Highlights
-
•
Arbidol (ARB) is licensed in Russia and China for the treatment of influenza and other viral infections.
-
•
ARB inhibits a large panel of viral pathogens, enveloped or not.
-
•
ARB displays a dual binding activity to lipid membranes and to viral or cellular proteins.
-
•
It blocks viral endocytosis and replication in membranous intracellular compartments.
Keywords: Arbidol, Influenza, Hepatitis C virus, Entry, Fusion, Antiviral therapy
Abstract
Arbidol (ARB) is a Russian-made small indole-derivative molecule, licensed in Russia and China for prophylaxis and treatment of influenza and other respiratory viral infections. It also demonstrates inhibitory activity against other viruses, enveloped or not, responsible for emerging or globally prevalent infectious diseases such as hepatitis B and C, gastroenteritis, hemorrhagic fevers or encephalitis. In this review, we will explore the possibility and pertinence of ARB as a broad-spectrum antiviral, after a careful examination of its physico-chemical properties, pharmacokinetics, toxicity, and molecular mechanisms of action. Recent studies suggest that ARB’s dual interactions with membranes and aromatic amino acids in proteins may be central to its broad-spectrum antiviral activity. This could impact on the virus itself, and/or on cellular functions or critical steps in virus-cell interactions, thereby positioning ARB as both a direct-acting antiviral (DAA) and a host-targeting agent (HTA). In the context of recent studies in animals and humans, we will discuss the prospective clinical use of ARB in various viral infections.
1. Introduction
Arbidol (ARB) has been administered for decades in Russia and China against influenza, with no major adverse effects reported. Its vast potential as a broad-spectrum antiviral agent, defined through in vitro and in vivo studies, lends hope for its clinical use against various infectious diseases that are at present not therapeutically controlled. However, evidence for beneficial effects in humans, especially in the perspective of long-term administration in chronic diseases, remains equivocal. This could be attributable to a relative lack of standardized animal studies and controlled clinical trials in healthy and infected subjects. In addition to influenza and pathogenic human respiratory viruses, ARB shows mainly in vitro inhibitory activity against the hepatitis B virus (HBV), hepatitis C virus (HCV), chikungunya virus (CHIKV), reovirus, Hantaan virus and coxsackie virus B5.
In this paper, we update current knowledge about ARB, linking its physico-chemical properties to its molecular mode of action, toxicity and possible pharmaceutical forms. We will outline recent studies on the molecular and cellular mechanisms by which ARB may inhibit several steps of viral life cycles, and discuss how ARB is emerging as both a direct-acting agent (DAA) and a host-targeting agent (HTA).
2. Overview of ARB: history, initial clinical studies in Russia and China, toxicity
ARB, or ethyl-6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2 [(phenylthio)methyl]-indole-3-carboxylate hydrochloride monohydrate, is a small indole derivative (Fig. 1 A). It is also called umifenovir. Its invention is attributed to a joint consortium of Russian scientists from the Chemical–Pharmaceutical Scientific Research Institute of Russia, the Scientific Research Institute of Medical Radiology in Obninsk and the Leningrad-Pasteur Scientific Research Institute for epidemiology and microbiology, some 40 years ago, as described in:
arbidol.net/robert-nikolaevich-glushkov.html
arbidol.org/1973-4-arbidol-invented-WAY-To-THE DISCOVERY.pdf
arbidol.org/article1.html.
Fig. 1.
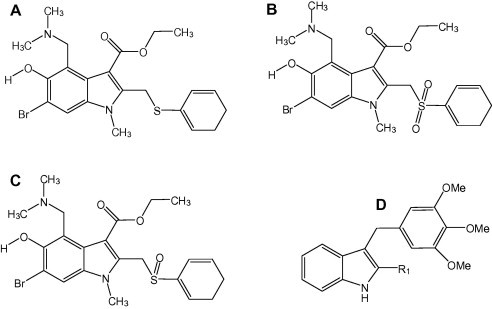
Chemical structures of arbidol (A), sulfonyl-arbidol (B), and sulfinyl-arbidol (C). In D, structure of a prototypic aryl-thio-indole molecule, as synthesized by La Regina et al. (2013).
One of the first descriptions of its chemical synthesis was published in 1993 (Trofimov et al., 1993), and modified later on Miller and Bergeron (1994). The drug is manufactured by Moscow-based Masterlek™, a subsidiary of Pharmstandard Group (see below), and by Shijiazhuang No.4 Pharmaceutical™ in China (http://www.sjzsiyao.com/products_detail/&productId=46.html).
ARB has been marketed for 20 years in Russia and has been used since 2006 in China for the prophylaxis and treatment of human pulmonary diseases caused by influenza A and B viruses and other human pathogenic respiratory viruses, as reviewed in Boriskin et al., 2008, Brooks et al., 2004. It is also used to prevent flu epidemics in poultry in China (Berendsen et al., 2012), and is available from Chinese companies specialized in animal health products, such as:
The first reports on the clinical efficacy of ARB were published in Russian in the 1990s, in groups of students and industrial workers during epidemics of influenza A and outbreaks of acute respiratory diseases (Gagarinova et al., 1993, Obrosova-Serova et al., 1991). Later studies performed in servicemen reported the efficacy and cost-effectiveness of prophylactic or curative treatments of ARB against acute respiratory viral infections, where ARB was shown to decrease the febrile period (Shumilov et al., 2002, Shuster et al., 2004). When the information is available, the duration of ARB treatment varies from 5 to 20 days.
Chinese clinical studies with similar design (patient inclusion criteria, ARB doses and duration of administration) point to a comparable efficacy and tolerability of ARB (Wang et al., 2004). ARB efficacy compared well or even better with that of other commonly used antiviral molecules such as rimantadine (Roflual®), oseltamivir (Tamiflu®), ribavirin or interferon-alpha (Gatich et al., 2008, Kolobukhina et al., 2008, Kolobukhina et al., 2009, Leneva and Shuster, 2006). It potentiated the in vitro effect of rimantadine against influenza A and B viruses (Burtseva et al., 2007), enhanced the immunomodulatory properties of the anti-flu vaccine Vaxigrip®, administered in a cohort of 125 elderly patients (Semenenko et al., 2005), and had a beneficial effects on flu in patients with another infectious immunodeficiency (Glushkov et al., 1999).
Most of these studies point to a dual pharmacological action of ARB: a specific effect on respiratory viruses and an immune-stimulating effect, with induction of serum interferon and activation of phagocytes. Studies have also been conducted in children suffering from flu and other acute viral respiratory diseases (Beliaev et al., 1996, Drinevskiĭ et al., 1998). The latter study – and most documented one – was conducted on 158 children of 1–14 years old, infected with influenza A or B or both or with other respiratory viruses. Over a 5-day treatment, ARB was efficient at reducing the duration of infection and the occurrence of complications, and its immunomodulating action was again suggested. In 2002, Masterlek™, the company currently marketing ARB, sponsored a vast clinical trial conducted on 500 children from 6 to over 12 years old. ARB was given (i) either in prophylaxis twice a week for 3 weeks or once daily for 12 days; (ii) or in treatment thrice daily for 3 days. In all cases, ARB treatment led to a significant reduction of the duration of clinical signs, with no observed adverse effect or complications; see:
Interestingly, in studies comparing antivirals, most viral strains were sensitive to ARB, whereas several resistant variants were found with rimantadine (cf also the recent Iatsyshina et al., 2010, L’vov et al., 2013) (see also Section 7.).
Since 2004, ARB is also patented by Masterlek™ for its medicinal use as an antiviral agent against atypical pneumonia induced by the severe acute respiratory syndrome coronavirus (SARS-CoV); see:
Most recently, a double-blind, randomized, placebo-controlled phase IV clinical trial has been launched by Pharmstandard/Masterlek™, to assess whether ARB is effective in the treatment and prophylaxis of flu and common cold:
Two dosages will be evaluated: 800 mg/day for 5 days, or 200 mg/day for 10 days. Completion of this study is expected in 2015.
Apart from this recent trial and in spite of an abundance of studies in the 1990s, the overall language barrier renders difficult the precise evaluation of the number of subjects enrolled per study, the way clinical trials were designed, and subsequent statistical analyses performed. Moreover, an official Russian site exists for arbidol (arbidol.ru), where more information could be collected; but no English translation is available.
As to studies specifically addressing ARB toxicity issues, initial literature is mostly in Russian, when available. Acute toxicity data report oral LD50s of 340–400 mg/kg in mice, and >3000 mg/kg in rats and guinea pigs:
These values are also reported elsewhere:
http://arbidol.org/pre-1990-animal-human-test-results.pdf; http://arbidol.org/rat.html; (Loginova et al., 2009, Shi et al., 2007). Administered intravenously, ARB exhibited LD50s of 109 mg/kg in mice and 140 mg/kg in rats (Eropkin et al., 2009). On long-term per os administration of ARB in rats, guinea pigs, rabbits or dogs from 2 to over 6 months (with doses ranging from 25 to 125 mg/kg), no pathological changes were observed in animals. These doses would roughly correspond to 10- to 50-fold the therapeutic doses in humans.
ARB is also reported not to induce embryo toxicity in pregnant female rats, nor alter the reproductive function of animals, over a 20 day-administration period of 500 mg/kg doses (http://arbidol.org/rat.html). Recent data from a Chinese group showed a good tolerability of ARB administered to rats per os, at daily doses ranging from 80 to 320 mg/kg over a 4-week period (Wang et al., 2010). But in fact this study assessed the toxicity of a 1:2.5 combination of ARB with acetaminophen, which renders difficult to precisely evaluate the toxicity of each molecule individually. In healthy male volunteers receiving a single 200 mg-dose of ARB, an excellent tolerability was reported (Liu et al., 2009).
From these data, it appears that ARB is a well-tolerated molecule with a high therapeutic index, when administered on periods ranging from a few days to a month. To date, however, no studies have addressed the long-term administration of ARB, for example in the context of chronic infections.
3. ARB bioavailability, pharmacokinetics and metabolism
As an indole derivative, ARB is expected to be poorly soluble in aqueous media. This is of major repercussion on its bioavailability, forms of administration and pharmacokinetics. Efforts to improve ARB water solubility were undertaken, through the chemical grafting of acrylamide polymers to the ARB molecule (Eropkin et al., 2009). Antiviral properties of these complexes were maintained compared to the parent molecule. They also displayed a better in vitro pharmacological index than ARB, defined as IC50/VIC50 (VIC, virus-inhibiting concentration). However these polymers were not further developed. ARB is soluble in hot glycerol, where it remains soluble down to 23 °C. It can then be diluted into aqueous media and administered in vitro or in vivo (Brooks et al., 2012). However no pharmacokinetic nor metabolite studies were performed from this mode of administration.
A very selective, sensitive and accurate method of detection of ARB from human plasma by high-pressure liquid chromatography was designed, and allowed to conclude that no interference existed between ARB and its expected metabolites (Metz et al., 1998). Studies based upon this HPLC method then evaluated the pharmacokinetic parameters of ARB after oral or intravenous administration in rats. Comparable plasma elimination half-lives (t 1/2) and maximum concentrations (C max) were obtained for oral doses six times higher than those injected; however this was only performed on a small number of animals (Liu et al., 2007a). The drug is manufactured in Russia and China as tablets or capsules, each containing ARB as its active ingredient. Initial Russian studies in humans revealed that the main site of ARB metabolism is the liver. ARB was rapidly distributed in tissues and organs with maximal accumulation in liver (3.1% w/w), pituitary gland, kidneys, lymphatic nodes and thyroid, adrenal gland, bone marrow, lungs, plasma, thymus and spleen (less than 1% each) (Glushkov, 1992); see:
Plasma C max was reached within ∼1 h or ∼1.5 h after a 50 mg- or 100 mg-dose, respectively, and t 1/2 was between 17 and 21 h. About 40% of the total intake dose of ARB was excreted unchanged within 48 h via feces. More recent studies reported much shorter plasma C max and t 1/2 in Chinese healthy volunteers, concluding that Russian and Chinese populations differed in ARB elimination rates (Liu et al., 2007b, Liu et al., 2009). However doses administered differed, and technological improvements, especially in detection sensitivity, might also explain such discrepancies. Single doses of 200 mg of ARB administered to healthy volunteers from dispersible tablets or capsules were found bioequivalent and well tolerated (Liu et al., 2009). Pharmacokinetics of oral single vs multiple doses of ARB were compared in healthy subjects, from plasma samples analyzed by HPLC (Metz et al., 1998, Sun et al., 2013). C max increased linearly with the intake dose for single dose administrations, peaking at 2.16 μg/ml for a 800 mg-dose (Sun et al., 2013). ARB exhibited little accumulation with repeated doses, and the pharmacokinetic profile differed from that observed after single dosage.
Based on ARB’s chemical structure, several metabolites can be predicted (Fig. 1A): oxidation at the sulfur atom, loss of the 4-(dimethylamino)methyle group and N-demethylation, conjugation at the 5-hydroxyl moiety. In a pioneering study in rat urine, the formation of sulfone and sulfoxide forms was confirmed after HPLC, from an oral administration of an ARB/starch suspension (Anisimova et al., 1995). Glucuronide or sulfate conjugations were also observed at position 5, and after demethylation of the (dimethylamino)methyle group (see also Liu et al., 2012). In human urine, after administration of a single 300 mg-dose of ARB to healthy subjects, 17 metabolites could be identified, of which the major ones were ARB glucuronide and sulfoxide-ARB (or sulfinyl-ARB; Fig. 1B) glucuronide (Wang et al., 2008). Similar metabolites as identified in rats were observed.
ARB could also be glucuronidated in vitro, using purified human liver microsomes; this study also revealed that the microsomal (recombinant) UDP-glucuronosyl-transferase (UGT) 1A9 was the major UGT isoform involved in ARB glucuronidation (Song et al., 2013). Conversely, ARB was found to inhibit UGT1A9 (ibid.; Liu et al., 2013). Since UGT1A9 is involved in the metabolism of several drugs (e.g. acetominophen, diclofenac), potential drug-drug interactions that could lead to adverse effects should be carefully examined if ARB is administered with other molecules. A more complete picture could be obtained from a study in healthy volunteers receiving a single oral dose of 200 mg ARB, where urine, feces and plasma metabolites were analyzed (Deng et al., 2013). Most of the metabolites were sulfoxidated, dimethylamine N-demethylated, glucuronide- or sulfate-conjugated. About 32.4% of the total intake dose of ARB was excreted unchanged via feces, as previously reported (Boriskin et al., 2008). Sulfinyl-ARB (Fig. 1B) was the major circulating component detected in plasma, followed by unmetabolized ARB, N-demethyl-sulfinyl-ARB and sulfonyl-ARB (Fig. 1C). Urine samples contained mainly glucuronide and sulfate conjugates. In vitro experiments using human liver, intestine and kidney microsomes, and recombinant enzymes, showed that ARB was metabolized by human microsomes from liver and intestines but not from kidney. In these organs, CYP3A4 was identified as a key metabolic enzyme of ARB.
Still, questions remain about the pharmacokinetic properties of ARB metabolites and their potential antiviral activity. The following parameters were reported (Deng et al., 2013): tmax for ARB and dimethylamine N-demethylated ARB were comparable (1.4 and 1.5 h, respectively), while it was much longer for sulfinyl- and sulfonyl-ARB (13 and 19 h). Plasma elimination half-lives (t 1/2) of all metabolites were longer than that of ARB (26.3, 25, 25.7 and 15.7 h for N-demethylated, sulfinyl-, sulfonyl-ARB and ARB, respectively). Exposure to metabolites is therefore greater than that to ARB, and the main metabolite, sulfinyl-ARB, is expected to accumulate on repeated ARB doses. Along these lines, sulfinyl-ARB was reported to contribute for some of the pharmacological activities associated with ARB, and sulfonyl-ARB could inhibit protein kinase C ([IC50] = 7.78 μM) (Demin et al., 2010). Therefore the potency and duration effect for the agent may be underestimated by measuring only ARB concentrations.
It is also predictable, based upon in vitro data with microsomes, that some of the in vivo metabolites could occur in cell cultures, especially in studies addressing the antiviral effect of ARB on hepatotropic viruses using liver-derived cell lines. This could explain why ARB demonstrated greater efficacy under pre-incubation conditions, where metabolites could already be produced and exert specific effects (see below Section 4.). However, a recent study directly addressing the in vitro antiviral properties of sulfinyl- and sulfonyl-ARB against the Chikungunya alphavirus showed only moderate to weak activity as compared to that of the parent molecule (Delogu et al., 2011). This was reinforced by the observation that pre-incubation of cells with ARB prior to infection did not improve antiviral effect, pointing to a minor role (if any) of ARB metabolites against Chikungunya infection, at least in vitro. In any case, further investigations will be necessary to: (i) understand the importance of metabolites in the efficacy and safety of ARB, due to their high plasma exposure and long elimination half-lives; (ii) assess their stability in circulation, tissue binding and storage properties.
4. Broad-spectrum antiviral activity of ARB
ARB has been shown to display antiviral in vitro and/or in vivo activity against a number of enveloped or non-enveloped RNA or DNA viruses, including influenza viruses A, B and C (Leneva et al., 2005), respiratory syncytial virus, SARS-CoV, adenovirus, parainfluenza type 5, poliovirus 1, rhinovirus 14, coxsackievirus B5, hantaan virus, Chikungunya virus, HBV and HCV [reviewed in Boriskin et al., 2008, Brooks et al., 2004, Brooks et al., 2012, Liu et al., 2013a] (see also Table 1 ).
Table 1.
Viruses against which ARB has demonstrated antiviral activity. Virion type: E, enveloped; NE, non-enveloped. References in bold report animal studies of ARB antiviral activity. See text for details and abbreviations.
| Family | Virus | Virion type | In vitro IC50 (μM) | In vivo (mg/kg/day) | DAA/HTA | References |
|---|---|---|---|---|---|---|
| Orthomyxoviridae | Influenza | E | 2.5–16 | A/H3N2 2–50 | Both | Brooks et al., 2012, Fediakina et al., 2005, Fediakina et al., 2011, Leneva and Shuster, 2006, Leneva et al., 2010, Liu et al., 2013b, Loginova et al., 2008, Shi et al., 2007 |
| A/H3N2 12 | A/H1N1 100 | |||||
| B 13.3 | A/H1N1 90–180 | |||||
| A 15–30 | ||||||
| Paramyxoviridae | RSV | E | 16 | – | nd/HTA | Shi et al. (2007) |
| no IC50 | 10–50 | nd/HTA | Brooks et al. (2012) | |||
| Picornaviridae | Poliovirus 1 | NE | 0.41 | – | nd/HTA | Brooks et al. (2012) |
| Rhinovirus 14 | NE | 12.2 | – | nd/HTA | idem | |
| Coxsackie B5 | NE | 5 | 50 | Both | Zhong et al. (2009) | |
| Bunyaviridae | Hantaan | E | 2 | 5–20 | Both | Deng et al., 2009, Wei et al., 2013 |
| Rhabdoviridae | VSV | E | 14 | – | nd/HTA | Blaising et al. (2013) |
| Reoviridae | Reovirus T1L | NE | 10 | – | nd/HTA | Blaising et al. (2013) |
| Togaviridae | Chikungunya | E | 12.2 | – | not DAA/HTA | Delogu et al. (2011) |
| Hepadnaviridae | HBV | E | DNA replic 43 HBsAg 90 | – | nd/HTA | Zhao et al. (2006) |
| Flaviviridae | HCV | E | 2–11.3 | – | Both | Blaising et al., 2013, Boriskin et al., 2006, Boriskin et al., 2008, Haid et al., 2009, Pécheur et al., 2007, Teissier et al., 2011 |
4.1. Respiratory viruses
Numerous reports in Russian describe the antiviral potency of ARB against human or avian influenza A viruses, and notably the highly pathogenic H5N1 (Fediakina et al., 2005, Leneva and Shuster, 2006) and the pandemic 2009 H1N1 subtype (Fediakina et al., 2011). In vitro studies report IC50s in the 2.5–16 μM range, and state an effect of ARB comparable to that of ribavirin, but superior to that of rimantadine, with rimantadine-resistant strains sensitive to ARB (Burtseva et al., 2007, Romanovskaia et al., 2009, Leneva et al., 2010, Fediakina et al., 2011). A few studies state a potentiating effect of ARB and rimantadine (or amantadine) on influenza A and B viruses (Leneva et al., 2005, Burtseva et al., 2007). However adamantane antivirals are scarcely used against influenza viruses, due to low barrier to resistance. In these studies, accessible information does not allow to evaluate the stage of the viral life cycle targeted by ARB nor its mode of action. Shi and coworkers showed a greater inhibitory effect on influenza A H1N1 when ARB was added before infection or when it was pre-incubated with the virus (Shi et al., 2007), suggesting that membrane impregnation and/or metabolites could underlie ARB antiviral activity (see Section 6.). ARB demonstrated similar in vitro antiviral activity as the reference drug ribavirin (Virazole®) against the respiratory syncytial virus (RSV), an enveloped virus of the Paramyxoviridae family (Leneva et al., 2002). ARB was most efficient when added before infection (Shi et al., 2007), with an IC50 of 16 μM.
Recently, Tannock and coworkers reported a potent antiviral activity of ARB on several virus families responsible of respiratory infections in animals and humans, in particular on influenza A H3N2 (IC50 12 μM), and the non-enveloped Picornaviridae poliovirus 1 and rhinovirus 14 (Brooks et al., 2012; see also Brooks et al., 2004). Concerning RSV, only a reduction in plaque size and not in number could be observed, hampering the estimation of an IC50. In this study, ARB was added to cells as an aqueous glycerol solution, instead of the classical dilution from DMSO in other studies (Leneva et al., 2002, Shi et al., 2007). This might explain the discrepancy of antiviral effect on RSV.
ARB also displayed an inhibitory effect on the coxsackievirus B5, another member of the Picornaviridae family responsible for a variety of pathologies including respiratory infections, myocarditis or encephalitis (Zhong et al., 2009). ARB was most active on the virus itself (virucidal test) or when added after infection, through the inhibition of late stages of viral replication. Indeed it was shown to prevent the viral RNA synthesis in a dose-dependent manner, with maximal effect obtained at 5 μM.
One study in Russian describes in vitro antiviral activity of ARB against the SARS-CoV, when added after viral infection and at high concentration (95 μM) (Khamitov et al., 2008). Depending on the cell type, ARB CC50 was reported to vary between 20 and ∼200 μM (e.g. Boriskin et al., 2006, Brancato et al., 2013, Brooks et al., 2012, Shi et al., 2007). The dose exhibiting anti-SARS-CoV activity may likely be cytotoxic.
Studies conducted on mouse-adapted flu models showed that ARB was effective when administered orally at doses from 15 to 30 mg/kg (Loginova et al., 2008, Leneva et al., 2010), or up to 100 mg/kg (Shi et al., 2007), especially when given in prophylaxis before infection. Extrapolated to humans, these doses would correspond to ∼1–6 g per day, not evaluated clinically in terms of safety.
Recently, ARB was found to be effective in vivo against two influenza A H1N1 strains, responsible for seasonal or pandemic flu (Liu et al., 2013b). Reductions in lung viral titers and lesions were observed for oral doses of 90–180 mg/kg/day, and secretion of lung and macrophage cytokines was modulated, indicating an inhibitory effect of ARB on virus-induced inflammation. However, no effect on interferon-alpha was observed, in line with (Brooks et al., 2012) but contrary to initial reports (Glushkov, 1992). Brooks et al. (2012) reported a minor effect of ARB on flu A-infected mice at doses from 2 to 50 mg/kg/day. Discrepancies between results from different groups might come from: (i) bioavailability issues, due to differences in solvents used to solubilize ARB (DMSO vs glycerol); (ii) animal models of flu, using viruses and viral strains adapted or not adapted to mice; (iii) doses administered to animals. However, an overall anti-flu effect of ARB in vivo seems apparent.
Mice with RSV-induced pneumonia were responsive to ARB at 10–50 mg/kg/day doses, with an observable but not significant reduction in lung infectious titers as compared to untreated animals (Brooks et al., 2012). While this study points to a potential promising effect of ARB against RSV in vivo, the limited of global studies addressing the effect of ARB against RSV should invite moderation and a call for additional studies.
One study addressed the antiviral effect of ARB in mice infected with the coxsackievirus B5 (Zhong et al., 2009). Mice developed interstitial pneumonia and myocarditis, and some received ARB orally for 6 days. At a dose of 50 mg/kg, the drug prolonged survival and reduced viral propagation in lungs and heart. Although this result completes the picture of the broad-spectrum antiviral activity of ARB, it must again be taken with caution, since it is the sole study on this virus, conducted on a small number of animals and with a high dose of ARB.
4.2. Viruses causing hemorrhagic fever and encephalitis
Recently, Chinese studies demonstrated antiviral activity of ARB against the Hantaan virus, an enveloped virus from the Bunyaviridae family (Deng et al., 2009, Wei et al., 2013), causing an often lethal hemorrhagic fever with renal syndrome (HFRS). In vitro, ARB was more efficient when added before infection, with an IC50 in the 2 μM range. A direct virucidal effect was noted only for ARB concentrations over 15 μM. In vivo, it was able to increase the survival rate, reduce histopathological changes and viral loads in the lethal model of intracranially-infected suckling mice. Also, serum levels of TNF-alpha were modulated. Since these studies were performed by only one research group, with a limited number of animals, they should be reproduced by others before concluding to a beneficial effect of ARB against hantavirus infection. However ARB efficacy compared well in vivo with that of ribavirin, the reference treatment for such a disease (Wei et al., 2013).
Viruses from the Rhabdoviridae family are known to induce neurological disorders, encephalitis or, more recently reported, hemorrhagic fever (Grard et al., 2012). The only study addressing the effect of ARB against a virus of this family was conducted in our laboratory on the vesicular stomatitis virus (VSV) (Blaising et al., 2013). This enveloped RNA virus mainly infects cattle and pigs, causing oral lesions, anorexia and lethargy. ARB was shown to inhibit in vitro VSV infection in a very similar concentration range as that already shown to affect influenza A or RSV infection [IC50 of 14 (Blaising et al., 2013), 12 (Brooks et al., 2012) or 16 μM (Shi et al., 2007), respectively]. Again, ARB displayed optimal antiviral activity when incubated with cells before infection.
4.3. Non-enveloped Reoviridae
This family of double-stranded RNA viruses comprises animal and human pathogens, such as the rotavirus, a major agent of gastroenteritis in children. We recently addressed the potential of ARB against the mammalian reovirus T1L strain (Blaising et al., 2013). This virus is a prototypic member of the Orthoreovirus genus, which infects a wide variety of host species without causing a significant pathology in humans. In spite of this, reovirus has proven to be a useful model for studying viral pathogenesis. In vitro, ARB inhibited reovirus infection in the 10 μM range, but interestingly, did not exert any effect on infectious subvirion particles (ISVPs), intermediates of reovirus infection (Chandran et al., 2002) that could also directly infect cells via a different entry mechanism from that of reovirus (Martinez et al., 1996). This points to the molecular mechanisms of action of ARB (detailed in Section 6.).
4.4. Chikungunya virus (CHIKV) infection
This alphavirus is an enveloped single-stranded RNA virus from the Togaviridae family, loosely related to Flaviviridae (see below HCV). It is responsible for recent outbreaks of a rheumatological disease. Some neurological complications were described, together with meningo-encephalitis. ARB demonstrated potent in vitro activity against CHIKV infection (Delogu et al., 2011). ARB did not show virucidal activity, contrary to data on respiratory viruses (Shi et al., 2007, Zhong et al., 2009), and displayed the highest efficiency when preincubated with cells 24 h before infection (IC50 ∼ 7.5 μM). In this study, the main metabolites sulfinyl- and sulfonyl-ARB were assayed and exhibited only weak antiviral activity, with IC50s > 55 μM. ARB activity was not improved when a 12 h-preincubation with cells was performed, suggesting that metabolites or degradation products are not responsible for ARB antiviral action. Taken together, these results suggest an interference of the parent molecule ARB with early steps of the viral life cycle, such as cell binding and entry.
4.5. Hepatitis viruses
ARB and derivatives demonstrated in vitro efficiency against the hepatitis B virus (HBV), an enveloped DNA virus from the Hepadnaviridae family (Chai et al., 2006, Zhao et al., 2006). ARB prevented HBV DNA replication with an IC50 of 45 μM, and reduced the production of the virion surface antigen HBsAg at 90 μM; however the 50% cytotoxic concentration was 140 μM, suggesting that inhibitory concentrations are most likely cytotoxic. This work will be further discussed below in the section structure/activity relationship (SAR; Section 5.).
We showed that ARB exerts in vitro antiviral activity against the hepatitis C virus (HCV) (reviewed in Boriskin et al. (2008)), a member of the Flaviviridae family of enveloped viruses. More specifically, ARB was most efficient when incubated with cells before infection and left during infection (Pécheur et al., 2007). As already shown with other viruses, ARB also displayed virucidal activity (Haid et al., 2009, Pécheur et al., 2007). In the 10 μM range, ARB inhibited HCV entry, fusion in in vitro and in cellulo studies (Blaising et al., 2013, Haid et al., 2009, Teissier et al., 2011), and replication on longer times of cell treatment (Boriskin et al., 2006, Sellitto et al., 2010). However, as previously reported in the case of influenza A infection in vivo (Brooks et al., 2012), ARB was not found to induce interferon antiviral responses in vitro against HCV (Boriskin et al., 2006). From these studies, ARB molecular mechanisms of action were proposed (see Section 6 below).
5. ARB structure–activity relationship (SAR)
Several studies aimed at gaining a better understanding of the structural features of ARB important for its broad antiviral activity, improving ARB therapeutic index, or identifying novel lead compounds active against emergent viruses. Compounds derived from the chemical structure of ARB were synthesized and assayed against various influenza A and B viruses (Brancato et al., 2013). The amine in position 4 and the hydroxyl moiety in position 5 were found important for ARB antiviral action, whereas the presence or absence of Br in position 6 had little effect (see Fig. 1A). Insertion of a methyl group between the indole ring and 5-hydroxyl considerably increased antiviral potency of the resulting compound. This molecule was shown to directly bind HA2, with a greater affinity than ARB.
The presence or absence of the 6-Bromo group had also no influence on HBV or HCV infections (Sellitto et al., 2010, Zhao et al., 2006). More specifically, the introduction of particular azote-based heterocyclic groups at position 4 improved anti-HBV activity (Zhao et al., 2006), while it had little effect against HCV (Sellitto et al., 2010). Replacement of the S-phenyl group at position 2 by a phenyl-sulfonyl decreased the cytotoxicity and increased the anti-HBV activity of the compound (Chai et al., 2006, Zhao et al., 2006), while removal of this group was without any influence against HCV (Sellitto et al., 2010). The 5-hydroxy group was found dispensable against HCV. Thus, it appears that different substituents of the ARB molecule play a role in the antiviral activity, depending on the virus considered, the cellular model used and the test conditions.
The combination of in vitro, in cellulo and in silico analyses will help refine the SAR of ARB. In particular, in silico molecular docking studies allowed the precise identification of amino-acid(s) involved in ARB (or derivative) interaction with HA2 (Nasser et al., 2013). Also, three-dimensional quantitative SAR (3D-QSAR) helped design novel anti-HBV compounds based upon a 5-hydroxy-1H-indole-3-carboxylate skeleton, and predict their antiviral potency (Chai et al., 2011). This type of approach is also now conceivable to study the potential interactions of ARB with HCV envelope glycoproteins and clarify structural requirements for antiviral activity, since the 3D-structure of HCV E2 has recently been released (Kong et al., 2013).
6. Molecular mechanisms of ARB antiviral action
ARB’s broad-spectrum antiviral activity suggests that the molecule acts on common critical step(s) of virus-cell interactions. Evidence indicates that ARB directly exerts a virucidal effect, and can then be considered as a direct-acting antiviral (DAA). Most studies also report an effect of ARB on one or several stages of the viral life cycle, such as cell entry (attachment, internalization) and replication. ARB could therefore also act as a host-targeting agent (HTA). In the following section, we will examine the mechanisms by which ARB could exert such dual antiviral activity (recapitulated in Table 1).
6.1. ARB binds to both lipids and protein residues
ARB is an indole-based hydrophobic molecule susceptible to formation of supramolecular arrangements through aromatic stacking interactions with selective amino-acid residues of proteins (phenylalanine, tyrosine, tryptophan). By liquid-state NMR analysis, we showed that ARB displays interfacial properties and intercalates in the shallow layer above the glycerol backbone of phospholipids (Teissier et al., 2011). It is even conceivable that ARB could locally become more concentrated in viral or cellular membranes.
It was also shown that ARB interacts with aromatic residues within the viral glycoprotein involved in membrane interactions and destabilization necessary for fusion, aka the fusion protein (Leneva et al., 2009 for influenza hemagglutinin; Teissier et al., 2011 for HCV E2). This could therefore underlie the virucidal (DAA) effect of ARB, interacting with the viral lipid envelope and/or with key residues within structural proteins of virions (required for cellular receptor/captor recognition and/or membrane fusion). This effect has been described for enveloped [influenza A H1N1 virus (Shi et al., 2007); Hantaan virus (Deng et al., 2009); HCV (Haid et al., 2009, Pécheur et al., 2007)] and non-enveloped viruses [coxsackie virus B5 (Zhong et al., 2009)], consistent with ARB’s dual physico-chemical properties. ARB could also locally impair viral attachment to cell plasma membrane by stabilizing the membrane, and/or by masking key residues in a viral protein involved in receptor recognition, in a sort of DAA + HTA effect. This would have consequences on viral entry.
As shown by fluorescence spectroscopy and surface plasmon resonance analyses, ARB affinity for lipid membranes is even more pronounced at acidic pH, the optimal pH for the fusion step of several enveloped viruses, influenza viruses and HCV in particular (Fig. 2 ) (Haid et al., 2009, Pécheur et al., 2007, Teissier et al., 2010, Teissier et al., 2011). This interaction with phospholipids may perturb membrane fluidity, thereby rendering the lipid bilayer less prone to fusion. Inhibition of viral entry and membrane fusion occurred in the 10 μM range, in agreement with ARB affinity for membranes and the concentration range achieved in healthy volunteers (Sun et al., 2013).
Fig. 2.
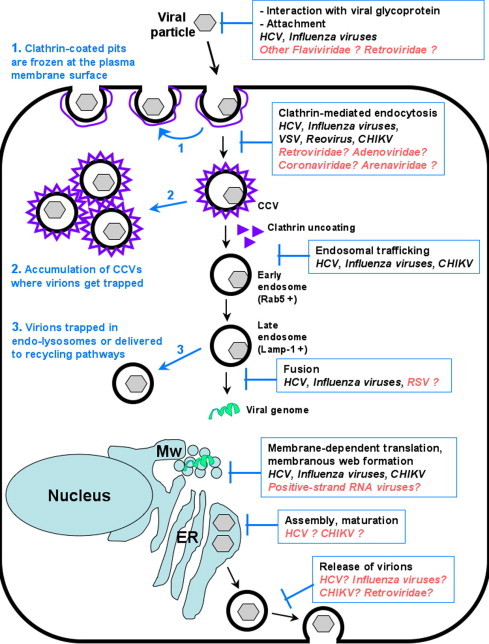
Broad-spectrum activity of ARB and its molecular mechanisms of action at the cellular level. The different steps of the viral life cycle inhibited by ARB are indicated in blue boxes. Potential effect of ARB on other viruses or families of viruses are mentioned in orange. Blue arrows and text indicate the consequences of ARB on cellular pathways and virions. For clarity and regarding current knowledge about the molecular mechanisms of ARB, we only show the clathrin-dependent endocytosis pathway. MW, membranous web, ER, endoplasmic reticulum.
Mechanistically, the dual binding capacity of ARB to lipids and proteins might also underlie alterations of protein/protein and/or protein/lipid interactions at other stages of the viral life cycles, such as replication, assembly and budding. For a number of viruses, in particular in the Flaviviridae family, replication occurs in a subcellular compartment called the membranous web (Heaton and Randall, 2011, Moradpour et al., 2007). The membranous web is an emanation of the endoplasmic reticulum induced by viral proteins such as HCV NS4B (Gouttenoire et al., 2010, Romero-Brey et al., 2012). Since the web is created and maintained through interactions between viral and cellular proteins and lipids, it is plausible that ARB could impair viral replication through its ability to bind proteins and lipids. Concerning viral assembly and budding, intracellular membranes are obligate partners of nucleocapsids during packaging of enveloped viruses, and for the secretion of newly assembled viral particles. In the case of HCV, viral assembly is concomitant to the assembly of lipoproteins, giving rise to lipo-viro particles (Bartenschlager et al., 2011). ARB could therefore interfere with these processes through its physico-chemical dual interactions with lipids and proteins.
6.2. ARB inhibition of viral entry
Recently, we provided molecular details of how ARB inhibits virus entry into target cells, in a study based on live-cell confocal imaging, using HCV as a model of an enveloped virus (Blaising et al., 2013) (Fig. 2). First, ARB was found to drastically impede virion attachment to cell plasma membrane. ARB subsequently impaired the release of clathrin-coated pits (CCPs) from the plasma membrane, resulting in a slowing of clathrin-coated vesicle (CCV) intracellular trafficking. The net result was an intracellular accumulation of CCVs containing trapped virions. ARB was also shown to affect clathrin-mediated endocytosis (CME) by impeding dynamin-2-induced membrane scission, and thereby CCP formation. Lastly, ARB inhibited fusion between endocytic vesicles and endosomes and hindered viral intracellular trafficking. Virions were not properly delivered to Rab5-positive endosomal compartments where fusion occurs and/or Rab5 was not recruited to virion-containing vesicles. As a result, fusion was greatly impaired and virions trafficked to endo-lysosomal Lamp-1-positive compartments for degradation.
Overall, the data suggest that ARB’s dual interactions with lipids and proteins may alter several aspects of intracellular trafficking with and maturation in endosomal compartments. ARB may impede the recruitment and/or disassembly of machineries required for proper endosomal trafficking and viral entry. Thus, ARB inhibition of key actors of intracellular trafficking may be a likely explanation of its broad-spectrum antiviral activity (Table 1, Fig. 2), and suggest that ARB acts as an HTA.
In most studies, ARB exerted a maximal antiviral effect when used before infection, indicating an activity on early stages of viral infection and/or the requirement for ARB to impregnate cells. In the current state of the literature, ARB was shown to be active against viruses that enter cells by routes requiring at least one of these features: acidification, Rab5, dynamin-2, actin. Reovirus, VSV and HCV hijack CME (respectively: Boulant et al., 2013, Johannsdottir et al., 2009, Meertens et al., 2006), HBV also most likely enters via this pathway (Yan et al., 2012). CHIKV entry is mainly achieved via CME (Leung et al., 2011), but alternative clathrin-independent pathways have been described, dependent upon pH, dynamin-2, Rab5 and actin cytoskeleton integrity (Bernard et al., 2010).
Viruses such as influenza or hantaan can enter through clathrin-dependent and -independent endocytotic pathways that have acidification in common (reviewed in Mercer and Helenius, 2009, Lozach et al., 2010). RSV entry is achieved by macropinocytosis and, as shown for HCV, the intracellular trafficking of virions is Rab5-dependent (Krzyzaniak et al., 2013). In the Picornaviridae family, group B coxsackieviruses entry in epithelial cells occurs via a complex process, combining caveolin-dependent endocytosis with features of macropinocytosis such as dependence upon Rab5 (Coyne et al., 2007). Also in this family, poliovirus 1 relies on an actin- and tyrosine kinase-dependent endocytic pathway to invade its target cells (Brandenburg et al., 2007), and rhinovirus 14 entry is pH-dependent and likely achieved by macropinocytosis (Khan et al., 2010). Concerning SARS-CoV entry, the only consensus feature is its dependence on acidification in internal cell compartments (Inoue et al., 2007, Wang et al., 2008).
Apart from lipid membranes, it is therefore conceivable that ARB acts on several cellular targets common to the life cycle of various viruses. Studies directly addressing ARB as an HTA and its interactions with proteins of intracellular trafficking are not available at present, but from our work with HCV, Rab5, dynamin-2 and elements of the clathrin coat could be potential targets (Blaising et al., 2013). It is also conceivable that elements of the cytoskeleton could be targeted; indeed, molecules based on an aryl-thio-indole skeleton (Fig. 1D), closely related to ARB chemically, are inhibitors of tubulin polymerization, and thereby potent anticancer agents (La Regina et al., 2013).
6.3. ARB inhibition of viral fusion
ARB was reported to inhibit influenza- and HCV-mediated membrane fusion (Leneva et al., 2009, Teissier et al., 2011). In vitro studies showed that ARB increases the stability of the influenza virus hemagglutinin (HA) and hinders low pH structural reorganizations necessary for HA to adopt its fusiogenic conformation, thus blocking infection at the viral fusion step (Leneva et al., 2009; see also below Section 7.). Concerning HCV, fusion inhibition is dose-dependent but does not depend on the HCV genotype or on the lipid composition of target membranes (liposomes); it predominantly prevails at low pH (Boriskin et al., 2006, Haid et al., 2009, Pécheur et al., 2007, Teissier et al., 2011). ARB was found to directly interact with peptides from the HCV E2 glycoprotein (Teissier et al., 2011) and within a pocket of the influenza HA2 subunit of hemagglutinin (Nasser et al., 2013), thereby exerting its effect as a DAA. Interestingly, these peptides and pocket contain aromatic residues such as tyrosines and tryptophans, which could engage in aromatic stacking interactions with ARB molecules, as described above. ARB may therefore inhibit fusion by impairing conformational changes in viral fusion proteins during initiation of fusion (DAA activity) and by increasing membrane rigidity, rendering membranes refractory to the destabilization that is required for fusion (HTA activity).
6.4. ARB inhibition of viral replication, assembly and budding
ARB was shown to inhibit HCV replication in replicon systems, i.e. a cell culture context where virus replicates without any production of infectious viral particles (Boriskin et al., 2006, Sellitto et al., 2010). A progressive decline in both viral protein and RNA expression was observed in ARB-treated cells, and cells could be cured of replicating viral RNA after 10 weeks of ARB treatment (Boriskin et al., 2006). Since HCV modulates lipid metabolism (Bassendine et al., 2013) and creates a lipid-rich internal membrane environment favorable for virus replication (i.e. the membranous web), ARB could therefore impregnate these membranes to impede the formation and maintenance of the membranous web and in turn viral replication.
CHIKV replication takes place in the host cell cytoplasm and is associated with cytoplasmic membrane alterations (Solignat et al., 2009). Replication complexes are attached to the membrane of modified endosomes and lysosomes to form organelles characteristic of alphavirus replication called type 1 cytopathic vacuoles. These vacuoles consist in vesicles of 0.6–2.0 μm in diameter harboring numerous spherules (Grimley et al., 1968), which are positive for lysosomal markers (Kujala et al., 2001). The vacuoles produce viral RNA until cell death. As already described for HCV, lipid bilayers are therefore essential for CHIKV replication. It is thus plausible that ARB may also impede the formation and stability of these vacuoles, thereby perturbing CHIKV replication.
In the absence of studies aimed at addressing the potential interactions between ARB and cellular proteins involved in viral replication, one cannot exclude that such interactions might occur, as already suggested at the viral entry/maturation stage. To date, no report has been made concerning an effect of ARB on viral assembly; however, further investigations are still needed to address this question directly. Concerning viral budding, a recent study supports the notion that ARB could inhibit influenza virus egress because viral RNAs accumulate in cells at later stages of infection (Brooks et al., 2012). ARB impregnation of cellular membranes and/or the targeting of proteins involved in intracellular trafficking that relate to viral morphogenesis/budding could again underlie this observation.
7. Viral resistance to ARB
In spite of its usage in Russia and China for several years in flu, ARB does not seem to generate a high degree of viral resistance. Epidemic strains of influenza A/H1N1 and A/H3N2 isolated in Russia in 2008–2009 revealed resistance to oseltamivir and/or rimantadine, but were all sensitive to ARB (Burtseva et al., 2009). The 2009 pandemic swine influenza A/H1N1 was found largely resistant to rimantadine, but had retained its sensitivity to oseltamivir (Tamiflu®) and ARB (Iatsyshina et al., 2010). In 2011–2012, influenza A/H3N2 and B viruses were found to be the cause of a vast epidemic in Russia; all tested strains were sensitive to oseltamivir, zanamivir (Relenza®) and arbidol, but resistant to rimantadine (L’vov et al., 2013).
However resistance to ARB of various strains of influenza viruses has been reported, in particular in a population of influenza B (Burtseva et al., 2007). In a study aimed at understanding the anti-influenza mechanism of action of ARB, Leneva and colleagues isolated seven viral mutants from the influenza A/H7N7 “Weybridge” strain, that were refractory to ARB doses above 38 μM (Leneva et al., 2009). All mutants exhibited a single mutation in the HA2 subunit of the influenza hemagglutinin, the subunit involved in membrane fusion. This translated functionally into a 0.2-unit increase in the pH required to induce HA2 conformational changes. ARB was found to directly interact with HA2, thereby increasing its stability to pH and impeding fusion in endosomes during virus infection. Using an elegant proteomic approach, this interaction was further investigated by Nasser and coworkers, and found confined to one peptide encompassing HA2 residues 104–120. This region contains the ARB already identified mutation resistance K117R (Leneva et al., 2009, Nasser et al., 2013). Taken together, these data reveal that resistance of influenza viruses to ARB mainly arises from mutations in the HA2 fusion protein, consistent with ARB antiviral activity related to membrane fusion.
Addressing ARB antiviral mechanism of action against CHIKV, Delogu and coworkers isolated a mutant virus adapted to ARB at 56 μM (Delogu et al., 2011). This virus was characterized by a single mutation in the E2 viral envelope glycoprotein, in a region most likely involved in cell-surface receptor recognition, and maybe indirectly to membrane fusion. Clearly, additional studies on ARB resistance in the context of other viral infections are warranted.
8. Conclusion and perspectives
In conclusion, the broad-spectrum activity of ARB may arise through duality of function: a capacity to interact with both membranes and with viral and/or cellular proteins. ARB therefore has features of both a DAA and a HTA. These interactions would impede cellular processes and pathways that are hijacked by several viruses to infect their host cells. Regarding HCV, we have shown that ARB inhibits most steps of HCV entry, from attachment to internalization, until the final step of membrane fusion. ARB also inhibits HCV replication, which may arise via alteration of intracellular membrane-protein structures essential for intracellular trafficking (e.g. clathrin coat components, elements of the cytoskeleton) and virus replication (e.g. membranous web), and could hinder membrane rearrangements necessary for the viral budding step. The broad-spectrum activity and the cellular mechanisms affected by ARB are summarized in Fig. 2. Through these effects, ARB could display an antiviral activity on viruses that hijack similar cellular pathways or have overlapping life cycles. In particular, endocytosis is used by several viruses and viral families including human immunodeficiency virus (von Kleist et al., 2011), Adenoviridae, Arenaviridae, Coronaviridae, Togaviridae to achieve productive infection (Table 1). Moreover, all positive-strand RNA viruses of eukaryotes are known to reorganize intracellular membranes to create specific virus replication organelles. For these reasons, efforts should be pursued in order to determine the potential inhibitory effect of ARB on a large class of viruses. A better understanding of its molecular mechanisms of action would also contribute to refine the conditions at which it could be given in long-term regimens against chronic infections (e.g. hepatitis B or C). Indeed current data on toxicity issues are insufficient to evaluate the safety of ARB in chronic administration. Nevertheless, most studies point to a good tolerability of this molecule. In the present state of our knowledge, ARB could therefore constitute a cost-effective pharmacological approach, affordable for emerging countries in urgent need for effective antiviral therapies.
Acknowledgments
We thank Steeve Boulant for his invaluable contribution to live-cell imaging of HCV infection in Blaising et al., 2013. J.B. is the recipient of a doctoral Grant from the Rhône-Alpes region (ARC 1 Santé), and E-I. P. is supported by ANRS (Agence Nationale pour la Recherche sur le SIDA et les hépatites virales).
References
- Anisimova O.S., Frolova L.V., Chistyakov V.V., Ermachenkov I.A., Golovanova I.V., Zotova S.A., Pleshkova A.P., Yadrovskaya V.A., Sheinker Y.N. Study of metabolism of the antiviral drug arbidol by mass spectrometry, thin-layer and high-performance liquid chromatography. Pharm. Chem. J. 1995;29:78–82. [Google Scholar]
- Bartenschlager R., Penin F., Lohmann V., André P. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 2011;19:95–103. doi: 10.1016/j.tim.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Bassendine M.F., Sheridan D.A., Bridge S.H., Felmlee D.J., Neely R.D. Lipids and HCV. Semin. Immunopathol. 2013;35:87–100. doi: 10.1007/s00281-012-0356-2. [DOI] [PubMed] [Google Scholar]
- Beliaev A.L., Burtseva E.I., Slepushkin A.N., Beliaeva N.A. Arbidole – a new drug for prevention of influenza and acute viral respiratory infections in children [Russian] Vestn. Ross. Akad. Med. Nauk. 1996;8:34–37. [PubMed] [Google Scholar]
- Berendsen B.J., Wegh R.S., Essers M.L., Stolker A.A., Weigel S. Quantitative trace analysis of a broad range of antiviral drugs in poultry muscle using column-switch liquid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2012;402:1611–1623. doi: 10.1007/s00216-011-5581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard E., Solignat M., Gay B., Chazal N., Higgs S., Devaux C., Briant L. Endocytosis of chikungunya virus into mammalian cells: role of clathrin and early endosomal compartments. PLoS ONE. 2010;5:e11479. doi: 10.1371/journal.pone.0011479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaising J., Lévy P.L., Polyak S.J., Stanifer M., Boulant S., Pécheur E.I. Arbidol inhibits viral entry by interfering with clathrin-dependent trafficking. Antiviral Res. 2013;100:215–219. doi: 10.1016/j.antiviral.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Boriskin Y.S., Leneva I.A., Pécheur E.I., Polyak S.J. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr. Med. Chem. 2008;15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- Boriskin Y.S., Pécheur E.I., Polyak S.J. Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection. Virol. J. 2006;3:56. doi: 10.1186/1743-422X-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant S., Stanifer M., Kural C., Cureton D.K., Massol R., Nibert M.L., Kirchhausen T. Similar uptake but different trafficking and escape routes of reovirus virions and ISVPs imaged in polarized MDCK cells. Mol. Biol. Cell. 2013;24:1196–1207. doi: 10.1091/mbc.E12-12-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato V., Peduto A., Wharton S. Design of inhibitors of influenza virus membrane fusion: synthesis, structure-activity relationship and in vitro antiviral activity of a novel indole series. Antiviral Res. 2013;99:125–135. doi: 10.1016/j.antiviral.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Brandenburg B., Lee L.Y., Lakadamyali M., Rust M.J., Zhuang X., Hogle James M., Hogle J.M. Imaging poliovirus entry in live cells. PLoS Biol. 2007;5(7):e183. doi: 10.1371/journal.pbio.0050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks M.J., Burtseva E.I., Ellery P.J., Marsh G.A., Lew A.M., Slepushkin A.N., Crowe S.M., Tannock G.A. Antiviral activity of arbidol, a broad-spectrum drug for use against respiratory viruses, varies according to test conditions. J. Med. Virol. 2012;84:170–181. doi: 10.1002/jmv.22234. [DOI] [PubMed] [Google Scholar]
- Brooks M.J., Sasadeusz J.J., Tannock G.A. Antiviral chemotherapeutic agents against respiratory viruses: where are we now and what’s in the pipeline? Curr. Opin. Pulmonary Med. 2004;10:197–203. doi: 10.1097/00063198-200405000-00009. [DOI] [PubMed] [Google Scholar]
- Burtseva E.I., Shevchenko E.S., Leneva I.A., Merkulova L.N., Oskerko T.A., Shliapnikova O.V., Zaplatnikov A.L., Shuster A.M., Slepushkin A.N. Rimantadine and arbidol sensitivity of influenza viruses that caused epidemic morbidity rise in Russia in the 2004–2005 season [Russian] Vopr. Virusol. 2007;52:24–29. [PubMed] [Google Scholar]
- Burtseva E.I., Shevchenko E.S., Beliakova N.V., Oskerko T.A., Kolobukhina L.V., Merkulova L.N., Vartanian R.V., Prilipov A.G., Rotanov M., Zaplatnikov A.L. Monitoring of the sensitivity of epidemic influenza virus strains isolated in Russia to etiotropic chemical agents [Russian] Vopr. Virusol. 2009;54:24–28. [PubMed] [Google Scholar]
- Chai H., Zhao Y., Zhao C., Gong P. Synthesis and in vitro anti-hepatitis B virus activities of some ethyl 6-bromo-5-hydroxy-1H-indole-3-carboxylates. Bioorg. Med. Chem. 2006;14:911–917. doi: 10.1016/j.bmc.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Chai H., Liang X.X., Li L., Zhao C.S., Gong P., Liang Z.J., Zhu W.L., Jiang H.L., Luo C. Identification of novel 5-hydroxy-1H-indole-3-carboxylates with anti-HBV activities based on 3D QSAR studies. J. Mol. Model. 2011;17:1831–1840. doi: 10.1007/s00894-010-0873-7. [DOI] [PubMed] [Google Scholar]
- Chandran K., Farsetta D.L., Nibert M.L. Strategy for nonenveloped virus entry: a hydrophobic conformer of the reovirus membrane penetration protein micro 1 mediates membrane disruption. J. Virol. 2002;76:9920–9933. doi: 10.1128/JVI.76.19.9920-9933.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne C.B., Shen L., Turner J.R., Bergelson J.M. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2:181–192. doi: 10.1016/j.chom.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delogu I., Pastorino B., Baronti C., Nougairede A., Bonnet E., de Lamballerie X. In vitro antiviral activity of arbidol against Chikungunya virus and characteristics of a selected resistant mutant. Antiviral Res. 2011;90:99–107. doi: 10.1016/j.antiviral.2011.03.182. [DOI] [PubMed] [Google Scholar]
- Demin, A.V., Martianov, V.A., Shuster, A.M., 2010. Protein kinase C inhibitors exhibiting an anti-inflammatory, anti-allergic and anti-asthma effect. Russian Patent, WO/2010/064958.
- Deng H.Y., Luo F., Shi L.Q., Zhong Q., Liu Y.J., Yang Z.Q. Efficacy of arbidol on lethal hantaan virus infections in suckling mice and in vitro. Acta Pharmacol. Sin. 2009;30:1015–1024. doi: 10.1038/aps.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P., Zhong D., Yu K., Zhang Y., Wang T., Chen X. Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans. Antimicrob. Agents Chemother. 2013;57:1743–1755. doi: 10.1128/AAC.02282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinevskiĭ V.P., Osidak L.V., Natsina V.K., Afanas’eva O.I., Mil’kint K.K., Danini G.V., Ispolatova A.V., Koreniako I.E., Karelina N.N., Marinich I.G., Boldasov V.K. Chemotherapeutics for treatment of influenza and other viral respiratory tract infections in children [Russian] Antibiot. Khimioter. 1998;43:29–34. [PubMed] [Google Scholar]
- Eropkin M.Y., Solovskii M.V., Smirnova M.Y., Bryazzhikova T.S., Gudkova T.M., Konovalova N.I. Synthesis and biological activity of water-soluble polymer complexes of arbidol. Pharm. Chem. J. 2009;43:563–567. doi: 10.1007/s11094-010-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fediakina I.T., Leneva I.A., Iamnikova S.S., L’vov D.K., Glushkov R.G., Shuster A.M. Sensitivity of influenza A/H5 viruses isolated from wild birds on the territory of Russia to arbidol in the cultured MDCK cells [Russian] Vopr. Virusol. 2005;50:32–35. [PubMed] [Google Scholar]
- Fediakina I.T., Shchelkanov M.I., Deriabin P.G., Leneva I.A., Gudova N.V., Kondrat’eva T.V., L’vov D.K. Susceptibility of pandemic influenza virus A 2009 H1N1 and highly pathogenic avian influenza virus A H5N1 to antiinfluenza agents in cell culture [Russian] Antibiot. Khimioter. 2011;56:3–9. [PubMed] [Google Scholar]
- Gagarinova V.M., Ignat’eva G.S., Sinitskaia L.V., Ivanova A.M., Rodina M.A., Tur’eva A.V. The new chemical preparation arbidol: its prophylactic efficacy during influenza epidemics. Zh. Mikrobiol. Epidemiol. Immunobiol. 1993;5:40–43. (in Russian) [PubMed] [Google Scholar]
- Gatich R.Z., Kolobukhina L.V., Vasil’ev A.N., Isaeva E.I., Burtseva E.I., Orlova T.G., Voronina F.V., Kol’tsov V.D., Malinovskaia V.V. Viferon suppositories in the treatment of influenza in adults. Antibiot. Khimioter. 2008;53:13–17. (Russian) [PubMed] [Google Scholar]
- Glushkov R.G. Monograph: arbidol. antiviral, immunostimulant, interferon inducer. Drugs Future. 1992;17(12) [Google Scholar]
- Glushkov R.G., Gus’kova T.A., Krylova L.Iu., Nikolaeva I.S. Mechanisms of arbidole’s immunomodulating action. Vestn. Ross. Akad. Med. Nauk. 1999;3:36–40. (Russian) [PubMed] [Google Scholar]
- Gouttenoire J., Penin F., Moradpour D. Hepatitis C virus nonstructural protein 4B: a journey into unexplored territory. Rev. Med. Virol. 2010;20:117–129. doi: 10.1002/rmv.640. [DOI] [PubMed] [Google Scholar]
- Grard G., Fair J.N., Lee D., Slikas E. A novel rhabdovirus associated with acute hemorrhagic fever in central Africa. PLoS Pathogens. 2012;8(9):e1002924. doi: 10.1371/journal.ppat.1002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P.M., Berezesky I.K., Friedman R.M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J. Virol. 1968;2:1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haid S., Pietschmann T., Pécheur E.I. Low pH-dependent hepatitis C virus membrane fusion depends on E2 integrity, target lipid composition, and density of virus particles. J. Biol. Chem. 2009;284:17657–17667. doi: 10.1074/jbc.M109.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton N.S., Randall G. Dengue virus and autophagy. Viruses. 2011;3:1332–1341. doi: 10.3390/v3081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatsyshina S.B., Minenko A.N., Kushakova T.E., Praded M.N., Kudriavtseva A.V., Shipulin G.A., Maleev V.V., Pokrovskiĭ V.I. Pandemic influenza A/H1N1 (sw2009) in Russia: epidemiology, diagnosis, clinical picture, and treatment. Ter. Arkh. 2010;82:10–14. (Russian) [PubMed] [Google Scholar]
- Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., Hattori T., Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsdottir H.K., Mancini R., Kartenbeck J., Amato L., Helenius A. Host cell factors and functions involved in vesicular stomatitis virus entry. J. Virol. 2009;83:440–453. doi: 10.1128/JVI.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamitov R.A., Loginova S.I., Shchukina V.N., Borisevich S.V., Maksimov V.A., Shuster A.M. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr. Virusol. 2008;53:9–13. (Russian) [PubMed] [Google Scholar]
- Khan A.G., Pickl-Herk A., Gajdzik L., Marlovits T.C., Fuchs R., Blaas D. Human rhinovirus 14 enters rhabdomyosarcoma cells expressing ICAM-1 by a clathrin-, caveolin-, and flotillin-independent pathway. J. Virol. 2010;84:3984–3992. doi: 10.1128/JVI.01693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolobukhina L.V., Malinovskaia V.V., Gatich R.Z., Merkulova L.N., Burtseva E.I., Isaeva E.I., Parshina O.V., Guseva T.S., Orlova T.G., Voronina F.V. Evaluation of the efficacy of wiferon and arbidol in adult influenza. Vopr. Virusol. 2008;53:31–33. (Russian) [PubMed] [Google Scholar]
- Kolobukhina L.V., Merkulova L.N., Shchelkanov M.Iu., Burtseva E.I., Isaeva E.I., Malyshev N.A., L’vov D.K. Efficacy of ingavirin in adults with influenza. Ter. Arkh. 2009;81:51–54. (Russian) [PubMed] [Google Scholar]
- Kong L., Giang E., Nieusma T., Kadam R.U. Hepatitis C virus E2 envelope glycoprotein core structure. Science. 2013;342:1090–1094. doi: 10.1126/science.1243876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzaniak M.A., Zumstein M.T., Gerez J.A., Picotti P., Helenius A. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PLoS Pathogens. 2013;9:e1003309. doi: 10.1371/journal.ppat.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala P., Ikaheimonen A., Ehsani N., Vihinen H., Auvinen P., Kaariainen L. Biogenesis of the Semliki Forest virus RNA replication complex. J. Virol. 2001;75:3873–3884. doi: 10.1128/JVI.75.8.3873-3884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Regina G., Bai R., Rensen W.M. Toward highly potent cancer agents by modulating the C-2 group of the arylthioindole class of tubulin polymerization inhibitors. J. Med. Chem. 2013;56:123–149. doi: 10.1021/jm3013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leneva I.A., Sokolova M.V., Fediakina I.T., Khristova M.L., Fadeeva N.I., Gus’kova T.A. Study of the effect of antiviral drugs on the reproduction of the respiratory syncytial virus by enzyme immunoassay. Vopr. Virusol. 2002;47:42–45. (Russian) [PubMed] [Google Scholar]
- Leneva I.A., Fediakina I.T., Gus’kova T.A., Glushkov R.G. Sensitivity of various influenza virus strains to arbidol. Influence of arbidol combination with different antiviral drugs on reproduction of influenza virus A. Ter. Arkh. 2005;77:84–88. (Russian) [PubMed] [Google Scholar]
- Leneva I.A., Shuster A.M. Antiviral etiotropic chemicals: efficacy against influenza A viruses A subtype H5N1. Vopr. Virusol. 2006;51:4–7. (Russian) [PubMed] [Google Scholar]
- Leneva I.A., Russell R.J., Boriskin Y.S., Hay A.J. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res. 2009;81:132–140. doi: 10.1016/j.antiviral.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Leneva I.A., Fediakina I.T., Eropkin M.I., Gudova N.V., Romanovskaia A.A., Danilenko D.M., Vinogradova S.M., Lepeshkin A.I., Shestopalov A.M. Study of the antiviral activity of Russian anti-influenza agents in cell culture and animal models. Vopr. Virusol. 2010;55:19–27. (Russian) [PubMed] [Google Scholar]
- Leung J.Y., Ng M.M., Chu J.J. Replication of alphaviruses: a review on the entry process of alphaviruses into cells. Adv. Virol. 2011;2011:249640. doi: 10.1155/2011/249640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.Y., Wang S., Yao W.F., Wu H.Z., Meng S.N., Wei M.J. Pharmacokinetic properties and bioequivalence of two formulations of arbidol: an open-label, single-dose, randomized-sequence, two-period crossover study in healthy Chinese male volunteers. Clin. Ther. 2009;31:784–792. doi: 10.1016/j.clinthera.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Liu D.Y., Yang Z.Q. Characteristics of human infection with avian influenza viruses and development of new antiviral agents. Acta Pharmacol. Sin. 2013;34:1257–1269. doi: 10.1038/aps.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Xiong H.R., Lu L., Liu Y.Y., Luo F., Hou W., Yang Z.Q. Antiviral and anti-inflammatory activity of arbidol hydrochloride in influenza A (H1N1) virus infection. Acta Pharmacol. Sin. 2013;34:1075–1083. doi: 10.1038/aps.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen X.H., Zhang Y.Y., Liu W.T., Bi K.S. Determination of arbidol in rat plasma by HPLC–UV using cloud-point extraction. J. Chromatogr. B. 2007;856:273–277. doi: 10.1016/j.jchromb.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Liu X., Huang Y.W., Li J., Li X.B., Bi K.S., Chen X.H. Determination of arbidol in human plasma by LC–ESI-MS. J. Pharm. Biomed. Anal. 2007;43:371–375. doi: 10.1016/j.jpba.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Liu X., Li H., Bi K.S., Chen X.H., Cai H., Cai B.C. Identification of metabolites of arbidol by ultra-high performance liquid chromatography tandem mass spectrometry. Yao Xue Xue Bao. 2012;47:1521–1526. (Chinese) [PubMed] [Google Scholar]
- Liu X., Huang T., Chen J.X. Arbidol exhibits strong inhibition towards UDP-glucuronosyltransferase (UGT) 1A9 and 2B7. Pharmazie. 2013;68:945–950. [PubMed] [Google Scholar]
- Loginova S.I., Borisevich S.V., Maksimov V.A., Bondarev V.P., Nebol’sin V.E. Therapeutic efficacy of Ingavirin, a new domestic formulation against influenza A virus (H3N2) Antibiot. Khimioter. 2008;53:27–30. (Russian) [PubMed] [Google Scholar]
- Loginova S.I., Borisevich S.V., Maksimov V.A., Bondarev V.P. Toxicity estimation of unspecific medicinal antiviral agents for prophylaxis and therapy of hazard and especially hazard viral infections. Antibiot. Khimioter. 2009;54:11–14. (Russian) [PubMed] [Google Scholar]
- Lozach P.Y., Mancini R., Bitto D., Meier R., Oestereich L., Överby A., Pettersson R., Helenius A. Entry of bunyaviruses into mammalian cells. Cell Host Microbe. 2010;7:488–499. doi: 10.1016/j.chom.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’vov D.K., Burtseva E.I., Kolobukhina L.V., Feodoritova E.L., Shevchenko E.S. Development of the influenza epidemic in season 2011–2012 in some areas of Russia: results of activity of the influenza etiology and epidemiology center of the ivanovsky institute of virology. Vopr. Virusol. 2013;58:15–20. (Russian) [PubMed] [Google Scholar]
- Martinez C.G., Guinea R., Benavente J., Carrasco L. The entry of reovirus into L cells is dependent on vacuolar proton-ATPase activity. J. Virol. 1996;70:576–579. doi: 10.1128/jvi.70.1.576-579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J., Helenius A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- Meertens L., Bertaux C., Dragic T. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J. Virol. 2006;80:11571–11578. doi: 10.1128/JVI.01717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz R., Muth P., Ferger M., Kukes V.G., Vergin H. Sensitive high-performance liquid chromatographic determination of arbidol, a new antiviral compound, in human plasma. J. Chromatogr. A. 1998;810:63–69. doi: 10.1016/s0021-9673(97)01006-6. [DOI] [PubMed] [Google Scholar]
- Miller L., Bergeron R. Preparative liquid chromatographic isolation of unknown impurities in Arbidol and SI-5. J. Chromatogr. A. 1994;658:489–496. [Google Scholar]
- Moradpour D., Penin F., Rice C.M. Replication of Hepatitis C virus. Nature Rev. Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- Nasser Z.H., Swaminathan K., Muller P., Downard K.M. Inhibition of influenza hemagglutinin with the antiviral inhibitor arbidol using a proteomics based approach and mass spectrometry. Antiviral Res. 2013;100:399–406. doi: 10.1016/j.antiviral.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Obrosova-Serova N.P., Burtseva E.I., Nevskiĭ I.M., Karmanova R.I., Nazarov V.I., Pitkenen A.A., Slepushkin A.N. The protective action of arbidol during a rise in respiratory diseases in 1990. Vopr. Virusol. 1991;36:380–381. (Russian) [PubMed] [Google Scholar]
- Pécheur E.I., Lavillette D., Alcaras F., Molle J., Boriskin Y.S., Roberts M., Cosset F.L., Polyak S.J. Biochemical mechanism of hepatitis C virus inhibition by the broad-spectrum antiviral arbidol. Biochemistry. 2007;46:6050–6059. doi: 10.1021/bi700181j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovskaia A.A., Durymanov A.M., Sharshov K.A. Investigation of susceptibility of influenza viruses A (H1N1), the cause of infection in humans in April–May 2009, to antivirals in MDCK cell culture. Antibiot. Khimioter. 2009;54:41–47. (Russian) [PubMed] [Google Scholar]
- Romero-Brey I., Merz A., Chiramel A., Lee J.Y., Chlanda P., Haselman U., Santarella-Mellwig R., Habermann A., Hoppe S., Kallis S., Walther P., Antony C., Krijnse-Locker J., Bartenschlager R. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathogens. 2012;8:e1003056. doi: 10.1371/journal.ppat.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto G., Faruolo A., de Caprariis P., Altamura S., Paonessa G., Ciliberto G. Synthesis and anti-hepatitis C virus activity of novel ethyl 1H-indole-3-carboxylates in vitro. Bioorg. Med. Chem. 2010;18:6143–6148. doi: 10.1016/j.bmc.2010.06.058. [DOI] [PubMed] [Google Scholar]
- Semenenko T.A., Sel’kova E.P., Gotvianskaia T.P., Gaĭdarenko A.D., Polezhaeva N.A., Evseeva L.F., Nikolaeva O.G. Characteristics of the immune status in specific and nonspecific prophylaxis of influenza in elderly persons. Zh. Mikrobiol. Epidemiol. Immunobiol. 2005;6:24–28. (Russian) [PubMed] [Google Scholar]
- Shi L., Xiong H., He J., Deng H., Li Q., Zhong Q., Hou W., Cheng L., Xiao H., Yang Z. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch. Virol. 2007;152:1447–1455. doi: 10.1007/s00705-007-0974-5. [DOI] [PubMed] [Google Scholar]
- Shumilov V.I., Shuster A.M., Lobastov S.P., Shevtsov V.A., Mednikov B.L., Piiavskiĭ S.A., Litus V.I. Efficacy of arbidol in prophylaxis and treatment of acute respiratory viral infections in servicemen. Voen. Med. Zh. 2002;323(51–3):96. (Russian) [PubMed] [Google Scholar]
- Shuster A.M., Shumilov V.I., Shevtsov V.A., Mar’in G.G., Kozlov V.N. Arbidol used in the prophylaxis of acute respiratory viral infections and their complications in servicemen. Voen. Med. Zh. 2004;325(44–5):80. (Russian) [PubMed] [Google Scholar]
- Solignat M., Gay B., Higgs S., Briant L., Devaux C. Replication cycle of chikungunya: a re-emerging arbovirus. Virology. 2009;393:183–197. doi: 10.1016/j.virol.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.H., Fang Z.Z., Zhu L.L., Cao Y.F., Hu C.M., Ge G.B., Zhao D.W. Glucuronidation of the broad-spectrum antiviral drug arbidol by UGT isoforms. J. Pharm. Pharmacol. 2013;65:521–527. doi: 10.1111/jphp.12014. [DOI] [PubMed] [Google Scholar]
- Sun Y., He X., Qiu F., Zhu X., Zhao M., Li-Ling J., Su X., Zhao L. Pharmacokinetics of single and multiple oral doses of arbidol in healthy Chinese volunteers. Int. J. Clin. Pharmacol. Ther. 2013;51:423–432. doi: 10.5414/CP201843. [DOI] [PubMed] [Google Scholar]
- Teissier E., Penin F., Pécheur E.I. Targeting cell entry of enveloped viruses as an antiviral strategy. Molecules. 2010;16:221–250. doi: 10.3390/molecules16010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier E., Zandomeneghi G., Loquet A., Lavillette D., Lavergne J.P., Montserret R., Cosset F.L., Bockmann A., Meier B.H., Penin F., Pécheur E.I. Mechanism of inhibition of enveloped virus membrane fusion by the antiviral drug arbidol. PLoS ONE. 2011;6:e15874. doi: 10.1371/journal.pone.0015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofimov F.A., Tsyshkova N.G., Zotova S.A., Grinev A.N. Synthesis of a new antiviral agent, arbidole. Pharm. Chem. J. 1993;27:75–76. [Google Scholar]
- von Kleist L., Stahlschmidt W., Bulut H. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell. 2011;146:471–484. doi: 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Wang M.Z., Cai B.Q., Li L.Y., Lin J.T., Su N., Yu H.X., Gao H., Zhao J.Z., Liu L. Efficacy and safety of arbidol in treatment of naturally acquired influenza. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26:289–293. (Chinese) [PubMed] [Google Scholar]
- Wang M., Shu B., Bai W.X., Liu J., Yao J., Pan W.N., Pan Y.Y. A 4-week oral toxicity study of an antiviral drug combination consisting of arbidol and acetaminophen in rats. Drug Chem. Toxicol. 2010;33:244–253. doi: 10.3109/01480540903311050. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen X., Li Q., Zhong D. Metabolite identification of arbidol in human urine by the study of CID fragmentation pathways using HPLC coupled with ion trap mass spectrometry. J. Mass Spectrom. 2008;43:1099–1109. doi: 10.1002/jms.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F., Li J.L., Ling J.X. Establishment of SYBR green-based qPCR assay for rapid evaluation and quantification for anti-Hantaan virus compounds in vitro and in suckling mice. Virus Genes. 2013;46:54–62. doi: 10.1007/s11262-012-0834-6. [DOI] [PubMed] [Google Scholar]
- Yan H., Zhong G., Xu G., He W., Jing Z. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Zhao Y., Chai H., Gong P. Synthesis and in vitro anti-hepatitis B virus activities of some ethyl 5-hydroxy-1H-indole-3-carboxylates. Bioorg. Med. Chem. 2006;14:2552–2558. doi: 10.1016/j.bmc.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Zhong Q., Yang Z., Liu Y., Deng H., Xiao H., Shi L., He J. Antiviral activity of Arbidol against Coxsackie virus B5 in vitro and in vivo. Arch. Virol. 2009;154:601–607. doi: 10.1007/s00705-009-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


