Abstract
Zika fever, a mosquito-borne infectious disease caused by Zika virus (ZIKV), is an epidemic disease for which no effective therapy has been established. The recent outbreaks of ZIKV in Brazil and French Polynesia have been linked to a considerable increase in the incidence of fetal microcephaly and other diseases such as Guillain-Barre syndrome. Because there is currently no specific therapy or vaccine, the early exploitation of a method to prevent expansion of ZIKV is a high priority. To validate commonly used antiviral drugs, we evaluated the effect of ribavirin, a drug used to treat hepatitis C with interferon-β (IFN-β), on ZIKV replication. In mammalian cells, we observed an inhibitory effect of ribavirin on ZIKV replication and ZIKV-induced cell death without cytotoxic effect. Furthermore, we found that STAT1-deficient mice, which lack type I IFN signaling, were highly sensitive to ZIKV infection and exhibited lethal outcome. Ribavirin abrogated viremia in ZIKV-infected STAT-1-deficient mice. These data suggest that the inhibition of viral RNA-dependent RNA polymerases may be effective for treatment of ZIKV infection. Our data provide a new insight into the mechanisms for inhibition of ZIKV replication and prevention of Zika fever.
Keywords: Zika virus, Ribavirin, STAT1-deficient mice
Highlights
-
•
Ribavirin inhibits ZIKV replication in mammalian cells.
-
•
Ribavirin prevents ZIKV-induced apoptosis and cell death.
-
•
Ribavirin administration abrogates viremia in ZIKV-infected STAT1-deficient mice.
-
•
Leading to a prolonged survival.
1. Introduction
ZIKV is an arthropod-born virus (arbovirus) of genus Flavivirus, which includes Dengue virus (DENV), West Nile virus, Yellow fever virus, and Japanese encephalitis virus (Lanciotti et al., 2008, Lazear and Diamond, 2016, Solomon, 2016). ZIKV was isolated for the first time from a Rhesus monkey in the Zika forest of Uganda in 1947 (Dick, 1952a, Dick, 1952b). ZIKV is transmitted to humans by mosquitoes, especially Aedes aegypti and Aedes albopictus. Symptoms of infection include fever, skin rash, arthralgia, and conjunctivitis (Hayes, 2009), but up to 80% of patients are asymptomatic (Duffy et al., 2009, Petersen et al., 2016, Slavov et al., 2016). ZIKV infection of humans by viruses of the African and Asian lineages was of marginal importance until the end of 20th century. In 2007, however, the first outbreak was reported in Yap Island in Micronesia, where the virus was transmitted by Aedes aegypti (Lanciotti et al., 2008). A widespread epidemic of the Asian lineage of ZIKV was reported in New Caledonia and French Polynesia from 2013 to 2015. ZIKV reached Brazil in 2013, and shortly thereafter, the virus spread among other countries in South and Central America (Faria et al., 2016). In 2015, the number of cases of pregnancy-associated microcephaly suddenly increased in Brazil, in close association with a concurrent outbreak of ZIKV. A growing body of evidence indicates that ZIKV infection in pregnant women causes congenital abnormalities and fetal demise (Brasil et al., 2016, Li et al., 2016, Miner et al., 2016, Sarno et al., 2016, Ventura et al., 2016). However, the mechanism underlying these phenomena remains unknown, and no therapy for these cases has been established.
Type I interferon receptor (Ifnar1)-deficient mice sustain high ZIKV loads in the brain and spinal cord and die of infection, whereas wild-type mice are resistant to ZIKV (Lazear et al., 2016). Furthermore, ZIKV-infected pregnant mice of SJL strain yield pups with congenital fetal abnormalities (Cugola et al., 2016). SJL mice carry mutations in the Tyk2 gene, which is critical for type I IFN signaling (Izumi et al., 2015). These studies indicate that ZIKV is transmissible to the fetus, leading to defects in fetal development, and suggest that the type I IFN response plays an important role in ZIKV phylaxis in mice.
The antiviral drug ribavirin has been used for treatment of hepatitis C virus (HCV) infection in combination with type I IFN (Abdel-Hady et al., 2014, Bansal et al., 2015). Ribavirin exerts its antiviral effect by inhibiting viral RNA-dependent RNA polymerases (Bougie and Bisaillon, 2003, Maag et al., 2001, Toltzis et al., 1988), inhibiting mRNA cap formation (Goswami et al., 1979), and up-regulating genes related to IFN signaling (Feld et al., 2007, Zhang et al., 2003), as well as other mechanisms. Like ZIKV and DENV, HCV is a member of family Flaviviridae. Recent work showed that ribavirin inhibited DENV replication in Vero cells (Rattanaburee et al., 2015), suggesting that the drug might also be an effective inhibitor of ZIKV replication.
In this study, we investigated the effects of ribavirin and IFN-β on ZIKV replication. We have found that the combination of ribavirin and IFN-β, as well as ribavirin or IFN-β alone inhibited ZIKV replication in vitro. They could inhibit the viral replication in Vero cells (a monkey cell line), SH-SY5Y cells (a human cell line), and C6/36 cells (a mosquito cell line). Moreover, we found that mice carrying a null mutation in the gene encoding STAT1, which is essential for type I IFN signaling, were highly susceptible to lethal infection with ZIKV. Finally, we found that administration of ribavirin inhibited in the early stage of viremia in ZIKV-infected STAT1-deficient mice. Taken together, these results indicate that ribavirin potently inhibits ZIKV replication both in vitro and in vivo, and administration of RNA polymerase inhibitors may be effective treatment strategy for the prevention of ZIKV infection.
2. Materials and methods
2.1. Virus and cells
Zika virus, MR 766 (Uganda, 1974), was kindly provided by the Rockefeller University (New York, NY, USA). Vero cells (African green monkey kidney) and SH-SY5Y cells were maintained at 37 °C in 5% CO2 in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 50 U/mL penicillin, and 50 μg/mL of streptomycin. C6/36 cells (Aedes albopictus) were maintained at 28 °C in 5% CO2 in 10% complete MEM, which consists of Eagle's MEM (Nissui, Tokyo, Japan) with L-alanyl-glutamic acid (Gibco GlutaMAX, Thermo Fisher Scientific, Waltham, MA, USA), non-essential amino acids (Thermo Fisher Scientific), sodium bicarbonate (Thermo Fisher Scientific), and 10% FBS (Hyclone FBS, Thermo Fisher Scientific). ZIKV was subsequently propagated on C6/36 cells in 2% complete MEM.
2.2. Mice
STAT1-deficient mice (Meraz et al., 1996) were kindly provided by Akihiko Yoshimura (Keio University, Tokyo, Japan). Male STAT1-deficient mice aged 7–9 weeks were used throughout this study. Mice were kept in specific pathogen–free facilities in the Division of Laboratory Animal Science of Oita University (Oita, Japan). All experiments using these mice were approved by and performed according to the guidelines of the Oita University Animal Ethics Committee.
2.3. Reagents
Ribavirin was purchased from Nacalai Tesque (Kyoto, Japan). Ganciclovir was purchased from Wako (Osaka, Japan). Recombinant human IFN-β was purchased from PeproTech (Rocky Hill, NJ, USA).
2.4. Real-time RT-PCR assay
Confluent Vero cells, SH-SY5Y cells, and C6/36 cells in 12-well culture plates (Costar, Corning, NY, USA) were infected with ZIKV diluted in 2% complete MEM at a multiplicity of infection (MOI) of 5 × 10−5 (Vero cells, C6/36 cells), or 5 × 10−4 (SH-SY5Y cells). Following 1 h adsorption at 37 °C, 2% complete MEM containing ribavirin (2.5–100 μg/mL) and/or IFNβ (0.05–5 U/mL) was added on the cells. Subsequently, cells were incubated for 4 days at 37 °C, 5% CO2 in a humidified environment. Total RNA was isolated from Vero cells, SH-SY5Y cells, and C6/36 cells using TRIzol reagent (Thermo Fisher Scientific), and cDNA was generated from 0.5 μg of total RNA using a Verso cDNA Kit (Thermo Fisher Scientific). Real-time RT-PCR was performed using ZIKV primers; ZIKV_qpF and ZIKV_qpR (Table 1 ) (Lanciotti et al., 2008). Amplification of the target fragment was performed on a LightCycler96 (Roche, Basel, Switzerland) using a KAPA SYBR FAST qPCR Kit (Kapa Biosystems, Wilmington, MA, USA). All data were normalized against the corresponding level of the mRNA encoding β-actin, or 40S ribosomal protein S17 (AAEL004175) expression (Leming et al., 2014). Amplification conditions were as follows: 45 cycles of 95 °C for 5 s and 60 °C for 30 s. Primers for ZIKV, β-actin, and 40S ribosomal protein S17 were purchased from FASMAC (Kanagawa, Japan). The sequences of primers are listed in Table 1.
Table 1.
Primers list used in this study.
| Primer name | Sequence | Descriptions |
|---|---|---|
| ZIKV_qpF | TTGGTCATGATACTGCTGATTGC | Real-time RT-PCR |
| ZIKV_qpR | CCTTCCACAAAGTCCCTATTGC | Real-time RT-PCR |
| ZIKV ENV_qpF | GCTGGDGCRGACACHGGRACT | RT-PCR, Real-time RT-PCR |
| ZIKV ENV_qpR | RTCYACYGCCATYTGGRCTG | RT-PCR, Real-time RT-PCR |
| beta-actin_qpF | GGGAATGGGTCAGAAGGACT | Real-time RT-PCR |
| beta-actin_qpF | CTTCTCCATGTCGTCCCAGT | Real-time RT-PCR |
| human beta-actin_qpF | CGGCATCGTCACCAACTG | Real-time RT-PCR |
| human beta-actin_qpR | AACATGATCTGGGTCATCTTCTC | Real-time RT-PCR |
| 40s ribosomal protein s17_qpF | CGATGGATTTCCACACGAAC | Real-time RT-PCR |
| 40s ribosomal protein s17_qpR | GCTTCATCAGATGTGTCACGA | Real-time RT-PCR |
2.5. Viral titration and plaque assays
To determine the viral titer, confluent Vero cells in 12-well culture plates were infected with serial dilutions of ZIKV in 2% complete MEM. Following 1 h adsorption at 37 °C, 2% methylcellulose (MP Biomedicals, Aurora, OH, USA) was layered on the cells. Subsequently, cells were incubated for 4 days at 37 °C, 5% CO2 in a humidified environment, fixed in 10% formalin (Wako) and then stained with methylene blue (Wako) to count the plaques. To examine the effect of ribavirin, IFN-β, and ganciclovir on ZIKV replication, Vero cells were infected with ZIKV at an MOI of 5 × 10−5, 5 × 10−4 as described above, and then the methylcellulose containing ribavirin (10–1000 μg/mL), IFN-β (2.5–10 U/mL), or ganciclovir (0.5–2 μM or 10–100 μg/mL) was layered. Four days after, the plates were photographed.
2.6. Detection of apoptosis and cell death by microscopic and flow cytometric analysis
Confluent Vero cells in 12-well culture plates were infected with ZIKV diluted in 2% complete MEM at an MOI of 5 × 10−3. Following 1 h adsorption at 37 °C, the 2% complete MEM containing ribavirin (15.6–2000 μg/mL) was added. Four days after treatment, cells were observed under a microscope (Biorevo BZ-9000, Keyence, Osaka, Japan) and photographed. And then the cells were trypsinized and collected along with the supernatants, and cell viability was determined by staining with FITC–Annexin V (Biolegend, San Diego, CA, USA) and the Zombie Red Fixable Viability kit (Biolegend) staining. Data were acquired using a FACS BD LSRFortessa X-20 (BD Biosciences, Franklin Lakes, NJ, USA) and analysed using a FlowJo software (Tree Star, Inc., Ashland, OR, USA).
2.7. Evaluation of ribavirin in vivo using ZIKV-infected STAT1-deficient mice
STAT1-deficient mice were subcutaneously infected in the dorsal flank with 0.2 mL PBS containing ZIKV (1.0 × 104 plaque-forming units). To assess the inhibitory effect on viremia, ribavirin (15 mg) was administered intraperitoneally for 3 consecutive days post-infection. To measure viremia, RT-PCR was performed as follows: total RNA was isolated from the same amount of blood serum using a QuickGene RNA Tissue Kit SII (RT-S2) (KURABO, Osaka, Japan). The purified RNA was subjected to RT-PCR using a SuperScript III One-Step RT-PCR System, Platinum Taq (Thermo Fisher Scientific), using ZIKV primers; ZIKV ENV_qpF and ZIKV ENV_qpR (Table 1) (Faye et al., 2008). Amplification conditions were as follows: 50 °C for 30 min for reverse transcription; 94 °C for 2 min for denaturation; and 40 cycles of 94 °C for 30 s, 53 °C for 30 s, and 68 °C for 60 s for amplification. For quantitative analysis, cDNA was generated from the equivalent amount of total RNA from blood serum using a Verso cDNA Kit. Real-time RT-PCR was performed using ZIKV primers; ZIKV ENV_qpF and ZIKV ENV_qpR (Table 1). Primers for ZIKV were purchased from FASMAC. The sequences of primers are listed in Table 1. To analyse the survival rate, ribavirin (2 mg or 10 mg) was intraperitoneally administered daily starting on the day of infection.
2.8. Measurement of viral load in the brain
The brain samples were homogenized in 2% complete MEM. The homogenates were clarified by centrifugation. The titration was performed by plaque assay using the supernatant of tissue homogenates. The viral titer was adjusted for sample volume and tissue weight to calculate the titer as PFU/g.
2.9. Statistical analysis
We used the unpaired Student's t-test and log-rank test to evaluate the statistical significance of differences.
3. Results
3.1. Ribavirin and IFN-β potently inhibit ZIKV replication in Vero cells
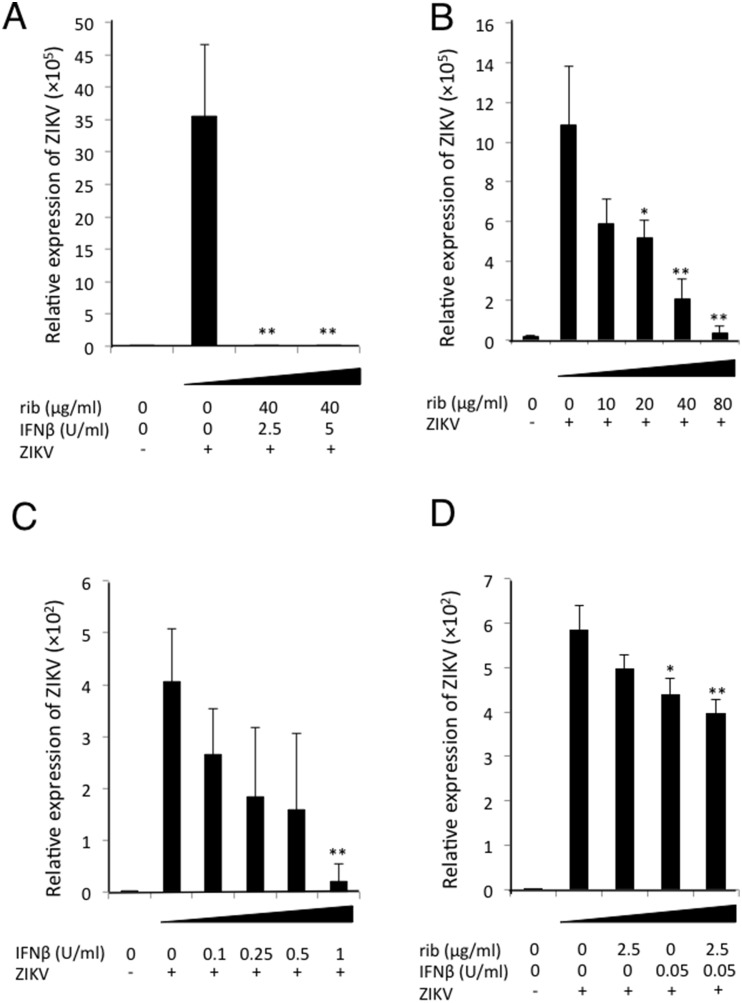
In general, ribavirin is prescribed in combination with type I IFN to patients with HCV. To examine whether the combination of ribavirin and IFN-β inhibits ZIKV replication, viral suppression assays in Vero cells were performed. RT-PCR assays revealed that 40 μg/mL of ribavirin together with 2.5 U/mL of IFN-β could inhibit the viral replication completely (Fig. 1 A). Next, we assessed the antiviral effect of each reagent on ZIKV replication. Treatment with 40 μg/mL of ribavirin could inhibit ZIKV replication not completely, but significantly, while treatment with 80 μg/mL of ribavirin could inhibit the replication almost completely (Fig. 1B). On the other hand, treatment with 1 U/mL of IFN-β could almost completely suppress the viral replication (Fig. 1C). Furthermore, low amount of ribavirin (2.5 μg/mL) or IFN-β (0.05 U/mL) slightly suppressed the viral replication, but no synergistic effect was observed (Fig. 1D).
Fig. 1.
Ribavirin inhibits ZIKV replication in Vero cells. (A–D) Vero cells were infected with ZIKV at an MOI of 5 × 10−5. After 1 h adsorption, 2% complete MEM containing both ribavirin and IFN-β (A, D), ribavirin (B) or IFN-β (C) was added on the cells. Cells were incubated for 4 days, total RNA was isolated from Vero cells, and expression of ZIKV RNA was analysed by quantitative RT-PCR assay. The relative amount of viral RNA expression normalized against the corresponding level of β-actin mRNA is shown. ‘ZIKV -’ represents uninfected cells. Data represent mean values ± S.D. of three independent experiments. **, P < 0.01. *, P < 0.05.
3.2. Ribavirin inhibits ZIKV replication in human and mosquito cells
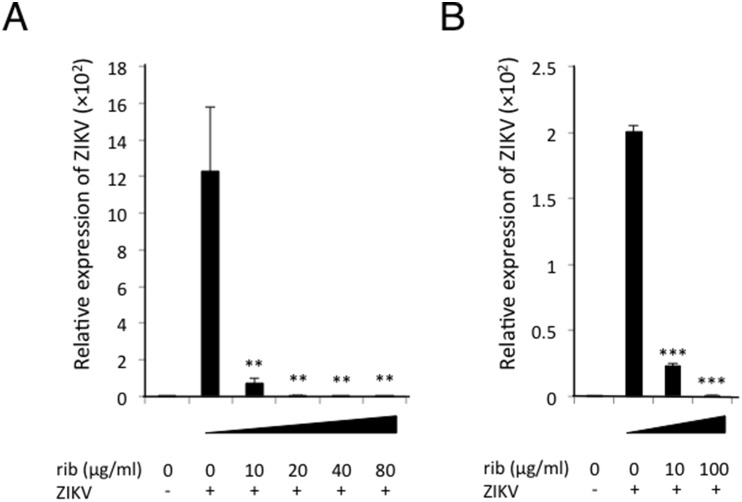
Since Vero cells are defective in the production of type I IFN, we examined the antiviral effect of ribavirin on cell lines that normally produce type I IFN. We infected ZIKV to SH-SY5Y cells, a human neuroblastoma cell line, and C6/36 cells, an Aedes albopictus cell line, and then treated the cells with ribavirin. Although 10 μg/mL of ribavirin partially suppressed the viral replication in Vero cells, it could strongly inhibit the replication in SH-SY5Y cells (Fig. 2 A). Moreover, treatment of C6/36 cells with 10 μg/mL of ribavirin significantly inhibited ZIKV replication (Fig. 2B). These results suggest that ribavirin more efficiently alleviates ZIKV replication in immune sufficient human cells and mosquito cells.
Fig. 2.
Ribavirin inhibits ZIKV replication in human neuroblastoma and mosquito cells. (A, B) SH-SY5Y cells (A) and C6/36 cells (B) were infected with ZIKV at an MOI of 5 × 10−4 and MOI of 5 × 10−5, respectively. Ribavirin was added on the cells followed by 4 days culture in vitro. The expression of ZIKV RNA was normalized by β-actin (A) or 40S ribosomal protein S17 mRNA (B). ‘ZIKV -’ represents uninfected cells. Data represent mean values ± S.D. of three independent experiments. ***, P < 0.001. **, P < 0.01.
3.3. Ribavirin prevents ZIKV-induced apoptosis and cell death
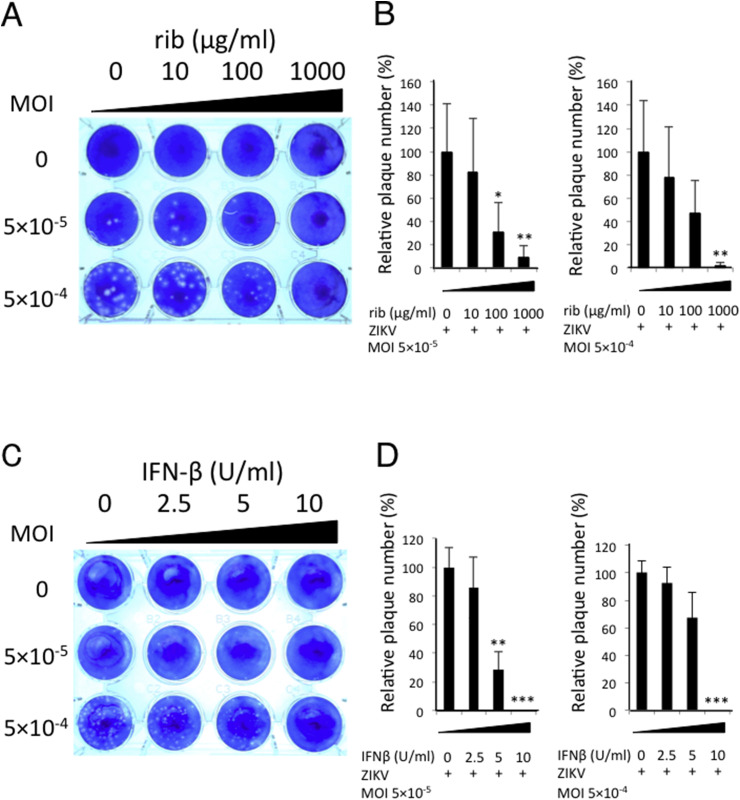
ZIKV infection of human neural progenitor cells causes an increase in caspase-3 activation, followed by cell death (Tang et al., 2016). Hence, we tested whether ribavirin would inhibit ZIKV-induced apoptosis and cell death. First, we infected Vero cells with ZIKV in the presence of various concentrations of ribavirin and performed plaque forming assays. Ribavirin inhibited plaque formation partially at a concentration of 10 μg/mL or 100 μg/mL, and almost completely at 1000 μg/mL, (Fig. 3 A and B). Similarly, 2.5 or 5 U/mL of IFN-β partially and 10 U/mL of IFN-β completely suppressed the plaque formation (Fig. 3C and D). These results suggest that both ribavirin and IFN-β can effectively prevent ZIKV-induced cell death in vitro.
Fig. 3.
Ribavirin inhibits ZIKV-induced apoptosis and cell death of Vero cells. (A–D) Vero cells were infected with ZIKV at an MOI of 5 × 10−4 or MOI of 5 × 10−5. After 1 h adsorption, 2% methylcellulose containing ribavirin (A, B) or IFN-β (C, D) was layered on the cells. Cells were incubated for 4 days, fixed in 10% formalin, stained with methylene blue, and photographed (A, C). Data are representative of four (A) or three (C) independent experiments. Relative plaque numbers to untreated control were graphed (B, D). Data represent mean values ± S.D. of four (B) or three (D) independent experiments. ***, P < 0.001. **, P < 0.01. *, P < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
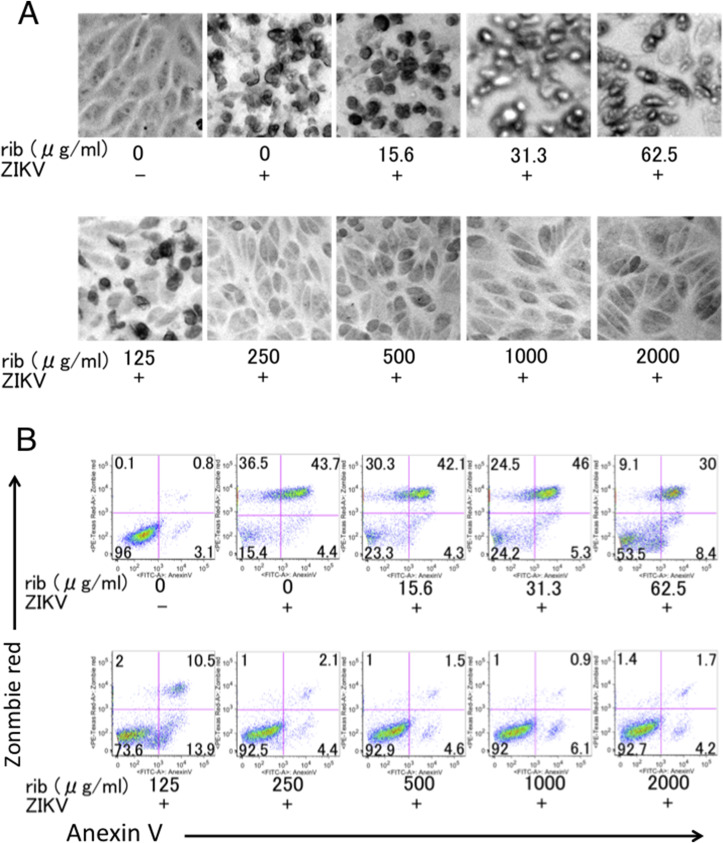
Confocal microscopic analysis revealed that uninfected Vero cells attached to the surface of culture dishes, while ZIKV-infected Vero cells detached from the dishes in the absence of ribavirin (Fig. 4 A). However, ribavirin allowed the ZIKV-infected Vero cells to attach to the dishes at concentrations of 250 μg/mL and above. ZIKV-induced cell death could be suppressed with no cytotoxicity in the presence of up to 2000 μg/mL of ribavirin. In addition, we quantified apoptotic and dead cells by flow cytometry using a fluorescent conjugate of annexin V and the amine-reactive fluorescent dye Zombie Red. As shown in Fig. 4B, annexin V+ apoptotic cells and Zombie Red+ dead cells could be detected in 3.1% and 0.9% of uninfected Vero cells, respectively. On the other hand, the proportions of dead cells in ZIKV-infected Vero cells increased to 80.2%. Consistent with the microscopic observation, the ratio of dead cells began to reduce at concentrations of 62.5 μg/mL and 125 μg/mL of ribavirin though the proportions of apoptotic cells were slightly increased. The ratio of apoptotic and dead cells in the presence of 250 μg/mL of ribavirin was almost comparable to those in uninfected cells. No cytotoxicity was observed even in the presence of 2000 μg/mL of ribavirin. Taken together, these results suggest that ribavirin prevents ZIKV-induced apoptosis and cell death without cytotoxicity.
Fig. 4.
Analysis of ZIKV-induced apoptosis and cell death by confocal microscopy and flow cytometry. (A, B) Vero cells were infected with ZIKV at MOI of 5 × 10−3. Following 1 h of adsorption at 37 °C, cells were cultured with 2% MEM containing ribavirin (15.6–2000 μg/mL). Four days post-infection, cells were observed by microscopy (A), or stained with annexin V and Zombie Red and analysed by flow cytometry (B). ‘ZIKV -’ represents uninfected cells. Data are representative of two independent experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
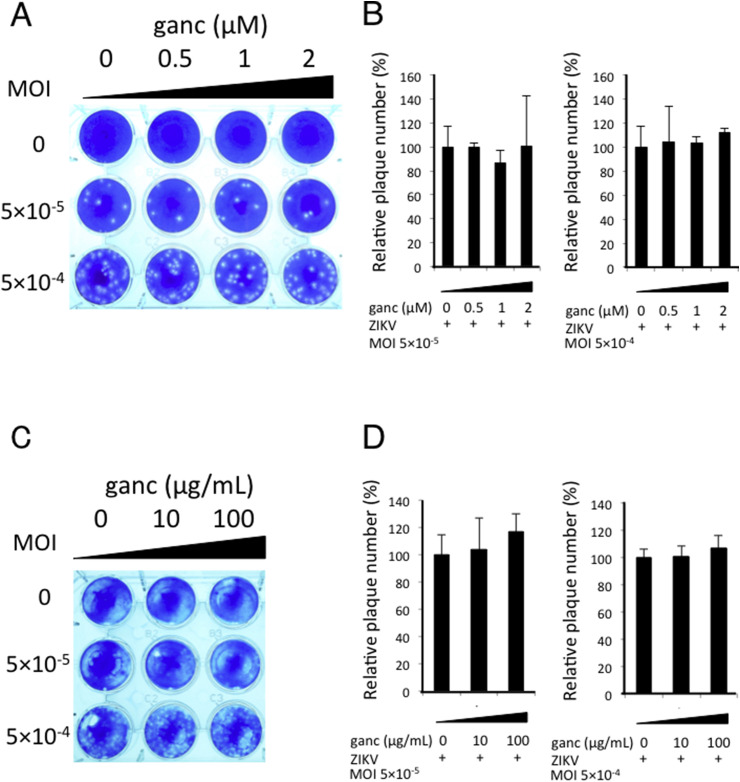
3.4. Ganciclovir can not prevent ZIKV-induced cell death
Ganciclovir is a potent inhibitor of herpes family of viruses, including cytomegalovirus (Crumpacker, 1996). The mechanism by which ganciclovir interrupt the replicative virus is selective and potent inhibition of viral DNA polymerase (Matthews and Boehme, 1988). It has been reported that in the plaque reduction assay, ganciclovir inhibited plaque formation at a concentration of 0.5 μM effectively (Tanaka et al., 2004). As expected, ganciclovir could not inhibit the plaque formation by ZIKV (Fig. 5 A and B). Moreover, the inhibitory effect of plaque formation was not observed even at a high concentration of ganciclovir (100 μg/mL) (Fig. 5C and D). These data suggest that DNA polymerase inhibitor is not effective against ZIKV.
Fig. 5.
Ganciclovir has no effect on ZIKV-induced apoptosis and cell death. (A–D) Vero cells were infected with ZIKV at an MOI of 5 × 10−4 or MOI of 5 × 10−5. After 1 h adsorption, 2% methylcellulose containing ganciclovir were layered on the cells. Cells were incubated for 4 days, fixed in 10% formalin, stained with methylene blue, and photographed (A, C). Data are representative of four (A) or three (C) independent experiments. Relative plaque numbers to untreated control were graphed (B, D). Data represent mean values ± S.D. of four (A) or three (D) independent experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Ribavirin administration abrogates viremia of ZIKV-infected STAT1-deficient mice
Finally, we investigated whether ribavirin can control the ZIKV replication in vivo.
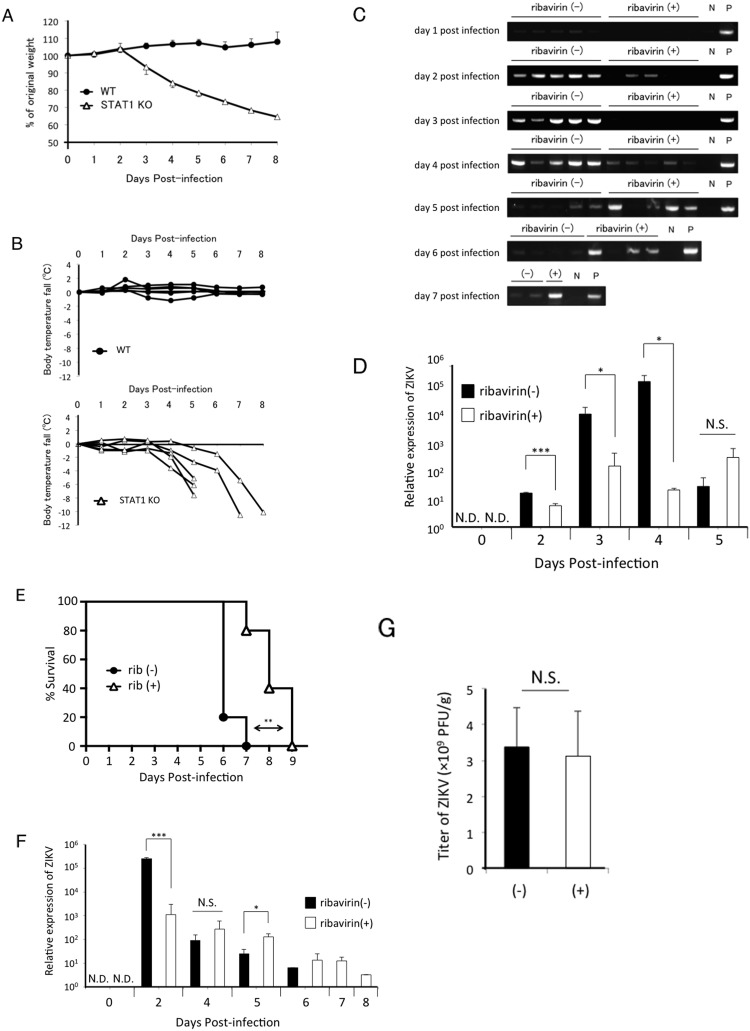
Wild-type mice are resistant to ZIKV infection, whereas Ifnar1-deficient mice are highly susceptible to the infection (Lazear et al., 2016). Consistent with previous reports, no viremia was detected in wild-type mice (data not shown). Because STAT1 functions as a signal transducer in the IFNAR1 pathway, we attempted to establish a ZIKV-infected mouse model using STAT1-deficient mice. An acute weight loss was observed in ZIKV-infected STAT1-deficient mice from day 3 post infection, and died between 6 and 9 day post infection losing 30–40% of original body weight (Fig. 6 A). Moreover, the viral infection resulted in significant hypothermia from day 4 post infection (Fig. 6B). Two days prior to the lethal outcome, the mice displayed abnormal behavior such as ataxic gait and shivering. Moreover, we analysed viremia by RT-PCR assay (Fig. 6C). An intense viremia began to be observed by day 2 post infection, however, interestingly, it started to decrease by day 5 post infection and subsequently, the mice died by day 8 post infection. We also quantified the viremia by real-time RT-PCR assay revealing that the level of viremia on day 3 post infection was more than a thousand times greater than that on day 1 post infection (Fig. 6D). This suggests rapid and robust virus replication in STAT1-deficient mice. These results indicate that STAT1-deficient mice are useful as a mouse model of lethal infection with ZIKV.
Fig. 6.
Analysis of viremia and survival of ZIKV-infected STAT1-deficient mice. STAT1-deficient mice were subcutaneously infected with 1.0 × 104 PFU of ZIKV per mouse. (A) The average of body weight (n = 6) and (B) individual rectal temperature curves (n = 5) were shown. (C) 15 mg of ribavirin (ribavirin +) (n = 5) or PBS (ribavirin −) (n = 5) was administered intraperitoneally for 3 consecutive days post-infection. Blood samples were collected daily from infected mice, and mRNA was extracted. Expression of ZIKV RNA was analysed by RT-PCR. N: no template control. P: positive control using viral diluent as a template. Data are representative of two independent experiments. (D) The quantitative expression levels of Zika virus. Blood serum samples in (C) were measured by real-time RT-PCR assay (n = 3 or 4 per group). The bar graph represents the mean and standard deviation values. N.S., not significant; ***, P < 0.001. *, P < 0.05. (E) 10 mg of ribavirin (rib +) (n = 5) or PBS (rib −) (n = 5) was administered intraperitoneally everyday. Survival rates were monitored. **, P < 0.01. (F) The quantitative expression levels of Zika virus. Blood serum samples in (E) were measured by real-time RT-PCR assay as shown (D). (G) The titration of brains on day 5 post infection in (C) was performed by plaque assay. The bar graph represents the mean ± S.D. N.S., not significant.
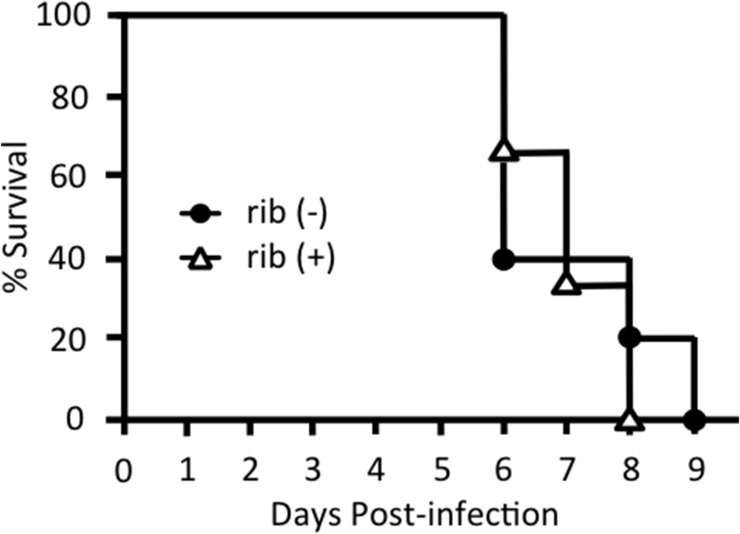
We then attempted to prevent ZIKV viremia in STAT1-deficient mice by treating them with ribavirin. We administrated 15 mg of ribavirin intraperitoneally to ZIKV-infected STAT1-deficient mice for 3 consecutive days after infection. Although ribavirin treatment suppressed the viremia until 4 days after infection, RT-PCR bands were observed 2 days after discontinuation of ribavirin administration (day 5 post infection) (Fig. 6C). Ribavirin treatment significantly suppressed the levels of viremia on day 2 and 3 post infection compared to those of untreated mice (less than one-tenth and one-thousandth, respectively), however, the levels of viremia were comparable between two groups on day 5 post infection (Fig. 6D). On the other hand, ribavirin treatment failed to suppress the viral load in the brain on day 3 (data not shown) and day 5 post infection (Fig. 6G). Moreover, daily administration of 10 mg of ribavirin intraperitoneally to ZIKV-infected STAT1-deficient mice significantly prolonged the survival time (Fig. 6E). Consistent with Fig. 6D, an intense viremia on day 2 post infection was inhibited by ribavirin but the low levels of viremia sustained until the death of these mice (Fig. 6F). In previous reports, administration of approximately 1–2 mg of ribavirin in DENV-infected mice and ZIKV-infected mice failed to reduce viremia in AG129 mice (Schul et al., 2007, Chang et al., 2011, Julander et al., 2017). Thus, we evaluated the effect of low doses of ribavirin in our mouse model, however, 2 mg of ribavirin could not suppress the viremia and not prolong the survival. (Suppl. Fig. 1). These data indicate that high-doses of ribavirin efficiently abrogate ZIKV viremia in the early stage of infection, leading to a prolonged survival of STAT1-deficient mice.
4. Discussion
In healthy adults, severe cases of ZIKV infection are uncommon, and about 80% of infections are asymptomatic (Duffy et al., 2009, Petersen et al., 2016, Slavov et al., 2016). However, ZIKV infection of pregnant women results in microcephaly and other developmental disorders in newborns, representing a major public health problem. To resolve the issue, rapid development of drugs that are useful for the prevention of Zika fever is necessary. Because it takes several years to develop a new drug, we tested the widely used antiviral drugs to examine the efficacy against ZIKV. As both HCV and ZIKV belong to family Flaviviridae, we hypothesized that ribavirin, a drug used to treat hepatitis C, would exert suppressive effects on ZIKV. Although it has been reported that ribavirin cannot inhibit ZIKV replication in Vero cells (Julander et al., 2017), we found that ribavirin potently inhibited ZIKV replication not only in Vero cells but also in human neuroblastoma cell line (Fig. 1, Fig. 2A). In addition, IFN-β also inhibited ZIKV replication in Vero cells, though the combination did not exert synergistic effect (Fig. 1D). Since most of Zika-endemic areas are developing countries and IFN-β is very expensive, a clinical application of IFN-β is difficult for such areas. In this study, we examined the efficacy of ribavirin against ZIKV infection because it is an affordable drug.
Ribavirin, whose safety has already been demonstrated, is widely used as a therapeutic drug for hepatitis C. Because ribavirin inhibits viral RNA-dependent RNA polymerases (Bougie and Bisaillon, 2003, Maag et al., 2001, Toltzis et al., 1988) and mRNA cap formation (Goswami et al., 1979). Indeed, ribavirin could inhibit the replication of DENV, Middle East respiratory syndrome (MERS) coronavirus, and severe acute respiratory syndrome (SARS) coronavirus (Falzarano et al., 2013a, Falzarano et al., 2013b, Rattanaburee et al., 2015, Koren et al., 2003), which suggests that ribavirin could be effective against RNA viruses. Thus, it is reasonable to predict that ribavirin could also suppress the replication of ZIKV, which is a single-stranded positive-sense RNA virus.
The blood concentration of ribavirin reaches approximately 24 μg/mL following intravenous injection of 1000 mg ribavirin to humans (Koren et al., 2003). Our data indicated that 20 μg/mL of ribavirin could almost completely inhibit ZIKV replication in human SH-SY5Y cells (Fig. 2A) suggesting possible application of ribavirin for Zika fever. However, it should be used in short-term treatment, because long-term oral administration of ribavirin causes hemolytic anemia as a frequent side effect (Bausch et al., 2010, Brochot et al., 2010, Koren et al., 2003, Poynard et al., 1998, Russmann et al., 2006), while no major side effects were reported in short-term treatment for acute Lassa fever with several intravenous shots of 1000 mg of ribavirin (Bausch et al., 2010, McCormick et al., 1986).
It is argued that ribavirin is contraindicated in pregnant patients because of its teratogenic activity in hamster and rat embryos (Ferm et al., 1978). Recently new drugs, ledipasvir and sofosbuvir, were adopted as therapeutic drugs for hepatitis C. Although there are no adequate data regarding the risk in human pregnancy, no adverse developmental outcomes of these drugs were observed in animal studies (Highlights, 2013, Highlights, 2014). A recent study reported that sofosbuvir inhibits Zika virus infection (Bullard-Feibelman et al., 2017), though it would be difficult to use the drugs in developing countries because of their expensiveness.
Generally, ZIKV can infect mainly primates but not wild-type mice. A few mouse models were reported, so far. For example, Ifnar1-deficient mice that lack type I IFN receptors are highly susceptible to ZIKV infection (Lazear et al., 2016). Furthermore, SJL mice that are defective for type I IFN signaling can be infected with ZIKV. It has been reported that the vertical infection could be occurred by trans-placental transmission in ZIKV-infected pregnant SJL mice (Cugola et al., 2016). However, we failed to confirm the ZIKV infection in SJL mice, in which viremia and lethal outcome were not observed (data not shown). In this study, we found that STAT1-deficient mice, which lack type I IFN signaling, were highly sensitive to ZIKV infection and exhibited lethal outcome. The viremia was observed 2–4 days after infection, however the levels of viremia decreased 5 days after infection without treatment for unknown reasons. Using this mouse model, we have demonstrated that ribavirin could successfully control ZIKV viremia and prolong the survival time significantly (Fig. 6C, E). However, ZIKV load in the brains were comparable between ribavirin-treated and untreated groups on day 5 post infection (Fig. 6G) and the low levels of viremia observed until the death of the mice treated with ribavirin (Fig. 6F), which suggests that ribavirin could not completely eliminate ZIKV. These results account for the lethal outcome in ZIKV-infected STAT1-deficient mice, however the mechanism underlying the prolonged mouse survival is still uncertain. We have shown that administration of ribavirin for the first three days after infection failed to prolong the survival of infected mice, even though they were treated with 15 mg of ribavirin (Fig. 6C). Schul et al. treated DENV-infected mice with 75 mg/kg of ribavirin for 3 day-consecutive administration. On the contrary, continuous administration of 10 mg of ribavirin throughout the course of infection could successfully prolong the survival (Fig. 6E). Thus the treatment regimens are critical for the control of ZIKV. On the other hand, daily administration of 2 mg (lower dose) of ribavirin, which resembles the doses used by other groups (Schul et al., 2007, Chang et al., 2011, Julander et al., 2017), could not prolong the survival of ZIKV-infected STAT1-deficient mice (Supplementary Fig. 1). The data suggests that daily administration of a high concentration of ribavirin is required to prolong the survival time of ZIKV-infected STAT1-deficient mice. Although low doses of ribavirin could inhibit viral replication judged by RT-PCR assay (Fig. 1), high doses of ribavirin were required for the suppression of apoptosis of infected cells judged by plaque assay (Fig. 3). Thus, even the viremia judged by RT-PCR assay appeared to be suppressed, apoptosis of cerebral nerve cell might be induced. The mechanism underlying lethal outcome of ZIKV-infected STAT1-deficient mice remains to be solved.
Competing financial interests
The authors have no competing financial interests to declare.
Acknowledgements
We thank Chiharu Aoki for excellent secretarial assistance, Ichiro Kurane for providing essential materials and members of Kobayashi's lab for valuable discussions. This work was supported by a Grant-in-Aid for Young Scientists (Start-up) (Grant No. 15H06512), Grant-in-Aid for Scientific Research (B) (Grant No. 26305014), Suzuken Memorial Foundation and GSK Japan Research Grant 2016.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.antiviral.2017.08.007.
Contributor Information
Naganori Kamiyama, Email: kamiyama@oita-u.ac.jp.
Takashi Kobayashi, Email: takashik@oita-u.ac.jp.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Fig. S1.
Low doses of ribavirin cannot prolong the survival time of ZIKV-infected STAT1-deficient mice. STAT1-deficient mice were subcutaneously infected with 1.0 × 104 PFU of ZIKV per mouse and 2 mg of ribavirin (rib +) (n = 3) or PBS (rib −) (n = 5) was administered intraperitoneally everyday. Survival rates were monitored.
References
- Abdel-Hady M., Bansal S., Davison S.M., Brown M., Tizzard S.A., Mulla S., Barnes E., Davies P., Mieli-Vergani G., Kelly D.A. Treatment of chronic viral hepatitis C in children and adolescents: UK experience. Arch. Dis. Child. 2014;99:505–510. doi: 10.1136/archdischild-2013-304601. [DOI] [PubMed] [Google Scholar]
- Bansal S., Singal A.K., McGuire B.M., Anand B.S. Impact of all oral anti-hepatitis C virus therapy: a meta-analysis. World J. Hepatol. 2015;7:806–813. doi: 10.4254/wjh.v7.i5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausch D.G., Hadi C.M., Khan S.H., Lertora J.J.L. Review of the literature and proposed guidelines for the use of oral ribavirin as postexposure prophylaxis for Lassa fever. Clin. Infect. Dis. 2010;51:1435–1441. doi: 10.1086/657315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougie I., Bisaillon M. Initial binding of the broad spectrum antiviral nucleoside ribavirin to the hepatitis C Virus RNA polymerase. J. Biol. Chem. 2003;278:52471–52478. doi: 10.1074/jbc.M308917200. [DOI] [PubMed] [Google Scholar]
- Brasil P., Pereira J.P., Jr., Raja Gabaglia C., Damasceno L., Wakimoto M., Ribeiro Nogueira R.M., Carvalho de Sequeira P., Machado Siqueira A., Abreu de Carvalho L.M., Cotrim da Cunha D., Calvet G.A., Neves E.S., Moreira M.E., Rodrigues Baião A.E., Nassar de Carvalho P.R., Janzen C., Valderramos S.G., Cherry J.D., Bispo de Filippis A.M., Nielsen-Saines K. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochot E., Castelain S., Duverlie G., Capron D., Nguyen-Khac E., François C. Ribavirin monitoring in chronic hepatitis C therapy: anaemia versus efficacy. Antivir. Ther. 2010;15:687–695. doi: 10.3851/IMP1609. [DOI] [PubMed] [Google Scholar]
- Bullard-Feibelman K.M., Govero J., Zhu Z., Salazar V., Veselinovic M., Diamond M.S., Geiss B.J. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antivir. Res. 2017;137:134–140. doi: 10.1016/j.antiviral.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Schul W., Butters T.D., Yip A., Liu B., Goh A., Lakshminarayana S.B., Alonzi D., Reinkensmeier G., Pan X., Qu X., Weidner J.M., Wang L., Yu W., Borune N., Kinch M.A., Rayahin J.E., Moriarty R., Xu X., Shi P.Y., Guo J.T., Block T.M. Combination of α-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antivir. Res. 2011;89:26–34. doi: 10.1016/j.antiviral.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C.S. Ganciclovir. N. Engl. J. Med. 1996;335:721–729. doi: 10.1056/NEJM199609053351007. [DOI] [PubMed] [Google Scholar]
- Cugola F.R., Fernandes I.R., Russo F.B., Freitas B.C., Dias J.L.M., Guimarães K.P., Benazzato C., Almeida N., Pignatari G.C., Romero S., Polonio C.M., Cunha I., Freitas C.L., Brandão W.N., Rossato C., Andrade D.G., Faria D. de P., Garcez A.T., Buchpigel C.A., Braconi C.T., Mendes E., Sall A.A., Zanotto P.M., Peron J.P., Muotri A.R., Beltrão-Braga P.C. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick G.W. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Dick G.W. Zika virus (II). Pathogenicity and physical properties. Trans. R. Soc. Trop. Med. Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- Duffy M.R., Chen T.H., Hancock W.T., Powers A.M., Kool J.L., Lanciotti R.S., Pretrick M., Marfel M., Holzbauer S., Dubray C., Guillaumot L., Griggs A., Bel M., Lambert A.J., Laven J., Kosoy O., Panella A., Biggerstaff B.J., Fischer M., Hayes E.B. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci. Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzarano D., de Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P., Brining D., Bushmaker T., Martellaro C., Baseler L., Benecke A.G., Katze M.G., Munster V.J., Feldmann H. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat. Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria N.R., Azevedo, Rdo S., Kraemer M.U., Souza R., Cunha M.S., Hill S.C., Thézé J., Bonsall M.B., Bowden T.A., Rissanen I., Rocco I.M., Nogueira J.S., Maeda A.Y., Vasami F.G. da S., Macedo F.L., Suzuki A., Rodrigues S.G., Cruz A.C., Nunes B.T., Medeiros D.B. de A., Rodrigues D.S., Nunes Queiroz A.L., da Silva E.V., Henriques D.F., Travassos da Rosa E.S., de Oliveira C.S., Martins L.C., Vasconcelos H.B., Casseb L.M., Simith, Dde B., Messina J.P., Abade L., Lourenço J., Alcantara Carlos Junior, de Lima M.M., Giovanetti M., Hay S.I., de Oliveira R.S., Lemos Pda S., de Oliveira L.F., de Lima C.P., da Silva S.P., de Vasconcelos J.M., Franco L., Cardoso J.F., Vianez-Júnior J.L., Mir D., Bello G., Delatorre E., Khan K., Creatore M., Coelho G.E., de Oliveira W.K., Tesh R., Pybus O.G., Nunes M.R., Vasconcelos P.F. Zika virus in the Americas: early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye O., Faye O., Dupressoir A., Weidmann M., Ndiaye M., Alpha, Sall A. One-step RT-PCR for detection of Zika virus. J. Clin. Virol. 2008;43:96–101. doi: 10.1016/j.jcv.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Feld J.J., Nanda S., Huang Y., Chen W., Cam M., Pusek S.N., Schweigler L.M., Theodore D., Zacks S.L., Liang T.J., Fried M.W. Hepatic gene expression during treatment with peginterferon and ribavirin: identifying molecular pathways for treatment response. Hepatology. 2007;46:1548–1563. doi: 10.1002/hep.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferm V.H., Willhite C., Kilham L. Teratogenic effects of ribavirin on hamster and rat embryos. Teratology. 1978;17:93–101. doi: 10.1002/tera.1420170117. [DOI] [PubMed] [Google Scholar]
- Goswami B.B., Borek E., Sharma O.K., Fujitaki J., Smith R.A. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem. Biophys. Res. Commun. 1979;89:830–836. doi: 10.1016/0006-291x(79)91853-9. [DOI] [PubMed] [Google Scholar]
- Hayes E.B. Zika virus outside Africa. Emerg. Infect. Dis. 2009;15:1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlights of Prescribing Information. Sovaldi (Sofosbuvir) Tablets, for Oral Use Initial U.S. Approval. 2013. [Google Scholar]
- Highlights of Prescribing Information. Harvoni (Ledipasvir and Sofosbuvir) Tablets, for Oral Use Initial U.S. Approval. 2014. [Google Scholar]
- Izumi K., Mine K., Inoue Y., Teshima M., Ogawa S., Kai Y., Kurafuji T., Hirakawa K., Miyakawa D., Ikeda H., Inada A., Hara M., Yamada H., Akashi K., Niho Y., Ina K., Kobayashi T., Yoshikai Y., Anzai K., Yamashita T., Minagawa H., Fujimoto S., Kurisaki H., Shimoda K., Katsuta H., Nagafuchi S. Reduced Tyk2 gene expression in β-cells due to natural mutation determines susceptibility to virus-induced diabetes. Nat. Commun. 2015;6:6748. doi: 10.1038/ncomms7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander J.G., Siddharthan V., Evans J., Taylor R., Tolbert K., Apuli C., Stewart J., Collins P., Gebre M., Neilson S., Van Wettere A., Lee Y.M., Sheridan W.P., Morrey J.D., Babu Y.S. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antivir. Res. 2017;137:14–22. doi: 10.1016/j.antiviral.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren G., King S., Knowles S., Phillips E. Ribavirin in the treatment of SARS: a new trick for an old drug? CMAJ. 2003;168:1289–1292. [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R.S., Kosoy O.L., Laven J.J., Velez J.O., Lambert A.J., Johnson A.J., Stanfield S.M., Duffy M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Diamond M.S. Zika virus: new clinical syndromes and its emergence in the western hemisphere. J. Virol. 2016;90:4864–4875. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Govero J., Smith A.M., Platt D.J., Fernandez E., Miner J.J., Diamond M.S. A mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leming M.T., Rund S.S., Behura S.K., Duffield G.E., O'Tousa J.E. A database of circadian and diel rhythmic gene expression in the yellow fever mosquito Aedes aegypti. BMC Genomics. 2014;15:1128. doi: 10.1186/1471-2164-15-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Xu D., Ye Q., Hong S., Jiang Y., Liu X., Zhang N., Shi L., Qin C.-F., Xu Z. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016;19:120–126. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Maag D., Castro C., Hong Z., Cameron C.E. Hepatitis C virus RNA-dependent RNA polymerase (NS5B) as a mediator of the antiviral activity of ribavirin. J. Biol. Chem. 2001;276:46094–46098. doi: 10.1074/jbc.C100349200. [DOI] [PubMed] [Google Scholar]
- Matthews T., Boehme R. Antiviral activity and mechanism of action of ganciclovir. Rev. Infect. Dis. 1988;10(Suppl. 3):S490–S494. doi: 10.1093/clinids/10.supplement_3.s490. [DOI] [PubMed] [Google Scholar]
- McCormick J.B., King I.J., Webb P.A., Scribner C.L., Craven R.B., Johnson K.M., Elliott L.H., Belmont-Williams R. Lassa fever: effective therapy with ribavirin. N. Engl. J. Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- Meraz M.A., White J.M., Sheehan K.C., Bach E.A., Rodig S.J., Dighe A.S., Kaplan D.H., Riley J.K., Greenlund A.C., Campbell D., Carver-Moore K., DuBois R.N., Clark R., Aguet M., Schreiber R.D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- Miner J.J., Cao B., Govero J., Smith A.M., Fernandez E., Cabrera O.H., Garber C., Noll M., Klein R.S., Noguchi K.K., Mysorekar I.U., Diamond M.S. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E.E., Staples J.E., Meaney-delman D., Fischer M., Ellington S.R., Callaghan W.M., Jamieson D.J. Interim guidelines for pregnant women during a Zika virus outbreak - United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2016;65:30–33. doi: 10.15585/mmwr.mm6502e1. [DOI] [PubMed] [Google Scholar]
- Poynard T., Marcellin P., Lee S.S., Niederau C., Minuk G.S., Ideo G., Bain V., Heathcote J., Zeuzem S., Trepo C., Albrecht J. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- Rattanaburee T., Junking M., Panya A., Sawasdee N., Songprakhon P., Suttitheptumrong A., Limjindaporn T., Haegeman G., Yenchitsomanus P. Inhibition of dengue virus production and cytokine/chemokine expression by ribavirin and compound A. Antivir. Res. 2015;124:83–92. doi: 10.1016/j.antiviral.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Russmann S., Grattagliano I., Portincasa P., Palmieri V.O., Palasciano G. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr. Med. Chem. 2006;13:3351–3357. doi: 10.2174/092986706778773059. [DOI] [PubMed] [Google Scholar]
- Sarno M., Sacramento G.A., Khouri R., do Rosário M.S., Costa F., Archanjo G., Santos L.A., Nery N., Vasilakis N., Ko A.I., de Almeida A.R. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schul W., Liu W., Xu H.Y., Flamand M., Vasudevan S.G. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J. Infect. Dis. 2007;195:665–674. doi: 10.1086/511310. [DOI] [PubMed] [Google Scholar]
- Slavov S.N., Otaguiri K.K., Kashima S., Covas D.T. Overview of Zika virus (ZIKV) infection in regards to the Brazilian epidemic. Braz. J. Med. Biol. Res. 2016;49:e5420. doi: 10.1590/1414-431X20165420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T. Flavivirus encephalitis and other neurological syndromes (Japanese encephalitis, WNV, Tick borne encephalits, Dengue, Zika virus) Int. J. Infect. Dis. 2016;45:24. [Google Scholar]
- Tanaka M., Kodaira H., Nishiyama Y., Sata T., Kawaguchi Y. Construction of recombinant herpes simplex virus type I expressing green fluorescent protein without loss of any viral genes. Microbes Infect. 2004;6:485–493. doi: 10.1016/j.micinf.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Tang H., Hammack C., Ogden S.C., Jin P., Wen Z., Qian X., Li Y., Yao B., Shin J., Zhang F., Lee E.M., Christian K.M., Didier R.A., Song H., Ming G.L. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toltzis P., O'Connell K., Patterson J.L. Effect of phosphorylated ribavirin on vesicular stomatitis virus transcription. Antimicrob. Agents Chemother. 1988;32:492–497. doi: 10.1128/aac.32.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura C.V., Maia M., Bravo-Filho V., Góis A.L., Belfort R. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;387:228. doi: 10.1016/S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Jamaluddin M., Wang S., Tian B., Garofalo R.P., Casola A., Brasier A.R. Ribavirin treatment up-regulates antiviral gene expression via the interferon-stimulated response element in respiratory syncytial virus-infected epithelial cells. J. Virol. 2003;77:5933–5947. doi: 10.1128/JVI.77.10.5933-5947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]