Key Points
Question
Is mycophenolate a well-tolerated and beneficial treatment of morphea?
Findings
In this multicenter cohort study that included 77 patients with predominantly severe, recalcitrant morphea, 87% had either improved or stable disease after 9 to 12 months of treatment with mycophenolate. Mycophenolate and mycophenolic acid were well tolerated, with gastrointestinal adverse effects occurring most commonly (31%) and cytopenia (4%) and infection (3%) occurring less frequently.
Meaning
These findings suggest that mycophenolate could be a well-tolerated and beneficial therapy for recalcitrant, severe morphea.
Abstract
Importance
First-line systemic therapy for morphea includes methotrexate with or without systemic corticosteroids. When this regimen is ineffective, not tolerated, or contraindicated, a trial of mycophenolate mofetil (MMF) or mycophenolic acid (MPA)—referred to herein as mycophenolate—is recommended; however, evidence to support this recommendation remains weak.
Objective
To evaluate the effectiveness and tolerability of mycophenolate for the treatment of morphea.
Design, Setting, and Participants
A retrospective cohort study was conducted from January 1, 1999, to December 31, 2018, among 77 patients with morphea from 8 institutions who were treated with mycophenolate.
Main Outcomes and Measures
The primary outcome was morphea disease activity, severity, and response at 0, 3 to 6, and 9 to 12 months of mycophenolate treatment. A secondary outcome was whether mycophenolate was a well-tolerated treatment of morphea.
Results
There were 61 female patients (79%) and 16 male patients (21%) in the study, with a median age at disease onset of 36 years (interquartile range, 16-53 years) and median diagnostic delay of 8 months (interquartile range, 4-14 months). Generalized morphea (37 [48%]), pansclerotic morphea (12 [16%]), and linear morphea of the trunk and/or extremities (9 [12%]) were the most common subtypes of morphea identified. Forty-one patients (53%) had an associated functional impairment, and 49 patients (64%) had severe disease. Twelve patients received initial treatment with mycophenolate as monotherapy or combination therapy and 65 patients received mycophenolate after prior treatment was ineffective (50 of 65 [77%]) or poorly tolerated (21 of 65 [32%]). Treatments prior to mycophenolate included methotrexate (48 of 65 [74%]), systemic corticosteroids (42 of 65 [65%]), hydroxychloroquine (20 of 65 [31%]), and/or phototherapy (14 of 65 [22%]). After 3 to 6 months of mycophenolate treatment, 66 of 73 patients had stable (n = 22) or improved (n = 44) disease. After 9 to 12 months of treatment, 47 of 54 patients had stable (n = 14) or improved (n = 33) disease. Twenty-seven patients (35%) achieved disease remission at completion of the study. Treatments received in conjunction with mycophenolate were frequent. Mycophenolate was well tolerated. Gastrointestinal adverse effects were the most common (24 [31%]); cytopenia (3 [4%]) and infection (2 [3%]) occurred less frequently.
Conclusions and Relevance
This study suggests that mycophenolate is a well-tolerated and beneficial treatment of recalcitrant, severe morphea.
This cohort study evaluates the use of mycophenolate mofetil and mycophenolic acid for the treatment of morphea.
Introduction
Morphea (localized scleroderma) is a rare sclerosing disorder of the skin and subcutaneous tissue that affects patients of all ages.1,2,3 Patients with extensive involvement unresponsive to phototherapy, with facial involvement, or with involvement traversing joints require systemic treatment to prevent disease progression and its associated morbidity. Experts recommend treatment with methotrexate (MTX) for these patients.2,3,4,5,6 This recommendation is based on a randomized, double-blind, placebo-controlled clinical trial in a pediatric population comparing the combination of prednisone for 3 months and MTX for 12 months vs prednisone alone for 3 months.7 Those who received MTX demonstrated improved skin scores and were less likely to experience disease relapse compared with those who received placebo. In addition, several small retrospective studies and a prospective study have further supported the value and safety of MTX, with or without systemic corticosteroids (SCSs), for the treatment of morphea.8,9,10,11,12,13,14,15 For some patients, treatment with MTX is ineffective, not well tolerated, or contraindicated. Studies suggest that approximately 25% to 30% of patients with morphea will not show clinical improvement with MTX.7,12 In this subset of patients, a trial of mycophenolate mofetil (MMF) or mycophenolic acid (MPA)—referred to herein as mycophenolate—is recommended, although evidence supporting this practice is based on small case series.2,4,5
The recommendation for the use of mycophenolate for the treatment of morphea is primarily extrapolated from literature demonstrating the effectiveness of MMF for individuals with systemic sclerosis. Mycophenolate mofetil has demonstrated efficacy in systemic sclerosis–related interstitial lung disease and skin thickening as measured by the modified Rodnan skin score.16,17,18,19 Because morphea and systemic sclerosis share a similar pathogenesis, it is hypothesized that mycophenolate may also be a beneficial treatment for patients with morphea.
There are few data supporting the benefit of MMF or MPA for the treatment of morphea. Two retrospective studies on the use of MMF were conducted for a total of 17 patients with morphea20,21; all but 1 patient had a favorable response to treatment. Fourteen patients were treated concomitantly with SCSs (oral prednisone, intravenous methylprednisolone, or both). Six patients were treated concomitantly with MTX. To expand on these data, we performed a multicenter review of patients with morphea who have been treated with mycophenolate.
Methods
Study Population and Design
A multicenter retrospective cohort study was conducted from January 1, 1999, to December 31, 2018, among patients from Brigham and Women’s Hospital, Cleveland Clinic, Duke University, Northwestern University, New York University Langone Medical Center, Oregon Health & Science University, University of Pennsylvania, and University of Texas Southwestern. A search of electronic health records for patients with an International Classification of Diseases, Ninth Revision, or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, diagnosis of morphea or localized scleroderma who were also prescribed MMF or MPA was conducted at each of the involved sites. Data were uploaded into REDCap at each institution; REDCap entries were reviewed in detail for discrepancies by 1 of us (M.A.). Inclusion criteria were patients who were evaluated by a dermatologist at any of the involved medical centers, aged 1 to 88 years, classified as having active morphea of any subtype, treated with MMF or MPA, and were followed up for at least 3 months. To avoid potential confounding, patients with concomitant eosinophilic fasciitis or lichen sclerosus were excluded from this case series. Eighty patients were identified, and 77 were eligible for analysis. One patient was eliminated as an outlier because this patient reported an onset of morphea at birth, and 2 patients had inadequate documentation available. This study was approved by the Brigham and Women’s Hospital, Cleveland Clinic, Duke University, Northwestern University, New York University Langone Medical Center, Oregon Health & Science University, University of Pennsylvania, and University of Texas Southwestern Institutional Review Boards. A waiver of informed consent was granted by the institutional review board committees because data were deidentified.
Definitions
Definitions were generated to standardize disease activity, severity, and response across each institution. These definitions were modeled after the Localized Scleroderma Cutaneous Assessment Tool and a recent publication.21,22 A detailed list of definitions that was used to categorize patients is available in the eMethods in the Supplement. Disease activity was categorized as active, inactive, or disease remission (DR). Disease remission was defined by the presence of stable disease for 6 months or more. Disease severity was categorized as mild, moderate, or severe. Disease response was categorized as improved, stable, or progressive. A beneficial response (BR) was defined as stable or improved disease (Box). The primary end point of this study was to determine disease response for patients with morphea who were treated with mycophenolate. Disease severity and activity were recorded prior to initiation of mycophenolate. Because disease severity was in part determined by metrics that do not typically fluctuate with treatment (eg, body surface area), it was recorded only prior to initiation of mycophenolate. Disease activity and response after 3 to 6 months and 9 to 12 months of treatment were recorded. These time points were selected because mycophenolate may take 3 to 4 months to become effective. In addition, mycophenolate is typically started at a low dose and titrated upward for several months. Patients receiving MMF and/or MPA were included in this study. If a patient achieved DR and was able to discontinue therapy, the disease activity was recorded 6 months after discontinuation.
Box. Disease Activity, Severity, and Responsea.
Disease activity
Active
Erythematous border
Development of new lesions
Expansion of existing lesions
Inactive
No change in lesion number, size, or associated erythema
Disease remission
Inactive disease ≥6 months
Disease severity
Mild
BSA, 0%-5%
Involvement of ≤2 anatomical sites
Absence of associated functional impairments
Moderate
BSA, 6%-10%
≥4 Morphea plaques that are >3 cm and involve at least 2 of 7 anatomical sites
Absence of associated functional impairments
Severe
BSA, >10%
Morphea involving the face or crossing joints
The presence of associated functional impairments
Disease response
Improved
Absence of new or expanding lesions and 1 of the following:
Decreased erythema
Decreased induration
Improvement of an associated functional impairment
Stable
No change in:
Erythema
Induration
Size
Number
Progressive
New or expanding lesions
Increased erythema
Increased induration
Progression of associated functional impairments
Beneficial response
Stable or improved disease
Statistical Analysis
Descriptive statistics were used to summarize patients’ demographic and clinical characteristics. To compare patients with response and patients without response at 3 to 6 months and 9 to 12 months, t tests were used for continuous data, and the Wilcoxon rank-sum test was used when the normality assumption was questionable; χ2 tests and Fisher exact tests were used when appropriate for categorical data. Odds ratios were calculated using univariable logistic regression. Multivariable logistic regression was considered but deemed inappropriate owing to low counts in some categories. All P values were from 2-sided tests and results were deemed statistically significant at P < .05. Analyses were performed using R, version 3.6.0 (R Project for Statistical Computing).23
Results
Demographic and Clinical Characteristics
Seventy-seven patients were eligible for analysis (Table 1). There were 61 female patients (79%) and 16 male patients (21%); 66 patients (86%) were white. The median age at onset of morphea was 36 years (interquartile range [IQR], 16-53 years). The median diagnostic delay was 8 months (IQR, 4-14 months). Generalized morphea (37 [48%]), pansclerotic morphea (12 [16%]), and linear morphea of the trunk and/or extremities (9 [12%]) were the most common subtypes. Forty-one patients (53%) had an associated functional impairment; joint contracture was the most common impairment (21 [51%]). Forty-nine patients (64%) had severe disease, which was expected because more than 80% of patients had inherently more severe subtypes (generalized, pansclerotic, or linear morphea). In addition, because this study evaluated a treatment that is typically used as a second-line or third-line agent, it was anticipated that most patients would have severe disease.
Table 1. Patient Demographic and Clinical Characteristics.
| Characteristic | Value (N = 77) |
|---|---|
| Age at onset, y | |
| Median (IQR), y | 36 (16-53) |
| Range | 2-85 |
| Sex, No. (%) | |
| Female | 61 (79) |
| Male | 16 (21) |
| Race/ethnicity, No. (%) | |
| White non-Hispanic, non-Latino | 66 (86) |
| Hispanic or Latino | 4 (5) |
| Diagnosis delay, mo | |
| Median (IQR) | 8 (4-14) |
| Range | 1-120 |
| Disease duration prior to starting mycophenolate, mo | |
| Median (IQR) | 16 (8-48) |
| Range | 0-249 |
| Started MMF as first line therapy, No. (%) | 12 (16) |
| Morphea subtype, No. (%) | |
| Circumscribed | 7 (9) |
| Linear | |
| Trunk or extremities | 9 (12) |
| Head or neck | 6 (8) |
| Generalized | 37 (48) |
| Pansclerotic | 12 (16) |
| Mixed | 6 (8) |
| Functional impairment, No. (%) | 41 (53) |
| Decreased joint extension or contraction | 21/41 (51) |
| Peripheral sensory neuropathy | 10/41 (24) |
| Arthralgia or pain | 7/41 (17) |
| Hair loss | 6/41 (15) |
| Headaches | 5/41 (12) |
| Anxiety or depression | 2/41 (5) |
| Limb-length discrepancy | 1/41 (2) |
| Vision impairment or loss | 1/41 (2) |
| Autoantibodies evaluated, No. (%) | 57 (74) |
| Positive ANA | 24/57 (42) |
| Disease severity, No. (%) | |
| Mild | 11 (14) |
| Moderate | 17 (22) |
| Severe | 49 (64) |
Abbreviations: ANA, antinuclear antibody; IQR, interquartile range; MMF, mycophenolate mofetil.
Autoimmune Serologic Findings
Autoimmune serologic findings were evaluated for 57 patients (74%). Twenty-four of these 57 patients (42%) had a positive antinuclear antibody; 14 of those with a positive antinuclear antibody (58%) had generalized morphea. Antinuclear antibody titers ranged from 1:40 to 1:1280.
Initial Treatment With MMF
Twelve patients (16%) received first-line treatment with mycophenolate as monotherapy or combination therapy. Mycophenolate was considered a first-line agent if the patient had not previously been treated with topical or systemic therapies. Two of these patients had circumscribed disease, 5 had generalized disease, 2 had linear disease, and 3 had pansclerotic disease. Two patients had mild disease, 4 had moderate disease, and 6 had severe disease. Three of 12 patients received mycophenolate monotherapy, 8 of 12 received concurrent SCS, and 1 of 12 received concurrent SCS and MTX.
Addition of MMF
Sixty-five patients (84%) received the addition of mycophenolate as monotherapy or combination therapy after the typical standard of care treatments failed. Treatments used prior to initiation of mycophenolate are outlined in Table 2. Forty-eight of these 65 patients (74%) were treated with MTX. The median maximum dose of MTX was 20 mg/wk (IQR, 15-25 mg/wk). The median duration of MTX use was 12 months (IQR, 6-33 months). The median total cumulative dose of MTX was 814 mg (IQR, 315-2001 mg). Systemic corticosteroids were used for 42 patients (65%). Oral prednisone was the most common SCS used (29 of 65). Oral prednisone and intravenous methylprednisolone (9 of 65) and intravenous methylprednisolone (4 of 65) were used less frequently. Seventeen patients received a one-time tapering course of SCSs, 9 received 2 or more tapering courses of SCSs, and 8 received continuous SCSs. The median duration of SCS use was 10 months (IQR, 4-24 months). Twenty patients (31%) received hydroxychloroquine (HCQ). Phototherapy was used for 14 patients (22%); UV-A1 therapy was the most frequently used form of phototherapy (7 [11%]). Topical therapies used prior to MMF included corticosteroids (26 [40%]), tacrolimus (9 [14%]), and calcitriol (7 [11%]).
Table 2. Treatments Used Prior to Initiation of Mycophenolate Mofetil.
| Treatment prior to mycophenolatea | Value (n = 65) |
|---|---|
| Methotrexate, No. (%) | 48 (74) |
| Maximum dose of MTX, mg/wk | |
| Median (IQR) | 20 (15-25) |
| Range | 2.5-30 |
| Duration of MTX use, mo | |
| Median (IQR) | 12 (6-33) |
| Range | 0.75-156 |
| Total cumulative dose of MTX, mg | |
| Median (IQR) | 814 (315-2001) |
| Range | 30-12 000 |
| Systemic corticosteroids, No. (%) | 42 (65) |
| IVMP | 4 (6) |
| Prednisone | 29 (45) |
| IVMP and prednisone | 9 (14) |
| Duration of systemic corticosteroid use, mo | |
| Median (IQR) | 10 (4-24) |
| Range | 1.5-100 |
| Topical therapy, No. (%) | |
| Corticosteroids | 26 (40) |
| Tacrolimus | 9 (14) |
| Calcitriol | 7 (11) |
| Phototherapy, No. (%) | 14 (22) |
| UV-A1 | 7 (11) |
| Narrowband UV-B | 3 (5) |
| PUVA | 2 (3) |
| Narrowband UV-B and UV-A1 | 1 (2) |
| UV-A1 and PUVA | 1 (2) |
| Hydroxychloroquine | 20 (31) |
| Duration of hydroxychloroquine use, mo | |
| Median (IQR) | 24 (7.5-46.5) |
| Range | 1-108 |
Abbreviations: IQR, interquartile range; IVMP, intravenous methylprednisolone; MTX, methotrexate; PUVA, psoralen and UV-A.
Other treatments included intralesional kenalog, topical pimecrolimus, minocycline, doxycycline, tocilizumab, psoralen and UV-A therapy, and azathioprine.
Fifty of 65 patients (77%) initiated mycophenolate because prior treatments were ineffective; 35 of these 50 patients (70%) initiated mycophenolate because MTX was ineffective. Twenty-one of 65 patients (32%) started mycophenolate owing to intolerance of their previous therapy.
Initial or Additional Treatment With MMF
Almost all participants were treated with MMF. Seven received MPA; 5 of those 7 patients started therapy with MMF but were transitioned to MPA owing to gastrointestinal (GI) adverse events (AEs). The median duration of disease prior to initiation of mycophenolate was 16 months (IQR, 8-48 months). The median maximum dose of MMF was 2.0 g/d (IQR, 2.0-3.0 g/d; range, 0.3-4.0 g/d). Doses of MPA were converted to doses of MMF (720 mg MPA = 1000 mg MMF). A total of 21 patients (27%) received 3.0 g/d or more, 38 (49%) received 2.0 to 2.9 g/d, 15 (20%) received 1.0 to 1.9 g/d, and 3 (4%) received less than 1.0 g/d. The median duration of treatment with mycophenolate at the time of the study was 13.5 months (IQR, 8.0-24.0 months). Only 10% of patients (8 of 77) received mycophenolate as monotherapy. Of 69 patients, 16 (23%) received concurrent topical therapy, and 53 (77%) received concurrent systemic therapy; SCSs were the most frequent systemic therapy (41% [28 of 69]). Methotrexate was used by 8 of 69 patients (12%), and SCS plus MTX was used by 10 of 69 patients (15%). Of the 38 patients who received concurrent SCS, 7 received intravenous methylprednisolone, and the remainder received oral prednisone. The maximum median dose of prednisone was 30 mg/d (IQR, 10-40 mg/d). Duration of prednisone use was variable: 15 patients received 1 tapering dose, 2 patients received 2 or more tapering doses, and 13 patients used prednisone continuously. The maximum median dose of concurrent MTX was 25 mg/wk (IQR, 20-25 mg/wk), and the duration of concurrent MTX use was 11.5 months (IQR, 4-21 months). Concurrent HCQ was used by 5 patients (median duration, 12 months [IQR, 9-47 months]). Concurrent phototherapy was used by 2 patients.
Disease Activity After 3 to 6 Months and 9 to 12 Months of MMF
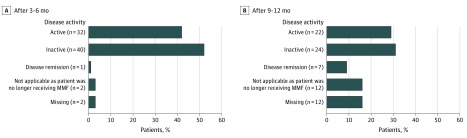
All participants had active disease prior to initiating mycophenolate. After 3 to 6 months of therapy, 73 patients were available for analysis (11 patients received mycophenolate therapy initially, and 62 received additional mycophenolate therapy). Three of 11 patients (27%) receiving mycophenolate therapy initially had active disease compared with 29 of 62 patients (47%) who received mycophenolate as additional therapy; the odds of active disease at 3 to 6 months with initial therapy was 0.43 (95% CI, 0.07-2.02) times the odds of active disease with additional mycophenolate therapy (P = .33). One patient with mild, circumscribed disease achieved DR at 3 to 6 months; this patient received mycophenolate treatment initially as monotherapy. After 9 to 12 months, 53 patients were available for analysis (8 received mycophenolate therapy initially, and 45 received additional mycophenolate therapy). Of those who received mycophenolate therapy initially, 3 of 8 patients (38%) had active disease compared with 19 of 45 patients (42%) who received mycophenolate as additional therapy, but the odds ratio was not statistically significant (0.82; 95% CI, 0.11-4.85) (Figure). Seven patients achieved DR after 9 to 12 months of treatment; which increased to 27 patients at completion of the study. Six of 12 patients who initially received mycophenolate therapy achieved DR; 5 of 6 responders were treated concurrently with MTX or SCS. Twelve of 27 patients who achieved DR with mycophenolate previously received MTX, which failed. Of the 27 patients who achieved DR, 10 continued to receive treatment with mycophenolate, and 17 were able to discontinue treatment. Fifteen of 27 patients who achieved DR were concomitantly treated with MTX and/or SCS. Of the 17 patients who were able to discontinue mycophenolate, 15 remained in remission 6 months after discontinuation.
Figure. Disease Activity at 3 to 6 Months and 9 to 12 Months.
A, Disease activity after 3 to 6 months. After 3 to 6 months, 73 patients were available for analysis (11 received mycophenolate mofetil [MMF] initially, and 62 received additional MMF therapy). One patient with mild, circumscribed disease achieved disease remission at 3 to 6 months. B, Disease activity after 9 to 12 months. After 9 to 12 months, 53 patients were available for analysis (8 received MMF initially, and 45 received additional MMF therapy. Seven patients achieved disease remission at 9 to 12 months.
Disease Response After 3 to 6 Months and 9 to 12 Months of Mycophenolate
Sixty-six of 73 participants (90%) had stable disease (2 received mycophenolate therapy initially, and 20 received additional mycophenolate therapy) or improved disease (8 received mycophenolate therapy initially, and 36 received additional mycophenolate therapy) after 3 to 6 months of treatment, constituting a BR (Table 3). Forty-seven of 54 participants (87%) had stable disease (1 received mycophenolate therapy initially, and 13 received additional mycophenolate therapy) or improved disease (7 received mycophenolate therapy initially, and 26 received additional mycophenolate therapy) after 9 to 12 months of treatment. There were 4 patients who had stable or improved disease at 3 to 6 months, but they transitioned to progressive disease at 9 to 12 months; 3 of 4 patients received concurrent treatments (1 received MTX, SCS, and HCQ; 1 received SCS; and 1 received HCQ), and all patients received 2 to 3 g/d equivalent of MMF. Only 7 of 54 patients (13%) were refractory to treatment with mycophenolate.
Table 3. Disease Response to Treatment With Mycophenolatea.
| Disease response | Patients, No./total No. (%) | ||
|---|---|---|---|
| Initial treatment with mycophenolate | Addition of mycophenolate | All | |
| At 3-6 mob | |||
| Progressive disease | 1/11 (9) | 6/62 (10) | 7/73 (10) |
| Stable disease | 2/11 (18) | 20/62 (32) | 22/73 (30) |
| Improved disease | 8/11 (73) | 36/62 (58) | 44/73 (60) |
| Beneficial responsec | 10/11 (91) | 56/62 (90) | 66/73 (90) |
| At 9-12 mod | |||
| Progressive disease | 0/8 (0) | 7/46 (15) | 7/54 (13) |
| Stable disease | 1/8 (13) | 13/46 (28) | 14/54 (26) |
| Improved disease | 7/8 (88) | 26/46 (57) | 33/54 (61) |
| Beneficial response | 8/8 (100) | 39/46 (85) | 47/54 (87) |
Initial patient population included 80 patients; 3 were excluded from analysis (1 still received treatment but for <3-6 months, 1 outlier, and 1 no data available).
Four patients were excluded from analysis (2 with an adverse event, 1 lost to follow-up, and 1 no data at 3-6 months).
Stable disease plus improved disease.
Twenty patients were excluded from analysis (8 still received treatment but for <9-12 months, 5 had an adverse event, 1 experienced insurance difficulties, 1 had a desire to conceive, 1 treatment ineffective, and 4 lost to follow-up).
Participants were stratified by BR at 3 to 6 months and 9 to 12 months to determine whether specific factors were associated with response to mycophenolate (eTable in the Supplement). After 3 to 6 months, BR was seen in 93% of patients (41 of 44) with deep morphea and 77% of patients (23 of 30) with superficial morphea. Compared with patients with superficial morphea, those with deep morphea had an 11.2-fold greater likelihood of BR, although the 95% CI (1.77-219.00) suggested significant imprecision. This association did not hold true after 9 to 12 months of treatment. After 9 to 12 months, dosing of mycophenolate per day was associated with a BR (47 of 54 patients [87%]; P = .01). Of the 47 patients who experienced a BR, 1 patient (2%) received less than 1.0 g/d of MMF, 9 (19%) received 1.0 to 1.9 g/d, 25 (53%) received 2.0 to 2.9 g/d, and 12 (26%) received 3.0 g/d or more. Disease severity, presence of functional impairment, morphea subtype, sex, race/ethnicity, anatomical location of morphea, antinuclear antibody positivity, and age at disease onset had no association with response to mycophenolate.
Safety of MMF and MPA
Most participants (73 of 77) were treated with MMF. Five patients experienced GI AEs and were transitioned to MPA. Two patients were initially treated with MPA and experienced no AEs. Thirty-four patients (44%) experienced an AE while taking mycophenolate; 21 of 34 patients were treated with concurrent systemic therapies (17 received SCS, 6 received MTX, 6 received HCQ, 2 received doxycycline, and 1 received intravenous immunoglobulin; some patients were receiving multiple concurrent therapies). The most common AE was GI distress (24 of 77 [31%]); 13 of 24 patients who experienced GI distress were treated with concurrent systemic therapies. Three patients (4%) developed cytopenia (1 with anemia and leukopenia and 2 with leukopenia). Two patients (3%) who were concurrently taking SCS developed infections. Many other AEs were reported (dizziness, palpitations, fatigue, alopecia, hirsutism, insomnia, depressive mood, vivid dreams, xerostomia, and transaminitis). There were no reports of malignant neoplasms or death.
Twelve participants discontinued mycophenolate secondary to an AE (7 with GI distress, 2 with infection, 2 with cytopenia, and 3 with fatigue; some patients experienced more than 1 AE). Of those who discontinued secondary to GI distress, all were treated with MMF initially, and only 1 patient was switched to MPA. Four patients discontinued mycophenolate to conceive.
Discussion
Previously, 2 retrospective reviews with a total of 17 patients sought to evaluate response to treatment with MMF for morphea. All but 1 of these patients had a favorable response to MMF.20,21 The present study expands on these data. With 77 patients from 8 institutions, this is the largest study to date evaluating the response and safety of mycophenolate for morphea, to our knowledge.
This study included patients with predominantly moderate to severe morphea. Most patients (77%) initiated mycophenolate because the prior treatment was ineffective. A BR was seen in 90% of patients (66 of 73) after 3 to 6 months and 87% (47 of 54) after 9 to 12 months of treatment. Seven patients achieved DR after 9 to 12 months of treatment; this increased to 27 patients at completion of the study. These data suggest that severe, recalcitrant morphea may have a BR associated with mycophenolate. Furthermore, although patients with morphea typically show signs of response after 3 to 4 months of therapy with mycophenolate, this study suggests that continued improvement in disease control may occur beyond typical trial durations. Beneficial response to treatment with mycophenolate was associated with doses of 2 g/d or more equivalent MMF. This finding is similar to the goal dose of MMF for systemic sclerosis (ie, 3 g/d).16
Methotrexate and SCS are currently recommended as first-line systemic agents for the treatment of morphea.2,3,4,5,6 To our knowledge, head-to-head comparison studies evaluating treatment of morphea with mycophenolate vs MTX have not been performed. In a pediatric randomized clinical trial evaluating MTX vs placebo for 12 months with SCS for the first 3 months, treatment response was seen in approximately two-thirds of the MTX group.7 In the present study, treatment response to mycophenolate was seen in more than 85% of patients. Almost half of the patients in this study (35 of 77) initiated mycophenolate because MTX was ineffective; despite this, only 7 of 54 patients (13%) were refractory to treatment with mycophenolate. Tolerability of MTX and mycophenolate appear to be similar. In the aforementioned randomized clinical trial, 56.5% of patients in the MTX group experienced mild AEs, with none requiring discontinuation of treatment.7 Comparatively, 44% of individuals in the present study reported an AE; 12 individuals discontinued secondary to an AE.
In this study, 12 patients initially received mycophenolate therapy alone or in combination. Three of these 12 patients received mycophenolate monotherapy; 2 of 3 had a BR, and 1 of 3 had progressive disease. Eight of these 12 patients experienced a BR after 9 to 12 months (2 received mycophenolate monotherapy and 6 received SCS). Half of these 12 patients achieved DR; concurrent treatment with SCS and/or MTX occurred for 5 of 6 patients. One patient with mild disease achieved DR while receiving mycophenolate monotherapy. Two patients discontinued mycophenolate owing to ineffectiveness. Overall, the data suggest that mycophenolate as monotherapy may be beneficial for mild morphea. Furthermore, mycophenolate, used in combination with SCS and/or MTX, may be beneficial for moderate to severe morphea. More studies are needed to compare the effectiveness of mycophenolate with MTX as first-line therapy for morphea.
Forty-four percent of patients in this study experienced an AE, which is higher than the 18.5% AE rate previously reported.24 The source of this discrepancy is GI distress; the present study reported GI distress in 24 of 34 patients (71%), and Omair et al24 reported GI distress in 48% of patients. More than half of the patients in this study with GI distress were treated with concurrent systemic therapies, which may have compounded their symptoms. The rate of infection in this study (3%) was less frequent than than in the study by Omair et al24 (37.8%). Four individuals discontinued treatment with mycophenolate owing to an associated infection (n = 2) or cytopenia (n = 2). There were no serious AEs. More than three-fourths of patients in this study were treated with concurrent systemic therapies. These data suggest that mycophenolate, alone or in combination with other systemic therapies, is a well-tolerated treatment option for morphea.
Limitations and Strengths
This study has some limitations, including its retrospective nature, lack of a control group, presence of potential confounding variables (ie, concurrent systemic treatments), and missing data. The sample size of this study is small; this limitation is difficult to overcome because the aim of this study was to evaluate a therapy that is used as a second-line or third-line agent for an already uncommon skin disease. The natural course of morphea is for any 1 plaque to burn out over the course of 3 to 5 years; it is difficult to determine whether improvement was secondary to mycophenolate or to the natural course of the disease. More important, 35% of the patients had either pansclerotic or linear morphea, subtypes that do not typically follow the aforementioned timeline. Participants in this study had persistently active disease with a long duration prior to initiation of mycophenolate, making spontaneous remission less likely. A strength of this study is that patients with morphea from 8 institutions across the United States participated.
Conclusions
The findings of this study suggest that mycophenolate mofetil or mycophenolic acid, used alone or in combination with other systemic therapies, is a well-tolerated and beneficial treatment of severe, recalcitrant morphea. In this study, 77% of participants received prior systemic treatment that failed, 64% had severe disease, and subsequently more than 85% had either improved or stable disease after treatment with mycophenolate.
eMethods.
eTable. Demographic and Clinical Characteristics Stratified by Disease Response After 3-6 and 9-12 Months of Treatment with Mycophenolate Mofetil
Footnotes
Abbreviation: BSA, body surface area.
Definitions for disease activity were extrapolated from the Localized Scleroderma Cutaneous Assessment Tool and a recent publication.21,22 Each subtype of morphea tends to have its own inherent severity. For instance, circumscribed plaque morphea is typically characterized by localized disease with minimal associated functional impairment. Alternatively, linear and pansclerotic morphea more frequently affect high-risk locations (head or neck, joints) and may cause deep sclerosis, heralding a higher risk of associated functional impairments. For this reason, definitions for disease severity were generated in an attempt to correspond with the definitions provided for each subtype of morphea: circumscribed plaque morphea (mild), generalized morphea (moderate), and linear or pansclerotic morphea (severe). Additional information regarding definitions is available in the eMethods in the Supplement.
References
- 1.Fett N, Werth VP. Update on morphea, part I: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64(2):217-228. doi: 10.1016/j.jaad.2010.05.045 [DOI] [PubMed] [Google Scholar]
- 2.Fett N, Werth VP. Update on morphea, part II: outcome measures and treatment. J Am Acad Dermatol. 2011;64(2):231-242. doi: 10.1016/j.jaad.2010.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol. 2011;65(5):925-941. doi: 10.1016/j.jaad.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 4.Fett N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31(4):432-437. doi: 10.1016/j.clindermatol.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 5.Li SC, Torok KS, Pope E, et al. ; Childhood Arthritis and Rheumatology Research Alliance (CARRA) Localized Scleroderma Workgroup . Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res (Hoboken). 2012;64(8):1175-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fett NM. Morphea: evidence-based recommendations for treatment. Indian J Dermatol Venereol Leprol. 2012;78(2):135-141. doi: 10.4103/0378-6323.93628 [DOI] [PubMed] [Google Scholar]
- 7.Zulian F, Martini G, Vallongo C, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1998-2006. doi: 10.1002/art.30264 [DOI] [PubMed] [Google Scholar]
- 8.Cox D, O’ Regan G, Collins S, Byrne A, Irvine A, Watson R. Juvenile localised scleroderma: a retrospective review of response to systemic treatment. Ir J Med Sci. 2008;177(4):343-346. doi: 10.1007/s11845-008-0217-0 [DOI] [PubMed] [Google Scholar]
- 9.Fitch PG, Rettig P, Burnham JM, et al. Treatment of pediatric localized scleroderma with methotrexate. J Rheumatol. 2006;33(3):609-614. [PubMed] [Google Scholar]
- 10.Uziel Y, Feldman BM, Krafchik BR, Yeung RS, Laxer RM. Methotrexate and corticosteroid therapy for pediatric localized scleroderma. J Pediatr. 2000;136(1):91-95. doi: 10.1016/S0022-3476(00)90056-8 [DOI] [PubMed] [Google Scholar]
- 11.Weibel L, Sampaio MC, Visentin MT, Howell KJ, Woo P, Harper JI. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155(5):1013-1020. doi: 10.1111/j.1365-2133.2006.07497.x [DOI] [PubMed] [Google Scholar]
- 12.Zulian F, Vallongo C, Patrizi A, et al. A long-term follow-up study of methotrexate in juvenile localized scleroderma (morphea). J Am Acad Dermatol. 2012;67(6):1151-1156. doi: 10.1016/j.jaad.2012.03.036 [DOI] [PubMed] [Google Scholar]
- 13.Christen-Zaech S, Hakim MD, Afsar FS, Paller AS. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59(3):385-396. doi: 10.1016/j.jaad.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 14.Kreuter A, Gambichler T, Breuckmann F, et al. Pulsed high-dose corticosteroids combined with low-dose methotrexate in severe localized scleroderma. Arch Dermatol. 2005;141(7):847-852. doi: 10.1001/archderm.141.7.847 [DOI] [PubMed] [Google Scholar]
- 15.Kroft EB, Creemers MC, van den Hoogen FH, Boezeman JB, de Jong EM. Effectiveness, side-effects and period of remission after treatment with methotrexate in localized scleroderma and related sclerotic skin diseases: an inception cohort study. Br J Dermatol. 2009;160(5):1075-1082. doi: 10.1111/j.1365-2133.2008.09017.x [DOI] [PubMed] [Google Scholar]
- 16.Derk CT, Grace E, Shenin M, Naik M, Schulz S, Xiong W. A prospective open-label study of mycophenolate mofetil for the treatment of diffuse systemic sclerosis. Rheumatology (Oxford). 2009;48(12):1595-1599. doi: 10.1093/rheumatology/kep295 [DOI] [PubMed] [Google Scholar]
- 17.Mendoza FA, Nagle SJ, Lee JB, Jimenez SA. A prospective observational study of mycophenolate mofetil treatment in progressive diffuse cutaneous systemic sclerosis of recent onset. J Rheumatol. 2012;39(6):1241-1247. doi: 10.3899/jrheum.111229 [DOI] [PubMed] [Google Scholar]
- 18.Stratton RJ, Wilson H, Black CM. Pilot study of anti-thymocyte globulin plus mycophenolate mofetil in recent-onset diffuse scleroderma. Rheumatology (Oxford). 2001;40(1):84-88. doi: 10.1093/rheumatology/40.1.84 [DOI] [PubMed] [Google Scholar]
- 19.Vanthuyne M, Blockmans D, Westhovens R, et al. A pilot study of mycophenolate mofetil combined to intravenous methylprednisolone pulses and oral low-dose glucocorticoids in severe early systemic sclerosis. Clin Exp Rheumatol. 2007;25(2):287-292. [PubMed] [Google Scholar]
- 20.Martini G, Ramanan AV, Falcini F, Girschick H, Goldsmith DP, Zulian F. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology (Oxford). 2009;48(11):1410-1413. doi: 10.1093/rheumatology/kep244 [DOI] [PubMed] [Google Scholar]
- 21.Mertens JS, Marsman D, van de Kerkhof PC, et al. Use of mycophenolate mofetil in patients with severe localized scleroderma resistant or intolerant to methotrexate. Acta Derm Venereol. 2016;96(4):510-513. doi: 10.2340/00015555-2297 [DOI] [PubMed] [Google Scholar]
- 22.Arkachaisri T, Vilaiyuk S, Torok KS, Medsger TA Jr. Development and initial validation of the localized scleroderma skin damage index and physician global assessment of disease damage: a proof-of-concept study. Rheumatology (Oxford). 2010;49(2):373-381. doi: 10.1093/rheumatology/kep361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2014. [Google Scholar]
- 24.Omair MA, Alahmadi A, Johnson SR. Safety and effectiveness of mycophenolate in systemic sclerosis: a systematic review. PLoS One. 2015;10(5):e0124205. doi: 10.1371/journal.pone.0124205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable. Demographic and Clinical Characteristics Stratified by Disease Response After 3-6 and 9-12 Months of Treatment with Mycophenolate Mofetil