Highlights
-
•
Amylmetacresol/2,4-dichlorobenzyl alcohol lozenge showed virucidal effects against parainfluenza virus and cytomegalovirus.
-
•
Hexylresorcinol lozenge showed virucidal effects against parainfluenza virus.
-
•
Mean reductions in viral titre were significantly greater compared with their respective placebo lozenge.
-
•
Peak virucidal effects were observed following 1 min of incubation in vitro.
Abbreviations: AMC, amylmetacresol; ASTM, American Society for Testing and Materials; ATCC, American Type Culture Collection; CMV, cytomegalovirus; DCBA, 2,4-dichlorobenzyl alcohol; EBV, Epstein–Barr virus; HEPES, 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid; MEM, minimum essential medium; PIV, parainfluenza virus; PIV3, parainfluenza virus type 3; RPMI, Roswell Park Memorial Institute; RSV, respiratory syncytial virus; RTI, respiratory tract infection; SARS-CoV, severe acute respiratory syndrome coronavirus; TCID50, tissue culture infectious dose 50%
Keywords: 2,4-Dichlorobenzyl alcohol; Amylmetacresol; Cytomegalovirus; Hexylresorcinol; Parainfluenza virus; Respiratory tract infection
Abstract
Most respiratory tract infections are self-limiting and caused by viruses, and do not warrant antibiotic treatment. Despite this, patients with respiratory tract infections often receive antibiotics, fuelling the rise of antibiotic resistance. Therefore, there is a need to encourage patients to try alternative non-antibiotic therapies, which ideally treat the symptoms and the cause. Lozenges containing amylmetacresol and 2,4-dichlorobenzyl alcohol (AMC/DCBA lozenges) as well as lozenges containing hexylresorcinol have been shown to provide effective symptomatic relief for sore throat. In this study, we investigated whether these lozenges also have virucidal effects in vitro against two viruses associated with respiratory tract infections, parainfluenza virus type 3 and cytomegalovirus. Both viruses were incubated with AMC/DCBA lozenge, placebo lozenge or the active ingredients (AMC/DCBA) as free substances, and parainfluenza virus type 3 was incubated with hexylresorcinol lozenge, placebo lozenge or hexylresorcinol as a free substance. Virucidal effects were observed with the active lozenges and the active ingredients as free substances against both parainfluenza virus type 3 and cytomegalovirus. Mean reductions in viral titre were significantly greater compared with placebo lozenge and peak effects were observed for the shortest incubation time, 1 min. These findings suggest that AMC/DCBA lozenge and hexylresorcinol lozenge have the potential to have local antiviral effects in patients with sore throat due to viral respiratory tract infections. Use of such over-the-counter treatments for self-limiting respiratory tract infections may satisfy patients’ desire for an anti-infective medication and reduce the demand for antibiotics.
1. Introduction
Respiratory tract infections (RTIs) are the most common illnesses to affect humans (Denny, 1995). Typical symptoms include sore throat, rhinitis, cough and fever (Dasaraju and Liu, 1996, Eccles, 2007). Most RTIs are caused by a viral infection (Denny, 1995), for example, the symptom of sore throat is reported to be caused by viruses in 85–95% of cases in adults, 95% in children under 5 years and 70% in children aged 5–15 years (Worrall, 2011). Viruses associated with RTIs include orthomyxoviruses (influenza), paramyxoviruses (parainfluenza viruses [PIV], respiratory syncytial virus [RSV]), coronaviruses, picornaviruses, adenoviruses and herpes viruses (cytomegalovirus [CMV], Epstein–Barr virus [EBV]) (Collier and Oxford, 2000).
Although RTIs are generally self-limiting and have a predominantly viral cause, patients visiting healthcare professionals often expect antibiotics (or are perceived to expect antibiotics) and receive inappropriate antibiotic treatment (van der Velden et al., 2013). In Europe, one-quarter of those who took antibiotics in the last year did so to provide symptom relief, and there remains much consumer confusion about the effects of antibiotics with 49% of Europeans falsely believing that antibiotics kill viruses and 41% believing they were effective against colds and flu (European Commission, 2013). Physicians respond to sore throat patients’ demands (actual and perceived) by prescribing an antibiotic in approximately 60% of cases (Barnett and Linder, 2014, Gulliford et al., 2014) but only about 5–15% of sore throats in adults are caused by a bacterial infection (Shulman et al., 2012). This unnecessary use of antibiotics drives the development of antibiotic resistance (Goossens et al., 2005), which is an increasingly serious threat to global public health (WHO, 2014). Therefore, there is a need to encourage patients with RTIs to try alternative treatments, while antibiotics should be reserved for patients with a serious illness or those at increased risk of complications (Essack and Pignatari, 2013).
An ideal treatment would provide the symptomatic relief that patients seek as well as treat the cause. Locally delivered formats such as lozenges and sprays are useful as they enable active ingredients to reach the site of infection directly; the localized delivery means that side effects are lower compared with systemically acting treatments (Farrer, 2011). Lozenges containing the antiseptics and local anaesthetics amylmetacresol (AMC) and 2,4-dichlorobenzyl alcohol (DCBA) or hexylresorcinol have been developed to treat the symptoms of sore throat (Buchholz et al., 2009, Foadi et al., 2014, McNally et al., 2010, McNally et al., 2012, Wade et al., 2011). These lozenges have demonstrated statistically significant reductions in sore throat symptoms in placebo-controlled clinical trials (McNally et al., 2010, McNally et al., 2012, Wade et al., 2011). AMC/DCBA lozenges have demonstrated antibacterial effects in vivo (Richards et al., 1989) and in vitro (Richards and Xing, 1993). AMC/DCBA lozenges have also been shown to have some virucidal effects in vitro on three enveloped viruses – RSV, influenza A virus and severe acute respiratory syndrome coronavirus (SARS-CoV) (Oxford et al., 2005).
This study investigated the in vitro virucidal activity of AMC/DCBA alone and in a lozenge on two other enveloped viruses that can cause sore throat and respiratory illness – PIV type 3 (PIV3) and CMV (Bisno, 2001). It also assessed the in vitro virucidal activity of hexylresorcinol alone and in a lozenge on PIV3. To our knowledge, this is the first study to examine the virucidal effects of throat lozenges on these viruses.
2. Material and methods
The American Society for Testing and Materials (ASTM) international standard method E1052-11 for “Standard Test Method to Assess the Activity of Microbicides against Viruses in Suspension” was followed.
The challenge viruses, PIV3 (strain C243 ATCC VR-93) and CMV (strain ATCC-2011-8 ACTT VR-1788) were obtained from the American Type Culture Collection (ATCC). PIV3 was propagated in Vero cells (ATCC CCL-81) and CMV in MRC-5 cells (ATCC CCL-171). The test substances were honey and lemon AMC/DCBA lozenge, cherry menthol hexylresorcinol lozenge, placebo lozenge, AMC/DCBA as free active substances and hexylresorcinol as a free active substance (Table 1 ). A positive control (sodium hypochlorite) and negative control (dilution medium) were also tested.
Table 1.
Test substances and controls.
| Test substance | Contact time |
|---|---|
| AMC/DCBA lozenge | 1, 5 and 10 min |
| Strepsils® Honey and Lemon lozenge, containing 0.6 mg AMC and 1.2 mg DCBA, dissolved in 4.5 mL of artificial saliva⁎ | |
| |
| Placebo lozenge for AMC/DCBA experiments | 1 and 10 min |
| Placebo lozenge, dissolved in 4.5 mL artificial saliva⁎ | |
| |
| AMC/DCBA as free active substances | 1 and 10 min |
| 0.6 mg AMC and 1.2 mg DCBA dissolved in 4.5 mL artificial saliva⁎ | |
| Hexylresorcinol lozenge | 1, 5 and 10 min |
| Strepsils Extra Cherry lozenge, containing 2.4 mg hexylresorcinol and 4 mg menthol, dissolved in 4.5 mL of artificial saliva⁎ | |
| |
| Placebo lozenge for hexylresorcinol experiments | 1 and 10 min |
| Placebo lozenge, dissolved in 4.5 mL artificial saliva⁎ | |
| |
| Hexylresorcinol as a free active substance | 1 and 10 min |
| 2.4 mg hexylresorcinol as a free active substance, dissolved in 4.5 mL artificial saliva⁎ | |
| Positive control | 1 min |
| Sodium hypochlorite, 1000 ppm (1500 ppm for the hexylresorcinol experiments) | |
| Negative control | 10 min |
| Dilution medium (MEM + 3 μg/mL trypsin for the PIV3 experiments and MEM + 5% fetal bovine serum for the CMV experiments) | |
AMC, amylmetacresol; CMV, cytomegalovirus; DCBA, 2,4-dichlorobenzyl alcohol; MEM, minimum essential medium; PIV3, parainfluenza virus type 3.
4.2 g sodium bicarbonate, 0.5 g sodium chloride, and 0.2 g potassium carbonate was added to ∼900 mL of sterile deionized water. 3 g of bovine serum albumin was added to the solution and the pH adjusted to 6.5 ± 0.5. Sterile deionized water was added to reach a total volume of 1000 mL.
A 2.7 mL aliquot of each test substance (Table 1) was transferred into a 50 mL conical tube. Then 0.3 mL of the virus stock (titre of 106–108 TCID50/mL for PIV3 and 105.5–107.5 TCID50/mL for CMV) was added and mixed immediately (by vortexing) at ambient room temperature (21 °C). Different contact times (1, 5 or 10 min) were used for each test (Table 1). Upon completion of the contact time, the reaction mixture was immediately mixed (by vortexing) with an equal volume of neutralizer (RPMI medium + 10% newborn calf serum + 1% HEPES + 1% NaHCO3 for the AMC/DCBA and PIV3 experiments, MEM for the hexylresorcinol and PIV3 experiments and MEM + 10% fetal bovine serum + 1% HEPES + 1% NaHCO3 for the AMC/DCBA and CMV experiments). The quenched sample was serially diluted 10-fold with dilution medium. Serial dilutions were inoculated onto host cells in a 24-well plate (1 mL inoculum per well, n = 4 per dilution). The inoculated plates were incubated at 36 ± 2 °C in 5 ± 1% CO2 for 7 days for the AMC/DCBA and PIV3 experiments, 5–9 days for the hexylresorcinol and PIV3 experiments and 14 days for the AMC/DCBA and CMV experiments. After the incubation period, the titre of infectious virus was determined by TCID50 assay using the Spearman-Karber formula. The results from the negative control was used as the input viral load and compared with the test substances to evaluate the viral reduction by each test substance. Each experiment (for each test substance, each control and each contact time) was run in triplicate. The mean reductions in viral titre were compared between the test substances and the placebo lozenge using the two-sided Student t-test.
Additional control tests were also conducted to assess the effectiveness of the neutralizer and cytotoxicity of the test substances. A 2.7 mL aliquot of the test substance (each lozenge and each combination of free active substances) was mixed with 0.3 mL of dilution medium, incubated at ambient room temperature (21 °C) for each contact time, and then an equal volume of neutralizer was added. Following serial dilution of the reaction mixture in dilution medium, 100 μL of a low titred virus stock (containing no more than approximately 5000 units of virus) was added to 4.5 mL of each dilution and incubated at ambient room temperature (21 °C) for at least 10 min. These were then inoculated onto the host cells which were assessed for the presence of infectious virus at the end of the incubation period. To evaluate cytotoxicity, the condition of the host cells was recorded at the end of the incubation period.
The viability of the host cells and sterility of the cell culture medium were tested by inoculating control cells with medium during the incubation period of the study. The titre of the virus stock was confirmed by inoculating virus stock onto the host cells.
3. Results
3.1. Effects of AMC/DCBA on PIV3
AMC/DCBA lozenge exhibited a 2.43–2.68 log10 (mean 2.51 log10) reduction in viral titre at 1 min, 2.18–2.68 log10 (mean 2.51 log10) reduction at 5 min, and 2.43–3.40 log10 (mean 2.92 log10) reduction at 10 min (Fig. 1 ). Placebo lozenge exhibited 0–0.43 log10 (mean 0.14 log10) reduction at 1 min and 1.18–1.43 log10 (mean 1.35 log10) reduction at 10 min (Fig. 1). AMC/DCBA as free active substances exhibited 4.18–4.56 log10 (mean 4.31 log10) reduction at 1 min and 4.18–4.56 log10 (mean 4.43 log10) reduction at 10 min (Fig. 1).
Fig. 1.
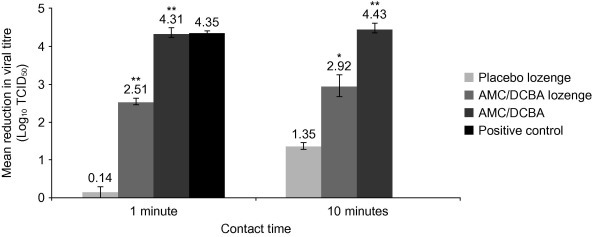
Effects of AMC/DCBA on PIV3. AMC/DCBA, amylmetacresol/2,4-dichlorobenzyl alcohol; PIV3, parainfluenza virus type 3; TCID50, tissue culture infectious dose 50%. *p < 0.01 and **p < 0.001 compared with placebo lozenge for the same contact time. Positive control was tested at 1 min contact time only (not 10 min). Results for 5 min contact time with AMC/DCBA lozenge not shown. The error bars indicate the standard error of the mean.
The positive control exhibited a complete inactivation of the virus within 1 min (Fig. 1).
The negative control did not exhibit any negative effect on the titre of virus or cell viability (data not shown). Neither the AMC/DCBA lozenge nor AMC/DCBA as free active substances exhibited any direct cell cytotoxicity within the assay (data not shown).
3.2. Effects of hexylresorcinol on PIV3
Hexylresorcinol lozenge exhibited a 4.80–5.30 log10 (mean 5.05 log10) reduction in viral titre at 1 min, ⩾5.22 log10 reduction at 5 and 10 min (Fig. 2 ). Placebo lozenge exhibited 1.55–2.55 log10 (mean 2.13 log10) reduction at 1 min and 1.80–2.55 log10 (mean 2.22 log10) reduction at 10 min (Fig. 2). Hexylresorcinol as a free active substance exhibited ⩾5.22 log10 reduction at 1 and 10 min (Fig. 2).
Fig. 2.
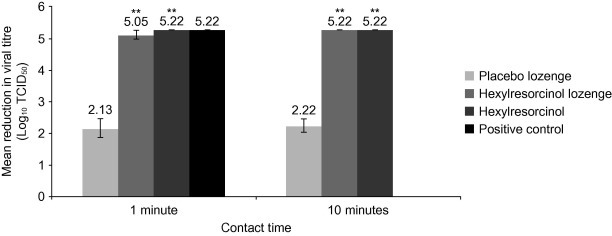
Effects of hexylresorcinol on PIV3. PIV3, parainfluenza virus type 3; TCID50, tissue culture infectious dose 50%. **p < 0.001 compared with placebo lozenge for the same contact time. Positive control was tested at 1 min contact time only (not 10 min). Results for 5 min contact time with hexylresorcinol lozenge not shown. The error bars indicate the standard error of the mean.
The effects of the positive control were similar to that of hexylresorcinol lozenge and hexylresorcinol as a free active substance (Fig. 2). The negative control did not exhibit any negative effect on the titre of virus or cell viability (data not shown). Neither the hexylresorcinol lozenge nor hexylresorcinol as a free active substance exhibited any direct cell cytotoxicity within the assay (data not shown).
3.3. Effects of AMC/DCBA on CMV
Both AMC/DCBA lozenge and AMC/DCBA as free active substances, as well as the positive control, exhibited a ⩾4.92 log10 reduction in viral titre at all time-points assessed (Fig. 3 ). Placebo lozenge exhibited 0.5–0.75 log10 (mean 0.58 log10) reduction at 1 min and 0.75–1.00 log10 (mean 0.83 log10) reduction at 10 min (Fig. 3).
Fig. 3.
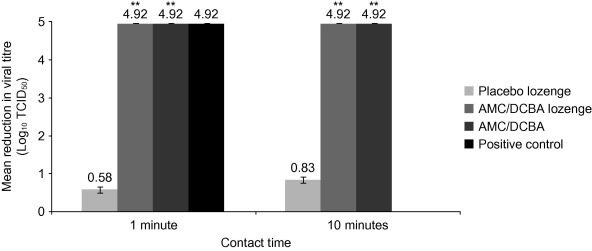
Effects of AMC/DCBA on CMV. AMC/DCBA, amylmetacresol/2,4-dichlorobenzyl alcohol; CMV, cytomegalovirus; TCID50, tissue culture infectious dose 50%. **p < 0.001 compared with placebo lozenge for the same contact time. Positive control was tested at 1 min contact time only (not 10 min). Results for 5 min contact time with AMC/DCBA lozenge not shown. The error bars indicate the standard error of the mean.
The negative control did not exhibit any negative effect on the titre of virus or cell viability (data not shown). Neither the AMC/DCBA lozenge nor AMC/DCBA as free active substances exhibited any direct cell cytotoxicity within the assay (data not shown).
4. Discussion
This study helps to further elucidate the action of long-standing active ingredients used for the relief of sore throat by showing that AMC/DCBA and hexylresorcinol exhibit virucidal activity, hexylresorcinol against PIV3 and AMC/DCBA against PIV3 and CMV in vitro.
There are four subtypes of PIV (PIV1, PIV2, PIV3 and PIV4), all of which can cause RTIs, although less is known about PIV4 (Henrickson, 2003). The prevalence and seasonality of PIV is reported to vary. In a study in China from 2009 to 2011, 3.7% of children and adults with RTI were positive for PIV (2.1% were positive for PIV3), with seasonal peaks of PIV3 and PIV1 occurring from April to July and September to November (Liu et al., 2013). In a study in Brazil from 2000 to 2010, 1.6% of children and adults with influenza-like illness were positive for PIV3, with the prevalence peaking in the second half of each year (Freitas, 2013). In contrast, in the USA from 1990 to 2004, seasonal peaks of PIV3 occurred in the spring, sometimes with a second smaller peak during the autumn (Fry et al., 2006). PIV can cause the symptom of sore throat (Bisno, 2001); in the study in China, 19% of patients with parainfluenza virus infection had the symptom of pharyngeal discomfort (Liu et al., 2013).
CMV, a member of the herpes virus family, infects and establishes latency in approximately 70% of the global population (Emery, 2012). Although primary CMV infection in healthy adults is usually asymptomatic or associated with a mild mononucleosis-like syndrome, CMV can cause severe, life-threatening disease and mortality in neonates and in immunocompromised adults (Lancini et al., 2014). CMV can also cause sore throat (Bisno, 2001). In a study of hospitalized non-immunocompromised adults with confirmed or presumed CMV, 9% had pharyngitis (Bonnet et al., 2001).
In this study, both the active lozenges and the free active substances (AMC/DCBA and hexylresorcinol) exhibited virucidal activity within 1 min, with minimal further virucidal effects beyond 1 min. A greater virucidal effect against PIV3 was observed with AMC/DCBA as free active substances than with AMC/DCBA lozenge, whereas hexylresorcinol as a free active substance had a similar effect to that of hexylresorcinol lozenge. Possible reasons include the binding of AMC and/or DCBA by an excipient in the AMC/DCBA lozenge, and/or interference or possible protective effect from an excipient in the lozenge. In sore throat treatments, hexylresorcinol is routinely used at a higher dose than either AMC or DCBA, and this may also be a factor.
This difference in virucidal effect between AMC/DCBA as free active substances and AMC/DCBA lozenge was seen with PIV3 but not with CMV, suggesting that the mechanism of virucidal action of AMC/DCBA lozenge is different for the two viruses. Also, the placebo lozenges for both the AMC/DCBA and hexylresorcinol experiments appeared to exert some virucidal activity, potentially due to the presence of tartaric acid in the placebo lozenge (for the AMC/DCBA experiments), osmotic pressure changes or stickiness induced by the sugar in the placebo lozenge (for both the AMC/DCBA and hexylresorcinol experiments) (Oxford et al., 2005) or due to the action of other excipients (e.g., menthol in the placebo lozenge for the hexylresorcinol experiments).
This study adds to the previous observations of in vitro virucidal activity of AMC/DCBA lozenge against RSV, influenza A virus and SARS-CoV by Oxford and colleagues (2005). These data suggest that AMC/DCBA and hexylresorcinol lozenges have the potential to inactivate free virus during the infection cycle, although the limitation of both studies is that the observed in vitro effects may not occur in vivo.
Phenols (AMC and hexylresorcinol) and alcohols (DCBA) are thought to disrupt lipid membranes, and alcohols can also cause rapid denaturation of proteins (McDonnell and Russell, 1999). The virucidal activity of these substances is likely to be due to their effects on viral lipid membranes or protein-lipid interaction (Oxford et al., 2005). Consistent with this theory, AMC/DCBA lozenge has thus far been shown to exert virucidal activity against enveloped viruses but not as yet against non-enveloped viruses (Oxford et al., 2005).
5. Conclusions
The findings of this study as well as previous research (Oxford et al., 2005) suggest that AMC/DCBA lozenge and hexylresorcinol lozenge have the potential to have local antiviral effects in patients with sore throat due to viral RTIs. In addition, both lozenges have demonstrated efficacy against sore throat symptoms in clinical trials (McNally et al., 2010, McNally et al., 2012, Wade et al., 2011). Taken together, these data support the use of these lozenges as a first-line treatment for sore throat caused by viral RTIs.
Further investigation into the mechanisms of action and the clinical effects of AMC/DCBA and hexylresorcinol for the control of RTIs is warranted. Use of products containing such active ingredients, which not only relieve symptoms but also help treat the infectious cause of RTIs, should help to curb patient demand for antibiotics by satisfying their need for treatment of infection and hence reduce the burden of antibiotic resistance.
Funding source
This study was funded by Reckitt Benckiser Healthcare Ltd. The authors, who are employees of Reckitt Benckiser Healthcare Ltd, were involved in the study design, analysis and interpretation of data, and took the decision to submit this article for publication. Medical writing assistance for this article was funded by Reckitt Benckiser Healthcare Ltd.
Conflicts of interest
Adrian Shephard and Stela Zybeshari are employees of Reckitt Benckiser Healthcare Ltd.
Acknowledgments
This study was conducted by MicroBioTest Division of Microbac Laboratories, Inc. (Sterling, VA, USA). Statistical analyses were conducted by Bartosz Jenner of Reckitt Benckiser (Hull, East Yorkshire, UK). Medical writing assistance was provided by Papia Das of Elements Communications Ltd (Westerham, Kent, UK).
Glossary
- Enveloped viruses
Viruses that have an outer lipid membrane that contains viral proteins; non-enveloped viruses lack this membrane
- Serial dilution
The repeated dilution of a solution where the dilution factor is usually constant, for example, a 10-fold serial dilution involves diluting a solution 1:10 so it is one-tenth the concentration of the original solution, then diluting 1:10 again so that it is one-hundredth of the original solution, and so on
- Spearman–Karber formula
A formula used to determine viral titre: m = xk + (d/2) − d∑pi, where m = log10 of the titre relative to the test volume; xk = log10 of the smallest dosage which induces infection in all cultures; d = log10 of the dilution factor; pi = the proportion of positive results at each dilution
- TCID50
The amount of virus required to kill 50% of inoculated tissue culture cells
References
- Barnett M.L., Linder J.A. Antibiotic prescribing to adults with sore throat in the United States, 1997–2010. JAMA Intern. Med. 2014;174(1):138–140. doi: 10.1001/jamainternmed.2013.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisno A.L. Acute pharyngitis. N. Engl. J. Med. 2001;344(3):205–211. doi: 10.1056/NEJM200101183440308. [DOI] [PubMed] [Google Scholar]
- Bonnet F., Neau D., Viallard J.F., Morlat P., Ragnaud J.M., Dupon M., Legendre P., Imbert Y., Lifermann F., Le Bras M., Beylot J., Longy-Boursier M. Clinical and laboratory findings of cytomegalovirus infection in 115 hospitalized non-immunocompromised adults. Ann. Med. Interne (Paris) 2001;152(4):227–235. [PubMed] [Google Scholar]
- Buchholz V., Leuwer M., Ahrens J., Foadi N., Krampfl K., Haeseler G. Topical antiseptics for the treatment of sore throat block voltage-gated neuronal sodium channels in a local anaesthetic-like manner. Naunyn Schmiedebergs Arch. Pharmacol. 2009;380(2):161–168. doi: 10.1007/s00210-009-0416-x. [DOI] [PubMed] [Google Scholar]
- Collier L.H., Oxford J.S. Oxford University Press; 2000. Human Virology; pp. 231–232. [Google Scholar]
- Dasaraju P.V., Liu C. Infections of the respiratory system. In: Baron S., editor. fourth ed. University of Texas Medical Branch at Galveston; Galveston (TX): 1996. (Medical Microbiology). Chapter 93. [PubMed] [Google Scholar]
- Denny F.W., Jr. The clinical impact of human respiratory virus infections. Am. J. Respir. Crit. Care Med. 1995;152(4 Pt 2):S4–S12. doi: 10.1164/ajrccm/152.4_Pt_2.S4. [DOI] [PubMed] [Google Scholar]
- Eccles R. Mechanisms of symptoms of the common cold and influenza. Br. J. Hosp. Med. (Lond.) 2007;68(2):71–75. doi: 10.12968/hmed.2007.68.2.22824. [DOI] [PubMed] [Google Scholar]
- European Commission. Special Eurobarometer 407, antimicrobial resistance report. November 2013. Available at: http://ec.europa.eu/health/antimicrobial_resistance/docs/ebs_407_en.pdf. Last accessed September 2015.
- Emery V.C. Cytomegalovirus: recent progress in understanding pathogenesis and control. QJM. 2012;105(5):401–405. doi: 10.1093/qjmed/hcr262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essack S., Pignatari A.C. A framework for the non-antibiotic management of upper respiratory tract infections: towards a global change in antibiotic resistance. Int. J. Clin. Pract. 2013;67(Suppl. 180):4–9. doi: 10.1111/ijcp.12335. [DOI] [PubMed] [Google Scholar]
- Farrer F. Sprays and lozenges for sore throats. S. Afr. Pharm. J. 2011;78(4):26–31. [Google Scholar]
- Foadi N., de Oliveira R.C., Buchholz V., Stoetzer C., Wegner F., Pilawski I., Haeseler G., Leuwer M., Ahrens J. A combination of topical antiseptics for the treatment of sore throat blocks voltage-gated neuronal sodium channels. Naunyn Schmiedebergs Arch. Pharmacol. 2014;387(10):991–1000. doi: 10.1007/s00210-014-1016-y. [DOI] [PubMed] [Google Scholar]
- Freitas F.T. Sentinel surveillance of influenza and other respiratory viruses, Brazil, 2000–2010. Braz. J. Infect. Dis. 2013;17(1):62–68. doi: 10.1016/j.bjid.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A.M., Curns A.T., Harbour K., Hutwagner L., Holman R.C., Anderson L.J. Seasonal trends of human parainfluenza viral infections: United States, 1990–2004. Clin. Infect. Dis. 2006;43(8):1016–1022. doi: 10.1086/507638. [DOI] [PubMed] [Google Scholar]
- Goossens H., Ferech M., Vander Stichele R., Elseviers M., ESAC Project Group Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- Gulliford M.C., Dregan A., Moore M.V., Ashworth M., van Staa T., McCann G., Charlton J., Yardley L., Little P., McDermott L. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open. 2014;4(10):e006245. doi: 10.1136/bmjopen-2014-006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrickson K.J. Parainfluenza viruses. Clin. Microbiol. Rev. 2003;16(2):242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancini D., Faddy H.M., Flower R., Hogan C. Cytomegalovirus disease in immunocompetent adults. Med. J. Aust. 2014;201(10):578–580. doi: 10.5694/mja14.00183. [DOI] [PubMed] [Google Scholar]
- Liu W.K., Liu Q., Chen D.H., Liang H.X., Chen X.K., Huang W.B., Qin S., Yang Z.F., Zhou R. Epidemiology and clinical presentation of the four human parainfluenza virus types. BMC Infect. Dis. 2013;13:28. doi: 10.1186/1471-2334-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12(1):147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally D., Simpson M., Morris C., Shephard A., Goulder M. Rapid relief of acute sore throat with AMC/DCBA throat lozenges: randomised controlled trial. Int. J. Clin. Pract. 2010;64(2):194–207. doi: 10.1111/j.1742-1241.2009.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally D., Shephard A., Field E. Randomised, double-blind, placebo-controlled study of a single dose of an amylmetacresol/2,4-dichlorobenzyl alcohol plus lidocaine lozenge or a hexylresorcinol lozenge for the treatment of acute sore throat due to upper respiratory tract infection. J. Pharm. Pharm. Sci. 2012;15(2):281–294. doi: 10.18433/j31309. [DOI] [PubMed] [Google Scholar]
- Oxford J.S., Lambkin R., Gibb I., Balasingam S., Chan C., Catchpole A. A throat lozenge containing amyl meta cresol and dichlorobenzyl alcohol has a direct virucidal effect on respiratory syncytial virus, influenza A and SARS-CoV. Antivir. Chem. Chemother. 2005;16(2):129–134. doi: 10.1177/095632020501600205. [DOI] [PubMed] [Google Scholar]
- Richards R.M., Cowie G., McCague G.J. In vivo investigations of the antibacterial activity of lozenges and mouthwashes on the aerobic bacterial flora of the mouth and throat. Pharm. J. 1989;242:659–663. [Google Scholar]
- Richards R.M., Xing D.K. In vitro evaluation of the antimicrobial activities of selected lozenges. J. Pharm. Sci. 1993;82(12):1218–1220. doi: 10.1002/jps.2600821207. [DOI] [PubMed] [Google Scholar]
- Shulman S.T., Bisno A.L., Clegg H.W., Gerber M.A., Kaplan E.L., Lee G., Martin J.M., Van Beneden C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012;55(10):1279–1282. doi: 10.1093/cid/cis847. [DOI] [PubMed] [Google Scholar]
- van der Velden A., Duerden M.G., Bell J., Oxford J.S., Altiner A., Kozlov R., Sessa A., Pignatari A.C., Essack S.Y. Prescriber and patient responsibilities in treatment of acute respiratory tract infections — essential for conservation of antibiotics. Antibiotics. 2013;2(2):316–327. [Google Scholar]
- Wade A.G., Morris C., Shephard A., Crawford G.M., Goulder M.A. A multicentre, randomised, double-blind, single-dose study assessing the efficacy of AMC/DCBA Warm lozenge or AMC/DCBA Cool lozenge in the relief of acute sore throat. BMC Fam. Pract. 2011;12:6. doi: 10.1186/1471-2296-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance, 2014 Summary. Available at: http://apps.who.int/iris/bitstream/10665/112647/1/WHO_HSE_PED_AIP_2014.2_eng.pdf?ua=1. Last accessed September 2015.
- Worrall G. Acute sore throat. Can. Fam. Physician. 2011;57(7):791–794. [PMC free article] [PubMed] [Google Scholar]


