Short abstract
Fibroepithelial polyps (FEPs) are a rare, benign disease in the urinary system. We present a clinical case of a 34-year-old woman with a symptomatic bladder tumor. Cystoscopy showed a mulberry-like mass with calcification in the bladder trigone. After transurethral resection, histopathology showed an FEP in the bladder with calcification and squamous cell metaplasia. The patient was discharged from hospital 3 days after surgery. We review the recent literature to summarize the clinical manifestations, treatments, and prognosis of bladder FEPs.
Keywords: Bladder, fibroepithelial polyp, squamous cell metaplasia, hematuria, cystoscopy, transurethral resection
Introduction
Fibroepithelial polyps (FEPs) of the bladder are a rare, benign disease, which most commonly occurs in the ureter1 and is extremely rare in the urinary bladder.2–7 There have only been seven cases of bladder FEPs published in the English literature over recent decades.4,6–11 This lesion is usually discovered in children, and it has rarely been reported in adults.5 Hematuria, dysuria, and infections of the urinary tract are the most common symptoms of FEPs.1 Because most mulberry-like lesions, particularly in the bladder, suggest rhabdomyosarcomas, these lesions need to be differentiated from rhabdomyosarcomas.8 Transurethral resection (TUR) is recommended for FEPs, regardless of their pathological type. Cystoscopy and abdominal computed tomography (CT) show similar results between bladder FEPs and carcinoma.
We report here a rare case of a symptomatic mulberry-like FEP in the bladder with calcification and squamous cell metaplasia in a woman. The bladder FEP resembled bladder carcinoma. To the best of our knowledge, bladder FEPs with calcification and squamous cell metaplasia are extremely rare.
Case presentation
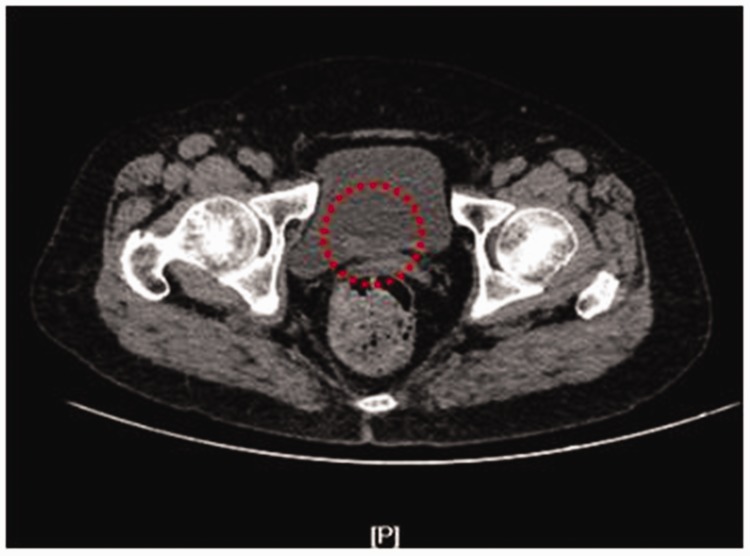
A 34-year-old woman presented to the First Hospital of Jilin University (Changchun, China) with the main complaint of hematuria for 1 month. A physical examination was unremarkable. Furthermore, the patient’s medical history was unremarkable. While urinary erythrocyte levels were elevated, urinalysis, routine blood, liver function, and kidney function were nonspecific. Ultrasound of the urinary system showed a small mass lesion of 20 × 10 mm that arose from the triangle area of the bladder. Abdominal CT showed an irregular mass (2.0 × 1.0 cm) that arose from the bladder (Figure 1). Cystoscopy showed a mulberry-like lump (2.0 × 1.0 cm) with a narrow stalk (Figure 2). The lump arose from the triangle area of the bladder. Additionally, bilateral ureteric orifices showed clear efflux. The rest of the bladder mucosa was normal in appearance.
Figure 1.
Abdominal computed tomography scan. The red circle indicates a hypodense mass (2.0 × 1.0 cm) arising from the bladder.
Figure 2.
Cystoscopic image. A mulberry-like mass (2.0 × 1.0 cm) with calcification arises from the triangle area of the bladder.
The patient underwent TUR of the bladder tumor. Subsequent histopathology showed a bladder FEP with calcification and squamous cell metaplasia (Figure 3). The postoperative course was uneventful and the patient was discharged from hospital 3 days after surgery. The patient did not receive any chemoradiotherapy. At a 6-month follow-up, cystoscopy showed no recurrence.
Figure 3.
Hematoxylin and eosin staining with different magnifications shows a bladder FEP with squamous cell metaplasia. A: 4×, B: 10×, C: 20×, and D: 100×.
This study was based on a clinical case and the patient provided consent for publication of the examination images.
Discussion
FEPs are extremely rare non-malignant tumors and usually originate from the mesoderm. They can grow anywhere in the urinary tract, but are extraordinarily rare in the bladder (approximately <1%).1–6 To the best of our knowledge, only seven cases of bladder FEPs have been published in the English literature.4,6–11 All of these cases are listed in Table 1.
Table 1.
Previous cases of bladder FEPs.
| Ref. no. | Age, years | Sex | Complaint | Size, cm | Treatment | Follow-up | Outcome | Characteristics |
|---|---|---|---|---|---|---|---|---|
| 6 | 35 | M | Hematuria | NR | NR | NR | NR | NR |
| 7 | 6 | M | Hematuria | 1 × 0.5 | TUR | 6 months | No | No |
| 8 | 40 | F | Dysuria | Approximately 3.0 | TUR | NR | NR | DICER1-positive |
| 9 | 2 | M | Hematuria | NR | TUR | 1 year | No | No |
| 10 | 11 | F | Hematuria | 2.4 × 2.0 | OS | NR | NR | NR |
| 4 | 14 | M | Hematuria | 1.8 × 1.5 | TUR | NR | NR | NR |
| 11 | 30 | M | Examination | 1.8 | NR | NR | NR | Urothelial carcinoma |
Ref. no. = reference number, F = female, M = male, NR = not recorded, TUR = transurethral resection, OS = open surgery.
These seven cases of bladder FEP were found in five male patients and two female patients. The incidence of FEPs in men is slightly higher than that in women. These seven patients ranged in age from 2 to 40 years. There were four pediatric patients and the average age of the patients was 19.7 years. Among the seven patients, five presented with hematuria and one had dysuria. One patient was admitted only on the basis of examination results. In five cases, the sizes of the FEPs were described. The size of FEPs ranged from 1.0 to 3.0 cm. A small size of FEP enables transurethral minimally invasive surgery to be performed. Therefore, TUR was commonly recommended in most cases of bladder FEPs. After the operation, five patients were not followed up. Two patients were followed up without recurrence. In one case, bladder FEPs eventually developed into urothelial cancer in a 30-year-old male patient. Findings in this case suggested that bladder FEPs can progress to malignancy.11 Bladder FEPs can also transform into urinary carcinomas. There has been no epidemiological investigation of bladder FEPs because of the lack of clinical cases. However, based on the analysis above, we can conclude the following. First, bladder FEPs usually affect children. Second, hematuria is the main clinical symptom of FEPs. Third, in extreme conditions, benign bladder FEPs can progress to urothelial cancer.
The pathogenesis of bladder FEPs remains unclear. Chronic infectious, obstructive, traumatic, and congenital factors may be the causes of bladder FEPs. Eckstein et al.8 proposed that DICER1 mutations play an important role in the pathogenesis of urinary FEPs. Lam et al.12 stated that the FEPs are congenital lesions. Nevertheless, the fact that congenital FEPs do not show any urinary symptoms for decades is remarkable. In our case, the female patient showed hematuria until middle age. Therefore, congenital factors are less likely to lead to bladder FEPs in adults. Regardless of the cause of bladder FEPs, this type of disease is benign and usually does not lead to recurrence.2–6,10
As mentioned above, painless gross hematuria is the most common clinical symptom of bladder FEPs. Dysuria may also occur when the size of the polyp is large. Bladder FEPs can mimic bladder carcinoma in clinical symptoms. When FEPs are located in the neck of the bladder or urethra, they can cause severe dysuria. However, when the mass is located in the ureter, it is more likely to induce painless gross hematuria.
Metaplasia refers to the transformation of one differentiated cell type into another differentiated cell type.13,14 Our case is rare because the FEP originated from the bladder and developed into squamous cell metaplasia. In the bladder, the main manifestation of metaplasia is transitional epithelium. The normal bladder epithelium may transform into squamous epithelium. Chronic inflammation, irritative stimuli, and infection can lead to metaplasia.14 Furthermore, squamous metaplasia is generally considered as preneoplastic and can develop into squamous carcinoma.13,14 If bladder FEPs are accompanied by cancer, most researchers recommend further radical cystectomy.15,16
Because of a lack of evidence, there is no consensus on the standard imaging diagnosis of bladder FEPs. Ultrasound is a preliminary examination used in patients with hematuria. A CT examination cannot be used to make a definite diagnosis of bladder lesions. An intravenous urogram is useful for detecting ureteral FEPs. An example of this detection is that a filling defect with smooth edges could be found in intravenous urograms of urinary FEPs.17 Magnetic resonance imaging may be a useful imaging examination for urinary FEPs. Bladder FEPs present as peripheral, smooth, space-occupying lesions and mild hyperintensity in T2-weighted imaging. Magnetic resonance imaging is mainly used for staging of FEPs. Differentiating bladder FEPs from transitional bladder carcinoma by imaging findings alone is difficult. Additionally, cystoscopy results can only be analyzed from the morphology of tumors. However, the final diagnosis of bladder FEPs is based on postoperative pathology.
Histologically, bladder FEPs are covered by a normal layer of transitional epithelial cells.18 In our patient, squamous cell metaplasia was observed in the transitional epithelium. Furthermore, bladder FEPs usually have a fibrovascular core. There is no specific expression factor in immunohistochemistry for FEPs. Eckstein et al.8 reported that desmin, smooth muscle actin, estrogen receptor, and CD56 are positive in bladder FEPs.
The main differential diagnosis of bladder FEPs includes rhabdomyosarcoma, transitional cell carcinoma and urinary bladder polypoid cystitis. Bladder FEPs are commonly mistaken for transitional cell carcinoma, usually resulting in more surgical treatment.19 Rhabdomyosarcoma usually has a subepithelial cambium, which is composed of small, round, blue cells. However, rhabdomyosarcoma also has obvious mucoid and edema areas with scattered large atypical cells.10 Indwelling catheters or fistulas can lead to polypoid cystitis, which is a polypoid inflammatory lesion of the bladder covered by reactive urothelium.3 Identifying the characteristic structural pattern of FEPs is a good way to prevent misdiagnosis.
Traditionally, treatments of these lesions include open exploration and resection.20 With the progress of medical technology, a transurethral operation has become more acceptable for bladder FEPs. Compared with traditional open surgery, TUR has the benefits of less trauma and faster recovery. Therefore, TUR is highly recommended as the first choice for bladder and urethral FEPs. After the operation, there is no consensus on long-term follow-up in patients with FEPs. However, bladder FEPs might progress into bladder carcinoma. Therefore, cystoscopy is recommended if the patient has urinary symptoms.14
Conclusion
Bladder FEPs are a rare, benign lower urinary tract disease. Hematuria is the most common symptom for bladder FEPs. Differential diagnosis between bladder carcinoma and FEPs is difficult. CT findings, cystoscopy findings, and clinical symptoms of bladder FEPs and bladder carcinoma are similar. Pathology is the standard means to differentiate between these two conditions. However, bladder FEPs may undergo squamous cell metaplasia and further malignant transformation. For treatment, TUR is the most effective operation. The prognosis is good in most patients with bladder FEPs. However, long-term follow-up is still necessary and regularly cystoscopy is usually recommended.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was funded by the Science and Technology Program of Jilin Province, grant number 20180101167JC, and the Youth Foundation of The First Hospital of Jilin University JDYY102019001.
ORCID iD
References
- 1.Williams TR, Wagner BJ, Corse WRet al. Fibroepithelial polyps of the urinary tract. Abdom Imaging 2002; 27: 217–221. [DOI] [PubMed] [Google Scholar]
- 2.Musselman P, Kay R. The spectrum of urinary tract fibroepithelial polyps in children. J Urol 1986; 136: 476–477. [DOI] [PubMed] [Google Scholar]
- 3.Al-Ahmadie H, Gomez AM, Trane Net al. Giant botryoid fibroepithelial polyp of bladder with myofibroblastic stroma and cystitis cystica et glandularis. Pediatr Dev Pathol 2003; 6: 179–181. [DOI] [PubMed] [Google Scholar]
- 4.Zachariou AG, Manoliadis IN, Kalogianni Pet al. A rare case of bladder fibroepithelial polyp in childhood. Arch Ital Urol Androl 2005; 77: 118–120. [PubMed] [Google Scholar]
- 5.Tsuzuki T, Epstein JI. Fibroepithelial polyp of the lower urinary tract in adults. Am J Surg Pathol 2005; 29 460–466. [DOI] [PubMed] [Google Scholar]
- 6.Young R. Fibroepithelial polyp of the bladder with atypical stromal cells. Arch Pathol Lab Med 1986; 110: 241–242. [PubMed] [Google Scholar]
- 7.Agarwal S, Sharma D, Pandey Set al. Benign fibroepithelial bladder polyp: a rare cause of childhood haematuria. BMJ Case Rep 2018; 2018: bcr-2018-226050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckstein M, Agaimy A, Woenckhaus Jet al. DICER1 mutation-positive giant botryoid fibroepithelial polyp of the urinary bladder mimicking embryonal rhabdomyosarcoma. Hum Pathol 2019; 84: 1–7. [DOI] [PubMed] [Google Scholar]
- 9.Natsheh A, Prat O, Shenfeld OZet al. Fibroepithelial polyp of the bladder neck in children. Pediatr Surg Int 2008; 24: 613–615. [DOI] [PubMed] [Google Scholar]
- 10.Lum D, Upadhyay V, Smith Aet al. Botryoid fibroepithelial polyp of the urinary bladder. A clinicopathological case report including frozen section findings. Histopathology 2007; 51: 704–707. [DOI] [PubMed] [Google Scholar]
- 11.Mamoon N, Hameed Z, Ilahi Fet al. The nested variant of urothelial carcinoma arising in a fibroepithelial polyp: report of a case and review of literature. Indian J Urol 2014; 30: 105–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam JS, Bingham JB, Gupta M. Endoscopic treatment of fibroepithelial polyps of the renal pelvis and ureter. Urology 2003; 62: 810–813. [DOI] [PubMed] [Google Scholar]
- 13.Akdaš A, Türkeri L. The impact of squamous metaplasia in transitional cell carcinoma of the bladder. Int Urol Nephrol 1991; 23: 333–336. [DOI] [PubMed] [Google Scholar]
- 14.Benelli A, Varca V, Vaccaro Cet al. Keratinizing squamous metaplasia of the bladder: our experience and current approaches. Urologia 2018: 1–4. doi: 10.1177/0391560318810197. [DOI] [PubMed] [Google Scholar]
- 15.De Berardinis E, Busetto GM, Giovannone Ret al. Recurrent transitional cell carcinoma of the bladder: a mixed nested variant case report and literature review. Can Urol Assoc J 2012; 6: E57–E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasco MJ, Daignault S, Bradley Det al. Nested variant of urothelial carcinoma: a clinicopathologic and immunohistochemical study of 30 pure and mixed cases. Hum Pathol 2010; 41: 163–171. [DOI] [PubMed] [Google Scholar]
- 17.Xu C, Zhang Z, Ye Het al. Imaging diagnosis and endoscopic treatment for ureteral fibroepithelial polyp prolapsing into the bladder. J Xray Sci Technol 2013; 21: 393–399. [DOI] [PubMed] [Google Scholar]
- 18.Macksood MJ, Roth DR, Chang CHet al. Benign fibroepithelial polyps as a cause of intermittent ureteropelvic junction obstruction in a child: a case report and review of the literature. J Urol 1985; 134: 951–952. [DOI] [PubMed] [Google Scholar]
- 19.Murtaza B, Akmal M, Mahmood Aet al. Bladder outlet obstruction in a 5 years boy. J Coll Physicians Surg Pak 2011; 21: 780–781. [PubMed] [Google Scholar]
- 20.He L, Li S, Zheng Cet al. Rare symptomatic bladder leiomyoma: case report and literature review. J Int Med Res 2018; 46: 1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]