Highlights
-
•
IBV nonstructural protein 5 was expressed in E. coli for antibody ELISA detection.
-
•
Nsp5-ELISA sensitivity was 98.1% and specificity 95%, compared to IDEXX.
-
•
Chickens infected or vaccinated showed similar antibody profiles by nsp5 or IDEXX.
Keywords: Ibv, Nonstructural protein 5 (nsp5), Enzyme-linked immunosorbent assay
Abstract
Infectious bronchitis virus (IBV) continues to be one of the most important poultry pathogens worldwide. The current commercially available enzyme-linked immunosorbent assay (ELISA) kits for IBV specific antibody detection are mostly based on the whole virion, and few serological tests based on nonstructural proteins of IBV have been developed. Herein, an alternative indirect ELISA for detection of IBV antibody was developed with IBV nonstructural protein 5 (nsp5) produced by Escherichia coli. Using an indirect immunofluorescence assay (IFA) and a commercial ELISA kit as reference, we optimized the nsp5-ELISA and determined its cut-off as 0.12. The diagnostic sensitivity (DSN), specificity (DSP) and accuracy of the nsp5-ELISA were 93.11%, 95.38% and 93.33%, respectively, compared with IFA in 660 field serum samples, and were 98.11%, 95.00% and 97.62%, respectively, compared with the commercial IBV ELISA kit (IDEXX) in 126 field sera samples. Furthermore, a time course of IBV specific antibody level detected by nsp5-ELISA following IBV infection and vaccination is consistent with that of IBV antibody detected by the commercial ELISA kit. The results presented in this study indicate that nsp5-ELISA has the potential to serve as a rapid, reliable and cost-effective method for IBV antibody detection. This study is the first to report the development of an nsp-based ELISA to detect an antibody to IBV.
1. Introduction
Infectious bronchitis (IB) is an acute and highly contagious respiratory disease (Cavanagh, 2003) caused by infectious bronchitis virus (IBV), a prototype of the Coronaviridae family. IBV infects chickens of all ages and causes significant economic losses to chicken farms. In addition to infection of the respiratory system, IBV also infects the reproductive system, which can lead to the low production and poor quality of eggs. Some of the virus strains have a tropism to chicken kidney cells and cause nephritis that can lead to the deaths of the infected chickens (Reddy et al., 2015). Currently, mutations are frequently noted in the IBV genome, leading to variation and drift of viral antigens that can pose challenges to diagnostic tests. IBV emerged more than 70 years ago and continues to be one of the most important poultry pathogens worldwide (Cook, 2016).
With the emergence of many new IBV genotypes/serotypes, diagnoses of IBV are considered more important for IB control in recent years. Among the diagnosis methods, chicken embryo culture has remained the method of choice to obtain field IBV isolates. RT-PCR is the first choice to detect IBV for many laboratories since it is rapid, sensitive and specific (Nguyen et al., 2013). Currently, Real-time PCR is a widely used method for the quantitative detection of IBV (Fellahi et al., 2016). Gene sequences and phylogenetic analyses are currently applied to determine the variation of newly isolated IBV. ELISA based on whole virions as well as recombinant S1 (spike protein 1 subunit) and N proteins (Nucleoprotein) could provide a rapid and large-scale detection method for IBV infection (Bronzoni et al., 2005, Lin et al., 2012, Gibertoni et al., 2005). However, few IBV detection methods are developed based on the nonstructural proteins (nsps).
Generally, nsps encode proteins that are important for viral replication and infection of the host. The high conservation of nsps might provide an advantage in the detection of virus infection because many RNA viruses, including IBV, mutate easily and either have no or a reduced cross-reaction to the original viruses (de Wit et al., 2011). Furthermore, nsps are present in the infected cells but not in the virions. After immunization with an inactivated vaccine prepared from purified virions, the animal would not produce any or very low levels of antibodies to virus nsps. Nsps have the potential to be used for differential diagnoses between vaccination with inactivated vaccine and natural infection. Nsp ELISA has become an essential part of the vaccination-based control and serosurveillance policy in many FMD- (foot-and-mouth disease-) endemic countries. Nsp-ELISA could provide long term (up to 3 years) and sensitive detection for FMDV infection (Elnekave et al., 2015). However, as of yet, there are no applications using nsps to detect IBV infection.
Because of the variation of IBV, there are many serotypes of IBV with little cross-reaction between different serotypes/variants. Rapid and reliable detection methods are needed to monitor the epidemiology of IBV in farms. The purpose of this study was to develop an ELISA to detect IBV nsp antibodies and evaluate its use in differential diagnoses, epidemiological investigations or vaccination monitoring.
2. Materials and methods
2.1. Viruses and chickens
The IBV strain SC021202 was identified as a nephropathogenic strain by our research group (Zhou et al., 2004) and was adapted to the DF-1 cell line. This cell line was cultured in Dulbecco’s Minimum Essential Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, USA).
One-month-old and three-month-old specific-pathogen-free (SPF) chickens were purchased from Shennong Company, Zhejiang, China, and reared in SPF isolators. The three-month-old chickens were used to prepare negative serum and positive serum, and the one-month-old chickens were used to prepare IBV infected or vaccinated-serum samples.
2.2. Serum samples
2.2.1. Reference negative and positive sera
Reference negative sera were collected from different ages of SPF chickens, which were confirmed to be free of IBV antibodies by an immunofluorescence assay (IFA).
To prepare the reference positive serum to IBV nsp5, three-month-old SPF chickens were immunized subcutaneously with an emulsion mixture containing equal volume of complete Freund’s adjuvant and purified nsp5 (1 mg/chicken in 0.5 ml volume). The purified nsp5 was prepared as described in 2.4. Two booster immunizations with the same dose of antigen emulsified with equal volume of incomplete Freund’s adjuvant were administered to the chickens at 2-week intervals. Ten days following the third immunization, the sera of immunized chickens were collected, and the titers of the sera were examined by IFA and then stored at −20 °C until future use.
Positive serum against avian influenza virus (AIV), Newcastle disease virus (NDV) and infectious bursal disease virus (IBDV) from SPF chickens were provided by the IDEXX Company (IDEXX laboratory in Beijing).
2.2.2. Serum samples from IBV infected chickens and vaccinated chickens
Ten one-month-old chickens in one group were infected with SC021202; sera were collected from the wing veins of the infected chickens every three days during the first month post infection and every week afterward.
In parallel experiments, chickens of the same age were vaccinated with attenuated H52 or inactivated M41 vaccines, in two separate groups, both vaccines were purchased from Yibang CLt. Qingdao, China. Sera were collected as described above.
2.2.3. Clinical serum samples
Six hundred and sixty field serum samples were randomly obtained from chicken flocks in different areas of Zhejiang and Shandong Provinces, China during 2012–2015.
2.3. Construction of the recombinant pGEX-4T-1-nsp5 plasmid vector
Total RNA from the allantoic fluid of SPF chicken embryonated eggs infected with IBV SC021202 was extracted by using MiniBest Viral RNA Extraction Kit Ver.4.0 (Takara, China) and reverse transcribed using RevertAid First Strand cDNA Synthesis Kit (Fermentas, Canada). Based on the genomic sequence of IBV isolate SC021202, the following primer pair were used to amplify a 921 bp sequence of the nsp5 gene: forward primer 5′- CGGAATTCGCTGGTTTTAAGAAGCTAGTTTC −3′ contained the EcoRI site, and reverse primer 5′- GCGTCGACTCACTGTAGTCTGACAC −3′ contained the SalI site. The purified PCR product was digested with EcoRI and SalI and cloned into the prokaryotic expression vector pGEX-4T-1 (Amersham, USA). Then, the recombinant plasmid (pGEX-4T-1-nsp5) was transformed into E. coli BL21 (DE3) strain (Amersham, USA) and clones containing the correct nsp5 coding sequence were identified by PCR and restriction analysis, and then confirmed by DNA sequence analysis.
2.4. Antigen preparation
The resulting E. coli BL21 (DE3) clones containing the recombinant plasmid (pGEX-4T-1-nsp5) were picked and cultured at 37 °C for subsequent expression of recombinant nsp5. Bacteria harboring the pGEX-4T-1 vectors without the insert were used as a negative control. The recombinant E. coli was induced by a final concentration of 0.1 mM isopropylthio-b-d-thiogalactoside (IPTG) for 5 h at 37 °C. Then, the cells were pelleted and resuspended in PBS and lysed by sonication on ice. The lysate was centrifuged at 12,000g for 10 min at 4 °C to collect the supernatant, which was loaded into a GSTrap FF affinity column (Amersham, USA) according to the manufacturer’s protocol. After washing twice with 0.01 M phosphate-buffered saline (PBS) at pH 7.4, the GST-fused nsp5 protein was eluted with an elution buffer (50 mM Tris–HCl, 10 mM glutathione reduced, pH 8.0). The collected samples were identified by SDS-PAGE and Western blot assays as previously described (Sambrook and Russell, 2001). The concentration of the recombinant nsp5 was determined by a BCA Protein Assay Kit (Thermo Scientific, USA) according to the manufacturer’s protocol. The sample was then stored at −70 °C.
2.5. SDS-PAGE and Western blot analysis
Protein samples were mixed with 4 × loading buffer and boiled for 10 min. The denatured protein solution was separated on a 12% discontinuous SDS-PAGE gel. Protein gels were either stained with Coomassie brilliant blue G-250 (Bio-Rad, USA) or transferred onto a 0.45 μm nitrocellulose membrane (Millipore, USA). The membrane was blocked with blocking buffer (5% skim milk and 0.05% Tween-20 dissolved in PBS) at 37 °C for 1 h and then incubated with serum samples diluted in blocking buffer. After washing three times with wash buffer (0.05% Tween-20 dissolved in PBS, PBST), the membrane was incubated at 37 °C for 45 min with a peroxidase-conjugated affinipure goat anti-chicken IgG (H + L) (KPL, USA) diluted 1:5000 in blocking buffer. Following an additional three washes, detection was performed using TMB Peroxidase Substrate (KPL, USA).
2.6. IFA
IFA was used as the reference method to detect the presence of antibodies to IBV. The 80% confluent monolayer of DF-1 cells cultured in 96-well plates was infected with IBV SC021202. Uninfected cells were used as negative control. The cells were then fixed in a 1:1 v/v mixture of methanol and acetone for 30 min at −20 °C. After washing with PBST, the plates were blocked for 1 h at 37 °C with blocking buffer. Serum samples (100 μl) diluted either 1:20 or 1:100 in blocking buffer were added to the wells of the plates and incubated for 1 h at 37 °C. Following three washes with PBST, FITC-conjugated goat anti-chicken IgG (KPL, USA) diluted 1:200 was added and incubated for 45 min at 37 °C. After washing, the positive signal was examined under an IX71 inverted fluorescence research microscope (Olympus Optical Co. Ltd.). Serum samples that gave positive signals at a dilution of 1:20 or higher were designated as IBV antibody positive.
2.7. ELISA procedure
Briefly, 96-well microtiter plates (Canada JET Biochemicals Int’l. Inc.) were coated with 0.5 μg/100 μl rGST-nsp5 protein in 0.01 M PBS (pH7.4) at 4 °C overnight. After washing with PBST three times, the plates were blocked with 200 μl blocking buffer for 2 h at 37 °C, and then incubated with 100 μl serum samples diluted in blocking buffer for 1 h at 37 °C. Following five washes with PBST, the plates were further incubated with 100 μl HRP-conjugated goat anti-chicken IgG (KPL, USA) in blocking buffer for 1 h at 37 °C. Then, the plates were washed, and the colorimetric reaction was developed by incubating the plates with 100 μl chromogenic substrate for 10 min at 37 °C. Color development was stopped with 50 μl 2 M H2SO4, and an optical density at 450 nm (OD450) was recorded using an ELx800 universal microplate reader (Bio-Tek Instruments, Inc., USA).
2.8. Optimization of ELISA working conditions
Using the procedure described above, the optimum concentration of purified recombinant nsp5 as the coating antigen and dilution of serum samples as primary antibodies were determined by checkerboard titration procedures (Crowther, 2000). Briefly, the nsp5 was immobilized onto 96-well microtiter plates using serial two-fold dilutions from 7.69 μg/ml to 0.91 μg/ml. Correspondingly, reference chicken IBV nsp5-positive serum and negative control serum were diluted in serial two-fold dilutions from 1:25 to 1:1600. To determine the optimal conjugate dilution in the nsp5-ELISA, the HRP-labeled goat anti-chicken IgG (KPL, USA) was added to the plate at the following dilutions: 1:1000, 1:2000, 1:4000 and 1:8000. The coating buffer was optimized from distilled water at pH 6.5 (H2O), 0.9% NaCl at pH 7.0 (NS), 0.01 M phosphate-buffered saline at pH 7.4 (PBS), 0.05 M Tris–HCl buffer at pH 8.5 (TB), 0.05 M carbonate–bicarbonate buffer at pH 9.6 (CB), and 0.1 M NaOH solution at pH 13 (NaOH). Subsequently, different assay parameters and the concentration of component reagents of the nsp5-ELISA were optimized, including selection of blocking buffer, diluent for serum samples, determination of exposure time for serum samples and the conjugate and reaction time for chromogenic substrate. The conditions that gave the highest OD450 ratio between positive and negative serum (P/N value) with the positive control serum yielding an absorbance near 1.5 were scored as optimal working conditions.
2.9. Application of nsp5 ELISA
Six hundred and sixty field serum samples were tested by nsp5-ELISA in duplicate to set a negative–positive cut-off value for this assay. IFA was used as the reference to compare with the nsp5-ELISA. The S/P ratios of 660 field serum samples obtained from the nsp5-ELISA were compared with those obtained from the IFA results. A receiver-operating characteristic analysis (ROC) was performed, and a cut-off point was determined so that the diagnostic sensitivity (DSN) and specificity (DSP) were maximized while the sum of false negative and false positive results was minimized. The S/P value was calculated with the following formula: S/P = (OD450 of sample-OD450 of negative control)/(OD450 of reference positive control-OD450 of negative control).
Compared with the detection by the reference method IFA, the DSN and DSP and accuracy of the nsp5-ELISA were calculated using the following formulas: DSN = TP/(TP + FN) × 100, DSP = TN/(TN + FP) × 100 and Accuracy = (TP + TN)/total number of serum samples tested × 100, where TP, FN, TN and FP represented true-positives, false-negatives, true-negatives and false-positives, respectively.
To detect the specificity of the nsp5-ELISA, positive sera against AIV, NDV and IBDV were tested according to the nsp5-ELISA procedure. Each sample was tested in duplicate, and the S/P ratios were calculated.
For further validation of this assay, 126 of the above 660 serum samples were randomly selected and tested by both the nsp5-ELISA and a commercial ELISA kit (IDEXX, USA). The DSN, DSP and accuracy of the nsp5-ELISA compared with the commercial ELISA kit for the 126 serum samples were calculated using the above-mentioned formula. The discrepant samples were further confirmed by IFA.
2.10. Reproducibility experiments
Evaluation of assay reproducibility within and between runs was performed as described by Jacobson (Jacobson, 1998). Fifteen field serum samples (10 IFA positive samples, 5 IFA negative samples) were selected for the reproducibility experiments. For intra-assay (within-plate) reproducibility, three replicates of each serum sample were analyzed within the same plate. For inter-assay (between-run) reproducibility, three replicates of each sample were run in different plates. The mean S/P ratio, standard deviation (SD) and coefficient of variation (CV) were calculated.
2.11. Detection of infected and vaccinated serum samples by nsp5-ELISA
Sera from the SC021202-infected, attenuated H52 and inactivated M41 vaccinated groups were tested for IBV antibody detection with nsp5-ELISA under optimized conditions and compared to the detection with the commercial ELISA kit (IDEXX, USA).
3. Results
3.1. Identification of recombinant nsp5
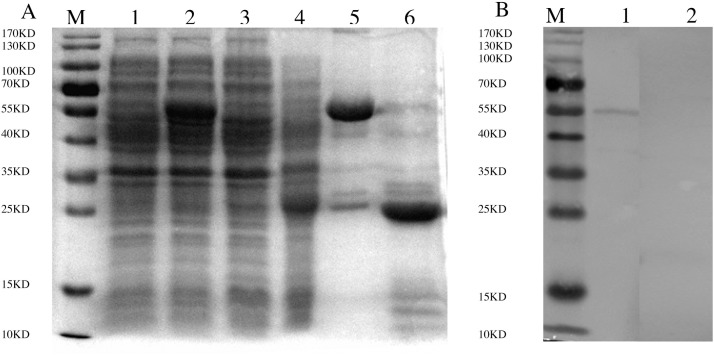
The successful construction of recombinant pGEX-4T-1-nsp5 plasmid was confirmed by PCR and sequence analysis, and this recombinant plasmid was then transformed and expressed in E. coli. The recombinant nsp5 protein was expressed in a soluble form and purified by affinity chromatography. As shown in Fig. 1 A, recombinant GST-fused nsp5 were eluted from a GSTrap FF affinity column with several degraded bands including GST; only the 60 kDa-rGST-nsp5 protein reacted strongly with chicken anti-IBV serum in Western blot analysis, whereas the degraded product including the GST did not react with anti-IBV serum (Fig. 1B). The data show that the recombinant IBV nsp5 fused with GST was purified and the recombinant protein has a good immune reaction with a specific antibody to IBV.
Fig. 1.
(A) Expression and purification of recombinant GST-fused nsp5 analyzed by SDS-PAGE. M, standard protein marker; lane 1, uninduced E.coli transformed with PGEX-4T-1/nsp5; lane 2, induced E.coli transformed with PGEX-4T-1/nsp5; lane 3, uninduced E.coli transformed with PGEX-4T-1; lane 4, induced E. coli transformed with PGEX-4T-1; lane 5, purified GST-nsp5 protein; lane 6, purified GST. (B) Western blotting analysis of purified GST-nsp5. Positive serum to IBV SC021202 were used as the primary antibody. M, standard protein marker; lane 1, purified GST-nsp5; lane 2, GST.
3.2. Optimization of the nsp5-ELISA procedure
In the checkerboard ELISAs, the optimal concentration of nsp5 antigen and the dilution for serum samples were set at 3.64 μg/ml and 1:200, respectively (Fig. 2 a and b), based on the standard that the OD450 value of positive serum was close to 1.5 and the OD450 ratio between positive and negative sera (P/N value) was highest with the lowest background. Using the same standard, the optimal dilution of the conjugate was defined as 1:1000 (Fig. 2c). After the above-mentioned conditions were fixed, the coating buffer was optimized. It was found that the use of PBS as a coating buffer gave the highest P/N value compared with other coating buffers (Fig. 2d). PBS containing 10% newborn bovine serum (NBS) and PBS containing 5% skim milk and 0.05% Tween-20 were optimized as the blocking buffer and diluent for serum samples, respectively (Fig. 2e and f). The optimal exposure times for serum samples and the conjugate was determined to be 60 min and 45 min, respectively; these times showed that the highest P/N values in nsp5-ELISA (Fig. 2g and h). Finally, the best reaction time for chromogenic substrate was defined as 10 min (data not shown).
Fig. 2.
Optimization of ELISA working conditions. (a) Concentration of coating antigen. (b) Dilution of serum sample. (c) Dilution of conjugates. (d) Coating buffers. H2O, distilled water; NS, physiological saline (0.9% NaCl); PBS, 0.01 M phosphate-buffered saline pH 7.4; TB, 0.05 M Tris–HCl buffer, pH 8.5; CB, 0.05 M carbonate–bicarbonate buffer pH 9.6; NaOH, 0.1 M NaOH solution, pH 13. (e) Blocking buffers (PBS dissolved). SM1, 2% skim milk; SM2, 5% skim milk; BSA1, 0.1% BSA; BSA2, 0.5% BSA; BSA3, 1% BSA; NBS1, 5% new-born calf serum; NBS2, 10% new-born calf serum. (f) Serum diluent buffer (PBS dissolved). PBST1, 0.05% Tween-20; PBST2, 0.1% Tween-20; BSA, 0.1% BSA; BSAT, 0.1% BSA and 0.05% Tween-20; SM, 5% skim milk; SMT, 5% skim milk and 0.05% Tween-20. (g) Exposure time of serum sample. (h)Conjugate exposure time. The vertical dotted lines indicate selected condition. Ab, antibody/serum sample.
3.3. Determination of the nsp5-ELISA cut-off
Based on the ROC analysis of the nsp5-ELISA, the S/P ratios of IFA-negative serum samples and IFA-positive serum samples varied from a minimum of −0.386 to a maximum of 0.131 and from a minimum of 0.0696 to a maximum of 2.731, respectively, as defined by IFA (Fig. 3 ). A cut-off S/P ratio of 0.12 for nsp5-ELISA was found to give an optimal result with a DSN of 93.1%, a DSP of 95.4% and an accuracy of 93.3% compared with the results from other cut-offs (Table 1 ). Thus, samples with S/P ratios less than or equal to 0.12 were considered negative, and those with ratios of more than 0.12 were considered positive.
Fig. 3.
Frequency plots of the number of IFA positive (n = 595) and negative (n = 65) samples of field sera and the natural log of S/P ratios obtained from the nsp5 ELISA demonstrating two overlapping populations. The number in parentheses is the S/P ratio. The negative–positive cut-off was defined as 0.12 with an OD450 value of a positive serum control close to 1.5 for the nsp5 ELISA, at which the DSN and DSP of the assay were greater than 90%.
Table 1.
Evaluation of the nsp5-ELISA with selected cut-offs.a
| S/P | False positive | False negative | DSN | DSP | Accuracy |
|---|---|---|---|---|---|
| 0.12 | 41 | 3 | 93.1% | 95.4% | 93.3% |
| 0.11 | 31 | 10 | 94.8% | 84.6% | 93.8% |
| 0.10 | 20 | 15 | 96.6% | 76.9% | 94.7% |
Detection with a total of 660 samples. DSN, diagnostic sensitivity, DSP, diagnostic specificity.
3.4. Evaluation of the nsp5-ELISA
The specificity of the nsp5-ELISA was evaluated by testing the reactivity of antibodies against AIV, NDV and IBDV, respectively with selected cut-offs. The S/P ratios of standard positive sera against AIV, NDV and IBDV are shown in Table 2 . The S/P ratios of all antisera to the above mentioned viruses were significantly less than the cut-off value of 0.12. These data revealed that there was no cross-reactivity between the IBV GST-fused nsp5 antigen and antibodies against AIV, NDV, and IBDV, indicating that the recombinant GST-fused nsp5 antigen is specific for IBV antibody.
Table 2.
Specificity of the nsp5 ELISA to antisera against AIV, NDV and IBDV.a
| Antisera | OD450 value(mean ± SD) | S/P ratio value(mean ± SD) |
|---|---|---|
| AIV | 0.132 ± 0.003 | 0.005318 ± 0.0017 |
| NDV | 0.155 ± 0.002 | 0.01861 ± 0.0013 |
| IBDV | 0.135 ± 0.004 | 0.006795 ± 0.0021 |
Detection with nsp5-ELISA in optimized condition with a cut-off of 0.12.
With a cut-off of 0.12, out of 660 serum samples, 557 and 103 serum samples were classified as positive and negative, respectively, by nsp5-ELISA, while 595 and 65 were classified as positive and negative, respectively, when the same samples were analyzed by the reference, IFA. Nsp5-ELISA showed 93.3% accuracy compared to the IFA in the 660 serum samples (Table 3 ).
Table 3.
Comparison of the nsp5 ELISA with IFA for 660 field serum samples.
| nsp5 ELISA results a | IFA results |
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 554 | 3 | 557 |
| Negative | 41 | 62 | 103 |
| Total | 595 | 65 | 660 |
The negative–positive cut-off was 0.12. By calculation, the DSN, DSP and accuracy of the nsp5 ELISA was 93.11%, 95.38% and 93.33%, respectively, compared with IFA.
To further evaluate nsp5 ELISA, we compared it with the commercial IBV ELISA kit in the analysis of 126 field sera. The results are shown in Table 4 . Of the 126 samples, 105 samples were positive for antibodies to IBV, and 21 were negative by nsp5-ELISA at a cut-off of 0.12. By the IDEXX kit, 106 out of the 126 samples were positive and 20 samples were negative. There were 104 positive samples and 19 negative samples that were judged by both methods (Table 4). Compared to the IDEXX ELISA kit, the DSN, DSP and accuracy of the nsp5-ELISA were 98.11%, 95.00% and 97.62%, respectively (Table 4). The three discrepant samples with both ELISAs were further confirmed by IFA. The results showed that the two positive samples as determined by the IDEXX ELISA kit were both negative by IFA, while the one sample determined as negative by the IDEXX ELISA kit was positive by IFA. These results indicated that, in the case of the three samples, the results by nsp5-ELISA were similar to those by IFA rather than those by IDEXX kit.
Table 4.
Comparison of nsp5-ELISA and the commercial IBV ELISA kit (IDEXX, USA) with 126 field serum samples.a
| nsp5 ELISA results | IDEXX IBV kit results |
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 104 | 1 | 105 |
| Negative | 2 | 19 | 21 |
| Total | 106 | 20 | 126 |
The negative–positive cut-off was 0.12. By calculation, the DSN, DSP and accuracy of the nsp5 ELISA was 98.11%, 95.0% and 97.62%, respectively compared with the IBV IDEXX ELISA kit.
3.5. Reproducibility of nsp5 ELISA
The reproducibility of the test was determined by comparing S/P ratios of each field serum sample in several tests. The intra-assay CV of 10 positive and 5 negative serum samples tested ranged from 3% to 12% (Table 5 ), whereas the inter-assay CV of these serum samples ranged from 7% to 16% (Table 6 ). These data showed that the nsp5-ELISA was reproducible and yielded a low and acceptable variation.
Table 5.
The coefficients of variation of positive sera within the same run.a
| Number of antisera |
Ib (S/P value) |
IIb (S/P value) |
IIIb (S/P value) |
X | SD | CV (%) |
|---|---|---|---|---|---|---|
| 1 | 0.944 | 1.181 | 0.970 | 1.032 | 0.130 | 12.62 |
| 2 | 0.624 | 0.766 | 0.668 | 0.686 | 0.073 | 10.62 |
| 3 | 0.254 | 0.320 | 0.307 | 0.294 | 0.035 | 11.93 |
| 4 | 0.479 | 0.523 | 0.562 | 0.521 | 0.041 | 7.94 |
| 5 | 0.451 | 0.472 | 0.507 | 0.477 | 0.028 | 5.91 |
| 6 | 0.299 | 0.328 | 0.371 | 0.333 | 0.036 | 10.86 |
| 7 | 0.398 | 0.364 | 0.424 | 0.395 | 0.030 | 7.14 |
| 8 | 0.449 | 0.506 | 0.396 | 0.451 | 0.055 | 12.22 |
| 9 | 0.479 | 0.438 | 0.396 | 0.438 | 0.042 | 9.51 |
| 10 | 0.404 | 0.427 | 0.404 | 0.412 | 0.013 | 3.27 |
| 11 | 0.099 | 0.085 | 0.098 | 0.094 | 0.008 | 8.70 |
| 12 | 0.052 | 0.048 | 0.042 | 0.047 | 0.005 | 1 0.91 |
| 13 | 0.094 | 0.077 | 0.082 | 0.084 | 0.009 | 10.83 |
| 14 | 0.049 | 0.050 | 0.052 | 0.050 | 0.002 | 2.99 |
| 15 | 0.051 | 0.044 | 0.044 | 0.046 | 0.004 | 8.84 |
The negative–positive cut-off was 0.12.
Three repeated experiments with different batches of antigens.
Table 6.
The coefficients of variation of positive and negative sera between runs.a
| Number of antisera |
Ib (S/P value) |
IIb (S/P value) |
IIIb (S/P value) |
X | SD | CV (%) |
|---|---|---|---|---|---|---|
| 1 | 1.132 | 0.944 | 0.852 | 0.976 | 0.143 | 14.63 |
| 2 | 0.910 | 0.958 | 0.790 | 0.886 | 0.086 | 9.78 |
| 3 | 0.290 | 0.254 | 0.280 | 0.275 | 0.019 | 6.82 |
| 4 | 0.504 | 0.479 | 0.398 | 0.460 | 0.055 | 11.97 |
| 5 | 0.522 | 0.451 | 0.394 | 0.455 | 0.064 | 14.09 |
| 6 | 0.339 | 0.299 | 0.265 | 0.301 | 0.037 | 12.24 |
| 7 | 0.366 | 0.348 | 0.284 | 0.333 | 0.043 | 13.02 |
| 8 | 0.491 | 0.449 | 0.425 | 0.455 | 0.033 | 7.33 |
| 9 | 0.563 | 0.479 | 0.530 | 0.524 | 0.042 | 7.99 |
| 10 | 0.416 | 0.404 | 0.531 | 0.451 | 0.070 | 15.53 |
| 11 | 0.072 | 0.099 | 0.097 | 0.089 | 0.015 | 16.71 |
| 12 | 0.060 | 0.055 | 0.069 | 0.061 | 0.007 | 11.88 |
| 13 | 0.098 | 0.094 | 0.084 | 0.092 | 0.008 | 8.31 |
| 14 | 0.088 | 0.075 | 0.099 | 0.088 | 0.012 | 14.07 |
| 15 | 0.073 | 0.059 | 0.063 | 0.065 | 0.007 | 11.51 |
The negative–positive cut-off was 0.12.
Three repeated experiments with different batches of antigens.
3.6. Time course of IBV nsp5 antibody level following IBV infection and vaccination
To further evaluate the application of the nsp5-ELISA, one-month-old SPF chickens were infected with the IBV SC021202 strain or inoculated with M41 or H52 vaccines. Sera from infected or vaccinated chickens on different days post infection/vaccination were collected and tested for specific antibody by nsp5-ELISA. The results were also compared with those from the IDEXX ELISA kit. As shown in Fig. 4 A, antibodies to nsp5/IBV in SC021202-infected chickens can be detected at 6 days post inoculation by both assays. The antibodies then dramatically increased and reached a maximal level 12 days post infection. Although only one infected chicken survived 21 days post infection, nsp5/IBV antibodies of the remaining chicken remained at a high level until 154 days post infection. In contrast, seroconversion in the inactivated M41 (Fig. 4B) and attenuated H52 (Fig. 4C) vaccinated groups began at 9 and 12 days post inoculation, respectively, which was delayed compared to those in the SC021202 infected group. In the inactivated M41 immunized group, antibody levels declined below the IBV IDEXX cut-off at 95 days and 99 days (nsp5) post inoculation, while in the attenuated H52 immunized group, the corresponding antibody levels declined below the respective cut-off 135 days (IBV/IDEXX) and 140 days (nsp5) post infection (Fig. 4C). The data showed that a time course of the IBV nsp5 antibody level following IBV infection and vaccination, which were detected by the nsp5-ELISA, is consistent with those of IBV antibodies detected by the commercial ELISA kit and that the nsp5 antibody levels decreased earlier in the inactivated M41 immunized group than in the attenuated H52 and SC021202 infected groups.
Fig. 4.
Comparative detection of antibodies to IBV in SC021202 infected (A), M41 inactivated vaccine (B) and H52 attenuated vaccine (C) immunized chickens by nsp5 ELISA and IDEXX IBV ELISA. The blue dotted lines indicate the cut-off of nsp5 ELISA; the black dotted lines indicate the cut-off of IDEXX IBV ELISA. In the IBV SC021202 infected group, 21 days post infection, only one chicken survived; therefore, there is no standard deviation indicated after 21 d.p.i. in A. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
ELISA has been widely used in IBV serological profiling for its characteristics of simplicity, rapidness, sensitivity and being suitable for large-scale use. In previous established ELISA assays, the whole virion or recombinant subunits of structural proteins were used as the antigens for IBV antibody detection (Bronzoni et al., 2005, Lin et al., 2012, Gibertoni et al., 2005). In this study, we first developed a nonstructural protein nsp5-based ELISA for IBV antibody detection.
Nsp5 is the main protease (Mpro) of Coronavirus, also called 3C-like proteinase (3CLpro), and it lyses ORF-1ab-encoding polyprotein into 12 nonstructural proteins (nsp5-nsp16) (Anand et al., 2003). The mutation of the Coronavirus nsp5 enzyme’s key active sites led to a loss of nsp5 enzymatic activity and impacted viral rescue (Stobart et al., 2012). Thus, the nsp5 of SARS-CoV is considered a drug target for anti-virus therapy. Recently, we found that nsp5 was clearly expressed in IBV-infected DF-1 cells at the beginning of infection, which was evidenced with both mRNA and protein levels analyzed by real-time PCR and IFA, respectively. However, the expression decreased dramatically after 24 h post infection (data not shown), suggesting that IBV nsp5 might be important for the early infection of IBV to host cells. Recombinant GST-fused nsp5 reacted with antiserum to IBV in a Western blot analysis (Fig. 1B) in this study, and antiserum to nsp5 reacted with a different tropism of IBV, including nephropathogenic SC01202, Mass-type H120 and proventriculitis-associated ZJ971 infected DF-1 cells (data not shown). The above findings indicate that IBV nsp5 is important for virus infection, and it has good immunogenicity and serological-reactivity, which enabled the use of this nsp5 as an antigen to detect IBV antibody.
For the establishment of the nsp5-ELISA, we first optimized the nsp5-ELISA and determined its cut-off as 0.12 with IFA as reference method. With the cut-off of 0.12, the nsp5-ELISA showed quite high sensitivity (93.1%), specificity (95.4%) and accuracy (93.3%) in the antibody detection of the samples compared with those of the detections with other cut-offs (Table 1). When compared to the reference IFA and the commercial IBV ELISA kit, the nsp5-ELISA showed 93.1% sensitivity, 95.4% specificity, 93.3% accuracy with IFA (Table 3), and 98.1% sensitivity, 95.0% specificity, 97.6% accuracy with IDEXX ELISA kit (Table 4), respectively, suggesting that nsp5-ELISA is a reliable assay for the detection of IBV antibody.
To further confirm the potential application of nsp5-ELISA in IBV antibody detection, we analyzed the time course of the IBV nsp5 antibody level following IBV infection and vaccination and also compared the results with those from the IDEXX IBV ELISA kit. In an IBV SC021202-infected group, antibodies to nsp5 of IBV in infected chickens could be detected as early as 6 days after infection (Fig. 4A), while nsp5 antibody was positive on 9 and 12 days post inoculation in the inactivated M41 (Fig. 4B) and attenuated H52 (Fig. 4C) vaccinated group, respectively, indicating that the virulent isolate SC021202 elicited nsp5 antibody more easily in the host, in contrast to the M41 and H52 vaccines. Nsp5/IBV antibodies remained at high levels for at least 154 days in the SC021202-infected group (Fig. 4A). In the H52 vaccinated groups, the antibody levels gradually decreased below the cut-off 135 days (IBV-IDEXX) and 140 days (nsp5) post vaccination. In the inactivated M41 vaccinated group, nsp5/IBV antibodies gradually decreased approximately 77 days post inoculation and became negative 95–98 days post vaccination. The above data suggest that the nsp5 antibody in IBV infected/vaccinated chickens is consistent with IBV antibody. Furthermore, in virulent and attenuated IBV infected chickens, the nsp5/IBV positive antibodies persisted longer than those in inactive vaccinated chickens. Therefore, in this case, nsp5 might not be a very suitable factor for differential detection between infection and attenuated IBV vaccination. However, the nsp5-ELISA could detect IBV specific antibody 6 days post infection in a virulent IBV infected group earlier than the detection in attenuated and inactivated vaccination groups, suggesting that nsp5-ELISA is suitable to detect the early infection of virulent IBV.
As was previously documented, most serological studies including the IDEXX ELISA kit use viral particles of IBV as an antigen for detection of IBV infection. However, preparation of purified virions for use as an ELISA coating antigen is time-consuming and expensive, while recombinant protein expressed in vitro has more advantages as serodiagnostic antigen that it allow better standardization of the tests, reduce the costs of production and purification, and is easy to prepare with large amount (Lugovskaya et al., 2006). In this study, we used GST-fused nsp5 expressed by E. coli as the coating antigen to establish an nsp5-ELISA to detect IBV antibody. The nsp5-ELISA was proved to be sensitive and specific for IBV antibody detection. To exclude the interference of GST tag, we compared the data obtained by the nsp5-ELISA (GST-fused nsp5 as a coating antigen) with the data obtained after removing the GST background in a parallel experiment (GST as ELISA coating antigen). The results showed that there is no significant difference between the two sets of data (data not shown), indicating that the GST-fused nsp5 is as efficient as the coating antigen for the nsp5-ELISA. Additionally, homology analysis reveals that the nsp5 protein of IBV strain SC021202 used in this study is 89.1%-99.7% homologous to different serotypes of reference strains (data not shown). Therefore, the nsp5-ELISA has potential to detect antibodies induced by IBV of different serotypes.
Generally, nonstructural proteins are present in the infected cells or tissues but not inside the virions. After immunization with inactivated vaccine, the host is expected to produce minimal or no antibodies to nonstructural proteins. In this study, we detected relatively high levels of antibody to IBV nsp5 in the inactivated M41 vaccinated group, which might be due to virions of the vaccine that may not be completely purified, but further confirmation is needed.
In summary, we have developed an ELISA with IBV nsp5 that is equally as sensitive as the commercial ELISA kit that uses the whole IBV virion as coating antigen. The nsp5-ELISA has the potential to serve as a rapid, reliable and cost-effective method for IBV antibody detection. This report describes the first study to develop an nsp-based ELISA to detect specific antibody to IBV.
Competing interests
No conflicts of interest are declared.
Acknowledgments
This work was supported by National Program on Key Research Project of China (2016YFD0500800), National Natural Science Foundation of China (31302104) and China Agriculture Research System (CARS-41-K11).
Contributor Information
Jing Lei, Email: milly0923@126.com.
Tingting Shi, Email: 2512463349@qq.com.
Dongnan Sun, Email: 446422431@qq.com.
Kaikun Mo, Email: 1505187337@qq.com.
Yan Yan, Email: yyan1512@163.com.
Yulan Jin, Email: jinyulan@zju.edu.cn.
Min Liao, Email: liaomin4545@zju.edu.cn.
Jiyong Zhou, Email: jyzhou@zju.edu.cn.
References
- Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- Bronzoni R.V., Fatima M., Montassier S., Pereira G.T., Gama N.M., Sakai V., Montassier H.J. Detection of infectious bronchitis virus and specific anti- viral antibodies using a Concanavalin A-Sandwich-ELISA. Viral Immunol. 2005;18:569–578. doi: 10.1089/vim.2005.18.569. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K. Keynote Speakers; 2016. Abstracts, Progranne and Show Guide of 19th World Veterinary Poultry Association Congress; pp. 65–69. [Google Scholar]
- Crowther J.R. The ELISA Guidebook 149 (III–IV) 2000. Methods in molecular biology; pp. 1–413. [DOI] [PubMed] [Google Scholar]
- de Wit J.J., Cook J.K., Van der Heijden H.M. Infectious bronchitis virus variants: a review of the history, current situatuion and control measures. Avian Pathol. 2011;29:71–93. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnekave E., Shilo H., Gelman B., Klement E. The longevity of anti NSP antibodies and the sensitivity of a 3ABC ELISA – A 3 years follow up of repeatedly vaccinated dairy cattle infected by foot and mouth disease virus. Vet. Microbiol. 2015;178:14–18. doi: 10.1016/j.vetmic.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Fellahi S., Harrak M.E., Kuhn J.H., Sebbar G., Bouaiti el A., Khataby K., Fihri O.F., Houadfi M.E., Ennaji M.M. Comparison of SYBR green I real-time RT-PCR with conventional agarose gel-based RT-PCR for the diagnosis of infectious bronchitis virus infection in chickens in Morocco. BMC Res. Notes. 2016;9:231. doi: 10.1186/s13104-016-2037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibertoni A.M., Maria de Fátima S.M., Sena J.A., Givisiez P.E., Furuyama C.R., Montassier H.J. Development and application of a Saccharomyces cerevisiae-expressed nucleocapsid protein-based enzyme-linked immunosorbent assay for detection of antibodies against infectious bronchitis virus. J. Clin. Microbiol. 2005;43:1982–1984. doi: 10.1128/JCM.43.4.1982-1984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson R.H. Validation of serological assays for diagnosis of infectious diseases. Rev. Sci. Technol. 1998;17:469–526. doi: 10.20506/rst.17.2.1119. [DOI] [PubMed] [Google Scholar]
- Lin K.H., Lin C.F., Chiou S.S., Hsu A.P., Lee M.S., Chang C.C., Chang T.J., Shien J.H., Hsu W.L. Application of purified recombinant antigenic spike fragments to the diagnosis of avian infectious bronchitis virus infection. Appl. Microbiol. Biotechnol. 2012;95:233–242. doi: 10.1007/s00253-012-4143-8. [DOI] [PubMed] [Google Scholar]
- Lugovskaya N.N., Scherbakov A.V., Yakovleva A.S., Tsyvanyuk M.A., Mudrak N.S., Drygin V.V., Borisov A.V. Detection of antibodies to avian infectious bronchitis virus by a recombinant nucleocapsid protein-basedenzyme-linked immunosorbent assay. J. Virol. Methods. 2006;135:292–296. doi: 10.1016/j.jviromet.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.T., Kwon H.J., Kim I.H., Hong S.M., Seong W.J., Jang J.W., Kim J.H. Multiplex nested RT-PCR for detecting avian influenza virus, infectious bronchitis virus and Newcastle disease virus. J. Virol. Methods. 2013;188:41–46. doi: 10.1016/j.jviromet.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Reddy V.R., Theuns S., Roukaerts I.D., Zeller M., Matthijnssens J., Nauwynck H.J. Genetic characterization of the belgian nephropathogenic infectious bronchitis virus (NIBV) reference strain B1648. Viruses. 2015;7:4488–4506. doi: 10.3390/v7082827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D.W. Molecular Cloning: A Laboratory Manual. Third ed. 2001. SDS-polyacrylamide gel electrophoresis of proteins, staining SDS-polyacrylamide gels and immunoblotting; pp. A840–A4854. [Google Scholar]
- Stobart C.C., Lee A.S., Lu X., Denison M.R. Temperature-sensitive mutants and revertants in the coronavirus nonstructural protein 5 protease (3CLpro) define residues involved in long-distance communication and regulation of protease activity. J. Virol. 2012;86:4801–4810. doi: 10.1128/JVI.06754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.Y., Zhang D.Y., Ye J.X., Cheng L.Q. Characterization of an avian infectious bronchitis virus isolated in China from chickens with nephritis. J. Vet. Med. B Infect. Dis. Vet. Public. Health. 2004;51:147–152. doi: 10.1111/j.1439-0450.2004.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]