Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) has continued spreading since its emergence in 2012 with a mortality rate of 35.6%, and is a potential pandemic threat. Prophylactics and therapies are urgently needed to address this public health problem. We report here the efficacy of a vaccine consisting of chimeric virus-like particles (VLP) expressing the receptor binding domain (RBD) of MERS-CoV. In this study, a fusion of the canine parvovirus (CPV) VP2 structural protein gene with the RBD of MERS-CoV can self-assemble into chimeric, spherical VLP (sVLP). sVLP retained certain parvovirus characteristics, such as the ability to agglutinate pig erythrocytes, and structural morphology similar to CPV virions. Immunization with sVLP induced RBD-specific humoral and cellular immune responses in mice. sVLP-specific antisera from these animals were able to prevent pseudotyped MERS-CoV entry into susceptible cells, with neutralizing antibody titers reaching 1: 320. IFN-γ, IL-4 and IL-2 secreting cells induced by the RBD were detected in the splenocytes of vaccinated mice by ELISpot. Furthermore, mice inoculated with sVLP or an adjuvanted sVLP vaccine elicited T-helper 1 (Th1) and T-helper 2 (Th2) cell-mediated immunity. Our study demonstrates that sVLP displaying the RBD of MERS-CoV are promising prophylactic candidates against MERS-CoV in a potential outbreak situation.
Keywords: Middle East respiratory syndrome coronavirus, Receptor binding domain, CPV, Virus-like particles, Immune response
Highlights
-
•
We constructed a chimeric parvovirus virus-like particles displaying the MERS-CoV receptor-binding domain on the surface.
-
•
We found that the chimeric virus-like particles induced RBD-specific, neutralizing antibody responses in mice.
-
•
Splenocytes from immunized mice had considerably higher secretion of both Th1- and Th2-type cytokines.
-
•
Our results reveal that chimeric virus-like particles induce both specific humoral and cell-mediated immunity.
1. Introduction
Middle East respiratory syndrome coronavirus (MERS-CoV) infections can cause severe and possibly lethal acute respiratory disease in humans. A total of 630 deaths from 1769 cases of MERS-CoV were reported between September 2012 to June 2016, a mortality rate of 35.6%. Of considerable concern, imported cases of MERS have been being reported in countries worldwide, which raise concerns of a potential pandemic. Vaccination is the best means to control and prevent disease, but a licensed prophylaxis is not currently available against MERS-CoV.
The surface of MERS-CoV particles is composed of the spike (S) glycoprotein, which plays a crucial step in the viral life cycle by interacting with the dipeptidyl peptidase 4 (DPP4) molecules on the target cell to initiate viral docking and entry. Studies have shown that the receptor-binding domain (RBD) is a major antigenic determinant for the induction of neutralizing antibodies (Du et al., 2013a, Du et al., 2013b, Mou et al., 2013). Therefore, a candidate expressing the RBD of MERS-CoV is a plausible design for an effective vaccine.
Virus-like particles (VLP) are an excellent platform for epitope presentation because they have been shown to efficiently interact with antigen-presenting cells and display heterologous epitopes at high density (Barcena and Blanco, 2013). Parvovirus-like particles expressing the major structural protein VP2 in insect cells are very stable and highly immunogenic (Lopez de Turiso et al., 1992, Martinez et al., 1992). Moreover, canine parvovirus (CPV) does not cause disease in human, therefore parvovirus-like particles is a safe expression platform. Parvovirus VLP has been successfully used for the expression of foreign antigens and induction of robust B-, T-cell responses (Miyamura et al., 1994, Casal et al., 1999, Lo-Man et al., 1998, Xu et al., 2014, Rueda et al., 1999).
In this study, we evaluated the efficacy of a chimeric CPV VLP expressing the RBD of MERS-CoV. The structural integrity of these VLP was confirmed by electron microscopy and the immunogenicity of parvovirus-like VLP was evaluated in a mouse model of infection.
2. Methods
2.1. Construction of recombinant baculovirus
Nucleotides encoding spike glycoprotein residues 367–606 of the MERS-CoV RBD (GenBank accession no. KF600645) were codon-optimized for the highest expression levels possible in insect cells and biochemically synthesized (Sangon Biotech, China). Three repeat flexible linkers (Gly4Ser)3 were cloned to the C-terminus of RBD. The RBD and linkers were subcloned into the SalI-NotI sites of pFastBac1-VP2, to generate pFastBac1-RBD-VP2. pFastBac1-RBD-VP2 was then transformed into E. coli DH10Bac cells, and recombinant bacmids containing the RBD insert were confirmed by PCR.
Recombinant baculoviruses were generated by recombinant bacmids transfected into Sf9 insect cells in the presence of the Liposome 2000 transfection reagent, following the Bac-to-Bac Expression Systems manual (Invitrogen, USA). Supernatants containing recombinant baculovirus were harvested 5 days after transfection as viral stocks, which are designated Bac-RBD-VP2. The titers of these baculovirus stocks were determined using a rapid titration kit (BacPakBaculovirus Rapid Titer Kit; Clontech, USA).
2.2. Indirect immunofluorescence assay
Expression of RBD-VP2 protein was confirmed by indirect immunofluorescence as previously described (Feng et al., 2014). Briefly, Sf9 insect cells were maintained at 27 °C in TNM-FH medium supplemented with 10% FBS and infected with Bac-RBD-VP2 at a multiplicity of infection (MOI) of 3. Control cells were infected with baculovirus pFastBac1. At 48 h post infection, the culture plates were fixed with 4% paraformaldehyde at room temperature for 20 min, washed with PBS-0.05% Tween 20 (PBST), and then incubated with mouse anti-MERS-S polyclonal antibody or mouse anti-VP2 monoclonal antibody containing 1% bovine serum albumin at 37 °C for 1 h. After washing with PBST, the cells were stained with FITC-tagged goat anti-mouse IgG and Evans Blue (diluted 1:500 in PBST) at 37 °C for 1 h. After washing, the cells were examined under a fluorescent microscope.
2.3. Preparation and purification of chimeric VLP
Sf9 insect cells were maintained as suspension cultures in serum-free SF900II medium (Life technologies, USA) at 27 °C, with shaking at 120 rpm. Sf9 cells were infected with recombinant baculovirus at a MOI of 3. Cells were harvested 96 h after infection, washed with PBS, and lysed with 25 mM bicarbonate solution on ice for 10 min. After centrifugation at 12,000 × g for 10 min, the cell pellet is removed and purified chimeric VLP was obtained by a quarter volume of saturated ammonium sulfate precipitation for 20 min on ice, and then subsequently centrifugated at 12,000 g for 20 min. The viral pellet is resuspended in PBS and dialyzed overnight.
2.4. Western blot
Samples of purified sVLP, purified recombinant RBD proteins, CPV VLP and lysate from pFastBac1 infected cells were transferred onto a polyvinylidene fluoride (PVDF) membrane (Immobilin-P, Millipore, USA) after SDS-PAGE for Western blotting with mouse anti-RBD or -VP2 monoclonal antibodies.
2.5. Characterization by electron and immunoelectron microscopy
sVLP were loaded onto grids, kept at room temperature for 5 min, stained with 1% sodium phosphotungstate, and then examined directly under a transmission electron microscope (TEM). For immunoelectron microscopy (IEM), the sVLP were loaded onto formvar-coated grids after removal of excess sample solution and incubated with mouse anti-RBD monoclonal antibody. After washing with PBS, the grids were stained with gold-tagged goat anti-mouse IgG antibody (Sigma-Aldrich, SaintLouis, MO, USA). After another wash with PBS, the grids were observed under the TEM.
2.6. Hemagglutination (HA) test
HA tests were carried out as previously described (Feng et al., 2014). Briefly, 25 μL of 2-fold serial dilutions of sVLP were made in PBS, and then an additional 25 μL of PBS was added to each well. 50 μL of 1% (V/V) pig erythrocytes were then added to each well for 1 h at 4 °C. The HA titer was determined by the highest dilution of the sVLP that agglutinated the erythrocytes.
2.7. Immunization studies
Thirty-two BALB/c mice (6-week-old, female) were randomized into four groups. All groups were vaccinated intramuscularly (IM) in the gastrocnemius muscle. Mice in group 2 were given 10 μg of sVLP; mice in group 3 were given 10 μg of sVLP and mixed with 50 μg Alum adjuvant (Thermo, USA) per animal; mice in group 4 were given 10 μg of sVLP and mixed with 50 μg polyriboinosinic acid [poly(I:C)] adjuvant (Sigma-Aldrich, Saint Louis, MO, USA), and the mice in group 1 were given PBS as control. Identical vaccinations were then repeated at 14-days after the initial administration for all groups. Blood samples were obtained from the orbital vein of mice on days 14 and 28 post-vaccination.
2.8. RBD-specific antibody measurement in the sera of mice
RBD-specific IgG antibodies from the sera of immunized and control mice were measured by indirect ELISA as described previously (Wang et al., 2016). Briefly, 96-well microtiter plates (Corning Costar, USA) were pre-coated with 100 μL of purified RBD antigen at a final concentration of 1 μg/mL and incubated overnight. After the plates were blocked with skimmed milk for 2 h at 37 °C, 100 μL of 2-fold serially diluted serum samples were added and incubated at 37 °C for 1 h. After washing with PBST, 100 μL of HRP-labeled goat antibody against mouse IgG (Millipore, USA) was added (diluted 1:4000), and incubated at 37 °C for 1 h. After washing, 100 μL of the substrate 3, 3′, 3, 5′-tetramethylvenzidine (TMB) (Sigma, USA) was added to each well, incubated for 30 min, and then stopped with 50 μL of 2 M H2SO4. Optical density values were measured using a 96-wells ELISA plate reader at a wavelength of 450 nm (Bio-Rad, USA).
2.9. Pseudotyped virus neutralization assay
The preparation of MERS-pseudotyped virus and determination of neutralizing antibody titers were performed as described previously (Li et al., 2015). 10 μg pcDNA3.1-MERS-S plasmids and 10 μg pNL4-3.luc.RE plasmids were co-transfected into 293 T cells. The supernatant was harvested after 48 h, centrifuged to remove the cell debris, and stored at −80 °C. In the neutralization assay with MERS-pseudotyped virus, mixtures containing equal volumes of serially diluted mice sera and 100 × TCID50 of pseudotyped virus were incubated at 37 °C for 30 min, before incubation on Huh 7 cells. Samples were assayed in quadruplicate. After incubation for 4 h, the medium was replaced with DMEM containing 10% fetal bovine serum. After 48 h incubation at 37 °C, the luciferase activity of the samples were measured using Infinite M200. The 50% neutralization dose (ND50) was calculated using Graphpad Prism.
2.10. Flow cytometry assays
One week after the second vaccination, inguinal lymph nodes were collected from immunized mice. Cell suspensions were stained with anti-CD11c, anti-CD80, and anti-CD86 monoclonal antibodies (BD Biosciences, Franklin, USA) at 4 °C for 30 min. After two washes with PBS, flow cytometery was performed on LSR-II flow cytometer (BD Bioscience) and the data analyzed by FlowJo software.
2.11. ELISpot assays
Splenocytes harvested at two weeks after the second immunization from inoculated mice were plated at a density of 2 × 105 cells per well in a 96-well ELISpot plate (Mabtech AB, Sweden), which had been pre-coated to detect IFN-γ, IL-2 or IL-4 expression. Purified RBD antigen was added at a final concentration of 10 μg/mL for stimulating cytokine production, and the rest of the assay was carried out according to manufacturer instructions. Spot forming cells (SFCs) were counted using an automated ELISpot reader (AID ELISPOT reader-iSpot, AID GmbH, GER).
2.12. Laboratory facility and ethics statement
The environment and housing facilities for mice is in accordance with the National Standards of Laboratory Animal Requirements of China (GB 14925-2001). Animal studies were strictly conducted with the recommendations of the Veterinary Institute at the Academy of Military Medical Sciences and approved by the Animal Welfare and Ethics Committee (permit number SCXK-2012-017).
3. Results
3.1. Expression and assembly of sVLP displaying RBD
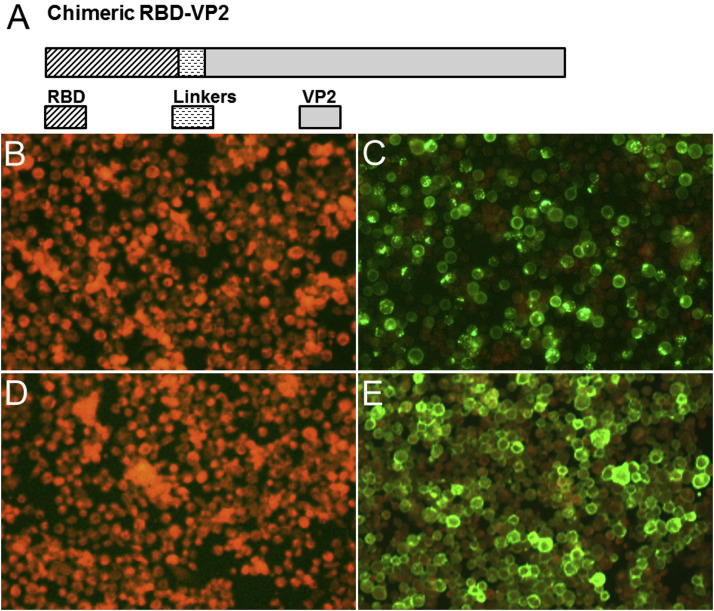
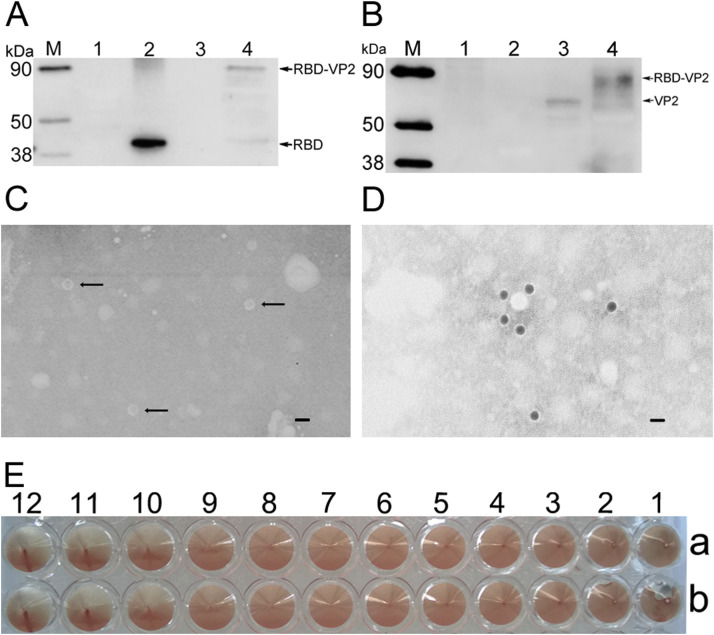
The strategy for the design of sVLP is shown in (Fig. 1 A), in which MERS-RBD was fused to VP2 of CPV to generate chimeric VLPs. Immunofluorescence assay confirmed the expression of recombinant RBD-VP2 in Sf9 cells (Fig. 1B, C, D and E), which was also detected by Western blotting of the purified sVLP with anti-RBD monoclonal antibody (Fig. 2 A). Immunoblotting with anti-VP2 monoclonal antibody showed detection in CPV VLP and sVLP samples (Fig. 2B), suggesting that the RBD is assembled with CPV-VP2 in the sVLP.
Fig. 1.
Construction of fused MERS-CoV RBD with VP2 and identification of recombinant RBD-VP2. (A) Schematic diagram for the construction of MERS-CoV RBD and VP2, the MERS-CoV RBD is the spike glycoprotein residues 367–606, and the linker is Gly4Ser. (B, C, D, E) IFA detection of expression of the chimeric baculoviruses in Sf9 infected cells. Cells were infected with the recombinant baculoviruses in C, E, and were mock infected in B, D. Cells were detected with anti-RBD monoclonal antibody in B and C, and anti-VP2 monoclonal antibody in D and E.
Fig. 2.
Characterization and identification of sVLP. (A, B) Western blot analyses of sVLP. Lane 1 lysate from pFastBac1 infected cells served as a control; lane 2 purified RBD recombinant protein expressed in prokaryotic expression system; lane 3 purified VLP of CPV VP2; lane 4 sVLP, analyzed by WB using (A) anti-RBD monoclonal antibody and (B) anti-VP2 monoclonal antibody. (C, D) TEM and IEM analysis of sVLP. (C) sVLP produced by infected Sf9 cells and purified by ammonium sulfate precipitation were stained with 1% sodium phosphotungstate. Bar = 20 nm. (D) The sVLP were incubated with anti-RBD monoclonal antibody and probed using a gold-labeled goat anti-mouse IgG antibody. (E) sVLP hemagglutinates pig erythrocytes.
In order to obtain sVLP morphology, sVLP was subjected to negative staining electron microscopy. sVLP with a diameter of ∼25 nm were observed (Fig. 2C), which is slightly larger than VP2 preparations (approximately 20 nm in diameter) (Lopez de Turiso et al., 1992). This demonstrates that RBD fused with VP2 self-assemble into chimeric VLP (Gilbert et al., 2006). Immunoelectron microscopy was performed with a gold-tagged antibody against the RBD. Results show that multiple gold particles bound to sVLP (Fig. 2D), demonstrating that the RBD is displayed on the surface of viral particles. Subsequently, hemagglutination of purified sVLP was investigated, and it was shown that sVLP could hemagglutinate pig erythrocytes and the HA titer reached as high as 1: 210 (Fig. 2E).
3.2. sVLP induce humoral immune responses in mice
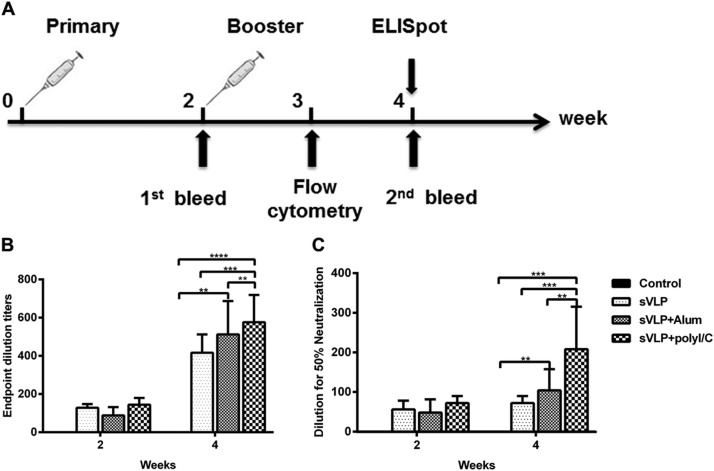
To evaluate the immunogenicity of chimeric VLP, mice were immunized IM with sVLP, sVLP in combination with 50 μg Alum adjuvant, sVLP in combination with 50 μg poly(I: C) adjuvant, or given PBS (Fig. 3 A). Mouse sera were collected two weeks after each immunization and analyzed for RBD-specific antibody responses by IgG ELISA. RBD-specific antibody responses were detected in all sVLP vaccinated mice at two weeks after the first injection. IgG ELISA results show that immunized mice were able to induce a robust humoral response specific to RBD after the first vaccination, and all mice showed enhanced antibody responses against RBD after receiving the second immunization (Fig. 3B), with the highest IgG antibody titer reaching 1: 640 in poly(I:C) adjuvanted group.
Fig. 3.
Mice immunization procedure, RBD-specific antibody and neutralizing antibodies against MERS-CoV infection. (A) BALB/c (n = 8) were vaccinated IM with 10 μg of sVLP, 10 μg of sVLP and 50 μg of Alum, or 10 μg of sVLP and 50 μg of poly(I:C) adjuvant and the control group was treated with PBS. Blood samples were collected from the orbital vein of mice before immunization and two weeks after each immunization. (B) ELISA results show that immunized mice were able to induce a robust humoral response specific to RBD, with serum IgG titers reaching 1:640 after the second immunization. (C) Immunized mice serum titrations were determined with MERS-pseudotyped virus on Huh 7 cells. The titers were determined as the highest serum dilutions giving a 50% reduction of luciferase activity and are expressed as means+/-SD.
We then evaluated the mouse sera for the ability to inhibit MERS-pseudotyped virus entry into Huh7 cells (Zhao et al., 2013, Perera et al., 2013). Sera from the control group did not show any neutralization activity, whereas sera from sVLP and groups with Alum and poly(I:C) adjuvant-immunized mice were neutralizing after the first vaccination and the neutralization activity was boosted after the second immunization (Fig. 3C). The neutralizing antibody titer was highest in mice injected with poly(I:C) adjuvanted sVLP at a titer of 1: 320.
3.3. Immunization with sVLP activates more DCs
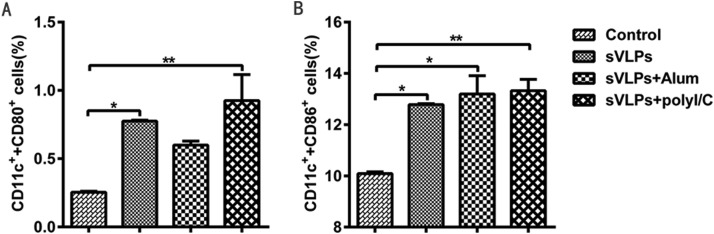
To determine whether sVLP is able to stimulate cytokine secretion by dendritic cells (DCs), the percentages of DCs in lymph nodes were analyzed by flow cytometry. A greater number of DCs were detected in all three immunized groups compared with the control (Fig. 4 ). In addition to CD11c+ and CD80+ (Fig. 4A), the percentage of CD11c+ and CD86+ (Fig. 4B) were considerably higher in mice injected with poly(I:C) adjuvanted sVLP, suggesting that poly(I:C) is able to activate more DCs in lymph nodes to generate a robust immune response.
Fig. 4.
Flow cytometry for recruitment and/or activation of DCs in lymph nodes from immunized mice. Inguinal lymph nodes were isolated from mice each group 7 days after the second immunization and were stained with mouse anti-CD11c, anti-CD80, and anti-CD86 monoclonal antibodies. Double-positive (A) CD11c+CD80+, and (B) CD11c+CD86+ cells were plotted. The data represent the means+/-standard deviation (SD) of double-positive cell percentages. Statistical analysis between the four groups were analyzed by one-way ANOVA (*p < 0.05, **p < 0.01).
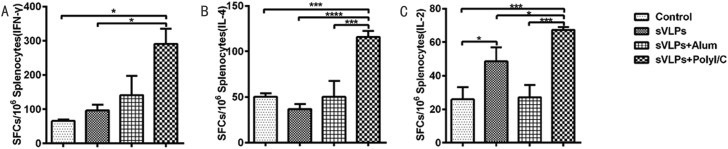
3.4. sVLP induce cell-mediated immune responses in mice
The cell-mediated immune responses were evaluated with mouse splenocytes using IFN-γ, IL-2 and IL-4 ELISpot assays. The poly(I:C) adjuvanted sVLP group had substantially higher numbers of IFN-γ, IL-2 and IL-4 secreting cells when compared with other groups. Immunization with sVLP resulted in increased IL-2 secreting cells only. However, spot-forming cells (SFCs) produced from immunization with sVLP in combination with Alum adjuvant does not appear to have a difference with the control group (Fig. 5 ).
Fig. 5.
Enzyme-linked immunospot assays of IFN-γ, IL-4 and IL-2 secretion in immunized mice. Splenocytes were isolated from mice and stimulated with purified RBD. Splenocytes secreting (A) IFN-γ or (B) IL-4 and (C) IL-2 were quantitated using ELISpot assay. The data represent the means+/-standard deviation (SD), with units of SFCs per million splenocytes. Statistical analysis between the four groups were analyzed by one-way ANOVA (*p < 0.05, ***p < 0.001, ****p < 0.0001).
4. Discussion
Previous approaches to MERS-CoV vaccine candidates included recombinant viral vectors, DNA vaccines, as well as protein-based platforms. A DNA vaccine, which was developed by expressing the synthetic consensus anti-spike protein has shown immunogenicity in mice, nonhuman primates (NHPs) and camels (Muthumani et al., 2015), and another DNA plasmid vaccine that expresses the MERS-CoV S glycoprotein has entered Phase I clinical trials (Clinical Trials.gov, 2016). Additionally, delivery of the S antigen by way of DNA vaccination, nanoparticles, recombinant viral vectors such as modified vaccinia Ankara or adenovirus or RBD-based subunit vaccines have shown immunogenicity and efficacy in mice and/or NHPs against MERS-CoV challenge (Du et al., 2013b, Muthumani et al., 2015, Wang et al., 2015, Coleman et al., 2014, Song et al., 2013, Volz et al., 2015, Kim et al., 2014, Lan et al., 2014, Lan et al., 2015).
The incorporation of foreign epitopes into the wild-type VLP can result in excellent immunity and are safe for human use (Grgacic and Anderson, 2006). VLP derived from hepatitis B virus (Ye et al., 2013), Newcastle disease virus (Schmidt et al., 2014, Cullen et al., 2015), and parvovirus (Gilbert et al., 2004, Gilbert et al., 2006) have been shown to be suitable candidates for the expression of foreign epitopes. In the present study, we demonstrated that the recombinant CPV VP2 linked to MERS RBD results in self-assembly into sVLP which display the RBD on the surface, and showed that this vaccine candidate is able to induce robust B- and T-cell responses in mice.
The lack of an animal model that recapitulates the symptoms of human infections with MERS-CoV constitutes a challenge in evaluating the efficacy of vaccines against MERS-CoV (Modjarrad, 2016, van Doremalen and Munster, 2015), and current animal models (including Ad5-DPP4 transgenic mice or nonhuman primate models) are too costly to obtain. We therefore decided to first test our vaccine candidate and regimens for immunogenicity and safety in the readily available BALB/c mouse model to assess specific immune responses before advancing the best-performing candidate into a suitable animal model for MERS-CoV.
Both specific humoral and cell-mediated immunity is essential for effective vaccination against many pathogens (Slifka and Amanna, 2014). In our study, mice that received sVLP produced a Th1-biased response with high levels of IL-2 secretion, whereas mice immunized with sVLP + poly(I:C) produced substantial IFN-γ, IL-2 and IL-4 responses, representing mixed Th1 and Th2 responses. In contrast, mice that received sVLP + Alum did not result in a detectable cellular immune response, likely because Alum is known to be a poor cellular immune response inducer (Maughan et al., 2015). In contrast, poly(I:C) is a double-stranded RNA polymer that acts as a molecular mimic of viral infection and is recognized by Toll-like receptor 3, resulting in expression of IFN-α/β and Th1- and Th2- related cytokines (Ichinohe et al., 2005). Furthermore, poly(I:C) has been used in many clinical trials with good results (Okada et al., 2011, Rahimian et al., 2015). The percentage of CD11c and CD86, CD11c and CD80 double-positive cells was notably increased in mice received regimes containing sVLP, which suggested that sVLP could efficiently recruit or activate DCs in lymph nodes, which is important for effective stimulation of the innate immune response and as a link to the adaptive immune response (Grgacic and Anderson, 2006). In addition to CD11c+ and CD80+, CD11c+ and CD86+ were highest in mice injected with poly(I:C) adjuvanted sVLP, the IgG levels was highest in the poly(I:C) adjuvanted group. In summary, poly(I:C) adjuvanted sVLP is a promising vaccine regimens for the next stage of pre-clinical testing in larger animal models, and the dosage, route of administration and intervals of immunization will need to be further evaluated and optimized to maximize chances of a successful vaccination.
Acknowledgments
This work was supported by the National Science and Technology Major Project of the ministry of Science and Technology of China (No. 2015ZX09102025 and 2014ZX09102044-007), the China Postdoctoral Science Foundation (2015M580592) and the Open Project Grant (2014SKLRD-O01) from the State Key Lab of Respiratory Disease, Guangzhou institute of Respiratory Disease. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Songtao Yang, Email: yst62041@163.com.
Xianzhu Xia, Email: xiaxzh@cae.cn.
References
- Barcena J., Blanco E. Design of novel vaccines based on virus-like particles or chimeric virions. Subcell. Biochem. 2013;68:631–665. doi: 10.1007/978-94-007-6552-8_21. [DOI] [PubMed] [Google Scholar]
- Casal J.I., Rueda P., Hurtado A. Parvovirus-like particles as vaccine vectors. Methods. 1999;19(1):174–186. doi: 10.1006/meth.1999.0843. [DOI] [PubMed] [Google Scholar]
- Clinical Trials.gov . 2016. Phase I, Open Label Dose Ranging Safety Study of GLS-5300 in Healthy Volunteers. [Google Scholar]
- Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32(26):3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen L.M., Blanco J.C., Morrison T.G. Cotton rat immune responses to virus-like particles containing the pre-fusion form of respiratory syncytial virus fusion protein. J. Transl. Med. 2015;13(1):350. doi: 10.1186/s12967-015-0705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Zhao G., Kou Z., Ma C., Sun S., Poon V.K. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J. Virol. 2013;87(17):9939–9942. doi: 10.1128/JVI.01048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Kou Z., Ma C., Tao X., Wang L., Zhao G. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PLoS One. 2013;8(12):e81587. doi: 10.1371/journal.pone.0081587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H., Hu G.Q., Wang H.L., Liang M., Liang H., Guo H. Canine parvovirus VP2 protein expressed in silkworm pupae self-assembles into virus-like particles with high immunogenicity. PLoS One. 2014;9(1):e79575. doi: 10.1371/journal.pone.0079575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L., Toivola J., Lehtomäki E., Donaldson L., Käpylä P., Vuento M. Assembly of fluorescent chimeric virus-like particles of canine parvovirus in insect cells. Biochem. Biophys. Res. Commun. 2004;313(4):878–887. doi: 10.1016/j.bbrc.2003.11.176. [DOI] [PubMed] [Google Scholar]
- Gilbert L., Toivola J., Valilehto O., Saloniemi T., Cunningham C., White D. Truncated forms of viral VP2 proteins fused to EGFP assemble into fluorescent parvovirus-like particles. J. Nanobiotechnol. 2006;4:13. doi: 10.1186/1477-3155-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgacic E.V., Anderson D.A. Virus-like particles: passport to immune recognition. Methods. 2006;40(1):60–65. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T., Watanabe I., Ito S., Fujii H., Moriyama M., Tamura S. Synthetic double-stranded RNA poly(I: C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 2005;79(5):2910–2919. doi: 10.1128/JVI.79.5.2910-2919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Okada K., Kenniston T., Raj V.S., AlHajri M.M., Farag E.A. Immunogenicity of an adenoviral-based Middle East Respiratory Syndrome coronavirus vaccine in BALB/c mice. Vaccine. 2014;32(45):5975–5982. doi: 10.1016/j.vaccine.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Deng Y., Chen H., Lu G., Wang W., Guo X. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS One. 2014;9(11):e112602. doi: 10.1371/journal.pone.0112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Yao Y., Deng Y., Chen H., Lu G., Wang W. Recombinant receptor binding domain protein induces partial protective immunity in rhesus macaques against Middle East respiratory syndrome coronavirus challenge. EBioMedicine. 2015;2(10):1438–1446. doi: 10.1016/j.ebiom.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wan Y., Liu P., Zhao J., Lu G., Qi J. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell Res. 2015;25(11):1237–1249. doi: 10.1038/cr.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo-Man R., Rueda P., Sedlik C., Deriaud E., Casal I., Leclerc C. A recombinant virus-like particle system derived from parvovirus as an efficient antigen carrier to elicit a polarized Th1 immune response without adjuvant. Eur. J. Immunol. 1998;28(4):1401–1407. doi: 10.1002/(SICI)1521-4141(199804)28:04<1401::AID-IMMU1401>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Lopez de Turiso J.A., Cortes E., Martinez C., Ruiz de Ybanez R., Simarro I., Vela C. Recombinant vaccine for canine parvovirus in dogs. J. Virol. 1992;66(5):2748–2753. doi: 10.1128/jvi.66.5.2748-2753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C., Dalsgaard K., Lopez de Turiso J.A., Cortes E., Vela C., Casal J.I. Production of porcine parvovirus empty capsids with high immunogenic activity. Vaccine. 1992;10(10):684–690. doi: 10.1016/0264-410x(92)90090-7. [DOI] [PubMed] [Google Scholar]
- Maughan C.N., Preston S.G., Williams G.R. Particulate inorganic adjuvants: recent developments and future outlook. J. Pharm. Pharmacol. 2015;67(3):426–449. doi: 10.1111/jphp.12352. [DOI] [PubMed] [Google Scholar]
- Miyamura K., Kajigaya S., Momoeda M., Smith-Gill S.J., Young N.S. Parvovirus particles as platforms for protein presentation. Proc. Natl. Acad. Sci. U. S. A. 1994;91(18):8507–8511. doi: 10.1073/pnas.91.18.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modjarrad K. MERS-CoV vaccine candidates in development: the current landscape. Vaccine. 2016;34:2982–2987. doi: 10.1016/j.vaccine.2016.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H., Raj V.S., van Kuppeveld F.J., Rottier P.J., Haagmans B.L., Bosch B.J. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J. Virol. 2013;87(16):9379–9383. doi: 10.1128/JVI.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumani K., Falzarano D., Reuschel E.L., Tingey C., Flingai S., Villarreal D.O. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci. Transl. Med. 2015;7(301) doi: 10.1126/scitranslmed.aac7462. 301ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Kalinski P., Ueda R., Hoji A., Kohanbash G., Donegan T.E. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-cytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R.A., Wang P., Gomaa M.R., El-Shesheny R., Kandeil A., Bagato O. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. Bull. Eur. les Mal. Transm. = Eur. Commun. disease Bull. 2013;18(36) doi: 10.2807/1560-7917.es2013.18.36.20574. pii=20574. [DOI] [PubMed] [Google Scholar]
- Rahimian S., Fransen M.F., Kleinovink J.W., Christensen J.R., Amidi M., Hennink W.E. Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation. J. Control Release. 2015;203:16–22. doi: 10.1016/j.jconrel.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Rueda P., Hurtado A., del Barrio M., Martinez-Torrecuadrada J.L., Kamstrup S., Leclerc C. Minor displacements in the insertion site provoke major differences in the induction of antibody responses by chimeric parvovirus-like particles. Virology. 1999;263(1):89–99. doi: 10.1006/viro.1999.9911. [DOI] [PubMed] [Google Scholar]
- Schmidt M.R., McGinnes-Cullen L.W., Kenward S.A., Willems K.N., Woodland R.T., Morrison T.G. Modification of the respiratory syncytial virus f protein in virus-like particles impacts generation of B cell memory. J. Virol. 2014;88(17):10165–10176. doi: 10.1128/JVI.01250-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka M.K., Amanna I. How advances in immunology provide insight into improving vaccine efficacy. Vaccine. 2014;32(25):2948–2957. doi: 10.1016/j.vaccine.2014.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F., Fux R., Provacia L.B., Volz A., Eickmann M., Becker S. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J. Virol. 2013;87(21):11950–11954. doi: 10.1128/JVI.01672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Munster V.J. Animal models of Middle East respiratory syndrome coronavirus infection. Antivir. Res. 2015;122:28–38. doi: 10.1016/j.antiviral.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz A., Kupke A., Song F., Jany S., Fux R., Shams-Eldin H. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J. Virol. 2015;89(16):8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Shi W., Joyce M.G., Modjarrad K., Zhang Y., Leung K. Evaluation of candidate vaccine approaches for MERS-CoV. Nat. Commun. 2015;6:7712. doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zheng X., Gai W., Zhao Y., Wang H., Wang H. MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular imminity in rhesus macaques. Oncotarget. 2016 doi: 10.18632/oncotarget.8475. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Guo H.C., Wei Y.Q., Dong H., Han S.C., Ao D. Self-assembly of virus-like particles of canine parvovirus capsid protein expressed from Escherichia coli and application as virus-like particle vaccine. Appl. Microbiol. Biotechnol. 2014;98(8):3529–3538. doi: 10.1007/s00253-013-5485-6. [DOI] [PubMed] [Google Scholar]
- Ye X., Ku Z., Liu Q., Wang X., Shi J., Zhang Y. Chimeric virus-like particle vaccines displaying conserved enterovirus 71 epitopes elicit protective neutralizing antibodies in mice through divergent mechanisms. J. Virol. 2013;88(1):72–81. doi: 10.1128/JVI.01848-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Du L., Ma C., Li Y., Li L., Poon V.K. A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV. Virology J. 2013;10:266. doi: 10.1186/1743-422X-10-266. [DOI] [PMC free article] [PubMed] [Google Scholar]