Abstract
background and purpose
Celastrol, a quinone methide triterpene isolated from the root extracts of Tripterygium wilfordii, can greatly induce the gene expression activity of heme oxygenase-1 (HO-1) to achieve disease prevention and control. HO-1 induction was recently shown to result in anti-HCV activity by inducing type I interferon and inhibiting hepatitis C virus (HCV) NS3/4A protease activity. The aim of the present study is to evaluate the anti-HCV activity of celastrol and characterize its mechanism of inhibition.
Methods
The anti-HCV activity of celastrol was evaluated using the HCV subgenomic replicon and HCVcc infection systems. The anti-HCV mechanism of celastrol targeting HO-1 expression was clarified using specific inhibitors against several signaling pathways. The transcriptional regulation of celastrol on target gene expression was determined using promoter-based reporter activity assay. The synergistic effect of celastrol and a numbers of clinically used anti-HCV drugs was determined via a drug combination assay.
Results
Celastrol inhibited HCV replication in both the HCV subgenomic and HCVcc infection systems with EC50 values of 0.37 ± 0.022 and 0.43 ± 0.019 μM, respectively. Celastrol-induced heme oxygenase 1 (HO-1) expression promoted antiviral interferon responses and inhibition of NS3/4A protease activity, thereby blocking HCV replication. These antiviral effects were abrogated by treatment with the HO-1-specific inhibitor SnMP or silencing of HO-1 expression by transfection of shRNA, which indicates that HO-1 induction contributes to the anti-HCV activity of celastrol. JNK mitogen-activated protein kinase and nuclear factor erythroid 2-related factor 2 (Nrf2) were confirmed to be involved in the inductive effect of celastrol on HO-1 expression. Celastrol exhibited synergistic effects in combination with interferon-alpha, the NS5A inhibitor daclatasvir, and the NS5B inhibitor sofosbuvir.
Conclusion
Celastrol can serve as a potential supplement for blocking HCV replication. Targeting the JNK/Nrf2/HO-1 axis presents a promising strategy against HCV infection.
Keywords: HCV, Celastrol, Heme oxygenase 1, JNK mitogen-activated protein kinase
Graphical abstract
Highlights
-
•
Celastrol inhibits HCV replication.
-
•
Celastrol induces HO-1 production.
-
•
Celastrol induces interferon-α production and inhibits HCV NS3/4A protease.
-
•
Celastrol synergistically inhibits HCV replication in combination with IFN-α, sofosbuvir or daclatasvir.
1. Introduction
Hepatitis C virus (HCV) is an enveloped single-stranded positive-sense RNA virus belonging to the Hepacivirus genus of the Flaviviridae family (Brass et al., 2006). The genome of the virus includes 9600 base pairs encoding 4 structural proteins (C, E1, E2 and p7) and 6 nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A and NS5B) (Brass et al., 2006). HCV infection is a risk factor of chronic liver diseases, including cirrhosis, fibrosis and hepatocellular carcinoma. HCV-infected patients have been estimated to number up to 200 million worldwide (Alter, 2007). In the past, the standard of care (SOC) for HCV infection is treatment with a combination of pegylated interferon (PEG-IFN-α) and ribavirin (RBV); however, the efficacy of this treatment is only 40%–50% in patients infected with genotype 1 HCV(Ghany et al., 2009). As well, this SOC therapy is associated with several adverse effects, such as anemia, headache, fatigue and depression (Schoggins et al., 2011). In 2011, telaprevir and boceprevir, the first DAAs were approved by the US Food and Drug Administration (FDA) and found to exhibited higher rates of sustained virologic responses (SVRs) in patients infected with genotype 1 HCV when combined with PEG-IFN-α, which provided a new SOC for the treatment of chronic HCV infection (Kiser et al., 2012). Despite the increasing efficacy of the treatment modes developed for HCV infection, however, undesirable side effects continue to be observed during therapy (Kiser et al., 2012). The FDA recently approved the two-agent combo Harvoni and the four-agent combo Viekira Pak for distribution; these treatments could achieve SVRs up to 95% in patients infected with genotype 1 HCV (Koretz, 2014). Unfortunately, while these antiviral agents achieve higher rates of SVR while avoiding the adverse effects often induced by IFN (Koretz, 2014), they also exist the risk to reactivate the hepatitis B virus (HBV) in treating patients with HCV and HBV co-infection (Yeh et al., 2017). Moreover, these two drugs relatively more expensive than other drugs. Thus, development of a more safety and cost-effective substitute for treatment is an important endeavor.
Celastrol is a quinone methide triterpene isolated from Tripterygium wilfordii, a medicinal plant used to treat a range of illnesses including inflammation, swelling, fever, sores, and pain in India, Japan, China, Korea, and other Asian countries (Ju et al., 2015, Lee et al., 2015, Youn et al., 2014). Celastrol is a meal supplement that is widely used in herbal medicine because of its diverse biological activities, which include anti-inflammation, anti-cancer, hepatoprotection, and anti-microbial properties (Ju et al., 2015, Lee et al., 2015). Several reports have demonstrated that celastrol exhibits inhibitory effects on human immunodeficiency virus (Youn et al., 2014), dengue virus (Yu et al., 2017a), and other severe acute respiratory syndrome-associated coronaviruses (Ryu et al., 2010). Celastrol can induce heme oxygenase-1 (HO-1) gene expression via nuclear factor erythroid 2-related factor 2 (Nrf2) activation to prevent circulatory failure prevention and protect against myocardial ischemia (Der Sarkissian et al., 2014). HO-1 induction was recently shown to result in anti-HCV activity by inducing type I interferon and inhibiting HCV NS3/4A protease activity (Lehmann et al., 2010, Zhu et al., 2010).
HO-1 is an inducible and rate-limiting enzyme that degrades heme to produce biliverdin, carbon monoxide (CO) and ferrous iron (Fe3+) via the heme catabolic pathway (Maines, 2005). The enzyme and its metabolites exhibit protective effects against oxidative stress-induced tissue damage upon exposure to multiple stimuli such as viral and bacterial products including lipopolysaccharides, cytokines, oncogenes, mitogens, and various growth factors (Farombi and Surh, 2006, Paine et al., 2010). Upon HCV infection, down-regulation of HO-1 expression has been observed in HCV-infected patients and HCV core protein expression although the biological role of HO-1 suppression and the detailed mechanism of HCV against HO-1 expression were unclear (Abdalla et al., 2004). HO-1 is a detoxifying enzyme that is mainly regulated by Nrf2 (Shan et al., 2006). Activation of HO-1 expression occurs when Nrf2 binds to the antioxidant response element (ARE) of the HO-1 promoter region. By contrast, BACH1 suppresses HO-1 expression by competing with the ARE binding site (Shan et al., 2006). Under normal conditions, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH-associated protein (Keap1)-mediated proteasome degradation (Shan et al., 2006). Upregulating Nrf2/ARE-dependent HO-1 expression to achieve anti-inflammatory and anti-oxidative stress functions is mediated by several host factors, including the mitogene-activated protein kinase (MAPK) molecules p38 MAPK, extracellular signal-regulated kinase 1 and 2 (ERK1/2), and c-Jun N-terminal kinase (JNK) (Paine et al., 2010). In this study, our data revealed that celastrol significantly inhibits HCV replication, and that the anti-HCV effect of celastrol was attenuated by the HO-1 specific inhibitor tin mesoporphyrin (SnMP) or HO-1 gene expression silencing. Celastrol-mediated HO-1 induction contributed to the anti-HCV action through inducing antiviral IFN response and inhibiting HCV NS3 protease activity. Moreover, the JNK/Nrf2/ARE axis was the critical pathway involved in celastrol-mediated HO-1 induction against HCV replication. Combinations of celastrol and IFN-α, sofosbuvir or daclatasvir were tested to determine their ability to enhance of anti-HCV activity.
2. Materials and methods
2.1. Cell culture and virus
Human hepatoma (Huh-7), Ava5 (Huh-7 cells containing HCV genotype 1b subgenomic RNA replicon cells) (Blight et al., 2000), and Huh7.5/J6/JFHEMCVIRESRlucNeo (Huh-7 cells harboring HCV genotype 2a subgenomic RNA and renilla luciferase reporter gene) obtained from Apath LLC (St. Louis, MO) were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 1% non-essential amino acids, and 1% antibiotic–antimycotic in a 5% CO2 atmosphere at 37 °C. Huh-7.5 cells stably expressing the pEF/JFH1-Rz/N plasmid were grown to produce cell culture-derived infectious HCV particles (HCVcc), and the conditional medium was collected to harvest viral particles according to a protocol described previously (Kato et al., 2007).
2.2. Reagents
Celastrol (PubChemCID:122724) was purchased from Fusol-Material Co., Ltd (Tainan, Taiwan). IFN-α-2a (Roferon©-A) was purchased from Roche Ltd. An HO-1 specific inhibitor [tin mesoporphyrin (SnMP)], and MAPK-specific inhibitors (SP600125, SB203580, and PD98059) were purchased from Sigma (St. Louis, MO). Daclatasvir and sofosbuvir was purchased from Shanghai Haoyuan Chemexpress Co., Ltd. The final concentration of DMSO in all reactions was maintained at 0.1%.
2.3. Cell cytotoxicity assay
Ava5 cells were seeded in 96-well plates at a density of 5 × 104 cells per well and treated with celastrol at the indicated concentrations. After 3 days incubation, the cell viability was determined by the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA) according to the manufacturer's instructions.
2.4. Plasmid construction
pHO-1-Luc (Hill-Kapturczak et al., 2003) and p3xARE-Luc (Liao et al., 2008) vectors were used to measure the transcriptional activity of HO-1 and Nrf2, respectively. pISRE-Luc, a reporter vector containing firefly luciferase under the control of an IFN-stimulated response element (ISRE), was used to measure IFN response-dependent transcriptional activity (Stratagene, Agilent Technologies, CA, USA). All cloned DNA fragments were verified by DNA sequencing.
2.5. Western blotting
Western blotting was performed as described previously (Lee et al., 2014). In brief, 20 μg of cell lysates was analyzed by SDS-PAGE, and then transferred to a PVDF membrane. The membranes were probed with anti-HCV NS5B (1:5000; Abcam, Cambridge, MA, USA), anti-HO-1 (1:3000; Abcam), anti-Nrf2 (1:3000; GeneTex, CA, USA), anti-phospho-ERK1/2 (1:1000; Cell Signaling Technology, Inc. Danvers, MA, USA), anti-phospho-p38 (1:1000; Cell Signaling), anti-phospho-JNK (1:1000; Cell Signaling), anti- ERK1/2 (1:1000; Cell Signaling), anti-p38 (1:1000; Cell Signaling), or anti-JNK (1:1000; Cell Signaling)antibody. A loading control was determined using anti-GAPDH antibody (1:10000; GeneTex).
2.6. Quantification of HCV RNA and cellular mRNAs
RNA isolation and quantitative real-time RT-PCR (qRT-PCR) were performed as described previously (Lee et al., 2014). Relative mRNA levels were determined by normalization against the cellular endogenous glyceraldehyde-3-phosphate dehydrogenase (gapdh) gene. The primers used in the study are listed in Table 1 .
Table 1.
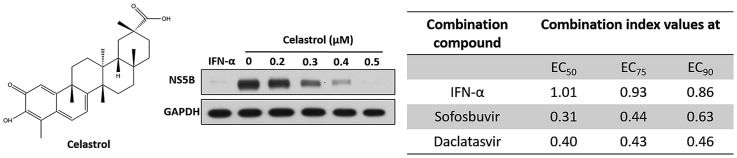
Celastrol synergistically or additively inhibits HCV replication when combined with IFN-α, sofosbuvir, daclatasvir or telaprevir. Standard deviations denote the mean ± SD of three independent experiments.
| Combination compound | Combination index values at |
||
|---|---|---|---|
| EC50 | EC75 | EC90 | |
| IFN-α | 1.01 ± 0.02 | 0.93 ± 0.04 | 0.86 ± 0.01 |
| Sofosbuvir | 0.31 ± 0.05 | 0.44 ± 0.02 | 0.63 ± 0.01 |
| Daclatasvir | 0.40 ± 0.06 | 0.43 ± 0.07 | 0.46 ± 0.02 |
| Telaprevir | 1.03 ± 0.04 | 0.99 ± 0.08 | 0.97 ± 0.03 |
2.7. Transfection and luciferase activity assay
To evaluate the transcriptional regulation of HO-1, Nrf2, or IFN response by celastrol, the Ava5 cells were seed on the 24-wells plates at a density of 4 × 105 cells per well. After 24 h incubation, the 0.5 μg of promoter-driven firefly luciferase plasmids, including pHO-1-Luc, p3xARE-Luc, or pISRE-Luc, were respectively transfected into pre-seeded Ava5 cells using T-Pro™ transfection reagent (Ji-Feng Biotechnology Co., Ltd. Taiwan), according to the manufacturer's instructions. The transfected cells were then treated with celastrol at various concentrations for 3 days. Cell lysates were prepared for the luciferase activity assay and Western blotting using the Bright-Glo Luciferase Assay System (Promega, Madison, WI, USA).
2.8. NS3/4A activity assay
The cell-based NS3/4A activity assay was performed as described previously (Lee et al., 2014). In brief, the Huh-7 cells were seed on the 24-wells plates at a density of 4 × 105 cells per well. After 24 h incubation, the cells were co-transfected with the NS3/4A protease reporter vector pEG(DEΔ4AB)SEAP and NS3/4A expression vector pNS3/4A, followed by celastrol treatment with or without SnMP or 3 days. The 0.6 μM telaprevir treatment server as the positive control. Supernatants were harvested for the SEAP activity assay using the Phospha-Light assay kit. Each transfection mixture contained 0.1 μg of the firefly luciferase expression vector (pFLuc) as a transfection control for normalization against SEAP activity.
2.9. Quantification of extracellular interferon alpha (IFN-α)
Cells were seeded in 24-well plates at a density of 4 × 104 cells/well and treated with various concentrations of celastrol for 3 days. The cell culture medium was harvested to measure IFN-α concentration using a human IFN-α ELISA kit (USCNK Life Science Inc., USA) according to the manufacturer's protocol. Absorbance was detected at 450 nm using an Epoch microplate spectrophotometer.
2.10. Nuclear fraction preparation
Ava5 cells were seeded in a 10-cm dish at a density of 1.4 × 106 cells per 10-cm dish, and treated with increasing concentrations of celastrol for 3 days. The cells were then collected for preparation of a nuclear fraction as described previously (Lee et al., 2014).
2.11. Analysis of drug synergism
Ava5 cells were treated with serially diluted celastrol (0, 0.05, 0.1, or 0.2 μM) in combination with serially diluted IFN-α (7.5, 15, 30, or 60 U/ml), telaprevir (0.075, 0.15, 0.3, and 0.6 μM) sofosbuvir (10, 20, 40, or 80 nM), or daclatasvir (0.5, 1, and 2 pM). After 3 days of incubation, HCV RNA levels were determined by qRT-PCR. Multiple drug combination data were analyzed using CalcuSyn2™ software (Biosoft, Cambridge, UK) which compares single and multiple drug dose effects and determines the combination index (CI) value. The effect of a multiple drug combination is presented as antagonism (CI > 1), additivity (CI = 1), or synergism (CI < 1).
2.12. Statistical analysis
Data were presented as the mean ± standard deviation of at least three independent experiments. Statistical significance was analyzed using Student's t-test. A significant difference was considered as *P < 0.05.
3. Results
3.1. Celastrol inhibits HCV replication
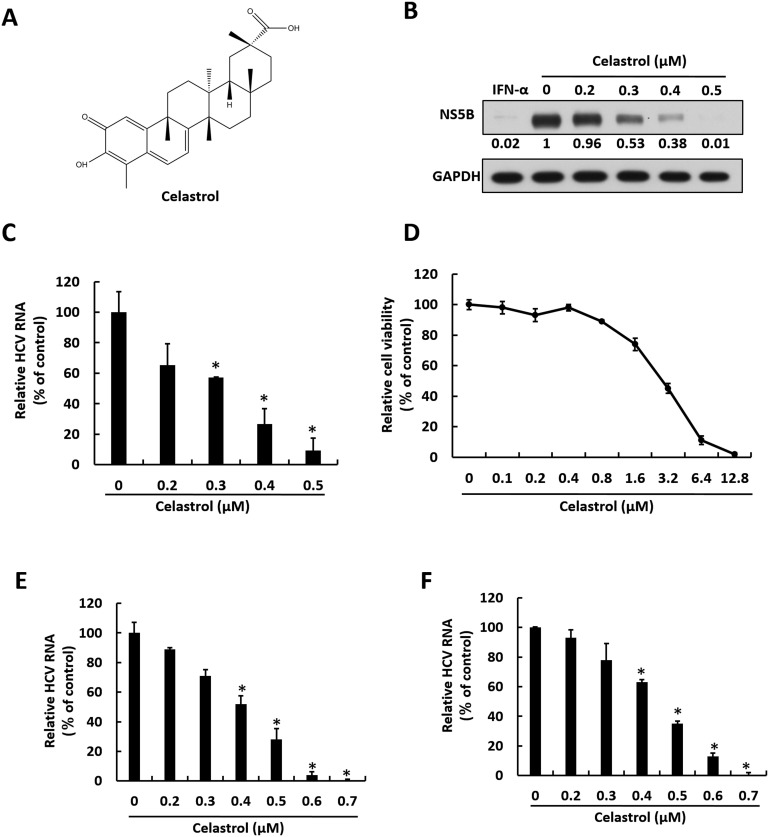
Celastrol is a quinone methide triterpene (Fig. 1 A) possessing anti-DENV activity (Lee et al., 2015). To discover whether celastrol exhibits anti-HCV activity, we first treated HCV subgenomic replicon Ava5 cells with celastrol at increasing concentrations for 3 days. Western blotting assay and qRT-PCR analysis were performed to determine HCV protein and RNA levels under celastrol treatment, respectively. The cytotoxic effect of celastrol on Ava5 cells was also tested using the MTS assay. The results indicated that celastrol dose-dependently reduced HCV protein synthesis (Fig. 1B) and RNA replication with a 50% effective concentration (EC50) of 0.37 ± 0.022 μM (Fig. 1C) without cytotoxicity at effective antiviral concentrations (50% cytotoxicity concentration: CC50 = 4.83 ± 0.32 μM) (Fig. 1D). Based on the results collected from HCV replicon, the selectivity index of celastrol against HCV replication approximates 13. The JFH1 replicon and HCVcc infectious assay was performed to confirm the anti-HCV activity of celastrol. Here, Huh7.5/J6/JFHEMCVIRESRlucNeo replicon cells and JFH1-infected Huh-7 cells were treated with increasing concentrations of celastrol for 3 days. As shown in Fig. 1E and F, qRT-PCR analysis revealed that celastrol dose-dependently reduced HCV RNA levels and fully inhibited HCV replication at a concentration of 0.7 μM.
Fig. 1.
Celastrol suppresses HCV replication. (A) Structure of celastrol. (B–C) Celastrol inhibits HCV RNA and protein synthesis in HCV genotype 1b subgenomic replicon cells. Ava5 cells were treated with 0, 0.2, 0.3, 0.4, or 0.5 μM of celastrol for 3 days. (B) Viral protein level were determined by Western blotting with anti-HCV NS5B antibody, using GAPDH as the internal control. Band intensity was quantified by densitometric scanning and presented as fold-values. (C) RNA levels of HCV were analyzed by qRT-PCR, and viral RNA was normalized against the GAPDH mRNA level. (D) Cytotoxicity was analyzed by MTS assay after incubation of celastrol for 3 days. (E–F) Celastrol inhibits HCV RNA and protein synthesis in HCV genotype 1b subgenomic replicon cells and HCVcc-infected cells. Huh7.5/J6/JFHEMCVIRESRlucNeo replicon (E) and HCV-infected Huh-7.5 cells (F) were treated with 0, 0.2, 0.3, 0.4, 0.5, 0.6, or 0.7 μM of celastrol for 3 days. RNA levels of HCV were analyzed by qRT-PCR, and viral RNA was normalized against the GAPDH mRNA level. Error bars denote the mean ± SD of three independent experiments. *P < 0.05.
3.2. Celastrol inhibits HCV replication through the induction of HO-1 expression
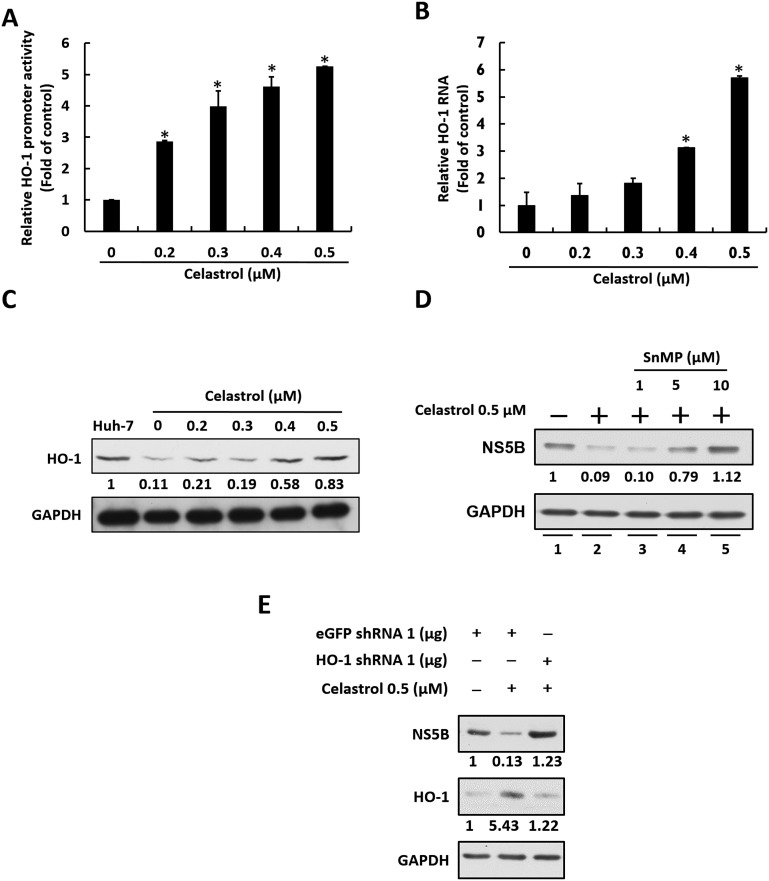
Celastrol was recently shown to induce on HO-1 gene expression for antiviral activity (Youn et al., 2014). We first examined whether celastrol could induce HO-1 expression by determining HO-1 promoter activity, as well as HO-1 RNA and protein levels, in Ava5 cells in the presence of celastrol. Ava5 cells were transfected pHO-1-Luc containing a firefly luciferase gene driven by the HO-1 promoter. Then, the transfected-cells were treated with celastrol at increasing concentrations for 3 days and subjected to luciferase activity assay. The results indicated that celastrol significantly induced the HO-1 promoter activity in a concentration-dependent manner (Fig. 2 A). As expected, the HO-1 RNA and protein levels were also dose-dependently induced by celastrol (Fig. 2B and C). To investigate whether celastrol-induced HO-1 expression is involved in the anti-HCV activity of celastrol, Ava5 cells were co-treated with a fixed concentration of celastrol and increasing concentrations HO-1 specific inhibitor SnMP for 3 days. Western blotting assay was preformed to determine the restorative effect of SnMP on HCV protein synthesis upon celastrol treatment. As shown in Fig. 2D, celastrol inhibited HCV protein synthesis compared with non-celastrol treated cells (lanes 1 and 2) By contrast, SnMP treatment dose-dependently attenuated the suppressive effect of celastrol on HCV protein synthesis (lanes 3–5). As expect, the HO-1 specific shRNA mediated HO-1 gene silencing also can attenuate the suppressive effect of celastrol on HCV protein synthesis (Fig. 2E). These results reveal that celastrol inhibited HCV replication is correlated with HO-1 induction.
Fig. 2.
Celastrol inhibits HCV replication by stimulating HO-1 expression. (A) Celastrol induces HO-1 promoter activity. Ava5 cells were transfected with pHO-1-Luc followed by celastrol treatment. Luciferase activity was determined after 3 days. (B–C) Celastrol induces HO-1 RNA and protein levels upon HCV replication. Ava5 cells were incubated with celastrol for 3 days, after which total RNA and protein were collected. RNA (B) and protein (C) levels of HO-1 were determined by qRT-PCR and Western blotting, respectively. Band intensity was quantified by densitometric scanning and presented as fold-values. (D) HO-1 inhibitor SnMP attenuates the inhibitory effect of celastrol on HCV replication. Ava5 cells were co-treated with a fixed concentration of celastrol and 1, 5, or 10 μM of SnMP for 3 days. HCV protein synthesis was then evaluated by Western blotting. (E) HO-1 specific shRNA attenuates the inhibitory effect of celastrol on HCV replication. Ava5 cells were transfected with 1 μg of HO-1 shRNA, and then treated with 0.5 μM of celastrol for 3 days. HCV protein synthesis was evaluated by Western blotting. Error bars denote the means ± SD of three independent experiments. *P < 0.05.
3.3. Celastrol induces antiviral IFN response in Ava5 cells
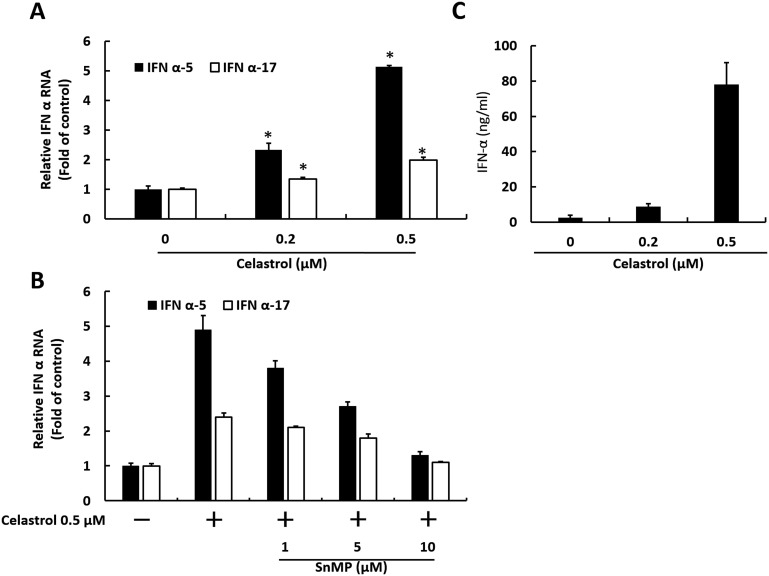
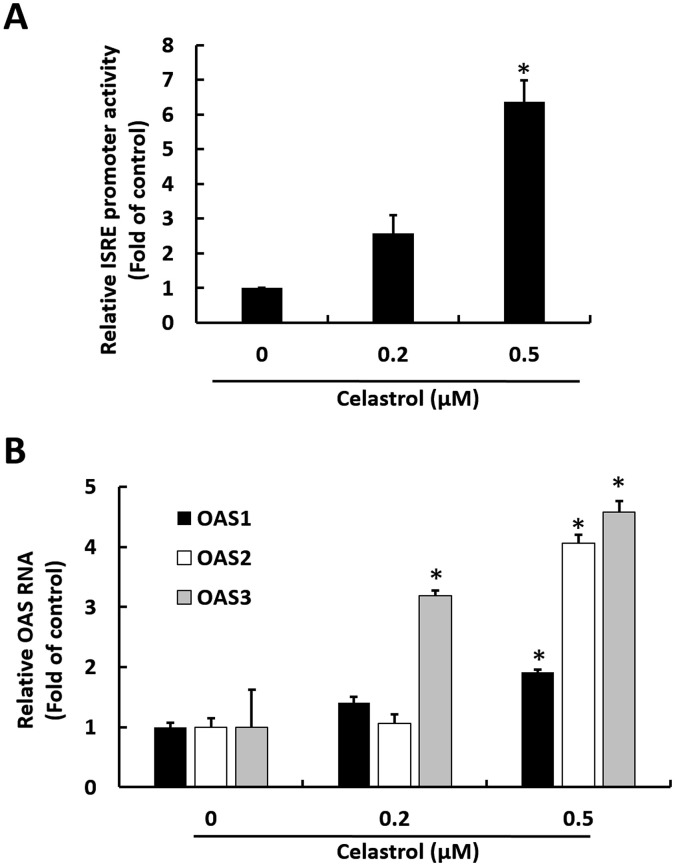
HO-1 induction has been reported to induce IFN responses against virus replication (Lehmann et al., 2010). To investigate whether anti-HCV effect of celastrol is mediated by antiviral IFN responses, we first measured IFN-α gene expression in Ava5 cells upon celastrol treatment at increasing concentrations for 3 days. The results of qRT-PCR analysis indicated that IFN-α-5 and IFN-α-17 RNA levels were gradually induced by celastrol (Fig. 3 A). By contrast, the inductive effect of celastrol on IFN-α-5 and IFN-α-17 RNA levels was significantly attenuated by HO-1 inhibitor SnMP in a concentration-dependent manner (Fig. 3B). We next measured IFN-α protein secretion levels in the culture medium after celastrol treatment. As expected, ELISA results indicated that IFN-α protein secretion levels were gradually induced by celastrol after 3 days of treatment (Fig. 3C). An antiviral effect on cells may be attributed to the interaction of IFN-α and cell surface IFN-α receptors, leading to the activation of ISRE and upregulation of downstream antiviral genes (Yu et al., 2017b). To evaluate whether celastrol-induced IFN-α could activate downstream antiviral genes, ISRE activity and the expression of three critical IFN-mediated antiviral genes, including 2′-5′-oligoadenylate synthetase 1–3 (OAS1–3), were measured. Ava5 cells were transfected with ISRE-driven firefly luciferase reporter plasmid followed by treatment with celastrol for 3 days. Luciferase assay results indicated that the ISRE promoter activity was increased by approximately 2.8–6.5-fold by celastrol (Fig. 4 A). OAS1–3 gene expression levels were significantly upregulated approximately 1.8 ± 0.04, 3.9 ± 0.06, and 4.7 ± 0.09-fold by celastrol, respectively (Fig. 4B).
Fig. 3.
Celastrol induces antiviral IFN responses through HO-1 induction. (A) Celastrol induces IFN RNA levels upon HCV replication. Ava5 cells were treated with 0, 0.2, or 0.5 μM of celastrol for 3 days. RNA levels of IFN-α-5 and IFN-α-17 were measured by qRT-PCR. (B) HO-1 inhibitor antagonizes the inductive effect of celastrol on IFN expression levels. Ava5 cells were treated with 0.5 μM of celastrol and increasing concentrations of SnMP for 3 days. RNA levels of IFN-α-5 and IFN-α-17 were analyzed by qRT-PCR. (C) Celastrol induces IFN secretion upon HCV replication. Ava5 cells were treated with 0, 0.2, or 0.5 μM of celastrol for 3 days. The supernatant was then collected to analyze the secretion level of IFN-α by ELISA. Error bars denote the mean ± SD of three independent experiments. *P < 0.05.
Fig. 4.
Celastrol increases IFN-mediated antiviral genes expression. (A) Celastrol stimulates ISRE activity. Ava5 cells were transfected with 0.5 μg of pISRE-Luc followed by 0, 0.2, or 0.5 μM of celastrol and incubated for 3 days. Luciferase activity represented ISRE activity. (B) Celastrol stimulates the expression level of antiviral genes. Ava5 cells were treated with 0, 0.2, or 0.5 μM of celastrol for 3 days, and RNA levels of OAS1, OAS2 and OAS3 were analyzed by qRT-PCR. Error bars denote the mean ± SD of three independent experiments. *P < 0.05.
3.4. Celastrol inhibits HCV NS3/4A protease activity in Huh-7 cells
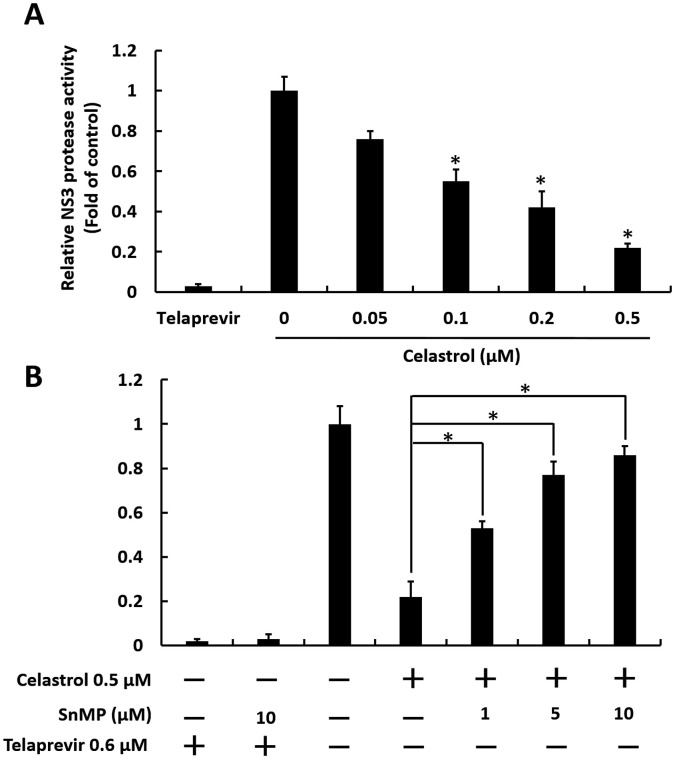
Another proposed anti-HCV action of HO-1 induction is inhibition of NS3/4A protease activity (Zhu et al., 2010). Therefore, we performed a cell-based HCV protease assay to examine the ability of celastrol to target HCV NS3/4A protease activity. Huh-7 cells were co-transfected with pEG(DE4AB)SEAP reporter and HCV pNS3/4 protease expression plasmids, followed by celastrol treatment for 3 days. As shown in Fig. 5 A, HCV NS3/4A protease activity decreased by approximately 1.2–4-fold in comparison with that of the control by celastrol. By contrast, the inhibitory effect of celastrol on HCV NS3/4A protease activity was dose-dependently attenuated by HO-1 inhibitor SnMP (Fig. 5B). These data indicate that targeting HCV NS3/4A protease is an alternative anti-HCV action of celastrol.
Fig. 5.
Celastrol suppresses HCV protease activity via upregulating HO-1 activity. (A) Celastrol inhibits HCV protease activity. Ava5 cells were transfected with pEG(DE4AB)SEAP and pNS3/4 followed by 0, 0.05, 0.1, or 0.2 μM of celastrol for 3 days. The 0.6 μM telaprevir treatment server as the positive control. EGFP activity served as the internal control, and SEAP activity represented HCV NS3/4A protease activity. (B) HO-1 inhibitor attenuates the inhibitory effect of celastrol on HCV protease activity. Ava5 cells were transfected with pEG(DE4AB)SEAP and pNS3/4 followed by 0.5 μM of celastrol and the indicated concentrations of SnMP for 3 days. The 0.6 μM telaprevir treatment server as the positive control. EGFP activity served as the internal control, and SEAP activity represented HCV NS3/4A protease activity. Error bars denote the mean ± SD of three independent experiments. *P < 0.05.
3.5. Celastrol induces Nrf2 nuclear translocation in Ava5 cells
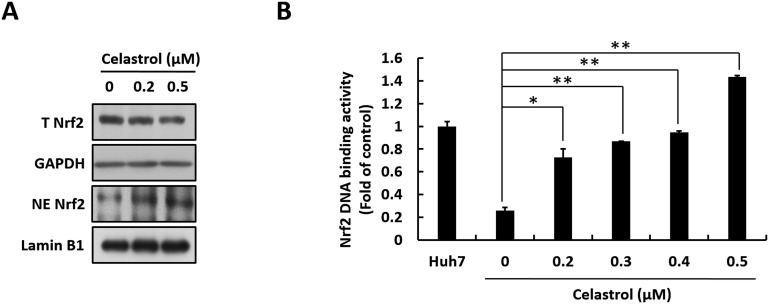
Nrf2 functions as an important upstream regulator in the mediation of HO-1 expression by binding to the ARE response element (Farombi and Surh, 2006). To investigate whether celastrol-induced HO-1 induction is mediated by Nrf2 activation, we first investigated Nrf2 nuclear translocation. Ava5 cells were treated with increasing concentrations of celastrol for 3 days, after which the total cells lysate and nuclear fraction were harvested and subjected to Western blotting assay. As shown in Fig. 6 A, total Nrf2 protein levels were not affected by celastrol (upper panel). By contrast, nuclear Nrf2 protein levels significantly accumulated upon celastrol treatment at a concentration of 0.5 μM, which is the effective dosage against HCV replication. We then examined the Nrf2-mediated ARE activation caused by celastrol. Here, Ava5 cells were transfected with ARE-driven firefly luciferase reporter plasmid. The transfected-cells were treated with increasing concentrations of celastrol for 3 days. As expected, ARE-driven firefly luciferase activity was elevated by celastrol in a concentration-dependent manner (Fig. 6B). Considering these results, the Nrf2-ARE-HO-1 axis can be concluded to be strongly associated with the anti-HCV activity of celastrol.
Fig. 6.
Celastrol induces HO-1 expression level by upregulating Nrf2 activation. (A) Celastrol increases the nuclear accumulation of Nrf2. Ava5 cells were incubated with celastrol for 3 days, and the total cell lysate and cellular nuclear fraction were harvested. Total Nrf2 (T Nrf2) and nuclear Nrf2 (NE Nrf2) levels were analyzed by Western blotting using anti-Nrf2 antibody. Lamin B1 served as the internal control for the nuclear fraction, and GAPDH served as internal control for the total cell lysate. (B) Celastrol increases Nrf2-mediated ARE transactivation. Ava5 cells were transfected with 0.5 μg of p2xARE-Luc followed by celastrol treatment for 3 days, and then harvest to analyze their luciferase activity. Here, luciferase activity was used to represent ISRE activity. Error bars denote the mean ± SD of three independent experiments. *P < 0.05.
3.6. JNK MAPK is involved in Nrf2-mediated HO-1 induction by celastrol
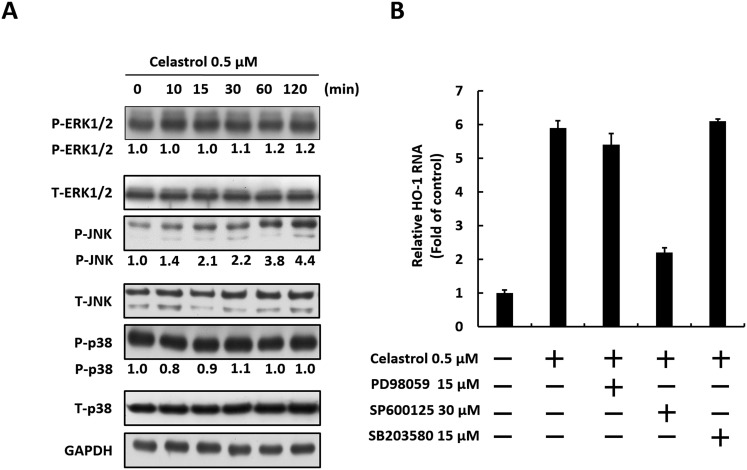
Activation of the MAPK signaling cascade, which includes p38, ERK1/2, and JNK, has been reported to be involved in HO-1 induction resulting in anti-HCV activity (Huang et al., 2006, Paine et al., 2010, Pei et al., 2012, Yano et al., 2009). To investigate whether MAPKs are involved in anti-HCV effect of celastrol, Ava5 cells were treated with 0.5 μM celastrol for 0–120 min. The phosphorylation levels of p38, ERK1/2, and JNK were then measured by Western blotting with phospho-specific antibodies. As shown in Fig. 7 A, the JNK phosphorylation levels were elevated by celastrol in a time-dependent manner compared with that at the time point of 0 min. By contrast, celastrol showed no significant effect on ERK/1/2 or p38 phosphorylation at any time point. To clarify the role of JNK in the HO-1 induction of celastrol, we used specific inhibitors against ERK1/2 (PD98059), JNK (SP600125), and p38 (SB203580), to measure HO-1 RNA expression. As shown in Fig. 7B, HO-1 RNA expression levels were induced by celastrol compared with non-celastrol treated cells and the JNK inhibitor SP600125 significantly reduced the HO-1 inductive effect of celastrol. By contrast, the ERK1/2 inhibitor PD98059 and p38 inhibitor SB203580 showed no significant effect on celastrol-induced HO-1 induction. These results suggest that the anti-HCV effect of celastrol is associated with JNK-mediated HO-1 induction in Ava5 cells.
Fig. 7.
Celastrol induces JNK-MAPK activation to stimulate Nrf2-HO-1 pathway. (A) Celastrol increases JNK phosphorylation. Ava5 cells were incubated with 0.5 μM of celastrol, and the cell lysate was collected at the indicated time points (0–120 min). The levels phosphorylated and total proteins of ERK1/2, JNK and P38 were analyzed by Western blotting with specific antibodies. Band intensity was quantified by densitometric scanning and presented as fold-values relative to the time point of 0 min (defined as 1) following normalization against the GAPDH protein level. (B) JNK inhibitor antagonizes the inductive effect of celastrol on HO-1 expression. Ava5 cells were incubated with 0.5 M of celastrol and a MAPK inhibitor, including ERK1/2 (PD98059), JNK (SP600125), or p38 (SB203580). After 3 days treatment, HO-1 RNA levels were analyzed by qRT-PCR. Error bars denote the mean ± SD of three independent experiments.
3.7. Celastrol synergistically or additively inhibits HCV replication when combined with IFN-α, sofosbuvir, daclatasvir or telaprevir
To determine whether celastrol can enhance the anti-HCV activity of several of clinically used anti-HCV drugs, such as IFN-α, the NS3/4A inhibitor telaprevir, the NS5B inhibitor sofosbuvir (Lam et al., 2012) and the NS5A inhibitor daclatasvir (Lee et al., 2011a). Ava5 cells were co-treated with each drug and celastrol at various concentration ratios for 3 days. The synergistic effect of each combination was evaluated by Calcusyn 2.1 as described by Chou and Talalay (1984). The CI values for the EC50, EC75, and EC90 of IFN-α ranged from 1.01 to 0.86, those of telaprevir ranged from 1.03 to 0.97, sofosbuvir ranged from 0.31 to 0.63 and daclatasvir is ranging from 0.4 to 0.46 (Table 1). No significant cytotoxicity was observed in any combination treatment, as assessed by a colorimetric MTS assay (data not shown). These findings reveal that celastrol may serve as a dietary supplement for enhancing the therapeutic effect of clinically used anti-HCV drugs.
4. Discussion
The type I IFN system presents important innate immunity to effectively block virus replication (Jones et al., 2005, Morrison et al., 2013). HCV infection has been reported to inhibit antiviral IFN responses that facilitate virus replication by promoting the HCV NS3/4A protease-mediated cleavage of mitochondrial antiviral-signaling protein (MAVS)/TIR-domain-containing adapter-inducing interferon-β (TRIF) (Gokhale et al., 2014). In the present study, we found that a natural product celastrol could effectively inhibit HCV NS3/4A protease activity and enhance IFN-mediated antiviral gene expression through HO-1 induction (Fig. 3, Fig. 4, Fig. 5). The HO-1 metabolite biliverdin has been proven to be a blocker of HCV NS3/4A protease activity. Therefore, the regulatory effect of celastrol on the mitochondria-mediated INF signaling pathway against virus replication presents promising prospects for future investigations.
Our data revealed that Nrf-2-mediated HO-1 induction contributed to the anti-HCV activity of celastrol based on the accumulation of nuclear Nrf-2 and enhancement of Nrf-2 binding activity on the ARE response element (Fig. 6). Given that the activation of Nrf2 nuclear translocation is regulated by Keap1-dependent ubiquitination and Bach1, a competitor of Nrf2 for binding to ARE in the HO-1 promoter region (Maines, 2005), future studies should be performed to determine whether celastrol alters the expression levels of Keap-1 or Bach1 for regulating HO-1 induction. Knowledge in this area will help provide alternative targets for screening anti-HCV agents. We further found that the HO-1 inductive effect of the celastrol was also associated with JNK activity (Fig. 7). However, several kinases are involved in JNK activation, including mitogen-activated protein kinase kinase 4 (MKK4), MKK7 and mixed-lineage kinases (MLKs) (Wasserman et al., 2010). Future studies should examine the effect of celastrol on the kinases involved in JNK activation comprehensively describe the relationship between celastrol and HO-1 induction. Several studies have indicated that celastrol exhibits anti- inflammatory activity by inhibiting of nuclear factor-κB (NF-κB) and downstream cycloxygenase-2 (COX-2) (Ding et al., 2013, Joshi et al., 2016). On the basis of earlier findings on a promising tactic against HCV infection via down-regulation of NF-κB-mediated COX-2 expression (Chen et al., 2013, Lee et al., 2011b), we propose that the inhibitory effect of celastrol on NF-κB-mediated COX-2 expression may, at least in part contribute to its anti-HCV activity. Hence, more work is necessary to elucidate additional signaling pathways involved in the anti-HCV activity of celastrol.
Drug combination therapy is considered to be a promising approach to increase therapeutic efficacy and decrease drug resistance in comparison with mono-drug therapy (Hajj et al., 2015). The two-agent combo Harvoni and the four-agent combo Viekira Pak, which could achieve the SVRs of up to 95% in patients infected with genotype 1 HCV. However, the low fidelity of HCV polymerase during viral replication may lead to the emergence of drug resistance, which poses a major challenge in treating HCV infection (Lee et al., 2014). Targeting host factors could be an alternative strategy to eliminate drug resistance in HCV therapeutic regimens because the mutation rate of the host genome is lower than that of the RNA virus genome (Liao et al., 2008). In this study, we showed that celastrol can be considered as a suitable candidate for HCV therapy to minimize the risk of drug resistance by targeting host HO-1 signaling pathway and synergistically inhibiting HCV replication in combination with clinically used drugs against different viral targets (Table 1). Further studies are warranted to clarify the potential clinical relevance of our findings.
In summary, the data indicated that celastrol efficiently inhibited HCV replication via the induction of the JNK/Nrf2/HO-1 axis, which may represent a therapeutic target for the future development and discovery of anti-HCV drugs. Celastrol exhibited synergetic effects on anti-HCV activity in combination with IFN, sofosbuvir or daclatasvir. These results reveal that celastrol may serve as a dietary supplement for enhancing therapeutic effects of the anti-HCV drugs currently available.
Acknowledgments
We are grateful to Dr. Charles Rice (Rockefeller University and Aapth, LCC, USA) for kindly supporting Con1b replicon plasmid, Human hepatoma cell; Huh-7 and HCV subgenomic replicon containing cell line; Ava5, Huh7.5/J6/JFHEMCVIRESRlucNeo HCV replicon cells and Dr. T. Wakita (National Institute of Infectious Diseases, Japan) for providing the JFH1 plasmid. This work was supported by a grant from the Ministry of Science and Technology, Taiwan, (MOST104-2320-B-037-025-MY3; MOST105-3111-Y-043-006), Kaohsiung Medical University, Taiwan (KMU-PT105009), Kaohsiung Medical University under Aim for the Top Universities Grant (KMU-TP105H02), the National Sun Yat-Sen University-KMU Joint Research Project (NSYSU-KMU 106-I007).
References
- Abdalla M.Y., Britigan B.E., Wen F., Icardi M., McCormick M.L., LaBrecque D.R., Voigt M., Brown K.E., Schmidt W.N. Down-regulation of heme oxygenase-1 by hepatitis C virus infection in vivo and by the in vitro expression of hepatitis C core protein. J. Infect. Dis. 2004;190(6):1109–1118. doi: 10.1086/423488. [DOI] [PubMed] [Google Scholar]
- Alter M.J. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 2007;13(17):2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blight K.J., Kolykhalov A.A., Rice C.M. Efficient initiation of HCV RNA replication in cell culture. Sci. (New York, N.Y.) 2000;290(5498):1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- Brass V., Moradpour D., Blum H.E. Molecular virology of hepatitis C virus (HCV): 2006 update. Int. J. Med. Sci. 2006;3(2):29–34. doi: 10.7150/ijms.3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.J., Tseng C.K., Chang F.R., Yang J.I., Yeh C.C., Chen W.C., Wu S.F., Chang H.W., Lee J.C. Aqueous extract of the edible Gracilaria tenuistipitata inhibits hepatitis C viral replication via cyclooxygenase-2 suppression and reduces virus-induced inflammation. PloS One. 2013;8(2) doi: 10.1371/journal.pone.0057704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T.C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Der Sarkissian S., Cailhier J.F., Borie M., Stevens L.M., Gaboury L., Mansour S., Hamet P., Noiseux N. Celastrol protects ischaemic myocardium through a heat shock response with up-regulation of haeme oxygenase-1. Br. J. Pharmacol. 2014;171(23):5265–5279. doi: 10.1111/bph.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q.H., Cheng Y., Chen W.P., Zhong H.M., Wang X.H. Celastrol, an inhibitor of heat shock protein 90beta potently suppresses the expression of matrix metalloproteinases, inducible nitric oxide synthase and cyclooxygenase-2 in primary human osteoarthritic chondrocytes. Eur. J. Pharmacol. 2013;708:1–7. doi: 10.1016/j.ejphar.2013.01.057. [DOI] [PubMed] [Google Scholar]
- Farombi E.O., Surh Y.J. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J. Biochem. Mol. Biol. 2006;39(5):479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- Ghany M.G., Strader D.B., Thomas D.L., Seeff L.B. American Association for the Study of Liver Diseases, Diagnosis, management, and treatment of hepatitis C: an update. Hepatol. Baltim. Md. 2009;49(4):1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale N.S., Vazquez C., Horner S.M. Hepatitis C virus. Strategies to evade antiviral responses. Future Virol. 2014;9(12):1061–1075. doi: 10.2217/fvl.14.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajj R., Milet A., Toulorge D., Cholet N., Laffaire J., Foucquier J., Robelet S., Mitry R., Guedj M., Nabirotchkin S., Chumakov I., Cohen D. Combination of acamprosate and baclofen as a promising therapeutic approach for Parkinson's disease. Sci. Rep. 2015;5:16084. doi: 10.1038/srep16084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Kapturczak N., Sikorski E., Voakes C., Garcia J., Nick H.S., Agarwal A. An internal enhancer regulates heme- and cadmium-mediated induction of human heme oxygenase-1. Am. J. Physiol. Ren. Physiol. 2003;285:F515–F523. doi: 10.1152/ajprenal.00137.2003. [DOI] [PubMed] [Google Scholar]
- Huang Y., Chen X.C., Konduri M., Fomina N., Lu J., Jin L., Kolykhalov A., Tan S.L. Mechanistic link between the anti-HCV effect of interferon gamma and control of viral replication by a Ras-MAPK signaling cascade. Hepatol. Baltim. Md. 2006;43(1):81–90. doi: 10.1002/hep.21011. [DOI] [PubMed] [Google Scholar]
- Jones M., Davidson A., Hibbert L., Gruenwald P., Schlaak J., Ball S., Foster G.R., Jacobs M. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 2005;79(9):5414–5420. doi: 10.1128/JVI.79.9.5414-5420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi Vikram, Venkatesha Shivaprasad H., Ramakrishnan Chandrasekaran, Nanjaraj Urs Ankanahalli N., Hiremath Vilas, Moudgil Kamal D., Velmurugan Devadasan, Vishwanath Bannikuppe Sannanaik. Celastrol modulates inflammation through inhibition of the catalytic activity of mediators of arachidonic acid pathway: secretory phospholipase A 2 group IIA, 5-lipoxygenase and cyclooxygenase-2. Pharmacol. Res. 2016;113:265–275. doi: 10.1016/j.phrs.2016.08.035. [DOI] [PubMed] [Google Scholar]
- Ju Sung Mi, Youn Gi Soo, Cho Yoon Shin, Choi Soo Young, Park Jinseu. Celastrol ameliorates cytokine toxicity and pro-inflammatory immune responses by suppressing NF-κB activation in RINm5F beta cells. BMB Rep. 2015;48(3):172–177. doi: 10.5483/BMBRep.2015.48.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Matsumura T., Heller T., Saito S., Sapp R.K., Murthy K., Wakita T., Liang T.J. Production of infectious hepatitis C virus of various genotypes in cell cultures. J. Virol. 2007;81(9):4405–4411. doi: 10.1128/JVI.02334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser J.J., Burton J.R., Anderson P.L., Everson G.T. Review and management of drug interactions with boceprevir and telaprevir. Hepatol. Baltim. Md. 2012;55(5):1620–1628. doi: 10.1002/hep.25653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretz R.L. ACP Journal Club: review: telaprevir, boceprevir, simeprevir, or sofosbuvir improves response in HCV type 1. Ann. Intern. Med. 2014;161(10):JC11. doi: 10.7326/0003-4819-161-10-201411180-02011. [DOI] [PubMed] [Google Scholar]
- Lam A.M., Espiritu C., Bansal S., Micolochick Steuer H.M., Niu C., Zennou V., Keilman M., Zhu Y., Lan S., Otto M.J., Furman P.A. Genotype and subtype profiling of PSI-7977 as a nucleotide inhibitor of hepatitis C virus. Antimicrob. Agents Chemother. 2012;56(6):3359–3368. doi: 10.1128/AAC.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Ma H., Hang J.Q., Leveque V., Sklan E.H., Elazar M., Klumpp K., Glenn J.S. The hepatitis C virus NS5A inhibitor (BMS-790052) alters the subcellular localization of the NS5A non-structural viral protein. Virology. 2011;414(1):10–18. doi: 10.1016/j.virol.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.C., Chen W.C., Wu S.F., Tseng C.K., Chiou C.Y., Chang F.R., Hsu S.H., Wu Y.C. Anti-hepatitis C virus activity of Acacia confusa extract via suppressing cyclooxygenase-2. Antivir. Res. 2011;89(1):35–42. doi: 10.1016/j.antiviral.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Lee J.C., Tseng C.K., Young K.C., Sun H.Y., Wang S.W., Chen W.C., Lin C.K., Wu Y.H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014;171:237–252. doi: 10.1111/bph.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Jin Young, Lee Byung Ho, Kim Nam Doo, Lee Joo Young. Celastrol blocks binding of lipopolysaccharides to a Toll-like receptor4/myeloid differentiation factor2 complex in a thiol-dependent manner. J. Ethnopharmacol. 2015;172:254–260. doi: 10.1016/j.jep.2015.06.028. [DOI] [PubMed] [Google Scholar]
- Lehmann Elisabeth, El-Tantawy Walid Hamdy, Ocker Matthias, Bartenschlager Ralf, Lohmann Volker, Hashemolhosseini Said, Tiegs Gisa, Sass Gabriele. The heme oxygenase 1 product biliverdin interferes with hepatitis C virus replication by increasing antiviral interferon response. Hepatology. 2010;51(2):398–404. doi: 10.1002/hep.23339. [DOI] [PubMed] [Google Scholar]
- Liao B.C., Hsieh C.W., Liu Y.C., Tzeng T.T., Sun Y.W., Wung B.S. Cinnamaldehyde inhibits the tumor necrosis factor-alpha-induced expression of cell adhesion molecules in endothelial cells by suppressing NF-kappaB activation: effects upon IkappaB and Nrf2. Toxicol. Appl. Pharmacol. 2008;229:161–171. doi: 10.1016/j.taap.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Maines M.D. The heme oxygenase system: update 2005. Antioxid. Redox Signal. 2005 Nov-Dec;7(11–12):1761–1766. doi: 10.1089/ars.2005.7.1761. [DOI] [PubMed] [Google Scholar]
- Morrison J., Laurent-Rolle M., Maestre A.M., Rajsbaum R., Pisanelli G., Simon V., Mulder L.C., Fernandez-Sesma A., García-Sastre A. Dengue virus co-opts UBR4 to degrade STAT2 and antagonize type I interferon signaling. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine A., Eiz-Vesper B., Blasczyk R., Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010;80(12):1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Pei R., Zhang X., Xu S., Meng Z., Roggendorf M., Lu M., Chen X. Regulation of hepatitis C virus replication and gene expression by the MAPK-ERK pathway. Virol. Sin. 2012;27(5):278–285. doi: 10.1007/s12250-012-3257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y.B., Park S.J., Kim Y.M., Lee J.Y., Seo W.D., Chang J.S., Park K.H., Rho M.C., Lee W.S. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg. Med. Chem. Lett. 2010;20(6):1873–1876. doi: 10.1016/j.bmcl.2010.01.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins J.W., Wilson S.J., Panis M., Murphy M.Y., Jones C.T., Bieniasz P., Rice C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y., Lambrecht R.W., Donohue S.E., Bonkovsky H.L. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006;20(14):2651–2653. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- Wasserman T., Katsenelson K., Daniliuc S., Hasin T., Choder M., Aronheim A. A novel c-Jun N-terminal kinase (JNK)-binding protein WDR62 is recruited to stress granules and mediates a nonclassical JNK activation. Mol. Biol. Cell. 2010;21(1):117–130. doi: 10.1091/mbc.E09-06-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M., Ikeda M., Abe K., Kawai Y., Kuroki M., Mori K., Dansako H., Ariumi Y., Ohkoshi S., Aoyagi Y., Kato N. Oxidative stress induces anti-hepatitis C virus status via the activation of extracellular signal-regulated kinase. Hepatol. Baltim. Md. 2009;50(3):678–688. doi: 10.1002/hep.23026. [DOI] [PubMed] [Google Scholar]
- Yeh M.L., Huang C.F., Hsieh M.H., Ko Y.M., Chen K.Y., Liu T.W., Lin Y.H., Liang P.C., Hsieh M.Y., Lin Z.Y., Chen S.C., Huang C.I., Huang J.F., Kuo P.L., Dai C.Y., Yu M.L., Chuang W.L. Reactivation of hepatitis B in patients of chronic hepatitis C with hepatitis B virus infection treated with direct acting antivirals. J. Gastroenterol. Hepatol. 2017 Feb 23 doi: 10.1111/jgh.13771. [DOI] [PubMed] [Google Scholar]
- Youn G.S., Kwon D.J., Ju S.M., Rhim H., Bae Y.S., Choi S.Y., Park J. Celastrol ameliorates HIV-1 Tat-induced inflammatory responses via NF-kappaB and AP-1 inhibition and heme oxygenase-1 induction in astrocytes. Toxicol. Appl. Pharmacol. 2014;280(1):42–52. doi: 10.1016/j.taap.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Yu J.S., Tseng C.K., Lin C.K., Hsu Y.C., Wu Y.H., Hsieh C.L., Lee J.C. Celastrol inhibits dengue virus replication via up-regulating type I interferon and downstream interferon-stimulated responses. Antivir. Res. 2017;137:49–57. doi: 10.1016/j.antiviral.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.S., Wu Y.H., Tseng C.K., Lin C.K., Hsu Y.C., Chen Y.H., Lee J.C. Schisandrin A inhibits dengue viral replication via upregulating antiviral interferon responses through STAT signaling pathway. Sci. Rep. 2017;7:45171. doi: 10.1038/srep45171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Zhaowen, Wilson Anne T., Luxon Bruce A., Brown Kyle E., Mathahs M. Meleah, Bandyopadhyay Sarmistha, McCaffrey Anton P., Schmidt Warren N. Biliverdin inhibits hepatitis C virus nonstructural 3/4A protease activity: mechanism for the antiviral effects of heme oxygenase? Hepatology. 2010;52(6):1897–1905. doi: 10.1002/hep.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]