Short abstract
Background
We retrospectively reviewed the data of three patients with intravenous leiomyomatosis (IVL), one of whom had intracardiac leiomyomatosis, and analyzed their clinical symptoms, preoperative assessment findings, operative approaches, and recurrence.
Case presentation: The present study describes three cases of IVL extending into the inferior vena cava, even as far as the right atrium and ventricle, and discusses the imaging findings and differential diagnosis of this tumor entity. The three patients, two of whom were diagnosed during the first operation and one of whom had a giant pelvic mass, were surgically treated with complete tumor resection, hysterectomy, and bilateral salpingo-oophorectomy. The pathological examination findings were suggestive of IVL. The duration of time from the first myomectomy or hysterectomy to IVL occurrence ranged from 2 to 18 months. No signs of recurrence were observed during follow-up. Computed tomography and magnetic resonance imaging played a vital role in the diagnostic process and presurgical assessment.
Conclusion
In clinical practice, IVL should be considered before surgery for a broad ligament myoma or giant pelvic mass. Surgery should always aim for complete tumor excision and include hysterectomy and bilateral salpingo-oophorectomy. Vascular reconstruction computed tomography is a good choice for diagnosis and follow-up.
Keywords: Intravenous leiomyomatosis (IVL), intracardiac leiomyomatosis (ICL), broad ligament leiomyoma, giant pelvic mass, hysterectomy, bilateral salpingo-oophorectomy
Introduction
Intravenous leiomyomatosis (IVL) is a rare uterine neoplasm characterized by nodular masses of benign smooth muscle cells growing within veins. The incidence of IVL is low; fewer than 300 patients with IVL were reported before 2018.1 IVL was first reported by Birch-Hirschfeld in 1896.2 Norris and Parmley3 subsequently described the detailed diagnostic criteria for IVL and proposed an evidence-based theory of the origin of IVL; namely, that IVL might arise either from the wall of veins within the myometrium or develop as a result of an unusual extensive vascular invasion from a myometrial leiomyoma. Lam et al.4 reported that IVL intruded into the systemic venous circulation mainly via two routes: one was through the uterine vein (the main approach), and the other was through the ovarian vein. Although histologically benign, IVL can directly lead to various degrees of vascular occlusion and high mortality. This is mainly because this smooth muscle tumor can extend into the inferior vena cava, right-side cardiac chambers, and even the pulmonary artery.5 This extension of IVL into the right side of the heart is called intracardiac leiomyomatosis (ICL). As a rare and severe form of IVL, ICL can lead to heart blockade resulting in death.6
IVL reportedly occurs in women aged 28 to 80 years5 (median age, 45 years), and most are in the perimenopausal stage. Generally, patients with IVL have a history of uterine myomas7 or hysterectomy. The cytogenetics and molecular biology of patients with IVL have rarely been described. Quade et al.8 reported that gene rearrangement at chromosomes 12 and 14 [t(12;14)(q15;q24)] or inappropriate secretion of gonadal sex steroids (because patients with IVL always have a high estrogen level) might play important roles in the pathogenesis of IVL.
In this study, we retrospectively reviewed and analyzed the medical records of three patients with IVL, including one with ICL, who underwent surgical treatment in our hospital during the past 5 years.
Case report
Case 1
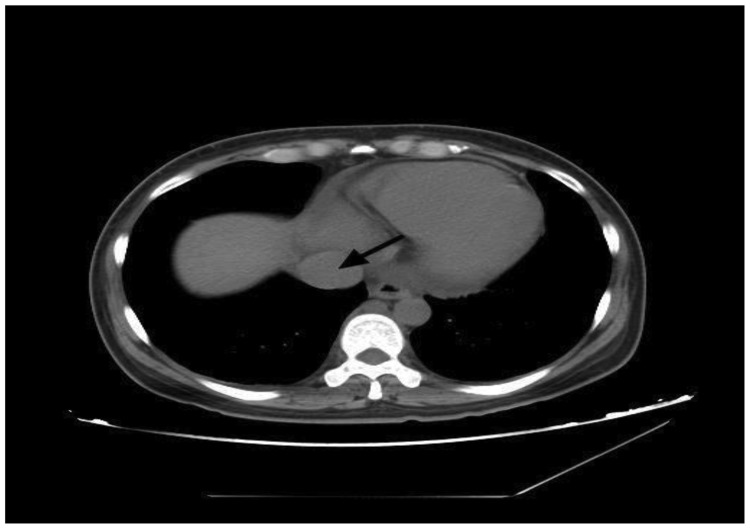
A 34-year-old woman (gravida 4, parity 1) was admitted because of acute swelling in both legs and paroxysmal dyspnea for 3 months. She had no history of smoking, thromboembolism, or hypercoagulopathy in her medical record. She had undergone total abdominal hysterectomy and left salpingo-oophorectomy 18 months previously for treatment of a broad ligament myoma, and the pathological examination findings were suspicious for IVL. Computed tomography (CT) showed a large multilobulated mass protruding through the inferior vena cava, occupying most of the right atrial cavity, and extending through the tricuspid valve into the right ventricle (Figure 1). Abdominal magnetic resonance imaging revealed an 8-cm pelvic tumor, which was suspected to be a uterine fibroid, extending up to the inferior vena cava. The patient was diagnosed with ICL before the operation. The first-stage surgery involved abdominal tumor resection and right salpingo-oophorectomy under general anesthesia. Intraoperatively, a convoluted and vascularized tumor mass was observed growing along the left ovarian vein and extending upward along the inferior vena cava, and it was contiguous with the left pelvic wall and involved the retroperitoneum. Considering a possible hemorrhage volume of about 6000 mL, a second-stage surgery involving removal of the right atrial and ventricular neoplasms was performed 2 weeks later. The subsequent pathologic examination findings confirmed ICL. No relapse occurred for 4 years postoperatively.
Figure 1.
Computed tomography findings of Case 1. An axial image of an abdominal and pelvic computed tomography scan at the level of the superior vena cava is shown. An intravascular tumor was seen in the vena cava (black arrow).
Case 2
A 40-year-old woman (gravida 1, parity 1) was admitted with abdominal distention and bilateral leg edema. Abdominal ultrasound revealed a huge tumor of about 30 × 25 cm with a clear boundary, and it was suspected to have originated from the uterus. A CT scan confirmed these ultrasonic findings, which were highly suggestive of IVL. The tumor invaded the left iliac crest, the common iliac vein, and even the inferior vena cava (Figure 2(a)). Thus, a combined vascular and gynecologic operation was performed. During the surgical operation, a huge myoma originating from the isthmic portion of the uterus was observed; it invaded the pelvic venous plexus and reached the hepatic portal vein. The tumor was resected completely, and bilateral salpingo-oophorectomy was performed. The intraoperative blood loss volume was about 5300 mL. The subsequent pathologic examination findings further confirmed our diagnosis (Figure 2(b)). No recurrence was found for 5 years postoperatively.
Figure 2.
Computed tomography and pathological findings of Case 2. (a) Axial image of a contrast-enhanced abdominal and pelvic computed tomography scan at the level of the left renal vein. An intravascular tumor was observed within the inferior vena cava (black arrow). (b) Hematoxylin–eosin staining of an intravenous leiomyoma thrombus.
Case 3
A 40-year-old woman (gravida 3, parity 2) was admitted with IVL. She had undergone myomectomy and unilateral oophorectomy at another hospital 2 months previously. During the operation, a 10-cm myoma was seen along the broad ligament, and a tumor thrombus was found in the right ovarian vessels. The postoperative pathological examination confirmed IVL. Postoperative ultrasonography revealed no thrombus in the inferior vena cava. The patient presented to our department for the second-stage surgery with no symptoms. Vascular reconstruction CT showed a tumor in the inferior vena after a 2-month interval (Figure 3(a)). We performed total abdominal hysterectomy, left salpingo-oophorectomy, and tumor thrombectomy by angiotomy (Figure 3(b)). The intraoperative blood loss volume was about 2000 mL, and the pathologic examination confirmed IVL. The patient was clinically well at 2 months postoperatively.
Figure 3.
Computed tomography and gross findings of Case 3. (a) Vascular reconstruction computed tomography showed a leiomyoma located in the inferior vena cava (white arrow). (b) Surgically removed leiomyoma that had been located in the inferior vena cava.
Discussion
Early diagnosis of IVL is difficult. Because most patients with IVL are asymptomatic and the tumor extension remains inside the small vessels of the myometrium in the early stage, the tumor is difficult to detect by any imaging method. Early diagnosis of IVL is achieved only when patients have symptoms of a hysteromyoma, such as abdominal pain, irregular bleeding, and an abdominal mass. When the tumor intrudes into the vena cava, it causes several symptoms such as limb swelling, ascites, and liver enlargement, which are indicative of caval obstruction. As the IVL continues to progress, it can evolve into ICL, which manifests as complete obstruction of the tricuspid valve or massive embolism of the pulmonary artery; this may result in congestive heart failure, syncope, and even sudden death. The symptoms of paroxysmal dyspnea and limb swelling in our first patient led to a preoperative diagnosis of ICL, which was further confirmed by CT.
Our literature review revealed 69 patients with IVL.9–12 Among them, 35 patients had pelvic masses (1.5–24 cm), including 27 patients with masses extending into the broad ligament. Thus, the incidence of a pelvic mass involving the broad ligament is as high as 77%. In the present study, both the first and third patients initially underwent surgical treatment of a broad ligament myoma. The postoperative pathologic examination findings were suspicious for IVL. Therefore, the possibility of IVL should be considered before surgery for a broad ligament leiomyoma. The second patient had developed a giant abdominal mass. To the best of our knowledge, this is the largest pelvic mass reported in a patient with IVL to date. Thus, IVL should also be considered before surgery for a giant pelvic mass.
A retrospective analysis of patients with IVL revealed that about 64% of patients had previously undergone hysterectomy, and the time before the occurrence of IVL ranged from 6 months to 20 years.13 This time interval for the third patient in our study was only 2 months. Ultrasound after the first operation revealed no tumor thrombus in the pelvic vessels; 2 months later, however, the tumor had already extended into the inferior vena cava. For the first patient, the recurrence interval was 18 months with no follow-up. Both patients were suspected to have IVL during the first operation. Close postoperative monitoring may prevent the occurrence of ICL.
Clinically, ultrasonography is widely used to assess the condition of the uterus as well as to provide information on the intravascular thrombus in patients with IVL. Doppler ultrasound can be used to visualize blood flow within the tumor thrombus. However, it is difficult to visualize small leiomyomas in small vessels, especially in asymptomatic patients. Echocardiography is typically chosen as the first method for ICL imaging. The following two echocardiographic presentations in a female patient should alert clinicians to the possibility of ICL: (1) a right atrial mass with caval involvement and (2) an intravascular and intra-atrial mass not attached to the endothelial surface or endocardium but instead freely mobile within the inferior vena cava and right-sided cardiac chambers. The mass is typically long and serpentine or worm-like.14 Because of the high readability and large field of view, imaging methods such as CT and magnetic resonance imaging are always used for IVL diagnosis and determination of the extent of tumor involvement. Vascular reconstruction CT imaging can clearly show the location, size, and full-scale extension pathway of IVL lesions, indicating its great clinical significance in the establishment of a well-prepared surgical plan to achieve a good outcome.
Surgical removal of a tumor remains the most effective method of tumor treatment. One-stage or two-stage surgery can be chosen according to several factors,15 such as the patient’s tolerance of the surgical procedure, the tumor anatomy, and the surgeon’s specialty. Both one-stage and two-stage surgery have their advantages and disadvantages.16,17 For example, one-stage surgery always results in a longer operation time with a high incidence of complications, such as bleeding. In contrast, two-stage surgery is more likely to be adopted because of its shorter operation time, reduced risk of bleeding, and safer resection of intracardiac tumor masses despite the potential risk of the second episode of general anesthesia. Among the present cases, we only performed a two-stage surgery in one patient because of the potential for massive blood loss and the patient’s poor tolerance of surgery. Because of the tumor invasion into the vessels, more blood loss will occur during the operation. Thus, clinical assessment is essential before the operation, including assessment of the form and size of the tumor mass, its adjacent vessels, and the extent of its involvement.
Successful treatment of IVL is highly dependent on whether the tumor can be completely excised. Thus, bilateral salpingo-oophorectomy is commonly recommended. However, IVL recurrence has been reported in postmenopausal women, suggesting that the efficacy of drug therapy is controversial. Treatment with a gonadotropin-releasing hormone agonist is reportedly an effective method to shrink a myoma.18 However, if the treated myoma initially decreases in size but later grows, surgical intervention is necessary.19 Considering the traditional treatment of leiomyoma and the higher expressions of estrogen receptor and progesterone receptor in patients with IVL, hormonal therapy is widely used. Adjuvant tamoxifen20 and medroxyprogesterone21 have been prescribed in a limited number of patients who have undergone complete tumor resection, while some papers report minimal or no response.22
The long-term follow-up is indispensable after complete resection of IVL. Some patients have reportedly shown continued growth of an incompletely excised intravenous tumor during a time period ranging from 7 months to 15 years after surgery, leading to a high recurrence rate of 30%.23 Du et al.10 reported a recurrence rate of 16.6% in 18 patients with IVL in 2011, suggesting that a young age at onset and the initial size of the tumor may be predisposing factors for recurrence. Surgery is still the most effective treatment for recurrent IVL.
In conclusion, IVL is a rare disease that often occurs in perimenopausal women with symptoms of phlebothrombosis or a right atrial mass. Early detection and accurate diagnosis are imperative for appropriate treatment. Surgery is still the best therapeutic option for IVL.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethical approval
The study was approved by the institutional review board of Beijing Friendship Hospital, Capital Medical University.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Informed consent was obtained from all patients enrolled in the study.
ORCID iD
References
- 1.Demirkiran F, Sal V, Kaya Uet al. Intravenous leiomyoma with extension to the heart: a case report and review of the literature. Case Rep Obstet Gynecol 2013; 2013: 602407. DOI: 10.1155/2013/602407. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Birch-Hirschfeld FV. Lehrbuch der pathologischen anatomie. 5th ed Leipzig: FCW Vogel, 1896. [Google Scholar]
- 3.Norris MH, Parmley TH. Mesenchymal tumors of the uterus. V. Intravenous leiomyomatosis. A clinical and pathologic study of 14 cases. Cancer 2010; 36: 2164–2178. [DOI] [PubMed] [Google Scholar]
- 4.Lam PM, Lo KW, Yu MYet al. Intravenous leiomyomatosis: two cases with different routes of tumor extension. J Vasc Surg 2004; 39: 465–469. DOI: 10.1016/j.jvs.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Li B, Chen X, Chu YDet al. Intracardiac leiomyomatosis: a comprehensive analysis of 194 cases. Interact Cardiovasc Thorac Surg 2013; 17: 132–138. DOI: 10.1093/icvts/ivt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi T, Shkrum MJ. A case report of sudden death from intracardiac leiomyomatosis. Am J Forensic Med Pathol 2018; 39: 119–122. DOI: 10.1097/PAF.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 7.Li RJ, Zhang CC, Yang Yet al. Echocardiographic study of intravenous leiomyomatosis with intracardiac extension: two case reports and review of the literature. Heart Lung Circ 2013; 22: 690–692. DOI: 10.1016/j.hlc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Quade BJ, Dal Cin P, Neskey DMet al. Intravenous leiomyomatosis: molecular and cytogenetic analysis of a case. Mod Pathol 2002; 15: 351–356. DOI: 10.1038/modpathol.3880529. [DOI] [PubMed] [Google Scholar]
- 9.Clement PB, Young RH, Scully RE. Intravenous leiomyomatosis of the uterus. A clinicopathological analysis of 16 cases with unusual histologic features. Am J Surg Pathol 1988; 12: 932–945. [PubMed] [Google Scholar]
- 10.Du J, Zhao X, Guo Det al. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 18 cases, with emphasis on early diagnosis and appropriate treatment strategies. Hum Pathol 2011; 42: 1240–1246. DOI: 10.1016/j.humpath.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Mulvany NJ, Slavin JL, Ostor AGet al. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 22 cases. Int J Gynecol Pathol 1994; 13: 1–9. [DOI] [PubMed] [Google Scholar]
- 12.Tang L, Lu B. Intravenous leiomyomatosis of the uterus: a clinicopathologic analysis of 13 cases with an emphasis on histogenesis. Pathol Res Pract 2018; 214: 871–875. DOI: 10.1016/j.prp.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Harris LM, Karakousis CP. Intravenous leiomyomatosis with cardiac extension: tumor thrombectomy through an abdominal approach. J Vasc Surg 2000; 31: 1046–1051. DOI: 10.1067/mva.2000.104601. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Shen Y, Sun Yet al. Intravenous leiomyomatosis with intracardiac extension: echocardiographic study and literature review. Tex Heart Inst J 2014; 41: 502–506. DOI: 10.14503/THIJ-13-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma G, Miao Q, Liu Xet al. Different surgical strategies of patients with intravenous leiomyomatosis. Medicine (Baltimore) 2016; 95: e4902. DOI: 10.1097/MD.0000000000004902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castelli P, Caronno R, Piffaretti Get al. Intravenous uterine leiomyomatosis with right heart extension: successful two-stage surgical removal. Ann Vasc Surg 2006; 20: 405–407. DOI: 10.1007/s10016-006-9024-0. [DOI] [PubMed] [Google Scholar]
- 17.Okada M, Miyoshi Y, Kato Get al. Successful one-stage surgical removal of intravenous leiomyomatosis with cardiac extension in an elderly patient. Gen Thorac Cardiovasc Surg 2012; 60: 153–156. DOI: 10.1007/s11748-011-0791-3. [DOI] [PubMed] [Google Scholar]
- 18.Tresukosol D, Kudelka A, Malpica Aet al. Leuproloide acetate and intravascular leiomyomatosis. Obstet Gynecol 1995; 86: 688–692. [DOI] [PubMed] [Google Scholar]
- 19.Khayata GM, Thwaini S, Aswad SG. Intravenous leiomyomatosis extending to the heart. Int J Gynaecol Obstet 2003; 80: 59–60. [DOI] [PubMed] [Google Scholar]
- 20.Guo X, Zhang C, Fang Let al. Echocardiographic characteristics of intravenous leiomyomatosis with intracardiac extension: a single-institution experience. Echocardiography 2011; 28: 934–940. DOI: 10.1111/j.1540-8175.2011.01472.x. [DOI] [PubMed] [Google Scholar]
- 21.Kullo IJ, Oh JK, Keeney GLet al. Intracardiac leiomyomatosis: echocardiographic features. Chest 1999; 115: 587–591. DOI: 10.1378/chest.115.2.587. [DOI] [PubMed] [Google Scholar]
- 22.Stolf NA, dos Santos GG, Haddad VL. Unusual abdominal tumors with intracardiac extension. Two cases with successful surgical resection. Rev Hosp Clin Fac Med Sao Paulo 1999; 54: 159–164. [DOI] [PubMed] [Google Scholar]
- 23.Moniaga NC, Randall LM. Uterine leiomyomatosis with intracaval and intracardiac extension. Gynecol Oncol Case Rep 2012; 2: 130–132. DOI: 10.1016/j.gynor.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]