Short abstract
Nocardia usually manifests as opportunistic infections in immunocompromised hosts. Here, we report a rare case of an immunocompetent patient with lymphocutaneous nocardiosis. The patient was a 34-year-old man presenting with fever, multiple scattered pustules on both upper limbs and several subcutaneous nodules on the left elbow and forearm. Skin biopsy of the subcutaneous nodule revealed suppurative inflammation of the lymph nodes. Pus cultures were finally identified as Nocardia brasiliensis. The patient fully recovered without relapse after receiving optimized antimicrobial therapy consisting of linezolid combined with sulfonamides. Nocardiosis is a rare opportunistic disease which may be fatal and usually affects immunocompromised hosts, resulting in suppurative and granulomatous inflammation. Nocardia has a long culture cycle, is difficult to diagnose, and is more likely to be neglected in healthy young people. The present case suggests that physicians should be aware that nocardiosis is a differential diagnosis to consider in patients with suppurative infection, especially when anti-infective treatment is ineffective.
Keywords: Nocardia brasiliensis, immunocompetent, lymphocutaneous nocardiosis, suppurative infection, opportunistic infection, antimicrobial therapy
Background
The genus Nocardia are actinomycetes widely distributed in the environment. Contact with humans usually manifests as an opportunistic infection in immunocompromised hosts, especially individuals who have undergone solid organ transplantation, patients with HIV/AIDS, or patients receiving long-term treatment with corticosteroids or immunosuppressive drugs.1,2 However, Nocardia species sometimes infect healthy individuals. Here, we report a case of lymphocutaneous nocardiosis caused by Nocardia brasiliensis in an immunocompetent young man.
Case presentation
In November 2015, a 34-year-old Chinese man who had fever with multiple pustules on both limbs for 5 days was referred to the Infectious Diseases Division of the First People's Hospital of Hangzhou.
Five days earlier, the patient was feverish with a high temperature of 39°C and experienced dizziness and nausea but no vomiting, chills, or cough and sputum. His fever was followed by erythematous swelling of the fingers and left wrist. The patient was otherwise healthy and remembered no episode of injury. The only relevant history was that he had been to a dental clinic 5 days earlier. Despite a 4-day course of antibiotic therapy (cefotaxime + ornidazole), his symptoms worsened. Erythematous swelling of the fingers and left wrist developed, accompanied by white maceration, shallow ulcers and pustules, with spreading subcutaneous nodules along both forearms. Considering the possibility of drug-resistant bacterial infection, treatment with vancomycin combined with piperacillin sulbactam was selected. However, the patient's symptoms did not improve significantly.
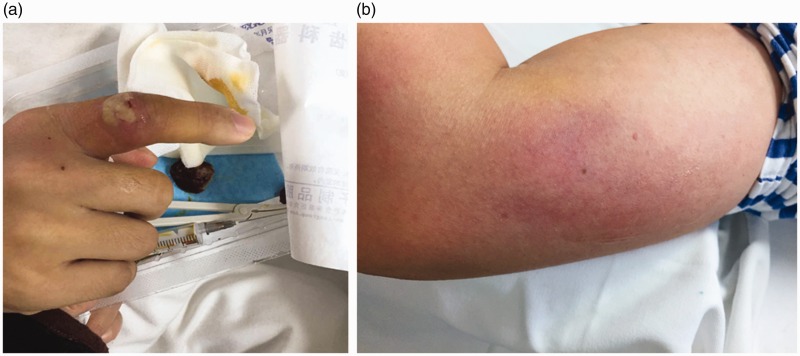
Physical examination revealed a heart rate of 100 beats/minute, blood pressure of 121/77 mmHg, respiratory rate of 20 breaths/minute, temperature of 38.2°C, numerous 1 × 2 cm pustules on the fingers and left wrist (Figure 1a), and several subcutaneous nodules on the left elbow and forearm. The largest nodule measured 4 × 5 cm (Figure 1b).
Figure 1.
(a) Clinical features at first visit. Erythematous swelling of the right index finger was observed, accompanied by white maceration and pustules. (b) Clinical features at first visit. Erythematous swelling of the left elbow observed, accompanied by a subcutaneous nodule approximately 4 × 5 cm in size.
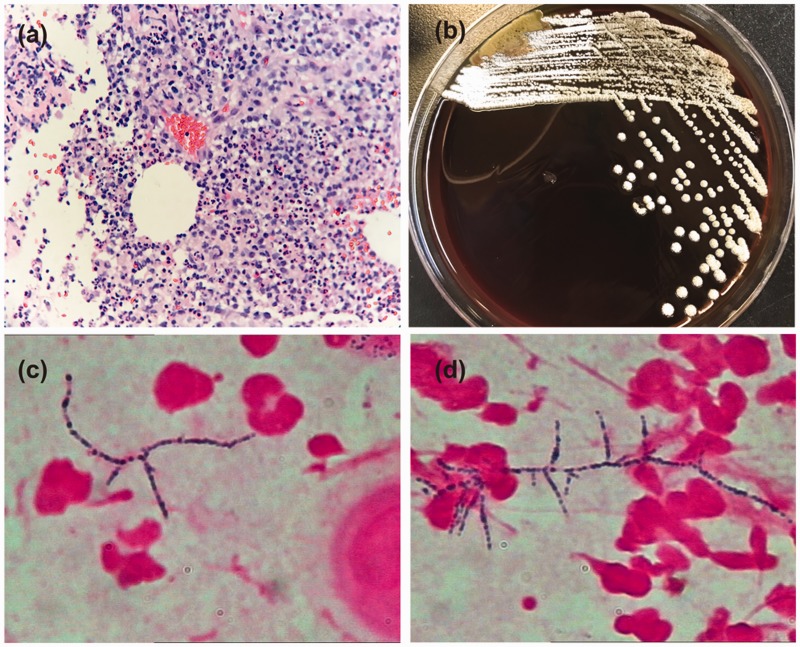
Laboratory investigations revealed a white blood cell count of 15.3 × 109/L (reference range: 3.5–9.5 × 109/L) (78.7% neutrophils), C-reactive protein of 64 mg/L (reference range: 0–8 mg/L), and normal procalcitonin. Urinalysis results were within normal limits. A purified protein derivative skin test for tuberculosis was negative. Antinuclear antibodies, rheumatoid factors, tumour markers, HIV and syphilis tests were all negative. Chest computed tomography showed no obvious abnormalities. Ultrasonography of the subcutaneous nodule on the right elbow suggested a swollen lymph node. Next, histology of a skin biopsy taken from the subcutaneous nodule of the left elbow was performed. The result showed abscess formation in the lymph nodes (Figure 2a). Pus cultures on blood agar plates grew heaped, chalky white colonies (Figure 2b). Gram staining revealed Gram-positive organisms; microscopically, the organisms were slender rods with many branches (Figure 2c–d). The organism was finally identified as Nocardia brasiliensis by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) and 16S rRNA gene sequencing. The diagnosis was amended and treatment was changed immediately to 600 mg linezolid every 12 hours combined with trimethoprim-sulfamethoxazole (TMP-SMX, 80/400 mg, three tablets three times a day) for 2 weeks. This treatment produced complete resolution of the lesions, and TMP-SMX monotherapy was continued for 6 weeks. There was no subsequent recurrence.
Figure 2.
(a) Histopathological findings. A skin biopsy taken from the largest subcutaneous abscess of the left elbow revealed a significant inflammatory cell infiltration, with abscess formation and giant cells in lymph node tissue (haematoxylin and eosin, ×200). (b) Colony culture. A small number of chalky white colonies formed on a blood agar plate. (c, d) A smear of the bacteria from a cultured colony. Gram-positive rods with long, sinuous branches were observed (Gram stain, ×1000).
Susceptibility testing was performed using the Etest method. We subcultured the N. brasiliensis isolate on sheep blood agar plates and, after 96 hours, inoculated the isolate into broth to a turbidity equivalent of 0.5 McFarland standard as measured using a densitometer (Densimat; bioMérieux, Marcy l’Etoile, France). The inoculum was spread on Mueller-Hinton agar. After incubating for 96 hours at 37°C in ambient air, the minimum inhibitory concentrations (MICs) were recorded as the values at which the inhibition zone intercepted the scale on the Etest strip (Bio-kont, Zhejiang, China). The MICs are shown in Table 1 and were interpreted according to the CLSI M24-A2 recommendations.3
Table 1.
Antimicrobial susceptibility testing results for Nocardia brasiliensis.
| Antimicrobial agent | MIC∗, µg/mL | Tentative interpretation |
|---|---|---|
| Imipenem | 32 | R |
| Gentamicin | 0.064 | S |
| Tobramycin | 0.064 | S |
| Cefotaxime | 256 | R |
| Cefepime | 256 | R |
| Ceftriaxone | 256 | R |
| Amoxicillin-clavulanic acid | 0.25 | S |
| Amikacin | 0.5 | S |
| Linezolid | 0.5 | S |
| Clarithromycin | 0.125 | S |
| Trimethoprim-sulfamethoxazole | 0.064 | S |
| Doxycycline | 0.125 | S |
| Ciprofloxacin | 32 | R |
| Minocycline | 0.064 | S |
I = intermediate, MIC = minimum inhibitory concentration, R = resistant, S = susceptible.
∗MICs were determined by the E-test method and interpreted according to the CLSI M24-A2 recommendations.3
Discussion
Nocardiosis is a rare opportunistic disease that is potentially fatal and mainly affects the lungs, skin and central nervous system, resulting in suppurative and granulomatous inflammation.4 The infection can be systemic or cutaneous.
Cutaneous nocardiosis can be divided into primary cutaneous nocardiosis and secondary cutaneous nocardiosis caused by haematogenous dissemination. In contrast to other types of nocardiosis, primary cutaneous disease can develop in immunocompetent hosts with a history of trauma and can be further divided into three major subtypes: (1) actinomycetoma, (2) superficial skin infection, and (3) lymphocutaneous.5 Currently, N. brasiliensis accounts for the majority of cutaneous infections. After skin infection, an abscess or localized cellulitis can form on the surface of the skin. If the infection spreads to regional lymph nodes, painful multiple erythematous nodules or satellite pustules may develop and be distributed along the lymphatic tract.5–7 This form of nocardiosis is called lymphocutaneous nocardiosis. Patients often have a recent history of trauma, and the lower limbs are affected quite commonly. Given the similar presentation of sporotrichosis, lymphocutaneous nocardiosis is often called sporotrichoid nocardiosis. Sporotrichosis is the main differential diagnosis.8,9
Nocardia species dwell in the soil, often invading the skin through open wounds during gardening activities. Another transmission route is through infected animals.5,7,8 So far, there have been only two cases reported in China of lymphocutaneous nocardiosis caused by N. brasiliensis in immunocompetent patients. One was reported by Chu et al.10 in 2017 and occurred in an 87-year-old woman following a wasp sting. Another case was reported by Zhang et al.11 in 2019 and occurred in a 42-year-old woman following an unknown insect bite. In this present case, the patient was finally diagnosed with the lymphocutaneous type of primary cutaneous nocardiosis caused by N. brasiliensis. This is the first report of this condition in a healthy young man in China. By contrast with previous reports, the patient had no history of trauma or garden work. Although he had a history of dental work before the onset, it was difficult to explain his cutaneous infection.
Considering that antibiotic sensitivity varies among Nocardia species, it is important to accurately discriminate these organisms at the species level.12 Identification of Nocardia species is often performed using molecular techniques such as PCR restriction enzyme analysis and 16S rRNA gene sequencing. MALDI-TOF MS-based identification is a potentially rapid and inexpensive alternative.13,14 In the current case, the isolate was identified by MALDI-TOF MS and this result was confirmed by 16S rRNA gene sequencing.
All cases of nocardial infection should receive treatment, including cases in immunocompetent patients with localized cutaneous disease.15 Nocardial infections frequently call for prolonged therapy. Monotherapy with TMP-SMX has evolved towards initial combination drug therapy for most forms of nocardiosis.16 For patients with central nervous system involvement, disseminated infection and serious disease, a three-drug regimen comprising TMP-SMX, amikacin, and either ceftriaxone or imipenem is recommended.17,18 For immunocompetent patients with all forms of cutaneous nocardiosis, TMP-SMX monotherapy or in combination with another antibiotic may be adequate. Mycetoma, some ocular infections, deep abscesses and rare cases with other body sites involvement may require surgical treatment.18
Antimicrobial susceptibility tests are especially important for guiding treatment choices. Multidrug resistant strains of N. brasiliensis are common. In a study in Spain, susceptibility testing of 39 strains of N. brasiliensis showed that the species was susceptible to TMP-SMX, linezolid and amikacin. Low resistance rates (7.7% to 12.8%) were observed against amoxicillin/clavulanic acid, tobramycin and cefotaxime, whereas high rates (56.4% to 82.1%) were recorded for imipenem, minocycline, ciprofloxacin and erythromycin.4 These results were similar to some past studies.19,20 Linezolid is a welcome and useful addition to the treatment options for nocardiosis and has excellent potential as a second-line agent. This drug may also be especially useful as initial therapy until susceptibility results for other potential agents are available, and it has become an attractive alternative to TMP-SMX , imipenem, or amikacin for empirical treatment.4,21
When we started treatment for the present case, the susceptibility results of this strain were not available. Referring to the results of antibiotic sensitivity from the above studies, we empirically chose linezolid and TMP-SMX as the initial combination therapy and achieved a good therapeutic result. Later, the susceptibility results showed that this isolate was sensitive to TMP-SMX, linezolid, amikacin, amoxicillin-clavulanic acid and clarithromycin, but resistant to Imipenem, cefotaxime and ciprofloxacin. Therefore, this strain was classified as multidrug resistant.
The duration of therapy for nocardiosis is variable and depends on the clinical condition and the patient's immune status. Three months of treatment is proposed for a primary cutaneous disease, and in cases of mild disease with a quick and complete improvement, a shorter treatment course of 1 month is possible. However, mycetoma requires more prolonged therapy.18 Six months to 1 year is recommended for visceral or systemic nocardiosis.13
Nocardia has a long culture cycle and is difficult to diagnose. It is more likely to be neglected in healthy young people. Therefore, nocardiosis should be excluded, especially for suppurative infections with poor therapeutic effects.
Acknowledgements
The authors wish to acknowledge the patient’s participation in this study.
Author contributions
SL and XX wrote the case report; MW and JZ took care of the patient; PC and JD took part in the drafting; SW conducted bacterial identification and antimicrobial susceptibility testing; JJ revised the manuscript and reviewed the final manuscript; all authors read and approved the final version of the manuscript.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. The patient understood that the text and any images published in the article will be freely available on the internet and may be seen by the general public.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (LQ18H070006) and the Department of Health of Zhejiang Province (2018KY566).
ORCID iDs
Shenghai Wu https://orcid.org/0000-0002-9923-1014
References
- 1.Haussaire D, Fournier PE, Djiguiba Ket al. Nocardiosis in the south of France over a 10-years period, 2004-2014. Int J Infect Dis 2017; 57: 13–20. [DOI] [PubMed] [Google Scholar]
- 2.Bafghi MF, Eshraghi SS, Heidarieh Pet al. Nocardiosis in immune disorder disease. Malays J Med Sci 2014; 21: 75–76. [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes—Second Edition: Approved Standard M24-A2. Wayne, PA, USA: CLSI, 2011. [PubMed] [Google Scholar]
- 4.Valdezate S, Garrido N, Carrasco Get al. Epidemiology and susceptibility to antimicrobial agents of the main Nocardia species in Spain. J Antimicrob Chemother 2017; 72: 754–761. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda H, Saotome A, Usami Net al. Lymphocutaneous type of nocardiosis caused by Nocardia brasiliensis: a case report and review of primary cutaneous nocardiosis caused by N. brasiliensis reported in Japan. J Dermatol 2008; 35: 346–353. [DOI] [PubMed] [Google Scholar]
- 6.Chen KW, Lu CW, Huang TCet al. Cutaneous manifestations of Nocardia brasiliensis infection in Taiwan during 2002-2012-clinical studies and molecular typing of pathogen by gyrB and 16S gene sequencing. Diagn Microbiol Infect Dis 2013; 77: 74–78. [DOI] [PubMed] [Google Scholar]
- 7.Atzori L, Pinna AL, Pau M. Cutaneous nocardiosis. SOJ Microbiol Infect Dis 2014; 2: 8. [Google Scholar]
- 8.Secchin P, Trope BM, Fernandes LAet al. Cutaneous nocardiosis simulating cutaneous lymphatic sporotrichosis. Case Rep Dermatol 2017; 9: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhde KB, Pathak S, McCullum IJet al. Antimicrobial-resistant nocardia isolates, United States, 1995-2004. Clin Infect Dis 2010; 51: 1445–1448. [DOI] [PubMed] [Google Scholar]
- 10.Chu L, Xu X, Ran Y. Primary cutaneous nocardiosis caused by Nocardia brasiliensis following a wasp sting. Clin Exp Dermatol 2017; 42: 416–419. [DOI] [PubMed] [Google Scholar]
- 11.Mu YZ, Liu Y, Wang YJet al. A case report and review of lymphocutaneous nocardiosis caused by Nocardia brasiliensis reported in China. Dermatol Ther 2019; 32: e13001. [DOI] [PubMed] [Google Scholar]
- 12.Bibi S, Irfan S, Zafar Aet al. Isolation frequency and susceptibility patterns of Nocardia species at a tertiary hospital laboratory in Karachi, Pakistan. J Infect Dev Ctries 2011; 5: 499–501. [DOI] [PubMed] [Google Scholar]
- 13.Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog 2018; 114: 369–384. [DOI] [PubMed] [Google Scholar]
- 14.Marin M, Ruiz A, Iglesias Cet al. Identification of Nocardia species from clinical isolates using MALDI-TOF mass spectrometry. Clin Microbiol Infect 2018; 24: 1342.e5–1342.e8. [DOI] [PubMed] [Google Scholar]
- 15.Burgert SJ. Nocardiosis: a clinical review. Infect Dis Clin Pract 1999; 8: 27–32. [Google Scholar]
- 16.Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc 2012; 87: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown-Elliott BA, Brown JM, Conville PSet al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev 2006; 19: 259–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection 2010; 38: 89–97. [DOI] [PubMed] [Google Scholar]
- 19.McTaggart LR, Doucet J, Witkowska Met al. Antimicrobial susceptibility among clinical Nocardia species identified by multilocus sequence analysis. Antimicrob Agents Chemother 2015; 59: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai CC, Liu WL, Ko WCet al. Multicenter study in Taiwan of the in vitro activities of nemonoxacin, tigecycline, doripenem, and other antimicrobial agents against clinical isolates of various Nocardia species. Antimicrob Agents Chemother 2011; 55: 2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moylett EH, Pacheco SE, Brown-Elliott BAet al. Clinical experience with linezolid for the treatment of nocardia infection. Clin Infect Dis 2003; 36: 313–318. [DOI] [PubMed] [Google Scholar]