Highlights
-
•
Black tea theaflavins show significant anti-herpes simplex virus type 1 (HSV-1) effect on both A549 and Vero cells.
-
•
Theaflavin is able to inhibit the entry of HSV-1 to its host by blocking the adsorption and penetration processes.
-
•
Application of these agents as natural topical remedy to prevent and treat HSV-1 infection and spreading is promising.
Keywords: Herpes simplex virus, HSV-1, Vero cells, A549 cells, Theaflavins, Natural product
Abstract
Tea is the second most consumed drink in the world. The beneficial effects of tea have been mostly attributed to its catechin content. Black tea is derived from the leaves of Camellia sinensis plant, and it is rich in theaflavin polyphenols, in particular theaflavin (TF1), theaflavin-3-monogallate (TF2A), theaflavin-3′-monogallate (TF2B), and theaflavin-3,3′-digallate (TF3). Vero and A549 cells were used to evaluate the effect of purified individual black tea theaflavins as anti-herpes simplex virus 1 agents. With the rise of HSV resistant strains, there is a critical need to develop novel antiherpesviral treatments. Results of the cytotoxicity assay tested by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium] showed that TF1, TF2, and TF3 are not toxic to Vero and A549 cells at a concentration up to 75 μM. The antiviral activity of the individual theaflavins was tested by plaque reduction assay, MTS assay, flow cytometric analysis and confocal microscopy observations. The results showed that TF1, TF2, and TF3 exhibit potent, dose-dependent anti-HSV-1 effect, with TF3 being the most efficient in both Vero and A549 cells. A concentration of 50 μM TF3 and above was sufficient to inhibit >99% of the production of HSV-1 viral particles. The anti-HSV-1 effect of TF3 is due to a direct effect on the virions, and treating Vero or A549 cells with TF3 for 1 h prior to infection, or treating the cells at different times post infection does not inhibit HSV-1 production. TF3 is stable at vaginal pH, indicating its potential to be a promising natural and affordable remedy against herpes simplex viral infections.
1. Introduction
Herpes simplex virus (HSV) infections are considered a significant worldwide health concern. HSV-1 is the main cause of oral infection, affecting the mouth and lips, while HSV-2 is mostly linked to genital herpes. It is estimated that approximately 45–98% of the world’s population are seropositive for HSV-1, while about 7% are seropositive for HSV-2 (Fatahzadeh and Schwartz, 2007). In the United States alone, about 40–63% of people are infected with HSV-1, and 16% are infected with HSV-2. The persistence of HSV-2 increases yearly, with 500,000 new cases in the United States (Fatahzadeh and Schwartz, 2007).
HSV viruses are enveloped, double stranded, DNA viruses made up of 152 kbp (HSV-1) and 155 kbp (HSV-2) in length (Dolan et al., 1998). The HSV life cycle begins when viral glycoproteins bind host epithelial cellular receptors. The viral glycoprotein D (gD) is the main determinant of cellular binding and fusion (Spear et al., 2006). Upon binding to a suitable host cell receptor such as nectin-1, nectin-2, herpes virus entry mediator (HVEM) or 3-O-sulfated (3-OS) heparan sulfate (HS), gD forms a fusion complex with gB, gH, and gL. This complex allows the viral envelope to fuse to the host cellular plasma membrane and consequently virus entry into the host cell (Spear et al., 2006, Subramanian and Geraghty, 2007). Next, the viral nucleocapsid is transported to the host cell nucleus where viral replication and transcription takes place (Garner, 2003). To outsmart the host’s immune system, the virus also achieves latent stage by infecting neuronal cells. While latent, the virus is dormant until re-stimulated into the lytic cycle. In this way, HSV is able to propagate itself for the lifetime of the infected host (Kramer et al., 2003).
Recently, a lot of attention has been given to the health properties found in tea (Dufresne and Farnworth, 2001, Sharangi, 2008). Black, green and oolong tea come from the same plant, Camellia sinensis. Black tea is the most consumed drink in the world (78%) after water, while green tea comprises only about 20% and 2% oolong tea (Kuroda and Hara, 1999). Black tea is rich in theaflavin polyphenols, in particular theaflavin (TF1), theaflavin-3-monogallate (TF2A), theaflavin-3′-monogallate (TF2B), and theaflavin-3,3′-digallate (TF3) (Fig. 1 ) (Li et al., 2013).
Fig. 1.
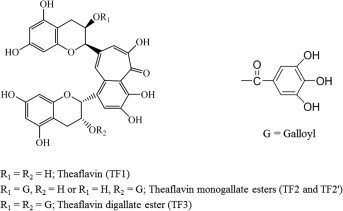
Chemical structures of purified black tea theaflavins, TF1, TF2 (TF2A and TF2B), and TF3.
Black tea theaflavins have been shown to contain antioxidant (Almajano et al., 2008), anti-cancer (Gosslau et al., 2011), anti-inflammatory (Zu et al., 2012), antimicrobial (Almajano et al., 2008, Friedman, 2007), and antiviral abilities, including bovine coronavirus, bovine rotavirus (Clark et al., 1998), HIV-1 (Liu et al., 2005, Yang et al., 2012), influenza (Zu et al., 2012), and HSV-1 (Cantatore et al., 2013). Previously, we have showed the antiviral efficacy of the major catechin constituent in green tea, Epigallocatechin gallate (EGCG) and its ester modification, palmitoyl-EGCG in combating HSV-1 infection in Vero cells (de Oliveira et al., 2013). Crude black tea extract has also been shown to contain anti-herpesviral activity and theaflavins may be the major antiviral players (Cantatore et al., 2013). Here, we want to determine the effect of purified individual black tea theaflavins against HSV-1 infection. Furthermore, theaflavins share many of the structural characteristics of green tea catechins, suggesting that it may also be an effective compound against HSV infections. Thus, application of these agents as natural topical remedy to prevent and treat HSV-1 infection and spreading is promising.
2. Materials and methods
2.1. Cell culture maintenance
Green monkey kidney cells (Vero) and human lung adenocarcinoma epithelial cells (A549) were kindly provided by Dr. Sandra Adams (Department of Biology and Molecular Biology, Montclair State University, Montclair, NJ 07043, USA). Vero cells were cultured until confluent in Dulbecco’s Minimal Essential Media (DMEM) with 5% Fetal Bovine Serum (FBS) and 1 μg/ml gentamicin at 37 °C and 5% CO2. A549 cells were cultured until confluent in 1× Ham’s F-12 K nutrient media, Kaighn’s modification with 2 mM l-glutamine, with 10% FBS and 1 μg/ml gentamicin at 37 °C and 5% CO2.
2.2. HSV-1 UL 46 virus maintenance
A recombinant strain of HSV-1, GHSV-UL46, expressing the tegument protein pUL46 gene fused to green fluorescent protein (GFP) was used for all viral assays (Willard, 2002). Passage of the virus was performed in T-75 flasks and cells were allowed to reach complete cytopathic effect (CPE). The media was then collected, centrifuged and the supernatant containing virus was stored in cryogenic vials at −80 °C.
2.3. Black tea theaflavins
Lipophilic Sephadex (LH-20) resin was purchased from Sigma–Aldrich (St. Louis, MO, USA). Ethyl acetate and acetone were purchased from Merck Chemical Co. (Damstadt, Germany). Black tea crude extract powder (theaflavins 28%) was obtained from DH Nutraceutical (Edison, NJ, USA).
2.4. Preparation of theaflavin (TF1), theaflavin-monogallate (TF2) and theaflavin-3,3′-digallate (TF3)
The specific theaflavin obtained from black tea crude extract were extracted and isolated as described in the previous study (Lo et al., 2006). Ethyl acetate fraction of black tea crude extract was subjected to the LH-20 column and eluted with 40% acetone solution. The TF1, TF2, and TF3 with distinguished reddish color were fractionated. The acetone solution in each fraction was concentrated by a rotary evaporator and lyophilized by a freeze dryer. Their purity (at least 98%) was confirmed by HPLC method (Li et al., 2013).
2.5. Preparation of black tea theaflavins solution
Purified TF1, TF2, and TF3 were each dissolved in DMSO to produce a stock concentration of 5 mM theaflavin solution. The stock solution was diluted in media accordingly to produce the desired concentrations of 12.5, 25, 50, and 75 μM.
2.6. Observation of cell morphology
Cell morphology was assessed using an AmScope IN200B microscope with an attached camera by comparing treated and untreated samples. Vero and A549 cells were grown in 60 mm dishes for 24 h, and treated with 12.5, 25, 50, and 75 μM of each theaflavin for 1 h. Unadsorbed theaflavin was aspirated and cells were washed with PBS. Fresh media was added to the dishes and cells were examined for morphological changes after 24 h of incubation at 37 °C and 5% CO2.
2.7. Determination of cytotoxicity
Vero and A549 cells were plated in 96 well plates and incubated for 24 h. Cultures of 70–80% confluency were treated with various concentrations of TF1, TF2, and TF3 (12.5, 25, 50, 75 μM) in triplicates for 1 h. Unadsorbed theaflavins were aspirated and fresh media was added to the wells. After incubation for an additional 24 h, cell viability/proliferation was determined using a tetrazolium reduction-based kit MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium] (MTS) (G5421, Promega Corp.) as previously described (de Oliveira et al., 2013).
Cytotoxicity was also determined after 72 h of treatment. Vero and A549 cells were plated in 96 well plates with a concentration of 1.0 × 105 cells per ml and a volume of 90 μl per well. Cells were treated with various concentrations of TF1, TF2, and TF3 (12.5, 25, 50, 75 μM) in triplicates. DMSO was used as control as previously described (Chiang et al., 2002). Cells were incubated for 3 days at 37 °C and 5% CO2, and cellular toxicity were determined using the MTS kit as described above.
2.8. Antiviral activity evaluation using MTS assay
The antiviral activity of TF1, TF2, and TF3 against HSV-1 was evaluated using MTS method. Vero and A549 cells were plated in 96 well plates with a concentration of 1.0 x 105 cells per ml and a volume of 70 μl per well. HSV-1 virions were treated with different concentrations of TF1, TF2, or TF3 (12.5, 25, 50, 75 μM) for 1 h at 37 °C prior to infection. DMSO was used as negative control. Cells were then infected with treated or untreated HSV-1 and allowed to adsorb for 1 h at 37 °C, 5% CO2. Unadsorbed virions were then aspirated and the cells were overlaid with medium. Cells were incubated for 3 days and antiviral activity was determined using the MTS kit (G5421, Promega Corp.). Viral inhibition rate was calculated as previously described (Chiang et al., 2002).
2.9. Viral titer determination using plaque reduction assay
Viral titer was determined by standard plaque assay. HSV-1 virions with serial dilution of 10−1–10−6 were plated onto confluent plates of Vero or A549 cells. The plaques forming units were observed 50 h later. HSV-1 virions were treated with different concentrations of TF1, TF2, or TF3 (12.5, 25, 50, 75 μM) for 1 h at 37 °C prior to infection. Vero and A549 cells were plated in 6 well plates and allowed to reach confluency. The cells were then infected with treated or untreated HSV-1 (1.2 × 106 PFU/mL) with serial dilution of 10−1–10−6, and allowed to adsorb for 1 h at 37 °C, 5% CO2. Unadsorbed virions were then aspirated and the cells were overlaid with a medium-containing agar. Cells were incubated for 50 h at 37 °C, 5% CO2 and then fixed with 12.5% formaldehyde in phosphate buffer for 48 h. Plaques were visualized and counted by staining them with crystal violet. Plaque assay was carried out in triplicate.
2.10. Flow cytometry antiviral study
Vero and A549 cells were grown in 60 mm dishes and allowed to reach confluency. HSV-1 virions were treated with different concentrations of TF1, TF2, or TF3 (25, 50, 75 μM) for 1 h at 37 °C, 5% CO2 prior to infection. The cells were then infected with treated or untreated HSV-1 (3.2 × 106 PFU/mL) for 1 h at 37 °C, 5% CO2. Unadsorbed virions were aspirated and cells were washed with PBS. Fresh media was added to the dishes and cells were incubated at 37 °C, 5% CO2 for an additional 12 h. Cells were then trypsinized and resuspended in PBS for flow cytometry analysis. Flow cytometry antiviral assay was carried out in triplicate.
2.11. Fluorescence confocal microscopy study
Vero and A549 cells were grown on glass cover slips placed in 6 well plates for 24 h. HSV-1 virions were treated with 75 μM TF3 for 1 h at 37 °C prior to infection. Cells were then infected with treated or untreated HSV-1 (1.2 × 106 PFU/mL) for 1 h at 37 °C, 5% CO2. Unadsorbed virions were aspirated and cells were washed with PBS. Fresh media was added to each well and cells were incubated at 37 °C, 5% CO2 for an additional 12 h. Cells were then stained with 300 μl of 300 nM DAPI (4,6-diamidino-2-phenylindole) for 5 min at room temperature in the dark. Next, cells were fixed with a 1:1 acetone/methanol solution for 20 min at −20 °C. The glass cover slips were then placed on to a glass slide with 20 μl of glycerol as the mounting media. Cells were analyzed using an Olympus FluoView™ FV-1000 confocal laser-scanning microscope (CLSM).
2.12. Binding assay
The binding assay was carried out at 4 °C, a temperature that allows HSV-1 virions to bind to cell receptors but not enter the cells. HSV-1 virions were treated with different concentrations of TF3 (50, 75 μM) for 1 h at 37 °C prior to infection. Vero and A549 cells were plated in 60 mm cell culture dishes and allowed to reach confluency. The cells were infected with treated or untreated HSV-1 (3.2 × 106 PFU/mL) with serial dilution of 10−1–10−6, and allowed to adsorb for 1 h at 4 °C. Unadsorbed virions were then aspirated and the cells washed with PBS twice. Plaque reduction assays were carried out as described in Section 2.9. Binding assay was carried out in triplicate.
2.13. Penetration assay
Vero and A549 cells were plated in 60 mm cell culture dishes and allowed to reach confluency. The cells were infected with treated or untreated HSV-1 (3.2 × 106 PFU/mL) with serial dilution of 10−1–10−6, and allowed to bind for 1 h at 4 °C. At this temperature, HSV-1 virions bind to cell receptors but do not penetrate the cells. Unbound virions were then aspirated and the cells were treated with different concentrations of TF3 (50, 75 μM) for 1 h at 37 °C, 5% CO2. At this temperature, HSV-1 virions are able to successfully penetrate the cells. Unadsorbed theaflavins were then aspirated and the cells washed with PBS twice. Plaque reduction assays were carried out as described in Section 2.9. Penetration assay was carried out in triplicate.
2.14. Flow cytometry binding/adsorption study
Vero and A549 cells were grown in 60 mm dishes and allowed to reach confluency. In order to study the ability of TF3 to inhibit HSV-1 binding and adsorption, virus and cell treatment with 75 μM TF3 was done at different time points of the viral infection. First, both HSV-1 virions (3.2 × 106 PFU/mL) and cells only were treated with 75 μM TF3 for 1 h at 37 °C prior to infection. Cells were also treated for 1 h at different times points post infection (0, 2, 4, 6, 8, 10 h). After treatment, the cells were washed with PBS, fresh media was added to the dishes and incubated at 37 °C, 5% CO2. At 12 h post infection, cells were trypsinized and resuspended in PBS for flow cytometry analysis.
2.15. Statistical analysis
All assays were performed in triplicates and the data analyzed using one way Analysis of Variance (ANOVA) (P < 0.05) by SPSS.
3. Results
3.1. Black tea theaflavins with concentrations up to 75 μM have no significant effect on Vero and A549 cell morphology and proliferation
Vero and A549 cells were exposed to 0, 12.5, 25, 50 and 75 μM of TF1, TF2, or TF3 for 1 h. Cell morphology was analyzed using phase contrast microscopy at 24 h post treatment. No significant changes in morphology were observed at any of the concentrations used (data not shown). Thus, treatments with TF1, TF2, or TF3 at concentrations up to 75 μM in Vero and A549 cells appear to have low to no cellular toxicity.
To determine whether Vero and A549 cells were viable and could proliferate after being treated with different concentrations of TF1, TF2, and TF3 for 1 h, followed by a 24 h TF-free incubation period, MTS [3(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium], a colorimetric kit that measures cellular metabolism was used. As seen in Fig. 2 , treatment of cells with 0, 12.5, 25, 50 and 75 μM of TF1, TF2 or TF3 did not significantly affect cell viability/proliferation. Although the cells responded to theaflavin treatments in a dose-dependent manner in Vero cells, cell proliferation was inhibited at a maximum of 12.60% for 75 μM TF1 and 6.83% for 75 μM TF2, but only 2.75% for 75 μM TF3. Exposure to all concentrations of TF1, TF2, and TF3 inhibited cells proliferation by less than 1.85% in A549 cells. The results suggested that cellular exposure to theaflavins up to 75 μM are not toxic in either Vero or A549 cells. MTS was also used to study the effect of TF1, TF2, and TF3 on Vero and A549 cells with a prolonged exposure time (3 days). The 50% cytotoxicity concentration (CC50) of TF1, TF2 and TF3 were 112.5, 100 and 112.5 μM, respectively. The results obtained showed that TF1, TF2, and TF3 are not toxic to Vero and A549 cells at a concentration of up to 75 μM even after 72 h exposure (data not shown).
Fig. 2.
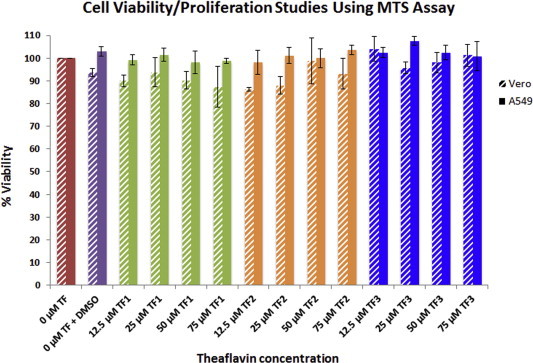
Cell viability and proliferation studies of Vero and A549 cells treated with different concentrations (0, 12.5, 25, 50, and 75 μM) of TF1, TF2, and TF3 using MTS assay. Results indicate the absorbance level of the mean of the results of triplicate tests on Vero (blue) and A549 (red) cells and the y-error bars represent standard deviation (SD). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Comparison of antiviral activity of TF1, TF2, and TF3 against HSV-1
To study the antiviral activity of TF1, TF2, and TF3 against HSV-1, Vero and A549 cells were infected with treated (0, 6.25, 12.5, 15, 20, 25, 35, 50, 60 and 75 μM TF1, TF2, TF3) or untreated HSV-1 for 1 h, followed by a 3-day incubation period. Half maximal effective concentration (EC50) and selective index (SI) were determined. MTS results showed that all three theaflavins possess antiviral activity against HSV-1, TF1 (EC50 = 50 μM; SI = 2.25), TF2 (EC50 = 25 μM; SI = 4), and TF3 (EC50 = 20 μM; SI = 5.625). Based on the SI values, the antiherpesviral activity of theaflavins are in the order of TF3 > TF2 > TF1. As TF3 was found to have the highest SI value, plaque reduction assay, flow cytometry assay, and fluorescence confocal microscopy studies were done to confirm TF3’s efficacy against HSV-1.
3.3. TF3 reduces HSV-1 plaque formation more effectively than TF1 or TF2
To study the effect of TF1, TF2, and TF3 on the production of HSV-1 viral plaques, Vero and A549 cells were infected with either treated (at concentrations 12.5, 25, 50, 75 μM of TF1, TF2, or TF3) or untreated HSV-1. As seen in Fig. 3, Fig. 4 , the effect of all three theaflavins was concentration-dependent. Treatments with 12.5 μM TF1, TF2 or TF3 did not show significant reduction of HSV-1 plaque formation. Exposure to 25 and 50 μM TF1 caused a 29.2 ± 5.8% and 77.2 ± 0.78% reduction in HSV-1 PFU in Vero cells and 12.84 ± 4.02% and 91.08 ± 0.12% in A549 cells respectively; exposure to 25 and 50 μM TF2 caused a 20.3 ± 0.45% and 89.0 ± 1.50% reduction in HSV-1 PFU in Vero and 13.88 ± 2.99% and 99.3 ± 0.01% in A549 cells respectively; and exposure to 25 and 50 μM TF3 caused a remarkably 93.56 ± 0.32% and >99% decrease in titer. Furthermore, exposure of 75 μM TF1, TF2, or TF3 all caused a >99% decrease in titer in both Vero and A549 cells.
Fig. 3.
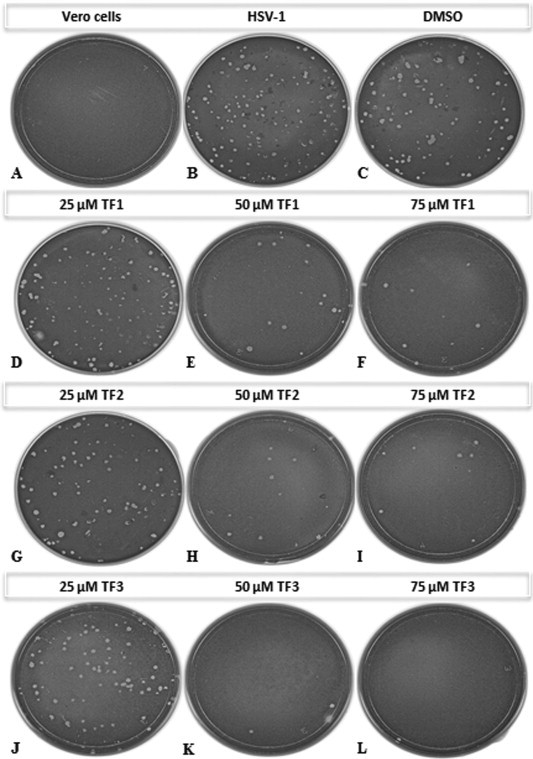
Plaque assay of HSV-1 treated or untreated with different concentrations of TF1, TF2, or TF3. (A) Vero cells only, (B) untreated HSV-1, (C) HSV-1 treated with DMSO, (D–F) HSV-1 treated with 25, 50, 75 μM TF1, (G–I) HSV-1 treated with 25, 50, 75 μM TF2, (J–L) HSV-1 treated with 25, 50, 75 μM TF3.
Fig. 4.
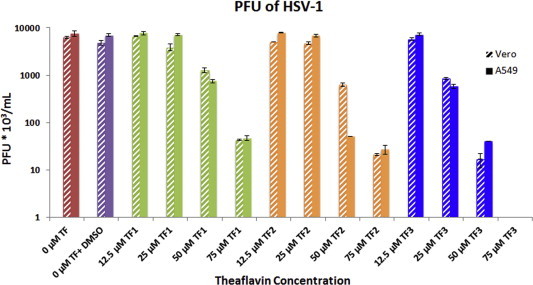
PFU of HSV-1 infected Vero and A549 cells treated or untreated with different concentrations (0, 12.5, 25, 50, and 75 μM) of TF1, TF2 or TF3. The numbers represent the mean of triplicate trials and the y-error bars represent SD.
While exposure of 75 μM of either TF1 or TF2 expressively reduces the HSV-1 viral titer, exposure of only 50 μM TF3 is sufficient to significantly inhibit the production of HSV-1 viral plaques (Fig. 3, Fig. 4). The plaque forming units were significantly different from the control (P < 0.05). Collectively, the results suggest that non-toxic concentrations of TF1, TF2 and TF3 can effectively inhibit HSV-1 viral plaque formation, while TF3 seems to be the most effective among the three.
3.4. Flow cytometry assay supports the antiviral efficacy of TF3 against HSV-1 infection of Vero and A549 cells
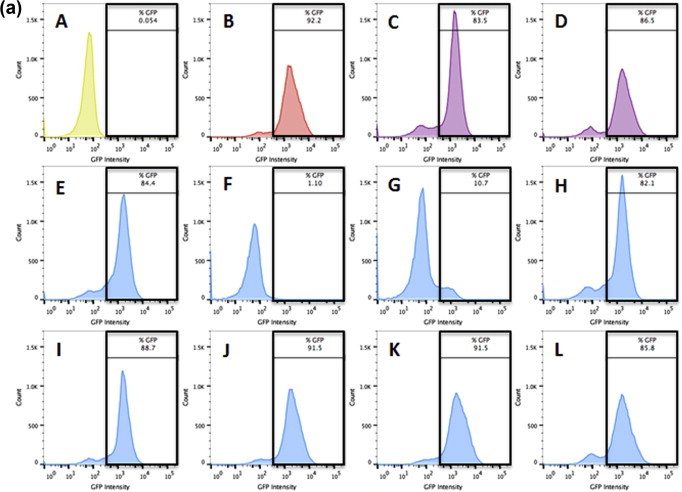
The antiviral efficacy of TF1, TF2, and TF3 against HSV-1 was also obtained from flow cytometry assay. The ability of HSV-1 to infect Vero (Fig. 5 a) or A549 (Fig. 5b) cells after being exposed to 25, 50, 75 μM of theaflavin was measured by the GFP intensity levels from the GHSV-UL46 HSV-1 strain. As seen in Fig. 5, when HSV-1 was treated with different concentrations (25, 50, 75 μM) of TF1 (D–F; D′–F′), TF2 (G–I; G′–I′), or TF3 (J–L; J′–L′), the infection was repressed at a dose-dependent manner. The higher the concentration of TF1, TF2 or TF3, the lower the GFP expression was observed in both Vero and A549 cells. Furthermore, the results of the flow cytometry assay confirm the effectiveness of theaflavins against HSV-1 infection in Vero and A549 cells, with TF3 being the most effective. The results further confirmed that 50 μM TF3 and above is sufficient to greatly inhibit HSV-1 viral infection in Vero and A549 cells (Fig. 5).
Fig. 5.
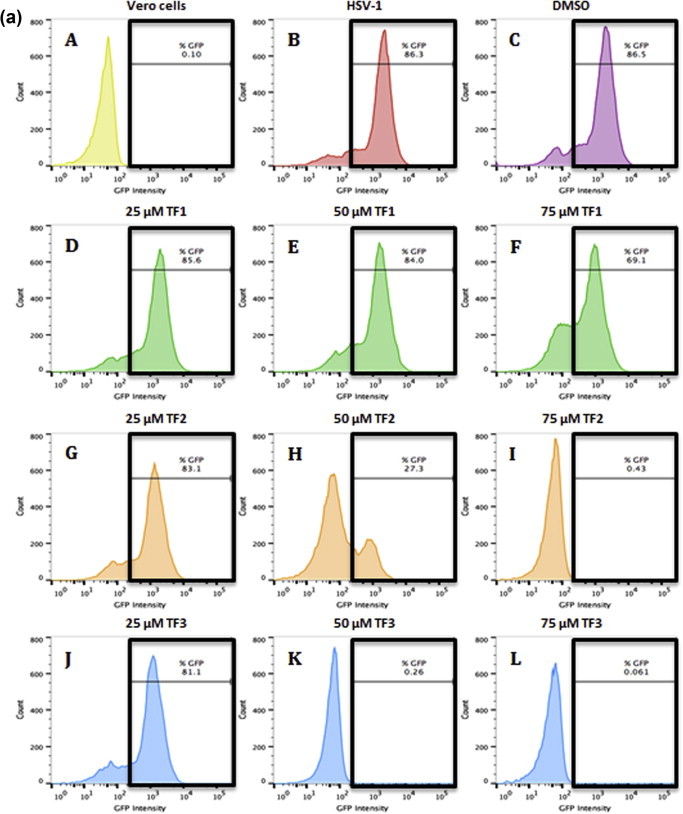
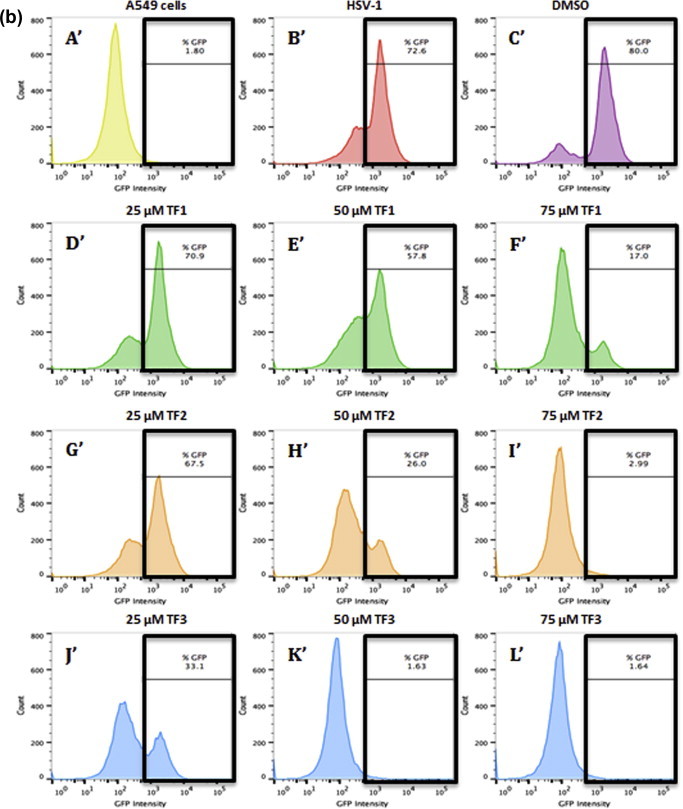
Antiviral effect of TF1, TF2, and TF3 against HSV-1 infected Vero (5a) and A549 (5b) cells as analyzed by flow cytometric system. The histograms depict GFP intensity from the GHSV-UL46 HSV-1 strain. (A) Vero cells only, (A′) A549 cells only (B, B′) untreated HSV-1, (C, C′) HSV-1 treated with DMSO, (D–F, D′–F′) HSV-1 treated with 25, 50, 75 μM of TF1, (G–I, G′–I′) HSV-1 treated with 25, 50, 75 μM of TF2, (J–L, J′–L′) HSV-1 treated with 25, 50, 75 μM of TF3.
3.5. Fluorescence confocal microscopy confirms the antiviral efficacy of TF3 against HSV-1 infection of Vero and A549 cells
To further determine the effect of theaflavins on the HSV-1 life cycle, Vero (A–D) and A549 (A′–D′) cells were infected with either treated (75 μM of TF3 for 1 h) or untreated HSV-1 (Fig. 6 ). The expression and localization of tegument-GFP was observed using a confocal microscope at 12 h post infection. Cells were stained with DAPI and the cell nucleus was analyzed for potential cytopathic effect. Upon infection, the pUL46-GFP tegument protein associates with cellular membranes and localizes throughout the cytoplasm as distinct puncta (Murphy et al., 2008, Willard, 2002). As shown in Fig. 6, a significant level of pUL46-GFP expression was observed in the untreated HSV-1 control (B; B′) and DMSO treated HSV-1 control (C; C′) in both Vero and A549 infected cells. Contrarily, 75 μM TF3 treated HSV-1 infected cells (D; D′) did not show GFP positive granules, but instead, it closely resembled the uninfected cells control (A; A′). Overall, the results indicate that treatment of HSV-1 with 75 μM TF3 significantly affect the HSV-1 life cycle in both Vero and A549 cells.
Fig. 6.
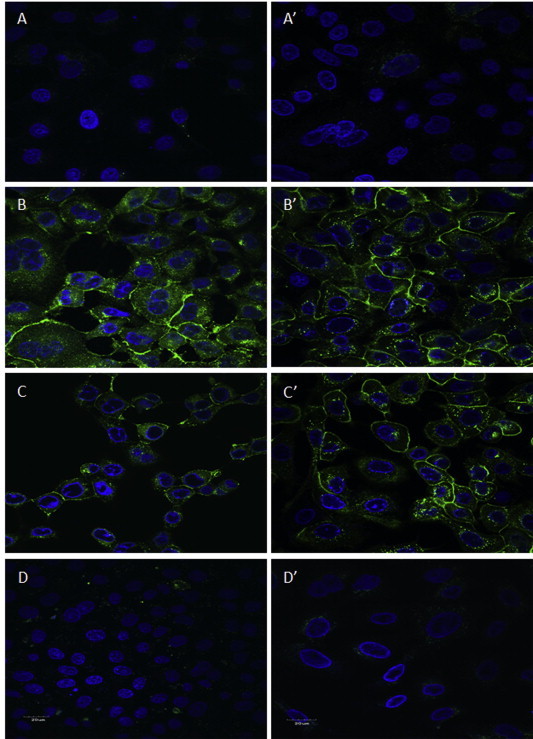
Fluorescence confocal microscopy observations (600×) of GFP expression and DAPI stain overlay of (A) Vero cells only, (A′) A549 cells only; HSV-1 infected Vero cells (B), HSV-1 infected A549 cells (B′); DMSO treated HSV-1 infected Vero cells (C), DMSO treated HSV-1 infected A549 cells (C′); 75 μM TF3 treated HSV-1 infected Vero cells (D), 75 μM TF3 treated HSV-1 infected A549 cells (D′).
3.6. TF3 inhibits viral attachment in Vero and A549 cells
To determine if treatment of HSV-1 with 75 μM TF3 disrupts virions from binding to Vero and A549 cellular receptors, a binding assay was performed. This assay is performed at 4 °C, a temperature that allows viral binding but not cellular penetration. Upon later placing the cells at 37 °C, virions are able to penetrate and complete its life cycle. If theaflavins disrupt viral binding, and consequently prevent viral penetration, no plaques should be observed. As seen in Fig. 7 , 50 and 75 μM TF3 treatment of HSV-1 in Vero and A549 infected cells are sufficient to stop the virus ability to bind and infect cells. While many plaques were seen in the cells infected with both untreated HSV-1 and DMSO treated HSV-1, no plaques were observed when HSV-1 was treated with 50 and 75 μM TF3. Together, this assay suggests that TF3 halters HSV-1 ability to complete its lytic cycle by preventing it from binding Vero and A549 cells.
Fig. 7.
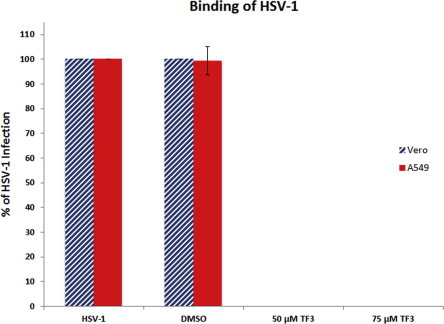
Binding assay of untreated or treated HSV-1 with 50 and 75 μM TF3 in infected Vero and A549 cells. The numbers represent the mean of triplicate trials and the y-error bars represent SD.
3.7. TF3 inhibits viral penetration in Vero and A549 cells
To determine if treatment of HSV-1 with 50 or 75 μM of TF3 disrupts the cellular penetration step of the HSV-1 life cycle, a penetration assay was performed. This assay is initially performed at 4 °C, which allows viral binding but prevents cellular penetration. The cells and virions are then exposed to 50 or 75 μM of TF3 at 37 °C for 1 h. At this temperature, the virions will penetrate the cells and complete its life cycle. If theaflavins disrupt viral penetration, no plaques should be observed. As seen in Fig. 8 , there was a substantial decrease in plaque formation (84.28 ± 5.82%, and 58.05 ± 12.01) in both Vero and A549 cells accordingly, when HSV-1 was exposed to 50 μM TF3 at the time of penetration. Furthermore, no plaques were seen with 75 μM TF3 treatment of HSV-1 in Vero infected cells (>99% decrease), while there was an 87.60 ± 1.37% decrease in plaque formation in A549 cells. Thus, this assay suggests that while 50 μM TF3 severely affects viral penetration, a concentration of 75 μM TF3 is needed to significantly halter HSV-1 ability to penetrate Vero and A549 cells.
Fig. 8.
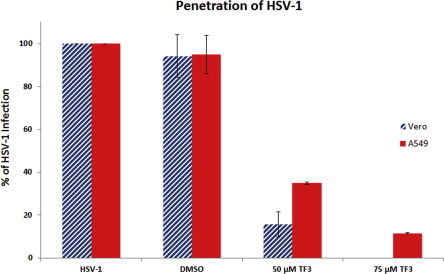
Penetration assay of untreated or treated HSV-1 with 50 and 75 μM TF3 in infected Vero and A549 cells. The numbers represent the mean of triplicate trials and the y-error bars represent SD.
3.8. Flow cytometry assay further confirms the ability of TF3 to inhibit HSV-1 binding/adsorption
To confirm the findings established by the binding assay, HSV-1 was exposed to 75 μM TF3 at different time points during its lytic cycle. GFP intensity from the GHSV-UL46 strain of HSV-1 was recorded from all the different treatments using a flow cytometer for both Vero (Fig. 9 a) and A549 (Fig. 9b) infected cells. Only pre-exposure (F; F′) and exposure at time 0 h (G; G′) of 75 μM TF3 to HSV-1 for 1 h showed an inhibitory effect against HSV-1, when compared to the controls: HSV-1 virus infected Vero and A549 cells (B; B′). Pre-exposure of 75 μM TF3 to Vero cells for 1 h (E; E′) or exposure of HSV-1 at 2, 4, 6, 8, or 10 h (H–L; H′–L′) post infection for 1 h did not have a significant inhibitory effect. The results confirm the findings established by the binding assay. TF3 seems to act directly on the HSV-1 viral particle, hindering its ability to bind/penetrate the cells. Contrarily, TF3 does not show any inhibitory effect once the virus has already penetrated the cells.
Fig. 9.
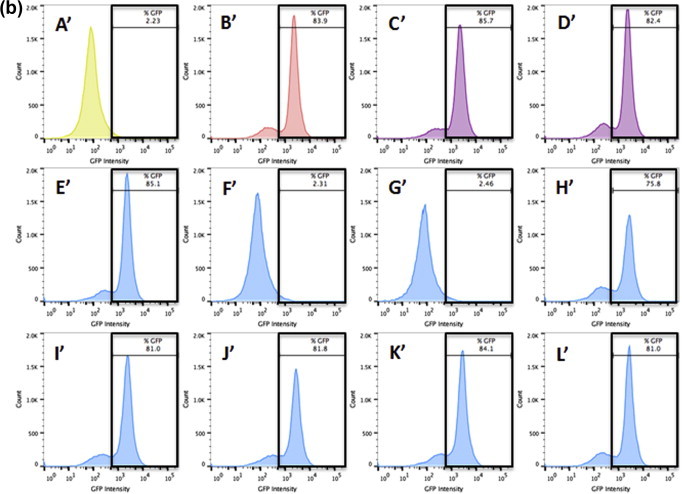
Flow cytometry analysis of binding/adsorption of untreated or 75 μM TF3 treated HSV-1 in infected Vero (9a) and A549 (9b) cells. The histograms depict GFP intensity from the GHSV-UL46 strain of HSV-1. (A) Vero cells only, (A′) A549 cells only (B, B′) Untreated HSV-1, (C, C′) DMSO treated HSV-1 at 2 h post infection, (D, D′) DMSO treated HSV-1 at 10 h post infection, (E, E′) Vero cells pre-treated with 75 μM TF3 for 1 h, (F, F′) HSV-1 pre-treated with 75 μM TF3 for 1 h, (G–L, G′–L′) HSV-1 treated with 75 μM TF3 for 1 h at 0, 2, 4, 6, 8, 10 h post infection.
4. Discussion
While there have been constant attempts to create a safe and effective vaccine against HSV infections, to date there have been little to no significant achievement (Bernstein and Stanberry, 1999, Quenelle et al., 2006). The current treatment of choice is nucleoside analogs, which targets the viral DNA polymerase and acts as chain terminators by preventing virus DNA elongation (Brady and Bernstein, 2004). Despite effective antiviral therapy, the virus has been shown to develop resistant strains, especially in immunocompromised hosts (Bacon et al., 2003). The virus acquires mutations in the thymidine kinase (TK) enzyme and/or the viral DNA polymerase gene and can thus bypass any antiviral effect the drug would have produced (Bacon et al., 2003, Piret and Boivin, 2011). Therefore, there is a need for new antiviral treatments that can substitute or complement currently used anti-HSV medicines.
The antiherpesviral activities of different natural compounds have been the focus of many studies due to their low toxicity and potent antiviral abilities (de Oliveira et al., 2013, Jassim and Naji, 2003). While green tea polyphenols have received the most attention in the past years, data presented in this study suggests that black tea theaflavins may be better candidates as a microbicide against HSV infections. Theaflavins have been reported to have no to low cytotoxicity effects on normal human lung fibroblast cells (MRC-5), MT-2 and CEM cells (Liu et al., 2005). Likewise, we show that TF1, TF2, and TF3 have low toxicity towards A549 and Vero cells at concentrations up to 75 μM.
GHSV-UL46, a recombinant HSV-1 virus with GFP gene fused to UL46 tegument protein, was used in this study. It served as a valuable tool to identify green fluorescence granules (viral particles) for fluorescence confocal microscopic observation. The green fluorescent protein can also be detected by a flow cytometer with a 488 nm blue laser, which provided quantitative information on HSV-1 infection.
Several experimental approaches used here demonstrated that TF1, TF2, and TF3 have a powerful inhibitory effect on the HSV-1 lytic cycle, with TF3 being the most effective. This is consistent with previous reports showing that black tea extract (80% TF) are able to inhibit HSV-1 infection (Cantatore et al., 2013) and reports showing that digallate dimers of EGCG, such as TF3 are more potent inhibitors of HSV infection (Isaacs et al., 2011). As seen here, treatment of virions with 50 μM and above of TF3 for 1 h and removed after 1 h adsorption caused a >99% reduction in infectivity in both Vero and A549 cells, as measured by plaque reduction assay (Fig. 3, Fig. 4) and flow cytometry (Fig. 5).
Moreover, the binding and penetration assay suggest that TF3 inhibits HSV-1 entry into target cells by interfering with the binding/adsorption of the virions to the cellular receptor (Fig. 7, Fig. 9, Fig. 9). TF3 did not show significant antiviral effect after the virus had already penetrated the cells, implying that this is a direct effect on the virions. Taken the fact that once in contact with an appropriate cell, HSV infections will readily persist, an effective microbicide should be able to target the virus prior to the first contact with the host cell. Thus, TF3 seems to be an ideal candidate to combat HSV prior to host cellular infection. Similar results were seen in a HIV-1 study, in which TF3 also targeted the viral entry step by disrupting the glycoprotein 41 6-HB core structure (Yang et al., 2012).
In summary, the results presented here indicate that black tea theaflavins have a strong HSV-1 inhibitory effect in both Vero and A549 infected cells, while TF3 displays the highest response. As seen in all the antiviral assays conducted throughout the study, TF1, TF2, and TF3 inhibit HSV-1 at a dose-dependent manner. The plaque reduction assay, flow cytometric antiviral assay, and fluorescence confocal microscopy study, demonstrated that TF3 exhibits the strongest effect against HSV-1. The results suggest that TF3 should be further explored for the potential application in the management of HSV-1 infections.
Furthermore, a major advantage of using theaflavins as a microbicide is that they have been shown to be stable at non-favorable conditions such as acidic pH, high temperature and high humidity (Li et al., 2013). This high stability allows it to be a suitable topical antiviral agent in vaginal pH (Jhoo et al., 2005). In addition, a recent study has shown that the combination of TF3 and lactic acid may be an effective way to inactivate HSV in the vaginal area, confirming the results seen here (Isaacs and Xu, 2013). Finally, the current market anti-herpetic medications are highly costly, making TF3 a plausible, affordable solution for treatment and prevention of HSV infections. In conclusion, the use of TF3 against HSV-1 infections could have significant health benefits, and future studies should focus on better understanding the molecular mechanism by which theaflavins act against HSV-1 in animal models.
Acknowledgments
A. O. and D. P. were supported by SHU Graduate Teaching Assistantship in the Department of Biological Sciences. L.H. L. was supported by Montclair State University Faculty Scholarship Program. T. C. was supported by Seton Hall University Annual Biological Sciences Departmental Research Fund and William and Doreen Wong Foundation.
References
- Almajano M.P., Carbó Rosa, Jimenez J.A.L., Gordon M.H. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 2008;108:55–63. [Google Scholar]
- Bacon T.H., Levin M.J., Leary J.J., Sarisky R.T., Sutton D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 2003;16:114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.I., Stanberry L.R. Herpes simplex virus vaccines. Vaccine. 1999;17:1681–1689. doi: 10.1016/s0264-410x(98)00434-4. [DOI] [PubMed] [Google Scholar]
- Brady R.C., Bernstein D.I. Treatment of herpes simplex virus infections. Antiviral Res. 2004;61:73–81. doi: 10.1016/j.antiviral.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Cantatore A., Randall S.D., Traum D., Adams S.D. Effect of black tea extract on herpes simplex virus-1 infection of cultured cells. BMC Complem. Altern. Med. 2013;13:139. doi: 10.1186/1472-6882-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang L.C., Chiang W., Chang M.Y., Ng L.T., Lin C.C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Res. 2002;55:53–62. doi: 10.1016/s0166-3542(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Clark K.J., Grant P.G., Sarr A.B., Belakere J.R., Swaggerty C.L., Phillips T.D., Woode G.N. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Vet. Microbiol. 1998;63:147–157. doi: 10.1016/S0378-1135(98)00242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira A., Adams S.D., Lee L.H., Murray S.R., Hsu S.D., Hammond J.R., Dickinson D., Chen P., Chu T.C. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem. Toxicol. 2013;52:207–215. doi: 10.1016/j.fct.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan A., Jamieson F.E., Cunningham C., Barnett B.C., McGeoch D.J. The genome sequence of herpes simplex virus type 2. J. Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne C.J., Farnworth E.R. A review of latest research findings on the health promotion properties of tea. J. Nutr. Biochem. 2001;12:404–421. doi: 10.1016/s0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- Fatahzadeh M., Schwartz R.A. Human herpes simplex labialis. Clin. Exp. Dermatol. 2007;32:625–630. doi: 10.1111/j.1365-2230.2007.02473.x. [DOI] [PubMed] [Google Scholar]
- Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner J.A. Herpes simplex virion entry into and intracellular transport within mammalian cells. Adv. Drug Deliv. Rev. 2003;55:1497–1513. doi: 10.1016/j.addr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Gosslau A., En Jao D.L., Huang M.T., Ho C.T., Evans D., Rawson N.E., Chen K.Y. Effects of the black tea polyphenol theaflavin-2 on apoptotic and inflammatory pathways in vitro and in vivo. Mol. Nutr. Food Res. 2011;55:198–208. doi: 10.1002/mnfr.201000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs C.E., Xu W. Theaflavin-3,3′-digallate and lactic acid combinations reduce herpes simplex virus infectivity. Antimicrob. Agents Chemother. 2013;57:3806–3814. doi: 10.1128/AAC.00659-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs C.E., Xu W., Merz G., Hillier S., Rohan L., Wen G.Y. Digallate dimers of (−)-epigallocatechin gallate inactivate herpes simplex virus. Antimicrob. Agents Chemother. 2011;55:5646–5653. doi: 10.1128/AAC.05531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassim S.A., Naji M.A. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- Jhoo J.W., Lo C.Y., Li S., Sang S., Ang C.Y., Heinze T.M., Ho C.T. Stability of black tea polyphenol, theaflavin, and identification of theanaphthoquinone as its major radical reaction product. J. Agric. Food Chem. 2005;53:6146–6150. doi: 10.1021/jf050662d. [DOI] [PubMed] [Google Scholar]
- Kramer M.F., Cook W.J., Roth F.P., Zhu J., Holman H., Knipe D.M., Coen D.M. Latent herpes simplex virus infection of sensory neurons alters neuronal gene expression. J. Virol. 2003;77:9533–9541. doi: 10.1128/JVI.77.17.9533-9541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y., Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat. Res. 1999;436:69–97. doi: 10.1016/s1383-5742(98)00019-2. [DOI] [PubMed] [Google Scholar]
- Li S., Lo C.Y., Pan M.H., Lai C.S., Ho C.T. Black tea: chemical analysis and stability. Food Funct. 2013;4:10–18. doi: 10.1039/c2fo30093a. [DOI] [PubMed] [Google Scholar]
- Liu S., Lu H., Zhao Q., He Y., Niu J., Debnath A.K., Wu S., Jiang S. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim. Biophys. Acta. 2005;1723:270–281. doi: 10.1016/j.bbagen.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Lo C.Y., Li S., Tan D., Pan M.H., Sang S., Ho C.T. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol. Nutr. Food Res. 2006;50:1118–1128. doi: 10.1002/mnfr.200600094. [DOI] [PubMed] [Google Scholar]
- Murphy M.A., Bucks M.A., O’Regan K.J., Courtney R.J. The HSV-1 tegument protein pUL46 associates with cellular membranes and viral capsids. Virology. 2008;376:279–289. doi: 10.1016/j.virol.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Piret J., Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011;55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle D.C., Collins D.J., Marciani D.J., Kern E.R. Effect of immunization with herpes simplex virus type-1 (HSV-1) glycoprotein D (gD) plus the immune enhancer GPI-0100 on infection with HSV-1 or HSV-2. Vaccine. 2006;24:1515–1522. doi: 10.1016/j.vaccine.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Sharangi A.B. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.) – a review. Food Res. Int. 2008;42:529–535. [Google Scholar]
- Spear P.G., Manoj S., Yoon M., Jogger C.R., Zago A., Myscofski D. Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry. Virology. 2006;344:17–24. doi: 10.1016/j.virol.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Subramanian R.P., Geraghty R.J. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2903–2908. doi: 10.1073/pnas.0608374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard M. Rapid directional translocations in virus replication. J. Virol. 2002;76:5220–5232. doi: 10.1128/JVI.76.10.5220-5232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Li L., Tan S., Jin H., Qiu J., Mao Q., Li R., Xia C., Jiang Z.H., Jiang S., Liu S. A natural theaflavins preparation inhibits HIV-1 infection by targeting the entry step: potential applications for preventing HIV-1 infection. Fitoterapia. 2012;83:348–355. doi: 10.1016/j.fitote.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Zu M., Yang F., Zhou W., Liu A., Du G., Zheng L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antiviral Res. 2012;94:217–224. doi: 10.1016/j.antiviral.2012.04.001. [DOI] [PubMed] [Google Scholar]


