Abstract
Objective
Cholangiocarcinoma is the second most common primary hepatobiliary malignancy with high incidence and recurrence rate. Ubiquitin-specific protease 8 (USP8) is recently reported to be involved in tumor progression. Herein, we aimed to investigate the effects of USP8 on the growth and metastasis abilities of cholangiocarcinoma cells.
Methods
The siRNA interference was used to knock down USP8 in cholangiocarcinoma cell lines QBC939 and RBE; Hucct-1 cells were transfected with pcDNA3.1-USP8 to up-regulate its expression. The effects of USP8 on cholangiocarcinoma were detected by cell function assays. We analyzed the expressions of USP8, Bcl2, Bax, cleaved caspase-3, cleaved caspase-9, Akt, p-Akt, Cyclin D1 and P70S6K by Western blot analysis.
Results
We demonstrated that knockdown of USP8 significantly inhibited the proliferation, migration and invasion of QBC939 and RBE cells in vitro, while USP8 overexpression showed significant promoting effects on Hucct-1 cells. Moreover, silencing of USP8 also promoted apoptosis in cholangiocarcinoma cells by regulating the Bcl-2/Bax axis and Caspase cascade; up-regulation of USP8 decreased apoptosis in Hucct-1 cells. Importantly, knockdown of USP8 inhibited activation of the Akt signaling pathway by decreasing the phosphorylation level of Akt and up-regulated p53 expression, while USP8 overexpression increased activation of the Akt signaling pathway in Hucct-1 cells. Further, IGF-1 could reverse the inhibitory effects of USP8 knockdown on the Akt signaling pathway and the proliferation of QBC939 and RBE cells.
Conclusion
Taken together, our findings suggest that USP8 exerts an oncogenic role in the progression of cholangiocarcinoma and may be a potential therapeutic target for cholangiocarcinoma treatment.
Keywords: cholangiocarcinoma, ubiquitin-specific protease 8, Akt signaling pathway
Introduction
Globally, cholangiocarcinoma is the second most common primary hepatobiliary malignancy after hepatocellular carcinoma, accounting for 10–15% of the incidence of hepatobiliary tumors.1–3 It is reported that the overall incidence of cholangiocarcinoma has gradually increased worldwide in recent decades, especially has the highest incidence in Southeast Asia.4,5 Cholangiocarcinoma is an aggressive tumor with no obvious clinical manifestations in the early stages, and most patients have advanced disease at the time of diagnosis.6,7 In addition, it is difficult to get into the anatomical location of cholangiocarcinoma, which poses a great challenge to the treatment of cholangiocarcinoma.6 Surgical treatment is the preferred treatment for cholangiocarcinoma, but it is effective only in the early-stage disease, and cholangiocarcinoma is not sensitive to chemotherapy.7,8 What is worse, the recurrence rate of cholangiocarcinoma patients is 50% within 2 years after surgery.9 In addition, more than two-thirds of patients are unable to undergo surgery, and the 5-year survival rate for cholangiocarcinoma is approximately 5–15%.10 The invasiveness and metastasis of cholangiocarcinoma seriously affect the survival of patients. Therefore, it is urgent to investigate the detailed pathogenesis of cholangiocarcinoma in order to identify novel therapeutic targets for cholangiocarcinoma treatment.
Degradation of proteins within cells is an extremely complex and highly ordered process, which is mainly accomplished by the ubiquitin system. Ubiquitination which promotes protein degradation is known to play key roles in controlling protein abundance to maintain normal cellular processes, including cell cycle, proliferation, apoptosis, differentiation, metastasis, signal transduction, and inflammatory immunity.11–14 Abnormal ubiquitin metabolic pathway can lead to many diseases, including cancer, which is caused by the acceleration or slowdown of the degradation of disease-related proteins as substrates in ubiquitin degradation pathway.13,14 It is generally known that ubiquitination is a reversible post-translational modification, which is regulated by ubiquitin ligases and deubiquitinases (DUBs).15 DUBs function to hydrolyze ubiquitin from the ubiquitinated substrates, and the ubiquitin-specific proteases (USPs) are identified to be the largest DUBs subfamily in humans.16 Increasing studies have revealed that USPs is correlated with tumorigenesis and cancer progression.17–20
Ubiquitin-specific protease 8 (USP8), also known as UBPY, is a member of USPs family and functions in endosomal sorting of proteins.21 It has been revealed the critical role of USP8 in some diseases, such as Cushing’s disease.22 Recently, emerging evidences show that USP8 is also involved in tumor progression.23,24 Byun et al find that downregulation of USP8 could selectively inhibit the viability of gefitinib-resistant non-small cell lung cancer (NSCLC) cells and promote apoptosis, suggesting that USP8 may function as a potential drug target for gefitinib-resistant NSCLC.24 Yan et al report that USP8 is upregulated in cervical squamous cell carcinoma tissues, which promotes cell proliferation and invasion and is related to poor prognosis.23 However, USP8 is revealed to be downregulated in breast cancer cells, and its high expression is associated with better prognosis of patients.25 It seems that the precise roles of USP8 may depend on the difference in tissue location. However, whether UPS8 is implicated in the progression of cholangiocarcinoma is still poorly understood. Therefore, we investigated the biological role of USP8 in cholangiocarcinoma. Our data demonstrate that USP8 functions an oncogenic role in the growth and invasion of cholangiocarcinoma cells, indicating that USP8 may act as a potential therapeutic target for cholangiocarcinoma treatment.
Materials and Methods
Cell Culture and Transfection
The human cholangiocarcinoma cell lines QBC939, RBEandHucct-1 obtained from Cell Bank of Type Culture of the Chinese Academy of Sciences (Shanghai, China) were cultured with RPMI-1640 medium (Hyclone, Logan, UT, USA) supplemented with 10%FBS and penicillin/streptomycin at 37°C with 5% CO2. After entering the logarithmic growth phase, cells were washed 3 times with PBS, digested with trypsin and planted into a 6-well plate. When the cell density in the well plate reached 50–60% confluence, the cells were transfected with siRNAs (50nM) using Lipofectamine 2000 (Invitrogen, USA). The siRNA targeting USP8 (si-USP8) was synthesized by OriGene (Beijing, China), and siRNA Negative Control (OriGene) was used as negative control (si-NC). The siRNA-USP8 sequences were 5ʹ-CCAAAGAGAAAGGAGCAAT-3ʹ (529–548). The pcDNA3.1-USP8 expressing vector was obtained from Ribobio (Guangzhou, China) and transfected into Hucct-1 cells using Lipofectamine 2000. IGF-1 was obtained from Abcam (Cambridge, UK) and cells were treated with IGF-1 (200 ng/mL) for 24 h.
qRT-PCR Analysis
Following being transfected with siRNAs for 24 h, total RNA was extracted from cells using an Ultrapure RNA Kit (CWBIO, Beijing, China) and reverse transcripted into cDNA by the HiFiScript cDNA Synthesis Kit (CWBIO). qRT-PCRs were performed using FastSYBR Mixture (CWBIO) to examine the expression of USP8. The reactions were as follows: 95°C for 5 min, 95°C for 30 s, 40 cycles, 60°C for 45 s, 72°C for 30 min. The 2−ΔΔCt method was used to calculate the relative expression of USP8. The primers used in this study were synthesized by GENEWIZ (Suzhou, China): β-actin forward: 5ʹ-CCCGAGCCGTGTTTCCT-3ʹ, reverse: 5ʹ-GTCCCAGTTGGTGACGATGC-3ʹ; USP8 forward: 5ʹ- CTGCTGTGGCTTCAGTTCCT-3ʹ, reverse: 5ʹ- GGAAATAATCCTGCTGTTGCTTGA-3ʹ.
Western Blot Analysis
Cells transfected with siRNAs for 48 h were lysed with RIPA lysate (CWBIO) to extract protein, and the concentration of proteins was determined using the BCA assay (CWBIO). Twenty micrograms of proteins were separated by 10% SDS PAGE gel and electrotransferred to polyvinylidene fluoride membranes (PVDF; Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk at room temperature for 1 h and then incubated with primary antibodies (1:1000; Proteintech, USA) overnight at 4°C. Following washing with TBST, the membranes were then with HRP-conjugated secondary antibodies (1:5000; Proteintech) at room temperature for 1 h. The protein bands were visualized using an ECL kit (Millipore) and quantified using the Image Lab software (Bio-Rad, USA).
CCK8 Assay
CCK8 assay was performed to examine cell proliferation when USP8 was knocked down. Cells transfected with siRNAs for 24 h were digested by trypsinase and seeded in a 96-well plate at a density of 3000 cells/well. Cell viability was measured at 0, 24, 48 and 72 h, respectively. Before detection, 10 μL of CCK8 reagent (Solarbio, Beijing, China) was added to each well, and cells were incubated at 37°C for 1 h. The absorbance at 450 nm was detected by a microplate reader.
Colony Formation Assay
Colony formation assay was performed to evaluate the clonogenic ability of QBC939 and RBE cells when USP8 was silenced. Following 24 h of transfection, cells were digested and planted in 35mm dishes (300 cells/dish). After being cultured with RPMI-1640 medium at 37°C for 1–2 weeks, cells were fixed with 4%paraformaldehyde for 30 min and stained with 0.1% crystal violet for another 30 min. The visible colonies were counted and captured.
Transwell Assay
Transwell chambers (8μm pore size; Millipore) were performed for cell migration and invasion assays, while chambers coated with Matrigel were needed for invasion. Cells (1 × 105 cells) after 24 h of transfection were planted into the upper chamber, and the lower chambers were filled with 500 μL of medium containing 10% FBS. Following incubation at 37°C for 24 h, the migrated or invaded cells were fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet for another 20 min.
Gelatin Zymogram Assay
Cholangiocarcinoma cells QBC939 and RBE transfected with siRNAs for 24 h were cultured in serum-free medium at 37°C for 24 h. The supernatant was collected and separated by 10% SDS-PAGE gel containing with 0.5mg/mL gelatin. After electrophoresis, the gel was eluted, stained with 0.25% Coomassie Brilliant Blue R250 for 4 h at room temperature. Then, the gel was scanned by the Image Scanner (Ahmad Sohm, USA) and analyzed by IMAGISOANT TL V2003 software after being decolorized.
Flow Cytometry
After 24 h of transfection, the cells were cultured in serum-free medium for 24 h. Cells were washed with PBS and centrifuged at 4°C. The cells were resuspended with binding buffer to adjust the cell density to 1–5 × 106/mL. One hundred microliters of cell suspension was stained with 5 μL Annexin V/FITC (Beyotime, Beijing, China) at room temperature for 5 min in dark and stained with 10 μL PI (Beyotime) for 5 min. A flow cytometer was performed to detected cell apoptosis, and the Flowjo software was used to analyze the results.
Statistical Analysis
Data are presented as mean ± SD of at least triplicate independent experiments and analyzed using GraphPad software 7.0 (GraphPad Inc., USA). Student’s t-test or one-way ANOVA followed by the LSD post hoc test was conducted to compare the differences between groups. P <0.05 indicates a statistically significant difference.
Results
USP8 Regulates Proliferation of Cholangiocarcinoma Cells
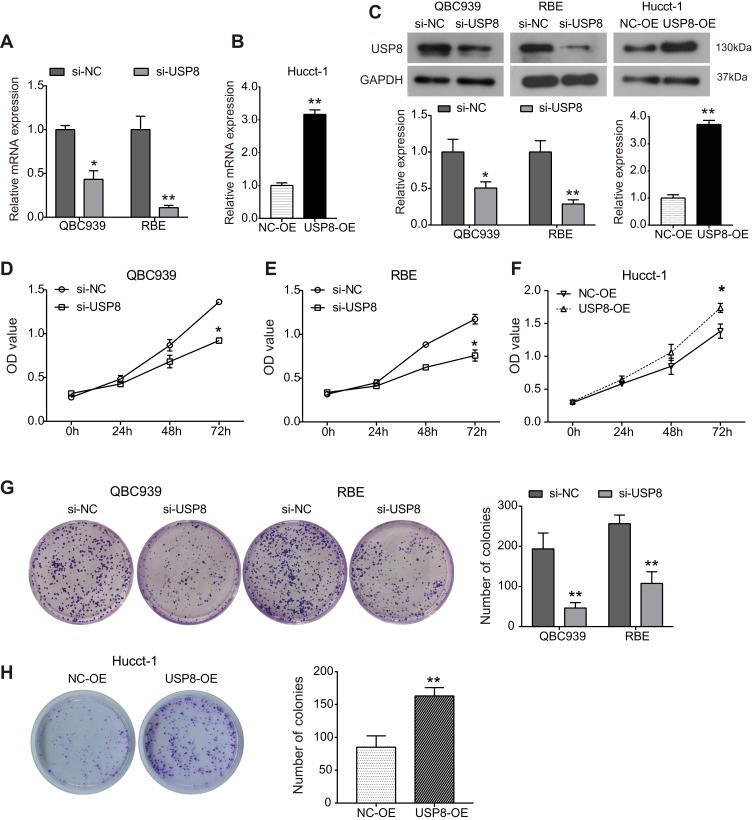
To investigate the biological function of USP8 in the progression of cholangiocarcinoma, QBC939 and QBE cells were transfected with siRNA targeting USP8 (si-USP8), siRNA-control was used as negative control (si-NC); Hucct-1 cells were transfected with pcDNA3.1-USP8 (USP8-OE), and a blank vector was used as negative control (NC-OE). As shown in Figure 1A–C, the expression of USP8 was markedly decreased in si-USP8-transfected cells compared with control cells at both mRNA and protein levels, and USP8 expression was markedly up-regulated in Hucct-1 cells transfected with pcDNA3.1-USP8. CCK8 assay was carried out to assess the effect of USP8 on cell proliferation. The outcomes showed that silencing of USP8 significantly inhibited the proliferation of QBC939 cells in comparison with control cells (Figure 1D). Similar inhibitory effect caused by si-USP8 on cell proliferation was also observed in RBE cells (Figure 1E). Consistently, upregulation of USP8 significantly enhanced the viability of Hucct-1 cells (Figure 1F). Consistent with the CCK8 results, the colony formation outcomes were also evident. As shown in Figure 1G, knockdown of USP8 resulted in significant decrease in the number of formed colonies in both QBC939 and QBE cells, indicating that down-regulation of USP8 inhibits clonogenic ability of cholangiocarcinoma cells. As indicated in Figure 1H, the clonogenic ability of Hucct-1 cells was significantly promoted by USP8 overexpression.
Figure 1.
USP8 regulates the growth of cholangiocarcinoma cells. Cholangiocarcinoma cell lines QBC939 and RBE were transfected with siRNA-USP8 to down-regulate its expression, and siRNA negative control was used as negative control group (si-NC); Hucct-1 cells were transfected with pcDNA3.1-USP8 (USP8-OE) to up-regulate its expression, and pcDNA3.1 vector was used as negative control (NC-OE). (A) RT-PCR assay was used to examine the relative expression of USP8 mRNA in QBC939 and RBE cells transfected with siRNAs for 24h. *P<0.05, **P<0.01 by Student’s t-test vs negative control; n = 3. (B) Expression of USP8 mRNA in Hucct-1 cells after transfected withpcDNA3.1-USP8. **P<0.01by Student’s t-test vs negative control; n = 3. (C) The protein expression of USP8 was detected using Western blot analysis. *P<0.05, **P<0.01by Student’s t-test vs negative control; n = 3. (D–E) CCK8 assay was performed to assess QBC939 (D), RBE (E) and Hucct-1 (F) cells proliferation after indicated treatment. *P<0.05 by Student’s t-test vs negative control; n = 3. (G and H) Colony formation assay was used to examine the clonogenic ability of cholangiocarcinoma cells. **P<0.01by Student’s t-test vs negative control; n = 3.
Down-Regulation of USP8 Inhibits Migration and Invasion Abilities of Cholangiocarcinoma Cells
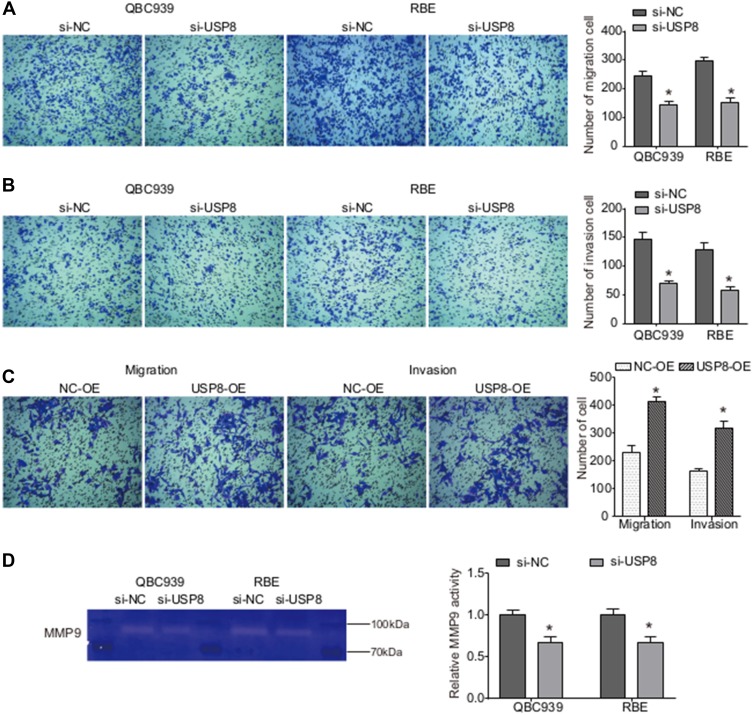
To get a preliminary understanding of the impact of USP8 on cholangiocarcinoma metastasis, a transwell assay was performed. As shown in Figure 2A, the number of migrating QBC939 and QBE cells was both significantly reduced by si-USP8 compared with the corresponding control cells. Moreover, the invasion assay demonstrated that decrease in USP8 expression inhibited the invasion abilities of both QBC939 and QBE cells (Figure 2B). However, USP8 overexpression significantly enhanced both migration and invasion abilities of Hucct-1 cells (Figure 2C).
Figure 2.
USP8 regulates the migration and invasion abilities of cholangiocarcinoma cells. (A and B) Following transfection of 24 h, Transwell assays were performed to assess the migration (A) and invasion (B) of QBC939 and RBE cells. *P<0.05by Student’s t-test vs negative control; n = 3. (C) Hucct-1 cells migration and invasion were assessed by Transwell assay. *P<0.05 by Student’s t-test vs negative control; n = 3. (D) The MMP9 activity was examined using gelatin zymogram assay when USP8 was knocked down. *P<0.05 by Student’s t-test vs negative control; n = 3.
It is generally known that MMP9 plays critical role in cell adhesion, tumor invasion and metastasis. Hence, we examined the MMP9 activity in cholangiocarcinoma cells after USP8 was silenced. Gelatin zymography analysis showed that knockdown of USP8 significantly inhibited the MMP9 activity in both QBC939 and QBE cells (Figure 2D). Collectively, down-regulation of USP8 may inhibit migration and invasion of cholangiocarcinoma cells by decreasing MMP9 activity.
Silencing of USP8 Promotes Apoptosis in Cholangiocarcinoma Cells
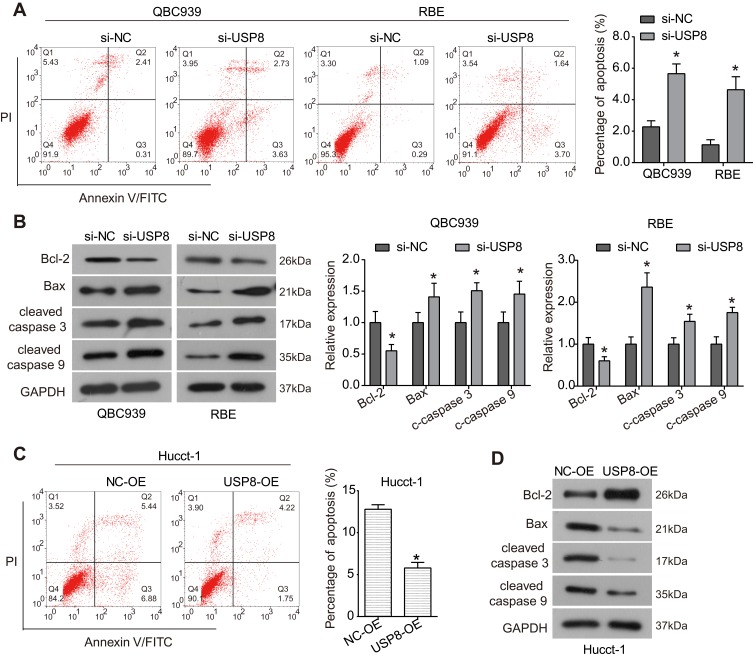
To investigate whether USP8 affects apoptosis in cholangiocarcinoma cells, flow cytometry assay was carried out. As demonstrated in Figure 3A, the percentage of apoptotic cells in QBC939 and QBE cells transfected with si-USP8 was sharply increased compared to the control group. The expression of apoptosis-related proteins was further examined by Western blot to understand the mechanism underlying the induced apoptosis. As shown in Figure 3B, the expression of anti-apoptotic protein Bcl-2 was significantly down-regulated by si-USP8 in QBC939 and QBE cells, while the expression of pro-apoptotic proteins Bax, cleaved Caspase 3 and cleaved Caspase 9 was up-regulated by knockdown of USP8 in comparison with the control group. However, up-regulation of USP8 significantly inhibited the percentage of apoptotic Hucct-1 cells (Figure 3C), up-regulated the expression of Bcl-2 and down-regulated the expression of Bax, cleaved Caspase 3 and cleaved Caspase 9 (Figure 3D). Taken together, these results indicate that USP8 may regulate apoptosis in cholangiocarcinoma cells through regulation of the Bcl-2/Bax axis and Caspase cascade.
Figure 3.
USP8 regulates apoptosis in cholangiocarcinoma cells. (A) Apoptosis in QBC939 and RBE cells transfected with siRNAs was examined using Flow cytometry assay. *P<0.05 by Student’s t-test vs negative control; n = 3. (B) After being transfected with siRNAs for 48 h, the expression of apoptosis-related proteins Bcl-2. Bax, cleaved Caspase 3 and cleaved Caspase 9 was examined using Western blot analysis. *P<0.05 by Student’s t-test vs negative control; n = 3. (C) Cell apoptosis of Hucct-1 cells was examined using Flow cytometry assay. *P<0.05 by Student’s t-test vs negative control; n = 3. (D) Expression of apoptosis-related proteins in Hucct-1 cells after indicated treatment.
Knockdown of USP8 Suppresses Activation of the Akt Signaling Pathway in Cholangiocarcinoma Cells
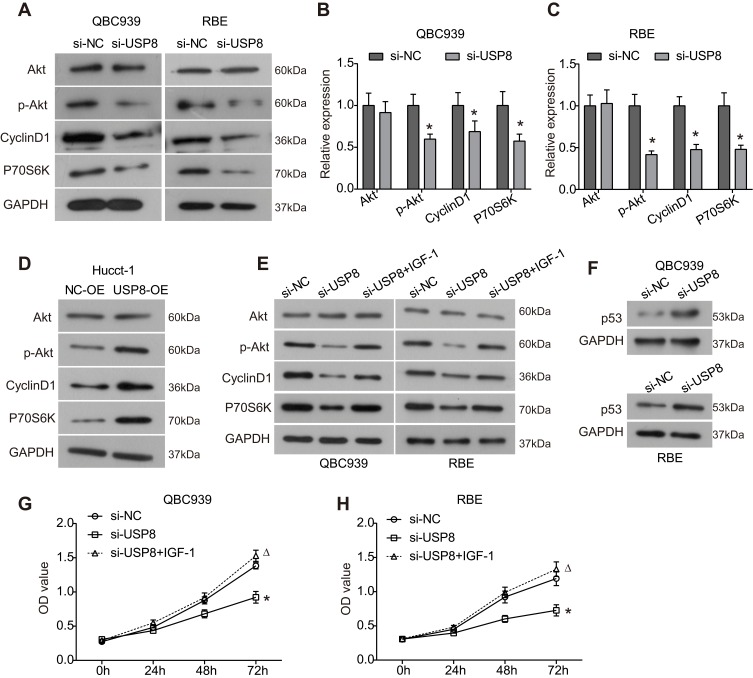
To determine whether UPS8 affects the Akt signaling pathway which plays critical roles in cellular physiological function and tumor progression, Western blot analysis was conducted. As presented in Figure 4A–C, downregulation of UPS8 resulted in significant decreased phosphorylation level of Akt (p-Akt) in QBC939 and RBE cells, while the expression of total Akt was not affected. Accordingly, the expression of downstream proteins Cyclin D1 and p70S6k was significantly downregulated by loss of USP8 in cholangiocarcinoma cells (Figure 4A–C). In Hucct-1 cells, the expression of p-Akt, Cyclin D1 and p70S6k was up-regulated by USP8 overexpression compared to the control group (Figure 4D). Moreover, IGF-1, the Akt pathway activator, significantly reversed the inhibitory effects of USP8 knockdown on the expression of p-Akt, Cyclin D1 and p70S6k (Figure 4E). Further, the inhibitory effect of si-USP8 on cell proliferation was significantly reversed by IGF-1 compared to the si-USP8 group (Figure 4G and H). Thus, these data indicated that the Akt signaling pathway is involved in the oncogenic role of USP8 in the progression of cholangiocarcinoma. Additionally, we also found that the expression of p53, the key tumor suppressor, was significantly up-regulated after USP8 was silenced in QBC939 and RBE cells (Figure 4F).
Figure 4.
Knockdown of USP8 inhibits activation of the Akt signaling pathway in cholangiocarcinoma cells. (A) After transfection of 48 h, the expression of key components of the Akt signaling pathway in QBC939 and RBE cells was examined using Western blot analysis. (B and C) Quantitative analysis of the results of Western blot analysis in QBC939 (B) and RBE cells (C). *P<0.05 by Student’s t-test vs negative control; n = 3. (D) Expression of the Akt signaling pathway-related proteins in Hucct-1 cells after indicated treatment. (E) Expression of the Akt signaling pathway-related proteins in QBC939 and RBE cells after indicated treatment. (F) Expression of p53 in QBC939 and RBE cells after USP8 was silenced. (G and H) CCK8 assay was used to assess the proliferation of QBC939 and RBE cells after indicated treatment.*P< 0.05 by ANOVA vs negative control; ΔP< 0.05 by ANOVA vs USP8-silenced group; n = 3.
Discussion
Invasion and metastasis of tumor cells are the main characteristics of malignant phenotype of tumors, and important factors affecting the prognosis of cancer patients. In addition, high recurrence rate is also a key factor affecting the prognosis of patients with cholangiocarcinoma. Therefore, inhibiting the metastatic activity of cholangiocarcinoma cells is one of the important therapeutic strategies for cholangiocarcinoma.
As mentioned above, as a member of the USP family, the largest deubiquitinase family, USP8 has been proved to be involved in the development of various tumors, including breast cancer,25 lung cancer24,26 and cervical squamous cell carcinoma.23 USP8 is revealed to play an oncogenic role in lung cancer and cervical squamous cell carcinoma, and its high expression related to poor prognosis,23,26 whereas USP8 is reported to might predict better survivals of breast cancer patients,25 these results suggest the different roles of USP8 in different tumors. In the present study, for the first time, we demonstrated that knockdown of USP8 significantly inhibited the proliferation, migration and invasion of cholangiocarcinoma cells, and decreased the MMP9 activity, while USP8 overexpression showed significant promoting effects, suggesting that USP8 functions as an oncogene in the growth and metastatic ability of cholangiocarcinoma.
Evading programmed death is one of the main features of tumor cells. Therefore, promoting tumor cell apoptosis may be an effective method to inhibit tumor growth. Jeong et al show that USP8 prevents extrinsic apoptosis by deubiquitylating and stabilizing the long isoform of FLICE-like inhibitory protein (FLIPL) which is a key regulator of death receptor-mediated apoptosis, and knockdown of USP8 could promote death receptor-induced apoptosis through downregulation of FLIPL and result in up-regulation of cleaved Caspase 8 and cleaved Caspase 3 in HeLa cells.27 Herein, we found that silencing of USP8 could induce apoptosis in cholangiocarcinoma cells, down-regulate the expression of Bcl-2, and up-regulate the expression of Bax, cleaved Caspase 3 and cleaved Caspase 9. As well known, Bcl-2/Bax and Caspase 9 are critical regulators involved intrinsic apoptosis pathway, and Caspase 3 is the key executioner in a series of apoptotic events.28 Collectively, USP8 regulates apoptosis in cholangiocarcinoma through regulating the intrinsic apoptosis pathway, and the effect of USP8 on extrinsic apoptosis will be further explored in the future.
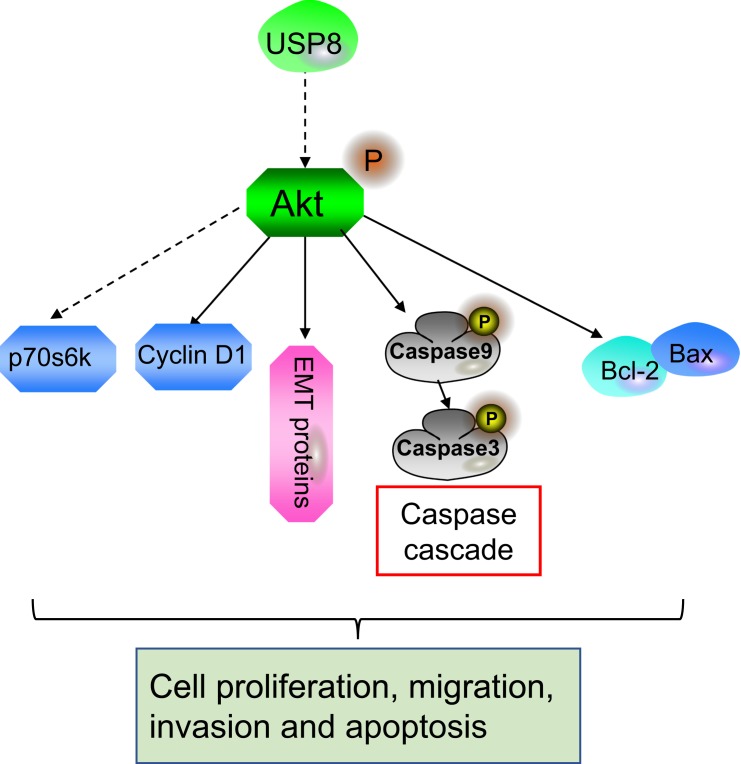
It has been revealed that USP8 can modulate intracellular transport of epidermal growth factor receptor (EGFR) and regulate its recycling through deubiquitination to regulate the stabilization of EGFR.29 Kim et al show that USP8 is over-expressed in lung adenocarcinomas, mutually correlated with overexpression of EGFR, and regulates the expression and half-life of EGFR.26 As generally known, EGFR is frequently dysregulated in multiple tumor types and plays a critical role in tumor progression.30 Therefore, the stabilization of EGFR signaling pathway may be involved in the tumor-promoting effect of USP8. Moreover, Panner et al report that USP8 is a downstream target of Akt, and the expression level of USP8 is negatively correlated with p-Akt level in glioblastoma multiforme xenografts.31 It has been widely known that the Akt signaling pathway plays critical roles in regulation of various cellular physiologies, as well as tumorigenesis and cancer development.32 The Akt signaling pathway is therefore considered a major target for cancer research.33 Herein, our data revealed that silencing of USP8 could markedly inhibit activation of the Akt signaling pathway by decreasing the phosphorylation of Akt in cholangiocarcinoma cells. Moreover, the inhibitory effects of USP8 knockdown on the Akt signaling pathway and cell proliferationwerereversed by IGF-1. Collectively, there may be a degenerative feedback loop of USP8-Akt (Figure 5), and the Akt signaling pathway may be involved in the tumor-promoting effects of USP8 in cholangiocarcinoma.
Figure 5.
The model of USP8 in the progression of cholangiocarcinoma cells.
Conclusion
In this study, we for the first time demonstrated that USP8 exerts an oncogenic role in the progression of cholangiocarcinoma, decrease in USP8 expression can significantly inhibit cell proliferation, migration and invasion, as well as promote apoptosis in cholangiocarcinoma cells. These findings suggest that USP8 might be a potential target for therapy of cholangiocarcinoma.
Funding Statement
This work was supported by the National Natural Science Foundation of China 81801163; Doctor Fund of Shandong Natural Science Foundation ZR201807060846; China Postdoctoral Science Foundation 2018M640636, and Jinan Clinical Medical Science and Technology Innovation Plan 201907065.
Disclosure
All authors declare that they have no conflicts of interest regarding this work.
References
- 1.Sumera R, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2017;15:95. doi: 10.1038/nrclinonc.2017.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spolverato G, Vitale A, Cucchetti A, et al. Can hepatic resection provide a long-term cure to patients with intrahepatic cholangiocarcinoma? Cancer. 2015;47(22):e51–e51. [DOI] [PubMed] [Google Scholar]
- 4.Bertuccio P, Bosetti C, Levi F, Decarli A, Negri E, Vecchia CL. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013;24(6):1667–1674. doi: 10.1093/annonc/mds652 [DOI] [PubMed] [Google Scholar]
- 5.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–125. doi: 10.1055/s-2004-828889 [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–517; discussion 517–509. doi: 10.1097/00000658-200110000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li JX, Ding XM, Han S, Wang K, Jiao CY, Li XC. mir-637 inhibits the proliferation of cholangiocarcinoma cell QBC939 through interfering CTSB expression. Eur Rev Med Pharmacol Sci. 2018;22(5):1265. [DOI] [PubMed] [Google Scholar]
- 8.Ruzzenente A, Conci S, Valdegamberi A, Pedrazzani C, Guglielmi A. Role of surgery in the treatment of intrahepatic cholangiocarcinoma. Eur Rev Med Pharmacol Sci. 2015;19(15):2892. [PubMed] [Google Scholar]
- 9.Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153(6):811–818. doi: 10.1016/j.surg.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noji T, Tsuchikawa T, Okamura K, et al. Resection and reconstruction of the hepatic artery for advanced perihilar cholangiocarcinoma: result of arterioportal shunting. J Gastrointest Surg. 2015;60(4):675–681. doi: 10.1007/s11605-015-2754-y [DOI] [PubMed] [Google Scholar]
- 11.Wertz IE, O’Rourke KM, Honglin Z, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430(7000):694–699. doi: 10.1038/nature02794 [DOI] [PubMed] [Google Scholar]
- 12.Yang Y. Regulation of apoptosis: the ubiquitous way. FASEB J. 2003;17(8):790–799. doi: 10.1096/fj.02-0654rev [DOI] [PubMed] [Google Scholar]
- 13.Trotman LC, Xinjiang W, Andrea A, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128(1):141–156. doi: 10.1016/j.cell.2006.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin X, Yang C, Fan P, et al. CDK5/FBW7-dependent ubiquitination and degradation of EZH2 inhibits pancreatic cancer cell migration and invasion. J Biol Chem. 2017;292(15):6269–6280. doi: 10.1074/jbc.M116.764407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J, Hu Q, Peng H, et al. The ubiquitin-specific protease USP8 deubiquitinates and stabilizes Cx43. J Biol Chem. 2018;9(1):jbc.RA117.001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695(1):189–207. doi: 10.1016/j.bbamcr.2004.10.003 [DOI] [PubMed] [Google Scholar]
- 17.Sajjad H, Ying Z, Galardy PJ. DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle. 2009;8(11):1688–1697. doi: 10.4161/cc.8.11.8739 [DOI] [PubMed] [Google Scholar]
- 18.Carrà G, Panuzzo C, Torti D, et al. Inhibition OF USP7 induces selective cancer cell death in chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2016;16:S49–S50. doi: 10.1016/j.clml.2016.07.07127521324 [DOI] [Google Scholar]
- 19.An T, Gong Y, Li X, et al. USP7 inhibitor P5091 inhibits Wnt signaling and colorectal tumor growth. Biochem Pharmacol. 2017;131(1):29–39. doi: 10.1016/j.bcp.2017.02.011 [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wang H, Tian L, Li H. Expression of USP7 and MARCH7 is correlated with poor prognosis in epithelial ovarian cancer. Tohoku J Exp Med. 2015;239(3):165–175. doi: 10.1620/tjem.239.165 [DOI] [PubMed] [Google Scholar]
- 21.Yeates EF, Tesco G. The endosomal-associated deubiquitinating enzyme USP8 regulates BACE1 ubiquitination and degradation. J Biol Chem. 2016;291(30):jbc.M116.718023. doi: 10.1074/jbc.M116.718023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faucz FR, Tirosh A, Tatsi C, et al. Somatic USP8 gene mutations are a common cause of pediatric cushing disease. J Clin Endocrinol Metab. 2017;102(8):2836–2843. doi: 10.1210/jc.2017-00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan M, Zhao C, Wei N, Wu X, Cui J, Xing Y. High expression of Ubiquitin-Specific Protease 8 (USP8) is associated with poor prognosis in patients with cervical squamous cell carcinoma. Med Sci Monit. 2018;24:4934–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byun S, Lee SY, Lee J, et al. USP8 is a novel target for overcoming gefitinib resistance in lung cancer. Clin Cancer Res. 2013;19(14):3894–3904. doi: 10.1158/1078-0432.CCR-12-3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han Q, Jun K, Yunfei C, Gang L. The expression of ubiquitin-specific peptidase 8 and its prognostic role in patients with breast cancer. J Cell Biochem. 2018;119(12):10051–10058. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Shiba-Ishii A, Nakagawa T, et al. Ubiquitin-specific protease 8 is a novel prognostic marker in early-stage lung adenocarcinoma. Pathol Int. 2017;67(6). doi: 10.1111/pin.12546 [DOI] [PubMed] [Google Scholar]
- 27.Jeong M, Lee EW, Seong D, et al. USP8 suppresses death receptor-mediated apoptosis by enhancing FLIPL stability. Oncogene. 2017;36(4):458–470. doi: 10.1038/onc.2016.215 [DOI] [PubMed] [Google Scholar]
- 28.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710 [DOI] [PubMed] [Google Scholar]
- 29.Kasahara K, Aoki H, Kiyono T, et al. EGF receptor kinase suppresses ciliogenesis through activation of USP8 deubiquitinase. Nat Commun. 2018;9(1). doi: 10.1038/s41467-018-03117-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciardiello F, Tortora G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer. 2003;39(10):1348–1354. doi: 10.1016/S0959-8049(03)00235-1 [DOI] [PubMed] [Google Scholar]
- 31.Panner A, Crane CA, Weng C, et al. USP8 links the PTEN-Akt-AIP4 pathway to the control of FLIPS stability and TRAIL sensitivity in glioblastoma multiforme. Cancer Res. 2010;70(12):5046. doi: 10.1158/0008-5472.CAN-09-3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igor V, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839 [DOI] [PubMed] [Google Scholar]
- 33.Kumar A, Rajendran V, Sethumadhavan R, Purohit R. AKT kinase pathway: a leading target in cancer research. Sci World J. 2013;2013(1):756134. doi: 10.1155/2013/756134 [DOI] [PMC free article] [PubMed] [Google Scholar]