Highlights
-
•
We established a method for simultaneous detection of ten viruses that infect ducks.
-
•
The method was based on multiplex PCR and MALDI-TOF MS.
-
•
The detection limits of the proposed method were 1.3–7.8 copies/μl for ten specific viruses.
-
•
The method was specific and sensitive for simultaneous detection.
Keywords: Duck, Viruses, Simultaneous detection, MALDI-TOF, Mass spectrometry
Abstract
Rapid screening of infectious viral diseases is the key to ensure healthy development of duck livestock industry. Currently routine viral detection methods are primarily used to detect up to 3 viruses. In this study, matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) was used for simultaneous detection and genotyping of ten viruses in duck, including Duck hepatitis A virus 1 (DHAV-1), DHAV-3, Duck astrovirus 1 (DAstV-1), DAstV-2, Duck reovirus 1 (DRV-1), DRV-2, Tembusu virus (TMUV), Avian influenza virus (AIV), Goose parvovirus (GPV) and Duck enteritis virus (DEV). The low detection limits of this proposed method for ten duck viruses ranged from 1.3 copies/μl to 7.8 copies/μl. The novel detection method with high sensitivity, good specificity and high throughput has the potential to be applied for disease diagnosis and surveillance.
1. Introduction
Duck viral diseases have posed substantial risks to duck industry worldwide. Classical duck viral diseases include duck viral enteritis (DVE), duck viral hepatitis (DVH), and reovirus infection in Muscovy ducks. Since 1996, various novel viral diseases have emerged in ducks, such as avian influenza (Guo et al., 1999), reovirus related Muscovy duck new liver disease and duck spleen necrosis disease (Chen et al., 2009; Hu et al., 2000; Liu et al., 2011), Tembusu virus (TMUV) related egg drops in ducks (Su et al., 2011), and goose parvovirus (GPV) related short beak and dwarfism syndrome (SBDS) in Pekin ducks (Ning et al., 2017). Among the diseases, DVH can be caused by three duck hepatitis A virus (DHAV) genotypes (Ding and Zhang, 2007; Fu et al., 2008) and two duck astroviruses (DAstVs) (Fu et al., 2009; Liu et al., 2014), originally called duck hepatitis virus type 2 (DHV-2) and duck hepatitis virus type 3 (DHV-3); duck reovirus (DRV) of 2 genotypes infect ducks (Ma et al., 2012; Wang et al., 2013).
In recent years, many efforts have been made to establish new molecular and serological diagnostic methods for detection of DHAV (Fu et al., 2008; Huang et al., 2012; Liu et al., 2010; Shen et al., 2015), DAstV (Liu et al., 2014), AIV (Yang et al., 2016), DRV (Yun et al., 2015), TMUV (Tang et al., 2012), GPV (Yang et al., 2017) and DEV (Plummer et al., 1998). Although some of them could identify multiple targets, cross reactivity greatly limited their capability for simultaneous diagnosis of these main infectious viruses. Shen et al (2015) reported that the VP3-DHAV-1 based ELISA method could detect antisera to both DHAV-1and DHAV-3, indicating the presence of cross reactivity. Besides, some multiple detection methods for pathogenic viruses in ducks were established, such as a multiplex PCR method for simultaneous detection of six duck viruses with a lower detection limit of 102 copies/μl (Wang et al., 2017) and a GenomeLab Gene Expression Profiler (GeXP) analyzer-based multiplex PCR assay for simultaneous detection of eleven duck viruses with a lower detection limit of 103 copies/μl (Zhang et al., 2015). Currently available techniques for virus detection, including viral isolation on embryos and cell cultures, molecular diagnostic methods (such as polymerase chain reaction, PCR), and serological diagnostic methods (such as enzyme-linked immunosorbent assay, ELISA), either are time-consuming, or require complex nucleic acid extraction, or have insufficient sensitivity.
Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) has been used to detect single nucleotide polymorphism (SNP) since 1990s (Haff and Smirnov, 1997; Ross et al., 1998). Recently, a multiplex PCR and MS based detection method has been reported in clinical virology and demonstrated as a promising method for high-throughput diagnostic or genotyping applications (Singhal et al., 2015). This method includes three procedures, multiplex PCR for amplification of target nucleic acids, single base extension for the mass probe and MS for identification of the products. This method has been successfully used in diagnosis of various viral infections, like foot and mouth disease (Peng et al., 2013b), human herpesvirus infection (Sjoholm et al., 2008) human papilloma virus infection (Peng et al., 2013a), and coronavirus infection (Xiu et al., 2017). They have shown their merits of high throughput, multiple-target detection and become one of the promising biomolecular techniques.
In this study, we report the development of a multiplex PCR and MALDI-TOF MS based method that is capable of simultaneously detecting 10 duck viruses, including DHAV-1, DHAV-3, DAstV-1, DAstV-2, DRV-1, DRV-2, TMUV, AIV, GPV, and DEV.
2. Materials and methods
2.1. Viral strains
The target duck-origin pathogens, including DHAV-1 isolate C80 (DQ864514) (Ding and Zhang, 2007), DHAV-3 isolate C-GY (EU352805) (Fu et al., 2008), DAstV-1 isolate D17 (MN149392) (Wang, 2015), DAstV-2 isolate SL4 (KF753806) (Liu et al., 2014), DRV-1 isolate 815-12 (KC508656) (Wang et al., 2013), DRV-2 isolate 091(JX478256) (Ma et al., 2012), TMUV isolate PS (KT876991) (Liang et al., 2016), GPV isolate JS1(KT935531) (Ning et al., 2017), and DEV (Jiang, 2011) were obtained from the College of Veterinary Medicine, China Agricultural University. The AIV subtype H9N2 (GQ373128) (Sun et al., 2010) was provided by Avian Influenza Lab at China Agricultural University. All these viruses were identified using RT-PCR/PCR prior to test. The non-target duck-origin pathogens, including E. coli, Salmonella and Riemerella anatipestifer were also obtained from the College of Veterinary Medicine, China Agricultural University.
2.2. Primer design
The VP1 gene of DHAV and GPV, the ORF2 gene of DAstV, the S4 segment of DRV-1, the S1 segment of DRV-2, the NS5 gene of TMUV, the M gene of AIV, and the UL6 gene of DEV were chosen to design to design the detection method. β-actin was chosen as an internal control. Primers used for plasmid construction (see Table S1 in the supplemental material) and virus detection (listed in Table 1 ) were designed using primer-blast website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome). For each target gene, sequences were downloaded from GenBank database and aligned by CLUSTALW (https://www.genome.jp/tools-bin/clustalw). The conserved regions were chosen to design detection primers. The Accession No. and the reference of each virus strain which was used for primer design could be found in Table S2. All the primers were synthesized and purified by Sangon Biotech (Shanghai, China).
Table 1.
Primers used in the simultaneous detection of 10 duck viruses using MALDI-TOF mass spectrometry.
| Virus | Targ et gene | PCR |
Mass Probe extension |
||||
|---|---|---|---|---|---|---|---|
| Forward primer sequence | Reverse primer sequence | Mass Probe sequence | Mass probe mass (Da) | Extension call | Extended Mass Probe mass (Da) | ||
| β-actina | – | ACGTTGGATGCAGCAGATGTGGATCAGCAAG | ACGTTGGATGCARGGGTGTGGGTGTTGGTAA | CCTCCATTGTCC | 3532.4 | A-β-actin | 3803.6 |
| AIV | M | ACGTTGGATGCTATCRTYCCRTCAGGCCCC | ACGTTGGATGGTCAGAGGTGACARGATTGGT | AAGCCGAGATCG | 3679.4 | C-AIV | 3926.6 |
| TMUV | NS5 | ACGTTGGATGCGCAATGGACCCAGGTATGA | ACGTTGGATGCGACCATTCCCCAGTCAGTC | AGAGCTGTGAGCT | 4014.6 | G-TMUV | 4327.8 |
| DHAV-1 | VP1 | ACGTTGGATGGGCCAACTCGACCAATTCCT | ACGTTGGATGGCTGGYTTYTTGAGACCCAT | CTGTTCATGTGGAC | 4269.8 | T-DHAV-1 | 4558 |
| GPV | VP1 | ACGTTGGATGGTACAAGACGGAGGAGCCAC | ACGTTGGATGATTRCCCACTCCATCGGCACC | CTGTGGCAGCATCTG | 4584 | A-GPV | 4855.2 |
| DRV-1 | S4 | ACGTTGGATGGGTBSACATGGTGTTTGACG | ACGTTGGATGCTCGGRRGCAACYGGTATCC | AGCTTCTCTCTGTCGA | 4823.2 | C-DRV-1 | 5070.4 |
| DAstV-2 | ORF2 | ACGTTGGATGCTTGTTTGGGCAGCCCTTTG | ACGTTGGATGCATCGATTGTGCGCTGTTGT | AATGGGCACGACAACA | 4908.2 | A-DAstV-2 | 5179.4 |
| DAstV-1 | ORF2 | ACGTTGGATGGCCGAGTAGGATCGAGGGTA | ACGTTGGATGTGGTGATTCCAYATGGTCGG | TCCCTAAGCAAAATTGA | 5162.4 | C-DAstV-1 | 5409.6 |
| DRV-2 | S1 | ACGTTGGATGTGTATTCACTATTCCGCCAG | ACGTTGGATGGCTATGTCAGCCATAAAGGA | TGCCATTGACGTTGCTAT | 5480.6 | G-DRV-2 | 5793.8 |
| DEV | UL6 | ACGTTGGATGTTCGGCTGCTACTTGCCTAC | ACGTTGGATGCGGCATTTACACGATACGGC | TAGCTCTGTCGTTACCACA | 5738.8 | T-DEV | 6027 |
| DHAV-3 | VP1 | ACGTTGGATGCTGACCRGGGTCAYGCATCT | ACGTTGGATGGTGCAAYCATRCADGGTGTT | TCTGGAGAAGTGATTCTGA | 5882.8 | C-DHAV-3 | 6130 |
The internal control.
2.3. Recombinant plasmid construction
The viral DNA/RNA were extracted using TIANamp Virus DNA/RNA Kit (Tiagen Biotech, Beijing, China) according to the manufacturer’s instruction. First, 1 μl of the extracted DNA/RNA was mixed with 10 pmol random primer N6 (TaKaRa, Dalian, China), and incubated at 70 °C for 5 min and then at 4 °C for 5 min. Then the mixture containing M-MLV reverse transcriptase (Promega, Madison, USA), 5 × Buffer, dNTPs, and Recombinant Ribonuclease Inhibitor (TaKaRa, Dalian, China) was added, the reaction mixture was incubated at 42 °C for 60 min and then at 94 °C for 5 min. For PCR section, 5 μl of the RT product was used to amplify target genes. 50 μl of PCR mixture contained 20 pmol of pair of primers, dNTPs, 10 μl of 5 × SF Buffer, 1 μl of Phanta Super-Fidelity DNA Polymerase (Vazyme Biotech Co., Ltd, Nanjing, China). PCR was performed at 94 °C for 5 min, followed by 40 cycles of at 94 °C for 30 s, at 55 °C for 30 s and at 72 °C for 45 s, and a final extension at 72 °C for 10 min. The target amplicons were purified by EasyPure Quick Gel Extraction Kit (TransGen Biotech, Beijing, China), and the purified targets were linked to Blunt TOPO vectors (Taihe Biotech, Beijing, China). Recombinant plasmids containing target gene of DHAV-1, DHAV-3, DAstV-1, DAstV-2, DRV-1, DRV-2, TMUV, AIV, GPV, and DEV were extracted using EasyPure Plasmid MiniPrep Kit (TransGen Biotech, Beijing, China). The concentration of plasmids was confirmed using NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, USA) to calculate the copies of each plasmid.
2.4. Method establishment and parameter optimization
Recombinant plasmids were used for method establishment. For each target, 2 μl of the plasmid was mixed with 2 μl of 2.5 × PCR mix (Intelligene Biosystems, Qingdao, China), 1 μl of PCR primers (0.5 μM each), and 1 μl ddH2O. PCR was performed at 95 °C for 15 min, followed by 35 cycles of 95 °C for 15 s, 56 °C for 30 s and 72 °C for 30 s, and a final extension at 72 °C for 10 min. After amplification, shrimp alkaline phosphatase (SAP) was used to digest excess dNTPs at 37 °C for 30 min, and then the SAP was inactivated at 85 °C for 5 min. The mass probe extension was performed using the Mass Probe Extension Kit (Intelligene Biosystems). Briefly, the reaction mixture was incubated with 1 μl of 10 × MPE buffer, 0.3 μl of MPE enzyme, 0.7 μl of MPE Termix, 1 μl of mass probe. The reaction procedure was 95 °C for 10 s, followed by 40 cycles of 95 °C for 30 s, 37 °C for 60 s and 72 °C for 60 s, and a final extension at 72 °C for 3 min. The products were desalted using Resin for 30 min, and centrifuged at 5000×gfor 2 min. The Focus Plate was pretreated with 1 μl of Nucleotide Matrix. 0.5 μl of each desalting product was mixed with 0.5 μl of Nucleotide Matrix, and the mixture was spotted on the surface of pretreated Focus Plate. Data acquisition and analysis were performed using Quan SNP software v 2.0 (Intelligene Biosystems).
Besides, to detect 10 viruses and the internal control simultaneously, 11 pairs of PCR primers were mixed to amplify the target genes of 10 viruses and the internal control, and the mixture of 11 mass probes were used to extend single base. The mixed plasmids of 10 viruses and internal control (104 copies/μl) were used as the template to optimize the concentration of mass probe.
2.5. Performance evaluation and clinical sample test
To evaluate the sensitivity of this proposed method, 10-fold serial dilutions of mixed plasmids (1010–100 copies/μl) were detected at the optimized experimental conditions to confirm the low detection limit. Ten clinical samples, including the duck livers containing DHAV-1, DHAV-3, DAstV-1, DRV-1, DRV-2, TMUV, GPV and DEV, the intestines containing DAstV-2, and the allantoic fluid containing the AIV subtype H9N2, were used to test the cross reactivity. These samples were confirmed with the presence of only one genotype virus in each sample using RT-PCR/PCR. The nucleotide extraction and reverse transcription were performed as described above. Besides, some bacteria, such as E. coli, Salmonella, and Riemerella anatipestifer, were used as the non-target pathogens to verify the specificity. The empty plasmids (Blunt TOPO vectors) were used as another internal control for the proposed method. Deionized water was used as the blank control.
Besides, to further verify the applicability of this proposed method for simultaneous detection, the clinical samples used for specificity test were artificially mixed for simulation of co-infection. Four types of mixture with different viruses were used to test this proposed method. Sample No. 1 (a mixture of DRV-1 and DRV-2) was used to simulate the identification of “White spot disease in Muscovy ducks”, “New liver disease in Muscovy”, and “Peking duck spleen necrosis disease”. Sample No. 2 (a mixture of DHAV-1, DHAV-3, DAstV-1, and DAstV-2) was used to simulate the sample of duck viral hepatitis. Sample No. 3 (a mixture of AIV, DRV-1, DEV, and GPV) was used to simulate the infection sample of both DNA and RNA viruses. Sample No. 4 was a mixture of all these ten viruses. Besides, we collected another 10 fecal swabs of newly born ducklings for viruses screening. The nucleotide extraction, reverse transcription, PCR, SAP digestion, and mass probe extension were performed as described above in the optimal conditions.
3. Results
3.1. MS-based method establishment
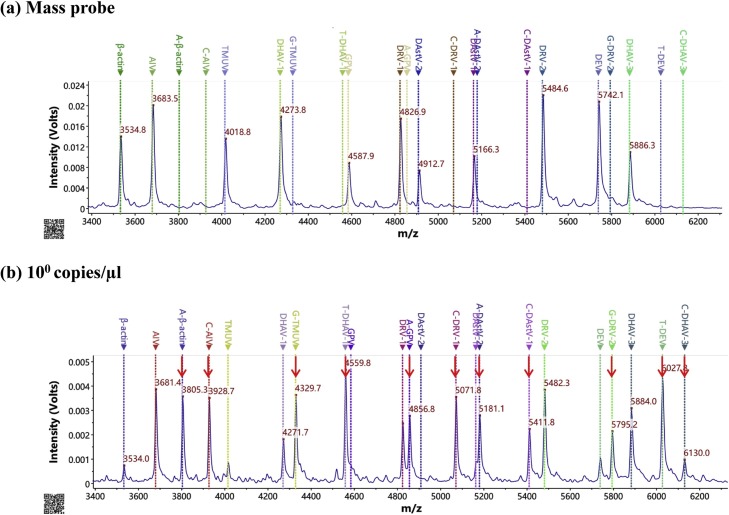
All the recombinant plasmids were constructed with a concentration of 1010 copies/μl. In this study, we first developed the MS-based method using the plasmid to detect DHAV-1, DHAV-3, DAstV-1, DAstV-2, DRV-1, DRV-2, TMUV, AIV, GPV, and DEV, respectively. As shown in Fig. S1, the lines marked as the virus name indicate the mass probe (such as DHAV-1), and the lines marked as the extended nucleotide-virus name indicate the extended mass probe (such as T-DHAV-1). When there is a peak at the extended mass probe line, it means the detection result is positive. All the 10 viruses and the internal control could be detected. Then, the 11-plex method was established to simultaneously detect the recombinant plasmids of 10 duck-origin pathogenic viruses and β-actin (Fig. 1 b). To achieve the best amplification signal of the MS-based reaction, the concentrations of mass probe were investigated. Based on the mass and the amplification efficiency, the concentrations were optimized. The optimization concentration of each extension primer in the primer mixture was 6.80 μM (β-actin), 6.00 μM (AIV), 7.20 μM (TMUV), 13.60 μM (DHAV-1), 6.00 μM (GPV), 10.00 μM (DRV-1), 6.80 μM (DAstV-2), 7.20 μM (DAstV-1), 15.60 μM (DRV-2), 12.00 μM (DEV), and 21.60 μM (DHAV-3), respectively (Fig. 1a).
Fig. 1.
Representative MALDI-TOF mass spectra of the mixed plasmids using the MS-based method. (a) primer; (b) 100 copies/μl. In the mass spectra, the marker lines noted with DHAV-1, DHAV-3, DAstV-1, DAstV-2, DRV-1, DRV-2, TMUV, AIV, GPV, DEV, and β-actin represent the mass probe and the marker lines noted with T-DHAV-1, C-DHAV-3, C-DAstV-1, A-DAstV-2, C-DRV-1, G-DRV-2, G-TMUV, C-AIV, A-GPV, T-DEV, and A-β-actin represent the extended mass probe in Table 1, respectively. The x-axis (m/z) represents the mass (Daltons) of extension primer and the y-axis represents the intensity (Volts). The red arrow indicates the positive target. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.2. Sensitivity and specificity evaluation
The recombinant plasmids of 1010–100 copies/μl were used to evaluate the sensitivity of the method proposed in this study. As shown in Fig. 1b, when the concentration of plasmids was 100 copies/μl, all the targets could be detected simultaneously. Based on the sensitivity test, the limit of detection (LOD) of the MS method is 1.5 copies for β-actin, 1.7 copies for AIV, 3.9 copies for TMUV, 4.0 copies for DHAV-1, 3.4 copies for GPV, 1.3 copies for DRV-1, 2.1 copies for DAstV-2, 1.3 copies for DAstV-1, 3.3 copies for DRV-2, 7.8 copies for DEV, and 7.3 copies for DHAV-3, respectively.
To evaluate the specificity of this MS-based method for the detection of 10 pathogenic viruses, the tests were carried out to detect virus separately at the optimal conditions. As shown in Fig. S2a–j, there were only two peaks for the specific viruses (T-DHAV-1, C-DHAV-3, C-DAstV-1, A-DAstV-2, C-DRV-1, G-DRV-2, G-TMUV, C-AIV, A-GPV, and T-DEV, respectively) and the internal control (A-β-actin), respectively. Furthermore, the specificity of this proposed method was also tested using non-target pathogens and the empty plasmids (Blunt TOPO vectors). Under the same optimal conditions, the controls yielded negative results for all the 10 viruses (Fig. S2k–n). The experimental results of specificity indicated that the method could accurately identify the target viruses without cross reactivity.
3.3. Clinical and simulated co-infection samples test
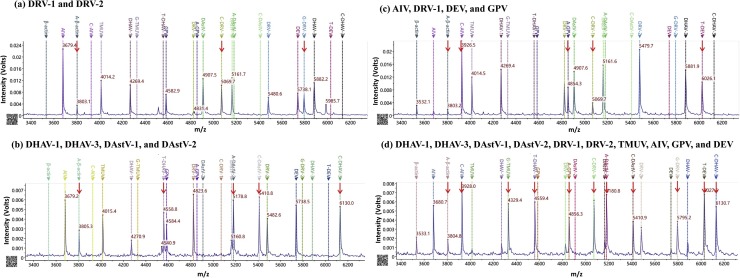
Ten clinical samples were collected and detected for these 10 viruses using RT-PCR/PCR. As shown in Fig. S2a–j, these 10 clinical samples were also detected using the proposed method, and the results were consistent with RT-PCR/PCR, verifying the validity and applicability of the MS-based method. For 4 simulated co-infection samples, as shown in Fig. 2 a, there were peaks at the position of C-DRV-1, G-DRV-2, and A-β-actin, indicating DRV-1 and DRV-2 were detected in sample No. 1. As shown in Fig. 2b, there were peaks at the position of C-DHAV-1, C-DHAV-3, C-DAstV-1, A-DAstV-2, and A-β-actin, indicating DHAV-1, DHAV-3, DAstV-1, and DAstV-2 were detected in sample No. 2. As shown in Fig. 2c, there were peaks at the position of C-AIV, C-DRV-1, T-DEV, A-GPV, and A-β-actin, indicating AIV, DRV-1, DEV, and GPV were detected in the sample No. 3. There were peaks at all of position of the extended mass probe in Fig. 2d, indicating DHAV-1, DHAV-3, DAstV-1, DAstV-2, DRV-1, DRV-2, TMUV, AIV, GPV, and DEV were simultaneously detected in sample No. 4. Besides, 10 fecal samples of newly born ducklings were negative for all viruses.
Fig. 2.
MALDI-TOF mass spectra of four simulated co-infection clinical samples. (a): The mass spectrum of sample No.1, indicating it is positive for DRV-1 and DRV-2; (b): The mass spectrum of sample No.2, indicating it is positive for DHAV-1, DHAV-3, DAstV-1, and DAst-2; (c): The mass spectrum of sample No.3, indicating it is positive for AIV, DRV-1, DEV, and GPV; (d): The mass spectrum of sample No.4, indicating it is positive for DHAV-1, DHAV-3, DAstV-1, DAstV-2, DRV-1, DRV-2, TMUV, AIV, GPV, and DEV. The x-axis (m/z) represents the mass (Daltons) of extension primer and the y-axis represents the intensity (Volts). The red arrow indicates the positive target. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
4. Discussion
In this study, we developed a MALDI-TOF MS based method for simultaneous detection of 10 pathogenic duck-origin viruses. Under the optimized reaction conditions, the proposed multiplex PCR and MS-based detection method showed no cross-reactions with each other and the non-target bacteria, indicating the developed MS-based method had a good specificity. The sensitivity test indicated that the lower detection limit of this method was determined as low as 100 copies/μl for the 11 recombinant plasmids, which was more sensitive and easier-to-read than the noted multiplex method (Wang et al., 2017; Zhang et al., 2015).
The VP1 region of DHAV and GPV, the ORF2 region of DAstV, the S4 segment of DRV-1, the S1 segment of DRV-2, the NS5 region of TMUV, the M gene of AIV, and the UL6 gene of DEV were chosen for target genes. To make the method more universal, the conserved regions of the target genes were chosen for primer and mass probe design. As recorded, DHAV-1, DHAV-3, DAstV-1, DAstV-2, DRV-1, DRV-2, TMUV, and AIV are RNA viruses, whereas GPV and DEV are DNA viruses (King, 2012). Therefore, we selected the TIANamp Virus DNA/RNA Kit for nucleic acid extraction and used M-MLV reverse transcriptase which has no impact on DNA for reverse transcription process in case of co-infection of DNA and RNA viruses.
The main merit of the proposed method is high throughput. This MS-based method could detect up to 30 targets in one reaction at the same time, and the maximum 384 samples could be analyzed within a single experiment (Peng et al., 2013a), which greatly improve the detection efficiency. On one hand, it could be used for diagnosis during the period of disease outbreaks. On the other hand, it could also be used for routine screening of pathogenic viruses in faces on the farm’s ground, eggs, and newly-born ducklings, providing an alternative diagnostic method for disease surveillance. Waterfowl are the main reservoir of various viruses, and several various detection panels could be customized according to the sequence of popular strains. The method could be very useful for large-scale epidemiological research studies in investigation of different viruses which is of interest. As co-infection clinical samples were not available for this study, the mock samples were prepared for simulation of clinical co-infection. Four types of mixtures were used to evaluate this method. As expected, the target pathogenic viruses could be identified accurately in all the samples, including RNA and DNA viruses.
In summary, a multiplex PCR and MALDI-TOF MS based method was developed to accurately detect 10 duck-origin pathogenic viruses simultaneously. The proposed MS-based method has shown its merits of good specificity, high sensitivity, and high throughput. Besides, this proposed method could be customized for different panels depending on the customers’ needs, making. it suitable for virus diagnosis and monitoring.
Declaration of Competing Interest
All authors have declared no conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31802219). The authors would like to thank Prof. Jinhua Liu at China Agricultural University for providing the H9N2 Avian influenza virus.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jviromet.2019.113723.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Chen S., Chen S., Lin F., Jiang B., Wang S., Cheng X., Zhu X., Zhang S., Li Z., Cheng Y. The primary study of pathogen of duck hemorrhagic-necrotic hepatitis. Chinese Agricultural Science Bulletin. 2009;25:28–31. [Google Scholar]
- Ding C., Zhang D. Molecular analysis of duck hepatitis virus type 1. Virology. 2007;361:9–17. doi: 10.1016/j.virol.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Fu Y., Pan M., Wang X., Xu Y., Xie X., Knowles N.J., Yang H., Zhang D. Complete sequence of a duck astrovirus associated with fatal hepatitis in ducklings. J. Gen. Virol. 2009;90:1104–1108. doi: 10.1099/vir.0.008599-0. [DOI] [PubMed] [Google Scholar]
- Fu Y., Pan M., Wang X., Xu Y., Yang H., Zhang D. Molecular detection and typing of duck hepatitis A virus directly from clinical specimens. Vet. Microbiol. 2008;131:247–257. doi: 10.1016/j.vetmic.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Guo Y.J., Wang M., Guo J.F., Dong J., Zhang Y. The complete nucleotide sequences of A/Goose/Guangdong/2/96 (H5N1) virus RNA segment 1-3 and 5. Chin J Exp Clin Virol. 1999;13:205–244. [PubMed] [Google Scholar]
- Haff L.A., Smirnov I.P. Single-nucleotide polymorphism identification assays using a thermostable DNA polymerase and delayed extraction MALDI-TOF mass spectrometry. Genome Res. 1997;7:378–388. doi: 10.1101/gr.7.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Chen S., Jiang B., Cheng X., Lin T., Li Y., Cheng Y. Discovery of the pathogen of Muscovy duck liver white spots disease. Fujian Journal of Animal Husbandry and Veteribary Medicine. 2000;22:1–3. [Google Scholar]
- Huang Q., Yue H., Zhang B., Nie P., Tang C. Development of a real-time quantitative PCR for detecting duck hepatitis a virus genotype C. J. Clin. Microbiol. 2012;50:3318–3323. doi: 10.1128/JCM.01080-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T.T. China Agricultural University; Beijing, China: 2011. Detection and Analysis of Pathogens Associated With Three Waterfowl Diseases. [Google Scholar]
- King A., Adams M.J., Carstens E.B., Lefkowitz E.J. Elsevier Academic Press Inc; San Diego: 2012. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. [Google Scholar]
- Liang T., Liu X., Cui S., Qu S., Wang D., Liu N., Wang F., Ning K., Zhang B., Zhang D. Generation of a reliable full-length cDNA of infectiousTembusu virus using a PCR-based protocol. Virus Res. 2016;213:255–259. doi: 10.1016/j.virusres.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Liu M., Zhang T., Zhang Y., Meng F., Li X., Hou Z., Feng X., Kong X. Development and evaluation of a VP1-ELISA for detection of antibodies to duck hepatitis type 1 virus. J. Virol. Methods. 2010;169:66–69. doi: 10.1016/j.jviromet.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Liu N., Wang F., Shi J., Zheng L., Wang X., Zhang D. Molecular characterization of a duck hepatitis virus 3-like astrovirus. Vet. Microbiol. 2014;170:39–47. doi: 10.1016/j.vetmic.2014.01.026. [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhang G., Huang Y., Ren G., Chen L., Gao J., Zhang D., Han B., Su W., Zhao J., Hu X., Su J. Isolation and characterization of a reovirus causing spleen necrosis in Pekin ducklings. Vet. Microbiol. 2011;148:200–206. doi: 10.1016/j.vetmic.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Ma G., Wang D., Shi J., Jiang T., Yuan Y., Zhang D. Complete genomic sequence of a reovirus isolate from Pekin ducklings in China. J. Virol. 2012;86:13137. doi: 10.1128/JVI.02512-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning K., Liang T., Wang M., Dong Y., Qu S., Zhang D. Genetic detection and characterization of goose parvovirus: implications for epidemiology and pathogenicity in Cherry Valley Pekin ducks. Infect. Genet. Evol. 2017;51:101–103. doi: 10.1016/j.meegid.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Peng J.P., Gao L., Guo J.H., Wang T., Wang L., Yao Q., Zhu H.J., Jin Q. Type-specific detection of 30 oncogenic human papillomaviruses by genotyping both E6 and L1 genes. J. Clin. Microbiol. 2013;51:402–408. doi: 10.1128/JCM.01170-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J.P., Yang F., Xiong Z.H., Guo J.H., Du J., Hu Y.F., Jin Q. Sensitive and rapid detection of viruses associated with hand foot and mouth disease using multiplexed MALDI-TOF analysis. J. Clin. Virol. 2013;56:170–174. doi: 10.1016/j.jcv.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Plummer P.J., Alefantis T., Kaplan S., O’Connell P., Shawky S., Schat K.A. Detection of duck enteritis virus by polymerase chain reaction. Avian Dis. 1998;42:554–564. [PubMed] [Google Scholar]
- Ross P., Hall L., Smirnov I., Haff L. High level multiplex genotyping by MALDI-TOF mass spectrometry. Nat. Biotechnol. 1998;16:1347–1351. doi: 10.1038/4328. [DOI] [PubMed] [Google Scholar]
- Shen Y., Cheng A., Wang M., Chen S., Jia R., Zhu D., Liu M., Sun K., Yang Q., Chen X. Development of an indirect ELISA method based on the VP3 protein of duck hepatitis A virus type 1 (DHAV-1) for dual detection of DHAV-1 and DHAV-3 antibodies. J. Virol. Methods. 2015;225:30–34. doi: 10.1016/j.jviromet.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Singhal N., Kumar M., Kanaujia P.K., Virdi J.S. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015:6. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoholm M.I.L., Dillner J., Carlson J. Multiplex detection of human herpesviruses from archival specimens by using matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2008;46:540–545. doi: 10.1128/JCM.01565-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Li S., Hu X., Yu X., Wang Y., Liu P., Lu X., Zhang G., Hu X., Liu D., Li X., Su W., Lu H., Mok N.S., Wang P., Wang M., Tian K., Gao G.F. Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS One. 2011;6:e18106. doi: 10.1371/journal.pone.0018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Pu J., Jiang Z., Guan T., Xia Y., Xu Q., Liu L., Ma B., Tian F., Brown E.G., Liu J. Genotypic evolution and antigenic drift of H9N2 influenza viruses in China from 1994 to 2008. Vet. Microbiol. 2010;146:215–225. doi: 10.1016/j.vetmic.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Tang Y., Diao Y., Yu C., Gao X., Chen L., Zhang D. Rapid detection of Tembusu virus by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP) Transbound. Emerg. Dis. 2012;59:208–213. doi: 10.1111/j.1865-1682.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- Wang D., Shi J., Yuan Y., Zheng L., Zhang D. Complete sequence of a reovirus associated with necrotic focus formation in the liver and spleen of Muscovy ducklings. Vet. Microbiol. 2013;166:109–122. doi: 10.1016/j.vetmic.2013.05.022. [DOI] [PubMed] [Google Scholar]
- Wang X.Y. China Agricultural University; Beijing, China: 2015. Molecular Identification and Biological Characterization of Newly Discovered Viruses Associated With Egg Drop of Ducks. [Google Scholar]
- Wang Y.J., Zhu S.Y., Hong W.M., Wang A.P., Zuo W.Y. A multiplex PCR for detection of six viruses in ducks. J. Virol. Methods. 2017;248:172–176. doi: 10.1016/j.jviromet.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Xiu L.S., Zhang C., Wu Z.Q., Peng J.P. Establishment and application of a universal coronavirus screening method using MALDI-TOF mass spectrometry. Front. Microbiol. 2017:8. doi: 10.3389/fmicb.2017.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Chen H., Wang Z., Yu X., Niu X., Tang Y., Diao Y. Development of a quantitative loop-mediated isothermal amplification assay for the rapid detection of novel goose parvovirus. Front. Microbiol. 2017;8:2472. doi: 10.3389/fmicb.2017.02472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Dai X., Chen H., Teng Q., Li X., Rong G., Yan L., Liu Q., Li Z. Development of blocking ELISA for detection of antibodies against H9N2 avian influenza viruses. J. Virol. Methods. 2016;229:40–47. doi: 10.1016/j.jviromet.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Yun T., Chen H., Yu B., Zhang C., Chen L., Ni Z., Hua J., Ye W. Development and application of an indirect ELISA for the detection of antibodies to novel duck reovirus. J. Virol. Methods. 2015;220:55–59. doi: 10.1016/j.jviromet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Zhang Y.F., Xie Z.X., Xie L.J., Deng X.W., Xie Z.Q., Luo S.S., Huang L., Huang J.L., Zeng T.T. GeXP analyzer-based multiplex reverse-transcription PCR assay for the simultaneous detection and differentiation of eleven duck viruses. BMC Microbiol. 2015;15:247. doi: 10.1186/s12866-015-0590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.