Abstract
Feline infectious peritonitis (FIP) is a feline coronavirus (FCoV)-induced fatal disease in wild and domestic cats. FCoV exists in two serotypes. Type I FCoV is the dominant serotype worldwide. Therefore, it is necessary to develop antiviral drugs against type I FCoV infection. We previously reported that type I FCoV is closely associated with cholesterol throughout the viral life cycle. In this study, we investigated whether U18666A, the cholesterol synthesis and transport inhibitor, shows antiviral effects against type I FCoV. U18666A induced cholesterol accumulation in cells and inhibited type I FCoV replication. Surprisingly, the antiviral activity of U18666A was suppressed by the histone deacetylase inhibitor (HDACi), Vorinostat. HDACi has been reported to revert U18666A-induced dysfunction of Niemann-Pick C1 (NPC1). In conclusion, these findings demonstrate that NPC1 plays an important role in type I FCoV infection. U18666A or other cholesterol transport inhibitor may be considered as the antiviral drug for the treatment of cats with FIP.
Keywords: Feline coronavirus, Cholesterol, U18666A, Histone deacetylase inhibitor, NPC1
1. Introduction
Coronaviruses are single-stranded positive-sense RNA viruses in the subfamily Coronavirinae of the family Coronaviridae, and cause respiratory and gastrointestinal diseases in mammals and birds (Su et al., 2016). Coronaviruses are important pathogens causing emerging and re-emerging infectious diseases. For example, outbreaks of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle-east respiratory syndrome coronavirus (MERS-CoV) occurred mainly in Asia in 2003 and 2012, respectively (de Wit et al., 2016). Feline infectious peritonitis (FIP) is known as a highly fatal disease caused by Coronaviruses, similar to SARS and MERS (Dandekar and Perlman, 2005, Pedersen, 2014).
Feline coronavirus (FCoV) belongs to Alphacoronavirus of the family Coronaviridae (de Groot et al., 2012). The FCoV virion is mainly composed of nucleocapsid (N), envelope, membrane, and peplomer spike (S) proteins (Motokawa et al., 1996). FCoV has been classified into types I and II according to the amino acid sequence of its S protein (Motokawa et al., 1995). Type II FCoV was previously suggested to be from recombination between type I FCoV and type II canine coronavirus (CCoV) (Herrewegh et al., 1998, Terada et al., 2014). Separate from these genotypes/serotypes, FCoV consists of two biotypes: low pathogenic feline enteric coronavirus (FECV: low-virulent FCoV) and high pathogenic FIP virus (FIPV: virulent FCoV) (Pedersen, 2014). FIPV can cause immune-mediated inflammatory disease with high mortality in domestic and wild felidae. Although antiviral drugs and vaccines against FIP have been investigated, no method has yet been established for practical use.
Serological and genetic surveys revealed that type I FCoV is dominant worldwide (Hohdatsu et al., 1992, Kummrow et al., 2005, Wang et al., 2014); therefore, antiviral drugs and vaccines need to be developed against type I FCoV infection. However, a few studies on type I FCoV have been performed because of its low replication ability in feline cell lines. We previously reported that type I FCoV is closely associated with cholesterol throughout the viral life cycle (Takano et al., 2016). We also confirmed that an increase in plasma membrane cholesterol enhances type I FCoV infection. These findings suggest that cell membrane cholesterol plays an important role in type I FCoV infection.
Cellular cholesterol is derived from de novo cholesterol biosynthesis and low density lipoprotein uptake (Simons and Ikonen, 2000). Biosynthesized or entrapped cholesterol is transported in cells and heterogeneously distributed in organelles. Association of the cholesterol biosynthesis and transport systems in cells with virus replication has been reported (Aizaki et al., 2008, Carette et al., 2011, Mackenzie et al., 2007, Zheng et al., 2003). Recent studies have shown that intracellular cholesterol synthesis and transport inhibitors potently reduced viral replication. For example, human hepatitis virus C (HCV) RNA replication is disrupted by HMG-CoA reductase inhibitors (Honda and Matsuzaki, 2011), and cholesterol transporter inhibitors inhibit replication of Ebola virus (Carette et al., 2011). However, it remains unclear whether cholesterol biosynthesis and intracellular transport inhibitor can suppress type I FCoV replication.
U18666A is a cationic amphiphilic drug (CAD) impairing cholesterol biosynthesis and intracellular transport (Cenedella, 2009). U18666A inhibits intracellular cholesterol biosynthesis by suppressing oxidosqualene cyclase (Cenedella et al., 2004). U18666A also inhibits cholesterol release from lysosomes through impairing the function of a cholesterol transporter, Niemann-Pick type C1 (NPC1) (Ko et al., 2001). It has been reported that U18666A suppresses replication of Ebola virus, dengue virus, and human hepatitis C virus (Elgner et al., 2016, Lu et al., 2015, Poh et al., 2012). However, no study on the influence of U18666A on FCoV infection has been reported.
In this study, we investigated whether U18666A inhibits FCoV infection.
2. Materials and methods
2.1. Cell cultures and viruses
Felis catus whole fetus (fcwf)-4 cells (kindly supplied by Dr. M. C. Horzinek of Universiteit Utrecht) were grown in Eagles' MEM containing 50% L-15 medium, 5% fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin. The type I FCoV KU-2 strain (FCoV-I KU-2) was isolated in our laboratory, and the type I FCoV Black strain (FCoV-I Black), the type I FCoV UCD-1 strain (FCoV-I UCD-1), and the type I FCoV UCD-4 strain (FCoV-I UCD-4) were kindly supplied by Dr. J. K. Yamamoto from the University of Florida. The type II FCoV WSU 79-1146 strain (FCoV-II 79-1146) was kindly provided by Dr. M. C. Horzinek of Universiteit Utrecht. The type II FCoV WSU 79-1683 (FCoV-II 79-1683) was kindly provided by Dr. A. J. McKeirnan from Washington State University. The type II CCoV 1-71 strain (CCoV-II 1-71) were supplied by Dr. E. Takahashi of the University of Tokyo. These viruses were grown in fcwf-4 cells at 37 °C.
2.2. Compounds
U18666A, Methyl-β-cyclodextrin (MbCD), Ro 48-8071, AY-9944, Amorolfine, Simvastatin, Clomiphene, and Alendronate were purchased from Sigma Aldrich (USA). Lovastatin, Fluvastatin, and Itraconazole were purchased from Wako Pure Chemical (Japan). Vorinostat was purchased from Cayman Chemical (USA). U18666A and MbCD were dissolved in water as 10 mM (U18666A) and 1 M (MbCD) stock, respectively. Other compounds were dissolved in dimethyl sulfoxide (DMSO) as 10 mM stock. On the day of the experiments, these compounds were diluted to the desired concentrations in maintenance medium.
2.3. Cytotoxic effects of compounds
The fcwf-4 cells were seeded on 96-well plates. The compounds were added in triplicate to the wells. After incubation for defined periods, the culture supernatants were removed, WST-8 solution (Kishida Chemical, Japan) was added, and the cells were returned to the incubator for 1 h. The absorbance of formazan produced was measured at 450 nm with a 96-well spectrophotometric plate reader, as described by the manufacturer. Percentage viability was calculated using the following formula: Cytotoxicity (%) = [(OD of compound-untreated cells - compound-treated cells)/(OD of compound-untreated cells)] × 100.
2.4. Detection of cellular cholesterol
The cellular cholesterol content of fcwf-4 cells was evaluated by the Cholesterol Cell Based Detection Assay Kit (Cayman chemical, USA) according to the manufacturer's instructions. Briefly, fcwf-4 cells were grown on an 8-well Lab-Tek Chamber Slide (Thermo Fisher Scientific, USA). Semi-confluent fcwf-4 cell monolayers were cultured in medium containing 2 μM U18666A at 37 °C for 9, 12, 16, or 24 h. After fixing and staining, filipin III-binding cells were analyzed using a Leica DM4B microscope and LAS X integrated imaging system (Leica Microsystems, Germany).
2.5. Measurement of cellular cholesterol
The amount of intracellular cholesterol was determined using the Amplex Red Cholesterol Assay Kit (Molecular Probes, USA) following the manufacturer's instructions.
2.6. Antiviral effects of compounds
Confluent fcwf-4 cell monolayers were cultured in medium containing compounds at the indicated concentrations in 24-well multi-plates at 37 °C for 24 h. Cells were washed and the virus (MOI 0.01) was adsorbed into the cells at 37 °C for 1 h. After washing, cells were cultured in carboxymethyl cellulose (CMC)-MEM or MEM. In the case of cells cultured in CMC-MEM, the cell monolayers were incubated at 37 °C for 72 h, fixed and stained with 1% crystal violet solution containing 10% buffered formalin, and the resulting plaques were then counted. The percentage of the plaque decrease or increase was determined using the following formula: Percentage of the plaque reduction (%) = [(plaque number of compound−treated cells)/(plaque number of compound−untreated cells)] × 100. In the case of cells cultured in MEM, the culture supernatants were collected 72 h post-infection, and virus titers were determined by the TCID50 assay.
2.7. Immunofluorescence assay
Fcwf-4 cells were grown on an 8-well Lab-Tek Chamber Slide (Thermo Fisher Scientific, USA). Semi-confluent fcwf-4 cell monolayers were cultured in medium containing 2 μM U18666A at 37 °C for 24 h. Cells were washed and the virus (MOI 0.01) was adsorbed into the cells at 37 °C for 1 h. After washing, cells were cultured in MEM. The cell monolayers were incubated at 37 °C. After 72 h, N protein levels were determined by an immunofluorescence assay (IFA), as described previously (Hohdatsu et al., 1991). For recognizing FCoV N protein, mAb E22-2 (mouse IgG1) prepared by our laboratory was used (Hohdatsu et al., 1991).
2.8. Time-of-U18666A addition experiment: pre virus infection
The confluent fcwf-4 cells were cultured in medium containing 2 μM U18666A for 3, 6, 9, 12, 16, or 24 h. After washing, the virus (MOI 0.01) was adsorbed into cells at 37 °C for 1 h. Cells were washed and cultured in CMC-MEM. The cell monolayers were incubated at 37 °C for 72 h, fixed and stained, and the resulting plaques were then counted. The percentage of the plaque reduction was measured with reference to the method described above.
2.9. Effects of HDAC inhibitor on U18666A-treated cells
Confluent fcwf-4 cell monolayers were cultured in medium containing 2 μM U18666A in 24-well multi-plates at 37 °C for 24 h. After washing, cells were cultured in medium containing Vorinostat at the indicated concentrations for 24 h. Cells were washed and the virus (MOI 0.01) was adsorbed into the cells at 37 °C for 1 h. Cells were washed and cultured in CMC-MEM. The cell monolayers were incubated at 37 °C for 72 h post-inoculation, fixed and stained, and the resulting plaques were then counted. The percentage of the plaque reduction was measured with reference to the method described above. The influence of Vorinostat on the intracellular distribution of cholesterol was investigated as described above. The influence on virus infection in Vorinostat-treated cells was confirmed by IFA as described above.
2.10. Statistical analysis
Data from only two groups were analyzed with the Student's t -test (Welch's t-test or Bartlett's test), and multiple groups were analyzed with a one-way ANOVA followed by Tukey's test.
3. Results
3.1. Antiviral effects of U18666A on FCoV-I KU2 and FCoV-II 79-1146
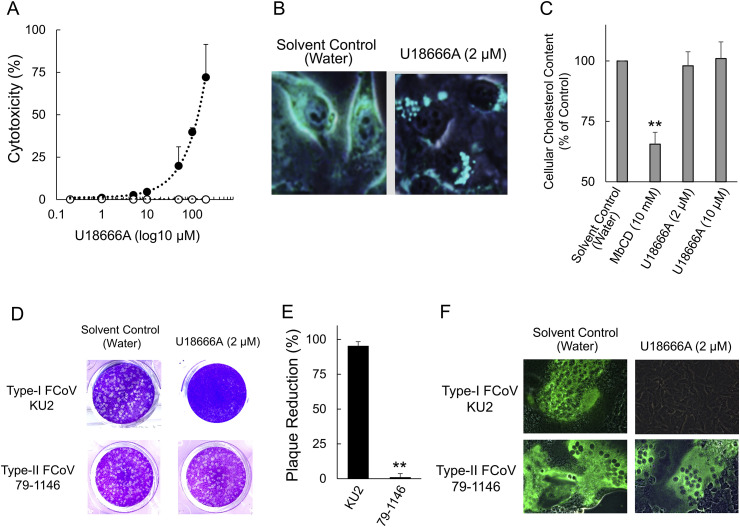
Cytotoxicity assay was performed to determine the non-toxic concentration of U18666A against fcwf-4 cells (Fig. 1 A). The 50% cytotoxic concentration (CC50) and the 10% cytotoxic concentration (CC10) value of U18666A were 97.6 ± 3.7 (mean ± SE) μM and 13.7 ± 1.1 (mean ± SE) μM, respectively. We investigated changes in the cellular cholesterol content of U18666A-treated fcwf-4 cells. U18666A caused accumulation of cholesterol as measured by filipin III staining (Fig. 1B). To determine whether U18666A affected the cellular cholesterol content, the level of cholesterol in fcwf-4 cells was measured. Although the level of cellular cholesterol significantly decreased in 10 mM MbCD-treated cells, neither 2 μM nor 10 μM U18666A decreased cellular cholesterol levels (Fig. 1C). In order to confirm the effects of the accumulation of cholesterol on FCoV infection, fcwf-4 cells were pretreated with U18666A, followed by virus inoculation. Pretreatment with U18666A demonstrated reduction of FCoV-I KU2 plaque formation (Fig. 1D and E). In contrast, the percentage of plaque reduction of FCoV-II 79-1146 was not affected by pretreatment with U18666A. We investigated the expression of viral proteins in order to further determine the effects of U18666A on FCoV infection. The N protein levels of FCoV-I KU-2 specifically decreased in fcwf-4 cells pretreated with U18666A (Fig. 1F). In contrast to FCoV-I KU2, pretreatment with U18666A did not affect the N protein levels of FCoV-II 79-1146 in fcwf-4 cells.
Fig. 1.
Antiviral effects of U18666A on FCoV-I KU2 and FCoV-II 79-1146. (A) Cytotoxic effects of U18666A in fcwf-4 cells. The black circles indicate treatment with U18666A, and the white circles indicate treatment with the solvent (water). Data represent four independent experiments. (B) U18666A induced accumulation of intracellular cholesterol. The cellular cholesterol content of cells was evaluated using a filipin-cholesterol stain. (C) Quantification of cellular cholesterol in fcwf-4 cells. The results are shown as mean ± SE. Data represent four independent experiments. **, p < 0.01 vs. solvent control. (D) Plaque reduction assay of FCoV in fcwf-4 cells treated with U18666A. (E) Inhibition of FCoV infection by U18666A in fcwf-4 cells. The results are shown as mean ± SE. Data represent four independent experiments. **, p < 0.01 vs. type I FCoV KU2. (F) Effects of U18666A on the viral protein. FCoV N protein was evaluated with IFA.
3.2. Differential effects of U18666A against FCoV serotypes I and II, and CCoV
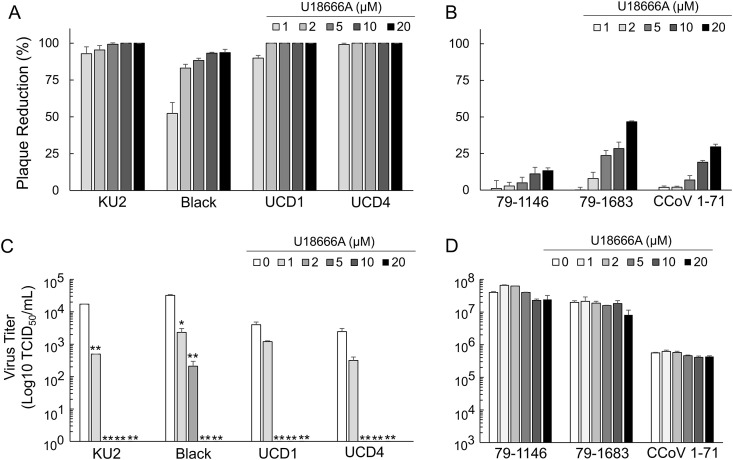
The relationship between the FCoV serotypes and antiviral effects of U18666A were investigated. The influence of U18666A on replication of 4 strains of type I FCoV (FCoV-I KU2, FCoV-I Black, FCoV-I UCD1, and FCoV-I UCD4), 2 strains of type II FCoV (FCoV-II 79-1146 and FCoV-II 79-1683), and 1 strain of type II CCoV (CCoV-II 1-71) in fcwf-4 cells was investigated. Pretreatment with U18666A reduced type I FCoVs plaque formation in a dose-dependent manner (Fig. 2 A). In contrast, the percentage of plaque reduction in type II FCoVs and type II CCoV was slightly affected by pretreatment with U18666A (Fig. 2B). The virus titers of type I FCoVs significantly decreased in the culture supernatant of cells pretreated with 2 μM or higher U18666A (Fig. 2C), and production of FCoV-I KU2, FCoV-I UCD1, and FCoV-I UCD4 into the culture supernatant was completely inhibited in cells pretreated with 2 μM U18666A. In contrast to type I FCoVs, pretreatment with U18666A did not affect the titer of type II FCoVs and type II CCoV in the supernatant of fcwf-4 cells (Fig. 2D).
Fig. 2.
Differential antiviral effects of U18666A against type I and II FCoV. (A) Plaque reduction of type I FCoV infected fcwf-4 cells treated with different concentrations of U18666A. (B) Plaque reduction of type II FCoV and type II CCoV infected fcwf-4 cells treated with different concentrations of U18666A. 79-1146: type II FIPV 79-1146. 79-1683: type II FECV 79-1683. (C) Virus titer of type I FCoV infected fcwf-4 cells treated with different concentrations of U18666A. **, p < 0.01 vs. 0 μM of U18666A. (D) Virus titer of type II FCoV and type II CCoV infected fcwf-4 cells treated with different concentrations of U18666A. 79-1146: type II FIPV 79-1146. 79-1683: type II FECV 79-1683.
3.3. Effects of exposure time on the antiviral activity of U18666A
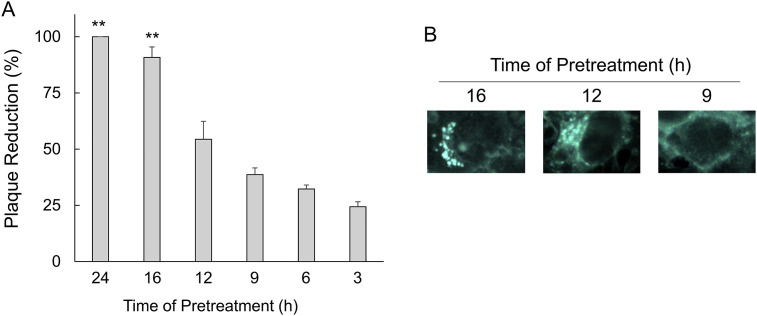
Pretreatment experiments were performed to determine the period at which U18666A exerted its antiviral effects. Pretreatment with U18666A significantly increased the degree of the reduction of FCoV-I KU2 plaque formation at 16 h prior to infection (Fig. 3 A). Cholesterol accumulation was observed in U18666A-pretreat cells by the reaction time (Fig. 3B). Cholesterol accumulation was noted in cells pretreated with U18666A for 16 h. Cholesterol accumulation were mostly lost in cells pretreated for 12 h, and completely disappeared in cells pretreated for 9 h.
Fig. 3.
Effects of exposure time on the antiviral activity of U18666A. (A) Plaque reduction of FCoV-I KU2 infected fcwf-4 cells pretreated with U18666A at different times. The results are shown as mean ± SE. Data represent four independent experiments. **, p < 0.01 vs pretreated with U18666A for 3 h. (B) Accumulation of intracellular cholesterol in fcwf-4 cells pretreated for the indicated time-points with U18666A.
3.4. Antiviral effects with compounds similar biochemical properties to U18666A
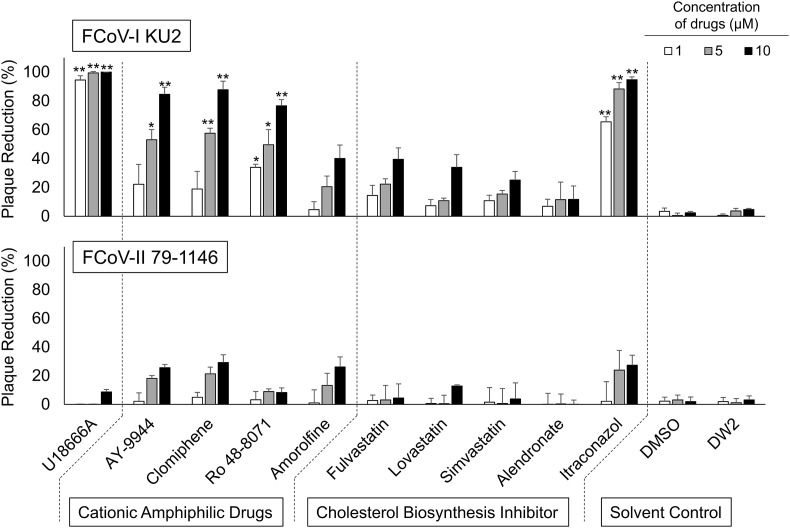
Inhibition of FCoV infection by compounds with biochemical properties similar to those of U18666A was investigated. Pretreatment with CADs, except Amorolfine, significantly increased the degree of reduction of FCoV-I KU2 plaque formation (Fig. 4 ). When cells were pretreated with cholesterol biosynthesis inhibitors, only itraconazole significantly inhibited FCoV-I KU2 plaque formation. No other cholesterol biosynthesis inhibitors showed significant inhibitory effects on FCoV-I KU2 plaque formation. No drug exhibited significant inhibitory effects on type II FCoV 79-1146 plaque formation. The selectivity index (SI) was calculated from the CC50 and half maximal effective concentrations (EC50) of each drug. For type I FCoV, the SI values of U18666A, AY-9944, Clomiphene, Ro 48-8071, and Itraconazole were high (Table 1 ). In contrast, for type II FCoV, the SI values of all drugs were low.
Fig. 4.
Effects of cationic amphiphilic drugs and cholesterol biosynthesis inhibitor on FCoV-I KU2 and FCoV-II 79-1146. The results are shown as mean ± SE. Data represent four independent experiments. **, p < 0.01 vs solvent control. *, p < 0.05 vs solvent control.
Table 1.
CC50, EC50, and SI value of Cationic Amphiphilic Drugs and Cholesterol Biosynthesis Inhibitor.
| Compounds | CC50 (μM) | FCoV-I KU2 |
FCoV-II 79-1146 |
||
|---|---|---|---|---|---|
| EC50 (μM) | SI | EC50 (μM) | SI | ||
| U18666A | 97.6 ± 3.7 | 0.2 ± 0.1 | 488.0 | >50.0 | <2.0 |
| AY-9944 | 66.7 ± 4.3 | 4.3 ± 0.1 | 15.4 | >50.0 | <1.3 |
| Clomiphene | 46.6 ± 10.8 | 3.7 ± 5.3 | 12.6 | 26.1 ± 8.9 | 1.8 |
| Ro 48-8071 | 248.7 ± 22.1 | 5.1 ± 0.4 | 48.8 | >100.0 | <2.5 |
| Amorolfine | 28.0 ± 3.5 | 13.7 ± 1.9 | 2.0 | 31.1 ± 2.2 | 0.9 |
| Fluvastatin | 74.4 ± 3.1 | 15.8 ± 2.2 | 4.7 | >50.0 | <1.5 |
| Lovastatin | 24.3 ± 1.9 | 25.9 ± 3.2 | 0.9 | >50.0 | <0.5 |
| Simvastatin | 9.6 ± 0.8 | 24.8 ± 0.3 | 0.4 | >50.0 | <0.2 |
| Alendronate | 153.3 ± 16.5 | 279.0 ± 33.4 | 0.5 | >100.0 | <1.5 |
| Itraconazol | 121.0 ± 5.0 | 0.7 ± 0.1 | 172.9 | 33.6 ± 3.6 | 3.6 |
Data are mean ± standard deviation.
3.5. Influence of HDACi on antiviral action of U18666A
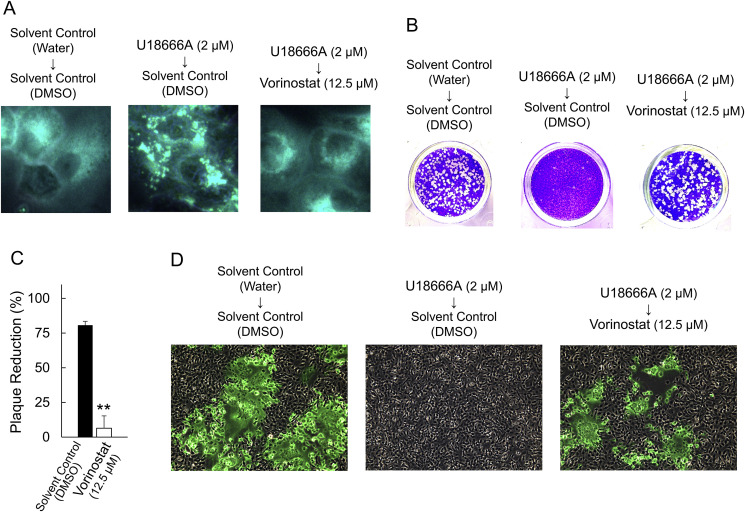
AY-9944, Clomiphene, Ro 48-8071, and Itraconazole, which significantly inhibited FCoV-I KU2 plaque formation, inhibit the function of cholesterol transporter NPC1, unlike the other drugs (Amorolfine, Fluvastatin, Lovastatin, Simvastatin, and Alendronate). Based on this, we assumed that inhibition of type I FCoV replication by U18666A is associated with reduction of NPC1 function, and investigated whether HDACi, which improves reduced NPC1 function, resolves U18666A-induced cholesterol accumulation and inhibition of type I FCoV replication. When an HDACi, Vorinostat, was added to U18666A-pretreated fcwf-4 cells, cholesterol accumulation were reduced (Fig. 5 A). The influence of Vorinostat on FCoV-I KU2 replication was investigated using plaque reduction assay. When Vorinostat was added to U18666A-pretreated fcwf-4 cells, the percentage of plaque reduction significantly decreased (Fig. 5B and C). The influence of Vorinostat on the N protein expression level in FCoV-infected cells was investigated using the fluorescent antibody method. The N protein levels of FCoV-I KU-2 specifically decreased in fcwf-4 cells pretreated with U18666A. On the other hand, the addition of Vorinostat to U18666A-pretreated fcwf-4 cells increased the level of FCoV-I KU-2 N protein (Fig. 5D).
Fig. 5.
Vorinostat reverts the U18666A-induced antiviral activity by cholesterol accumulation in fcwf-4 cells. (A) Vorinostat treatment reverts the effects of U18666A on cholesterol accumulation. (B) Vorinostat treatment reverts the effects of U18666A on plaque reduction of FCoV-I KU2. (C) Inhibition of antiviral effects of U18666A by Vorinostat. The results are shown as mean ± SE. Data represent four independent experiments. **, p < 0.01 vs solvent control. (D) Vorinostat treatment reverts the effects of U18666A on expression of FCoV-I KU2 N protein.
4. Discussion
Several studies have been made on the role of cholesterol in coronavirus infection. (Thorp and Gallagher, 2004, Nomura et al., 2004, Ren et al., 2008). However, the influence of the intracellular synthesis and transport systems of cholesterol has not been investigated. U18666A suppresses cholesterol biosynthesis and the function of cholesterol transporter NPC1. In this study, we investigated anti-FCoV activity of U18666A. U18666A induced intracellular cholesterol accumulation and strongly inhibited type I FCoV replication. In addition, treatment of cells with U18666A did not influence the cellular cholesterol level, suggesting that U18666A inhibits type I FCoV replication at a concentration suppressing cholesterol transporter but not influencing the synthesis system. In contrast to type I FCoV, no inhibitory effects of U18666A on type II FCoV replication were noted. We previously reported that type I FCoV infection is dependent on plasma membrane cholesterol, but type II FCoV does not (Takano et al., 2016). Our findings of the present and previous studies suggest that type I FCoV requires cholesterol to infect cells, but type II FCoV does not require cholesterol for its infection.
It was suggested that type II FCoV was arisen from a double recombination between type I FCoV and type II CCoV (Herrewegh et al., 1998, Terada et al., 2014). In FCoV-II 79-1146, the genomic region from ORF1b, ORF2 (encoding S protein), and ORF3c is derived from type II CCoV and the other regions are derived from type I FCoV (Herrewegh et al., 1998), suggesting that these genes are factors leading to differences in the virological properties between the FCoV serotypes. Of these genes, the homology of the ORF2 gene sequence between the serotypes is low similarity (45%) (Motokawa et al., 1996), suggesting that ORF2 is involved in the difference effects of U18666A between type I and II FCoV. S protein (encoded by ORF2) is an important protein for binding to the virus receptor and cell entry (Gallagher and Buchmeier, 2001). The physical properties of S protein are markedly different between types I and II FCoV. For example, type II FCoV S protein binds to the virus receptor, aminopeptidase N (APN), but type I FCoV S protein does not bind to APN (Hohdatsu et al., 1998). In addition, type I FCoV S protein binds to heparin, but type II FCoV S protein does not (de Haan et al., 2008). The differences in these S protein properties between the serotypes may also influence the differences in the effects of U18666A. To demonstrate this assumption, it is necessary to prepare a recombinant FCoV containing the type I FCoV ORF2 gene replacing the type II FCoV ORF2 gene. Confirmation of the influence of U18666A on replication of the recombinant FCoV is expected.
In this study, U18666A did not inhibit type II FCoV and type II CCoV production. On the contrary, it was previously reported that MβCD, a cholesterol extracting agents, inhibited type II CCoV but not type II FCoV (Pratelli and Colao, 2015, Takano et al., 2016). MβCD disrupts cholesterol- and caveolin-rich membrane domain (caveolae) (D'Alessio et al., 2005). Van Hamme et al. (2008) reported that Type II FCoV was shown to enter cells through caveolae-independent endocytosis. On the basis of this report and above, it is suggested that type II CCoV enters cells via caveolae.
The addition of U18666A 16 h before viral infection significantly inhibited type I FCoV replication. The duration of exposure to U18666A required for inhibiting type I FCoV replication was mostly consistent with that required for cholesterol accumulation in cells, strongly suggesting that when the cholesterol transporter is inhibited, type I FCoV replication is also inhibited. HCV replication is inhibited by U18666A. It has been reported that replicated virions were not released from endosomes when HCV-replicating cells were treated with U18666A for 16 h, i.e., HCV virions were accumulated with cholesterol in late endosomes (Elgner et al., 2016). Probably, type I FCoV replication is also inhibited by accumulation in late endosomes.
U18666A is an inhibitor of the cholesterol transporter NPC1 (Ko et al., 2001). U18666A binds NPC1 and block its function (Lu et al., 2015). In this study, AY-9944, Clomiphene, Ro 48-8071, and Itraconazole, which block NPC1 function similarly with U18666A (Shoemaker et al., 2013, Trinh et al., 2016), also exhibited type I FCoV replication-inhibitory effects, suggesting that type I FCoV replication was inhibited by blocking the NPC1 function. To verify this hypothesis, we investigated the influence of HDACi on the type I FCoV replication-inhibitory effects of U18666A. In contrast to U18666A, HDACi reverts NPC1 dysfunction (Pipalia et al., 2017). When the Vorinostat was added to U18666A-treated cells, intracellular cholesterol accumulation disappeared and type I FCoV replicability resumed. To our knowledge, reversion of U18666A-induced inhibition of virus replication by HDACi has not previously been reported. It is necessary to determine whether the same phenomenon is observed in Ebolavirus, dengue virus, and HCV.
It is suggested that breeds of cats can influence the susceptibility to FIP. Pesteanu-Somogyi et al. (2006) reported that the incidence of FIP among 13 breeds of cats. They showed that the incidence of FIP was the lowest in Siamese cats. Interestingly, the Siamese cat breed seems to have a genetic predisposition to NPC disease (Wenger et al., 1980). NPC1 dysfunction is a cause of NPC disease. These facts and the finding of our study suggest that the impaired NPC1 function is the cause of the low incidence of FIP in Siamese cats. That is to say, NPC1-deficient cats may be less sensitive to type I FCoV, which is dominant serotype in the field (Hohdatsu et al., 1992, Kummrow et al., 2005, Wang et al., 2014). Further investigation of type I FCoV sensitivity of Siamese cats is necessary.
U18666A strongly inhibited type I FCoV replication, but it did not inhibit type II FCoV replication. It was suggested that U18666A inhibits type I FCoV replication by suppressing the intracellular cholesterol transporter. The experiment using HDACi suggested that dysfunction of NPC1 is involved in the antiviral effects of U18666A. As type I FCoV is widely present in the field, U18666A may be applied as a therapeutic drug against FIP. It is necessary to investigate whether administration of U18666A exhibits therapeutic effects in cats with FIP and determine the cause of reversion of U18666A-induced inhibition of virus replication by HDACi.
Acknowledgements
This work was supported by JSPS KAKENHI (Grant-in-Aid for Scientific Research (B)) Grant Number JP16H05039.
References
- Aizaki H., Morikawa K., Fukasawa M., Hara H., Inoue Y., Tani H., Saito K., Nishijima M., Hanada K., Matuura Y., Lai M.M., Miyamura T., Wakita T., Suzuki T. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J. Virol. 2008;82:5715–5724. doi: 10.1128/JVI.02530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette J.E., Raaben M., Wong A.C., Herbert A.S., Obernosterer G., Mulherkar N., Kuehne A.I., Kranzusch P.J., Griffin A.M., Ruthel G., Dal Cin P., Dye J.M., Whelan S.P., Chandran K., Brummelkamp T.R. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenedella R.J. Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids. 2009;44:477–487. doi: 10.1007/s11745-009-3305-7. [DOI] [PubMed] [Google Scholar]
- Cenedella R.J., Jacob R., Borchman D., Tang D., Neely A.R., Samadi A., Mason R.P., Sexton P. Direct perturbation of lens membrane structure may contribute to cataracts caused by U18666A, an oxidosqualene cyclase inhibitor. J. Lipid Res. 2004;45:1232–1241. doi: 10.1194/jlr.M300469-JLR200. [DOI] [PubMed] [Google Scholar]
- D'Alessio A., Al-Lamki R.S., Bradley J.R., Pober J.S. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am. J. Pathol. 2005;166:1273–1282. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar A.A., Perlman S. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgner F., Ren H., Medvedev R., Ploen D., Himmelsbach K., Boller K., Hildt E. The intracellular cholesterol transport inhibitor U18666A inhibits the exosome-dependent release of mature hepatitis C virus. J. Virol. 2016;90:11181–11196. doi: 10.1128/JVI.01053-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R.J., Baker S.C., Baric R., Enjuanes L., Gorbalenya A.E., Holmes K.V., Perlman S., Poon L., Rottier P.J.M., Talbot P.J., Woo P.C.Y., Ziebuhr J. Family coronaviridae. In: King A., Adams M., Cartens E., Lefkowitz E., editors. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Academic Press; San Diego, CA: 2012. pp. 806–828. [Google Scholar]
- de Haan C.A.M., Haijema B.J., Schellen P., Schreur P.W., Te Lintelo E., Vennema H., Rottier P.J.M. Cleavage of group 1 coronavirus spike proteins: how furin cleavage is traded off against heparan sulfate binding upon cell culture adaptation. J. Virol. 2008;82:6078–6083. doi: 10.1128/JVI.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hamme E., Dewerchin H.L., Cornelissen E., Verhasselt B., Nauwynck H.J. Clathrin- and caveolae-independent entry of feline infectious peritonitis virus in monocytes depends on dynamin. J. Gen. Virol. 2008;89:2147–2156. doi: 10.1099/vir.0.2008/001602-0. [DOI] [PubMed] [Google Scholar]
- Herrewegh A.A., Smeenk I., Horzinek M.C., Rottier P.J., de Groot R.J. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 1998;72:4508–4514. doi: 10.1128/jvi.72.5.4508-4514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Sasamoto S., Koyama H. Antigenic analysis of feline coronaviruses with monoclonal antibodies (MAbs): preparation of MAbs which discriminate between FIPV strain 79-1146 and FECV strain 79-1683. Vet. Microbiol. 1991;28:13–24. doi: 10.1016/0378-1135(91)90096-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Okada S., Ishizuka Y., Yamada H., Koyama H. The prevalence of types I and II feline coronavirus infections in cats. J. Vet. Med. Sci. 1992;54:557–562. doi: 10.1292/jvms.54.557. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Izumiya Y., Yokoyama Y., Kida K., Koyama H. Differences in virus receptor for type I and type II feline infectious peritonitis virus. Arch. Virol. 1998;143:839–850. doi: 10.1007/s007050050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A., Matsuzaki Y. Cholesterol and chronic hepatitis C virus infection. Hepatol. Res. 2011;41:697–710. doi: 10.1111/j.1872-034X.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- Ko D.C., Gordon M.D., Jin J.Y., Scott M.P. Dynamic movements of organelles containing Niemann-Pick C1 protein: NPC1 involvement in late endocytic events. Mol. Biol. Cell. 2001;12:601–614. doi: 10.1091/mbc.12.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummrow M., Meli M.L., Haessig M., Goenczi E., Poland A., Pedersen N.C., Hofmann-Lehmann R., Lutz H. Feline coronavirus serotypes 1 and 2: seroprevalence and association with disease in Switzerland. Clin. Diagn. Lab. Immunol. 2005;12:1209–1215. doi: 10.1128/CDLI.12.10.1209-1215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F., Liang Q., Abi-Mosleh L., Das A., De Brabander J.K., Goldstein J.L., Brown M.S. Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. Elife. 2015;4 doi: 10.7554/eLife.12177. e12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J.M., Khromykh A.A., Parton R.G. Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host Microbe. 2007;2:229–239. doi: 10.1016/j.chom.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Motokawa K., Hohdatsu T., Aizawa C., Koyama H., Hashimoto H. Molecular cloning and sequence determination of the peplomer protein gene of feline infectious peritonitis virus type I. Arch. Virol. 1995;140:469–480. doi: 10.1007/BF01718424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa K., Hohdatsu T., Hashimoto H., Koyama H. Comparison of the amino acid sequence and phylogenetic analysis of the peplomer, integral membrane and nucleocapsid proteins of feline, canine and porcine coronaviruses. Microbiol. Immunol. 1996;40:425–433. doi: 10.1111/j.1348-0421.1996.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura R., Kiyota A., Suzaki E., Kataoka K., Ohe Y., Miyamoto K., Senda T., Fujimoto T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 2004;78:8701–8708. doi: 10.1128/JVI.78.16.8701-8708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. An update on feline infectious peritonitis: virology and immunopathogenesis. Vet. J. 2014;201:123–132. doi: 10.1016/j.tvjl.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesteanu-Somogyi L.D., Radzai C., Pressler B.M. Prevalence of feline infectious peritonitis in specific cat breeds. J. Feline Med. Surg. 2006;8:1–5. doi: 10.1016/j.jfms.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipalia N.H., Subramanian K., Mao S., Ralph H., Hutt D.M., Scott S.M., Balch W.E., Maxfield F.R. Histone deacetylase inhibitors correct the cholesterol storage defect in most Niemann-Pick C1 mutant cells. J. Lipid Res. 2017;58:695–708. doi: 10.1194/jlr.M072140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh M.K., Shui G., Xie X., Shi P.Y., Wenk M.R., Gu F. U18666A, an intra-cellular cholesterol transport inhibitor, inhibits dengue virus entry and replication. Antivir. Res. 2012;93:191–198. doi: 10.1016/j.antiviral.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Pratelli A., Colao V. Role of the lipid rafts in the life cycle of canine coronavirus. J. Gen. Virol. 2015;96:331–337. doi: 10.1099/vir.0.070870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Glende J., Yin J., Schwegmann-Wessels C., Herrler G. Importance of cholesterol for infection of cells by transmissible gastroenteritis virus. Virus Res. 2008;137:220–224. doi: 10.1016/j.virusres.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker C.J., Schornberg K.L., Delos S.E., Scully C., Pajouhesh H., Olinger G.G., Johansen L.M., White J.M. Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection. PLoS One. 2013;8:e56265. doi: 10.1371/journal.pone.0056265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Satomi Y., Oyama Y., Doki T., Hohdatsu T. Differential effect of cholesterol on type I and II feline coronavirus infection. Arch. Virol. 2016;161:125–133. doi: 10.1007/s00705-015-2655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y., Matsui N., Noguchi K., Kuwata R., Shimoda H., Soma T., Mochizuki M., Maeda K. Emergence of pathogenic coronaviruses in cats by homologous recombination between feline and canine coronaviruses. PLoS One. 2014;9:e106534. doi: 10.1371/journal.pone.0106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E.B., Gallagher T.M. Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J. Virol. 2004;78:2682–2692. doi: 10.1128/JVI.78.6.2682-2692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh M.N., Lu F., Li X., Das A., Liang Q., De Brabander J.K., Brown M.S., Goldstein J.L. Triazoles inhibit cholesterol export from lysosomes by binding to NPC1. Proc. Natl. Acad. Sci. U. S. A. 2016;114:89–94. doi: 10.1073/pnas.1619571114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.T., Chueh L.L., Wan C.H. An eight-year epidemiologic study based on baculovirus-expressed type-specific spike proteins for the differentiation of type I and II feline coronavirus infections. BMC Vet. Res. 2014;10:186. doi: 10.1186/s12917-014-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger D.A., Sattler M., Kudoh T., Snyder S.P., Kingston R.S. Niemann-Pick disease: a genetic model in Siamese cats. Science. 1980;208:1471–1473. doi: 10.1126/science.7189903. [DOI] [PubMed] [Google Scholar]
- de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.H., Plemenitas A., Fielding C.J., Peterlin B.M. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8460–8465. doi: 10.1073/pnas.1437453100. [DOI] [PMC free article] [PubMed] [Google Scholar]