Abstract
Coronaviruses (CoVs) are critical human and animal pathogens because of their potential to cause severe epidemics of respiratory or enteric diseases. In pigs, the newly emerged porcine deltacoronavirus (PDCoV) and re-emerged porcine epidemic diarrhea virus (PEDV) reported in the US and Asia, as well as the discovery of novel CoVs in wild bats or birds, has necessitated development of improved detection and control measures for these CoVs. Because the previous pancoronavirus (panCoV) RT-PCR established in our laboratory in 2007–2011 did not detect deltacoronaviruses (δ-CoVs) in swine fecal and serum samples, our goal was to develop a new panCoV RT-PCR assay to detect known human and animal CoVs, including δ-CoVs. In this study, we designed a new primer set to amplify a 668 bp-region within the RNA-dependent RNA polymerase (RdRP) gene that encodes the most conserved protein domain of α-, β-, γ-, and δ-CoVs. We established a one-step panCoV RT-PCR assay and standardized the assay conditions. The newly established panCoV RT-PCR assay was demonstrated to have a high sensitivity and specificity. Using a panel of 60 swine biological samples (feces, intestinal contents, and sera) characterized by PEDV, PDCoV and transmissible gastroenteritis virus-specific RT-PCR assays, we demonstrated that sensitivity and specificity of the newly established panCoV RT-PCR assay were 100%. 400 avian fecal (RNA) samples were further tested simultaneously for CoV by the new panCoV RT-PCR and a one-step RT-PCR assay with the δ-CoV nucleocapsid-specific universal primers. Four of 400 avian samples were positive for CoV, three of which were positive for δ-CoV by the conventional RT-PCR. PanCoV RT-PCR fragments for 3 of the 4 CoVs were sequenced. Phylogenetic analysis revealed the presence of one γ-CoV and two δ-CoV in the sequenced samples. The newly designed panCoV RT-PCR assay should be useful for the detection of currently known CoVs in animal biological samples.
Keywords: Coronaviruses, Pancoronavirus RT-PCR, Degenerate primers, RNA-dependent RNA polymerase (RdRP) gene
1. Introduction
Coronaviruses (CoVs) are positive-sense single-stranded RNA viruses that belong to the family Coronaviridae in the order Nidovirales. The family Coronaviridae is genetically and antigenically divided into four genera: Alphacoronavirus (α-CoV), Betacoronavirus (β-CoV), Gammacoronavirus (γ-CoV), and Deltacoronavirus (δ-CoV) (International Committee on Taxonomy of Viruses and King, 2012). The epidemiological investigation and phylogenetic analysis of CoVs revealed that bats are the likely natural host for the α-CoVs and β-CoVs, while birds are the suspect host for γ-CoVs and δ-CoVs (Bolles et al., 2011; Woo et al., 2012). To date, there are six CoVs that cause respiratory tract illness in humans, including α-CoVs, human CoV (HCoV)-NL63 and HCoV-229E, and β-CoVs HCoV-OC43, HKU1, the severe acute respiratory syndrome coronavirus (SARS-CoV), and the middle east respiratory syndrome coronavirus (MERS-CoV) (Lu et al., 2015). In pigs, there are also two α-CoVs and one δ-CoV that cause enteric disease: porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), and porcine deltacoronavirus (PDCoV), respectively (Jung et al., 2016). The other swine α-CoV, porcine respiratory coronarivurs (PRCV), and the swine β-CoV, porcine hemagglutinating encephalomyelitis virus (PHEV), induce respiratory and neurological diseases in pigs, respectively (Saif et al., 2012).5
Among animals, pigs are susceptible to different α-, β-, and δ-CoVs. In particular, porcine epidemic diarrhea (PED), induced by novel variants of the α-CoV PEDV has re-emerged or emerged with devastating impact on piglets in China since late 2010, in North (2013) and South (2014) Americas, and in Europe in 2015 (Jung and Saif, 2015; Langel et al., 2016; Lohse et al., 2017). More recently, the newly emerging δ-CoV, PDCoV, was identified in diarrheic pigs worldwide in 2014–2015, including the US, Canada, China, South Korea, and Thailand (Dong et al., 2016; Hu et al., 2016; Jung et al., 2015). In pigs, the newly emerged PDCoV and re-emerged PEDV reported in the US and Asia, as well as the discovery of novel CoVs in wild bats, birds or humans has necessitated improved detection and control measures for these CoVs (Jung et al., 2016; Lin et al., 2016).
Cross-species transmission plays a key role in CoV evolution, and novel epidemic CoVs can emerge unexpectedly (Hon et al., 2008; Huang et al., 2016). Thus, the development of molecular methods to detect a broad-range of CoVs, including previously unidentified CoVs, in biological samples from animals is a critical initial step to prepare for potential epidemics by newly emerging CoVs. Previously, a one-step RT-PCR with the pancoronavirus (panCoV)-specific forward (IN-2deg; GGGDTGGGAYTAYCCHAARTGYGA) and reverse (IN-4deg; TARCAVACAACISYRTCRTCA) universal primers targeting a 452-bp fragment of the polymerase gene was used to detect a bovine-like β-CoV in the diarrheic feces of a giraffe (Hasoksuz et al., 2007) and an α-CoV in mink feces (Vlasova et al., 2011). Our preliminary studies revealed that the previously established panCoV RT-PCR assay failed to detect the newly emerged PDCoV in the diarrheic pig feces, although the feces were positive for PDCoV RNA by PDCoV-specific RT-PCR. However testing for α-CoVs (PEDV and TGEV), β-CoV (bovine enteric CoV; BCoV), and γ-CoVs [avian infectious bronchitis (IBV), and turkey CoV (TCoV)] was positive.
Therefore, it was necessary to establish an updated panCoV RT-PCR assay to detect known α-, β-, γ-, and δ-CoVs, including PDCoV. In this study, we developed a new primer set designed to amplify a 668 bp-region within the RNA-dependent RNA polymerase (RdRP) gene of α-, β-, γ-, and δ-CoVs and established a one-step panCoV RT-PCR assay. This newly designed panCoV RT-PCR assay was further evaluated by detecting the CoVs in swine and avian fecal samples collected from the field.
2. Materials and methods
2.1. Viruses and viral RNA stocks
The PDCoV strain OH-FD22 was propagated in LLC-PK cells (Hu et al., 2015). Other viruses or viral RNA stocks were obtained after their inoculation into appropriate cell lines or embryonated turkey or chicken eggs as follows: PEDV (PC22A strain) was propagated in Vero cells (Oka et al., 2014); TGEV (virulent Miller-M6 strain) in ST cells (Saif et al., 2012); BCoV (Mebus strain) in human rectal tumor (HRT)-18 cells (Hasoksuz et al., 2002); TCoV (IN strain) in embryonated turkey eggs and IBV (Massachusetts strain) in embryonated chicken eggs (Ismail et al., 2001). Archival viral RNA samples from porcine rotavirus groups (Rota) A–C, porcine caliciviruses (noroviruses, sapoviruses, and St-Valerien-like viruses), human enteric coronavirus (HECoV, 4408 strain), Canine Coronaviruses (CCoV, UCD1 strain), Feline infectious peritonitis virus (FIPV, type 2 strain 79-1146), Feline coronavirus (FECoV, UCD strain), and bovine torovirus (BToV, B06143 strain) were identified previously by our laboratory, were also used to evaluate the specificity of the panCoV RT-PCR assay.
2.2. Samples and RNA extraction
Sixty clinical samples (30 intestinal contents from nursing piglets, and 20 feces and 10 sera from sows) were collected on different pig farms with diarrhea outbreaks during 2015–2016 in Henan, China, and were tested previously for PEDV, TGEV, PDCoV using the established virus-specific RT-PCR assays (Hu et al., 2015; Kim et al., 2000; Song et al., 2015). In these selected 60 swine clinical samples, 30 were positive for PEDV, PDCoV, or TGEV (one positive sample only included one CoV virus); the other 30 samples were negative for all 3 viruses (Table 1 ). The original fecal and intestinal samples were diluted 10-fold with phosphate-buffered saline (PBS), vortexed and centrifuged at 1847 × g at 4 °C for 20 min. The supernatant was collected and used to extract viral RNA. Porcine sera were used to extract viral RNA. The total RNA was extracted by RNAplus Reagent (TaKaRa, Japan) following the manufacturer’s instructions. The viral RNA was eluted with 50 μl of RNase-free water and was used as template for one-step panCoV RT-PCR.
Table 1.
Detection of porcine enteric coronaviruses (CoVs) in intestinal contents and fecal or serum samples of diarrheic piglets or sows in Henan, China by the newly designed panCoV RT-PCR assay compared with prior virus-specific RT-PCR.
| Samplesa | Pig age | Sample No. | virus-specific RT-PCR (% positive) |
PanCoV RT-PCR (% positive) | Sensitivity/Specififcity (%) | ||
|---|---|---|---|---|---|---|---|
| PDCoVb | PEDVc | TGEVd | CoVs | ||||
| Intestinal content | piglets | 30 | 6 | 14 | 2 | 22 | 100/100 |
| Feces | sow | 20 | 5 | 1 | 0 | 6 | 100/100 |
| Serum | sow | 10 | 2 | 0 | 0 | 2 | 100/100 |
| Total | 60 | 13 (21.7) | 15 (25.0) | 2 (3.3) | 30 | 100/100 | |
Samples were already tested for PEDV, PDCoV, and TGEV by virus-specific RT-PCR, and 30 samples were only positive for PEDV, or PDCoV, or TGEV, and the other 30 samples were negative for swine coronaviruses.
PDCoV was tested by virus-specific RT-PCR (Hu et al., 2015).
PEDV was tested by virus-specific RT-PCR (Song et al., 2015).
TGEV was tested by virus-specific RT-PCR (Kim et al., 2000).
Four hundred avian fecal RNA samples that originated from wild birds such as mallard and water fowl in Ohio, previously tested for the presence of avian influenza virus (AIV) (provided by Dr. Bowman, Ohio State University, Columbus, OH), were also used to screen for avian CoVs by using the new panCoV RT-PCR assay.
2.3. Primer design and synthesis for PanCoV RT-PCR assay
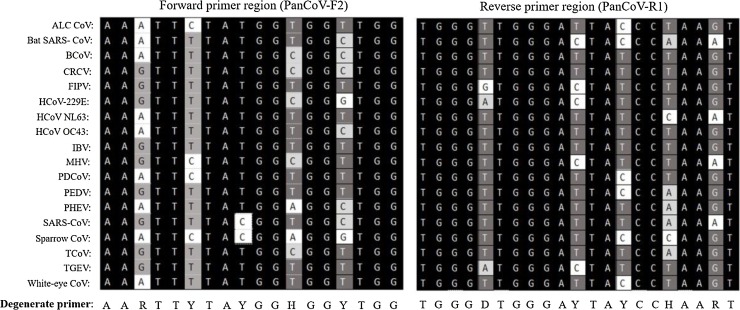
New primers were designed by targeting the RdRp gene that encodes the most conserved protein domain of α-, β-, γ-, and δ-CoVs. The RdRp sequences with full information on isolation/detection/submission of the CoVs were obtained from the GenBank database. The 18 representative CoVs were selected on the basis of their genus (Fig. 1 ). Multiple alignment of nucleotide and amino acid sequences of the RdRp gene from the 18 CoVs was performed by DNAStar 7.0 green (DNAstar, Madison, WI). Using the codon table of the International Union of Pure and Applied Chemistry (IUPAC) system for degenerate bases, a pair of consensus primers was designed and synthesized for amplification of a 668-bp fragment: panCoV- F2 (5′-AARTTYTAYGGHGGYTGG-3′) and panCoV- R1 (5′- GARCARAATTCATGHGGDCC-3′) (Fig. 1).
Fig. 1.
Design of degenerate primers for the new pancoronavirus RT-PCR assay based on alignment of nucleotide sequences of a conserved region within the RNA-dependent RNA polymerase gene of 18 coronaiviruses that are representative of α-, β-, γ-, and δ-CoVs. The forward (panCoV-F2) and reverse (panCoV-R1) primer sequences are shown at the bottom (Y = C/T, W = A/T, V = A/C/G, R = A/G, H = A/T/C, N = A/C/T/G). The 18 coronavirus sequences used in the current study are available from GenBank under the following accession numbers: Asian leopard cats (ALC) CoV, EF584908; bat SARS-CoV, DQ249235; bovine coronavirus (BCoV), AF391541; canine respiratory coronavirus (CRCV), JX860640; feline infectious peritonitis virus (FIPV), KC461235; human coronavirus (HCoV) 229E, AF304460; HCoV NL-63, AY567487; HCoV OC43, AY391777; infectious bronchitis virus (IBV), KC008600; murine hepatitis virus (MHV), X51939; porcine deltacoronavirus (PDCoV), JQ065043; porcine epidemic diarrhea virus (PEDV), NC_003436; porcine hemagglutinating encephalomyelitis virus (PHEV), NC_007732; SARS-CoV, AY864806; sparrow CoV, NC_016992; turkey coronavirus (TCoV), NC_010800; transmissible gastroenteritis virus (TGEV), DQ811789; white eye CoV, NC_016991.
2.4. Amplification conditions for one-step panCoVs RT-PCR assay
The one-step panCoV RT-PCR assay was performed with the QIAgen OneStep RT-PCR kit (Valencia, CA, USA) in a GeneAmp PCR system 9600 thermal cycler (Applied Biosystems, Foster City, CA, USA). The RT-PCR system (25 μL) included 5 × QIAGEN OneStep RT-PCR buffer (5 μL), dNTP (1 μL, final concentration of 400 μM of each dNTP), upstream and downstream primers (25 μmol/L, each 1 μL), RNAsin (40 μ/μl, 0.25 μL), enzyme mix (1 μL), RNA template (2 μL), with the final volume was made up with RNase-free water to 25 μL. The reaction was conducted with an initial reverse transcription step at 50 °C for 30 min, followed by PCR activation at 95 °C for 15 min, 35 cycles of amplification (40 s at 94 °C, 40 s at 52 °C, 1 min at 72 °C), and a final extension step at 72 °C for 10 min. Negative controls in this assay were a supernatant from uninfected LLC-PK cells and RNase-free water. PCR products were stained with ethidium bromide and visualized on agarose gels using UV-light.
The PCR products were purified using the QIAquick gel extraction kit (Valencia, CA, USA). Both strands of the PCR products were sequenced twice with an ABI Prism 3700 DNA analyzer (Applied Biosystems, Foster City, CA) by using the forward and reverse PCR primers. The sequences of the PCR products were compared with the known RdRp gene sequences of CoVs in the GenBank database.
2.5. Sensitivity and specificity of the panCoV RT-PCR assay
To determine the sensitivity of panCoV RT-PCR assay, the RdRp genes were amplified from PDCoV, PEDV and TGEV RNAs by using panCoV RT-PCR assay and the RT-PCR products were cloned into E. coli DH5 using the pMD18-T vector (Takara, China). The plasmids with the RdRp genes of PDCoV, PEDV and TGEV were confirmed by sequencing. The positive plasmid templates were diluted 10-fold (from 1 × 107 to 1 × 101 copies/μL) and amplified by panCoV PCR assays.
To determine the specificity of the panCoV RT-PCR assay, the RNA samples isolated from the CoVs (PEDV, TGEV, BCoV, TCoV, IBV, PDCoV, HECoV, CCoV, FIPV, FECoV) and some other enteric viruses, including BToV and porcine rotavirus (A–C) and porcine caliciviruses (noroviruses, sapoviruses, and St-Valerien-like viruses), were tested by using the newly designed panCoV RT-PCR assay.
2.6. Detection of CoV RNA in clinical samples by panCoV RT-PCR assay
Sixty swine intestinal contents, fecal or serum samples were retested for PDCoV, PEDV, or TGEV RNA in this experiment by virus-specific RT-PCR as published previously (Hu et al., 2015; Kim et al., 2000; Song et al., 2015). Of the 60 samples, 30 (22 intestinal contents from piglets, 6 fecal samples from sows, 2 serum samples from sows) were positive for either PEDV, TGEV, or PDCoV RNA. The other 30 intestinal contents or fecal or serum samples were negative for CoV RNA (Table 1). These samples were then tested by the new panCoV RT-PCR assay to evaluate sensitivity and specificity of the new panCoV. The avian fecal viral RNA samples were tested for CoV RNA by the panCoV RT-PCR assay, and were also tested for δ-CoV simultaneously using a one-step RT-PCR assay with the δ-CoV nucleocapsid-specific universal primers (UDCoVF: 5′-RYWGAYKSNTCNTGGTTYCA-3′ and UDCoVR: 5′- HGTGCCWGTRTARTARAAGG-3′) targeting a 194-bp fragment, with the same amplification conditions as the one-step panCoV RT-PCR assay (Vlasova and Saif, unpublished). Visually positive PCR products or DNA fragments were further sequenced by using the panCoV primers, and the sequence data were subjected to phylogenetic analysis according to our previously reported methods (Hu et al., 2015).
3. Results
3.1. Selection of the RdRp gene and design of a RT-PCR primer set for detection of panCoV
The most conserved regions of the RdRp gene of α-, β-, γ-, and δ-CoVs were selected as candidates to design PCR primers; among them, one primer set was selected because it had the lowest numbers of degenerate nucleotides (nt). Forward and reverse primers consisting of 18 and 21 nt, respectively, each contained 5 nt degenerate cores, and the expected amplicon size was 668 bp (Fig. 1).
3.2. Evaluation of specificity and sensitivity of the newly designed panCoV RT-PCR assay
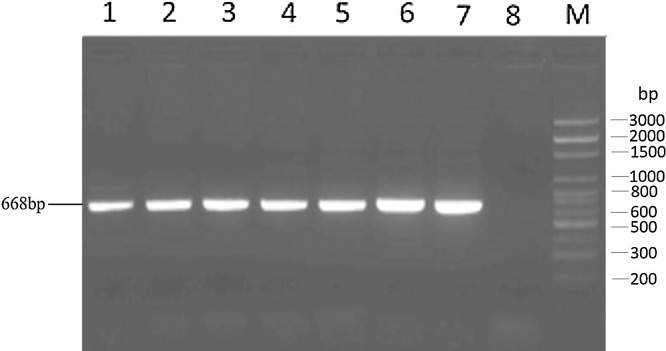
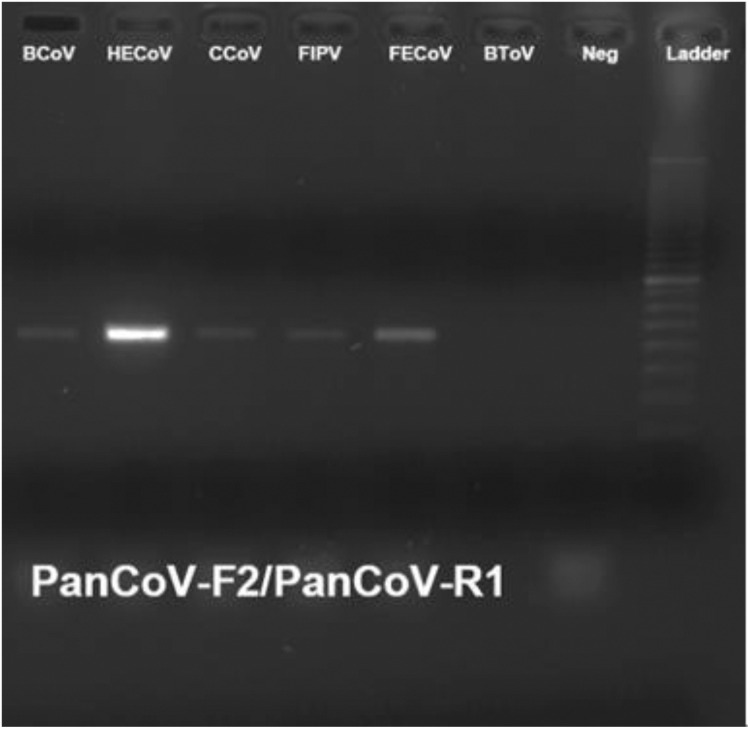
The reactivity of the newly designed panCoV RT-PCR assay was investiged by testing RNA samples isolated from the following selected animal α-, β-, γ-, and δ-CoVs: PEDV, TGEV, BCoV, TCoV, IBV, PDCoV, HECoV, CCoV, FIPV, and FECoV. The PCR amplicons of the expected size of 668-bp were observed for all the CoVs tested (Fig. 2, Fig. 3 ). Cell-culture grown PDCoV at different cell passages (5 and 80) was tested by panCoV RT-PCR assay and confirmed positive, regardless of cell-culture passage number. All the PCR amplicons were purified and sequenced, and the DNA sequences shared 100% homology with the analogous gene sequences available in GenBank (data not shown). No amplification products were observed for the negative controls (cell culture medium and water) and RNA samples from the following selected enteric viruses: BToV, porcine rotavirus (A–C) and porcine caliciviruses (noroviruses, sapoviruses, and St-Valerien-like viruses).
Fig. 2.
Gel electrophoresis results of PCR products by the newly designed panCoV one-step RT-PCR assay. The amplified bands correspond with the expected amplicon size (668 bp). Lane 1, transmissible gastroenteritis virus (TGEV, strain virulent Miller-M6); lane 2, porcine epidemic diarrhea virus (PEDV, strain PC22A); lane 3, porcine deltacoronavirus (PDCoV) (strain OH-FD22; cell culture passage number, 80); lane 4, PDCoV (strain OH-FD22; cell culture passage number, 5); lane 5, turkey coronavirus (strain IN); lane 6, infectious bursal disease virus (IBV, strain Massachusetts); lane 7, bovine enteric coronavirus (BCoV, strain Mebus); and lane 8, negative control; lane M, DNA ladder 3000 bp marker.
Fig. 3.
The specificity of the panCoV RT-PCR assays. The amplified bands correspond with the expected amplicon size (668 bp). BCoV: bovine enteric coronavirus (strain Mebus); HECoV: human enteric coronavirus (4408 strain), CCoV: canine coronaviruses (UCD1 strain); FIPV: feline infectious peritonitis virus (type 2 strain 79-1146), FECoV: feline coronavirus (UCD strain); BToV: bovine torovirus (B06143 strain); Neg: negative control; Ladder: DNA ladder 3000 bp marker.
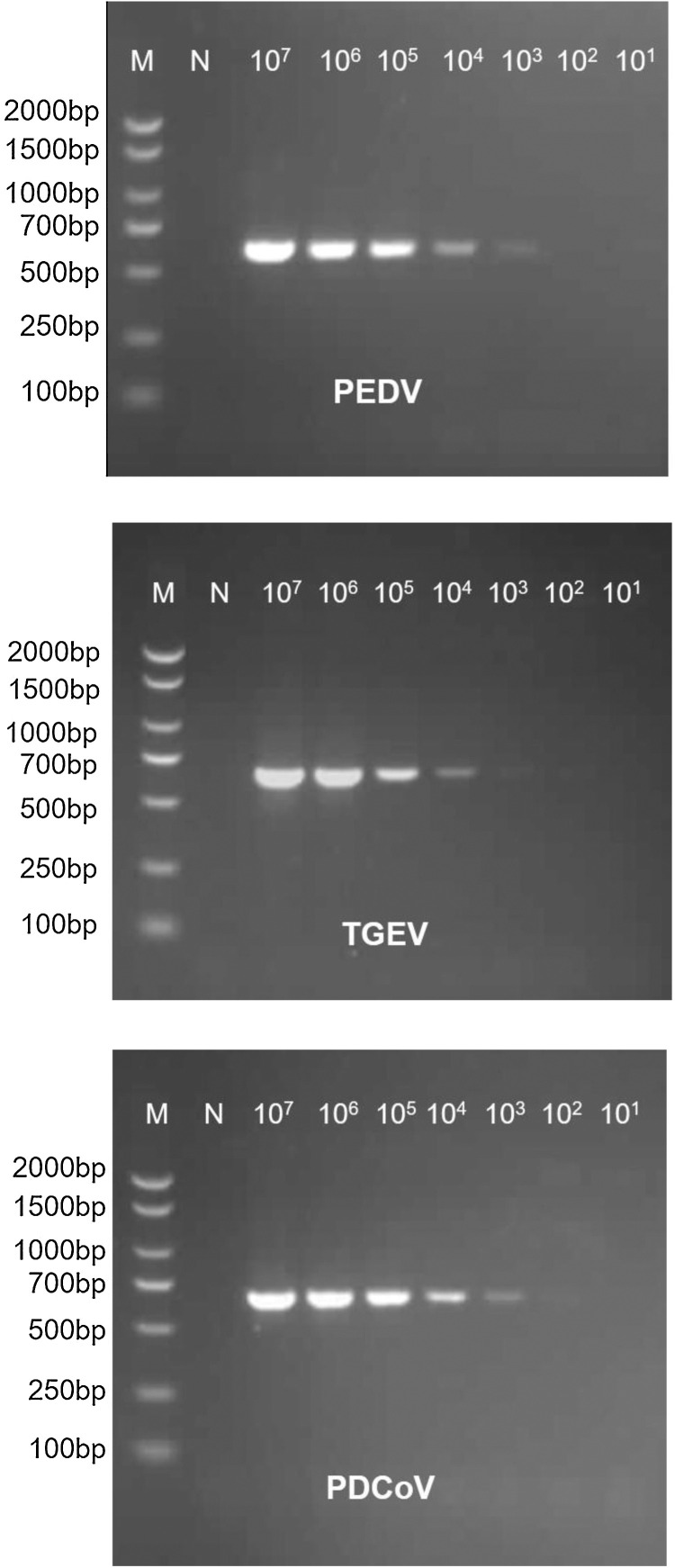
The sensitivity of the panCoV RT-PCR assay was determined using 10-fold dilutions of the recombinant plasmids including the RdRp genes of PDCoV, PEDV or TGEV (1 × 107–1 × 101 copies/μL of dilution). The detection sensitivity of panCoV RT-PCR was 1 × 103 copies/μL for PDCoV, 1 × 103 copies/μL for PEDV, and 1 × 104 copies/μL for TGEV (Fig. 4 ).
Fig. 4.
The sensitivity of the panCoV RT-PCR assays. The RdRp genes were amplified from recombinant plasmids that subcloned the RdRp genes of PDCoV, PEDV and TGEV into the pGEM-T Easy vector. The detection limit of the panCoV RT-PCR assay was determined through 10-fold dilutions of each plasmid (from 1 × 107 to 1 × 101 copies/μL) and amplified by panCoV RT-PCR assays. Lane M, DNA marker DL2000; Lane N: negative control; Lanes 107–101: recombinant plasmids of PDCoV, PEDV, and TGEV with 10-fold dilutions (1 × 107–1 × 101 copies/μL).
3.3. Use of the panCoV RT-PCR for swine fecal or blood samples
The 60 clinical samples from diarrheic pigs were tested by the newly designed panCoV RT-PCR to evaluate its performance (Table 1). By panCoV RT-PCR, 30 of 60 samples tested (50%) were positive for CoV RNA: 22 of 30 (73%) intestinal contents of diarrheic piglets; 6 of 20 (30%) fecal samples of diarrheic sows; and 2 of 10 (20%) serum samples of diarrheic sows (Table 1). The samples were also tested for PEDV, TGEV, or PDCoV RNA by virus-specific RT-PCRs. As shown in Table 1, sensitivity and specificity of the the new panCoV were 100%.
3.4. CoV detection by panCoV RT-PCR and phylogenetic analysis of the RdRp genes of CoVs
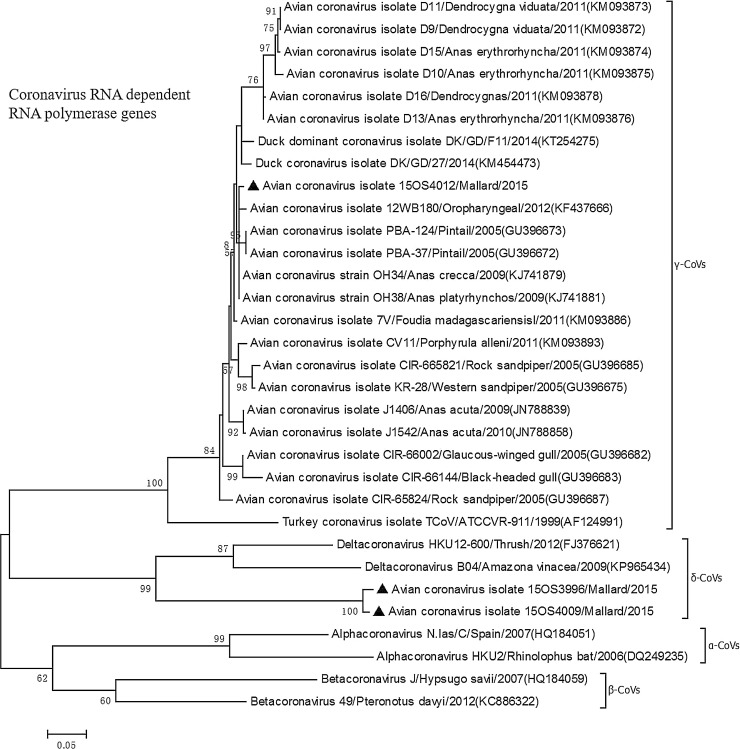
Four-hundred avian fecal RNA samples were tested for CoV RNA by the newly designed panCoV RT-PCR assay and also tested for δ-CoV using a one-step RT-PCR assay simultaneously. Only 1% (4 samples) from mallards were positive for CoV and 3 samples were positive for δ-CoV. Three of 4 CoV positive samples were sequenced, and the 3 RdRp gene nt sequences and others of seletected avian-original α-, β-, γ-, and δ-CoVs available in GeneBank were subjected to phylogenetic analysis (Fig. 5 ). The results revealed that one of the three was an avian γ-CoV, whereas the other two clustered with avian δ-CoVs. The 2 sequenced avian δ-CoV strains (15OS3996 and 15OS4009) had the closest relationship with wild avian δ-CoV strains from thrush, amazona vinacea and hypsugo savii. While the sequenced avian γ-CoV (strain 15OS4012) was clustered with the anas (duck)-original γ-CoVs.
Fig. 5.
Phylogenetic analysis of nucleotide (nt) sequences of RNA-dependent RNA polymerase (RdRP) gene of 3 coronaviruses (CoVs) identified in avian fecal samples in this study (indicated as triangles) with other RdRp gene nt sequences of seletected α-, β-, γ-, and δ-CoVs available in GeneBank. The reference sequences are indicated as strain names and accession numbers. The tree was constructed using the maximum likelihood method of the software MEGA6.06 (http://www.megasoftware.net). Bootstrap analysis was carried out on 1000 replicate data sets, and values were indicated adjacent to the branching points. The scale bar represents 0.05 nucleotide substitutions per site.
4. Discussion
Besides the known swine enteric CoVs, PEDV and TGEV, a new swine enteric CoV, PDCoV, emerged in domestic pigs in the US in 2014, and subsequently in other countries (Dong et al., 2015; Wang et al., 2014). The clinical signs of young piglets infected with PDCoV resemble those of PEDV or TGEV, which highlights the necessity for the differential molecular diagnosis of these three CoVs, especially in regions where PEDV and PDCoV co-exist, such as the current situation in the US, China, and South Korea (Dong et al., 2015; Lee et al., 2016; Wang et al., 2014). In our study, the newly designed panCoV RT-PCR primers had high specificity (100%) for the known animal α- (PEDV, TGEV, CCoV, FIPV, and FECoV), β- (BCoV and HECoV), γ- (TCoV and IBV), and δ- (PDCoV) CoVs tested.
We also evaluated its performance by testing porcine fecal and sera samples and comparing the positive rates with those reported by the conventional RT-PCR assay published previously (Hu et al., 2015; Kim et al., 2000; Song et al., 2015). In our study, we demonstrated sensitivity and specificity of our newly established panCoV RT-PCR assay to be 100%. The data indicate that the performance of the newly designed panCoV RT-PCR assay was comparable to that of the conventional virus-specific RT-PCR assay previously reported (Hu et al., 2015; Kim et al., 2000; Song et al., 2015). Because PDCoV could be of avian origin, we also tested the archival avian fecal RNA samples by the newly designed panCoV RT-PCR assay for the detection of γ- and δ-CoVs, and a one-step RT-PCR assay with the δ-CoV nucleocapsid-specific universal primers was used to detect δ-CoV simultaneously. We found that the newly designed panCoV RT-PCR assay also successfully detected avian γ- and δ-CoVs in avian fecal RNA samples, and the two assays had the same results for detection of the δ-CoV positive samples.
Phylogenetic analyses of the spike gene of PDCoV showed that PDCoV were closely related to CoVs isolated from the Asian leopard cats and Chinese ferret badgers (nt similarity ≥ 99.8%) and also wild birds (sparrow, magpie robin), implying the potential interspecies transmission of a δ-CoV between these wild mammals, birds, and pigs (Dong et al., 2007; Jung et al., 2016). CoVs are frequently detected in the feces of wild birds, which may act as reservoirs for avian γ- and δ-CoVs analyse (Duraes-Carvalho et al., 2015; Torres et al., 2017). Phylogenetic analysis revealed that two isolates clustered in the δ-CoVs genus and had the closest relationship with wild avian CoV strains, indicating the existence of δ-CoV in avian fecal samples from the US. The other one belonged to the γ-CoV genus. Complete genome sequencing of these 3 avian CoVs is needed to further characterize these new strains.
5. Conclusions
The newly established panCoV RT-PCR assay had specificity and sensitivity of 100% and reacted with the following animal α- (PEDV, TGEV, CCoV, FIPV, and FECoV), β- (BCoV and HECoV), γ- (TCoV and IBV), and δ- (PDCoV) CoVs tested, that was comparable to that of the virus-specific RT-PCR assays previously reported (Hu et al., 2015; Kim et al., 2000; Oka et al., 2014; Song et al., 2015). The newly designed panCoV RT-PCR assay also successfully detected avian γ- and δ-CoVs in avian fecal samples. Collectively, the newly designed panCoV RT-PCR assay should be useful for the detection of multiple animal CoVs, regardless of their genera, in animal biological samples.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
We thank X. Wang and M. Lee for technical assistance. This study was supported by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, the Ohio State University. This work was also supported by funds from the National Key Research and Development Program of China (2016YFD0500102-1) and National Natural Science Foundation of China (No. 31772773).
References
- Bolles M., Donaldson E., Baric R. SARS-CoV and emergent coronaviruses: viral determinants of interspecies transmission. Curr. Opin. Virol. 2011;1:624–634. doi: 10.1016/j.coviro.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B.Q., Liu W., Fan X.H., Vijaykrishna D., Tang X.C., Gao F., Li L.F., Li G.J., Zhang J.X., Yang L.Q., Poon L.L., Zhang S.Y., Peiris J.S., Smith G.J., Chen H., Guan Y. Detection of a novel and highly divergent coronavirus from asian leopard cats and Chinese ferret badgers in Southern China. J. Virol. 2007;81:6920–6926. doi: 10.1128/JVI.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N., Fang L., Yang H., Liu H., Du T., Fang P., Wang D., Chen H., Xiao S. Isolation, genomic characterization, and pathogenicity of a Chinese porcine deltacoronavirus strain CHN-HN-2014. Vet. Microbiol. 2016;196:98–106. doi: 10.1016/j.vetmic.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N., Fang L., Zeng S., Sun Q., Chen H., Xiao S. Porcine deltacoronavirus in mainland China. Emerg. Infect. Dis. 2015;21:2254–2255. doi: 10.3201/eid2112.150283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraes-Carvalho R., Caserta L.C., Barnabe A.C., Martini M.C., Ferreira H.L., Felippe P.A., Santos M.B., Arns C.W. Coronaviruses detected in Brazilian wild birds reveal close evolutionary relationships with beta- and deltacoronaviruses isolated from mammals. J. Mol. Evol. 2015;81:21–23. doi: 10.1007/s00239-015-9693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz M., Alekseev K., Vlasova A., Zhang X., Spiro D., Halpin R., Wang S., Ghedin E., Saif L.J. Biologic, antigenic, and full-length genomic characterization of a bovine-like coronavirus isolated from a giraffe. J. Virol. 2007;81:4981–4990. doi: 10.1128/JVI.02361-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz M., Sreevatsan S., Cho K.O., Hoet A.E., Saif L.J. Molecular analysis of the S1 subunit of the spike glycoprotein of respiratory and enteric bovine coronavirus isolates. Virus Res. 2002;84:101–109. doi: 10.1016/S0168-1702(02)00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon C.C., Lam T.Y., Shi Z.L., Drummond A.J., Yip C.W., Zeng F., Lam P.Y., Leung F.C. Evidence of the recombinant origin of a bat severe acute respiratory syndrome (SARS)-like coronavirus and its implications on the direct ancestor of SARS coronavirus. J. Virol. 2008;82:1819–1826. doi: 10.1128/JVI.01926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Jung K., Vlasova A.N., Chepngeno J., Lu Z., Wang Q., Saif L.J. Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States. J. Clin. Microbiol. 2015;53:1537–1548. doi: 10.1128/JCM.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Jung K., Vlasova A.N., Saif L.J. Experimental infection of gnotobiotic pigs with the cell-culture-adapted porcine deltacoronavirus strain OH-FD22. Arch. Virol. 2016;161:3421–3434. doi: 10.1007/s00705-016-3056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Liu W.J., Xu W., Jin T., Zhao Y., Song J., Shi Y., Ji W., Jia H., Zhou Y., Wen H., Zhao H., Liu H., Li H., Wang Q., Wu Y., Wang L., Liu D., Liu G., Yu H., Holmes E.C., Lu L., Gao G.F. A bat-derived putative cross-family recombinant coronavirus with a reovirus gene. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Committee on Taxonomy of Viruses and King A.M.Q. Academic Press; 2012. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. [Google Scholar]
- Ismail M.M., Cho K.O., Hasoksuz M., Saif L.J., Saif Y.M. Antigenic and genomic relatedness of turkey-origin coronaviruses, bovine coronaviruses, and infectious bronchitis virus of chickens. Avian Dis. 2001;45:978–984. [PubMed] [Google Scholar]
- Jung K., Hu H., Eyerly B., Lu Z., Chepngeno J., Saif L.J. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg. Infect. Dis. 2015;21:650–654. doi: 10.3201/eid2104.141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Hu H., Saif L.J. Porcine deltacoronavirus infection: etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus Res. 2016;226:50–59. doi: 10.1016/j.virusres.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015;204:134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L., Chang K.O., Sestak K., Parwani A., Saif L.J. Development of a reverse transcription-nested polymerase chain reaction assay for differential diagnosis of transmissible gastroenteritis virus and porcine respiratory coronavirus from feces and nasal swabs of infected pigs. J. Vet. Diagn. Invest. 2000;12:385–388. doi: 10.1177/104063870001200418. [DOI] [PubMed] [Google Scholar]
- Langel S.N., Paim F.C., Lager K.M., Vlasova A.N., Saif L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): historical and current concepts. Virus Res. 2016;226:93–107. doi: 10.1016/j.virusres.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Chung H.C., Nguyen V.G., Moon H.J., Kim H.K., Park S.J., Lee C.H., Lee G.E., Park B.K. Detection and phylogenetic analysis of porcine deltacoronavirus in Korean swine farms, 2015. Transbound. Emerg. Dis. 2016;63:248–252. doi: 10.1111/tbed.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.M., Saif L.J., Marthaler D., Wang Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016;226:20–39. doi: 10.1016/j.virusres.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse L., Krog J.S., Strandbygaard B., Rasmussen T.B., Kjaer J., Belsham G.J., Botner A. Experimental infection of young pigs with an early european strain of porcine epidemic diarrhoea virus and a recent US strain. Transbound. Emerg. Dis. 2017;64:1380–1386. doi: 10.1111/tbed.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Wang Q., Gao G.F. Bat-to-human: spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23:468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Saif L.J., Marthaler D., Esseili M.A., Meulia T., Lin C.M., Vlasova A.N., Jung K., Zhang Y., Wang Q. Cell culture isolation and sequence analysis of genetically diverse US porcine epidemic diarrhea virus strains including a novel strain with a large deletion in the spike gene. Vet. Microbiol. 2014;173:258–269. doi: 10.1016/j.vetmic.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J., Pensaert M.B., Sestak K., Yeo S.G., Jung K. Coronaviruses. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., editors. Diseases of Swine. Wiley-Blackwell; 2012. pp. 501–524. [Google Scholar]
- Song D., Huang D., Peng Q., Huang T., Chen Y., Zhang T., Nie X., He H., Wang P., Liu Q., Tang Y. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea viruses associated with outbreaks of severe diarrhea in piglets in Jiangxi, China 2013. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres C.A., Listorti V., Lupini C., Franzo G., Drigo M., Catelli E., Brandao P.E., Cecchinato M. Gamma and Deltacoronaviruses in quail and pheasants from Northern Italy1. Poult. Sci. 2017;96:717–722. doi: 10.3382/ps/pew332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova A.N., Halpin R., Wang S., Ghedin E., Spiro D.J., Saif L.J. Molecular characterization of a new species in the genus Alphacoronavirus associated with mink epizootic catarrhal gastroenteritis. J. Gen. Virol. 2011;92:1369–1379. doi: 10.1099/vir.0.025353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg. Infect. Dis. 2014;20:1227–1230. doi: 10.3201/eid2007.140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]