Abstract
Currently, no approved antiviral therapeutic is available for treatment or prevention of Ebola virus (EBOV) infection. In this study, we characterized an EBOV-glycoprotein (GP) pseudotyped HIV-1-based vector system in different cell cultures, including human umbilical vein endothelial cells (HUVECs) and human macrophages, for the screening of anti-EBOV-GP agent(s). Based on this system, we demonstrated that an aqueous extract (CHPV) from the Chinese herb Prunella vulgaris displayed a potent inhibitory effect on EBOV-GP pseudotyped virus (EBOV-GP-V)-mediated infection in various cell lines, including HUVEC and macrophage. In addition, our results indicated that CHPV was able to block an eGFP-expressing Zaire ebola virus (eGFP-ZEBOV) infection in VeroE6 cells. The anti-EBOV activity of CHPV was exhibited in a dose-dependent manner. At a 12.5 μg/ml concentration, the CHPV showed a greater than 80% inhibition of EBOV-GP-V and eGFP-EBOV infections. Likewise, our studies suggested that the inhibitory effect of CHPV occurred by binding directly to EBOV-GP-Vs and blocking the early viral events. Interestingly, our results have shown that CHPV was able to enhance the anti-EBOV activity of the monoclonal antibody MAb 2G4 against EBOV-GP. Overall, this study provides evidence that CHPV has anti-EBOV activity and may be developed as a novel antiviral approach against EBOV infection.
Keywords: Ebola virus (EBOV), Glycoprotein (GP), EBOV-GP pseudovirions, Chinese herb Prunella vulgaris, Monoclonal antibody MAb
Highlights
-
•
We characterized the infection of an EBOV-GP pseudotyped HIV-based vector in human endothelial cells and macrophages.
-
•
We identified an aqueous extract of Prunella vulgaris which displayed a potent inhibition of EBOV-GP mediated viral entry.
-
•
The inhibitory effect of Chinese herb P. vulgaris (CHPV) is through a directly binding to EBOV-GP.
-
•
The presence of CHPV enhances antiviral activity of a monoclonal antibody MAb against EBOV-GP.
-
•
This study provides the evidence that CHPV has potential to be developed as a new anti- EBOV agent.
1. Introduction
Ebola virus (EBOV) is an enveloped, negative-strand RNA virus belonging to the Filoviridae family that causes severe hemorrhagic fevers in humans and non-human primates, with case fatality rates as high as 90% (Wilson et al., 2001). EBOV was first identified in 1976, and since then over 20 reported natural Ebola virus outbreaks have occurred, with the 2014 Ebola outbreak in West Africa being the largest, causing over 11,000 deaths. Currently, there is still no effective treatment or vaccine for EBOV infection in humans; therefore, the development of new antiviral strategies is an urgent global health need.
The EBOV glycoprotein (GP), which comprises a surface unit GP1 and a transmembrane unit GP2, is an essential component of the viral envelope and plays a central role in viral entry (Misasi and Sullivan, 2014). EBOV GP1 is required for cell attachment and receptor interaction, while GP2 is required for membrane fusion. The EBOV-GP provides a potential target for therapeutic strategies to disrupt EBOV-GP-mediated virus entry and block the propagation of EBOV in vivo.
Given that filoviruses have a surface glycoprotein similar to other enveloped viruses and share a similar mechanism of entry with HIV-1, several well-defined compounds have been suggested for the potential inhibition of entry of filoviruses, i.e., lectins, glucosidase inhibitors, benzylpiperazine adamantine diamides, LJ-001, dUY11, selective estrogen receptor modulator (SERMS) and ion channel blockers (i.e., amiodarone and dronedarone) (Barton et al., 2014, Côté et al., 2011, Chang et al., 2013, Gehring et al., 2014, Johansen et al., 2013, Vincent et al., 2010, Wolf et al., 2010). However, most of these compounds have been proposed as antivirals based on in vitro or animal data, and investigations into their effectiveness and safety in humans are still required. Recently, a novel immunotherapeutic (ZMapp) consisting of a combination of monoclonal antibodies (c13C6, c2G4 and c4G7) and optimized from two previous antibody cocktails (MB-003 and ZMAb) was shown to be able to rescue 100% of rhesus macaques when treatment is initiated up to 5 days post–challenge (Murin et al., 2014, Pettitt et al., 2013, Qiu et al., 2014, Zhang et al., 2014). The monoclonal antibodies target the GP1-GP2 interface and the glycan cap, thereby inhibiting viral entry (Audet et al., 2014, Qiu et al., 2011, Qiu et al., 2012a). During the 2014 EBOV outbreak in Western Africa, ZMapp was administered to seven patients with Ebola virus disease (EVD) in the United States and several other countries, but the outcome was not considered to be statistically significant (McCarthy, 2014). Moreover, very little of the ZMapp antibodies is currently available (Lenny Bernstein, 2014). Thus, the lack of ZMapp and unavailability of experimental treatment in the most affected regions of the EBOV outbreak in Western Africa hinder its development in source-limited countries.
Traditional Chinese medicine holds a unique position among all traditional medicines because of its many years of history. Many aqueous extracts of traditional Chinese medicinal herbs have been proven to have antiviral activities (Zheng, 1990), and most of these are generally of low toxicity, cheap and readily accessible. Some Chinese herbs, including Prunella vulgaris, L. Panaxginseng C.A. Meyer, Andrographis paniculata, Lentinus edodes and Spirulina platensis, display inhibitory effects on the entry step of various viruses, such as HIV-1, Hepatitis B and HSV-1 (Chang et al., 1991, Fiore et al., 2008, HARADA, 2005, Hayashi et al., 1996a, Hayashi et al., 1996b, Hayashi, 2008, Kang et al., 2013, Lee et al., 2014, Li et al., 2001, Liu et al., 2002, Sato et al., 1996, Seubsasana et al., 2011, Tabba et al., 1989, Yao et al., 1992, Yoshida et al., 1988, Zhang et al., 2007). However, whether these herbs could also have inhibitory activities against EBOV infection remains unknown. In this study, we developed a sensitive EBOV-GP pseudotyped HIV-1-based vector system for screening anti-EBOV agent(s) and then investigated the antiviral mechanism of action. Based on this system, we identified an aqueous extract from the Chinese herb P. vulgaris (CHPV), which was able to inhibit EBOV-GP-V and eGFP-Ebola virus infections by binding to EBOV-GP and blocking viral entry. Interestingly, our results also showed that CHPV was able to enhance the anti-EBOV activity of the monoclonal antibody MAb 2G4 against EBOV-GP. Thus, this study provides evidence for the first time that CHPV, an aqueous extract from P. vulgaris, has potent anti-EBOV activity.
2. Materials and methods
2.1. Plasmid constructs
The EBOV-GP plasmid (pCAGGS-ZEBOV-GP) containing the gene of EBOV glycoprotein (GP) from the Mayinga strain was generated by cloning the full length of GP1,2 (nucleotides 142–2172; amino acid (aa) 1 to 676) into the eukaryotic expression vector pCAGGS (Wahl-Jensen et al., 2005). The Lentiviral vector encoding for Gaussia luciferase gene (pLenti-Basic-Gluc) was purchase from Target system Inc. The helper packaging plasmid pCMVΔ8.2 encoding for the HIV Gag-Pol, vesicular stomatitis virus G (VSV-G), and HIV-Envelope plasmids were described previously (Jayappa et al., 2015, Kobinger et al., 2001, Yao et al., 1998).
2.2. Cell culture, antibodies and chemicals
The human cervical epithelial cell (HeLa), TZM-b1 cells, human lung carcinoma cell (A549), human embryonic kidney cells (HEK293T), and kidney epithelial cells extracted from African green monkey (VeroE6) were cultured in Dulbecco's modified Eagle's medium. Two CD4+ T-lymphoid cell lines, Jurkat and C8166 T cells, were cultured in RPMI-1640 medium. All cell lines were supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin, in the exception of the VeroE6 cell line, which was cultured with 3% FBS. Human Umbilical Vein Endothelial cells (HUVECs) were cultured in EGM-2 Media (Lonza) containing FBS, hydrocortisone, hFGF-B, VEGF, R3-IGF-1, ascorbic acid, hEGF, GA-1000 and heparin. HUVECs Cells were cultured for 3–4 days and were passaged for no more than 7 generations for the experiments described. Human PBMC derived macrophages were prepared by dispensing fresh PBMCs into 24-well plates at 37 °C for 2 h. After gentle washing with DMEM, the adherent cells were cultured in DMEM containing 20% FBS and 10 ng/ml macrophage colony-stimulating factor (M-CSF; R&D systems) for 7 days. DNA transfection in HEK293T cells was performed with a standard calcium phosphate precipitation method.
The mouse monoclonal antibody (2G4) against EBOV glycoprotein and anti-HIVp24 monoclonal antibody were described previously (Ao et al., 2007, Qiu et al., 2011). The HIV-1 p24 ELISA Kit was obtained from the AIDS Vaccine Program of the Frederick Cancer Research and Development Center.
2.3. Preparation and purification of herb extracts
Ginsenoside, Spirulina polysaccharide, Lentinan and Diammonium glycyrrhizinate (DG) were obtained from Chinese companies (En Bang Biotech Co., Yuchang Biotech Inc., Luye Pharma Group Ltd. or China Tai-Tianqing Pharmaceutical Ltd.). Andrographolide was purchased from Sigma Inc. The dried fruitspikes of P. vulgaris L. (Labiatae) were purchased from Tong Ren Tang Health Pharmaceutical Co., Ltd. (Beijing, CN). The herb extraction process was based on methods described previously (Yao et al., 1992). Briefly, dried herbs were soaked overnight in deionized water at room temperature and then boiled for 1.5 h. After centrifugation (3000 g, 30 min), the supernatant was filtered through a 0.45 μm cellulose acetate membrane and lyophilized. The resulting dark brown residue was dissolved in deionized water and stored at −20 °C. A single symmetrical peak corresponding to a molecular weight of polysaccharides (approximately 10 kDa) in the aqueous extract from PV was detected by HPLC analysis, as described previously (Yao et al., 1992).
2.4. Viruses production, infection and inhibition experiments
EBOV-GP pseudovirions (EBOV-GP-Vs) were produced by transfecting HEK293T cells with pCAGGS-ZEBOV-GP (5 μg), pCMVΔ8.2 (7 μg) and pLenti-Basic-Gluc plasmids (3 μg). Meanwhile, VSV-G or HIV Env pseudovirions were produced from 293T cells transfected with VSV-G or HIV Env plasmid together with Δ8.2 and pLenti-Basic-Gluc plasmids. 48 hrs post-transfection, cell culture supernatants were collected and pseudovirions were purified from the supernatant by ultracentrifugation (32,000 rpm) for 2 h. The pelleted viruses were resuspended into DMEM medium and virus titers were quantified using an HIV-1 p24 ELISA assay.
The pseudovirions were quantified by measurement of Viral-associated HIV p24 level using ELISA assay. The same amounts of EBOV-GP-Vs, VSV-G or HIV Env pseudovirions (as adjusted by p24 levels) were incubated with target cells (at 0.1 × 105 per well (96 well plate) or 0.4 × 105 cells per well (24 well plate) for 2–4 h and washed with medium. After 24–72 h, the supernatants were collected and the viral infection was evaluated by measuring Gaussia luciferase (Gluc) activity. Briefly, 50ul of Coelenterazine substrate (Nanolight Technology) was added to 20ul of supernatant, mixed well and read in the luminometer (Promega, USA).
For testing of the anti-EBOV entry activity of Chinese herbs, various concentrations of each herb extract or compound were mixed with EBOV-GP-Vs or directly added into target cells at different time points. After 2–4 h of infection at 37 °C, the cells were washed twice with medium to remove excessive residue viruses and herb extract/compound. The anti-EBOV activity was evaluated by measuring the Gluc activity in the culture supernatant at different time points after infection. Similarly, to analysis the inhibitory effect of MAb 2G4 and CHPV, a series of diluted 2G4 alone or the combination of 2G4 with varying concentrations of CHPV were mixed with EBOV-GP-Vs and added to HEK293T cells or macrophages. After 2 h of incubation, the cells were washed and the Gluc activity in the cell culture supernatants was detected at 24 and 48 h post-infection.
In the macrophage studies, a continuous exposure to CHPV/pseudovirion treatment also was performed. Briefly, CHPV was added 1 h to cells prior to infection, followed by EBOV-GP-Vs infection with or without MAb 2G4. Then, infected cells were cultured in the same medium containing CHPV/EBOV-GP-Vs ± MAb 2G4 without washing. The Gluc activity in the cell culture supernatants was monitored at 24 and 48 h post-infection.
For the Zaire ebola virus (ZEBOV) infection, we utilized a recombinant ZEBOV expressing enhanced green fluorescent protein (eGFP) (named as eGFP-ZEBOV). This virus showed similar phenotypes to that of wild-type ZEBOV (wt-ZEBOV) in vitro, produced mild disease in rhesus macaques, and lethal in mice lacking signal transducer and activator of transcription 1, even that were moderately attenuated, compared with that caused by wt-ZEBOV (Ebihara et al., 2007). The virus stock was preparated, as described previously (Qiu et al., 2012a). To test the inhibitory effect of CHPV on eGFP-ZEBOV infection, we first added different amounts of CHPV or/and MAb 2G4 in VeroE6 cells for 10 min, then the cells were infected with approximately 100 PFU of eGFP-ZEBOV in the presence of CHPV or MAb 2G4. After 12 h of infection, the cells were washed and cultured with fresh medium without any drug. At 48 h of infection, cells were fixed with 10% phosphate-buffered formalin and fluorescent plaques were counted using an AID Fluorescent plate reader, as described previously (Patel et al., 2009).
2.5. Binding assay
For testing the binding of CHPV to EBOV-GP-Vs, EBOV-GP-Vs were mixed with CHPV, MAb 2G4 or medium alone and incubated for 10 min at 4 °C. The treated EBOV-GP-Vs were then collected by ultracentrifugation (Beckman SW41Ti rotor 22,000 rpm, 2 h). The pelleted pseudovirions were re-suspended in fresh DMEM and used to infect HEK293T cells and their infectivity was monitored by measurement of the Gluc activity in the supernatants after 48 h of infection.
To detect the viral attachment to the cells in the presence of CHPV, HEK293T cells in 24 -well plates were first cooled on ice for 15 min and EBOV-GP-Vs (P24 0.51 ng) were added in the absence or presence of indicated concentrations of CHPV. After 1 h incubation on ice, the cells were washed with cold PBS to remove unbound virus, lysed with RIPA lysis buffer and the virus attached to cells were detected with anti-p24 ELISA assay.
2.6. Cytotoxicity assay
The WST-1 cell proliferation assay (Roche) was used to determine the cytotoxicity of CHPV, as described earlier (Ao et al., 2011). Briefly, HUVECs were cultured at 4 × 103 cells/well in a 96-well plate and treated with CHPV for 2 or 48 h. After 48 h, WST-1 was added to the cultures at 10 μl/well and incubated at 37 °C for 4 h. Absorbance was measured at 490 nm using a microplate reader.
3. Results
3.1. Generation of an EBOV-GP pseudotyped HIV-1-based vector system
To generate EBOV-GP pseudovirions (EBOV-GP-Vs), the EBOV-GP-expressing plasmid (pCAGGS-ZEBOV-GP) was co-transfected with an HIV-based lentiviral vector that encoded the Gaussia luciferase gene and a packaging plasmid (pCMVΔR8.2) in HEK293T cells (Fig. 1 A). In parallel, HIV Env+ pseudovirions or viruses without envelope glycoprotein were included as controls. The Gaussia luciferase (Gluc) is a bioluminescent enzyme that can be secreted into the cell culture media, enabling the analysis of viral replication by direct measurement of Gluc activity in the supernatant.
Fig. 1.
Generation of an EBOV-GP-V pseudotyped virus system. (A) Schematic representation of the 3 co-transfected plasmids, including the lentiviral vectors encoding for the Gaussia luciferase (Gluc) gene, the HIV-1 helper construct Δ8.2 and the pCAGGS-ZEBOV-GP plasmid. (B) Western blot showing the GP1, preGP and P24 protein expression in virions and transfected cells. (C) Equal amounts of GP + or GP- virions (adjusted by P24) were used to infect HEK293T cells. After 48 h, the Gaussia Luciferase activity (Gluc) of the supernatants was measured and normalized as a percentage of the positive control (Gluc value of GP + as 100%) (D) Dose-dependent 2G4 (MAb) inhibition of EBOV-GP-V transduction in HEK293T cells. The same amount of EBOV-GP-V was incubated with the indicated concentration of 2G4 for 30 min and added to HEK293T cells for 4 h. The cells were washed and cultured in complete DMEM without antibody. Gluc activities in the supernatant were tested after 48 h of incubation. (E) Evaluating the transduction ability of EBOV-GP-V in different cell types. The results are the mean values ± standard deviations (SD) of three independent experiments.
To examine the expression and incorporation of EBOV-GP in the cells and the virus, both virus-producing cell and virus lysates were analyzed by SDS-PAGE and Western blot with a mouse anti-EBOV-GP1, as indicated in Fig. 1B. The preGP (110 kDa) and GP1 (120–130 kDa) were clearly detected in EBOV-GP-transfected cells (Fig. 1B, lane 1), with molecular masses corresponding to those previously described for EBOV-GP (Elliott et al., 1985, Volchkov et al., 1995, Volchkov et al., 1998). As expected, abundant GP1 was detected in EBOV-GP-Vs, indicating that EBOV-GP can be efficiently incorporated into the pseudovirions (Fig. 1B, lane 3). Meanwhile, the HIV capsid Gagp24 protein was detected in all of the cell lysates and the pelleted EBOV-GP-Vs and virions without envelope protein by rabbit anti-p24 antibodies (Fig. 1B, lower panel).
To test whether the EBOV-GP-V was able to infect HEK293T cells, equal amounts of EBOV-GP-Vs or viral particles without any envelope protein (as adjusted by HIV Gagp24) were incubated with HEK293T cells for 4 h. The Gluc activities in the supernatants were measured to evaluate the virus infectivity at 48 h post-infection. The results indicate that EBOV-GP-Vs were able to efficiently infect 293T cells, whereas viral particles without viral envelope protein did not produce any Gluc activity (Fig. 1C).
To further test whether the pseudovirion infection was mediated by EBOV-GP, a previously described monoclonal antibody (MAb), 2G4, which targets the Ebola virus glycoprotein (Qiu et al., 2011, Qiu et al., 2012b), was used to block the entry of EBOV-GP-V. Briefly, EBOV-GP-Vs were incubated with various dilutions of 2G4 for 30 min and then added onto HEK293T cells. At 48 h post-infection (p.i.), the Gluc activities were measured and the results showed that the EBOV-GP-V infection of HEK293T cells could be efficiently blocked by 2G4 in a dose-dependent manner (Fig. 1D). A 50% inhibition of HIV-1 infection was achieved when 2G4 was used at a concentration of 1.72 μg/mL. These results clearly indicated that EBOV-GP was responsible for the entry of pseudovirions and their infection.
The ability of EBOV-GP-V to infect other cell types was further analyzed in different cell lines, including HEK293T cells, CD4+ TZMb1 cells, human cervical epithelial cells (HeLa), CD4+ T-lymphoid cells (C8166 and Jurkat cells), HUVECs and human lung carcinoma cells (A549) (Fig. 1E). In parallel, the same amount of HIV-Env + pseudovirions (HIV-Env-Vs) and VSV-G pseudovirions (VSV-G–Vs) were used as controls. The Gluc activity was tested in the supernatant at 48 h p.i. As expected, the VSV-G–Vs efficiently infected all target cell lines, whereas HIV-Env-Vs only targeted the CD4+ T cells, including C8166, Jurkat and TZMb1 cells. In contrast, EBOV-GP-V was unable to induce detectable levels of Gluc activity in both the Jurkat and C8166 T-lymphoid cells. In agreement with the previously described EBOV-GP tropism range (Wool-Lewis and Bates, 1998), EBOV-GP-Vs were shown to be able to infect human HeLa cells, TZMb1 cells, human A549 cells and primary HUVECs. These results clearly distinguish the different tropisms of EBOV-GP from HIV and VSV-G envelope proteins.
3.2. Evaluation of different Chinese herbs as potential anti-EBOV-GP agents
The previous study demonstrated that EBOV-GP-Vs can mediate an EBOV-GP-specific viral entry and that viral replication can be easily detected by measuring Gluc activity in the supernatant. By using this system, we next tested the effect of different Chinese herbs on EBOV entry. A number of studies have suggested that the extracts of some Chinese herbs display a significant inhibitory effect on the entry and/or replication of different viruses (Table 1 ). Therefore, in our study, CHPV, ginsenoside, andrographolide, spirulina polysaccharide, lentinan and diammonium glycyrrhizinate extracted from P. vulgaris, as well as Panaxginseng, Andrographis paniculata, Spirulina platensis, Lentinus edodes and Liquorices, were assessed for potential anti-EBOV activity.
Table 1.
Antiviral activities of different extracts or components from Chinese herbs.
| Active extracts | Example of plant source | Antiviral actions | References |
|---|---|---|---|
| Aqueous extract of Prunella vulgaris | The purified bioactive extract from P. vulgaris L. fruitspikes. | Prevention of hiv attachment to CD4 receptors, and suppression of HIV-1 entry by disrupting the gp41 six-helix bundle formation. Inactivation of HSV-1 directly, blocked HSV-1 binding to Vero cells, and inhibited HSV-1 penetration into Vero cells. |
Liu et al., 2002; Tabba et al., 1989, Yao et al., 1992; Zhang et al., 2007 |
| Ginsenoside | Ginsenoside is a class of steroid glycosides, purified from Panaxginseng C.A. Meyer | Ginsenoside has effective anti-hepatitis B virus activity mediated by interrupting virus adsorption on host cell, DNA replication and secretion of hepatitis B surface antigen. |
Kang et al., 2013; Lee et al., 2014; Li et al., 2001 |
| Andrographolide | A major bioactive chemical constituent of Andrographis paniculata | Andrographolide was reported for anti-HSV-1 and anti-hiv activity in vitro. The inhibitory effects the compound on viral entry and replication steps. | Chang et al., 1991, Seubsasana et al., 2011 |
| Spirulina polysaccharide | A natural sulfated polysaccharide, isolated from Spirulina platensis | Spirulina polysaccharide was shown to target viral absorption, penetration stages and some replication stages after penetration into cells. |
Hayashi et al., 1996a, Hayashi, 2008; Hayashi et al., 1996b |
| Lentinan | A polysaccharide isolated from a common edible mushroom, Lentinus edodes | The sulfated lentinan exhibits a potent anti-HIV activity by suppressing viral adsorption to the cells and reverse transcriptase. | Yoshida et al., 1988 |
| Diammonium glycyrrhizinate(DG) | DG was extracted and purified from Liquorices (Radix glycyrrhizae) | Anti-hepatitis B virus by reducing transport to the membrane and sialylation of hepatitis B virus surface antigen. Anti-HIV by reduction of membrane fluidity lending to inhibition of fusion. The antiviral activity against SARS related coronavirus, respiratory syncytial virus, arboviruses, vaccinia virus and vesicular stomatitis virus, also have been revealed in vitro studies. |
Fiore et al., 2008, HARADA, 2005; Sato et al., 1996 |
Our cytotoxicity study showed that none of these extracts had obvious cytotoxic effects on HEK293T cells up to concentrations of 40 μg/mL (data not shown). We therefore used serial dilutions of CHPV from 0.625 μg/mL to 40 μg/ml. After 2 h of infection with EBOV-GP-Vs in the presence of each herb, the infected cells were washed to remove the residual pseudovirions and herb, after which the Gluc activity in the supernatant was measured at 48 h p.i. The results showed that in the presence of andrographolide or spirulina polysaccharide, EBOV-GP-V still replicated efficiently, suggesting that these herbs did not affect the replication of GP pseudotyped virus (Fig. 2 ). In contrast, CHPV showed a strong inhibitory effect on EBOV-GP-V infection, with an approximately 80% inhibition of EBOV-GP-V infection achieved when CHPV was used at 10 μg/mL (Fig. 2). The ginsenoside also displayed a modest antiviral effect at a higher concentration of 40 μg/ml. Thus, we continued to characterize the anti-EBOV activity of CHPV and the mode of its action.
Fig. 2.
Anti-EBOV-GP activity of several extracts of different Chinese herbs. Serial dilutions of CHPV, Ginsenoside, Andrographolide, Spirulina polysaccharide, Lentinan or Diammonium glycyrrhizinate were mixed with the same amount of EBOV-GP-V and immediately added to HEK293T cells in 24-well plates. At 2 h post-infection, the cells were washed and cultured in complete DMEM without herb extract or compound. The Gluc activity in the 48 h supernatants is presented as a percentage of the control activity (%), a ratio of Gluc activity in the presence of herb versus the absence of any herb.
3.3. CHPV interferes with the EBOV-GP-V entry step by acting on virus-like particles
To gain insight into the mechanism of how CHPV inhibits EBOV-GP-V infection, a consistent concentration of CHPV (10 μg/ml) was added to the HEK293T cell culture medium at various time points, including pre-, simultaneous and post-exposure of the cells to the EBOV-GP-pseudovirions. The results in Fig. 3 A show that when cells were pretreated with CHPV for 2 and 4 h and then removed before adding pseudovirions, there was no inhibitory effect on virus infection (Fig. 3A), suggesting that the inhibitory activity of CHPV did not act directly on cells to render them resistant to infection. Interestingly, when the cells were incubated simultaneously with EBOV-GP-V and CHPV, a strong inhibitory effect was achieved, with no negative impact on viral infection when CHPV was added at 2 or more hrs post-infection (Fig. 3A). All of these results revealed that CHPV blocks EBOV-GP-V replication in the early steps of the infection. This also led us to believe that the major inhibitory effect of CHPV may be through its action on the virus itself. As shown in Fig. 3B, EBOV-GP-Vs were first incubated with CHPV (10 μg/mL) for 10 min at 4 °C. The CHPV-treated viruses were pelleted by ultracentrifugation and washed once with media to remove residual unbound CHPV (Fig. 3B). As a control, EBOV-GP-Vs were incubated with the anti-EBOV-GP antibody 2G4 or media alone and were pelleted by ultracentrifugation. All pelleted viruses were used to infect HEK293T cells, and the Gluc activity was measured at 48 h post-infection. Similar to the pseudovirions incubated with 2G4, the pseudovirions pre-treated with CHPV also displayed a significant reduction of infection ability, when compared with the pseudovirions that were incubated with medium alone (Fig. 3C).
Fig. 3.
Characterization of the mechanism of CHPV anti-EBOV action. (A) A time-of-addition experiment was performed with EBOV-GP-V to identify the action target for CHPV. CHPV (10 μg/mL) was added at 6 h and 2 h prior to infection (washed away immediately before adding virus), during infection (0 h), and at 2 h, 4 h, 8 h, 12 h and 24 h post-infection. The mock-infected controls mean uninfected HEK293T cells, and the positive controls (PC) were HEK293T cells were infected with pseudovirions in the absence of CHPV. At 2 h post-infection, all of the cells were washed and cultured in complete DMEM. The Gluc activity was tested from the supernatant after a 48 h incubation, and the data are shown as a percentage of control activity (%), a ratio of Gluc activity in the presence of CHPV versus the absence of CHPV. (B, C) Schematic diagram of the evaluation of infection of EBOV–GP-V-bound CHPV. EBOV-GP-V was incubated for 10 min with CHPV, MAb or DMEM at 4 °C. The treated viruses were further concentrated by ultracentrifugation (100,000 g). The infectivity of the pelleted pseudovirions was tested by adding to HEK293T cells, and the Gluc activity after 48 h was shown as a relative ratio when compared with the virus incubated with DMEM. PC: EBOV-GP-V not treated with drug. (D) The same amount of CHPV was ultracentrifuged. The resulting supernatant and the pellet were incubated with EBOV-GP-V and added to HEK293T cells. The Gluc activity after 48 h is shown as a percentage of control activity (%). The results are the mean values ± standard deviations (SD) of three independent experiments. (E) CHPV blocks the attachment of EBOV-GP-V. The same amount of EBOV-GP-Vs (P24, 0.51 ng) and various concentration of CHPV were added to HEK293T cells on ice for 1 h. The sample without pseudovirions or CHPV treatment was used as a negative control. The cells were then washed with cold PBS to remove unbound virus and lysed by RIPA buffer. The viruses attached to cells were monitored by measuring the HIV p24 antigen levels by anti-p24 ELISA.
To exclude the possibility that the free soluble active constituents of CHPV could be pelleted by ultracentrifugation, rather than being associated with virus, we performed the same ultracentrifugation procedure with CHPV alone. After centrifugation, the supernatant or re-suspended pellet sample was added to HEK293T cells, followed by EBOV-GP-V infection. As shown in Fig. 3D, the CHPV in the supernatant after ultracentrifugation still showed strong antiviral activity compared with the EBOV-GP-Vs infection in the absence of CHPV. In contrast, the pellet portion did not exert any antiviral activity (Fig. 3D), indicating that the soluble active constituents of CHPV could not be pelleted. All of these results suggest that CHPV might be attached to the viral glycoproteins, consequently blocking viral entry. However, we could still not rule out the possibility that the presence of CHPV may also result in conformational changes of EBOV-GP.
To determine if CHPV directly interfered with the viral adsorption process by interaction with the virus, the cell attachment of EBOV GP-V was assessed by measurement of cell-associated pseudovirions by a p24 ELISA assay. Briefly, HEK293T cells were cooled on ice for 15 min and EBOV GP-V, in the presence or absence of CHPV (200, 20, 2 and 0.2 μg/mL), was added. After a 1 h incubation on ice, the cells were washed with cold PBS to remove unbound virus and lysed with RIPA buffer. The cell-bound pseudovirions were measured by detection of the p24 levels in the cell lysate. The results show that as the pseudovirions were incubated continuously with diluted CHPV, the amount of cell-associated p24 increased in a dose-dependent manner (Fig. 3E). At 20 ug/ml, CHPV could block approximately 80% of pseudovirion attachment. This result clearly indicates that CHPV could interfere with virus attachment to cells, consequently preventing virus entry.
3.4. Evaluation of the inhibitory effect of CHPV on EBOV-GP pseudovirion entry in HUVECs and human macrophages
As is well-documented, the dysregulation of endothelial cell functions in EBOV infection can induce a wide range of vascular effects that can cause changes in vascular permeability and hemorrhage (Dvorak et al., 1995, Kevil et al., 1998). Some studies suggest that EBOV-GP is the main determinant of vascular cell injury (Yang et al., 1998, Yang et al., 2000). As we demonstrated in Fig. 1E, HUVECs were extremely sensitive to EBOV. Therefore, we choose primary HUVECs as target cells to evaluate if CHPV could also protect HUVECs from EBOV-GP infection. Briefly, various concentrations of CHPV were mixed with EBOV-GP-V and added immediately onto HUVECs. At 2 h post-infection, the cells were washed and cultured in medium without the herb. As expected, upon an increase in the concentration of CHPV, the Gluc activities of EBOV-GP-V-infected HUVECs decreased in a dose-dependent manner (Fig. 4 A), indicating that the EBOV-GP-V infection in HUVECs was specifically blocked by CHPV. When 10 μg/mL of CHPV was used, less than 20% of Gluc activity could be detected. Meanwhile, Fig. 4B shows the cell viability after treatment with different concentrations of CHPV for 2 h or 48 h, as measured by the WST-1 cell proliferation assay. For the 2 h CHPV treatment group, we detected no cytotoxicity at a concentration of 320 μg/ml. For the 48 h continuous CHPV treatment group, the HUVECs grew normally at concentrations up to 40 μg/ml.
Fig. 4.
Evaluating the anti-EBOV effect and cytotoxicity of CHPV in vascular endothelial cells (HUVECs) and its anti-EBOV effect in macrophages. (A) The same amount of pseudovirions mixed with the indicated varying concentrations of CHPV were immediately added onto HUVECs for 2 h, then the cells were washed and cultured in DMEM without CHPV. The Gluc activities were tested from the supernatants after 48 h of incubation. (B) The cells were treated with CHPV for 2 or 48 h, after which the WST-1 cell proliferation assay was used to analyze cell proliferation. (C) Left 4 bars: CHPV was added into the macrophage culture 12 h before infection when EBOV-GP-V was added and continually cultured without washing; right 4 bars: EBOV-GP-V alone or mixed with CHPV (20 ug/ml) and immediately added onto cell culture, followed by washing of the cells after 4 h of incubation. The cells were then cultured in medium without CHPV. The supernatants of all samples were collected at 24 or 48 h post-infection and used to detect Gluc activity.
It is well known that macrophages are a major initial target cell for Ebola virus. Infected macrophages can attract more target cells, induce and increase vasodilatation, vascular permeability and disseminated intravascular coagulation by producing proinflammatory cytokines and chemokines (Bray and Geisbert, 2005). Therefore, it is necessary to evaluate the inhibitory effect of CHPV on EBOV-GP-V infection in macrophages. We first tested the effect of CHPV by incubating human primary macrophages with CHPV and EBOV-GP-V for 4 h. As shown in Fig. 4C (the 4 bars on the right side), the Gluc activity of supernatant from infected macrophages significantly decreased at 24 and 48 h in the presence of CHPV (20 μg/ml). We also infected macrophages in the presence of CHPV and maintained the macrophages in the medium containing EBOV-GP-Vs and CHPV for 48 h without washing. In this case, the EBOV-GP-Vs infection was inhibited by CHPV and the inhibition rate was as high as 95% compared with the positive control (Fig. 4C, left 4 bars), suggesting that the optimum inhibitory effect of CHPV could be obtained when there was a continuous presence of CHPV in the culture medium. Additionally, we observed no cytotoxic effect when 20 μg/ml of CHPV was present in the macrophages culture for 48 h (data not shown).
3.5. Analysis the EBOV entry inhibitory effect of MAb 2G4 and CHPV
As described above, both EBOV-GP-specific monoclonal antibodies 2G4 (Fig. 1D) and CHPV (Fig. 2, Fig. 3) could efficiently block the viral entry step. We next asked whether a stronger inhibitory effect could be achieved by combining CHPV and MAb 2G4 in relatively lower concentrations. In Fig. 5 A, 2G4 was serially diluted from 16 μg/ml to 0.32 μg/ml, and each concentration was combined with the indicated concentrations of CHPV (10 and 0.625 μg/mL). The results show that the viral infection was reduced approximately 80% when the concentration of CHPV was 10 μg/ml. Interestingly, a greater than 90% inhibitory effect was achieved when CHPV (10 μg/ml) was combined with 2G4 at concentrations above 1.6 μg/ml. The additive effect was more evident when a lower concentration (0.625 μg/ml) of CHPV was combined with different doses of 2G4. For example, when 1.6 μg/ml 2G4 and 0.625 μg/mL CHPV were added alone, 56% and 26% inhibition were obtained, respectively. When these two drugs were added together at the same concentration, 81% inhibition was observed. This clearly indicates that a much stronger inhibition could be achieved through the combined use of 2G4 and CHPV, as opposed to using each treatment alone. It is obvious that to achieve the same level of inhibition, the MAb dosage could be reduced in the presence of CHPV. A similar phenomenon was observed in macrophages: at 4 h post-infection with pseudovirions in the presence of CHPV, in the presence or absence of 2G4 in macrophages (Fig. 5B), the combination of CHPV (20 μg/ml) with 2G4 (1.6 μg/ml) could further decrease the infectivity of EBOV-GP-V compared with that of CHPV (20 μg/ml) or 2G4 (16 μg/ml) alone. Remarkably, when CHPV and 2G4 were added 1 h before infection, with the same amount of CHPV and 2G4 maintained during the course of infection, the infection of EBOV-GP-V was completely inhibited (Fig. 5C).
Fig. 5.
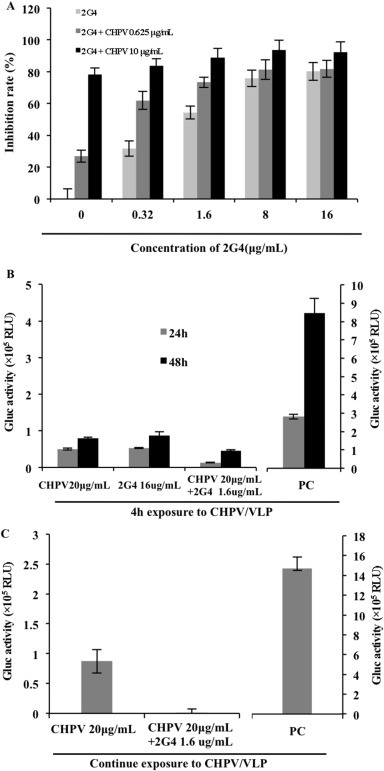
Analysis of the inhibitory effect of the combination of 2G4 and CHPV on EBOV entry. (A) Various concentrations of 2G4 alone or with CHPV (10 or 0.625 μg/ml) were added to HEK293T cells with the same amount of EBOV-GP-V. The anti-EBOV effect was displayed as percent inhibition, a ratio of Gluc activity in the presence of 2G4 or/and CHPV versus EBOV-GP-V alone. (B) The inhibitory effect of CHPV in the presence or absence of 2G4 in macrophages. The positive control (PC) was infected macrophages in the absence of CHPV or 2G4. EBOV-GP-V was mixed with CHPV (20 ug/ml) in the presence of absence of 2G4 (16 or 1.6 ug/ml) and immediately added onto the cell culture, followed by washing of the cells after 4 h of incubation. The cells were then cultured in medium without CHPV. The supernatants were collected at 24 and 48 h post-infection and used to detect Gluc activity. The major scales of the Y-axis on the left and on the right are different, with 0.5 units and 1 unit, respectively. (C) The same amounts of EBOV-GP-Vs in the presence or absence of 2G4 (1.6 ug/ml) were added onto CHPV (20 μg/mL)-preincubated (1 h) macrophages, and the same amount of CHPV with or without 1.6 ug/mL 2G4 was maintained during the course of infection. The positive control (PC) was infected macrophages in the absence of CHPV or 2G4. The supernatants were collected, and the Gluc activity was tested after 48 h of continuous culture without washing. The major scale base of the Y-axis is 0.5 units on the left and 2 units on the right.
3.6. Inhibition of CHPV against an eGFP-ZEBOV infection
Given that CHPV is able to block the EBOV-GP-mediated pseudovirion entry, the next question we asked was whether CHPV could block the ZEBOV infection. To address this question, we performed an eGFP-ZEBOV infection in the presence of CHPV or 2G4 antibody in VeroE6 cells. Briefly, different concentrations of CHPV or 2G4 antibody were added to VeroE6 cell cultures for 10 min, then the cells were infected by 100 PFU of eGFP-ZEBOV in the presence of same concentrations of CHPV or 2G4. After 12 h of infection, the cells were washed and cultured with fresh medium without any drug. At 48 h post-infection, we did not observe any CPHV-induced toxic effect on the cells, and the cells were fixed with 10% phosphate-buffered formalin and fluorescent plaques were counted using an AID fluorescent plate reader. Remarkably, results revealed that the presence of 6.25 ug/ml of CHPV reduced more than 50% of eGFP-ZEBOV infection, while in the presence of 25 ug/ml of CHPV, eGFP-ZEBOV infection was inhibited by approximately 80% (Fig. 6 A). In agreement with previous reports, 2G4 also showed a very strong blocking effect against Ebola virus infection (Audet et al., 2014, Qiu et al., 2011). At 0.6 μg/ml concentration of 2G4 antibody, approximately 80% of virus infection was blocked (Fig. 6A). These results provide strong evidence that CHPV is able to inhibit EBOV infection.
Fig. 6.
The inhibitory effect of CHPV on the eGFP-ZEBOV infection. The final treatment concentrations of CHPV were 2-fold dilutions beginning with 100 μg/ml down to 0.78 μg/ml, and 2G4 was started with 2.5 μg/ml followed by 2-fold dilutions down to 0.020 μg/ml. The indicated concentrations of CHPV or MAb alone were added to VeroE6 cells (A) or different concentrations of CHPV or MAb were mixed (as indicated) and added to VeroE6 cells (B) before exposure of the cells to 100 PFU of eGFP-ZEBOV. After incubating for12 h, the medium was changed with fresh medium. At 48 h later, cells were fixed with 10% phosphate-buffered formalin and fluorescent plaques were counted using an AID fluorescent plate reader. Error bars represent variation between triplicate samples, and the data of (A) and (B) are representative of results obtained in two independent experiments.
To further test whether the combined use of CHPV and 2G4 could enhance the anti-EBOV infection activity, we treated VeroE6 cells with 2G4 alone or 2G4 plus CHPV in VeroE6 cells during eGFP-ZEBOV infection, as described above. After 48 h of infection, the cells were fixed with 10% phosphate-buffered formalin and fluorescent plaques were counted using an AID fluorescent plate reader. Interestingly, the combined treatment of 2G4/CHPV potentiated inhibition against eGFP-ZEBOV infection (Fig. 6B), as results revealed that while treatment with 0.31 or 1.2 ug/ml of 2G4 alone reduced eGFP-ZEBOV infection to approximately 25% or 17%, respectively, the presence of 2G4/CHPV (0.31 ug/6.25 μg or 1.2 μg/25 ug/ml) reduced eGFP-ZEBOV infection to approximately 17% or 6%, respectively, compared with eGFP-ZEBOV infection in the absence of antibody and CHPV (Fig. 6B). This indicates that combined treatment with 2G4 and CHPV during EBOV infection could achieve a better inhibitory effect against viral infection.
4. Discussion
Due to the fact EBOV is classified as a biosafety level 4 pathogen, the study of its replication, as well as the testing of anti-EBOV drugs, requires biosafety level 4 containment (BSL-4) and procedures, significantly limiting the EBOV-related research activities of many microbiology laboratories. To overcome these limitations, several previous studies have examined the use of EBOV-GP pseudotyped viral vectors in a BSL-2 environment, including replication-defective MLV and HIV, replication-competent vesicular stomatitis virus and Newcastle disease virus, for investigation of EBOV-GP-mediated virus entry mechanism and for the screening of new therapeutic agent(s) (Basu et al., 2011, DiNapoli et al., 2010, Takada et al., 1997, Wen et al., 2013, Wong et al., 2010, Wool-Lewis and Bates, 1998, Yang et al., 1998, Yonezawa et al., 2005). In these studies, the entry of pseudotyped virus-like particles were monitored by using enzymes or biological markers expressed when the pseudovirions gained entry into the cells. In this study, we used a sensitive Ebola-GP-mediated HIV-based vector system, which contains a Gaussia luciferase (Gluc) gene as a reporter (Fig. 1A). Because Gluc can be secreted into the supernatant after being expressed, this assay makes it more practical to evaluate and quantify the level of EBOV-GP-mediated virus entry and can be used for anti-Ebola-GP drug screening in a BSL-2 environment. Our results demonstrate that the infection of EBOV-GP-V required a functional EBOV-GP-mediated cell entry, which was neutralized by MAb 2G4, which is specific for EBOV-GP (Fig. 1D).
By using this EBOV-GP-mediated virus entry system, we have tested several Chinese herb extracts and shown that the aqueous extract of P. vulgaris (CHPV) exhibited the most potent inhibitory activity against EBOV-GP virus entry out of all of the extracts tested (Fig. 2). Remarkably, CHPV could efficiently inhibit the infection of EBOV-GP pseudovirions in its primary target cell, the human macrophage. The inhibition rate reached as high as 99.5% when CHPV (20 ug/ml) was continually present in the culture medium (Fig. 5C). The same concentration of CHPV could inhibit approximately 80% of viral infectivity in HUVECs, even after cells were treated for only 2 h with CHPV and virus added soon after (Fig. 4). Moreover, our study has clearly shown that 1.25 ug/ml of CHPV reduced the wild type EBOV infection by 50%, while in the presence of 12.5 μg of CHPV, EBOV virus infection was inhibited by more than 80% (Fig. 6A). These observations provide convincing evidence for CHPV as a potential agent against EBOV that deserves further investigation in animal studies.
To investigate the CHPV anti-EBOV mechanism of action, a “time-of-addition” study was performed. Here, CHPV was identified as having blocked EBOV-GP-V infection, possibly by suppressing virus attachment through targeting the viral GP protein (Fig. 3). Previous studies demonstrated that aqueous extracts of PV can decrease the replication of herpes simplex virus (HSV) by preventing virus binding to cells (Chiu et al., 2004, Zhang et al., 2007) and inhibit HIV-1 infection through prevention of viral attachment to the CD4+ T cell receptor (Oh et al., 2011, Tabba et al., 1989, Yao et al., 1992), suggesting that CHPV targets an invariant component among these viruses. In fact, it was suggested that the antiviral activity of CHPV might be related to an anionic polysaccharide. Polysaccharide can form complexes with virus proteins, which is likely to occur mainly through the formation of salt linkages or ion pairs between oppositely charged groups on the polymers and the virus proteins, ultimately blocking virus entry (Izumi et al., 1994). For example, it is well established that sulfated polysaccharide can selectively interact with the polycationic V3 loop, which is a highly basic region on gp120 (Huang et al., 2005), to interfere with the attachment of HIV to cell-surface receptors (Harrop and Rider, 1998, Moulard et al., 2000, Vives et al., 2005). Whether a polyanionic property is responsible for the viral entry inhibition mechanism of CHPV remains to be determined. However, at this point, we also could not rule out the possibility that the presence of CHPV might also result in conformational changes in EBOV-GP and render it unable to interact with its receptor, which requires further investigation. The questions of the specificity of the antiviral activity of CHPV and whether CHPV can act against all viruses or only for enveloped viruses still remain unanswered. A previous report has shown that a polysaccharide from P. vulgaris (at 100 ug/ml) was active against the HIV-1 and herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), but was unable to inhibit cytomegalovirus (CMV), human influenza virus types A and B, poliovirus type 1 or vesicular stomatitis virus (VSV) (Xu et al., 1999). Consistently, our data also indicated that CHPV could not inhibit VSV-G pseudovirion infection (data not shown). Thus, CHPV action appears to be specific to certain virus species. In addition, our data also revealed that CHPV does not act on the cell surface or change its susceptibility to HIV (Yao et al., 1992) or EBOV (Fig. 3A). Thus, all of these observations suggest that there is a specificity of the antiviral effect of CHPV. However, the underlying molecular mechanism(s) requires further investigation.
Another interesting observation in this study is that the combination of the anti-EBOV antibody (2G4) with CHPV could enhance their anti-EBOV activity. The 2G4 antibody was first reported by Qiu et al. (Qiu et al., 2011), and it is a crucial component of the two antibody cocktails, ZMAb and ZMapp. Mab 2G4 shows great potential in blocking EBOV infection in vitro and in animal studies (Audet et al., 2014, Qiu et al., 2012a, Qiu et al., 2014, Qiu et al., 2013). Recently, ZMapp was approved in February 2015 for a clinical trial in Liberia and the United States (Al-Bayati et al., 2015). One disadvantage of using MAb as an anti-EBOV agent is its source limitation, especially for the resource-constrained geographic regions where the outbreaks of filovirus infection frequently occur. Therefore, the discovery of other agents capable of preventing EBOV virus infection or contributing to the enhancement of Mab-mediated anti-EBOV effects is needed. We investigated whether the presence of CHPV and 2G4 together would demonstrate a stronger anti-EBOV effect. The results showed that the combined use of lower concentrations of both 2G4 and CHPV could achieve the same anti-EBOV-GP efficiency as a high concentration of 2G4 alone (Fig. 5, Fig. 6B). The reason for this might be due to a combined effect through their different binding mechanisms. The 2G4 monoclonal antibody has been known to bind to the GP2 fusion loop, which is important for viral entry (Audet et al., 2014, Qiu et al., 2011). In contrast, the action of CHPV is speculated to target the GP1, due to the mucin-like domain in GP1 that is responsible for viral attachment (Lin et al., 2003, Simmons et al., 2003, Simmons et al., 2002, Takada et al., 2004) and the fact that CHPV exerts its effect during the attachment of virus to the cells. Additionally, we could not exclude the possibility that CHPV may also bind to the GP2 fusion region, which requires further investigation. This combined effect is significant, as (i) similar efficiencies would be achieved by using reduced amounts of antibody and CHPV, (ii) the combination of 2G4 and CHPV will reduce the likelihood of viral resistance and (iii) the therapeutic window could be extended by continuous use of CHPV in an infected individual. In addition, many studies have demonstrated that the aqueous extract contains many components having immunomodulatory effects (Ahn et al., 2003, Fang et al., 2005a, Fang et al., 2005b, Psotova et al., 2003, Ryu et al., 2000, Shin et al., 2001, Sun et al., 2005, Won et al., 2003). It is also possible that CHPV could be a combination of several active ingredients, which requires further investigation. Thus, aside from directly having an anti-EBOV effect, CHPV might also have great potential as an immunomodulator to suppress the severe upset and disorder of the immune cell proliferation and contraction caused by EBOV infection, further extending the antibody therapeutic window.
Overall, we demonstrated that CHPV acted as an EBOV entry inhibitor and could enhance the inhibitory activity of EBOV MAb. This natural plant extract merits further investigation as a promising antiviral due to its rich source, low costs, low cytotoxicity, wide acceptability and strong antiviral properties. Additionally, further detailed chemical research to identify and purify the active ingredient(s) will facilitate its mechanistic analyses and applications.
Acknowledgments
We thank Ms. K.N. Tran, X-l Tan, E. Mendoza, and T. Tian for technical supports. Dr. X-j Yao holds a Manitoba Research Chair Award from the Manitoba Health Research Council (MHRC). This work was supported by Canadian Institute of Health Research (CIHR) (HBF-131553)/Canadian HIV Vaccine Initiative (CHVI) Bridge Funding (201309OCB), CIHR/MHRC RPP grant (RPA-132176), National Natural Science Foundation of China (NNSFC, 81371863), and Canadian Foundation for AIDS Research (CANFAR grant# 023-013).
References
- Ahn S.C., Oh W.K., Kim B.Y., Kang D.O., Kim M.S., Heo G.Y., Ahn J.S. Inhibitory effects of rosmarinic acid on Lck SH2 domain binding to a synthetic phosphopeptide. Planta Med. 2003;69:642–646. doi: 10.1055/s-2003-41111. [DOI] [PubMed] [Google Scholar]
- Al-Bayati I., Teleb M., Lowder K., Salameh H.J. ZMapp: a new cocktail of monoclonal antibodies with much promise in containing the current Epidemic of ebola virus disease. Med. Sci. Rev. 2015;2:32–34. [Google Scholar]
- Ao Z., Huang G., Yao H., Xu Z., Labine M., Cochrane A.W., Yao X. Interaction of human immunodeficiency virus type 1 integrase with cellular nuclear import receptor importin 7 and its impact on viral replication. J. Biol. Chem. 2007;282:13456–13467. doi: 10.1074/jbc.M610546200. [DOI] [PubMed] [Google Scholar]
- Ao Z., Wang X., Bello A.J., Danappa Jayappa K., Yu Z., Fowke K., He X., Chen X., Li J., Kobinger G.P., Yao X. Characterization of anti-HIV activity mediated by R88-Apobec3G mutant fusion proteins in CD4+ T cells, PBMCs and macrophages. Hum. Gene Ther. 2011;22:1225–1237. doi: 10.1089/hum.2010.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet J., Wong G., Wang H., Lu G., Gao G.F., Kobinger G., Qiu X. Molecular characterization of the monoclonal antibodies composing ZMAb: a protective cocktail against Ebola virus. Sci. Rep. 2014;4 doi: 10.1038/srep06881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton C., Kouokam J.C., Lasnik A.B., Foreman O., Cambon A., Brock G., Montefiori D.C., Vojdani F., McCormick A.A., O'Keefe B.R. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob. Agents Chemother. 2014;58:120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Li B., Mills D.M., Panchal R.G., Cardinale S.C., Butler M.M., Peet N.P., Majgier-Baranowska H., Williams J.D., Patel I. Identification of a small-molecule entry inhibitor for filoviruses. J. Virol. 2011;85:3106–3119. doi: 10.1128/JVI.01456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M., Geisbert T.W. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int. J. Biochem. Cell Biol. 2005;37:1560–1566. doi: 10.1016/j.biocel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Côté M., Misasi J., Ren T., Bruchez A., Lee K., Filone C.M., Hensley L., Li Q., Ory D., Chandran K. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477:344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Warren T.K., Zhao X., Gill T., Guo F., Wang L., Comunale M.A., Du Y., Alonzi D.S., Yu W. Small molecule inhibitors of ER α-glucosidases are active against multiple hemorrhagic fever viruses. Antivir. Res. 2013;98:432–440. doi: 10.1016/j.antiviral.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R.S., Ding L., Gai-Qing C., Qi-Choa P., Ze-Lin Z., Smith K.M. Dehydroandrographolide succinic acid monoester as an inhibitor against the human immunodeficiency virus. Exp. Biol. Med. 1991;197:59–66. doi: 10.3181/00379727-197-43225. [DOI] [PubMed] [Google Scholar]
- Chiu L.C., Zhu W., Ooi V.E. A polysaccharide fraction from medicinal herb Prunella vulgaris downregulates the expression of herpes simplex virus antigen in Vero cells. J. Ethnopharmacol. 2004;93:63–68. doi: 10.1016/j.jep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- DiNapoli J.M., Yang L., Samal S.K., Murphy B.R., Collins P.L., Bukreyev A. Respiratory tract immunization of non-human primates with a Newcastle disease virus-vectored vaccine candidate against Ebola virus elicits a neutralizing antibody response. Vaccine. 2010;29:17–25. doi: 10.1016/j.vaccine.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H.F., Brown L.F., Detmar M., Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. pathology. 1995;146:1029. [PMC free article] [PubMed] [Google Scholar]
- Ebihara H., Theriault S., Neumann G., Alimonti J.B., Geisbert J.B., Hensley L.E., Groseth A., Jones S.M., Geisbert T.W., Kawaoka Y., Feldmann H. 2007, in vitro and in vivo characterization of recombinant Ebola viruses expressing enhanced green fluorescent protein. J. Infect. Dis. 2007;196:s313–S322. doi: 10.1086/520590. [DOI] [PubMed] [Google Scholar]
- Elliott L.H., Kiley M.P., McCormick J.B. Descriptive analysis of Ebola virus proteins. Virology. 1985;147:169–176. doi: 10.1016/0042-6822(85)90236-3. [DOI] [PubMed] [Google Scholar]
- Fang X., Chang R.C.-C., Yuen W.-H., Zee S.Y. Immune modulatory effects of Prunella vulgaris L. Int. J. Mol. Med. 2005;15:491–496. [PubMed] [Google Scholar]
- Fang X., Yu M.M.-S., Yuen W.-H., Zee S.Y., Chang R.C.-C. Immune modulatory effects of Prunella vulgaris L. on monocytes/macrophages. Int. J. Mol. Med. 2005;16:1109–1116. [PubMed] [Google Scholar]
- Fiore C., Eisenhut M., Krausse R., Ragazzi E., Pellati D., Armanini D., Bielenberg J. Antiviral effects of Glycyrrhiza species. Phytotherapy Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring G., Rohrmann K., Atenchong N., Mittler E., Becker S., Dahlmann F., Pöhlmann S., Vondran F.W., David S., Manns M.P. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J. Antimicrob. Chemother. 2014;69:2123–2131. doi: 10.1093/jac/dku091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARADA S. THE BROAD ANTI-VIRAL AGENT GLYCYRRHIZIN DIRECTLY MODULATES THE FLUIDITY OF PLASMA MEMBRANE AND HIV-1 ENVELOPE. BIOCHEM. J. 2005;392:191–199. doi: 10.1042/BJ20051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop H.A., Rider C.C. Heparin and its derivatives bind to HIV-1 recombinant envelope glycoproteins, rather than to recombinant HIV-1 receptor, CD4. Glycobiology. 1998;8:131–137. doi: 10.1093/glycob/8.2.131. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Hayashi T., Kojima I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: in vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res. Hum. Retroviruses. 1996;12:1463–1471. doi: 10.1089/aid.1996.12.1463. [DOI] [PubMed] [Google Scholar]
- Hayashi T. Studies on evaluation of natural products for antiviral effects and their applications. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2008;128:61–79. doi: 10.1248/yakushi.128.61. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Hayashi K., Maeda M., Kojima I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996;59:83–87. doi: 10.1021/np960017o. [DOI] [PubMed] [Google Scholar]
- Huang C.-c., Tang M., Zhang M.-Y., Majeed S., Montabana E., Stanfield R.L., Dimitrov D.S., Korber B., Sodroski J., Wilson I.A. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T., Hirata M., Takahashi K., Kokufuta E. Complexation of papain with strong polyanions and enzymatic activities of the resulting complexes. J. Macromol. Science—Pure Appl. Chem. 1994;31:39–51. [Google Scholar]
- Jayappa K.D., Ao Z., Wang X., Mouland A.J., Shekhar S., Yang X., Yao X. Human immunodeficiency virus type 1 Employs the cellular Dynein Light chain 1 protein for Reverse transcription through interaction with its integrase protein. J. Virol. 2015;JVI doi: 10.1128/JVI.03347-14. 03347–03314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen L.M., Brannan J.M., Delos S.E., Shoemaker C.J., Stossel A., Lear C., Hoffstrom B.G., DeWald L.E., Schornberg K.L., Scully C. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci. Transl. Med. 2013;5:190ra179. doi: 10.1126/scitranslmed.3005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L.-J., Choi Y.-J., Lee S.-G. Stimulation of TRAF6/TAK1 degradation and inhibition of JNK/AP-1 signalling by ginsenoside Rg3 attenuates hepatitis B virus replication. Int. J. Biochem. Cell Biol. 2013;45:2612–2621. doi: 10.1016/j.biocel.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Kevil C.G., Payne D.K., Mire E., Alexander J.S. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J. Biol. Chem. 1998;273:15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- Kobinger G.P., Weiner D.J., Yu Q.-C., Wilson J.M. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat. Biotechnol. 2001;19:225–230. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- Lee M.H., Seo D.J., Kang J.-H., Oh S.H., Choi C. Expression of antiviral cytokines in Crandell-Reese feline kidney cells pretreated with Korean red ginseng extract or ginsenosides. Food Chem. Toxicol. 2014;70:19–25. doi: 10.1016/j.fct.2014.04.034. [DOI] [PubMed] [Google Scholar]
- Lenny Bernstein B.D. U.S. company says; Washington Post: 2014. Ebola Test Drug's Supply 'exhausted' after Shipments to Africa. [Google Scholar]
- Li P., Hao X., Zhao C., Chang Y., Wang B., Wu H. Study on the antiviral activities of ginsenoside-Rg3 and-Rb3. Chin. J. Gerontol. 2001;21:215–216. [Google Scholar]
- Lin G., Simmons G., Pöhlmann S., Baribaud F., Ni H., Leslie G.J., Haggarty B.S., Bates P., Weissman D., Hoxie J.A. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 2003;77:1337–1346. doi: 10.1128/JVI.77.2.1337-1346.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Jiang S., Wu Z., Lv L., Zhang J., Zhu Z., Wu S. Identification of inhibitors of the HIV-1 gp41 six-helix bundle formation from extracts of Chinese medicinal herbs Prunella vulgaris and Rhizoma cibotte. Life Sci. 2002;71:1779–1791. doi: 10.1016/s0024-3205(02)01939-2. [DOI] [PubMed] [Google Scholar]
- McCarthy M. US signs contract with ZMapp maker to accelerate development of the Ebola drug. BMJ. 2014;349:g5488. doi: 10.1136/bmj.g5488. [DOI] [PubMed] [Google Scholar]
- Misasi J., Sullivan N.J. Camouflage and misdirection: the full-on assault of Ebola virus disease. Cell. 2014;159:477–486. doi: 10.1016/j.cell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulard M., Lortat-Jacob H., Mondor I., Roca G., Wyatt R., Sodroski J., Zhao L., Olson W., Kwong P.D., Sattentau Q.J. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 2000;74:1948–1960. doi: 10.1128/jvi.74.4.1948-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murin C.D., Fusco M.L., Bornholdt Z.A., Qiu X., Olinger G.G., Zeitlin L., Kobinger G.P., Ward A.B., Saphire E.O. Structures of protective antibodies reveal sites of vulnerability on Ebola virus. Proc. Natl. Acad. Sci. 2014;111:17182–17187. doi: 10.1073/pnas.1414164111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C., Price J., Brindley M.A., Widrlechner M.P., Qu L., McCoy J.A., Murphy P., Hauck C., Maury W. Inhibition of HIV-1 infection by aqueous extracts of Prunella vulgaris L. Virol. J. 2011;8:188. doi: 10.1186/1743-422X-8-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Tran K., Gray M., Li Y., Ao Z., Yao X., Kobasa D., Kobinger G.P. Evaluation of conserved and variable influenza antigens for immunization against different isolates of H5N1 viruses. Vaccine. 2009;27:3083–3089. doi: 10.1016/j.vaccine.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Pettitt J., Zeitlin L., Kim D.H., Working C., Johnson J.C., Bohorov O., Bratcher B., Hiatt E., Hume S.D., Johnson A.K. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci. Transl. Med. 2013;5:199ra113. doi: 10.1126/scitranslmed.3006608. [DOI] [PubMed] [Google Scholar]
- Psotova J., Kolář M., Soušek J., Švagera Z., Vičar J., Ulrichová J. Biological activities of Prunella vulgaris extract. Phytother. Res. 2003;17:1082–1087. doi: 10.1002/ptr.1324. [DOI] [PubMed] [Google Scholar]
- Qiu X., Alimonti J.B., Melito P.L., Fernando L., Ströher U., Jones S.M. Characterization of Zaire ebolavirus glycoprotein-specific monoclonal antibodies. Clin. Immunol. 2011;141:218–227. doi: 10.1016/j.clim.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Qiu X., Audet J., Wong G., Pillet S., Bello A., Cabral T., Strong J.E., Plummer F., Corbett C.R., Alimonti J.B. Successful treatment of Ebola virus–infected cynomolgus macaques with monoclonal antibodies. Sci. Transl. Med. 2012;4:138ra181. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- Qiu X., Fernando L., Melito P.L., Audet J., Feldmann H., Kobinger G., Alimonti J.B., Jones S.M. Ebola GP-specific monoclonal antibodies protect mice and guinea pigs from lethal Ebola virus infection. PLoS Negl. Trop. Dis. 2012;6:e1575. doi: 10.1371/journal.pntd.0001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Wong G., Audet J., Bello A., Fernando L., Alimonti J.B., Fausther-Bovendo H., Wei H., Aviles J., Hiatt E. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Wong G., Fernando L., Audet J., Bello A., Strong J., Alimonti J.B., Kobinger G.P. mAbs and Ad-vectored IFN-α therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci. Transl. Med. 2013;5:207ra143. doi: 10.1126/scitranslmed.3006605. [DOI] [PubMed] [Google Scholar]
- Ryu S.Y., Oak M.-H., Yoon S.-K., Cho D.-I., Yoo G.-S., Kim T.-S., Kim K.-M. Anti-allergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta Med. 2000;66:358–360. doi: 10.1055/s-2000-8531. [DOI] [PubMed] [Google Scholar]
- Sato H., Goto W., Yamamura J.-i., Kurokawa M., Kageyama S., Takahara T., Watanabe A., Shiraki K. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antivir. Res. 1996;30:171–177. doi: 10.1016/0166-3542(96)00942-4. [DOI] [PubMed] [Google Scholar]
- Seubsasana S., Pientong C., Ekalaksananan T., Thongchai S., Aromdee C. A potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells. Med. Chem. 2011;7:237–244. doi: 10.2174/157340611795564268. [DOI] [PubMed] [Google Scholar]
- Shin T.-Y., Kim Y.-K., Kim H.-M. Inhibition of immediate-type allergic reactions by Prunella vulgaris in a murine model. Immunopharmacol. Immunotoxicol. 2001;23:423–435. doi: 10.1081/iph-100107341. [DOI] [PubMed] [Google Scholar]
- Simmons G., Reeves J.D., Grogan C.C., Vandenberghe L.H., Baribaud F., Whitbeck J.C., Burke E., Buchmeier M.J., Soilleux E.J., Riley J.L. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology. 2003;305:115–123. doi: 10.1006/viro.2002.1730. [DOI] [PubMed] [Google Scholar]
- Simmons G., Wool-Lewis R.J., Baribaud F., Netter R.C., Bates P. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J. Virol. 2002;76:2518–2528. doi: 10.1128/jvi.76.5.2518-2528.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.-X., Qin F., Pan Y.-J. In vitro and in vivo immunosuppressive activity of Spica prunellae ethanol extract on the immune responses in mice. J. Ethnopharmacol. 2005;101:31–36. doi: 10.1016/j.jep.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Tabba H.D., Chang R.S., Smith K.M. Isolation, purification, and partial characterization of prunellin, an anti-HIV component from aqueous extracts of Prunella vulgaris. Antivir. Res. 1989;11:263–273. doi: 10.1016/0166-3542(89)90036-3. [DOI] [PubMed] [Google Scholar]
- Takada A., Fujioka K., Tsuiji M., Morikawa A., Higashi N., Ebihara H., Kobasa D., Feldmann H., Irimura T., Kawaoka Y. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J. Virol. 2004;78:2943–2947. doi: 10.1128/JVI.78.6.2943-2947.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A., Robison C., Goto H., Sanchez A., Murti K.G., Whitt M.A., Kawaoka Y. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. 1997;94:14764–14769. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M.R.S., Colpitts C.C., Ustinov A.V., Muqadas M., Joyce M.A., Barsby N.L., Epand R.F., Epand R.M., Khramyshev S.A., Valueva O.A. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc. Natl. Acad. Sci. 2010;107:17339–17344. doi: 10.1073/pnas.1010026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives R.R., Imberty A., Sattentau Q.J., Lortat-Jacob H. Heparan sulfate targets the HIV-1 envelope glycoprotein gp120 coreceptor binding site. J. Biol. Chem. 2005;280:21353–21357. doi: 10.1074/jbc.M500911200. [DOI] [PubMed] [Google Scholar]
- Volchkov V.E., Becker S., Volchkova V.A., Ternovoj V.A., Kotov A.N., Netesov S.V., Klenk H.-d. GP mRNA of ebola virus is Edited by the ebola virus polymerase and by T7 and Vaccinia virus polymerases 1. Virology. 1995;214:421–430. doi: 10.1006/viro.1995.0052. [DOI] [PubMed] [Google Scholar]
- Volchkov V.E., Feldmann H., Volchkova V.A., Klenk H.-D. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. 1998;95:5762–5767. doi: 10.1073/pnas.95.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl-Jensen V., Kurz S.K., Hazelton P.R., Schnittler H.-J., Ströher U., Burton D.R., Feldmann H. Role of Ebola virus secreted glycoproteins and virus-like particles in activation of human macrophages. J. Virol. 2005;79:2413–2419. doi: 10.1128/JVI.79.4.2413-2419.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Zhao B., Song K., Hu X., Chen W., Kong D., Ge J., Bu Z. Recombinant lentogenic Newcastle disease virus expressing Ebola virus GP infects cells independently of exogenous trypsin and uses macropinocytosis as the major pathway for cell entry. Virology J. 2013;10:331. doi: 10.1186/1743-422X-10-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J., Bosio C., Hart M. Ebola virus: the search for vaccines and treatments. Cell. Mol. Life Sci. CMLS. 2001;58:1826–1841. doi: 10.1007/PL00000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M.C., Freiberg A.N., Zhang T., Akyol-Ataman Z., Grock A., Hong P.W., Li J., Watson N.F., Fang A.Q., Aguilar H.C. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc. Natl. Acad. Sci. 2010;107:3157–3162. doi: 10.1073/pnas.0909587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won J., Hur Y.G., Hur E.M., Park S.H., Kang M., Choi Y., Park C., Lee K.H., Yun Y. Rosmarinic acid inhibits TCR-induced T cell activation and proliferation in an Lck-dependent manner. Eur. J. Immunol. 2003;33:870–879. doi: 10.1002/eji.200323010. [DOI] [PubMed] [Google Scholar]
- Wong A.C., Sandesara R.G., Mulherkar N., Whelan S.P., Chandran K. A forward genetic strategy reveals destabilizing mutations in the Ebolavirus glycoprotein that alter its protease dependence during cell entry. J. Virol. 2010;84:163–175. doi: 10.1128/JVI.01832-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool-Lewis R.J., Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 1998;72:3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.X., Lee S.H., Lee S.F., White R.L., Blay J. Isolation and characterization of an anti-HSV polysaccharide from Prunella vulgaris. Antivir. Res. 1999;44:43–54. doi: 10.1016/s0166-3542(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Yang Z.-y., Delgado R., Xu L., Todd R.F., Nabel E.G., Sanchez A., Nabel G.J. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279:1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- Yang Z.-y., Duckers H.J., Sullivan N.J., Sanchez A., Nabel E.G., Nabel G.J. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat. Med. 2000;6:886–889. doi: 10.1038/78645. [DOI] [PubMed] [Google Scholar]
- Yao X.-J., Mouland A.J., Subbramanian R.A., Forget J., Rougeau N., Bergeron D., Cohen E.A. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 1998;72:4686–4693. doi: 10.1128/jvi.72.6.4686-4693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X.-J., Wainberg M.A., Parniak M.A. Mechanism of inhibition of HIV-1 infection in Vitro by purified extract of Prunella vulgaris. Virology. 1992;187:56–62. doi: 10.1016/0042-6822(92)90294-y. [DOI] [PubMed] [Google Scholar]
- Yonezawa A., Cavrois M., Greene W.C. Studies of ebola virus glycoprotein-mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J. Virol. 2005;79:918–926. doi: 10.1128/JVI.79.2.918-926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida O., Nakashima H., Yoshida T., Kaneko Y., Yamamoto I., Matsuzaki K., Uryu T., Yamamoto N. Sulfation of the immunomodulating polysaccharide lentinan: a novel strategy for antivirals to human immunodeficiency virus (HIV) Biochem. Pharmacol. 1988;37:2887–2891. doi: 10.1016/0006-2952(88)90272-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y., But P.P.-H., Ooi V.E.-C., Xu H.-X., Delaney G.D., Lee S.H., Lee S.F. Chemical properties, mode of action, and in vivo anti-herpes activities of a lignin–carbohydrate complex from Prunella vulgaris. Antivir. Res. 2007;75:242–249. doi: 10.1016/j.antiviral.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li D., Jin X., Huang Z. Fighting Ebola with ZMapp: spotlight on plant-made antibody. Sci. China Life Sci. 2014;57:987–988. doi: 10.1007/s11427-014-4746-7. [DOI] [PubMed] [Google Scholar]
- Zheng M. Experimental study of 472 herbs with antiviral action against the herpes simplex virus. Zhong Xi Yi Jie He Za Zhi. 1990;10 39–41. [PubMed] [Google Scholar]


