Abstract
Rotavirus infection has emerged as an important cause of complications in organ transplantation recipients and might play a role in the pathogenesis of inflammatory bowel disease (IBD). 6-Thioguanine (6-TG) has been widely used as an immunosuppressive drug for organ recipients and treatment of IBD in the clinic. This study aims to investigate the effects and mode-of-action of 6-TG on rotavirus replication. Human intestinal Caco2 cell line, 3D model of human primary intestinal organoids, laboratory rotavirus strain (SA11) and patient-derived rotavirus isolates were used. We have demonstrated that 6-TG significantly inhibits rotavirus replication in these intestinal epithelium models. Importantly, gene knockdown or knockout of Rac1, the cellular target of 6-TG, significantly inhibited rotavirus replication, indicating the supportive role of Rac1 for rotavirus infection. We have further demonstrated that 6-TG can effectively inhibit the active form of Rac1 (GTP-Rac1), which essentially mediates the anti-rotavirus effect of 6-TG. Consistently, ectopic over-expression of GTP-Rac1 facilitates but an inactive Rac1 (N17) or a specific Rac1 inhibitor (NSC23766) inhibits rotavirus replication. In conclusion, we have identified 6-TG as an effective inhibitor of rotavirus replication via the inhibition of Rac1 activation. Thus, for transplantation patients or IBD patients infected with rotavirus or at risk of rotavirus infection, the choice of 6-TG as a treatment appears rational.
Keywords: Rotavirus, 6-Thioguanine, Rac1, Intestinal organoids
Highlights
-
•
6-TG inhibits rotavirus infection.
-
•
Rac1, the cellular target of 6-TG, supports rotavirus infection.
-
•
6-TG inhibits the active form of Rac1 (GTP-Rac1) to exert the anti-rotavirus effect.
1. Introduction
Rotavirus, a double stranded RNA (dsRNA) virus in the Reoviridae family, is a major cause of gastroenteritis, particularly in children younger than 5 years of age. As a major global health problem, this virus causes 114 million diarrhea episodes, 2.4 million hospitalizations, and an estimated 215,000 deaths worldwide annually (Grimwood and Buttery, 2007; Tate et al., 2016). Although rotavirus infection mainly occurs in developing countries, it also results in over 200 deaths and more than 87,000 hospital admissions in infants in European Union (Vesikari et al., 2007). Besides young children, organ transplantation patients are also susceptible to rotavirus infection irrespective of their age, causing long-term diarrhea and even death due to graft failure (Yin et al., 2015b). Although two global licensed rotavirus vaccines have been launched, no specific antiviral treatment is available.
A thio analogue of the naturally occurring purine base guanine, 6-thioguanine (6-TG), has been used in the clinic since the early 1950s (Munshi et al., 2014). 6-TG was initially developed to treat cancer; whereas currently it is widely used as an immunosuppressive agent in organ transplantation. It is also used as treatment for acute lymphoblastic leukemia in children and for autoimmune diseases (Bourgine et al., 2011). In particular, 6-TG is often used to treat inflammatory bowel disease (IBD) (de Boer et al., 2006). IBD including Crohn's disease (CD), ulcerative colitis (UC) and indeterminate colitis (IC) represent a heavy burden in Western countries (Kolho et al., 2012). Although the causes of exacerbations of IBD remain poorly characterized, gastrointestinal infections including rotavirus might induce flares in IBD (Masclee et al., 2013). Thus, preventing or treating rotavirus infection in these patients is of importance.
Upon ingestion, 6-TG is first metabolized into 6-thioguanosine monophosphate (6-TGMP), and subsequently into 6-thioguanosine diphosphate (6-TGDP), and finally into 6-thioguanosine triphosphate (6-TGTP) (Chouchana et al., 2012). Among these metabolites, 6-TGDP and 6-TGTP are able to compete with endogenous guanosine phosphates for Rac1 binding and to form 6-TGNP•Rac1 complexes. These complexes are in turn incapable to support the formation of the active configuration of Rac1, a process that Rac1 interacts with GTP. Thus, 6-TG indirectly provokes inhibition of Rac1-dependent signaling (Shin et al., 2016), which has substantial consequences for cellular physiology. As a member of the Ras superfamily of Rho GTPases, GTP-bound Rac1 mediates a myriad of cellular processes including actin reorganization and gene transcription. Intriguingly, IBD is characterized by hyperactivation of Rac1 in the phagocyte compartment. This is associated with reduced effector function of Rac1, which is sensitive to 6-TG treatment (Parikh et al., 2014). The inhibition of Rac1 resulting from 6-TG treatment restores innate immune functionality of phagocytes in IBD patients, contributing to disease remission (Parikh et al., 2014). Thus, Rac1 hyperactivation appears an important immunosuppressive effector in human pathophysiology, at least in the phagocyte compartment.
Given the clinical relevance and the potential in revealing mechanistic insight, we have investigated the effects and mechanism-of-action of 6-TG on rotavirus replication. To this end, we have demonstrated that 6-TG effectively combats rotavirus replication through inhibition of Rac1 activation.
2. Materials and methods
2.1. Viruses and reagents
Simian rotavirus SA11 strain and patient-derived rotavirus isolates (G1P[8]) were prepared as previously described (Yin et al., 2015a, 2016).
Stocks (0.1 mg/mL) of 6-TG (Sigma-Aldrich) were dissolved in alkali solution (1 M NaOH, 50 mg/mL), and NSC23766 (Merck Millipore) was dissolved in H2O (2 mM). All chemicals were stored in 25 μL aliquots and frozen at −80 °C.
2.2. Conventional enterocyte culture and human primary intestinal organoid culture
Human colon cancer cell line Caco2 and human embryonic kidney cell line 293 T (HEK 293 T) cells were grown in Dulbecco's modified Eagle medium (DMEM) (Invitrogen-Gibco, Breda, The Netherlands) supplemented with 20% (vol/vol) heat-inactivated fetal calve serum (FCS) (Hyclone, Lonan, Utah) and Penicillin (100 IU/mL)/streptomycin (100 mg/mL) (Invitrogen-Gibco) at 37 °C in a humidified 5% CO2 incubator. Rac1 knockout mouse embryonic fibroblast (MEF) cells were cultured in DMEM supplemented with 10% FCS, Penicillin (100 IU/mL)/streptomycin (100 mg/mL), l-Glutamine (Gibco® by Life Technologies), non-essential amino acids (Gibco® by Life Technologies) and sodium-pyruvate (Gibco® by Life Technologies). Caco2 cells with stable knockdown of Rac1 were generated by transduction of lentiviral shRNA (produced in HEK293T cells) targeting Rac1 and selected with puromycin (6 μg/mL) as described previously (Yin et al., 2016). The shRNA targeting sequences used in this study were listed in Table S1.
3D culture of human intestinal organoids was performed as previously described (Yin et al., 2015a).
2.3. Rotavirus inoculation and drug treatment
Inoculation and treatment of Caco2 cells and human intestinal organoids with SA11 and patient-derived rotavirus were performed as previously described (Yin et al., 2016).
2.4. Viability assay of cells or organoids
The viability of cells or organoids was determined by 3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Briefly, Caco2 cells (1 × 104 cells/well) or organoids were seeded into a 96-well culture plate and incubated with various concentrations of 6-TG or NSC23766 for 48 h, followed by adding 500 μg/mL of MTT solution to each well and incubation at 37 °C for 3 h. Subsequently, the medium was removed, and replaced with 100 μL DMSO and incubated at 37 °C for 50 min. Then, the absorbance was measured at 490 nm in an enzyme-linked immunosorbent assay reader (BIO-RAD). The effects of 6-TG, NSC294002, IFNα and ribavirin on host cell viability were determined by MTT assay (Fig. S1).
2.5. Transfection of plasmids
A constitutively active Rac1V12 and dominant inactive Rac1N17 plasmids were prepared as previously described (Apolloni et al., 2013). HEK 293 cells were seeded into 6-well plates for Rac activation assay or 48-well plates for infection assay with ∼70% confluence. The cells were washed with PBS, followed by adding 500 μL of Opti-MEM® reduced serum medium with 2 μg Rac1V12 or Rac1N17 plasmids and 10 μg polyethylenimine (PEI) per well of a 6-well plate, or 100 μL of Opti-MEM® reduced serum medium (Thermo Fisher Scientific) with 0.25 μg Rac1V12 or Rac1N17 plasmids and 1.25 μg polyethylenimine (PEI) (Sigma-Aldrich) per well of a 48-well plate. After 4–5 h of incubation, 2 mL or 0.5 mL of DMEM containing 10% FCS was added to each well of 6-well plate or 48-well plate, respectively. Transfected cells were infected with rotavirus for 24 h.
2.6. Quantitative real-time PCR (qRT-PCR)
Total cellular RNA was isolated using a NucleoSpin® RNA kit (MACHEREY-NAGEL, Düren, Germany) following the manufacturer's protocol, and quantified with a Nanodrop ND-1000 (Wilmington, DE, USA). cDNA was synthesized using the reverse transcription system from TAKARA according to manufacturer's instructions (TAKARA BIO INC). The resulting cDNA was diluted 1:10, and 2 μL of the diluted cDNA was used for qRT-PCR with primers listed in Table S1. All qRT-PCR experiments were performed by SYBR-Green-based (Applied Biosystems SYBR Green PCR Master Mix; Thermo Fisher Scientific Life Science) real-time PCR with the StepOnePlus System (Thermo Fisher Scientific Life Sciences). The expression of target mRNA was normalized to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. Gene expression analysis was performed by the ΔΔCT method (Yin et al., 2015a, 2016).
2.7. Rac activation assay
The levels of Rac1-bound GTP were detected using Rac interactive binding (CRIB) domain of PAK (aa56-272) as described previously (Fuhler et al., 2008). In brief, GST-PAKcrib protein was pre-coupled to glutathione-Sepharose beads (Sigma-Aldrich) for 45 min at 4 °C. Caco2 cells treated with various concentrations of 6-TG or NSC23766 (48 h, seeded in 6-well-plate) were lysed for 10 min in lysis buffer (50 mM Tris, pH 7.4, 10% glycerol, 200 mM NaCl, 1% NP-40, 2 mM MgCl2, 2 mM sodium orthovanadate, and protease inhibitors). Cell lysates were incubated with pre-coupled beads for 45 min. Then, agarose beads were washed 3 times with 1x lysis buffer, followed by boiling in Laemmli buffer, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 15%) analysis.
2.8. Western blotting
Cell lysates were subjected to SDS-PAGE, and proteins were transferred to PVDF membrane (Immobilon-FL). Rac1 monoclonal antibody (1:1000, rabbit; cell signaling) and SA11 rotavirus VP4 [1:1000, HS-2, a mouse monoclonal antibody (provided by Professor Harry Greenberg, Stanford University School of Medicine, USA)] were detected by Western blotting analysis. Detection of β-actin was served as loading control (1:1000, mouse monoclonal; Santa Cruz) as previously described (Versteeg et al., 2002).
2.9. Serial passaging of rotavirus with 6-TG treatment
To investigate whether rotavirus can develop resistance to 6-TG treatment, viruses were passaged in both MA104 and Caco2 cells in the absence of drug (vehicle control) or in the presence of gradually increasing concentrations of the drug (1000 ng/mL of 6-TG for passage 1–10 and 2000 ng/mL of 6-TG for passage 11–20). In brief, MA104 or Caco2 cells in 24-well plate were inoculated with 200 μL virus (MOI = 0.7) at 37 °C for 1 h, followed by adding 6-TG or without drug (as control). After 48 h, both cells and supernatant were harvested, subsequently frozen, thawed once, and centrifuged. The supernatant containing passaged viruses was stored at −80 °C until used for the next passage. Viruses were serially passaged by using 1 aliquot of viral stock from the preceding passage to infect fresh MA104 or Caco2 cells. The effect of each passage of virus (same titer) was quantified by qRT-PCR.
2.10. IC50 and CC50 calculation
IC50 and CC50 calculation were described previously (Yin et al., 2016).
2.11. Statistics
Data are presented as means, and statistical comparison between different groups was performed by Mann-Whitney test (two-tailed) using GraphPad Prism 5. Error bars represent the SEM, and P value <0.05 was considered statistically significant.
3. Results
3.1. 6-TG remarkably inhibits rotavirus replication
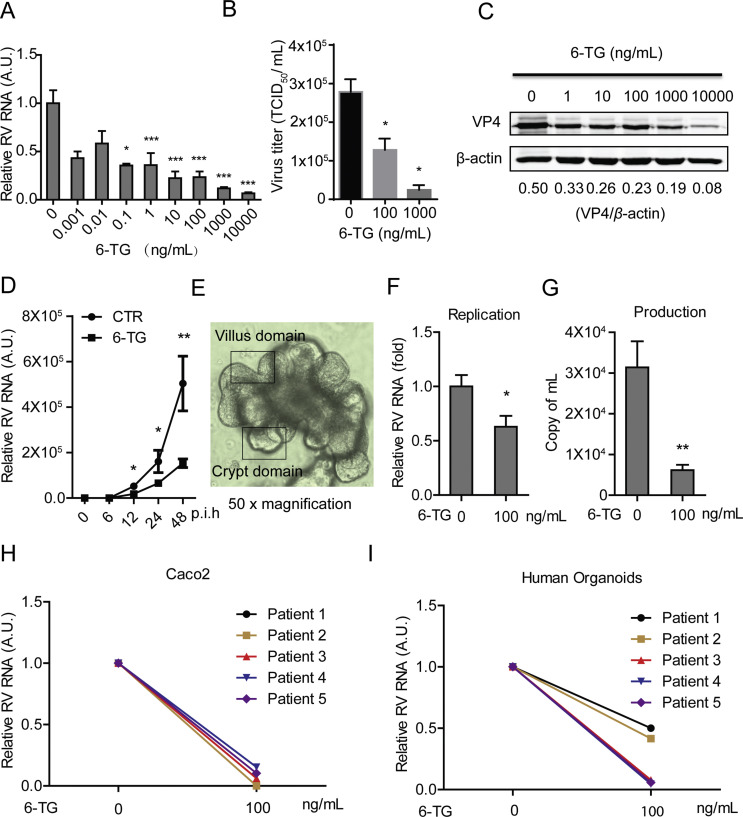
To assess the effects of 6-TG on rotavirus replication, we treated SA11 rotavirus-infected Caco2 cells with various concentrations (0.001–10000 ng/mL) of 6-TG for 48 h. This resulted in dose-dependent inhibition of rotavirus RNA replication (Fig. 1 A, n = 3–17, *P < 0.05, ***P < 0.001). The IC50 value of 6-TG against SA11 rotavirus was 3.0 × 10−13 M, which is substantially below the concentrations reached in patients undergoing 6-TG or azathioprine therapy. CC50 of 6-TG to Caco2 cells was 9.8 × 10−6 M and selectivity index (SI, CC50/IC50) was 3.3 × 107 (Table S2). This was further confirmed at the infectious virus production levels and protein levels of the rotavirus VP4 protein (Fig. 1B and C). To further verify the effect of 6-TG on rotavirus replication, SA11 rotavirus-infected Caco2 cells were treated with 6-TG at different time points (6, 12, 24 and 48 h), demonstrating that 6-TG significantly inhibited rotavirus RNA replication in time-dependent Manner (Fig. 1D, n = 4–8, *P < 0.05, **P < 0.01). These results were substantiated by experiments in human primary intestinal organoids, a model system that recapitulates many aspects of the intestinal epithelium, including the presence of a villus domain and a crypt domain (Fig. 1E) (Sato and Clevers, 2013). 6-TG significantly inhibited SA11 rotavirus replication and virus production in these organoids (Fig. 1F and G, n = 6, *P < 0.05, **P < 0.01). Our results were essentially repeated using five patient-derived rotavirus isolates in Caco2 cells but also in human intestinal organoids (Table S3). Treatment with 100 ng/mL 6-TG inhibited patient-derived isolates in both Caco2 cells (Fig. 1H) and human intestinal organoids (Fig. 1I). Hence, 6-TG significantly counteracts rotavirus replication at clinically relevant concentrations.
Fig. 1.
6-TG effectively inhibits rotavirus replication. (A) Treatment with 6-TG (48 h) significantly inhibited viral genomic RNA in SA11 rotavirus infected Caco2 cells (MOI = 0.7) in a dose-dependent manner (n = 6–17, means ± SEM, *P < 0.05, ***P < 0.001, Mann-Whitney test, A.U. denotes artificial unit). (B) Effects of 6-TG on the production of infectious viral particles determined by TCID50 method. Each bar represents the TCID50/mL (mean ± SEM) (n = 3, P < 0.05, Mann-Whitney test). (C) Treatment with 6-TG (48 h) significantly inhibited viral VP4 protein in SA11 rotavirus infected Caco2 cells in a dose-dependent manner (the densitometric analysis of immunoreactive bans in western bots was quantified by ImageJ software, and the ratio of VP4/β-actin was expressed in arbitrary units). (D) Treatment with 6-TG (6–48 h) significantly inhibited viral genomic RNA in SA11 rotavirus infected Caco2 cells (MOI = 0.7) in a time-dependent manner (n = 4–8, means ± SEM, *P < 0.05, **P < 0.01, Mann-Whitney test, p.i.h denotes hour of post infection). (E) The morphology of human primary small intestinal organoids with clear villus domain and crypt domain. (F) Treatment with 6-TG (48 h) significantly inhibited genomic RNA in SA11 rotavirus (MOI = 7) infected human intestinal organoids (n = 6, means ± SEM, *P < 0.05, Mann-Whitney test). (G) Treatment with 6-TG (48 h) significantly inhibited viral production in SA11 rotavirus infected human intestinal organoids (n = 6, means ± SEM, **P < 0.01, Mann-Whitney test). (H) Treatment with 100 ng/mL of 6-TG (48 h) inhibited viral RNA of patient-derived rotavirus isolates in Caco2 cells. (I) Treatment with 100 ng/mL of 6-TG (48 h) inhibited viral RNA of patient-derived rotavirus isolates in human intestinal organoids.
3.2. Development of resistance to 6-TG is uncommon for rotavirus
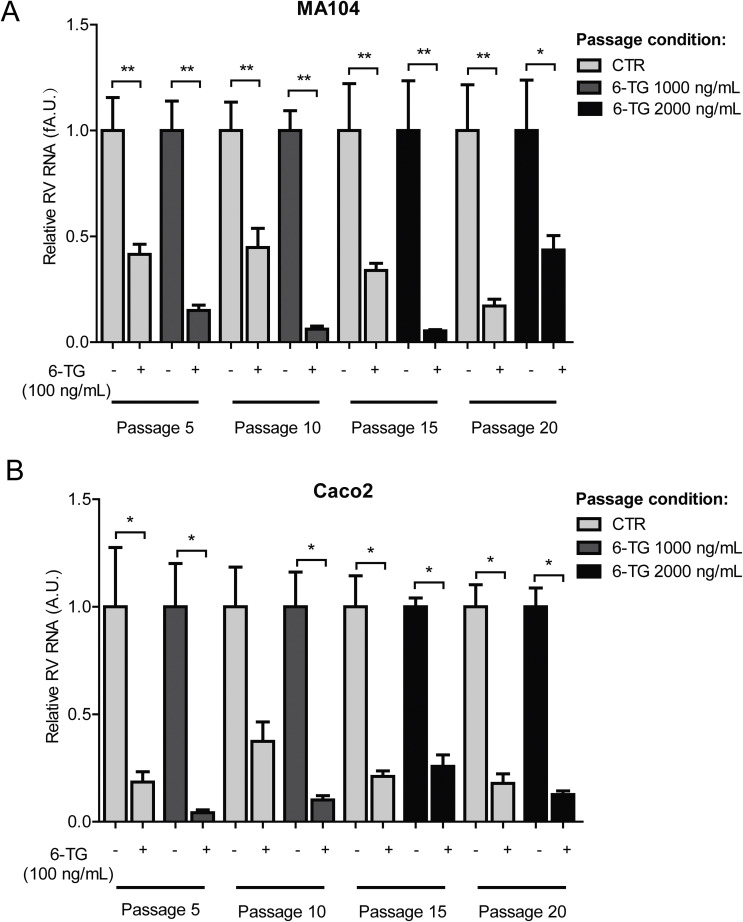
Rotavirus was serially passaged in the presence of escalating concentrations of 6-TG to assess the potential of drug resistance development. As a control, wild-type SA11 rotavirus was passaged in the absence of the drug. During the initial ten passages, infected cultures were exposed to 1000 ng/mL of 6-TG; whereas the drug concentration was increased to 2000 ng/mL at the subsequent later passages. After 20 passages, the effects of 6-TG (100 ng/mL) on each representative passage (both drug treated and untreated) of rotavirus was quantified by qRT-PCR. Our results indicate that rotavirus remains sensitive to 6-TG treatment in MA104 cells (Fig. 2 A) and Caco2 cells (Fig. 2B). Therefore, rotavirus does not easily develop 6-TG resistance, suggesting that the antiviral effects of 6-TG likely depend on a cellular target but not a direct interaction with the virus itself.
Fig. 2.
6-TG has a high barrier to drug resistance development. Rotavirus was serially passaged in MA104 or Caco2 cells exposed to no 6-TG (as control) and increasing concentrations of 6-TG until 20 passages (infected cultures were exposed to 1000 ng/mL of 6-TG in passage 1–10; whereas the drug concentration was increased to 2000 ng/mL at the subsequent later passages). The effect of 6-TG (100 ng/mL) on passage 5, 10, 15 and 20 (the drug treated and untreated) of rotavirus was quantified using qRT-PCR. 6-TG retained its anti-rotavirus activity even with continuous exposure to 6-TG for 20 passages in MA104 cells (A) and Caco2 cells (B).
3.3. Rac1, the cellular target of 6-TG, sustains rotavirus replication
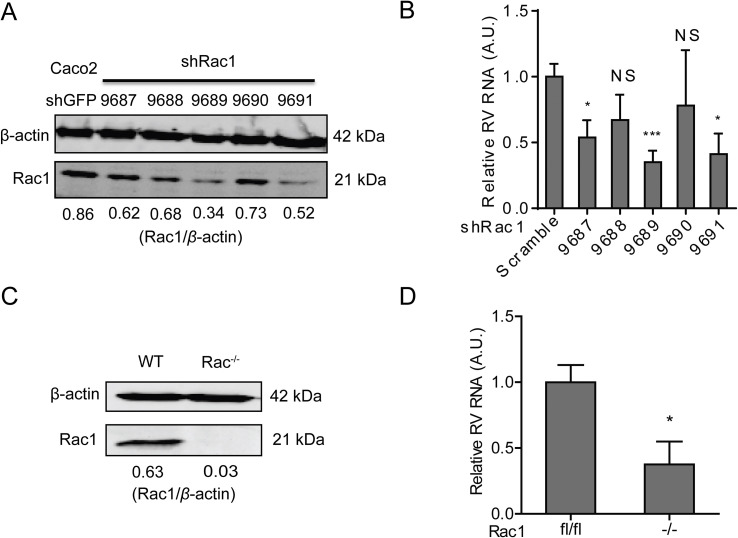
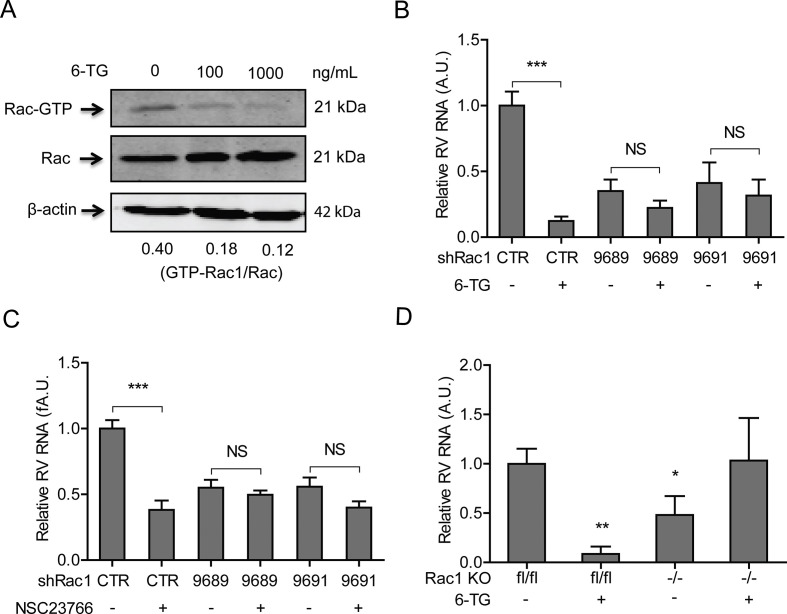
Although initially characterized as a nucleotide synthesis inhibitor, in the last decade it has become clear that many of the effects of 6-TG, at least in gastrointestinal pathophysiology, depend on its potency to inhibit Rac1 (Shin et al., 2016). Thus, we investigated the potential role of Rac1 on rotavirus replication. To this end, we performed a lentiviral RNAi-mediated loss-of-function assay to silence the Rac1 gene. Two (no. 9689 and 9691) out of five RNAi vectors showed potent knockdown of the target gene (Fig. 3 A). Importantly, these two RNAi vectors resulted in 65.0 ± 0.1% (n = 10, P < 0.001) and 61.8 ± 0.2% (n = 6, P < 0.05) reduction of SA11 rotavirus viral RNA, respectively (Fig. 3B). In apparent agreement, we obtained Rac1 knockout mouse embryonic fibroblasts cells (MEFs), and their status as a bona fide knockout was confirmed by western blot assay (Fig. 3C). Rotavirus replication in Rac1 knockout MEFs (−/−) was significantly less efficient (62.4 ± 0.2%; n = 4; P < 0.05), compared with the replication in wild type MEF (lf/lf) (Fig. 3D). Thus, decreased Rac1 levels correlate with increased resistance towards rotavirus replication.
Fig. 3.
Rac1, the drug target of 6-TG, sustains rotavirus replication. (A) Western blot assay detected Rac1 in transduced Caco2 cells transduced lentiviral RNAi vectors against Rac1 (The ratio of Rac1/β-actin was expressed in arbitrary units). (B) Three (No. 9687, 9689 and 9690) out of five lentiviral shRNA vectors inhibited rotavirus genomic RNA (n = 6–10, means ± SEM, *P < 0.05, ***P < 0.001, Mann-Whitney test). (C) Western blot assay confirmed knockout of Rac1 in Rac1 knockout (−/−) MEF cells. (D) SA11 rotavirus replication was significantly attenuated in Rac1 knockout (−/−) MEF cells (n = 4, means ± SEM, *P < 0.05, Mann-Whitney test).
3.4. Efficient rotavirus replication requires Rac1 activation
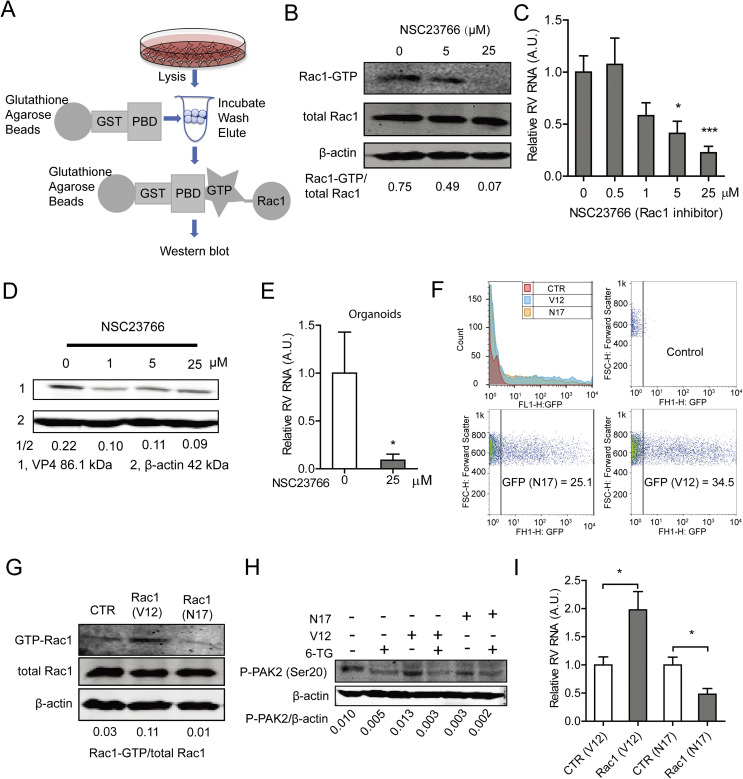
Rac1 acts as binary switch in cellular biochemistry, and it is only capable of provoking signaling in the active GTP-bound form (Shin et al., 2016). NSC23766, a specific GTP-Rac1 inhibitor was able to effectively inhibit GTP-Rac1 accumulation in Caco2 cells, as evident when tested in GTP-Rac1 specific pull-down assay (Fig. 4 A and B). Importantly, treatment with either 5 or 25 μM NSC23766 for 48 h resulted in 58.8 ± 0.1% (n = 8; P < 0.05) and 77.48 ± 0.1% (n = 10; P < 0.001) reduction on viral RNA levels, respectively (Fig. 4C). The IC50 value of NSC23766 against SA11 rotavirus was 1.1 × 10−8 M, the CC50 of NSC23766 to Caco2 cells was 3.1 × 10−4 M, and the SI was 2.8 × 104 (Table S2). The inhibitory effect of NSC23766 on the rotavirus infectious was further verified by a western blot assay, revealing that treatment with either 1, 5 or 25 μM NSC23766 significantly inhibits rotavirus VP4 protein synthesis in Caco2 cells (Fig. 4D). The effect of this drug was further confirmed by experiments in human primary intestinal organoids, which indicated 91.1 ± 0.1% (n = 6; P < 0.05) reduction of viral RNA in the organoids (Fig. 4E) following treatment with NSC23766.
Fig. 4.
The activation form of Rac1 is required for supporting rotavirus replication. (A) Schematic depicting the pull-down assay. (B) NSC23766 inhibited GTP-Rac1 detected by pulldown assay (The ratio of GTP-Rac1/Rac was expressed in arbitrary units). (C) Treatment with NSC23766 (48 h) significantly inhibited viral genomic RNA in SA11 rotavirus infected Caco2 cells in a dose-dependent manner (n = 8–10, means ± SEM, *P < 0.05, ***P < 0.001, Mann-Whitney test). (D) Treatment with NSC23766 (48 h) significantly inhibited viral VP4 protein in SA11 rotavirus infected Caco2 cells in a dose-dependent manner (The ratio of VP4/β-actin was expressed in arbitrary units). (E) Treatment with NSC23766 (48 h) significantly inhibited viral RNA in SA11 rotavirus infected human intestinal organoids (n = 6, means ± SEM, *P < 0.05, Mann-Whitney test). (F) Flow cytometric analysis of green fluorescence indicated the percentages of transduced cells with Rac1V12 and Rac1N17 plasmids. Median fluorescence identity (MFIs) of control, N17 and V12 are 3.43, 35.5 and 84.3, respectively. (G) Pull-down and western blot assays showed higher level of GTP-Rac1 with transduction of Rac1V12 but lower level of GTP-Rac1 with Rac1N17 plasmids (The ratio of GTP-Rac1/Rac was expressed in arbitrary units). (H) Detection of phospho-PAK2 indicated successful transduction of Rac1V12 and Rac1N17 plasmids. (I) Rac1V12 transduction facilitates but Rac1N17 inhibits rotavirus replication (n = 10, means ± SEM, *P < 0.05, Mann-Whitney test).
Next, we tested the effects of constitutively active and dominant negative forms of Rac1 on rotavirus replication. This was done by transfection of the active Rac1V12 plasmid or the dominant negative Rac1N17 plasmid. Expression of both plasmids was successful when tested by flow cytometry (Fig. 4F). Accordingly, Rac1V12-transfected cells displayed abundant GTP-Rac1, which in contrast is low in Rac1N17-transfected cells, as determined in a Rac pull-down assay (Fig. 4G). These results were confirmed by analyzing abundance of phospho-PAK2 status (Fig. 4H). Importantly, Rac1V12 promoted, but Rac1N17 inhibited rotavirus replication (Fig. 4I). Taken together, activation of Rac1 supports rotavirus replication.
3.5. 6-TG inhibits rotavirus via suppression of Rac1 GDP/GTP cycling
The inhibition of the activation of Rac1 by 6-TG was reported in many cell types (Fuhler et al., 2008; Shin et al., 2016; Tiede et al., 2003). Employing the Rac1 pull-down assay (Fig. 4A), we observed that 6-TG potently inhibited GTP-Rac1 accumulation; whereas corresponding western blots did not show reduced overall Rac1 levels in Caco2 cells following 6-TG treatment (Fig. 5 A). Functional studies using Rac1 knockdown Caco2 cells (Fig. 5B and C) were performed to demonstrate that both 6-TG and NSC23766 require Rac1 to combat rotavirus replication. In agreement, pharmacological Rac1 inhibitors did not inhibit rotavirus replication in Rac1 knockout MEF cells (Fig. 5D). Thus 6-TG inhibits rotavirus via suppression of Rac1 activation.
Fig. 5.
6-TG inhibits rotavirus via suppression of Rac1 activation. (A) Anti-rotavirus effect of 6-TG (100 ng/mL) was attenuated in Rac1 knockdown Caco2 cells (The ratio of VP4/β-actin was expressed in arbitrary units). (B) 6-TG inhibited GTP-Rac1 detected by pull-down assay. (C) Anti-rotavirus effect of NSC23766 (25 μg ng/mL) was attenuated in Rac1 knockdown Caco2 cells. (D) The anti-rotavirus effect of 6-TG (100 ng/mL) was attenuated in Rac1 knockout (−/−) MEF cells (n = 6–8, means ± SEM, *P < 0.05, **P < 0.01, Mann-Whitney test).
3.6. 6-TG has no combination effect with IFNα, but moderately antagonistic effect with ribavirin on rotavirus replication
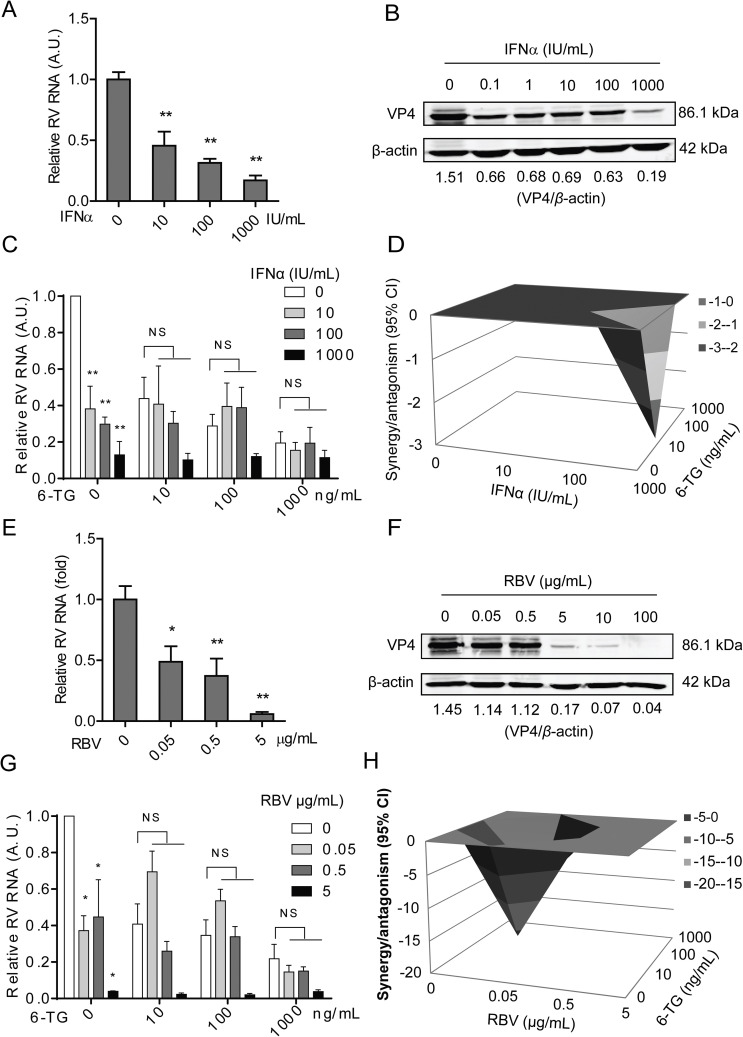
Interferon-alpha (IFNα) and ribavirin are widely used as general antivirals, being confirmed to significantly inhibit rotavirus replication in vitro (Yin et al., 2015a). Consistently, we again demonstrated the inhibitory effects of IFNα and ribavirin on rotavirus RNA (Fig. 6 A and 6E) and viral protein (Fig. 6B and F) in a dose-dependent manner. The IC50 value of IFNα against SA11 rotavirus was 3.1 × 10−5 IU, CC50 of IFNα to Caco2 cells was 18706 IU, and SI was 6.0 × 108 (Table S2). The IC50 value of ribavirin against SA11 rotavirus was 1.6 × 10−7 M, CC50 of ribavirin to Caco2 cells was 3.02 × 10−2 M and SI was 1.9 × 105 (Table S2). Next, we assessed the combinatory antiviral effects of 6-TG with IFNα or ribavirin. The combination of 6-TG and IFNα resulted in no combination (no synergy or antagonism) antiviral effect, with a synergy volume of −2.8 μM2% (Fig. 6C and D). However, the combination of 6-TG and ribavirin resulted in moderately antagonistic antiviral effect, with a synergy volume of −26.02 μM2% (Fig. 6G and H).
Fig. 6.
The effects of the combination of 6-TG with IFNα, or ribavirin on rotavirus replication. (A) Treatment with IFNα (48 h) significantly inhibited viral genomic RNA in SA11 rotavirus infected Caco2 cells in a dose-dependent manner (n = 4, means ± SEM, **P < 0.01, Mann-Whitney test). (B) Treatment with IFNα (48 h) remarkably inhibited viral VP4 protein in SA11 rotavirus infected Caco2 cells in a dose-dependent manner (The ratio of VP4/β-actin was expressed in arbitrary units). (C) Effect of the combination of various concentrations of 6-TG and IFNα on rotavirus replication in Caco2 cells. (D) Synergy plot representing the percentage of antiviral activity above/below the expected activity for the 6-TG-IFNα combination based on the data shown in C. (E) Treatment with ribavirin (48 h) significantly inhibited viral genomic RNA in SA11 rotavirus infected Caco2 cells in a dose-dependent manner (n = 4–7, means ± SEM, *P < 0.05, **P < 0.01, Mann-Whitney test). (F) Treatment with ribavirin (48 h) remarkably inhibited viral VP4 protein in SA11 rotavirus infected Caco2 cells in a dose-dependent manner (The ratio of VP4/β-actin was expressed in arbitrary units). (G) Effect of the combination of various concentrations of 6-TG and ribavirin on rotavirus replication in Caco2 cells. (H) Synergy plot representing the percentage of antiviral activity above/below the expected activity for the 6-TG-ribavirin combination based on the data shown in G.
4. Discussion
In this study, we have demonstrated that 6-TG effectively inhibits rotavirus replication via inhibition of the Rac1 activity. This is particularly interesting in view that rotavirus replication is especially an issue in organ transplantation recipients and in patients with IBD. 6-TG is a therapeutic option for both groups of patients. Based on the findings presented in the current study, the choice of 6-TG for these patients appears rational, in particular when they are at risk of rotavirus infection (Yin et al., 2015b).
Our results fit well with the momentum of studies that document antiviral activity of 6-TG. For instance, it has been reported that 6-TG can combat Middle East respiratory syndrome coronavirus infection by augmenting interferon responses (Cheng et al., 2015) and it has also reported that Simian virus 40 DNA replication is antagonized by 6-TG (Maybaum et al., 1987). Interestingly, it was reported that vaccinated IBD patients had lower titers of hepatitis B surface antibody (HBsAb), which might be influenced by the use of immunosuppressants including 6-TG (Watts et al., 2017).
As a 6-thiopurine (6-TP) prodrug, 6-TG is converted into pharmacologically active deoxy-6-thioguanosine phosphate (also called 6-thioguanine nucleotide) and 6-thioguanosine phosphate (6-TGNP). 6-TGNP can bind to Rac1 to form the 6-TGNP•Rac1 complex inactivating Rac1 (Shin et al., 2016). As a major player of the Rho family of small GTPases, Rac1 plays a vital role in various cellular signaling pathways to regulate a wide variety of cell functions including gene transcription, cell proliferation, apoptosis, motility, and redox signaling (D'Ambrosi et al., 2014). The expression of Rac1 is ubiquitous, but it has two conformational states including an inactive GDP-bound form and an active GTP-bound form (Bosco et al., 2009). It exerts biological functions mainly through activation of Rac1 (i.e. GTP-bound form) (Bosco et al., 2009). Many viruses interfere with or employ the conformational states of Rac1 to regulate their infection. At early stages of African swine fever virus (ASFV) infection, Rac1 is activated, and inhibition of Rac1 is able to suppress production of this virus (Quetglas et al., 2012). Rac1 is found to be activated during intracellular mature virus (MV) of Vaccinia virus entry (Mercer and Helenius, 2008).
Although rotavirus replication per se does not affect the activation of Rac1 (Fig. S4), we have demonstrated that the loss-of-function of Rac1 by gene knockdown or knockout significantly impairs rotavirus replication, which is in line with the previous finding that knockdown Rac1 could significantly inhibit Enterovirus 1 (EV1) infection (Karjalainen et al., 2008). More specifically, the activation of Rac1 is required as shown by the opposing effects of ectopic over-expression of the active or inactive forms of Rac1 on rotavirus replication. This mechanistically explains the potent anti-rotavirus effects of the GTP-Rac1 inhibitors, 6-TG and NSC23766. Of note, NSC23766 has been shown to inhibit the replication of several influenza viruses including a human virus strain from the 2009 pandemic and highly pathogenic avian virus strains (Dierkes et al., 2014).
Despite the absence of approved medications for treating rotavirus, the widely used general antivirals including ribavirin and IFNα have been studied on rotavirus in experimental models (Yin et al., 2015a). Here, we have evaluated the combinatory effects of 6-TG with IFNα or ribavirin. Consistently, we confirmed that ribavirin and IFNα inhibit rotavirus replication at both RNA and protein levels (Fig. 6A and B). We found increased potency of IFNα in the presence of 6-TG. However, whether the combination of IFNα and 6-TG could be used to treat rotavirus infected patients remains to be further investigated.
In conclusion, this study has demonstrated that 6-TG effectively inhibits rotavirus replication with a high barrier to drug resistance development. We further identified the active form of Rac1 as an important host factor supporting rotavirus replication. 6-TG exerts its anti-rotavirus effects via the specific inhibition of Rac1 activation. Herein, this study provided important references for clinicians to optimize medications for organ recipients or IBD patients who are infected with rotavirus or at risk of rotavirus replication. These results may also help the development of new anti-rotavirus therapies.
Conflicts of interest
All authors declare that they have no conflict of interest.
Author contributions
YY, SC, MH, WW, LX, WD, CQ and JS performed the experiments; GF and AV contributed to the scientific discussion and facilities; YY, MP and QP conceived the project and wrote the manuscript.
Acknowledgments
We would like to thank Professor Harry Greenberg (Stanford University School of Medicine, USA) for providing the mouse monoclonal antibody against rotavirus VP4 protein, Prof. Dr. Klemens Rottner and Dr. Anika Steffen (Helmholtz Centre for Infection Research) for providing the Rac1 lf/lf and Rac1 −/− MEFs cells, and Dr. Mauro Cozzolino (ITALIAN NATIONAL RESEARCH COUNCIL) for providing the active Rac1V12 and inactive Rac1N17 plasmids. This study was supported by the Dutch Digestive Foundation (MLDS) for a career development grant (No. CDG 1304 to Q. P.), the Erasmus MC Mrace grant (360525 to Q. P.), and the China Scholarship Council for funding PhD fellowship (201307720045 to Y. Y.).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.antiviral.2018.06.011.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Apolloni S., Parisi C., Pesaresi M.G., Rossi S., Carri M.T., Cozzolino M., Volonte C., D'Ambrosi N. The NADPH oxidase pathway is dysregulated by the P2X7 receptor in the SOD1-G93A microglia model of amyotrophic lateral sclerosis. J. Immunol. 2013;190:5187–5195. doi: 10.4049/jimmunol.1203262. [DOI] [PubMed] [Google Scholar]
- Bosco E.E., Mulloy J.C., Zheng Y. Rac1 GTPase: a “Rac” of all trades. Cell. Mol. Life Sci. 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgine J., Garat A., Allorge D., Crunelle-Thibaut A., Lo-Guidice J.M., Colombel J.F., Broly F., Billaut-Laden I. Evidence for a functional genetic polymorphism of the Rho-GTPase Rac1. Implication in azathioprine response? Pharmacogenetics Genom. 2011;21:313–324. doi: 10.1097/FPC.0b013e3283449200. [DOI] [PubMed] [Google Scholar]
- Cheng K.W., Cheng S.C., Chen W.Y., Lin M.H., Chuang S.J., Cheng I.H., Sun C.Y., Chou C.Y. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antivir. Res. 2015;115:9–16. doi: 10.1016/j.antiviral.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchana L., Narjoz C., Beaune P., Loriot M.A., Roblin X. Review article: the benefits of pharmacogenetics for improving thiopurine therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2012;35:15–36. doi: 10.1111/j.1365-2036.2011.04905.x. [DOI] [PubMed] [Google Scholar]
- D'Ambrosi N., Rossi S., Gerbino V., Cozzolino M. Rac1 at the crossroad of actin dynamics and neuroinflammation in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2014;8:279. doi: 10.3389/fncel.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer N.K., Reinisch W., Teml A., van Bodegraven A.A., Schwab M., Lukas M., Ochsenkuhn T., Petritsch W., Knoflach P., Almer S., van der Merwe S.W., Herrlinger K.R., Seiderer J., Vogelsang H., Mulder C.J., Dutch T.G.w.g. 6-Thioguanine treatment in inflammatory bowel disease: a critical appraisal by a European 6-TG working party. Digestion. 2006;73:25–31. doi: 10.1159/000091662. [DOI] [PubMed] [Google Scholar]
- Dierkes R., Warnking K., Liedmann S., Seyer R., Ludwig S., Ehrhardt C. The Rac1 inhibitor NSC23766 exerts anti-influenza virus properties by affecting the viral polymerase complex activity. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhler G.M., Drayer A.L., Olthof S.G., Schuringa J.J., Coffer P.J., Vellenga E. Reduced activation of protein kinase B, Rac, and F-actin polymerization contributes to an impairment of stromal cell derived factor-1 induced migration of CD34+ cells from patients with myelodysplasia. Blood. 2008;111:359–368. doi: 10.1182/blood-2006-11-060632. [DOI] [PubMed] [Google Scholar]
- Grimwood K., Buttery J.P. Clinical update: rotavirus gastroenteritis and its prevention. Lancet. 2007;370:302–304. doi: 10.1016/S0140-6736(07)61142-8. [DOI] [PubMed] [Google Scholar]
- Karjalainen M., Kakkonen E., Upla P., Paloranta H., Kankaanpaa P., Liberali P., Renkema G.H., Hyypia T., Heino J., Marjomaki V. A Raft-derived, Pak1-regulated entry participates in alpha2beta1 integrin-dependent sorting to caveosomes. Mol. Biol. Cell. 2008;19:2857–2869. doi: 10.1091/mbc.E07-10-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolho K.L., Klemola P., Simonen-Tikka M.L., Ollonen M.L., Roivainen M. Enteric viral pathogens in children with inflammatory bowel disease. J. Med. Virol. 2012;84:345–347. doi: 10.1002/jmv.23193. [DOI] [PubMed] [Google Scholar]
- Masclee G.M., Penders J., Pierik M., Wolffs P., Jonkers D. Enteropathogenic viruses: triggers for exacerbation in IBD? A prospective cohort study using real-time quantitative polymerase chain reaction. Inflamm. Bowel Dis. 2013;19:124–131. doi: 10.1002/ibd.22976. [DOI] [PubMed] [Google Scholar]
- Maybaum J., Bainnson A.N., Roethel W.M., Ajmera S., Iwaniec L.M., TerBush D.R., Kroll J.J. Effects of incorporation of 6-thioguanine into SV40 DNA. Mol. Pharmacol. 1987;32:606–614. [PubMed] [Google Scholar]
- Mercer J., Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Munshi P.N., Lubin M., Bertino J.R. 6-thioguanine: a drug with unrealized potential for cancer therapy. Oncol. 2014;19:760–765. doi: 10.1634/theoncologist.2014-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh K., Zhou L., Somasundaram R., Fuhler G.M., Deuring J.J., Blokzijl T., Regeling A., Kuipers E.J., Weersma R.K., Nuij V.J., Alves M., Vogelaar L., Visser L., de Haar C., Krishnadath K.K., van der Woude C.J., Dijkstra G., Faber K.N., Peppelenbosch M.P. Suppression of p21Rac signaling and increased innate immunity mediate remission in Crohn's disease. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3006763. 233ra253. [DOI] [PubMed] [Google Scholar]
- Quetglas J.I., Hernaez B., Galindo I., Munoz-Moreno R., Cuesta-Geijo M.A., Alonso C. Small rho GTPases and cholesterol biosynthetic pathway intermediates in African swine fever virus infection. J. Virol. 2012;86:1758–1767. doi: 10.1128/JVI.05666-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- Shin J.Y., Wey M., Umutesi H.G., Sun X., Simecka J., Heo J. Thiopurine prodrugs mediate immunosuppressive effects by interfering with Rac1 protein function. J. Biol. Chem. 2016;291:13699–13714. doi: 10.1074/jbc.M115.694422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate J.E., Burton A.H., Boschi-Pinto C., Parashar U.D. Global, regional, and national estimates of rotavirus mortality in children <5 Years of age, 2000-2013. Clin. Infect. Dis. 2016;62(Suppl. 2):S96–S105. doi: 10.1093/cid/civ1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiede I., Fritz G., Strand S., Poppe D., Dvorsky R., Strand D., Lehr H.A., Wirtz S., Becker C., Atreya R., Mudter J., Hildner K., Bartsch B., Holtmann M., Blumberg R., Walczak H., Iven H., Galle P.R., Ahmadian M.R., Neurath M.F. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J. Clin. Invest. 2003;111:1133–1145. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg H.H., Sorensen B.B., Slofstra S.H., Van den Brande J.H., Stam J.C., van Bergen en Henegouwen P.M., Richel D.J., Petersen L.C., Peppelenbosch M.P. VIIa/tissue factor interaction results in a tissue factor cytoplasmic domain-independent activation of protein synthesis, p70, and p90 S6 kinase phosphorylation. J. Biol. Chem. 2002;277:27065–27072. doi: 10.1074/jbc.M110325200. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Karvonen A., Prymula R., Schuster V., Tejedor J.C., Cohen R., Meurice F., Han H.H., Damaso S., Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- Watts A., Bennett W.E., Molleston J.P., Gupta S.K., Croffie J.M., Waseem S., McFerron B.A., Steiner S.J., Kumar S., Vanderpool C.P., Hon E.C., Bozic M.A., Subbarao G.C., Pfefferkorn M.D. The incidence of low seroimmunity to hepatitis B virus in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2017;65(5):551–554. doi: 10.1097/MPG.0000000000001580. [DOI] [PubMed] [Google Scholar]
- Yin Y., Bijvelds M., Dang W., Xu L., van der Eijk A.A., Knipping K., Tuysuz N., Dekkers J.F., Wang Y., de Jonge J., Sprengers D., van der Laan L.J., Beekman J.M., Ten Berge D., Metselaar H.J., de Jonge H., Koopmans M.P., Peppelenbosch M.P., Pan Q. Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antivir. Res. 2015;123:120–131. doi: 10.1016/j.antiviral.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Yin Y., Metselaar H.J., Sprengers D., Peppelenbosch M.P., Pan Q. Rotavirus in organ transplantation: drug-virus-host interactions. Am. J. Transplant. 2015;15:585–593. doi: 10.1111/ajt.13135. [DOI] [PubMed] [Google Scholar]
- Yin Y., Wang Y., Dang W., Xu L., Su J., Zhou X., Wang W., Felczak K., van der Laan L.J., Pankiewicz K.W., van der Eijk A.A., Bijvelds M., Sprengers D., de Jonge H., Koopmans M.P., Metselaar H.J., Peppelenbosch M.P., Pan Q. Mycophenolic acid potently inhibits rotavirus infection with a high barrier to resistance development. Antivir. Res. 2016;133:41–49. doi: 10.1016/j.antiviral.2016.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.