozoal agent, nitazoxanide was later identified as a first-in-class broad-spectrum antiviral drug and has been repurposed for the treatment of influenza. A Phase 2b/3 clinical trial recently published in The Lancet Infectious Diseases found that oral administration of nitazoxanide 600 mg twice daily for five days reduced the duration of clinical symptoms and reduced viral shedding compared to placebo in persons with laboratory-confirmed influenza. The same study also suggested a potential benefit for subjects with influenza-like illness who did not have influenza or other documented respiratory viral infection. From a chemical perspective, nitazoxanide is the scaffold for a new class of drugs called thiazolides. These small-molecule drugs target host-regulated processes involved in viral replication. Nitazoxanide is orally bioavailable and safe with extensive post-marketing experience involving more than 75 million adults and children. A new dosage formulation of nitazoxanide is presently undergoing global Phase 3 clinical development for the treatment of influenza. Nitazoxanide inhibits a broad range of influenza A and B viruses including influenza A(pH1N1) and the avian A(H7N9) as well as viruses that are resistant to neuraminidase inhibitors. It is synergistic with neuraminidase inhibitors, and combination therapy with oseltamivir is being studied in humans as part of ongoing Phase 3 clinical development. Nitazoxanide also inhibits the replication of a broad range of other RNA and DNA viruses including respiratory syncytial virus, parainfluenza, coronavirus, rotavirus, norovirus, hepatitis B, hepatitis C, dengue, yellow fever, Japanese encephalitis virus and human immunodeficiency virus in cell culture assays. Clinical trials have indicated a potential role for thiazolides in treating rotavirus and norovirus gastroenteritis and chronic hepatitis B and chronic hepatitis C. Ongoing and future clinical development is focused on viral respiratory infections, viral gastroenteritis and emerging infections such as dengue fever.
1. Introduction
In recent years, the desirability of new broad-spectrum antiviral drugs has been emphasized as a means of providing greater protection against a range of viruses while mitigating risks of resistance and reducing costs associated with developing drugs that are targeted to every specific virus (Shaw, 2011, Diarmond and Farzan, 2013, Debing et al., 2013, Zhou and Simmons, 2012). In view of the constant threat caused by new emerging strains of influenza and limitations of existing drugs, there is a particular need for drugs with new mechanisms of action that may be used alone or in combination with neuraminidase inhibitors to provide more optimal medical countermeasures against seasonal and pandemic influenza. In this context, the repurposing of nitazoxanide as a treatment of influenza could prove to be important.
Nitazoxanide or 2-(acetyloxy)-N-(5-nitro-2-thiazolyl) benzamide (Fig. 1 ) was first synthesized in the early 1970s on the scaffold of niclosamide in replacing one benzene ring, a 6-membered ring heterocycle, by a nitrothiazole, a 5-membered ring heterocycle (Rossignol and Cavier, 1975). Initially, nitazoxanide was developed as an oral anti-parasitic agent and studied for activity against protozoan and helminthic infections in vitro and/or in vivo in mice, cats, dogs and sheep (Cavier and Rossignol, 1982, Euzeby et al., 1980). It was first studied in humans for treatment of intestinal cestodes (Rossignol and Maisonneuve, 1984). Later, nitazoxanide was found effective in cell culture assays and in several animal models against the emerging intracellular protozoan, Cryptosporidium parvum (Blagburn et al., 1998, Theodos et al., 1998, Li et al., 2003, Baishanbo et al., 2006), and it was licensed in the United States (Alinia®, Romark Laboratories) as an Orphan Drug for treatment of diarrhea caused by C. parvum and Giardia intestinalis in adults and children at least 12 months of age (Ortiz et al., 2001, Amadi et al., 2002, Rossignol et al., 2001). It is the first and remains as the only FDA-approved treatment for Cryptosporidium infection. Nitazoxanide has also been widely commercialized in Latin America and in India where it is indicated for treating a broad spectrum of intestinal parasitic infections.
Fig. 1.
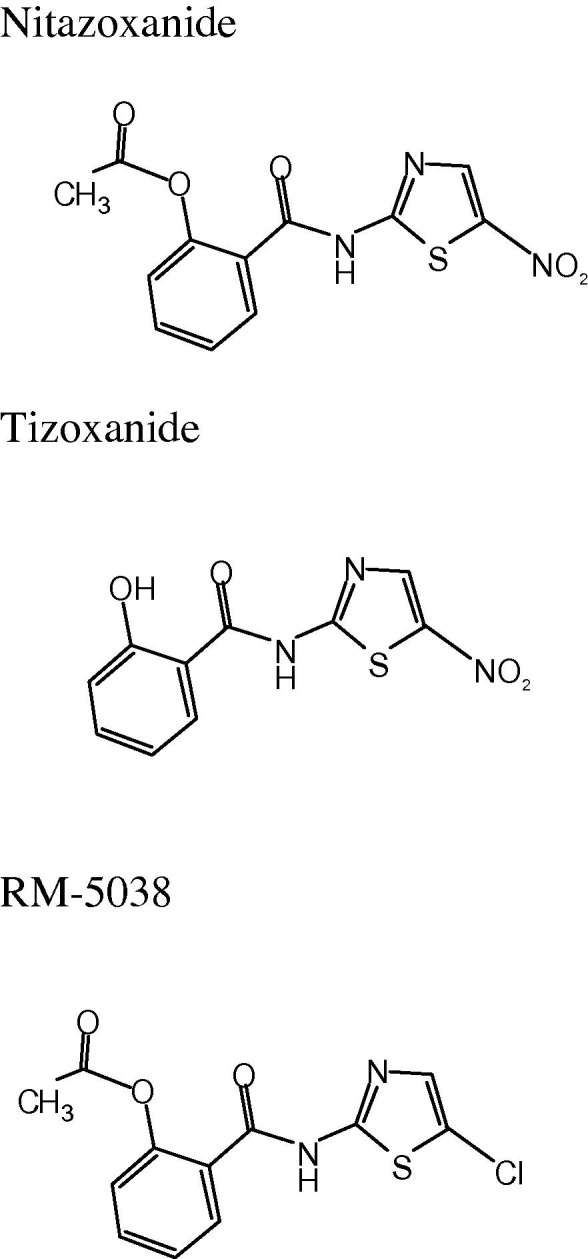
Chemical structures of nitazoxanide, tizoxanide and RM-5038.
In addition to its antiparasitic activity, nitazoxanide and its active circulating metabolite, tizoxanide (Fig. 1; Rossignol and Stachulski, 1999), are active in vitro against a broad range of obligate and facultative anaerobic gram positive and gram negative bacteria (Dubreuil et al., 1996, Mégraud et al., 1998, Pankuch and Appelbaum, 2006, Hecht et al., 2007, Finegold et al., 2009, Freeman et al., 2011) as well as replicating and non-replicating strains of Mycobacterium tuberculosis (De Carvalho et al., 2009). Animal studies and randomized clinical trials have confirmed the activity of the drug in treating Clostridium difficile disease (McVay and Rolfe, 2000, Musher et al., 2006, Musher et al., 2009). Hoffman et al. (2007) studied the mechanism of action of nitazoxanide against anaerobic bacteria and protozoa and showed that nitazoxanide inhibits pyruvate:ferredoxin oxidoreductase (PFOR) enzyme-dependent electron transfer reactions that are essential for anaerobic energy metabolism. Studies in M. tuberculosis have shown that nitazoxanide acts as an uncoupler disrupting membrane potential and intra-organism pH homeostasis (De Carvalho et al., 2011).
Having been licensed in the United States, nitazoxanide was subjected to extensive pharmacological testing for safety in animals and humans (Romark Laboratories, 2007). Recently, a clinical trial to evaluate cardiac safety in humans showed no effect on cardiac repolarization (Taubel et al., 2014). It is estimated that more than 75 million adults and children have been exposed to nitazoxanide in post-marketing experience for treating intestinal parasitic infections without any significant drug-related safety issues.
In clinical trials and post-marketing experience, nitazoxanide has been used primarily for treating intestinal infections. Treating systemic infections in animal models and in humans has been challenging because of the very poor solubility of the drug and its short elimination half-life. Following oral administration, nitazoxanide active pharmaceutical ingredient is absorbed from the intestinal tract and rapidly hydrolyzed by plasma esterases to form tizoxanide, its active circulating metabolite, which then is glucurono-conjugated in the liver. The drug is eliminated in urine and bile as tizoxanide and tizoxanide glucuronide. The half-life of tizoxanide in plasma is only approximately 1.3 h. More than 99.9% of circulating tizoxanide is bound to plasma proteins (Broekhuysen et al., 2000, Romark Laboratories, 2007).
In recent years, laboratory studies revealed broad-spectrum antiviral activity of nitazoxanide, rounding out a remarkably broad spectrum of activity for this drug and prompting efforts to develop nitazoxanide and other thiazolides as a new class of antiviral agents. A new controlled-release pharmaceutical formulation of nitazoxanide was developed to deliver drug systemically, and the drug is being repurposed as a new broad-spectrum agent with a novel mechanism of action for treatment of influenza. Here we review the antiviral activity of nitazoxanide and other thiazolides as reported in cell culture assays, animal studies and human clinical trials with an emphasis on influenza.
2. Influenza and other respiratory viruses
2.1. In vitro studies
Tizoxanide, the active circulating metabolite of nitazoxanide, inhibited replication of 16 strains of influenza A/H1N1, H3N2, H3N2v, H3N8, H5N9, H7N1, and one strain of influenza B (Table 1 ). The influenza viruses were sensitive to tizoxanide (IC50s ranging from 0.2 to 1.5 μg/mL, Table 1) using single step virus growth with high multiplicity of infection (5 PFU/cell) and multistep virus growth with low multiplicity of infection (0.001 PFU/cell). Antiviral activity was determined after 24 h in single step assays and after 24, 48 and 72 h for the multi-step assays by 50% tissue culture infectious dose (TCID50) and/or by hemagglutination assay. The IC50s for tizoxanide were similar when influenza A/H1N1/PR8 was cultured in different canine and human cell lines: MDCK cells, monocytic U937, T-lymphocytic Jurkat, and alveolar type II-like A549 (Rossignol et al., 2009a, Ashton et al., 2010, Belardo et al., 2011, Sleeman et al., 2014, Gubareva et al., 2014). Combinations of nitazoxanide with oseltamivir or zanamivir are synergistic when tested against influenza A/H1N1-PR8 and the avian A/H5N9. Combination index values ranged between 0.39 and 0.63, independent of multiplicity of infection used (Belardo et al., 2011). As observed for the hepatitis C virus (Korba et al., 2008b), tizoxanide had a high barrier to resistance to the influenza A virus. No decreased sensitivity was observed when viruses were passaged for 30 days in increasing concentrations (0.25, 0.50, 0.75 and 1.0 μg/mL) of tizoxanide (Belardo et al., 2011).
Table 1.
In vitro studies of tizoxanide against respiratory viruses.
| Strain | Cell line | EC50 (μg/mL) | SI1 | Reference |
|---|---|---|---|---|
| Orthomyxoviridae: influenza A and B | ||||
| H1N1 A/Puerto Rico/8/34 | MDCK | 0.3 | >50 | Rossignol et al., 2009a, Rossignol et al., 2009b |
| Monocytic U937 | 0.3 | >50 | Rossignol et al., 2009a, Rossignol et al., 2009b | |
| T-lymphocytic Jurkat | 0.3 | >50 | Rossignol et al., 2009a, Rossignol et al., 2009b | |
| Alveolar type II-like A549 | 0.3 | >50 | ||
| H1N1 A/Wisconsin/33 | MDCK | 0.5 | >100 | Rossignol et al., 2009a, Rossignol et al., 2009b |
| H1N1 A/Parma/24/09 (oseltamivir-R) | MDCK | 0.4 | >125 | Belardo et al. (2011) |
| H1N1 A/Goose/Italy/29624603 | MDCK | 1.5 | >33 | Belardo et al. (2011) |
| H1N1 A/California/04/p2009 | MDCK | <0.34 | Sleeman et al. (2014) | |
| H3N2v A/Ohio/88/2012 | MDCK | <0.27 | Sleeman et al. (2014) | |
| H3N2v A/Ohio/83/2012 | MDCK | <1.75 | Sleeman et al. (2014) | |
| H3N2 A/Washington/01/2007 | MDCK | <0.5 | Sleeman et al. (2014) | |
| H3N2 A/Texas/12/2007 | MDCK | <0.72 | Sleeman et al. (2014) | |
| H3N2 A/Firenze/7/03 | MDCK | 1.0 | >50 | Belardo et al. (2011) |
| H3N2 A/Parma/6/07 (amantadine-R) | MDCK | 0.3 | >166 | Belardo et al. (2011) |
| H3N8 A/Canine/Colorado-1/224986/06 | MDCK | 0.2 | 644 | Ashton et al. (2010) |
| H3N8 A/Canine/Colorado-3/3/06 | MDCK | 0.2 | 751 | Ashton et al. (2010) |
| H3N8 A/Canine/Colorado-4/2025974/07 | MDCK | 0.2 | 374 | Ashton et al. (2010) |
| H5N9 A/Chicken/Italy/9097/97 | MDCK | 0.5 | >100 | Rossignol et al., 2009a, Rossignol et al., 2009b |
| H7N1 A/Turkey/Italy/RA5563/99 | MDCK | 1.5 | >33 | Belardo et al. (2011) |
| Influenza B/Parma/3/04 | MDCK | 0.9 | >55 | Rossignol et al., 2009a, Rossignol et al., 2009b |
| Paramyxoviridae | ||||
| Parainfluenza Sendai virus (SeV) | 37RC | 0.5 | >100 | Rossignol and Santoro, (2014) |
| Respiratory syncytial virus (RSV) A-2 | HeLa ATCC | 0.3 | >166 | Rossignol and Santoro, (2014) |
| Coronaviridae | ||||
| Canine coronavirus S-378 (CCoV) | A72 | 1.0 | >50 | Rossignol and Santoro, (2014) |
| Picornavirdae | ||||
| Human rhinovirus type 2 | HeLa R19 | >50 | Rossignol and Santoro, (2014) | |
Selectivity index (IC50/50% cytotoxic concentration).
Tizoxanide also inhibited replication of parainfluenza Sendai virus (IC50 0.1 μg/mL), respiratory syncytial virus A2 (IC50 0.3 μg/mL), and canine coronavirus S-378 (IC50 1 μg/mL). The drug did not show activity in vitro against human rhinovirus type 2 (Table 1).
Peak and trough plasma concentrations of tizoxanide were 4.6 μg/mL and 0.8 μg/mL, respectively, during twice daily dosing of nitazoxanide controlled release tablets in a Phase 2b/3 clinical trial (Haffizulla et al., 2014). These concentrations are suitable for treating viral respiratory infections caused by influenza and other viruses as in vitro IC50s are typically between 0.1 and 1 μg/mL.
2.2. Mechanism of action
Studies of the mechanism of action of nitazoxanide against influenza viruses have shown that the drug blocks maturation of the viral hemagglutinin at the post-translational stage (Rossignol et al., 2009a). The drug had no effect on the other glycoprotein, neuraminidase, the target of oseltamivir and zanamivir, or the M2 protein, the target of amantadine, and it had no effect on viral infectivity, adsorption or entry into target cells (La Frazia et al., 2013, Rossignol et al., 2009a, Shi et al., 2014).
In peripheral blood mononuclear cells (PBMCs) stimulated by influenza virus, nitazoxanide potentiates the production of type 1 interferons (alpha and beta) produced by the host’s fibroblasts (Clerici et al., 2011). The significance of this activity is not fully understood, but it could contribute to the antiviral activity of nitazoxanide by interfering with maturation of the hemagglutinin glycoprotein or as another secondary mechanism of action.
2.3. Phase 2b/3 clinical trial
A Phase 2b/3 randomized, double-blind, placebo-controlled clinical trial was conducted in the United States to evaluate the safety and efficacy of nitazoxanide 300 mg controlled-release tablets in adults and adolescents at least 12 years of age with acute uncomplicated influenza. 624 subjects with fever, at least one respiratory symptom and one constitutional symptom of influenza were enrolled within 48 h of symptom onset and randomly assigned to receive treatment with placebo, 300 mg nitazoxanide or 600 mg nitazoxanide twice daily for five days. The primary efficacy analysis was time from first dose to alleviation of symptoms for subjects with laboratory-confirmed influenza at baseline.
Subjects receiving nitazoxanide experienced shorter times to alleviation of symptoms (600 mg: median 95.5 h, 95% CI 84.0 to 108.0, p = 0.008; 300 mg: median 109.1 h, 95% CI 96.1 to 129.5, p = 0.521) than subjects receiving the placebo (median: 116.7 h, 95% CI 108.1 to 122.1) (Fig. 2 ). The lack of statistical significance at the 0.05 level for the low dose group may have been due to lack of statistical power (Haffizulla et al., 2014).
Fig. 2.
Kaplan–Meier plot of time from first dose to alleviation of symptoms for subjects with confirmed influenza enrolled in a Phase 2b/3 clinical trial of nitazoxanide in subjects with uncomplicated influenza-like illness (Haffizulla et al., 2014, reprinted with permission from Elsevier).
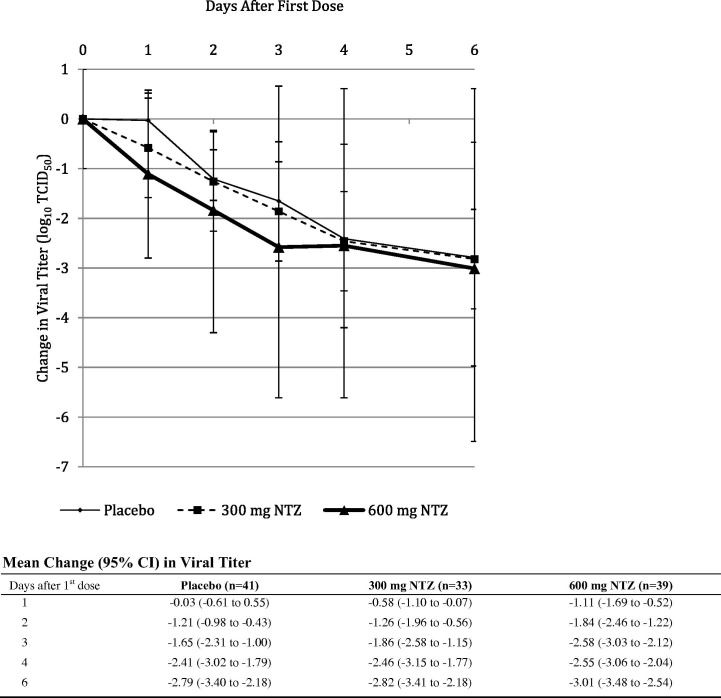
Influenza-infected subjects receiving nitazoxanide 600 mg also showed significant reductions (p = 0.0006) in TCID50 viral titers during treatment compared to subjects receiving placebo (Fig 3 ). No resistance was observed for influenza viruses collected from nitazoxanide-treated subjects, and no adverse effect on humoral immune response was observed. Adverse events were similar for the three treatment groups (Table 2 ) (Haffizulla et al., 2014).
Fig. 3.
Mean change in influenza TCID50 viral titer from baseline during Phase 2b/3 clinical trial in patients with uncomplicated influenza-like illness. Analysis of change in TCID50 viral titer for subjects with confirmed influenza that participated from whom daily nasopharyngeal swabs were collected. Statistical comparison using mixed model for repeated measures including baseline viral titer, treatment group and geographic location: p = 0.0006 for the difference between nitazoxanide 600 mg and placebo, p = 0.1553 for the difference between nitazoxanide 300 mg and placebo (Haffizulla et al., 2014, reprinted with permission from Elsevier).
Table 2.
Most common adverse events (>1% in any treatment group) reported in Phase 2b/3 clinical trial involving subjects with acute uncomplicated influenza-like illness (Haffizulla et al., 2014, reprinted with permission from Elsevier).
| Adverse event (no. and % of subjects reporting) | Placebo (n = 212) | Nitazoxanide 300 mg (n = 201) | Nitazoxanide 600 mg (n = 211) |
|---|---|---|---|
| Diarrhea | 7 (3%) | 4 (2%) | 17 (8%) |
| Headache | 24 (11%) | 12 (6%) | 17 (8%) |
| Bronchitis | 3 (1%) | 10 (5%) | 7 (3%) |
| Oropharyngeal pain | 7 (3%) | 5 (2%) | 10 (5%) |
| Abdominal pain | 7 (3%) | 4 (2%) | 8 (4%) |
| Vomiting | 2 (1%) | 3 (1%) | 8 (4%) |
| Chromaturia | – | 6 (3%) | 8 (4%) |
| Cough | 8 (4%) | 5 (2%) | 8 (4%) |
| Sinusitis | 8 (4%) | 6 (3%) | 3 (1%) |
| Nausea | 6 (3%) | 1 (<1%) | 6 (3%) |
| Pyrexia | 5 (2%) | 4 (2%) | 6 (3%) |
| Rhinorrhea | 7 (3%) | 5 (2%) | 4 (2%) |
| LFT abnormal | 4 (2%) | 5 (2%) | 5 (2%) |
| Wheezing | 3 (1%) | 2 (1%) | 5 (2%) |
| Nasal congestion | 5 (2%) | 3 (1%) | 5 (2%) |
| Insomnia | 4 (2%) | – | 5 (2%) |
| Chills | – | 4 (2%) | 1 (<1%) |
| Fatigue | 2 (1%) | 2 (1%) | – |
| Otitis media | – | 4 (2%) | 1 (<1%) |
| Dyspnea | 3 (1%) | 2 (1%) | 4 (2%) |
| Ear pain | 3 (1%) | 2 (1%) | 3 (1%) |
| Musculoskeletal stiffness | 6 (3%) | – | 3 (1%) |
| Constipation | – | 2 (1%) | – |
| Dry mouth | – | 2 (1%) | 1 (<1%) |
| Nasopharyngitis | – | 2 (1%) | 1 (<1%) |
| Blood triglycerides increase | – | 2 (1%) | – |
| Lipase increase | 2 (1%) | 2 (1%) | 1 (<1%) |
| Poor quality sleep | – | 2 (1%) | – |
| Respiratory tract congestion | – | 2 (1%) | – |
| Night sweats | 1 (<1%) | 2 (1%) | – |
Responses were similar for subjects infected with influenza A and influenza B. In contrast to neuraminidase inhibitors, treatment with nitazoxanide also significantly reduced the duration of flu-like symptoms (17.3 h reduction in time to alleviation of symptoms for 600 mg vs. placebo, p = 0.0208) in subjects with unknown viral etiology (Haffizulla et al., 2014).
This Phase 2b/3 clinical trial was designed according to current FDA guidance for the development of new drugs for treating influenza. From a regulatory perspective, placebo-controlled trials are required to clearly demonstrate drug-effect, and because of ethical considerations, placebo-controlled trials cannot be conducted in subjects with complicated illness or those at risk of complications. The trial design was generally consistent with previous clinical trials of oseltamivir and zanamivir, and while it is inherently difficult to compare results of this trial to clinical trials of neuraminidase inhibitors conducted fifteen to twenty years ago, reductions in symptom duration for the 600 mg dose group compared to the placebo (21.2 h) were in the range of those observed during similar trials of the neuraminidase inhibitors. Magnitude of treatment effect observed in different clinical trials could be influenced by study design (e.g., populations selected for analysis, use of acetaminophen or other symptom relief medications) and by characteristics of circulating influenza strains (e.g., pathogenicity, drug susceptibility) during a given trial. For example, this Phase 2b/3 trial enrolled subjects within 48 h of onset of symptoms while studies of the neuraminidase inhibitors typically enrolled subjects within 36 h of symptom onset. In subjects enrolled within 36 h of symptom onset, the median improvement in time to symptom alleviation for the nitazoxanide 600 mg treatment group was extended to 26.3 h.
2.4. Global Phase 3 clinical trial
A large global Phase 3 clinical trial is being conducted to support licensure of the nitazoxanide 300 mg controlled-release tablets for treatment of acute uncomplicated influenza (registered at ClinicalTrials.gov, number NCT01227421). Approximately 2000 subjects with influenza symptoms are being randomized to one of four treatment arms: placebo, nitazoxanide 600 mg, oseltamivir 75 mg, or nitazoxanide 600 mg plus oseltamivir 75 mg twice daily for five days. The primary efficacy endpoint is time from first dose to alleviation of symptoms, and co-primary efficacy analyses will compare time from first dose to alleviation of symptoms (i) for the nitazoxanide 600 mg group to that of the placebo group and (ii) for the nitazoxanide 600 mg plus oseltamivir 75 mg group to that of each of the other (monotherapy or placebo) groups. This study is being conducted under contract from the U.S. Department of Health and Human Services administered by the Biomedical Advanced Research and Development Authority (BARDA). This study is expected to provide important information related to the clinical benefit of nitazoxanide in treating uncomplicated influenza as well as the potential benefit of combining the drug with oseltamivir. To date, approximately 1600 subjects have been included in the trial over three flu seasons (one Northern Hemisphere and two Southern Hemisphere), and it is expected to be completed during the 2014/2015 influenza season in the Northern Hemisphere. Notably, the Phase 3 trial will be the largest clinical trial ever conducted for treatment of acute uncomplicated influenza, the first licensure-enabling clinical trial of a combination of drugs for treating influenza, and the first new registration-style clinical trial of oseltamivir that has been conducted in more than ten years.
Future clinical trials of nitazoxanide are expected to include patients at risk of influenza complications including those with co-morbidities such as asthma, chronic obstructive pulmonary disease (COPD) or diabetes, patients over 65 years of age and children under 12 years of age. A clinical trial in hospitalized patients with severe acute respiratory infection (SARI) has recently been initiated under the sponsorship the National Institute of Allergy and Infectious Diseases, National Institutes of Health (registered at ClinicalTrials.gov, number NCT02057757).
3. Other viral infections
3.1. Viral gastroenteritis caused by rotavirus and norovirus
3.1.1. In vitro studies
The activity of tizoxanide has been studied using eight strains of rotavirus cultured in three different cell lines (Table 3 ). Most of the work in molecular virology was carried out with two strains of rotavirus, the simian strain SA11-G3P(2) and the human strain Wa-G1P(8). Antiviral activity was observed using single-step virus growth at high m.o.i. (10 PFU/cell) and multi-step virus growth at a low m.o.i. (0.001 PFU/cell). In a single step virus growth assay using monkey kidney MA104 cells, tizoxanide inhibited replication of the simian SA11-G3P(2) and the human Wa-G1P(8) with IC50s of 0.5 and 1 μg/mL, respectively, while in a multistep assay, IC50s of 0.3 μg/mL were recorded for both viruses. 50% cytotoxic concentrations (CC50) in these cell lines were >50 μg/mL. Similar results were obtained using human gut-derived Caco-2 and HT-29 cells and with human G1, G2, G3, G4, G8 and G9 strains of the virus. Treatment of MA104 cells with 10 μg/mL of tizoxanide 3, 6 or 9 h before infection with rotavirus did not protect the cells from viral entry. Tizoxanide inhibited viral replication when cells were treated up to 6 h post-infection with optimal activity observed when tizoxanide was introduced between 0 and 3 h post-infection. (La Frazia et al., 2013, Rossignol et al., 2006).
Table 3.
In vitro studies of tizoxanide against a broad spectrum of non-respiratory viruses.
| Strain | Cell line | IC50 (μg/mL) | SI1 | Reference |
|---|---|---|---|---|
| Flavividae | ||||
| Hepatitis C virus (HCV) Genotype 1a | AVA52 | 0.09 | 56 | Korba et al. (2008a)3 |
| Hepatitis C virus (HCV) Genotype 2a | Huh7.52 | 0.06 | 100 | Korba et al. (2008a)3 |
| Dengue fever virus-2 (New Guinea strain) | Vero cells | 0.1 | 10 | Meneses et al. (2013) |
| Japanese encephalitis virus (JEV) | BHK-21 | 0.12 | 155 | Shi et al. (2014)4 |
| Yellow fever virus 17DD | Vero Cells | 0.06 | 35 | Meneses et al. (2013) |
| Hepadnaviridae | ||||
| Hepatitis B virus (HBV) | 2.2.15 | 0.06 | >172 | Korba et al. (2008a)3 |
| Retroviridae | ||||
| Human immunodeficiency virus (HIV) | PBMC | 0.5 | >100 | Tan et al., 20125 |
| Reoviridae | ||||
| Simian rotavirus A/SA11G3P{2} | MA104 | 0.3 | >100 | La Frazia et al. (2013)6 |
| Human rotavirus G1P[8] | MA104 | 1.0 | >50 | La Frazia et al. (2013)6 |
| Caliciviridae | ||||
| Norovirus | HG23 | 0.5 | 10 | Korba, personal communication |
Selectivity index (IC50/50% cytotoxic concentration).
HCV replicon-containing cell lines.
Supported by NIAID contract NO1-AI-30046 to Georgetown University Medical Center.
Supported by the Project of International Science and Technology Cooperation (No. 2010DFB33920), the Zhejiang Provincial Natural Science Foundation (No. Y110124) and the National Natural Science Foundation of China (No. 81371814).
Supported by the Damon Runyon Cancer Research Foundation (DRG 2008-09); the Charles A. King Trust, N.A., Bank of America, co-trustee; the National Natural Science Foundation of China (31100601); the National Key Basic Research Program (2012CB316503); and the Howard Hughes Medical Institute.
Supported by Romark Laboratories, L.C.
The IC50 and IC90 for tizoxanide in a norovirus G1 replicon-assay system using an HG-23 cell line were 0.5 and 1.2 μg/mL respectively (Korba, personal communication).
3.1.2. Mechanism of action
Studies have shown that tizoxanide inhibits the maturation of rotavirus viral protein 7 (VP7), a glycoprotein that forming the outer part of the virion and one of the six structural glycoproteins involved in rotavirus replication, alters viroplasm formation and interferes with viral morphogenesis (La Frazia et al., 2013).
3.1.3. Clinical trials
Two Phase 2 randomized, double-blind, placebo-controlled studies were conducted in two hundred (200) subjects with viral gastroenteritis caused by rotavirus or norovirus. Fifty adults and adolescents at least 12 years of age and 150 hospitalized children from 2 months to 11 years of age, 101 of them being from 2 to 11 months of age, were included in the two studies. Adults and adolescents at least 12 years of age received one 500 mg nitazoxanide tablet or one matching placebo tablet, twice daily for three consecutive days. Children under 12 years of age received a 20 mg/mL suspension or matching placebo administered orally at doses of 200 mg/10 mL (4–11 years of age), 100 mg/5 mL (1–3 years) and 50 mg/2.5 mL (<12 months) twice daily for three days. Nitazoxanide significantly reduced the duration of symptoms compared to placebo in adults and adolescents infected with rotavirus (p = 0.0052) as well as subjects infected with norovirus (p = 0.0295) (Rossignol and El-Gohary, 2006). Likewise, in children with rotavirus gastroenteritis, treatment with nitazoxanide significantly reduced the duration of symptoms compared to the placebo (p = 0.01) (Rossignol and El-Gohary, 2006). Another study conducted in 75 children aged from 28 days to 24 months hospitalized with rotavirus gastroenteritis also reported that nitazoxanide significantly reduced the duration of illness (Teran et al., 2009). Successful treatment of norovirus gastroenteritis with nitazoxanide was also reported for a 43-year-old patient with leukemia who had received chemotherapy and hematopoietic stem cell transplantation (HSCT) (Siddiq et al., 2011).
A large randomized, double-blind, placebo-controlled clinical trial supported by the Australian National Health Medical Research Council (NHMRC) is being conducted in 400 aboriginal children 3 months to 4 years of age hospitalized with diarrhea in the Northern Territory of Australia where severe diarrhea is caused by Cryptosporidium parvum, rotavirus and norovirus (registered at ClinicalTrials.gov, number NCT02165813). Kaufman et al. (2014) have recently identified nitazoxanide as a prospect for immediate clinical trials in immunocompromised patients infected with norovirus.
3.2. Hepatitis B virus
3.2.1. In vitro studies
Tizoxanide exhibited selective inhibition of intracellular HBV and extracellular virus production in 2.2.15 cells (Table 3). IC50s of 0.06, 0.02 and 0.38 μg/mL were recorded for tizoxanide, lamivudine and adefovir dipovoxil respectively with selectivity indices >100 (Korba et al., 2008a).
3.2.2. Clinical trials
In an open-label clinical trial, nitazoxanide was administered as one 500 mg tablet twice daily for 12 consecutive months in twelve adult subjects with chronic HBV; four were HBeAg-positive and eight were HBeAg-negative. Three of the four HBeAg positive patients became HBeAg-negative in an average of three months. Serum HBV DNA decreased in all HBeAg-positive patients and became negative in two of the four patients; more importantly, one patient became HBsAg negative after having cleared serum HBV DNA. Of the eight HBeAg-negative patients, seven became HBV DNA negative and two of these patients also became HBsAg-negative. These results suggest that further studies are warranted to determine the potential of nitazoxanide to cure HBV-infected patients (Rossignol and Keeffe, 2008).
3.3. Hepatitis C virus (HCV)
3.3.1. In vitro studies
Tizoxanide was first tested for activity against HCV genotype 1a in the HCV replicon-containing cell line, AVA5, and genotype 1b in the HCV replicon-containing cell line, Huh7.5 (Table 3). For genotype 1a, the IC50 and IC90 were 0.09 and 0.38 μg/mL, respectively, with a selectivity index (CC50/IC50) of 56. For genotype 1b, the IC50 and IC90 were 0.06 and 0.31 μg/mL, respectively, with a selectivity index (CC50/IC50) of 100. Combinations of tizoxanide with interferon α or with 2’C methylcytidine were synergistic (Korba et al., 2008a). The potential for resistance of HCV to tizoxanide was studied using an HCV genotype 1b replicon-containing cell line, which was subjected to increasing concentrations of tizoxanide for 24 consecutive weeks. HCV did not exhibit reduced susceptibility to tizoxanide, and viral genome sequencing did not show mutations characteristic of resistance. These studies suggested a host-directed mechanism of action and a high barrier to viral resistance (Korba et al., 2008b, Yon et al., 2011).
3.3.2. Mechanism of action
Mechanistic studies of the effect of tizoxanide on replication of hepatitis C virus in cell cultures have shown that it activates protein kinase R (PKR), an important part of innate immune response, in cells exposed to double-stranded RNA, which in turn results in phosphorylation of eukaryotic initiation factor 2α (eIF2-α), a gene known to block viral replication (Rossignol et al., 2008, Elazar et al., 2009).
More recent studies using bovine viral diarrhea virus (BVDV) as a surrogate for HCV infection, have shown that nitazoxanide inhibits replication of cytopathic and non-cytopathic BVDV by a mechanism that is likely to involve phosphorylation of PKR and eIF2-α. The authors also observed that nitazoxanide depletes ATP-sensitive intracellular Ca2+ stores resulting in mild endoplasmic reticulum (ER) stress, which disrupts N-linked glycosylation of BVDV structural proteins (Ashiru et al., 2014).
3.3.3. Clinical trials
Two hundred and eighty one (281) subjects infected with HCV genotype 4, three with genotype 1 and one with genotype 2, a total of 285 subjects were treated as part of a series of three clinical trials conducted in Egypt. The first was a randomized, double-blind, placebo-controlled clinical trial conducted in 47 treatment-naïve adults with chronic hepatitis C genotype 4. In this study, 4 of 23 subjects (17%) receiving one nitazoxanide tablet (500 mg) twice daily for 24 weeks experienced a sustained virologic response (SVR) with undetectable HCV RNA in serum six months after the end of treatment (median log10 reduction = 5.0) compared to none of the 24 subjects (0%) in the placebo treatment group (p = 0.049) (Rossignol et al., 2008). In a second study conducted in 120 adults with chronic hepatitis C genotype 4, administration of one 500 mg nitazoxanide tablet twice a day for 48 weeks with one 180 μg injection of peginterferon alfa-2a once weekly from week 13 to 48 with or without ribavirin increased SVR rates to 79% and 72% with and without ribavirin, respectively, compared to 50% obtained with one weekly 180 μg injection of peginterferon alfa-2a and ribavirin, the standard of care at that time (Rossignol et al., 2009b). In an open-label study in 44 adults with chronic hepatitis C (40 genotype 4, three genotype 1, one genotype 2), 80% of the subjects achieved SVR after treatment with nitazoxanide monotherapy for 4 weeks followed by nitazoxanide plus peginterferon alfa-2a without ribavirin for 36 weeks. All four subjects with genotype 1 and 2 experienced SVR (Rossignol et al., 2010). Importantly, nitazoxanide was well tolerated by subjects with chronic hepatitis C enrolled in each of these three Phase 2 studies with no significant additional side-effects or changes in laboratory safety values other than those caused by the combination of peginterferon alfa-2a and ribavirin carried out monthly during treatment.
While these clinical trials indicated improved responses when nitazoxanide was administered in combination with peginterferon for treatment of chronic hepatitis C, development was discontinued due to the development of new direct acting antiviral drugs. Combinations of nitazoxanide or other thiazolides with direct-acting antiviral drugs for treating chronic hepatitis C and/or HIV may be a path for future development.
3.4. Other Flaviviridae: dengue-2, yellow fever and Japanese encephalitis viruses
Tizoxanide has been shown to inhibit replication of three other Flaviviridae, dengue virus type 2, Japanese encephalitis virus (JEV) and yellow fever virus (YFV), in cell culture assays. Tizoxanide inhibited replication of dengue-2 and YFV in Vero cells with IC50s of 0.1 and 0.06 μg/mL, respectively and selectivity indices of 10 and 35 respectively, as shown in Table 3 (Meneses et al., 2013). Shi et al. (2014) reported that nitazoxanide inhibits replication of JEV in BHK-21 cells with an IC50 of 0.12 μg/mL and a selectivity index of 155. Nitazoxanide did not directly affect viral infectivity, adsorption or entry into target cells and was most effective when the cells were treated 2 h post-infection with a decrease in the antiviral activity when cells were treated at 5 and 10 h post-infection. These findings were consistent with studies using influenza virus and rotavirus (Rossignol et al., 2009a, La Frazia et al., 2013).
In mice challenged with a lethal dose of JEV, 50, 75 and 100 mg/kg of nitazoxanide were administered by oral gavage daily for 25 consecutive days. Mice survival was observed in 30%, 70% and 90% for the 50, 75 and 100 mg/kg/day, respectively, while untreated mice had all died by study day 9.
3.5. Human immunodeficiency virus (HIV)
A publication by Tan et al. (2012) reported that nitazoxanide and tizoxanide inhibited HIV replication with IC50s of approximately 0.5 μg/mL using a p24 enzyme-linked immunosorbent assay (ELISA) in PBMCs. The authors described a novel drug screening method used to identify strongly efficacious and synergistic drug pairs with the overall goal of expanding current antiretroviral repertoires. The authors reported that nitazoxanide synergizes with known HIV drugs such as integrase inhibitors, and nucleoside and non-nucleoside reverse transcription inhibitors, exerting its antiviral effect post-HIV-1 entry, but before or at reverse transcription.
4. New thiazolides
A large number of thiazolides closely related to the chemical structure of nitazoxanide have been synthesized and tested for activity against parasites, bacteria and viruses (Stachulski et al., 2011a, Stachulski et al., 2011b). Two compounds, nitazoxanide and RM-5038, have been selected for complete development. RM-5038 (Fig 1) is a new thiazolide derivative in which the nitro group on the 5-position of the thiazole ring of its chemical structure was replaced by chloride. RM-5038 is not active against anaerobic protozoa and bacteria, but it is active in cell culture and in vivo against Cryptosporidium parvum and has shown a broad spectrum of activity against HBV and HCV similar to nitazoxanide (Gargala et al., 2010, Gargala et al., 2013, Korba et al., 2008a). It is better absorbed from the gastro-intestinal tract than nitazoxanide and better tolerated in animal toxicology studies when administered in rats and dogs for 28 consecutive days.
5. Future directions
The safety of nitazoxanide and its broad-spectrum antiviral activity suggest a large number of alternative indications that could be explored for clinical development.
Over the next five years, clinical development of nitazoxanide is likely to focus primarily on viral respiratory infections. Clinical trials in special populations with influenza or influenza-like illnesses will be required to evaluate the benefit of the drug in hospitalized patients, pediatric patients and persons at risk of influenza complications. In view of the large amount of safety data for nitazoxanide in children, studies in pediatric patients under 12 years of age are likely to proceed quickly following completion of the ongoing Phase 3 trial. Studies in young children with influenza-like illness are likely to enroll large numbers with RSV infection. Specific studies in children with RSV infection may also be warranted.
There is presently no approved antiviral treatment for influenza in hospitalized patients or persons at risk of complications of flu. Furthermore, randomized controlled trials in these patient populations have proven to be difficult to design and conduct due to a number of factors including inability to use a placebo, underlying illnesses, drug efficacy, etc. If the synergistic activity of nitazoxanide and oseltamivir in cell cultures is confirmed by the ongoing Phase 3 clinical trial in subjects with acute uncomplicated influenza, that data may suggest a path for designing and conducting clinical trials of the combination compared to nitazoxanide monotherapy and oseltamivir monotherapy for treating patients hospitalized with influenza.
As new viral respiratory infections such as the MERS coronavirus emerge, development of animal models and perhaps clinical trials to study the activity of nitazoxanide are envisioned. Preclinical studies to evaluate efficacy of the drug against the MERS coronavirus are ongoing.
Given its history of use in treating a broad range of intestinal infections and data from Phase 2 clinical trials in patients with rotavirus and norovirus gastroenteritis, the rationale for additional studies of nitazoxanide against a broader range of infectious diarrheal illnesses including rotavirus and norovirus is strong. A study conducted in pediatric patients in Peru has shown that empiric treatment of diarrheal illnesses in children from that region significantly reduced the duration of illness compared to placebo (Rossignol et al., 2012). The ongoing study in aboriginal children supported by the Australian NHMRC is expected to provide additional data related to its use in treating diarrheal illnesses including viral gastroenteritis. Other similar studies may be expected in view of the growing recognition of the importance of Cryptosporidium as well as rotavirus and norovirus as causes of diarrhea in children. Studies of nitazoxanide for treatment of norovirus in immunocompromised patients have been suggested (Kaufman et al., 2014) and are likely in view of the need for a treatment in this population.
Aside from the advanced development of nitazoxanide for treating viral respiratory infections and viral gastroenteritis, the emergence of dengue fever and perhaps other viral diseases may provide paths for clinical development of nitazoxanide and other thiazolides.
Conflict of interest
Jean-François Rossignol is the inventor and has led the development of nitazoxanide and other thiazolides. He is an employee of and owns an equity interest in Romark Laboratories, L.C.
Acknowledgements
The global Phase 3 clinical trial of nitazoxanide 300 mg controlled-release tablets for treatment of influenza is funded under contract HHSO100201300004C with the U.S. Department of Health and Human Services administered through the Biomedical Advanced Research and Development Authority (BARDA).
References
- Amadi B., Mwiya M., Musuku J., Watuka A., Sianongo S., Ayoub A., Kelly P. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomized controlled trial. Lancet. 2002;360:1375–1380. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- Ashiru O., Howe J.D., Butters T.D. Nitazoxanide, an antiviral thiazolide, depletes ATP-sensitive intracellular Ca2+ stores. Virology. 2014;462–463:135–148. doi: 10.1016/j.virol.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Ashton L.V., Callan R.L., Rao S., Landoldt G.A. In vitro susceptibility of canine influenza A (H3N8) virus to nitazoxanide and tizoxanide. Vet. Med. Int. 2010;2010 doi: 10.4061/2010/891010. (article ID 891010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baishanbo A., Gargala G., Duclos C., François A., Rossignol J.F., Ballet J.J., Favennec L. Efficacy of nitazoxanide and paromomycin on biliary tract cryptosporidiosis in an immunosuppressed gerbil model. J. Antimicrob. Chemother. 2006;57:353–355. doi: 10.1093/jac/dki456. [DOI] [PubMed] [Google Scholar]
- Belardo, G., La Frazia, S., Cenciarelli, O., Carta, S., Rossignol, J.F., Santoro, M.G., 2011. Nitazoxanide, a novel potential anti-influenza drug, acting in synergism with neuraminidase inhibitors. Poster presented at: 49th Infectious Disease Society of America Annual Meeting, Oct 20–23, 2011, Boston, Massachusetts. https://idsa.confex.com/idsa/2011/webprogram/Paper31075.html.
- Blagburn B.L., Drain K.L., Land T.M., Kinard R.G., Moore P.H., Lindsay D.S., Patrick D.A., Boykin D.W., Tidwell R.R. Comparative efficacy evaluation of dicationic carbazole compounds, nitazoxanide and paromomycin against Cryptosporidium parvum infections in a neonatal mouse model. Antimicrob. Agents Chemother. 1998;42:2877–2882. doi: 10.1128/aac.42.11.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekhuysen J., Stockis A., Lins R.L., De Graeve J., Rossignol J.F. Nitazoxanide: pharmacokinetics and metabolism in man. Int. J. Clin. Pharmacol. Ther. 2000;38:387–394. doi: 10.5414/cpp38387. [DOI] [PubMed] [Google Scholar]
- Cavier R., Rossignol J.F. Etude de diverses associations d’anthelminthiques chez la souris. Rev. Méd. Vét. 1982;133:779–783. [Google Scholar]
- Clerici M., Trabattoni D., Pacei M., Biasin M., Rossignol J.F. The anti-infective nitazoxanide shows strong immuno-modulating effects [abstract] J. Immunol. 2011;186(155):21. [Google Scholar]
- De Carvalho L.P., Lin G., Jiang X., Nathan C. Nitazoxanide kills replicating and non-replicating Mycobacterium tuberculosis and evades resistance. J. Med. Chem. 2009;52:5789–5792. doi: 10.1021/jm9010719. [DOI] [PubMed] [Google Scholar]
- De Carvalho L.P., Darby C.M., Rhee K.Y., Nathan C. Nitazoxanide disrupts membrane potential and intra-bacterial pH homeostasis of Mycobacterium tuberculosis. ACS Med. Chem. Lett. 2011;2:849–854. doi: 10.1021/ml200157f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debing Y., Jochmans D., Neyts J. Intervention strategies for emerging viruses: use of antivirals. Curr. Opin. Virol. 2013;3:217–224. doi: 10.1016/j.coviro.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarmond M.S., Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol. 2013;13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil L., Houcke I., Mouton Y., Rossignol J.F. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob. Agents Chemother. 1996;40:2266–2270. doi: 10.1128/aac.40.10.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazar M., Liu M., McKenna S.A., Liu P., Gehrig E.A., Puglisi J.D., Rossignol J.F., Glenn J.S. The anti-hepatic C agent nitazoxanide induces phosphorylation of eIF2-alpha via PKR activation. Gastroenterology. 2009;137:1827–1835. doi: 10.1053/j.gastro.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Euzeby J., Prom T.S., Rossignol J.F. Experimentation des propriétés anthelminthiques de la nitazoxanide chez le chien, le chat et les ovins. Rev. Méd. Vét. 1980;131:687–696. [Google Scholar]
- Finegold S.M., Molitoris D., Vaisanen M.L. Study of the in vitro activities of rifaximin and comparator agents against 536 anaerobic intestinal bacteria from the perspective of potential utility in pathology involving bowel flora. Antimicrob. Agents Chemother. 2009;53:281–286. doi: 10.1128/AAC.00441-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J., Baines S.D., Todhunter S.L., Huscroft G.S., Wilcox M.H. Nitazoxanide is active against Clostridium difficile strains with reduced susceptibility to metronidazole. J. Antimicrob. Chemother. 2011;66:1407–1408. doi: 10.1093/jac/dkr077. [DOI] [PubMed] [Google Scholar]
- Gargala G., Le Goff L., Ballet J.J., Favennec L., Stachulski A.V., Rossignol J.F. Evaluation of new thiazolide/thiadiazolide derivatives reveals nitro-group-independent efficacy against Cryptosporidium parvum. Antimicrob. Agents Chemother. 2010;54:1315–1318. doi: 10.1128/AAC.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargala G., François A., Favennec L., Rossignol J.F. Activity of halogeno-thiazolides against Cryptosporidium parvum in experimentally infected immunosuppressed gerbils (Meriones unguiculatus) Antimicrob. Agents Chemother. 2013;57:2821–2823. doi: 10.1128/AAC.01538-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva, L.V., Marjuki, H., Mishin, V.P., Sleeman, K., Tamura, D., Chesnokov, A., De La Cruz, J., Villaneuva, J., Davis, T., 2014. Susceptibility of avian influenza A(H7N9) viruses to FDA approved and investigational antiviral drugs. Programme & Abstract Book, Influenza and Other Respiratory Virus Infections: Advances in Clinical Management. Third ISIRV-Antiviral Group Conference. Abstract P57, 83.
- Haffizulla J., Hartman A., Hoppers M., Resnick H., Samudrala S., Ginocchio C., Bardin M., Rossignol J.F. A randomized, double-blind, placebo controlled clinical trial of nitazoxanide in adults and adolescents with acute uncomplicated influenza. Lancet Infect. Dis. 2014;14:609–618. doi: 10.1016/S1473-3099(14)70717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht D.W., Galang M.A., Sambol S.P., Osmolski J.R., Johnson S., Gerding D.N. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob. Agents Chemother. 2007;51:2716–2719. doi: 10.1128/AAC.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P.S., Sisson G., Croxen M.A., Welch K., Harman W.D., Cremades N., Morash M.G. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob. Agents Chemother. 2007;51:868–876. doi: 10.1128/AAC.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S.S., Green K.Y., Korba B.E. Treatment of norovirus infections: moving antivirals from the bench to the bedside. Antiviral Res. 2014;105:80–91. doi: 10.1016/j.antiviral.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B.E., Mueller A.B., Farrar K., Gaye K., Mukerjee S., Ayers M.S., Rossignol J.F. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res. 2008;77:56–63. doi: 10.1016/j.antiviral.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Korba B.E., Elazar M., Lui P., Rossignol J.F., Glenn J.S. Studies of the potential for nitazoxanide or tizoxanide resistance in hepatitis C virus replicon-containing cell lines. Antimicrob. Agents Chemother. 2008;52:4069–4071. doi: 10.1128/AAC.00078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Frazia S., Ciucci A., Arnoldi F., Coira M., Gianferreti P., Angelini M., Belardo G., Burrone O., Rossignol J.F., Santoro G.M. Thiazolides, a new class of antiviral agents effective against rotavirus infection, target viral morphogenesis inhibiting viroplasm formation. J. Virol. 2013;83:11096–11101. doi: 10.1128/JVI.01213-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Brasseur P., Agnamey P., Lemeteil D., Favennec L., Ballet J.J., Rossignol J.F. Long-lasting anticryptosporidial activity of nitazoxanide in an immunosuppressed rat model. Folia Parasitol. (Praha) 2003;50:19–22. doi: 10.14411/fp.2003.003. [DOI] [PubMed] [Google Scholar]
- McVay C.S., Rolfe R.D. In vitro and in vivo activities of nitazoxanide against Clostridium difficile. Antimicrob. Agents Chemother. 2000;44:2254–2258. doi: 10.1128/aac.44.9.2254-2258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégraud F., Occhialini A., Rossignol J.F. Nitazoxanide, a potential drug to eradicate Helicobacter pylori with no cross-resistance to metronidazole. Antimicrob. Agents Chemother. 1998;42:2836–2840. doi: 10.1128/aac.42.11.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses, M.D.S., Duarte, R.S., Migowski, E.R., Ferreira, D.F., 2013. In vitro study on the effects of nitazoxanide on the replication of dengue virus and yellow fever virus. Poster Presented at the 28th International Conference on Antiviral Research (ICAR). Abstract #157, 101.
- Musher D.M., Logan N., Hamill R.J., DuPont H.L., Lentnek A., Gupta A., Rossignol J.F. Nitazoxanide in the treatment of Clostridium difficile colitis. Clin. Infect. Dis. 2006;43:421–427. doi: 10.1086/506351. [DOI] [PubMed] [Google Scholar]
- Musher D.M., Logan N., Bressler A.M., Johnson D.P., Rossignol J.F. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin. Infect. Dis. 2009;48:41–46. doi: 10.1086/596552. [DOI] [PubMed] [Google Scholar]
- Ortiz J.J., Ayoub A., Gargala G., Chegne N.L., Favennec L. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from northern Peru. Aliment. Pharmacol. Ther. 2001;15:1409–1415. doi: 10.1046/j.1365-2036.2001.01066.x. [DOI] [PubMed] [Google Scholar]
- Pankuch G.A., Appelbaum P.C. Activities of tizoxanide and nitazoxanide compared to those of five other thiazolides and three other agents against anaerobic species. Antimicrob. Agents Chemother. 2006;50:112–117. doi: 10.1128/AAC.50.3.1112-1117.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romark Laboratories, L.C. Alinia® (nitazoxanide) prescribing information. 2007.
- Rossignol J.F., Cavier R. Synthesis and antiparasitic activity of 2-benzamido nitrothiazoles. Chem. Abstr. 1975;83:28216n. [Google Scholar]
- Rossignol J.F., El-Gohary Y. Nitazoxanide in treatment of viral gastroenteritis: a randomized, double-blind, placebo-controlled clinical trial. Aliment. Pharmacol. Ther. 2006;24:1423–1430. doi: 10.1111/j.1365-2036.2006.03128.x. [DOI] [PubMed] [Google Scholar]
- Rossignol J.F., Keeffe E.B. Thiazolides: a new class of drugs for the treatment of chronic hepatitis B and C. Future Microbiol. 2008;3:539–545. doi: 10.2217/17460913.3.5.539. [DOI] [PubMed] [Google Scholar]
- Rossignol J.F., Maisonneuve H. Nitazoxanide in the treatment of Taenia saginata and Hymenolepis nana. Am. J. Trop. Med. Hyg. 1984;33:511–512. doi: 10.4269/ajtmh.1984.33.511. [DOI] [PubMed] [Google Scholar]
- Rossignol, J.F., Santoro M.G., 2014. Activity of thiazolides against other respiratory viruses than influenza. Programme & Abstract Book, Influenza and Other Respiratory Virus Infections: Advances in Clinical Management. Third ISIRV-Antiviral Group Conference. Abstract P54, 81.
- Rossignol J.F., Stachulski A. Synthesis and antibacterial activities of tizoxanide and its o-aryl glucuronide. J. Chem. Res. 1999:44–45. [Google Scholar]
- Rossignol J.F., Ayoub A., Ayers M.S. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized double-blind placebo-controlled study of nitazoxanide. J. Infect. Dis. 2001;184:103–106. doi: 10.1086/321008. [DOI] [PubMed] [Google Scholar]
- Rossignol J.F., Abu-Zekry M., Abeer H., Santoro M.G. Effect of nitazoxanide in treating severe rotavirus diarrhea: a randomized, double-blind, placebo-controlled trial. Lancet. 2006;368:124–129. doi: 10.1016/S0140-6736(06)68852-1. [DOI] [PubMed] [Google Scholar]
- Rossignol J.F., Kabil S.M., El-Gohary Y., Elfert A., Keeffe E.B. Clinical trial: randomized, double-blind, placebo-controlled study of nitazoxanide monotherapy for the treatment of patients with chronic hepatitis C genotype-4. Aliment. Pharmacol. Ther. 2008;28:574–580. doi: 10.1111/j.1365-2036.2008.03781.x. [DOI] [PubMed] [Google Scholar]
- Rossignol J.F., La Frazia S., Chiappa L., Ciucci A., Santoro M.G. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at post-translational level. J. Biol. Chem. 2009;284:29798–29808. doi: 10.1074/jbc.M109.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J.F., Elfert A., El-Gohary Y., Keefe E.B. Improved virologic response in chronic hepatitis C genotype-4. Patients given nitazoxanide, peginterferon, and ribavirin. Gastroenterology. 2009;136:856–862. doi: 10.1053/j.gastro.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Rossignol J.F., Elfert A., Keeffe E.B. Treatment of chronic hepatitis C using a 4-week lead-in with nitazoxanide before peginterferon plus nitazoxanide. J. Clin. Gastroenterol. 2010;44:504–509. doi: 10.1097/MCG.0b013e3181bf9b15. [DOI] [PubMed] [Google Scholar]
- Rossignol J.F., Lopez-Chegne N., Julcamoro L.M., Carrion M.E., Bardin M.C. Nitazoxanide for the empiric treatment of pediatric infectious diarrhea. Trans. R. Trop. Med. Hyg. 2012;106:167–173. doi: 10.1016/j.trstmh.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Shaw M.L. The host interactome of influenza virus presents new potential targets for antiviral drugs. Rev. Med. Virol. 2011;21:358–369. doi: 10.1002/rmv.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Wei J., Deng X., Li S., Qiu Y., Shao D., Li B., Zhang K., Xue F., Wang X., Ma Z. Nitazoxanide inhibits the replication of Japanese encephalitis virus in cultured cells and in a mouse model. Virol. J. 2014;11:10. doi: 10.1186/1743-422X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq D.M., Koo H.L., Adachi J.A., Viola G.M. Norovirus gastroenteritis successfully treated with nitazoxanide. J. Infect. 2011;63:394–397. doi: 10.1016/j.jinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman K., Mishin V.P., Guo Z., Garten R.J., Balish A., Fry A.M., Villanueva J., Stevens J., Gubavera V. Antiviral susceptibility of variant influenza A (H3N2)v viruses isolated in the United States during 2011–2013. Antimicrob. Agents Chemother. 2014;58:2045–2051. doi: 10.1128/AAC.02556-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachulski A.V., Pidathala C., Row E.C., Sharma R., Berry N.G., Iqbal M., Bentley J., Allman S.A., Edwards I.G., Helm A., Hellier J., Korba B.E., Semple J.E., Rossignol J.F. Thiazolides as novel antiviral agents: I. Inhibition of hepatitis B virus. J. Med. Chem. 2011;54:4119–4132. doi: 10.1021/jm200153p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachulski A.V., Pidathala C., Row E.C., Sharma R., Berry N.G., Iqbal M., Bentley J., Allman S.A., Edwards I.G., Helm A., Hellier J., Korba B.E., Semple J.E., Rossignol J.F. Thiazolides as novel antiviral agents: II. Inhibition of the hepatitis C virus. J. Med. Chem. 2011;54:8670–8680. doi: 10.1021/jm201264t. [DOI] [PubMed] [Google Scholar]
- Tan X., Hu L., Luquette L.J., 3rd, Gao G., Liu Y., Qu H., Xi R., Lu Z.J., Park P.J., Elledge J. Systematic identification of synergistic drug pairs targeting HIV. Nat. Biotechnol. 2012;30:1125–1130. doi: 10.1038/nbt.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubel J., Lorch U., Rossignol J.F., Ferber J., Camm A. Analyzing the relationship of QT interval and exposure to nitazoxanide, a prospective candidate for influenza antiviral therapy-A formal TQT study. J. Clin. Pharmacol. 2014 doi: 10.1002/jcph.300. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Teran C.G., Teran-Escalera C.N., Villarroel P. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single-blind controlled trial in Bolivian children. Int. J. Infect. Dis. 2009;13:518–523. doi: 10.1016/j.ijid.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Theodos C.M., Griffiths J.K., D’Onfro J., Fairfield A., Tzipori S. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 1998;42:1959–1965. doi: 10.1128/aac.42.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon C., Viswanathan P., Rossignol J.F., Korba B.E. Mutations in HCV non-structural genes do not contribute to resistance to nitazoxanide in replicon-containing cells. Antiviral Res. 2011;91:233–240. doi: 10.1016/j.antiviral.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Simmons G. Development of novel entry inhibitors targeting emerging viruses. Expert Rev. Anti. Infect. Ther. 2012;10:1129–1138. doi: 10.1586/eri.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]