Highlights
-
•
Artificial microRNAs designed against VEEV nsp-4 were found non-toxic in cell culture.
-
•
VEEV replication was effectively inhibited by all the artificial microRNAs in vitro.
-
•
Combination of multiple microRNAs in a single expression vector does not increase protective efficacy against VEEV infection.
Keywords: Artificial microRNA, VEEV, nsp-4, siRNA, Antiviral
Abstract
Venezuelan equine encephalitis virus is a member of the alphavirus family and genus togaviridae. VEEV is highly infectious in aerosol form and has been weaponized in the past making it a potential biothreat agent. At present, there are no FDA approved antiviral treatments or vaccines for VEEV. Artificial microRNAs are small molecules which are expressed through endogenous microRNA machinery by RNA polymerase II. These artificial microRNAs effectively inhibit gene expression and are non-toxic to the host cell. VEEV RNA dependent RNA polymerase (RdRp) is central to VEEV replication. Therefore, we hypothesize that targeted inhibition of VEEV RdRp using artificial microRNAs may efficiently inhibit VEEV replication. Five artificial microRNAs were tested in vitro in BHK cells. Three of these artificial miRNAs showed significant inhibition of VEEV replication. Further, these microRNAs were cloned into the expression vector in combination to see the synergistic effect on VEEV replication. Combination of more than one miRNA did not result in significant inhibition of virus replication. In conclusion, we have shown that RNAi through artificial microRNAs effectively inhibits VEEV replication and is significantly less toxic in comparison to siRNAs.
Venezuelan equine encephalitis virus (VEEV) is one of the most important New World pathogens of humans and equines, which has caused periodic outbreaks in Central and South America (Weaver et al., 2004). VEEV is an enveloped alphavirus with a non-segmented positive strand RNA genome of 11.4 kb (Paesseler and Weaver, 2009). VEEV infection causes a wide range of clinical symptoms ranging from unapparent disease to acute encephalitis in both humans and equines (Johnson and Martin, 1974). Mortality rates in equines have been estimated at 19–83% whereas mortality in humans is <1% (Walton et al., 1973, de la Monte et al., 1985).
Despite numerous efforts to develop antiviral therapies and vaccine countermeasures, currently there is no FDA approved antiviral drug or vaccine for use against VEEV infection. Short interfering RNAs (siRNAs) have shown immense potential to be used as an antiviral therapeutic for various human viral diseases. Human immunodeficiency virus, polio virus, hepatitis B virus, West Nile virus, severe acute respiratory syndrome associated coronavirus, foot and mouth disease virus, influenza virus and chikungunya virus have been successfully inhibited using virus specific siRNAs (Jacque et al., 2002, Ge et al., 2003, Hui et al., 2004, Tompkins et al., 2004, Wang et al., 2004, Giladi et al., 2003, Chen et al., 2004, Dash et al., 2008). Recently, it was shown that artificial microRNAs (miRNAs) which are small RNA molecules expressed under the backbone of endogeneous cellular miRNAs are more effective inhibitors of cellular genes (Maczuga et al., 2012). Artificial miRNAs are expressed though RNA polymerase II and are argued to be less cytotoxic than siRNAs due to their relatively low concentration inside the cells. Double stranded siRNAs can trigger interferon response which can cause cytotoxicity. Short hairpin RNA (shRNA) is transcribed through RNA polymerase III and can saturate dicer or other RNAi proteins which can lead to a reduced RNAi response and increased cellular toxicity. Hence artificial miRNAs have a clear advantage over siRNA or shRNAs in both in vitro and in vivo gene silencing (Boudreau et al., 2009, Xie et al., 2013). Artificial miRNAs have been studied as an antiviral approach for many viruses including adenoviruses, rabies virus, dengue virus and porcine reproductive and respiratory virus and has been shown to be an effective inhibitor of virus replication with minimal or no cytotoxicity (Ibrisimovic et al., 2013, Xia et al., 2013, Xie et al., 2013, Israsena et al., 2009).
In this study, we have targeted the viral non-structural protein-4 (nsp-4) region which encodes for the viral RNA dependent RNA polymerase and plays a critical role in the viral replication in the host cells. Previously, VEEV genome has been successfully targeted using siRNAs (O’brien, 2007). We also tested nsp-4 as a potential target for RNA interference studies using siRNAs. Four siRNAs were designed against the nsp-4 region and were tested for their antiviral efficiency. Similar to the previous report, siRNAs were found to be protective against VEEV infection and hence nsp-4 was chosen as the target for the design of artificial miRNAs (Supplementary Fig. 1). Artificial miRNA sequences were designed using Blockit RNAi Web Designer tool (Invitrogen Inc.). Top three high scoring artificial miRNAs were selected and the oligos were synthesized along with a negative control sequence which is not specific against nsp-4 sequence (Invitrogen Inc.) (Fig. 1 A). Artificial miRNAs were cloned into pcDNA™6.2-GW/EmGFP-miR vector (Invitrogen Inc.) according to the manufacturer’s protocol (Fig. 1B). To determine the toxicity of artificial miRNAs in in vitro experiments, we measured cell proliferation after artificial miRNA transfection using MTT assay. 100–400 ng of plasmid vector harboring artificial miRNAs were transfected in BHK-21 cells in each well of 96 well plates and incubated for 24 h. MTT assay (Invitrogen Inc.) was performed to evaluate the percentage of metabolically active cells. In our data, no significant toxic effect was observed due to the presence of artificial miRNAs in cells (Fig. 2 A). Antiviral efficacy against VEEV was tested in vitro in BHK-21 cells (ATCC Inc.) using TC-83 which is a vaccine strain of VEEV and approved to be used in BSL-2 laboratory. Moreover, the sequence of nsp-4 in TC-83 strain is identical to that of virulent VEEV strain. To assess the antiviral activity of artificial miRNAs against VEEV infection, BHK-21 cells were transfected with artificial miRNAs using lipofectamine 2000 transfection reagent (Life Technologies Inc.). Transfection was performed using 2 ug of total plasmid DNA per well of 12 well plates. Cells were incubated overnight after transfection and then infected with 0.1 multiplicity of infection (MOI) of TC-83. Cell supernatant and total cell lysates were harvested at 12 and 24 h post infection (p.i.). To estimate the amount of virus inhibition by artificial miRNAs, TCID50/ml (50% tissue culture infectious dose) was calculated from the cellular supernatant. TCID50 data suggested a significant inhibition of VEEV replication in presence of artificial miRNAs. The reduction of viral load in the cell supernatant was more pronounced at 12 h although significant inhibition was also observed at 24 h p.i. All the three artificial miRNAs showed protection against VEEV infection in vitro (Fig. 2B). Further, we performed real time PCR (RT-PCR) to estimate the reduction in the viral RNA load in the cells transfected with artificial miRNAs and infected with VEEV 12 h p.i. In cells treated with artificial miRNAs, the total viral RNA was significantly reduced with Mir-5 showing the maximum inhibition in viral RNA replication (Fig. 3 A). We then quantitated the total viral protein to understand whether the inhibition of viral RNA transcription is also reflected at the translational level. 20 ug of total cellular protein was loaded on 4–12% Bis–Tris gel followed by transfer on a nitrocellulose membrane. Western blot was performed with a monoclonal antibody specific to E2 glycoprotein of VEEV. Mir-5 treated BHK-21 cells again showed the least amount of virus presence at 24 h p.i. in comparison to negative miRNA control cells. Inhibition of viral protein was also observed in Mir-1 and Mir-3 (Fig. 3B). We also observed the presence of artificial miRNAs and virus using fluorescent microscopy. Artificial miRNAs vectors express a green fluorescent protein (EmGFP) which indicates the presence of artificial miRNAs. BHK-21 cells were transfected with artificial miRNAs and then infected with 0.1 MOI of TC-83 which express a cherry red protein (Atasheva et al., 2008). We found that cells which are positive (green) for Mir-1, Mir-3 or Mir-5 did not show the presence of TC-83 virus (red) at 24 h p.i. Cells which were not transfected with artificial miRNAs were all positive for VEEV infection which is shown in red color (Fig. 3C).
Fig. 1.
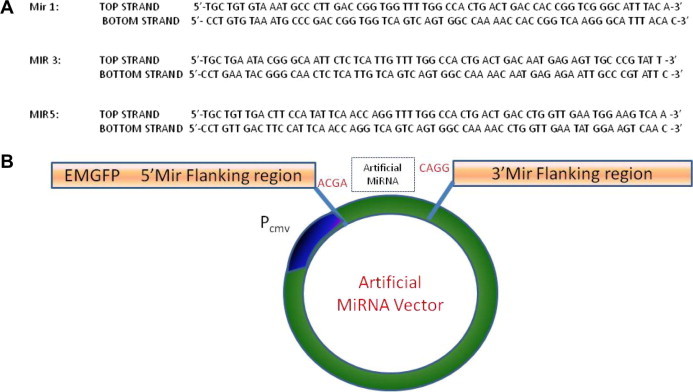
Design of artificial miRNA sequence and cloning strategy. (A) MiRNA sequences for cloning into artificial miRNA vector. Three sequences were designed against nsp-4 region of VEEV genome as target using Block-iT RNAi Designer, (Invitrogen). (B) Cloning strategy of artificial miRNAs in a vector with CMV promoter. An EmGFP gene is cloned before the 5’ flanking region. A four base over hank is present in the linearized vector which is used for directional cloning of the artificial miRNA sequences.
Fig. 2.
Toxicity and antiviral activity of artificial miRNAs in BHK-21 cells. (A) Cell proliferation assay to evaluate toxicity of artificial miRNAs in BHK cells. BHK cells were grown in 96 well plates and transfected with 100–400 ng of artificial miRNA. After 24 h of transfection MTT reagent was added to determine miRNA toxicity. No significant cellular cytotoxicity was seen. (B) Safety efficacy of artificial miRNAs in BHK-21 cells against VEEV infection. BHK cells were transfected with 2 μg miRNA per well of 12 well plates. After overnight transfection, cells were infected with 0.1 MOI of TC-83. Cell supernatant was collected at 12 and 24 h post infection. Virus titer estimation was performed using TCID50. Statistical significance was calculated using two tailed student t test, P < 0.001. Data is shown here is representative of one of the three experimental repeats.
Fig. 3.
Antiviral efficacy of artificial miRNAs in BHK-21 cells. (A) Viral RNA copy number by RT-PCR. BHK cells were treated with artificial miRNAs and infected with 0.1 MOI of TC-83. Total cellular RNA was isolated at 12 h p.i and RT-PCR was performed with specific primers for VEEV nsp-4 region. Fold changes were calculated by normalizing the values to GAPDH expression as endogenous control. (B) Western blot for VEEV E2 glycoprotein in BHK-21 cells treated with artificial miRNAs. Cells lysates was collected at 24 h p.i and probed for presence of VEEV E2 glycoprotein. A marked reduction in viral glycoprotein was observed in cells treated with Mir 1, 3 and 5 whereas an intense signal was observed in untreated cells. Beta–actin was used as loading control (C) Fluorescence microscopy images of BHK-21 cells transfected with artificial miRNA and green fluorescence indicates the expression of EmGFP along with artificial miRNAs. The cells were infected with TC-83 strain which express cherry red protein (TC-83 cherry red panel). In cells treated with Mir-1, 3 and 5 does not show any overlap in the presence of artificial miRNAs (green) and virus (red) (Merged Image). Cells treated with non-specific artificial miRNA shows overlapping green and red color indicated by white arrows. No overlap is evident in other artificial miRNA treatments. The cells in red are infected and which has a tendency to become rounded after infection which may appear smaller in comparison to uninfected cells. Further, few of the very small red spots are background cellular debris and may not represent live cells.
It has been previously shown that expressing more than one miRNA in one single artificial miRNA vector construct may provide better inhibition of virus (Israsena et al., 2009). We therefore cloned two artificial miRNAs together in the same expression vector. One of the miRNAs was used as a backbone and digested with BamH I and Xho I whereas the other miRNA used as insert was digested with Bgl II and Xho I to excise out the insert. The insert was then ligated into the vector backbone to generate a construct which express two miRNAs. Two recombinant plasmids vectors were generated, Mir15 with Mir-1 and Mir-5, and Mir-35 which contains single miRNA sequence of Mir-3 and Mir-5. We then investigated the synergistic effect of expressing two miRNAs through a single expression vector against VEEV infection in BHK-21 cells. Cells were transfected as previously described and cell lysates and supernatant were collected. Virus titer from cell supernatant at 12 h p.i. showed protection by both Mir-15 and Mir-35 in comparison to the negative control. Similarly at 24 h p.i, total viral load in supernatant was reduced in cells treated with Mir35.However, combination of two artificial miRNAs did not further reduce the viral load in comparison to single artificial miRNA treatment (Fig. 4 A). We further quantified the total viral protein from the cell lysates using western blot. In accordance with the virus titer data, reduction in total viral protein was observed in the cells treated with Mir-15 and Mir-35 but the combination treatment lead to a slight increase in the total viral protein in comparison to Mir-1, 3 and 5 treatment alone (Fig. 4B).
Fig. 4.
Antiviral effect of chained artificial miRNAs. (A) Inhibition of virus replication after treatment with combination of two artificial miRNAs. Mir 15 and Mir35 were generated by combining individual miRNAs in under one promoter. BHK-21 cells were transfected and supernatant was assayed for inhibition of virus replication by TCID50 at 12 and 24 h p.i. Virus titer indicated a modest inhibition by Mir 15 at 12 h p.i in virus replication in comparison to individual miRNAs whereas Mir-35 treatment showed a significant inhibition at 24 h p.i. Statistical significance was calculated bytwo tailed student t test, p < 0.01. Data is shown here is representative of one of three individual experimental repeats. (B) Western blot showing amount of viral envelop glycoprotein in the BHK-21 cell lysate upon after treatment of combined miRNAs. Western blot data corroborated with the virus titer data with increased presence of envelope glycoprotein in the cells indicating minimal inhibition of virus by combined artificial miRNAs.
These findings suggest that artificial miRNAs are effective in inhibiting VEEV replication by targeting the viral polymerase gene. Out of the three miRNAs tested, we found Mir-3 to be the most protective in reducing the total viral load in the cell supernatant. Combination of artificial miRNAs as a single construct did not confer increased antiviral activity compared to individual artificial miRNAs. This may have occurred due to relatively low concentrations of individual miRNAs when expressed together. However, artificial miRNAs were found to be non- toxic and effective inhibitors of VEEV replication in vitro. Due to their low toxicity without the need of chemical modifications as suggested for siRNAs (Bramsen and Kjems, 2011), these artificial miRNAs can be further tested in animal models for antiviral therapy against VEEV infection.
Acknowledgements
These studies were supported by Defense Threat Reduction Agency. Authors would also like to thank to Dr Ilya Frolov, Department of Microbiology, University of Alabama, Birmingham, AL, for kindly providing TC-83 strain of VEEV expressing cherry red protein.
Opinions expressed are those of the authors and should not be construed as official or reflecting the views of Uniformed Services University of Health Sciences, Department of Defense, Defense Threat Reduction Agency or Birla Institute of Technology and Science, Pilani, India.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.antiviral.2013.08.010.
Appendix A. Supplementary data
Inhibition of VEEV replication in vitro with siRNAs: Sequences of siRNA designed against nsp-4 region of VEEV. Negative siRNA: Scrambled RNA sequences 4G 7T 4C 6A (21 nt): 5’ TAGAGCGTAT TACGTATACC T 3’ Only 76% identical to 2 oryza sativa (rice) genes AP006523 AND AP008208 was used. B: Cell proliferation assay in vero cells showing protection by siRNA treatment from VEEV infection: Vero cells were grown in 96 well plates and transfected with 100ng of siRNA duplex targeted against nsp4 region of the virus genome. After 12hr of transfection cells were infected with VEEV (MOI=0.1). MTT assay was done to quantify the cell proliferation. Values ± SEM, P ⩽ 0.05.
References
- Atasheva S., Garmashova N., Frolov I., Frolova E. Venezuelan equine encephalitis virus capsid protein inhibits nuclear import in Mammalian but not in mosquito cells. J. Virol. 2008;82:4028–4041. doi: 10.1128/JVI.02330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau R.L., Martins I., Davidson B.L. Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol. Ther. 2009;17:169–175. doi: 10.1038/mt.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramsen J.B., Kjems J. Chemical modification of small interfering RNA. Methods Mol. Biol. 2011;721:77–103. doi: 10.1007/978-1-61779-037-9_5. [DOI] [PubMed] [Google Scholar]
- Chen W., Yan W., Du Q., Fei L., Liu M., Ni Z., Sheng Z., Zheng Z. RNA interference targeting VP1 inhibits foot-and-mouth disease virus replication in BHK-21 cells and suckling mice. J. Virol. 2004;78:6900–6907. doi: 10.1128/JVI.78.13.6900-6907.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P.K., Tiwari M., Santhosh S.R., Parida M., Lakshmana Rao P.V. RNA interference mediated inhibition of Chikungunya virus replication in mammalian cells. Biochem. Biophys. Res. Commun. 2008;376:718–722. doi: 10.1016/j.bbrc.2008.09.040. [DOI] [PubMed] [Google Scholar]
- de la Monte S., Castro F., Bonilla N.J., Gaskin de Urdaneta A., Hutchins G.M. The systemic pathology of Venezuelan equine encephalitis virus infection in humans. Am. J. Trop. Med. Hyg. 1985;34:194–202. doi: 10.4269/ajtmh.1985.34.194. [DOI] [PubMed] [Google Scholar]
- Ge Q., McManus M.T., Nguyen T., Shen C.H., Sharp P.A., Eisen H.N., Chen J. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc. Natl. Acad. Sci. USA. 2003;100:2718–2723. doi: 10.1073/pnas.0437841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi H., Ketzinel-Gilad M., Rivkin L., Felig Y., Nussbaum O., Galun E. Small interfering RNA inhibits hepatitis B virus replication in mice. Mol. Ther. 2003;8:769–776. doi: 10.1016/s1525-0016(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Hui E.K., Yap E.M., An D.S., Chen I.S., Nayak D.P. Inhibition of influenza virus matrix (M1) protein expression and virus replication by U6 promoter-driven and lentivirus-mediated delivery of siRNA. J. Gen. Virol. 2004;85:1877–1884. doi: 10.1099/vir.0.79906-0. [DOI] [PubMed] [Google Scholar]
- Ibrisimovic M., Kneidinger D., Lion T., Klein R. An adenoviral vector-based expression and delivery system for the inhibition of wild-type adenovirus replication by artificial microRNAs. Antiviral Res. 2013;97:10–23. doi: 10.1016/j.antiviral.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israsena N., Supavonwong P., Ratanasetyuth N., Khawplod P., Hemachudha T. Inhibition of rabies virus replication by multiple artificial microRNAs. Antiviral Res. 2009;84:76–83. doi: 10.1016/j.antiviral.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Jacque J.M., Triques K., Stevenson M. Modulation of HIV-1 replication by RNA interference. Nature. 2002;418:435–438. doi: 10.1038/nature00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.M., Martin D.H. Venezuelan equine encephalitis. Adv. Vet. Sci. Comp. Med. 1974;18:79–116. [PubMed] [Google Scholar]
- Maczuga P., Koornneef A., Borel F., Petry H., van Deventer S., Ritsema T., Konstantinova P. Optimization and comparison of knockdown efficacy between polymerase II expressed shRNA and artificial miRNA targeting luciferase and Apolipoprotein B100. BMC Biotechnol. 2012;12:42. doi: 10.1186/1472-6750-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien L. Inhibition of multiple strains of Venezuelan equine encephalitis virus by a pool of four short interfering RNAs. Antiviral Res. 2007;75:20–29. doi: 10.1016/j.antiviral.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paessler S., Weaver S.C. Vaccines for Venezuelan equine encephalitis. Vaccine. 2009;27(Suppl. 4):D80–85. doi: 10.1016/j.vaccine.2009.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins S.M., Lo C.Y., Tumpey T.M., Epstein S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl. Acad. Sci. USA. 2004;101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton T.E., Alvarez O., Jr., Buckwalter R.M., Johnson K.M. Experimental infection of horses with enzootic and epizootic strains of Venezuelan equine encephalomyelitis virus. J. Infect. Dis. 1973;128:271–282. doi: 10.1093/infdis/128.3.271. [DOI] [PubMed] [Google Scholar]
- Wang Z., Ren L., Zhao X., Hung T., Meng A., Wang J., Chen Y.G. Inhibition of severe acute respiratory syndrome virus replication by small interfering RNAs in mammalian cells. J. Virol. 2004;78:7523–7527. doi: 10.1128/JVI.78.14.7523-7527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C., Ferro C., Barrera R., Boshell J., Navarro J.C. Venezuelan equine encephalitis. Annu. Rev. Entomol. 2004;49:141–174. doi: 10.1146/annurev.ento.49.061802.123422. [DOI] [PubMed] [Google Scholar]
- Xia B., Song H., Chen Y., Zhang X., Xia X., Sun H. Efficient inhibition of porcine reproductive and respiratory syndrome virus replication by artificial microRNAs targeting the untranslated regions. Arch. Virol. 2013;158:55–61. doi: 10.1007/s00705-012-1455-z. [DOI] [PubMed] [Google Scholar]
- Xie P.W., Xie Y., Zhang X.J., Huang H., He L.N., Wang X.J., Wang S.Q. Inhibition of dengue virus 2 replication by artificial microRNAs targeting the conserved regions. Nucleic Acid Ther. 2013;23:244–252. doi: 10.1089/nat.2012.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inhibition of VEEV replication in vitro with siRNAs: Sequences of siRNA designed against nsp-4 region of VEEV. Negative siRNA: Scrambled RNA sequences 4G 7T 4C 6A (21 nt): 5’ TAGAGCGTAT TACGTATACC T 3’ Only 76% identical to 2 oryza sativa (rice) genes AP006523 AND AP008208 was used. B: Cell proliferation assay in vero cells showing protection by siRNA treatment from VEEV infection: Vero cells were grown in 96 well plates and transfected with 100ng of siRNA duplex targeted against nsp4 region of the virus genome. After 12hr of transfection cells were infected with VEEV (MOI=0.1). MTT assay was done to quantify the cell proliferation. Values ± SEM, P ⩽ 0.05.


