Abstract
The 31st International Conference on Antiviral Research (ICAR) was held in Porto, Portugal from June 11–15, 2018. In this report, volunteer rapporteurs provide their summaries of scientific presentations, hoping to effectively convey the speakers' goals and the results and conclusions of their talks. This report provides an overview of the invited keynote and award lectures and highlights of short oral presentations, from the perspective of experts in antiviral research. Of note, a session on human cytomegalovirus included an update on the introduction to the clinic of letermovir for the prevention of CMV infection and disease. The 31st ICAR successfully promoted new discoveries in antiviral research and drug development. The 32nd ICAR will be held in Baltimore, Maryland, USA, May 6–10, 2019.
Highlights
-
•
The 31st ICAR was held in Porto, Portugal, June 11–15, 2018.
-
•
This article provides an overview of the invited keynote and award lectures and highlights of short oral presentations.
-
•
ICAR provided an interdisciplinary forum to review recent developments in all areas of antiviral research.
-
•
The 32nd ICAR will be held in Baltimore, Maryland, USA, May 6–10, 2019.
1. Introduction
The International Society for Antiviral Research (ISAR) sponsors an annual international meeting, the International Conference on Antiviral Research (ICAR). The 31st ICAR was held at the Alfândega Conference Center in Porto, Portugal, from June 11–15, 2018. As in previous years (Andrei et al., 2017; Vere Hodge, 2013, 2014, 2015, 2017), the meeting provided an interdisciplinary forum at which investigators involved in basic, translational, and clinical research worldwide met to review recent developments in all areas of antiviral research, drug and vaccine development. The overarching goal of the conference is to drive the discovery of new antiviral therapies by fostering collaboration among scientists from the fields of basic virology, medicinal chemistry, pharmacology, animal models of disease and toxicology in academia and the pharmaceutical industry.
Once again we were honored by the scientists receiving ISAR awards of excellence. The Elion award lecture provided the perfect setting to discuss recent advances in anti-CMV treatment. The Women in Science (WIS) award lecture reminded us, once more, of the urgent needs to understand and effectively combat virus transmission in neglected populations. The intricate world of host-pathogen interactions and antiviral drug susceptibility was highlighted during the Prusoff lecture. The Holý award lecture recognized the synthesis of prodrugs as a hot topic in the field of medicinal chemistry.
Biological sciences are being revolutionized by big data, new genome sequencing and imaging technology. The antiviral field is no exception to this revolution. Next-generation sequencing is changing the way we recognize and diagnose the emergence of drug resistance and prevent treatment failure. Genomics and proteomics are helping the identification of host factors as new targets for drug development, and cryo-electron microscopy (cryo-EM) and tomography offer unprecedented, high-resolution images of virus particles. This year, ICAR recognized the need to discuss this revolution with a special session on Recent Technological Advances. How mutagenesis of the human genome may be used to decipher virus entry, or an excellent introductory lecture on cryo-electron microscopy and image reconstruction were among the highlights of this year's conference. Similarly, how new expertise may be applied to understand and predict the evolution of virus infections further recognized the role of new strategies to address long-standing unresolved questions.
We are positive that the 31st ICAR was another great annual gathering of antiviral researchers at all stages of their careers. This combination of experts from academia, industry, government, non-governmental organizations and other settings will continue to drive the progress of antiviral therapy. The complete 31st ICAR program is available at https://www.isar-icar.com/.
2. The ISAR awards
2.1. Gertrude Elion memorial award: Paul Griffiths, Center for virology, University College London medical School, London, UK
The winner of this year's Elion award was Paul Griffiths, whose award lecture focused on the development of a vaccine for the prevention of human cytomegalovirus (HCMV) infections in both infants (congenital) and for those receiving stem cell and solid organ transplants). For transplantation of a solid organ, the provision of an organ by a HCMV-positive donor (D+) to a HCMV-negative recipient (R-) results in the greatest chance of disease, of which viral load above a certain titer is the greatest predictor (the threshold concept). Paul presented the idea of viral peak shift – if a vaccine can shift the time when the peak viral titer occurs, then it can shift the onset of disease, and thus allow time for antiviral therapy to treat the virus before it turns into problematic and sometimes fatal disease.
Vaccination appears to induce both T- and B-cell responses, so its introduction to those with depleted immunity still has an effect. In studies to date, vaccination of R-patients resulted in a decreased incidence of re-infection. As for the mechanism of action for the antibodies produced as a result of the vaccine, that is still under investigation (could antibodies cause virus neutralization? Lysis of infected cells prior to shedding?). Paul's final thoughts revolved around the development of anti-HCMV drugs. Antivirals will still be necessary for the next 30–50 years upon introduction of the vaccine and mass vaccination of the population.
2.2. Antonín Holý memorial award: Chris Meier, University of Hamburg, Hamburg, Germany
In his lecture, Chris Meier nicely reviewed the efforts of his team to design and synthesize prodrugs of nucleoside monophosphates (NMPs), diphosphates (NDPs), and triphosphates (NTPs) (Meier, 2017). The use of prodrugs for the delivery of nucleotide-based antivirals is a hot topic in the field of medicinal chemistry. The reasons to mask nucleotides are clear: bypassing the phosphorylation step, due to limited metabolism of nucleoside analogues in the cell and efficient delivery of polar nucleotide analogues into the cells.
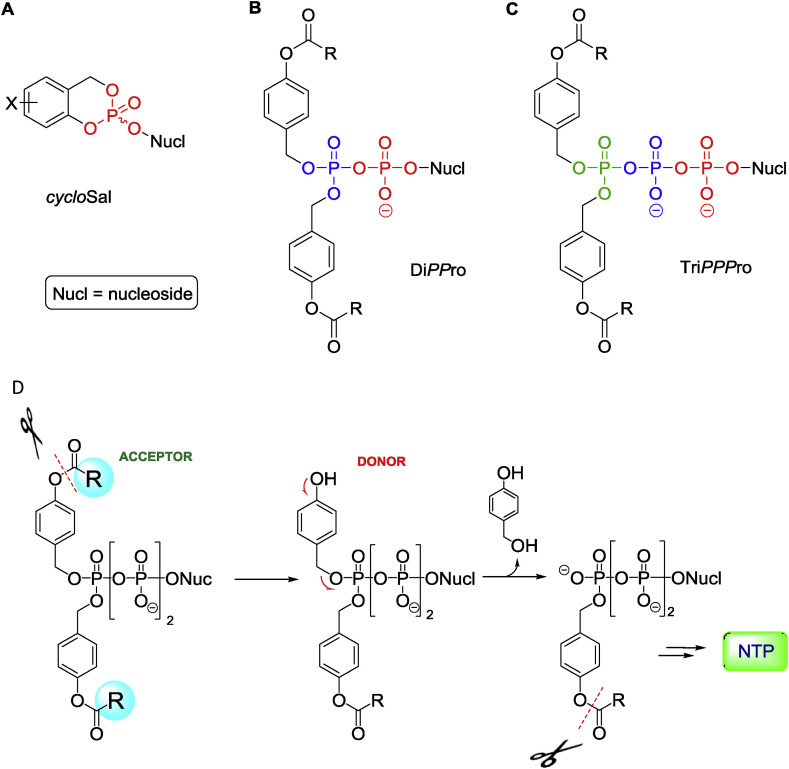
Chris first summarized the development of the cycloSal-approach for the intracellular delivery of NMPs (Fig. 1 A), in which salicyl alcohol was used as a cyclic bifunctional masking group, and release of the parent NMP was chemically driven (Meier and Balzarini, 2006). There are also numerous other types of NMP prodrugs, the most successful being the ProTide technology pioneered by Chris McGuigan (Cardiff, UK) and currently used for improved drug delivery (e.g. tenofovir alafenamide and sofosbuvir) (Mehellou et al., 2018; Slusarczyk et al., 2018).
Fig. 1.
Overview of nucleotide prodrugs developed by Chris Meier and colleagues. A) cycloSal-approach for delivery of nucleoside monophosphates (NMPs), B) DiPPro-approach for delivery of nucleoside diphosphates (NDPs), C) TriPPPro-approach for delivery of nucleoside triphosphates (NTPs). D) Schematic depiction of the TriPPPro-approach for the delivery of NTPs into cells. Figure kindly provided by Chris Meier.
Because NTPs are usually the antivirally active species, direct delivery of NTPs or NDPs would be beneficial, but chemically also quite challenging: “Direct delivery of triphosphate or diphosphate forms of the nucleoside analogues would be desirable but is impractical because of their instability during synthesis.” (Tan et al., 1999). Especially challenging, from the chemistry viewpoint, is the design of NDP and NTP prodrugs, for which the specific and selective removal of up to three or four masking groups, respectively, next to the phosphate anhydride bonds had to be achieved. It was expected that nucleophiles (e.g. intracellular water) would attack the phosphorus atom, leading to cleavage of the anhydride bond in fully masked NDPs and NTPs. The key idea of Chris's approach to avoid undesired cleavage was to keep the negative charge on the α- (for NDPs) or α- and β-phosphate (for NTPs) moieties, so that a nucleophile would not attack that position, due to electrostatic repulsion. This would lead to increased chemical stability of the phosphate anhydride bonds in the prodrugs of NDP and NTP prodrugs.
Chris next reported on the DiPPro-approach (Fig. 1B), that was developed for delivery of NDPs into cells, to bypass the second phosphorylation step (Meier, 2017). He especially mentioned the non-symmetric DiPPro-approach with controlled, stepwise removal of the prodrug moieties, that led to a highly selective delivery of NDPs (Weinschenk et al., 2015).
The ultimate challenge was the design and synthesis of NTP prodrugs, the so-called TriPPPro-approach (Fig. 1C). Two different routes -- phosphoramidite and H-phosphonate chemistries -- were used for the synthesis of target TriPPPro-compounds in good yields. As in the DiPPro-approach, TriPPPro-compounds bear two lipophilic groups at the terminal phosphate moiety (Gollnest et al., 2015, 2016). One can easily play with the masking groups, in order to tune lipophilicity and stability of the prodrug molecules. Longer alkyl chains increase the lipophilicity of the molecules, to compensate for the negative charges on the α- and β-phosphate moieties, and also enhance stability. Enzymatic hydrolysis of TriPPPro-compounds using porcine liver esterase (PLE) showed a selective formation of NTPs (Fig. 1D). Formation of the triphosphates was furthermore proven in primer-extension assays. Finally, Chris demonstrated several examples of TriPPPro-compounds derived from well-known antiviral agents, such as ddC (zalcitabine), d4T (stavudine), abacavir, and sofosbuvir. For example, the d4TTP-prodrug (stavudine triphosphate prodrug) was shown to release the active metabolite, d4TTP, and exhibited very good anti-HIV activity in TK-deficient cells. In studies in mice, application of a TriPPPro-derivative based on the sofosbuvir nucleoside led to a two-fold increase in the concentration of the corresponding triphosphate in the liver, compared to sofosbuvir.
2.3. The Women in Science award: A. Desiree LaBeaud, pediatric infectious diseases, Stanford University, Stanford, CA, USA
This year's Women in Science Awardee was A. Desiree LaBeaud. She began her talk by describing the breadth and attributes of dengue viral (DENV) and chikungunya (CHIKV) infections, which largely impact neglected populations. Of note, vector-borne diseases are estimated to account for 17% of all infectious diseases, with one million deaths per year. They are spreading largely due to the influences of modern life, including significant travel by up to 2 billion people each year, and increased niches that lead to mosquito breeding. These diseases are both endemic and epidemic. There are 65 million cases of dengue per year in Africa, and this year, Kenya is experiencing an outbreak of CHIKV. In Kenya alone, 10–20% of the children are exposed to DENV and CHIKV, though not all develop disease. Desiree noted the tremendous need for antivirals and vaccines to treat and prevent dengue and chikungunya disease as well as continued efforts to increase awareness and reduce breeding grounds for mosquitos.
Desiree presented findings on infection and disease for DENV and CHIKV at four urban and rural sites in Kenya. Her study includes two cohorts of children. The first is comprised of acutely ill children 1–17 years old (10 recruited per site per week) seen at illness onset and one month later. About 50% were also positive for malaria. The second cohort contains approximately 500 healthy children aged 1–12 years at each study site, visited every 6 months. Since the inception of the study, 5% of cases of fever were due to DENV infection and 3% were due to CHIKV infection. There is a stable source of virus in these communities, and outside of outbreaks, the incidence of disease is equal across all ages. However, during outbreaks the incidence of disease increases with increasing age, probably due to human movement. It was noted that both diseases have long-term effects. Of note, dengue patients are fully recovered at 1 month postinfection, and fare worse with malaria coinfection (Vu et al., 2017).
For the conclusion of the talk, Desiree described her work on mosquito population reduction as a strategy to reduce disease. Specifically, she noted that there were a number of containers with no purpose left in the communities that filled with stagnant water and drove the breeding of mosquitoes. She began a program involving children and mothers for collection and burial of these containers, as well as adding lids to used water containers. This intervention strategy had a major impact on reducing mosquito populations. Various activities and initiatives were introduced to the community including games with the children to collect unused containers.
2.4. William Prusoff young investigator award: Ester Ballana, IrsiCaixa - Institute for AIDS research, Barcelona, Spain
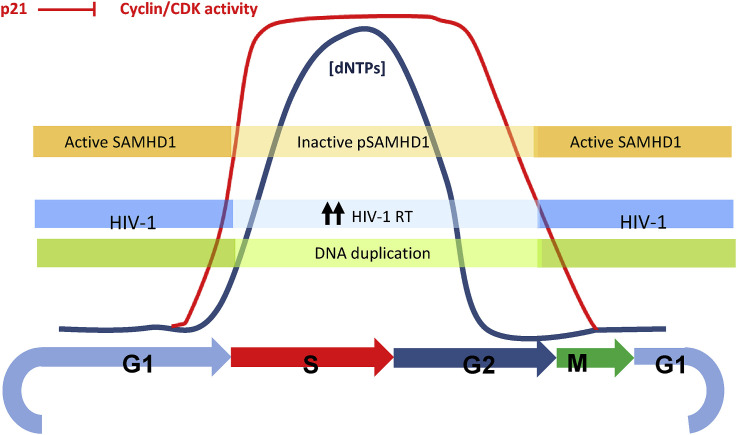
Ester Ballana began her lecture by introducing the role of host factors in modulating virus infections, in particular HIV-1. Restriction factors are host antiviral proteins that counteract or ‘restrict’ viral replication, and are considered a first line of defense against viruses or other incoming pathogens. Sterile-alpha-motif-and-histidine-aspartate-domain-containing protein 1 (SAMHD1) is a member of a unique group of restriction factors that limit retroviral replication at distinct stages of the viral life cycle (Ballana and Este, 2015). HIV-2 Vpx induces degradation of SAMHD1 by targeting it to the proteasome through addition of ubiquitin (Ub), bypassing SAMHD1 viral restriction. SAMHD1 is tightly linked to cell-intrinsic innate immune responses that direct antiviral defenses by triggering interferon (IFN) production and to the regulation of the cell cycle and cell proliferation (Fig. 2 ). Phosphorylation of SAMHD1 by cyclin-dependent kinases (CDK) is associated with deactivation of the restriction activity on HIV-1 replication in primary lymphoid and myeloid cells (Badia et al., 2016, 2017). Selective CDK4/6 inhibitors impede SAMHD1 phosphorylation, activating SAMHD1 function and blocking HIV-1 replication (Pauls et al., 2014).
Fig. 2.
SAMHD1 as a link between the cell cycle and HIV-1 susceptibility in primary cells. Regulation of intracellular dNTPs is essential for normal cellular metabolism. A balanced supply of each of the four canonical dNTPs is required for accurate genomic and mitochondrial DNA synthesis and repair; nucleotide metabolism is therefore precisely regulated during different stages of the cell cycle. Activation of SAMHD1 depends on cell cycle regulation. Cyclin-dependent kinases (CDK) phosphorylate SAMHD1, leading to its inactivation; in turn, p21, a natural CDK inhibitor promotes SAMHD1 activation. HIV-1 reverse transcriptase activity is favored by an inactive SAMHD1 and high dNTP levels. Figure kindly provided by Ester Ballana.
SAMHD1 restricts HIV-1 infection at the reverse transcriptase step in non-proliferating cells. The putative restriction mechanisms of SAMHD1 lie in its dNTP-triphosphohydrolase activity, which regulates the intracellular dNTP pool. Ester showed that SAMHD1 is a modulator of nucleotide analogue efficacy, whereas sensitivity to a non-nucleoside inhibitor (nevirapine) or to nucleoside phosphonates (cidofovir and tenofovir) is not affected. However, she also showed that SAMHD1 could either enhance or reduce the antiviral potency of anti-cancer analogues, an effect that was not dependent on the specific nucleotides targeted, but on their capacity to act as substrates or competitors for the catalytic site in SAMHD1 of the natural nucleotides. Additionally, selective CDK inhibitors also modify the antiviral activity of antimetabolites, an activity that is lost in SAMHD1-knockdown cells. She concluded that modulation of SAMHD1 function may constitute a promising target for the improvement of multiple therapies that employ nucleotide analogues or antimetabolites affecting the dNTP pool.
3. Cytomegalovirus
3.1. Neurodevelopmental sequelae of congenital CMV infection: role of virusiInduced inflammation. William Britt, Departments of pediatrics, microbiology, and neurobiology; School of Medicine, University of Alabama, Birmingham, Alabama, USA
Bill Britt presented information on congenital HCMV infections, whose long-term sequelae are primarily associated with damage to the central nervous system damage. The mechanisms of injury remain undefined, and it is still not understood why there is so much damage in infants with low levels of virus. Recent studies using animal models and autopsied patients indicated that the host inflammatory response plays a pivotal role in the development of the disease (specifically, auditory deficiencies).
Using murine cytomegalovirus (MCMV) as a model to define mechanisms of CNS disease, Bill's studies have demonstrated that host inflammatory responses are responsible for altered neurodevelopment in infected mice during the CNS development stage. Blocking inflammation with prednisolone or an anti-TNF antibody decreased the incidence, even though there were no major changes in virus replication/titers. The data indicate that antiviral therapy with anti-inflammatory agents could improve the long-term outcome of infants with congenital HCMV infections.
3.2. Treatment of congenital CMV infection: new populations, new regimens, and new drugs? David Kimberlin, University of Alabama, Birmingham, Alabama, USA
David Kimberlin presented information on infants with symptomatic CMV infections, which are straightforward to diagnose, since clinical manifestations drive the diagnosis, and asymptomatic infections, for which diagnosis is more difficult, and can only be achieved though broad-based screening. HCMV-induced hearing loss may not be present at birth, but can develop by 15 years of age (delayed onset). Data are only available for infants with symptomatic disease, with treatment started in the first month following diagnosis. Long-course ganciclovir (GCV) treatment resulted in a reduction of worsening/abnormal hearing loss; 6 months of therapy is superior to 6 weeks. The main adverse effect, neutropenia, was lessened with the use of valganciclovir, when compared to IV GCV. As additional drugs for the treatment of HCMV are developed, their use as monotherapies or in combination with valganciclovir for the treatment of congenital HCMV appears promising.
3.3. Letermovir: current state of the art. Randi Leavitt, clinical research, MSD, North Wales, PA, USA
Randi Leavitt presented data from the letermovir (LET) prophylaxis Phase 3 trial for haematopoietic stem cell transplant (HSCT) recipients (Marty et al., 2017). Because pre-emptive therapy is initiated only once viremia is detected, it is associated with an increased risk of overall mortality; prophylactic therapy is therefore preferred. Results using LET demonstrate a 23.5% decrease in therapy failure, which was primarily due to clinically significant levels of HCMV. In addition, by week 24, there was a 25.4% decrease in time to infection with LET vs. placebo. Finally, there was a significant decrease in adverse events (31.7%) when compared to placebo (in which no neutropenia was observed). A safer and more efficacious prophylactic agent for the prevention of HCMV infections is important for public health. Further research into the utility of LET is currently under way.
3.4. Letermovir resistance analysis in a clinical trial of cytomegalovirus prophylaxis for haematopoietic cell transplant recipients. Cameron Douglas, infectious disease research, Merck & Co., Inc., Kenilworth, NJ, USA
Cameron Douglas provided further information on LET resistance in a clinical trial of HCMV prophylaxis for HSCT recipients. CMV is a common and potentially life-threatening viral infection in this patient population. CMV R+ who undergo HSCT are at high risk for CMV reactivation, and CMV infection is associated with increased mortality. LET is a HCMV DNA terminase inhibitor with EC50 values against clinical isolates in the range of 0.7 nM–6.1 nM. LET is active against strains with mutations in UL97 and UL54 that confer resistance to DNA polymerase inhibitors. Based on the positive results from a Phase 3 clinical trial of HCMV prophylaxis for HSCT recipients (Marty et al., 2017), LET was approved in the EU for prophylaxis of CMV reactivation and disease in adult CMV R+ of an allogeneic HSCT. The recommended dosage is 480 mg once daily, initiated as early as day 0 and up to day 28 post-transplantation, and continued through day 100. The dose should be decreased to 240 mg once daily if it is administered with cyclosporine.
Cameron presented an analysis of LET resistance in Phase 3 trial subjects who experienced clinically significant CMV infection (csCMVI) with detectable DNAemia was presented. Total DNA was isolated from plasma at the time of csCMVi, CMV genes encoding pUL56 and pUL89 (subunits of the terminase complex) were amplified by PCR, and next-generation sequencing was performed. Genotyping was successfully carried out for 50 of 79 LET subjects with csCMVi, including 10 subjects with detectable CMV DNA on day 1 of prophylaxis. Replicate testing was performed to identify novel genotypic changes that were PCR artifacts, rather than true variants. A number of previously characterized natural genetic polymorphisms with no impact on LET susceptibility were also seen.
The remaining novel variants in UL56 and UL89 were characterized for their potential to confer resistance by recombinant phenotyping using the BAC system, which identified only three UL56 variants that conferred a LET EC50 shift in a cell-culture model of infection. These mutations (E237G, V236M and C325W) were from three subjects who received LET and experienced csCMVi. The pUL56 E237G mutation, associated with a modest 13-fold LET EC50 shift, was detected in 4.1% of the NGS reads from a subject with <151 CMV DNA copies/ml. Interestingly, a subject in the placebo arm also had this substitution at a low frequency (1.8% of the NGS reads). The pUL56 V236M mutant (30–50-fold LET EC50 shift) was detected in a subject who missed 5 of the first 10 doses of LET; this amino acid substitution was also detected in a subject enrolled in the Phase 2b trial who had received a suboptimal 60 mg LET dose. The UL56p C325W mutant (>8000-fold LET EC50 shift) was from a patient who was CMV DNAemic on day 1. Notably, recombinant viruses bearing the pUL56 substitutions E237G, V236M and C325W showed little to no change in their susceptibility to ganciclovir, cidofovir or foscarnet. Because the Phase 3 trial was conducted before there was information about LET-resistant mutations in UL51, this gene was not analyzed. Fitness studies with the terminase-mutant viruses showed no major growth impairment.
4. Recent technological advances
The session on recent technological advances presented two novel and robust experimental approaches that are being used to better understand viral replication and applied as tools for antiviral drug discovery.
4.1. Mutagenesis of the human genome to study virus entry. Thijn Brummelkamp, Netherlands Cancer Institute, Dept. of biochemistry, Amsterdam, The Netherlands
Thijn Brummelkamp described the use of haploid human cells in combination with insertional mutagenesis as a robust and potent tool to identify host factors used by viruses. He began by describing the approach, which is based on a derivative of a chronic myeloid leukemia (CML) cell line with a haploid karyotype (KBM7) and uses gene-trap retroviruses that contain a green fluorescent protein (GFP) marker in reverse orientation of the retroviral backbone. He described how gene inactivation by insertional mutagenesis allows the generation of null mutants for nonessential genes in this cell line, and how the gene-trap insertions can be mapped by deep sequencing. He and his group have applied this approach to identify host factors used by viruses from different families, and he presented several examples that illustrate the power of haploid genetic screens.
Thijn first described the identification of endo/lysosomal cholesterol transporter protein Niemann–Pick C1 (NPC1) as a host factor required for Ebola virus entry (Carette et al., 2011). Further study of NPC1 provided mechanistic evidence that Ebola glycoproteins directly bind to NPC1 inside the cell (Miller et al., 2012). He presented similar results for Lassa virus and the lysosome-associated membrane protein 1 (LAMP1), showing that both viruses need a cellular trigger to permit binding of the viral envelope protein to these intracellular receptors.
Applying the technology to the Picornaviridae, two distinct factors were identified: KREMEN1 and PLA2G16. Thijn showed how the cell surface molecule KREMEN1 was identified as the major entry receptor for Coxsackie virus A10 (CV-A10) and how KREMEN1 is required for CV-A10 pathogenesis (Staring et al., 2018). In addition, the phospholipase PLA2G16 was also recognized as a picornavirus host factor, which is required early during infection, enabling virion-mediated genome delivery into the cytoplasm. Remarkably, in the absence of PLA2G16 the incoming virions were subjected to an anti-bacterial clearance pathway (Staring et al., 2017).
4.2. A primer on cryo-EM and image reconstruction for antiviral drug development. Sarah Butcher, University of Helsinki, Helsinki, Finland
Sarah Butcher began by providing a detailed description of the basis of electron cryo-EM technique and how significant improvements in image reconstruction technologies have improved the number and quality of high-resolution structures in recent years. She described the pipeline of cryo-EM technology, which is based in the vitrification of a sample in an aqueous solution, followed by rapid immersion in liquid ethane, and finally imaging the “frozen-hydrated” sample in a specialized transmission electron microscope. She explained how the images are recorded and processed, generating an average three-dimensional representation which models the atomic coordinates of the object, eliminating the need for crystallization.
The second part of Sarah's talk focused on applications of cryo-EM to drug discovery, and she gave two examples. First, she described the use of cryo-EM to guide the design of a benzenesulfonamide derivative as an antiviral drug against Coxsackie B virus. In this case, atomic analysis of viral capsid proteins bound to the drug allowed identification of a new hydrophobic drug-binding pocket. Data on resistance mutations mapping to the binding pocket revealed by cryo-EM suggested that this newly revealed structure could be a potential target for the development of novel inhibitors. Next, she used respiratory syncytial virus (RSV) as an example of structural heterogeneity among viral particles. Electron cryo-tomographic characterization of RSV virions showed highly heterogeneous architectures, either spherical, filamentous, or a combination of both morphologies. Cryo-EM followed by sub-tomogram averaging revealed that different morphologies correlated with local ordering of the glycoprotein spikes, either in pre-fusion or post-fusion conformations (Liljeroos et al., 2013). To sum up, Sarah discussed the advantages and caveats of cryo-EM technology, highlighting its enormous potential, especially for resolving structures of not very small proteins (up to 0.1 MDa), but also pointing out some still unresolved issues, such as averaging and computational requirements and the need to develop standardized validation methods.
5. Viral hepatitis
5.1. Keynote address: hepatitis C virus: problem solved. and now? Jean-Michel Pawlotsky University of Paris-Est, Paris, France
Jean-Michel Pawlotsky told the revolutionary story of anti-hepatitis C virus (HCV) drug development, covering all relevant aspects, including the HCV medical burden, milestones of direct acting antiviral (DAA) discovery, the combination paradigm, handling of drug resistance, through the current standard of care in industrialized countries. He did not forget to state that huge efforts are still needed to identify all HCV patients, particularly in low-income countries, and treat them to potentially eliminate this disease worldwide. Currently we are far from achieving this goal …
Chronic HCV infection in the 1990s was a lethal disease in the long term leading to hepatocellular carcinoma (HCC) in undiagnosed and untreated patients. The first line of therapy, then based on nonspecific interferon-alpha (initially free, then pegylated) combined with ribavirin, was associated with a poor sustained virological response (SVR). But HCV encodes several enzymes and targetable proteins, including the serine protease NS3-4A, the polyfunctional NS5A protein and the NS5B polymerase. Therefore, drugs targeting these viral functions were soon developed and tested in clinic. Jean-Michel reminded us that the path was not without difficulties, and that many early drugs (e.g. BILN-2061, boceprevir, telaprevir) failed at a late stage of clinical evaluation, or were approved, but quickly replaced by better drugs.
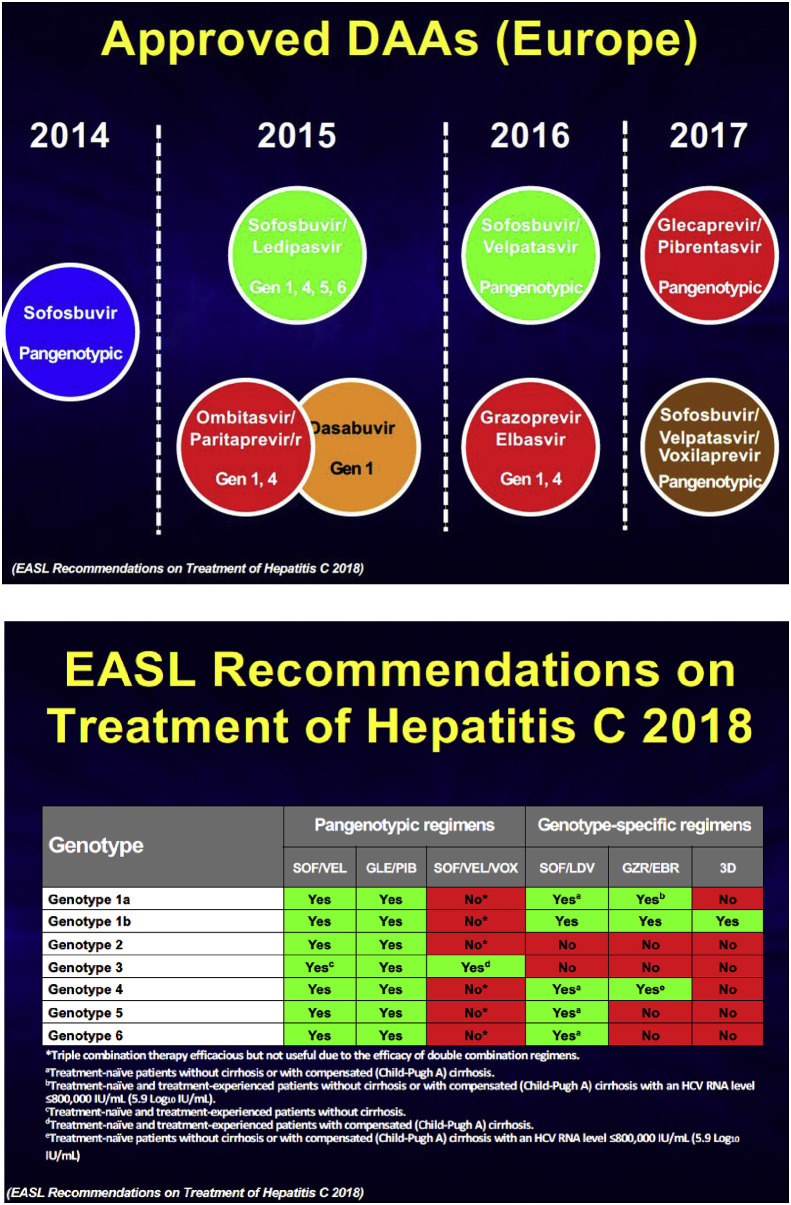
A major milestone was the approval in 2014 of sofosbuvir, a very potent nucleotide analogue targeting NS5B. This drug remained the backbone of combination therapy for a few years, but now many potent drugs targeting either NS3-4A, NS5A, or NS5B are approved and can be combined in bi or tri-therapy to reach virtually 100% SVR in most patients in a pan-genotypic manner (Fig. 3 ).
Fig. 3.
Timeline of the approval of HCV direct acting antivirals and the recommendations for treatment from the European Association for the Study of the Liver (EASL, 2018).
All patients benefit from a treatment leading to a cure, but the earlier they are treated, the greater the reduction in the risk of developing complications (decompensated cirrhosis, HCC). The World Health Organization's goal for 2030 is 90% of new infections prevented, 80% of eligible patients treated, and a 65% reduction in the mortality rate. Some countries such as Australia, Egypt, England, France, Germany, Japan, Spain and few others are on track with this road map, but many others need to improve their national action plans. So far it is estimated that only around 5% of patients have been treated worldwide. In China, which counts 10 million infected people, 20% have been diagnosed, but only 1–2% have been treated (www.polarisobservatory.org). “We know how to do it; let's make it happen.”
5.2. Mechanisms of immune dysfunction in chronic hepatitis B and possible immune therapies. Percy Knolle, Institute of molecular immunology and experimental oncology, Technische Universitaet Muenchen, Muenchen, Germany
Percy Knolle gave an excellent state-of-the-art lecture on the mechanisms of immune disfunction in chronic hepatitis B and possible immune therapies. Chronic HBV infection affects 250 million individuals worldwide, for whom the existing prophylactic vaccine is useless and a curative treatment option does not exist. Indeed, currently the sole therapeutic options rely on either peg-IFNα or nucleoside analogues, but those options lead to HBsAg loss (called a functional cure) in only 10% of patients. In contrast to hepatitis C, which can be cured with direct-acting antivirals, it is expected that immunotherapy of some sort will be necessary to functionally cure chronic HBV infections.
First, Percy reminded us that HBV infections occur in the context of a physiologic tolerogenic environment in the liver (i.e. high levels of IL-10, TGF-β, arginase production by liver macrophages and myeloid cells, LSEC, and hepatocytes). This may initially favor viral replication in the absence of primary innate cell-mediated inflammation, and renders possible the induction of T-cell exhaustion/attrition in a few patients (around 10% of immunocompetent adults) in a virologic context of high and persistent replication. In chronically infected patients, there is a long-lasting failure of HBV-specific immune responses that results in persistence. Yet in many patients, HBV infection remains acute and is cleared rapidly by strong and poly-epitopic T-cell responses. This implies that the infection can be defeated by immune responses, and that in chronically infected patients one would have to break the HBV-driven immune subversion to restore full control of the virus. Most therapeutic vaccination strategies have failed to restore immune control of infection (for a list of past and current trials, see Table 1 ).
Table 1.
Past and current trials of hepatitis B vaccines.
| Homologous vaccines | ||
| HepT cell | Peptide + adjuvant | Phase 1 |
| INO-1800 | DNA-vaccine | Phase 1 |
| CVI-HBV-002 | DNA-vaccine | Phase 1/2 |
| HB-110/100 | DNA-vaccine | Phase 1a |
| ppdpSC18 | DNA-vaccine | Phase 1/2a |
| HBO2-Vac-AND | DNA-vaccine | Phase 1/2a |
| Theravax | Protein + adjuvant | Phase 1ba |
| GS-4774 | Protein + adjuvant | Phase 2a |
| ePA-44 | Peptide + adjuvant | Phase 2a |
| ABX 203 | Protein | Phase 2/3a |
| TG1050 | Adeno vector vaccine | Phase 2 |
| Heterologous prime –boost vaccines | ||
| pSG2.HBs/MVAHBs | DNA-vaccine + MVA | Phase 1b/2a |
| TherVacB | Protein + MVA (broad) | Preclinical PoC |
Failed.
For the rest of his presentation, Percy focused on his own efforts to develop a therapeutic vaccination option based on a protein prime/MVA-vector boost strategy in patients who already receive a nucleoside analogue (NUC). NUC therapy reduces viremia, and therefore therapeutic vaccination occurs in a context of low serum virus titers. The TherVacB strategy, developed with Prof. Ulrike Protzer (Institute for Virology, Munich), relies first on protein priming with HBV surface antigen and core protein to induce neutralizing antibodies and to prime T cells. A boost with an MVA vector encoding various HBV epitopes (not disclosed) is then given, which expands HBV-specific T cells in liver parenchymal secondary/tertiary lymphoid structures called iMATEs (Huang et al., 2013).
To demonstrate the efficacy of this approach, Percy used an immune-competent mouse model that is transduced with an adenovirus-associated virus vector carrying the HBV genome [AAV-HBV (Dion et al., 2013);]. AAV-HBV launches a persistent infection in mice, which recapitulates the T-cell exhaustion phenotype. In this model, TherVacB induced HbsAg loss, anti-HBs antibody production (anti-HBs seroconversion), and effector HBs-specific intrahepatic CD8+ cells in iMATEs. Interestingly, the formation of iMATES was also favored by an “adjuvantation” procedure, based on CpG agonists (TLR9 ligand). At a more subcellular level and with an in-depth mechanistic effort, Percy presented unpublished data showing that iMATEs were induced by a TNFα/TNFR1 signaling pathway, leading to ROS-mediated mitochondria reshaping. If further preclinical validation of the TherVacB strategy is needed, a clinical evaluation of this patented approach is soon expected.
5.3. HBc and CAMs: a tale of a “Swiss-Army-knife” protein and antivirals. David Durantel, Cancer Research Center of Lyon (CRCL), INSERM, U1052, UMR_5286 CNRS/University of Lyon, Lyon, France
David Durantel's lecture summarized current efforts to develop molecules targeting the assembly of the HBV nucleocapsid, thereby preventing the neosynthesis of viral DNA. The core (HBc) is a structural protein that assembles a capsid around the pregenomic RNA, and reverse transcription then takes place to generate relaxed circular DNA (rcDNA). Core assembly modulators (CAMs) inhibit this process and the neosynthesis of rcDNA, similar to the action of nucleoside analogues (NUC). The HBc protein also has other regulatory functions in infected cells. David reminded us that, in cells with high levels of HBV replication, HBc is in the nucleus, where it binds to cccDNA and may contribute to its transcriptional activity or stability. HBc also binds to host DNA and may regulate host gene expression. Finally, as HBc is mainly an RNA-binding protein, it could bind to viral RNAs and regulate their fate. Due to the multifunctional character of HBc, CAMs might do more than initially envisaged.
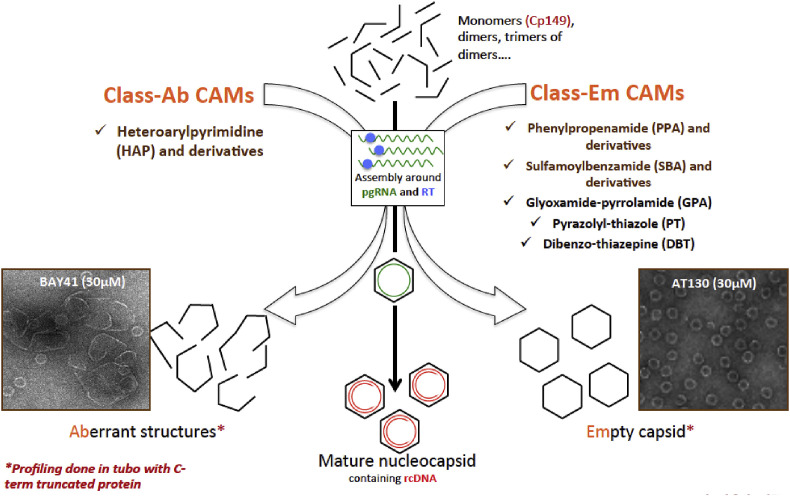
There are two main classes of CAMs that induce the formation of empty or aberrant capsids in vitro (Fig. 4 ). In each class, several scaffolds or chemotypes have been described. In cell culture models, they efficiently inhibit the production of virion progeny, with EC50 in the nanomolar range, and therapeutic indices as high as 10,000. CAMs also inhibit the secretion of RNA-containing particles and genome-free particles, which NUC do not. This is important, because many HBc-containing particles circulate in the blood of patients, with functions that are not yet fully understood.
Fig. 4.
Two main classes of HBV core assembly modulators (CAM) that induce the formation of empty capsid or the formation of aberrant capsid. Figure kindly provided by David Durantel.
David also reported a secondary mode of action of CAMs: they efficiently (at concentrations 10× the EC50) inhibit the formation of cccDNA when they are administered with the virus in animal models (Berke et al., 2017). Some CAMs inhibit the accumulation of intracellular vRNA and prevent HBeAg secretion. HBeAg is produced in large excess in the blood of patients and has immune-subversive properties. Seroconversion to anti-HBeAg status is an important clinical therapeutic endpoint, and some CAMs could accelerate this process.
David indicated that CAMs can be combined with NUC or Peg-IFN. Such combinations have been tested in preclinical animal models of HBV infection, and some were synergistic, which would be desirable to obtain in human. Finally, he presented some results from Phase 1 clinical trials (Table 2 ). In general, CAMs lead to a rapid decline of viremia, i.e. a 1- to 3-log10 reduction in 28 days of treatment. No severe adverse effects have been reported in the 4 trials for which results are available. These early results are interesting, but the long-term safety of CAMs and their superiority of CAMs over NUCs have yet to be proven, and one should remain cautious while waiting for the results of Phase 2 trials.
Table 2.
Drug development pipeline of HBV core assembly modulators (CAMs).
| Name | Class (MoA) | Phase | Company |
|---|---|---|---|
| NVR 3-778 | I (AT130-like) | 2 | Novira, Janssen, USA/Belgium |
| GLS4 | II (HAP-like) | 2 | HEC Pharma, China |
| RO7049389 | II (HAP-like) | 1b done→ 2 | Hoffmann-La-Roche, Switzerland |
| JNJ-6379 | I (?) (AT130-like) | 1b done→ 2 | Janssen, Belgium/USA |
| ABI-H0731 | II (?) | 1b/2a | Assembly Biosciences, USA |
| AB-423 | (?) | 1b | Arbutus Biopharma, USA |
| EP-027367 | (?) | IND | Enanta Pharmaceuticals, USA |
| AB-506 | (?) | IND | Arbutus Biopharma, USA |
| ABI-H2158 | (?) | IND | Assembly Biosciences, USA |
5.4. Hepatitis and retroviruses
Eva Riveira-Munoz of the Irsicaixa AIDS Research Institute, Barcelona, Spain, reported interesting results regarding the potentiation of the anti-HIV activity of known antimetabolites by a strategy of CDK4/6 inhibition, which targets SAMHD1 phosphorylation. SAMHD1, via its dNTPase activity, mediates an anti-HIV-1 activity by reducing the pool of dNTP. It also modulates the activity of antimetabolites such as pemetrexed (Pmtx), which have basal anti-HIV and anti-cancer activity. The dNTPase activity of SAMHD1 is inactivated by phosphorylation. The inhibition of SAMHD1 phosphorylation could therefore lead to more pronounced anti-HIV activity, due to dNTP deprivation. This study elegantly showed that CDK4/6 is involved in the phosphorylation of SAMHD1 and that its inhibition by palbociblib (Pb) prevented SAMHD1 phosphorylation and, in turn, potentiated the anti-HIV activity of Pmtx. Indeed the combination of Pb and Pmtx had an increased effect on HIV-1 replication, as well as an increased anti-cancer effect, with a combination index (CI) < 0.02. Such a combination could prove useful for therapeutic strategies.
Maria Pujantell, also of the Irsicaixa AIDS Research Institute in Barcelona, showed experimental evidence that A-to-I editing by the cellular adenosine deaminase, acting on RNA 1 (ADAR1), functions as a regulator of innate and antiviral immune function in HCV infection. ADAR1 has been reported as a key step in triggering innate immunity in response to foreign viral RNAs, including HIV-1 (Pujantell et al., 2017). Knockdown of ADAR1 enhanced expression of the RNA sensors RIG-I and MDA5, phosphorylation of STAT1 and expression and phosphorylation of IRF7, indicative of innate immune activation. Polymorphisms within the ADAR1 gene were found to be significantly associated with the clinical response to interferon therapy and advanced liver fibrosis in a cohort of 155 HCV- and HIV-1 coinfected patients (Pujantell et al., 2018). They concluded that ADAR1 regulates innate immune signaling and is an important contributor to the outcome of the HCV-host interactions.
Bingqian Qu of the Department of Infectious Diseases, University of Heidelberg, Germany, presented data on the anti-HBV activity of the NEDD8 activity enzyme (NAE) inhibitor MLN4924 (Pevonedistat). No anti-hepatitis delta virus activity was reported. MLN4924 was previously described as an anti-tumor drug (Lan et al., 2016). Neddylation is an important process for the activity of Cullin-Ring-ligases (CRL), the largest family of E3 ubiquitin ligases, which ubiquitinate about 20% of cellular proteins.
It was recently shown that HBV, via its HBx protein, co-opts a CRL to ubiquitinate and degrade an important HBV restriction factor, SMC5/6 (Decorsière et al., 2016). The rationale of the antiviral strategy was that inhibiting NAE would in turn inhibit the CRL, thereby stabilizing SMC5/6. In a relevant cell culture infectious model, MLN4924 inhibited the production of HBV virions with an EC50 as low as 62 nM, in the absence of toxicity in non-dividing cells. The cccDNA level was unchanged. The inhibitory phenotype included less accumulation of intracellular viral RNA. It remains to be determined whether reduced intracellular vRNA is due to a defect in transcription or to a post-transcriptional effect; in depth mechanistic studies are awaited to clarify these points. In vivo experiments are also planned to further validate this approach in pre-clinical models.
Tiffany Edwards of Saint Louis University, MO, USA, reported the development of HBV RNaseH inhibitors belonging to either the N-hydroxy-isoquinolinedione (HID) and N-hydroxy-pyridinedione (HPD) families. This work follows published efforts of this team to improve the efficacy and characterize the anti-RNAse activity of these compounds in vitro (Edwards et al., 2017; Lomonosova et al., 2017). As a recall, RNAse activity in HBV polymerase is crucial for the reverse transcription of pregenomic RNA into rcDNA within nucleocapsids. Until the effort of John Tavis and his team, no inhibitors of HBV RNAse were described. In 2017, the EC50 of HID and HPD (measured in HepG2 replicating HBV) ranged between 0.69 and 5 μM, with rather low therapeutic indices (not higher than 70). Authors undertook structure-activity relationship (SAR) studies to improve their compounds and reported here inhibitors with higher activity (EC50 at around 100 nM) and greater therapeutic index (up to 345). Interestingly, a synergy between one of the best modality and a nucleoside analogue, i.e. lamivudine, was also reported, thus pointing toward combination possibilities. Yet the best HBV RNAse inhibitors need to be further tested in other relevant in vitro infectious models (e.g. PHH), as well as in preclinical mouse models. The hit-to-lead optimization is ongoing.
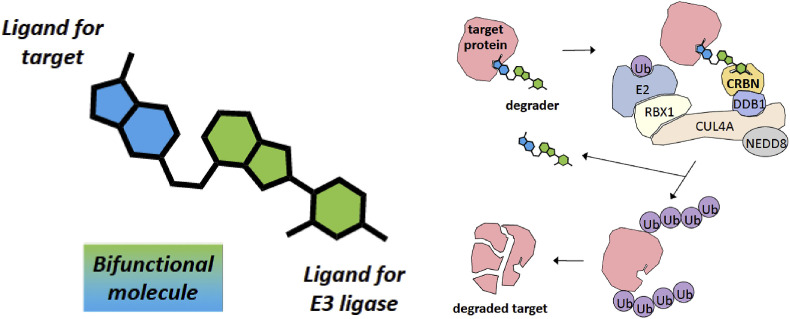
Melissanne de Wispelaere of Harvard Medical School, MA, USA, presented data on the development of bifunctional molecules that specifically bind to a target viral protein and induce its degradation after ubiquitination by Cullin-RING ligase (CLR) complexes (Cromm and Crews, 2017). The first step is to identify a small molecule that binds to a specific viral target, then link it to another moiety able to bind to an adapter of CLR (Fig. 5 ).
Fig. 5.
Development of bifunctional molecules that specifically bind to a target viral protein and induce its degradation. A small molecule ligand for a specific viral target is conjugated to a ligand for cereblon (CRBN), an adapter of Cullin-Ring-Ligase (CLR) complexes. Figure kindly provided by Melissanne de Wispelaere.
To exemplify this strategy, Melissanne presented data on the induced degradation of the HCV serine protease NS3-4A by two bifunctional molecules, DGY-03-81 and DGY-04-35, whose antiviral potency was only 10-fold lower than that of the parental NS3-4A protease inhibitor telaprevir. To demonstrate that the small molecules were recruiting the CLR complex to mediate degradation of NS3-4A, the cereblon-binding moiety in DGY-04-35 was modified to produce DGY-07-026, which cannot recruit cereblon and consequently does not mediate NS3-4A degradation. Degradation of NS3-4A could also be prevented by competition with lenalidomide, a cereblon ligand. Together, the experiments demonstrate that the small molecules were recruiting the CLR complex to mediate degradation of NS3-4A and thereby inhibit HCV.
Jinhong Chang of the Baruch Blumberg Institute, Doylestown, PA, USA, described novel benzamide compounds that modulate HBV nucleocapsid assembly in a distinct manner compared to other capsid assembly modulators (CAM), which are currently developed as anti-HBV in clinic. Starting from a chemical bank of around 20,000 molecules and using a home-made screening assay based on the AML12HBV10 cell line, they identified 8 novel chemotypes. The newly identified chemotypes also induce the formation of empty capsids, but the latter have a different mobility in an optimized, i.e. slow running, agarose capsid migration assay. Amongst the 8 molecules identified, BA-53038B was further studied. It showed an EC50 in the sub-μM range against WT HBV, remained active against F97L, V124A, V124F mutants, but was ineffective against V124W mutant, similar to other CAMs. This was indirect evidence that BA-53038B binds to the HAP pocket, but could induce different conformation change with surface altered charges. SAR study is yet to be done for a “hit to leader” strategy and to identify a drug candidate.
6. Virus evolution
6.1. Getting to the root of epidemic spread: an evolutionary perspective on pathogen emergence. Philippe Lemey, Department of microbiology and immunology, Rega Institute, KU Leuven, Belgium
Philippe Lemey presented three examples of viral molecular epidemiology, describing the evolution of HIV, Ebola, and Lassa fever viruses in a visually impressive keynote presentation. He explained how sequencing viral genomes, sometimes using “lab in a suitcase” portable devices, can provide phylodynamic and phylogeographic information about epidemics. The Bayesian Evolutionary Analysis Sampling Tree (BEAST) software (http://beast.community/) (Suchard et al., 2018) was employed to reconstruct epidemics by mutation mapping. The rate of HIV evolution was calculated and its introduction into the human population was traced back to Kinshasa (Democratic Republic of the Congo, former Belgian Congo) in the 1920s (Faria et al., 2014). From there, evidence of spread along transportation networks indicated a path from Kinshasa to Haiti, New York City, and San Francisco. This work also refuted the popular idea of a single “Patient Zero” (Worobey et al., 2016).
His second example was the 2014-16 West African Ebola epidemic, for which the genomic anatomy was determined by sequencing more than 5% of cases. Phylogeographic generalized linear modeling determined predictors of the outbreak in order of importance: origin population size, destination population size, geographic distance, within-country migration, and shared-border migration (Dudas et al., 2017). He concluded that this large epidemic consisted of a heterogeneous and spatially dissociated collection of transmission clusters of varying size, duration and connectivity.
The third example was the Lassa fever outbreak in Nigeria that still continues today. Philippe addressed the question of whether the increase in cases was due to a new virus strain that could have a greater potential for human-to-human transmission. By applying phylogenetic reconstruction and divergence dating to Lassa virus genomes obtained by collaborators using an in-field metagenomics approach, the increased incidence of human cases could be attributed to independent spillovers from multimammate rats, rather than human-to-human transmission. This was welcome news to the public health officials in Nigeria. In summary, Philippe observed that real-time epidemiology and surveillance of viral genomics can contribute to containment of zoonoses and epidemics.
6.2. Monitoring, predicting and altering the evolution of RNA virus populations. Marco Vignuzzi, Institut Pasteur, Paris, France
Marco Vignuzzi presented computational and experimental tools to study RNA virus evolution, and used compelling graphics, clear analogies, and animated features to demonstrate how his research group monitors, predicts, and experimentally alters RNA virus evolution. The high mutation frequency of RNA-dependent RNA polymerases (RdRp) and small genomes make RNA viruses a good system for study, since large virus populations create mutant spectra, or clouds of all possible mutations. This sequence space is almost infinitely large, approaching 4 × 106020 for a 10-kilobase genome.
Marco's group is interested in mapping the fitness landscape to identify “good neighborhoods” where highly fit, and potentially pathogenic, genotypes occur, represented as fitness peaks in the landscape, while valleys represent areas of deleterious mutations or loss of virulence. Their experiments begin with passing a virus (Coxsackie B virus in this example) five times in the presence of the mutagens ribavirin, 5-fluorouracil, 5-azacytidine, amiloride or Mn2+, resulting in variants at every position. The mutant swarm that ensued was evaluated by next-generation sequencing (NGS). The dimensions of this data set were then reduced by multi-dimensional scaling tools:
-
1)
PCA, capturing biological differences and strain separation, with mutations clustering by minority changes;
-
2)
Sparse PCA, to identify main contributors and determine which mutations drive the biological signal;
-
3)
SubMatrix Selection Singular Value Decomposition SMSSVD, which can detect overlaid signals particularly at low signal:noise ratios; and
-
4)
Non-linear dimension reduction IsoMap (2D IsoMap) to best visualize evolutionary trajectories, even between replicates of the same virus strain.
The analysis revealed that all biological signals could be captured in less than 12 dimensions, that were plotted to show the fitness landscape of the population. The conclusion was that minority variants are critical determinants of fitness phenotype. By identifying the “bad neighborhoods” of sequence space, they generated strains that were attenuated in vivo, raising the possibility of using these approaches to make vaccines. The approaches and tools described in this presentation are explained in an outstanding web page (Vignuzzilab.eu) in clear language for non-experts.
7. Medicinal chemistry
Nicky Hwang of the Baruch S. Blumberg Institute informed the audience about progress in developing the compound IHVR-19029, a derivative of the imino sugar deoxynojirimycin (DNJ). IHVR-19029 is an endoplasmic reticulum α-glucosidase I and II inhibitor, which efficiently blocks the replication of several hemorrhagic fever viruses, such as DENV, Ebola and Rift Valley fever virus, but suffers from a short plasma half-life and low oral bioavailability (Chang et al., 2013). As IHVR-19029 has four free hydroxyl groups on the DNJ moiety, they were used for making esters, in order to overcome these problems. Fully (tetra-acyl) and partially (di-acyl and mono-acyl) protected analogues of IHVR-19029 were prepared. The prodrugs retained antiviral activity against DENV, ZIKV, yellow fever and Ebola virus in cell-based assays, demonstrating an efficient release of the parent compound inside the cells after absorption (Ma et al., 2017). A pharmacokinetic study in mice demonstrated that the prodrug approach could lead to improved oral bioavailability, and an efficacy study in Ebola-infected mice showed improved protection.
Targeting the HBV core protein (Cp) represents an attractive and novel approach toward curative therapy. Zhengqiang Wang of the University of Minnesota, Minneapolis, MN, USA presented a novel chemotype of capsid assembly effectors (CAEs), for which the original hit was identified from high-throughput screening of commercial libraries. Subsequent extensive medicinal chemistry efforts, involving SAR and SPR studies, ADME and PK, led to the discovery of the compounds ZW-1042 and ZW-1066, which bind tightly to Cp and potently inhibit HBV replication in the nanomolar range (EC50 0.31 μM and EC50 0.11 μM, respectively). These compounds have improved oral bioavailability (F = 46% and 26%, respectively) without any observed cytotoxicity (CC50 > 100 μM).
Roberto Manganaro from Cardiff University, UK considered mimicking the heptad repeat B (HRB) region of the respiratory syncytial virus F protein as potential antiviral strategy.Synthetic peptides with the helical structure of the HRB region of the F protein were earlier reported as potent inhibitors of RSV fusion (Lambert et al., 1996). The HRB 488FDASISQVN496 fragment was selected as a suitable template for the rational design of α-helix mimics. Several chemoinformatic techniques were used to generate and evaluate a focused virtual library of compounds designed to mimic the hydrophobic interaction of the selected HRB fragment. The most promising compounds were then synthesized and evaluated for their activity against RSV-A2 and RSV-B in CPE-based assays in HEp-2 cells. The compounds were only weakly active against both RSV strains, so new analogues will therefore be synthesized in order to increase their antiviral activity.
Birgit Zonsics, also from Cardiff University, reported the discovery of novel small-molecule inhibitors of CHIKV. The viral genome encodes four non-structural proteins (nsP1-4) and nsP3 macro domain (known as an ADP-ribose binding module), which were selected as a target for the identification of novel inhibitors. Pharmacophore screening of the SPECS library, followed by docking and consensus rescoring, resulted in 26 compounds for subsequent biological evaluation. Of those, four exhibited activity against CHIKV, with the best compound having an EC50 3.4 μM and CC50 of 181 μM. Mechanism of action studies indicated an effect during the early stages of viral replication, probably on virus entry. Thirteen new analogues derived from the hit compound were then prepared and tested, but so far none has proved to be more potent than the hit. Synthesis of improved analogues and further mode-of-action studies are ongoing.
Marcella Bassetto, also from Cardiff University, reported on the application of several different computational approaches to the identification of novel inhibitors of ZIKV replication. Crystal structures of the NS2B-NS3 protease, NS3 helicase and NS5 methyltransferase were used to perform docking-based virtual screening of commercial and internal compound libraries. Among the in silico hits, one scaffold exhibited low-μM anti-ZIKV activity in cell-based assays. In parallel, a series of novel nucleoside analogues was designed to target a flavivirus-conserved hydrophobic pocket in proximity to the SAM binding site of the ZIKV MTase. Synthesis and evaluation of the new analogues led to the identification of a hit with an EC50 in the 10 μM range and no toxicity in a cell-viability assay. Finally, a homology model for the active complex of the NS5 polymerase was built and used to evaluate the potential binding of known broad-spectrum non-nucleoside inhibitors of viral polymerases. The best in silico hit was used as starting point for a shape-based screen of commercial small molecules. From the 7 compounds selected for in vitro evaluation, a third anti-ZIKV hit with an EC50 in the 10 μM range was identified. All three molecules will be further modified to increase their potencies.
Chenglong Zhao of the University of Hamburg, Germany, described the design and the synthesis of a series of non-symmetrical prodrugs of γ-alkyl-modified-d4T triphosphate (d4TTP) with the aim to deliver γ-alkyl-d4TTP into cells. Hydrolysis study of the prepared prodrugs in phosphate buffer solution (PBS, pH 7.3) demonstrated that their stability increased with the increasing length of the alkyl chain. In PBS, the half-lives of these prodrugs were around 200 h and in T-lymphocyte CEM cell extracts, the half-lives were dramatically reduced to 0.4 h - 5.2 h. It was shown that only the biodegradable prodrug moiety was cleaved. One of the compounds, alkyl-(C18H37)-d4TTP, was stable both in PBS (for at least 500 h) and, even more importantly and in contrast to d4TTP, in CEM extracts. This compound also exhibited very good antiviral activity in TK-deficient CEM/TK cell cultures infected with HIV-2. Moreover, the γ-alkyl-TTP counterpart was a substrate for HIV-RT but was not efficiently used by human DNA-Pol β in contrast to TTP itself. This may point to increased selectivity of the γ-modified nucleoside triphosphates towards the viral enzyme as compared to cellular polymerases.
8. Influenza and other respiratory viruses
8.1. Influenza
Davide Corti of the Humabs BioMed, Bellinzona, Switzerland, described the history of serotherapy and recent advances in the use of monoclonal antibodies to treat human diseases. New antibody engineering technologies have been developed to target conserved epitopes to highly variable viruses and to improve antibody effector functions. Multiple regions of specific viral targets, such as the stem of the influenza virus hemagglutinin can be targeted by specific antibodies and have the potential for prophylaxis and therapy of viral infections. Davide gave examples of the mechanism of action for neutralizing antibodies directed against influenza A virus, RSV, and ZIKV, and how antibodies can be used to identify protective antigens for the design of vaccines.
Ron Fouchier of the Department of Viroscience, Erasmus MC, Rotterdam, The Netherlands, described influenza virus infections in immunosuppressed patients, whose impaired immunity may cause prolonged virus infections and when combined with antiviral treatment can lead to the emergence of viruses with resistance mutations. Immunocompromised ferrets were used as a model to evaluate treatment strategies. Compared to immunocompetent ferrets they showed a prolonged presence of viral RNA and a greater total amount of virus shedding, and although oseltamivir treatment reduced shedding, it resulted in the emergence of neuraminidase inhibitor-resistant viruses. The mutations that arose in immunocompromised ferrets were similar to those seen in patients.
8.2. Other respiratory viruses
Xavier Saelens of the VIB Center for Medical Biotechnology, Department for Biomedical Molecular Biology, Ghent University, Ghent, Belgium, described treatment options available for patients infected with RSV. Promising new small-molecule antivirals that target the fusion protein or polymerase are being evaluated in the clinic. In addition, a number of human monoclonal antibodies, as well as single-domain antibodies, have been developed that target the RSV fusion and glycoprotein molecules. Although primarily intended for prophylactic use, some of these antibodies have a therapeutic effect in animal models. Polymerase inhibitors had greater potency than fusion protein-targeted antibodies in their studies. The evaluation of some of the small-molecule inhibitors in RSV challenge studies in humans demonstrated a therapeutic benefit, so long as the drug was administered as early as possible after RSV became detectable.
Carmen Mirabelli of KU Leuven, Leuven, Belgium, described the use of 3-dimensional human airway epithelial cells of bronchial origin (HuAEC) to study RSV infection and to evaluate RSV inhibitors. RSV-A replicates efficiently in HuAEC and viral RNA is shed for weeks after infection. Treatment with fusion inhibitors has an effect only when added at the time of infection, but replication inhibitors (both nucleoside and non-nucleoside) elicited a marked antiviral effect, even when treatment was delayed until one or three days after infection.
Brett Hurst of Utah State University, Logan, Utah, USA, described the use of human intravenous immunoglobulin (hIVIG) for the treatment of an enterovirus (EV)-D68 infection in mouse models of respiratory and neurological disease. In the respiratory model, hIVIG did not reduce lung virus titers or histopathologic evidence of injury of lung tissue, but reduced viremia and virus spread to the CNS. In a neurologic model, hIVIG prevented paralysis and mortality when administered within 48 h of infection. The work provides evidence for the use of hIVIG as a treatment of EV-D68 respiratory infections to prevent systemic viral spread.
Meehyein Kim of the Virus Research Group, Korea Research Institute of Chemical Technology, Daejeon, Republic of Korea, described the use of retinoic acid-inducible gene I (RIG-I) agonists for treatment of influenza virus infections. Kim gave examples of synthetic RNAs that have been modified to enhance their efficacy for use as RIG-I agonists in immunotherapy. Transfection of optimized RIG-I agonist RNA suppressed influenza virus protein expression. Intranasal immunization of mice with an inactivated influenza virus PR8 vaccine along with a RIG-1 agonist as adjuvant afforded complete protection against mouse-adapted PR8 infection, while only 40% of mice given vaccine alone survived challenge.
Lisa Evans DeWald of Emergent BioSolutions, Gaithersburg, MD, USA described the use of the iminosugar UV-4B as a host-targeted glucomimetic that inhibits glycosylation of viral envelope glycoproteins. Oral treatment with UV-4B protects mice against lethal infection with mouse-adapted, oseltamivir-sensitive and oseltamivir-resistant influenza A (H1N1 and H3N2) and B viruses. To examine the ability of influenza virus to develop resistance to UV-4B, virus was passaged in mice in the presence or absence of drug, and virus was isolated from the lungs to identify changes in the genome. Only 7 non-synonymous mutations were identified after five passages, with an absence of apparent viral escape mutants following sustained exposure to UV-4B.
9. Emerging infections
9.1. Ebola treatment: working in the dark under the spotlight. Michael Jacobs, Royal Free Hospital, London, UK
During the West African Ebola epidemic, he led the team that treated patients at the Royal Free Hospital in London, including a case of encephalomyelitic infection in a nurse who had earlier recovered from Ebola virus disease. Michael Jacobs noted that, in spite of an epidemic lasting nearly three years, with an estimated 28,000 cases, and considerable experience in administering experimental treatments to patients in Africa, the USA and Europe, there are still no therapies with proven efficacy. Part of the difficulty has been disagreement among experts over how experimental medications should ethically be administered and how trials should be designed to provide evidence of efficacy.
Mike therefore devoted part of his lecture to the current effort by the World Health Organization to facilitate the use of promising treatments in future infectious disease outbreaks, by means of a new “Ethical Framework for Monitored Emergency Use of Unregistered Interventions” (MEURI). It is intended to be applied when:
-
•
there is no proven effective treatment for a disease with high mortality;
-
•
clinical studies cannot be started immediately;
-
•
data are available that provide preliminary support for efficacy and safety; and
-
•
use is supported by a scientific committee on the basis of a favorable risk-benefit analysis.
Before a novel therapy is introduced, country authorities and an ethics committee must approve its use; adequate resources must be available to minimize risks; patients must give informed consent; and use of the drug must be monitored and data shared in a timely manner. Mike noted that, although the MEURI framework provides safeguards for patients, it does not include a reliable means of evaluating drug efficacy, which he considers is best obtained through randomized trials, although there is no pre-agreed trial design.
In the remainder of his lecture, Mike reviewed the course of illness in Ebola patients treated at the Royal Free Hospital, with reference to therapies that might be employed during the current outbreak in the Democratic Republic of the Congo. He commented that, although individual clinical histories do not provide evidence of efficacy, patient data may suggest a benefit. At present, the most promising therapy appears to be®ZMapp (Mapp Biopharmaceutical, Inc.), and data also support the use of the nucleotide analogue prodrug remdesivir (GS-5734, Gilead Sciences) under the MEURI framework. He also suggested that the triple human monoclonal antibody (mAb) combination REGN3470-3471-3479 (Regeneron Pharmaceuticals) could be used if ZMapp and remdesivir are not available. He expressed uncertainty about the efficacy and dosing of favipiravir, noting that it might be used if the drugs just listed are not available.
9.2. Alpha-ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication. Rolf Hilgenfeld, Institute of biochemistry and German Center for infection research, Lübeck, Germany
In the second plenary lecture of the session, Rolf Hilgenfeld described the activity of alpha-ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication. The main protease of coronaviruses and the 3C protease of enteroviruses share similar active-site architecture and a unique requirement for glutamine in the P1 position of the substrate. Rolf's group performed structure-based design of peptidomimetic alpha-ketoamides as inhibitors of main and 3C proteases. Close inspection of crystal structures of early lead compounds in complex with the target proteases revealed differences in the S2 pocket: it is small and covered by a lid in the HCoV-NL63 enzyme, large and covered in SARS-CoV Mpro, and large, but not covered in CVB3 3Cpro. The SARS-CoV Mpro also features high plasticity of the S2 site. The best near-equipotent inhibitors have P2 = cyclopentylmethyl or cyclohexylmethyl and display low- or sub-μM EC50 values against enteroviruses and alpha- and betacoronaviruses. In Huh7 cells, the compound DZL08 exhibits the highest activity of any antiviral described against the MERS-CoV; it is now under preclinical development.
9.3. Other presentations
Nam-Joon Cho of the Nanyang Technological University, Singapore, described the activity of a synthetic peptide that inhibits the replication of ZIKV and other mosquito-borne viruses and is capable of entering the central nervous system. The peptide selectively targets high-curvature phospholipid membranes, such as those enclosing susceptible viruses, and its composition has been engineered for high in vivo stability. In a mouse model of ZIKV infection, treatment starting three days after virus challenge protected against mortality and markedly reduced clinical symptoms. In addition to its systemic effects, the peptide crossed the blood-brain barrier (BBB), reducing viral loads in the brain and protecting against ZIKV-induced BBB injury. The findings support the potential of a brain-penetrating peptide for treating neurotropic viral infections.
Kristina Kovacikova of Leiden University Medical Center in the Netherlands described the identification of an inhibitor of CHIKV and the use of reverse genetics methods to identify the nsP1 protein as its target. Screening of more than 70 selenonucleoside and carbocyclic nucleoside analogues yielded a potent compound, LJ4277, with an EC50 of 0.2 μM and a selectivity index >1000. Selection for resistant mutants identified two mutations in nsP1 responsible for resistance to the compound. Based on these results and biochemical assays with purified alphavirus nsP1, Kristina and her colleagues hypothesize that LJ4277 directly affects alphavirus nsP1 methyl-transferase activity, in addition to an indirect effect on viral replication through inhibition of cellular S-adenosyl-L-homocysteine hydrolase.
Luděk Eyer of the Veterinary Research Institute in Brno, Czech Republic, reported that tick-borne encephalitis virus is strongly inhibited by 2′-C-methyl or 4′-azido modified nucleosides and by the imino-C-nucleoside BCX-4430 (BioCryst Pharmaceuticals), but escape mutants emerge relatively quickly during passage in the presence of each drug. Virus resistant to 2′-C-methylated nucleosides had the mutation S603T in the active site of the NS5 RNA polymerase and showed small plaques, a marked decrease in replicative fitness in vitro and the loss of neuroinvasiveness for mice. In contrast, virus resistant to BCX-4430 had a different NS5 mutation, E460D, and showed no change in plaque size or in vitro growth kinetics, but its neuroinvasiveness was also strikingly decreased. Structural studies and molecular docking identified the molecular basis of resistance of both mutant viruses.
Anna Płaszczyca of Heidelberg University, Germany, noted that the nonstructural protein 1 (NS1) protein of DENV is known to be essential for replication, but its molecular mechanisms remain elusive. Scatturo and colleagues previously reported a panel of NS1 mutants that abrogate DENV replication (Scaturro et al., 2015). Further characterization has revealed a novel interaction between NS1 and the NS4A-2K-NS4B precursor polyprotein and identified NS1 residues Gly161 and W168 as critical determinants. Alanine substitutions abrogated precursor protein binding and abolished RNA replication, but did not affect NS1 secretion. Electron microscopy-based studies suggest that the interaction between NS1 and the NS4A-2K-NS4B precursor is not necessary for the formation of the membranous replication organelle. The interface between NS1 and the NS4A- 2K-NS4B precursor may be a promising target for antiviral therapy.
9.4. Emerging infections and clinical evaluation of antivirals
Jinhong Chang of the Blumberg Institute, Doylestown, PA, USA, described efforts to determine the mechanism of action of the benzodiazepine compound BDAA, which inhibits the replication of yellow fever virus (YFV) in vitro and in hamsters (Guo et al., 2016). Examination of BDAA-resistant YFV found that the compound interacts with a three-residue motif centered at P219 of the NS4B protein. In addition to direct inhibition of viral replication, RNAseq analysis revealed that BDAA treatment also significantly enhances the levels of mRNAs specifying a broad range of cytokines, chemokines and ISGs. Using CRISPR/Cas9 knockout technology, Jinhong and colleagues demonstrated that MAVS, the adaptor of the RNA sensors RIG-I and MAD5, is essential for the enhancement of YFV-induced cytokine responses, but not for inhibition of YFV replication. The findings suggest that BDAA both impairs RNA replication and physically disrupts the replication complex, causing release of viral RNA, activation of RIG-I and/or MDA5 and stimulation of antiviral responses.
May Wang of Harvard Medical School, Boston, MA, USA described the activity of a novel small molecule, currently designated “3.3,” against Lassa fever virus (LFV). After attachment to the cell surface, LFV virions are endocytosed and transported to the late endosome/lysosome, where acidification promotes binding of the viral glycoprotein to its putative receptor, the cholesterol-binding integral membrane protein LAMP1. May and colleagues found that 3.3 competes with cholesterol for binding to the membrane distal domain (D1) of LAMP1: cholesterol strongly enhances the interaction of the viral GP with D1, while 3.3 blocks it. They therefore propose that binding of the LFV GP to LAMP1 is dependent on the presence of cholesterol in the D1 domain, and that its displacement by 3.3 prevents virus entry.
Merel Oeyen of the Rega Institute, KU Leuven, presented data on the activity against DENV and ZIKV of the novel peptide labyrinthopeptin A1, which was previously shown to inhibit HIV and herpes simplex virus (HSV) (Ferir et al., 2013). Anti-flaviviral activity was independent of the viral serotype/strain tested or the host cell line. In flavivirus-susceptible Vero, A549 and Raji DC-SIGN cells, the EC50 ranged from 0.8 to 1.6 μM; in the liver cell line Huh-7 the IC50 against DENV was 1.7 μM; while in the astroglial U87 and placental Jeg-3 cell lines the IC50 against ZIKV was 0.5–2.5 μM. The antiviral activity of LabyA1 was also confirmed in monocyte-derived dendritic cells infected with different DENV serotypes, using flow cytometry and qPCR. Mechanism of action studies suggest that LabyA1 interferes with the interaction between the viral envelope and cellular receptors that play a role in viral entry.
Justin Julander of Utah State University, Logan, UT, USA, described the evaluation of a human bispecific neutralizing antibody, FIT-1 (Wang et al., 2017), in mouse models of ZIKV disease. The human mAb ZKA190 binds to an epitope on DIII of the E protein and potently neutralizes multiple ZIKV strains, but escape mutants emerge in vitro and in vivo. When the binding specificity of ZKA190 and a second mAb targeting DII are combined in the bispecific antibody FIT-1, escape mutants are not seen. Its prophylactic efficacy was evaluated in a model of congenital ZIKV infection in AG129 mice, in which congenitally exposed pups are smaller than normal, have reduced head lengths and often show hearing loss. When dams were infected on day 7 of pregnancy and FIT-1 was administered 24 or 72 h after virus challenge, there was a significant improvement in dam survival, reductions in viral RNA in the placenta and fetus and in maternal spleen and brain samples, and a trend toward increased pup size and placental weight. The data suggested that earlier treatment was more effective. Adult male mice infected with ZIKV and treated with FIT-1 showed reduced virus levels in the testes, indicating that the antibody can reach “immune-privileged” sites, and that treatment might prevent sexual transmission.
Kristina Lanko of the Rega Institute, KU Leuven, described the development of new in vitro assays for the development of direct-acting antivirals against human parechoviruses (HPeV), which can cause sepsis-like illness and life-threatening neurological complications in infants. After finding that the viruses replicate well in the African green monkey kidney cell line BGM, she and colleagues analyzed the kinetics of replication of HPeV1 and HPev3 in these cells. Intracellular viral RNA levels peaked at 6 h postinfection for HPeV1 and at 12 h for HPeV3, but at 3 days postinfection, the extracellular genome copy numbers of both viruses were comparable. They then determined if certain compounds that inhibit entero/picornavirus replication also inhibit HPeV1/3 replication. Favipiravir and the nucleoside analogues 7-deaza-2′C-methyladenosine, 2C-methylcytidine, 2C-methylguanidine, 2C-methyladenosine were all active against HPeV1 and 3, with 2C-methylcytidine the most effective (EC90 10.5 μM). The enterovirus-specific inhibitors pleconaril, rupintrivir, and SMK-0213 did not inhibit HPeV.
Randall Lanier of Chimerix, Durham, NC, USA, gave what he noted was the first public presentation of data on the anti-norovirus activity of a novel purine nucleoside analogue, CMX521, which was originally identified by screening a compound library against a mouse-derived virus. The compound appears to target the active site of the RNA-dependent RNA polymerase, which is highly conserved among human and mouse noroviruses; it is therefore active against all viral genotypes. EC50 values range from 0.12 to <5 μM against a panel of diverse caliciviruses in transformed cell lines or replicons. In mice, orally administered CMX521 shows a dose-dependent inhibition of virus replication in gastrointestinal tissues, and labeled drug localizes to the gut. The compound is effective against human norovirus (HuNoV) GII.3 and GII.4 in human mucosal stem cell-derived organoids, suggesting that it will be broadly effective against diverse human noroviruses. Completed preclinical studies include animal toxicology, rate of formation and half-life of the active triphosphate in relevant cell types, resistance passaging, enterocyte transporter identification and in vivo distribution studies. CMX521 has now progressed to Phase 1 clinical development.
Valeria Cagno of the University of Geneva began her presentation by noting that an ideal strategy to fight viral infections is to develop broad-spectrum treatments that irreversibly inhibit infectivity. She then described the synthesis of sulfonic acid-decorated gold nanoparticles that mimic cellular attachment receptors (heparan sulfate proteoglycans) widely used by viruses. Viruses binding to these materials undergo structural deformations and an irreversible loss in infectivity, as shown by electron microscopy and molecular dynamic simulations. Nanomolar concentrations irreversibly inactivate HSV, human papilloma virus, RSV, DENV and ZIKV in vitro, without measurable cytotoxicity, and are also active ex vivo in HSV-2-infected human cervicovaginal histocultures and in vivo in RSV-infected mice. Nanoparticles in which the gold is replaced by a biocompatible sugar core show similar efficacy. A similar strategy is now being used to target viruses dependent on sialic acid receptors.
Brian Gentry of Drake University, Des Moines, Iowa, USA, discussed the metabolic activation of MBX-2168, a monohydroxymethyl methylenecyclopropane (MCPN) nucleoside analogue with a 6-ether moiety. MCPNs with ether or thioether substituents at the 6-position are known as broad-spectrum anti-herpesvirus agents (Komazin-Meredith et al., 2015). Synguanol and cyclopropavir are considered to be, respectively, first- and second-generation MCPNs. Their proposed mechanism of action involves sequential phosphorylation to a triphosphate (TP) form, which then inhibits the viral DNA polymerase. MBX-2168, a third-generation MCPN, is more potent and has a broader spectrum of antiviral activity, compared to the first and second generations. Increased potency against herpesviruses is not related to any observable increase in cytotoxicity.
The mechanism of action of MBX-2168 is similar to that of GCV, with two additional and distinct steps. GCV is converted to its monophosphate (MP) form by the CMV-encoded pUL97 protein kinase, followed by endogenous cellular phosphorylation to the active GCV-TP that inhibits viral DNA synthesis. In contrast, MBX-2168 was previously shown to be partially phosphorylated to the MBX-2168-MP by the cellular kinase TAOK3, allowing MBX-2168 to overcome UL97 resistance. Second, a butyl-ether moiety at the 6-position of the guanine ring must be enzymatically removed. To characterize this step, pentostatin, an inhibitor of adenosine deaminase (ADA) and adenosine-deaminase-like-protein-1 (ADAL1), was co-incubated with MBX-2168, which resulted in a reduction in its EC50 by >300-fold (0.02 μM vs. 6.47 μM). Further, the moiety at the 6-position of the guanine ring of MBX-2168 was not enzymatically removed by ADA, suggesting that it is removed by ADAL-1 following phosphorylation of the drug to a MP. Remarkably, when MBX-2168 was incubated with cellular extracts and an ADAL1-baculovirus construct, conversion of MBX-2168-MP to synguanol-MP was demonstrated, Future research will focus on the conversion of MBX-2168-MP to synguanol-MP by ADAL-1 in the presence and absence of 2-deoxycoformycin (dCF), a potent adenosine deaminase inhibitor, and on the conversion of MBX-2168 to its triphosphate in infected cells.
Liang Sun, of the Rega Institute, KU Leuven, described inhibitors of enterovirus A71, a major etiologic agent of hand, foot and mouth disease, which can cause severe neurological complications in young children. He and his colleagues reported earlier that a novel class of HIV inhibitors, tryptophan dendrimers, also blocks EV-A71 replication in the low-nM/high-pM range (Martinez-Gualda et al., 2017). Resistance selection, reverse genetics and cryo-EM showed that a single molecule of the prototype dendrimer, MADAL_0385, binds to the 5-fold vertex of the viral capsid. Receptor pull-down found that the dendrimer inhibits viral attachment to host cells by blocking interaction with at least two viral co-receptors, P-selectin glycoprotein ligand-1 and the glycosaminoglycan heparan sulfate, suggesting that its carboxyl group competes with receptors binding in the positively-charged cluster of the 5-fold vertex.
Bart Tarbet of Utah State University, Logan, UT, USA, described the activity of STF1019, a novel host-targeting antiviral inhibitor, in models of enterovirus D68 and 71 infections in AG129 mice. Although intranasal challenge of 4-week-old mice with EV-D68 does not cause mortality or weight loss, decreased lung function is observed by plethysmography, with elevated lung virus titers and proinflammatory cytokines. Following STF1019 treatment, only modest antiviral effects were observed, but mice given 100 or 200 mg/kg showed significant decreases in lung titers of IL-1α, IL-1β and IL-6. In the EV-D68 neurological model of infection of 10-day-old mice, treatment with STF1019 did not prevent weight loss, neurological signs or mortality. However, in the EV-71 neurological model, all mice treated with 200 mg/kg were protected from infection. These results demonstrate the utility of the EV-D68 and EV-71 mouse models to evaluate antivirals and the effectiveness of STF1019 as a potential therapy for EV-71 infection.
Julio César Villalvazo Guerrero of the Institute of Virology, Hannover Medical School, Germany started his presentation by noting that worldwide, 3.7 billion people under 50 years of age are infected with HSV-1, representing up to 87% of the population in Africa and 40–50% in the Americas. HSV is associated with significant disease, particularly in immunocompromised patients, including life-threatening infections of newborns and long-term disabling encephalitis and eye infections. Novel compounds targeting virus assembly could complement existing anti-HSV drugs in order to reduce the risk of emergence of drug-resistant viral mutants.
A reporter strain of HSV expressing GFP was employed in a phenotypic screening of about 19,000 compounds. Out of 61 hits, 27 compounds inhibited HSV plaque formation in Vero, HeLa and the human immortalized keratinocyte HaCaT cell lines, at EC50s ranging from 1.4 to 20 μM, while 50% cytotoxic concentrations (CC50) ranged from 15 to >200 μM. Some compounds showed higher selectivity indices than acyclovir, the gold standard for therapy of HSV. Experiments using a dual-color HSV strain expressing GFP in the tegument and red cherry in the capsid (Ivanova et al., 2016) were performed to identify which steps of the viral replication cycle were hindered. Some compounds inhibited nuclear capsid formation or capsid egress into the cytoplasm, while three (PANH_135, 173 and 174) reduced the number of HSV-1 capsids in the cytoplasm and blocked the binding of the C-terminal domain of pUL36 (the large essential tegument protein) to nuclear and cytoplasmic capsids. Time-of-addition studies showed that these compounds are still active when added 24 h after infection. Work is ongoing to elucidate their inhibitory potential for HSV-2, varicella zoster virus, CMV and Kaposi sarcoma HV and to determine the molecular and/or host targets.
Dana Wolf of The Hadassah Hospital and Hebrew University Faculty of Medicine, Jerusalem, Israel reported on artemisone, a novel, potent inhibitor of CMV. The anti-malarial artemisinin derivative artesunate had been shown to inhibit CMV in vitro via inhibition of CMV-induced NF-κB and Sp1 activation pathways and of the virus-modulated cell cycle progression state, both of which support efficient HCMV replication (Shapira et al., 2008).
Because of the inconsistent results obtained in the clinic with artesunate for the treatment of CMV disease, the development of novel artemisinin derivatives with enhanced antiviral activity was envisaged. Artemisone emerged as a potent inhibitor of CMV, including both laboratory-adapted strains and low-passage clinical isolates. Artemisone demonstrated ≥10-fold superior activity in epithelial cells and fibroblasts than artesunate and inhibited HCMV plaque formation, with EC50 values comparable to ganciclovir. Time-of-addition studies showed that the drug inhibits an early stage of the CMV replicative cycle and affects transcription of immediately-early (IE) genes; the effect is dependent on the multiplicity of infection (MOI). However, inhibition of a late stage of the replicative cycle was found to be independent of the MOI, suggesting a novel mechanism of action. Artemisone's inhibitory activity was specific to HCMV and the closely related rhesus macaque CMV, with no consistent activity against murine CMV or HSV-1.
Importantly, artemisone effectively hampered CMV infection, as determined by quantification of viral antigens by fluorescence microscopy and of viral mRNA, in a clinically relevant multicellular model of integral human placental tissues maintained in organ culture. This model closely recapitulates authentic viral infection, mirroring both the natural diversity of CMV-infected target cells and the characteristic cell-to-cell mode of viral spread observed in vivo (Oiknine-Djian et al., 2018). Based on these promising findings, the potential for clinical use of artemisone as a new CMV inhibitor was highlighted.
10. HIV treatment and cure
10.1. Transcriptional profiling of HIV latency. Angela Ciuffi, Institute of microbiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
Angela Ciuffi began her lecture by noting that, despite great efforts devoted to the identification of latency-reversal agents (LRAs) aimed at purging the HIV latent reservoir, only a small fraction of latently infected cells are effectively stimulated with LRAs, due to the heterogeneous nature of HIV-infected cells. To characterize this transcriptional heterogeneity, her team performed single-cell RNA sequencing (scRNA-seq) using the Fluidigm technology and a primary model of HIV latency. They analyzed the transcriptional profile of 224 latently infected CD4+T single cells, divided into three groups: untreated, histone deacetylase inhibitor vorinostat (SAHA)-treated and TCR-stimulated cells. Principal component analysis identified two different clusters in each of the three groups that correlated with differences in HIV expression: a “deep” stage CD4+ T-cell cluster, poorly responsive to external stimuli and difficult to reactivate, and an “inducible” stage CD4+ T-cell cluster, which was easier to stimulate and reactivate. They also found a 134-gene specific cellular signature that was associated with the success of HIV reactivation. In vivo analysis of single cells from HIV-infected individuals further confirmed the existence of the two cellular populations, which might reflect two distinct resting cellular states and display a different potential for cell stimulation and HIV reactivation.
10.2. Identification of a capsid-binding protein that recognizes the nuclear HIV capsid to promote CGAS-mediated innate immune activation in dendritic cells. Nicolas Manel, Institut Curie, PSL research University, Paris, France
In the first part of his lecture, Nicolas Manel gave an overview of the role of the DNA sensor cyclic GMP-AMP synthase (cGAS) in the induction of an innate immune response to HIV-2 or HIV-1 with Vpx infection in human monocyte-derived dendritic cells (DCs). Thanks to Vpx, HIV-2 infects DCs at high levels and activates innate immunity through sensing of DNA by cGAS-cGAMP-STING proteins (Lahaye et al., 2013). Manel's team was able to package cGAMP into viral particles (VLPs), transmit it into target cells, and induce an immune response. As he highlighted, this has implications for cancer immunotherapy, since cGAMP-VLPs induce anti-tumor T cell responses, and for vaccination, because cGAMP-VLPs could be delivered along with antigen by intramuscular injection, thereby exerting its immunomodulatory role.
The second part of the lecture was focused on depicting regulatory pathways that control sensing of HIV-2 by cGAS-STING. The identification of a previously unreported capsid-binding protein that binds directly to the HIV capsid, with more affinity for HIV-2 than HIV-1, represents an important contribution to the field. Manel and colleagues demonstrated by different approaches the existence of a nuclear protein that directly binds to the HIV capsid and that it is required for STING-IRF3-mediated interferon induction in response to HIV-2 in DCs. They also showed that association of this protein with cGAS is essential for the presence of cGAS in the nucleus of infected cells.
10.3. Strategies for an HIV cure: early lessons from “shock and kill” trials. Ole Schmeltz Søgaard, dept. of infectious diseases, Aarhus University Hospital, Aarhus, Denmark
Ole Søgaard reviewed the most important results obtained in HIV “shock and kill” trials, from their beginning to the present. They were conceived as a strategy to cure HIV infection, by means of the short-term administration of latency-reversing agents (LRA) that increase HIV-1 transcription, protein expression and plasma RNA. To induce virus reactivation, many pathways may be targeted by LRAs. Vorinostat provided the first direct measurement of the disruption of HIV-1 latency in vivo, with a mean 4.8-fold induction in unspliced cell-associated HIV-RNA that was not accompanied by a rebound of viral particles in plasma (Archin et al., 2012). The subsequent use of other LRAs such as panobinostat, which induces extracellular HIV-1 RNA and p24 production in plasma, further confirmed that it is possible to induce in vivo reactivation of HIV-1 (Wu et al., 2017). However, as Ole highlighted, none of these early trials, even those using potent LRAs such as romidepsin, were able to reduce the size of the latent reservoir, and once ART is interrupted, there is viral rebound. He therefore concluded that “shock” exists, but not “kill”. In the last part of his lecture, he reviewed newer studies that combined LRAs with therapeutic HIV-1 vaccines aimed at improving the killing of reactivated cells, and studies to test LRAs such as TLR7 and TLR9 agonists, which may also enhance antiviral immunity.
11. ANTIVIRALS satellite symposium
The ANTIVIRALS consortium is a Marie Skłodowska-Curie European Training Network (www.antivirals-etn.eu) that has trained 15 PhD students (early stage researchers, ESRs) since its start in 2015. Coordinator Frank van Kuppeveld of Utrecht University, The Netherlands, introduced the network and highlighted its special training elements. In particular, the close collaboration of academic and industrial partners was shown, in which both types of partners employed and trained the ESRs. He also mentioned the broad spectrum of courses in scientific and complementary skills that the network offered to its ESRs.
What the young researchers have learned during their ∼2.5 years of training was exemplified in the rest of the session. All gave 90-second presentations describing their projects and drawing the attention of the audience to their presentations at ICAR: 5 ESRs held oral presentations during the main program, and the remaining 10 presented posters.
Further examples of the achievements of the ESRs were given in two oral presentations. Lisa Bauer of Utrecht University showed how a new interdisciplinary research project was initiated by five PhD students from four different institutions, focusing on the mode of action of fluoxetine as an inhibitor of enterovirus 2C ATPase. The study brought together the disciplines of structure-based drug design and medicinal chemistry (Cardiff University & University of Vienna), structural biology (Aix-Marseille University) and molecular virology (Utrecht University) to create a basis for developing new molecules, based on the S-enantiomer of fluoxetine, hopefully without the side-effects of this anti-depressant.
Angelica Corcuera presented the results of her PhD research, performed at one of the ANTIVIRALS industrial partners, AiCuris. She studied the mechanism of action of various HBV capsid binders. Using a combination of size-exclusion chromatography, electron microscopy and fluorescence microscopy, she convincingly demonstrated that the mechanism of action of three new capsid-assembly modifiers was similar to sulfamoylbenzamide and phenylpropenamide, but distinct from heteroarylpyrimidine (HAP).
Acknowledgements
We thank all of the speakers of the 31st ICAR for their contribution to a successful meeting and all those who assisted in the making of this report.
References
- Andrei G., Carter K., Janeba Z., Sampath A., Schang L.M., Tarbet E.B., Vere Hodge R.A., Bray M., Este J.A. Highlights of the 30th international conference on antiviral research. Antivir. Res. 2017;145:184–196. doi: 10.1016/j.antiviral.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin N.M., Liberty A.L., Kashuba A.D., Choudhary S.K., Kuruc J.D., Crooks A.M., Parker D.C., Anderson E.M., Kearney M.F., Strain M.C., Richman D.D., Hudgens M.G., Bosch R.J., Coffin J.M., Eron J.J., Hazuda D.J., Margolis D.M. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia R., Pujantell M., Riveira-Muñoz E., Puig T., Torres-Torronteras J., Martí R., Clotet B., Ampudia R.M., Vives-Pi M., Esté J.A., Ballana E. The G1/S specific cyclin D2 is a regulator of HIV-1 restriction in non-proliferating cells. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005829. 1005810.1001371/journal.ppat.1005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia R., Pujantell M., Torres-Torronteras J., Menendez-Arias L., Marti R., Ruzo A., Pauls E., Clotet B., Ballana E., Este J.A., Riveira-Munoz E. SAMHD1 is active in cycling cells permissive to HIV-1 infection. Antivir. Res. 2017;142:123–135. doi: 10.1016/j.antiviral.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Ballana E., Este J.A. SAMHD1: at the crossroads of cell proliferation, immune responses, and virus restriction. Trends Microbiol. 2015;23:680–692. doi: 10.1016/j.tim.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Berke J.M., Dehertogh P., Vergauwen K., Van Damme E., Mostmans W., Vandyck K., Pauwels F. Capsid assembly modulators have a dual mechanism of action in primary human hepatocytes infected with hepatitis B virus. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00560-17. pii: e00560-00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette J.E., Raaben M., Wong A.C., Herbert A.S., Obernosterer G., Mulherkar N., Kuehne A.I., Kranzusch P.J., Griffin A.M., Ruthel G., Dal Cin P., Dye J.M., Whelan S.P., Chandran K., Brummelkamp T.R. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Warren T.K., Zhao X., Gill T., Guo F., Wang L., Comunale M.A., Du Y., Alonzi D.S., Yu W., Ye H., Liu F., Guo J.T., Mehta A., Cuconati A., Butters T.D., Bavari S., Xu X., Block T.M. Small molecule inhibitors of ER alpha-glucosidases are active against multiple hemorrhagic fever viruses. Antivir. Res. 2013;98:432–440. doi: 10.1016/j.antiviral.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromm P.M., Crews C.M. Targeted protein degradation: from chemical biology to drug discovery. Cell Chem. Biol. 2017;24:1181–1190. doi: 10.1016/j.chembiol.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decorsière A., Mueller H., van Breugel P.C., Abdul F., Gerossier L., Beran R.K., Livingston C.M., Niu C., Fletcher S.P., Hantz O., Strubin M. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature. 2016;531:386. doi: 10.1038/nature17170. [DOI] [PubMed] [Google Scholar]
- Dion S., Bourgine M., Godon O., Levillayer F., Michel M.L. Adeno-associated virus-mediated gene transfer leads to persistent hepatitis B virus replication in mice expressing HLA-A2 and HLA-DR1 molecules. J. Virol. 2013;87:5554–5563. doi: 10.1128/JVI.03134-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas G., Carvalho L.M., Bedford T., Tatem A.J., Baele G., Faria N.R., Park D.J., Ladner J.T., Arias A., Asogun D., Bielejec F., Caddy S.L., Cotten M., D'Ambrozio J., Dellicour S., Di Caro A., Diclaro J.W., Duraffour S., Elmore M.J., Fakoli L.S., Faye O., Gilbert M.L., Gevao S.M., Gire S., Gladden-Young A., Gnirke A., Goba A., Grant D.S., Haagmans B.L., Hiscox J.A., Jah U., Kugelman J.R., Liu D., Lu J., Malboeuf C.M., Mate S., Matthews D.A., Matranga C.B., Meredith L.W., Qu J., Quick J., Pas S.D., Phan M.V.T., Pollakis G., Reusken C.B., Sanchez-Lockhart M., Schaffner S.F., Schieffelin J.S., Sealfon R.S., Simon-Loriere E., Smits S.L., Stoecker K., Thorne L., Tobin E.A., Vandi M.A., Watson S.J., West K., Whitmer S., Wiley M.R., Winnicki S.M., Wohl S., Wolfel R., Yozwiak N.L., Andersen K.G., Blyden S.O., Bolay F., Carroll M.W., Dahn B., Diallo B., Formenty P., Fraser C., Gao G.F., Garry R.F., Goodfellow I., Gunther S., Happi C.T., Holmes E.C., Kargbo B., Keita S., Kellam P., Koopmans M.P.G., Kuhn J.H., Loman N.J., Magassouba N., Naidoo D., Nichol S.T., Nyenswah T., Palacios G., Pybus O.G., Sabeti P.C., Sall A., Stroher U., Wurie I., Suchard M.A., Lemey P., Rambaut A. Virus genomes reveal factors that spread and sustained the Ebola epidemic. Nature. 2017;544:309–315. doi: 10.1038/nature22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASL EASL recommendations on treatment of hepatitis C 2018. J. Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- Edwards T.C., Lomonosova E., Patel J.A., Li Q., Villa J.A., Gupta A.K., Morrison L.A., Bailly F., Cotelle P., Giannakopoulou E., Zoidis G., Tavis J.E. Inhibition of hepatitis B virus replication by N-hydroxyisoquinolinediones and related polyoxygenated heterocycles. Antivir. Res. 2017;143:205–217. doi: 10.1016/j.antiviral.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria N.R., Rambaut A., Suchard M.A., Baele G., Bedford T., Ward M.J., Tatem A.J., Sousa J.D., Arinaminpathy N., Pepin J., Posada D., Peeters M., Pybus O.G., Lemey P. HIV epidemiology. The early spread and epidemic ignition of HIV-1 in human populations. Science. 2014;346:56–61. doi: 10.1126/science.1256739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferir G., Petrova M.I., Andrei G., Huskens D., Hoorelbeke B., Snoeck R., Vanderleyden J., Balzarini J., Bartoschek S., Bronstrup M., Sussmuth R.D., Schols D. The lantibiotic peptide labyrinthopeptin A1 demonstrates broad anti-HIV and anti-HSV activity with potential for microbicidal applications. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnest T., de Oliveira T.D., Schols D., Balzarini J., Meier C. Lipophilic prodrugs of nucleoside triphosphates as biochemical probes and potential antivirals. Nat. Commun. 2015;6:8716. doi: 10.1038/ncomms9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnest T., Dinis de Oliveira T., Rath A., Hauber I., Schols D., Balzarini J., Meier C. Membrane-permeable triphosphate prodrugs of nucleoside analogues. Angew Chem. Int. Ed. Engl. 2016;55:5255–5258. doi: 10.1002/anie.201511808. [DOI] [PubMed] [Google Scholar]
- Guo F., Wu S., Julander J., Ma J., Zhang X., Kulp J., Cuconati A., Block T.M., Du Y., Guo J.T., Chang J. A novel benzodiazepine compound inhibits yellow fever virus infection by specifically targeting NS4B protein. J. Virol. 2016;90:10774–10788. doi: 10.1128/JVI.01253-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.R., Wohlleber D., Reisinger F., Jenne C.N., Cheng R.L., Abdullah Z., Schildberg F.A., Odenthal M., Dienes H.P., van Rooijen N., Schmitt E., Garbi N., Croft M., Kurts C., Kubes P., Protzer U., Heikenwalder M., Knolle P.A. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat. Immunol. 2013;14:574–583. doi: 10.1038/ni.2573. [DOI] [PubMed] [Google Scholar]
- Ivanova L., Buch A., Dohner K., Pohlmann A., Binz A., Prank U., Sandbaumhuter M., Bauerfeind R., Sodeik B. Conserved tryptophan motifs in the large tegument protein pUL36 are required for efficient secondary envelopment of herpes simplex virus capsids. J. Virol. 2016;90:5368–5383. doi: 10.1128/JVI.03167-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komazin-Meredith G., Cardinale S.C., Comeau K., Magalhaes K.J., Hartline C.B., Williams J.D., Opperman T.J., Prichard M.N., Bowlin T.L. TAOK3 phosphorylates the methylenecyclopropane nucleoside MBX 2168 to its monophosphate. Antivir. Res. 2015;119:23–27. doi: 10.1016/j.antiviral.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaye X., Satoh T., Gentili M., Cerboni S., Conrad C., Hurbain I., El Marjou A., Lacabaratz C., Lelievre J.D., Manel N. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity. 2013;39:1132–1142. doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Lambert D.M., Barney S., Lambert A.L., Guthrie K., Medinas R., Davis D.E., Bucy T., Erickson J., Merutka G., Petteway S.R., Jr. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2186–2191. doi: 10.1073/pnas.93.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan H., Tang Z., Jin H., Sun Y. Neddylation inhibitor MLN4924 suppresses growth and migration of human gastric cancer cells. Sci. Rep. 2016;6:24218. doi: 10.1038/srep24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeroos L., Krzyzaniak M.A., Helenius A., Butcher S.J. Architecture of respiratory syncytial virus revealed by electron cryotomography. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11133–11138. doi: 10.1073/pnas.1309070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonosova E., Daw J., Garimallaprabhakaran A.K., Agyemang N.B., Ashani Y., Murelli R.P., Tavis J.E. Efficacy and cytotoxicity in cell culture of novel alpha-hydroxytropolone inhibitors of hepatitis B virus ribonuclease H. Antivir. Res. 2017;144:164–172. doi: 10.1016/j.antiviral.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Wu S., Zhang X., Guo F., Yang K., Guo J., Su Q., Lu H., Lam P., Li Y., Yan Z., Kinney W., Guo J.T., Block T.M., Chang J., Du Y. Ester prodrugs of IHVR-19029 with enhanced oral exposure and prevention of gastrointestinal glucosidase interaction. ACS Med. Chem. Lett. 2017;8:157–162. doi: 10.1021/acsmedchemlett.6b00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gualda B., Sun L., Rivero-Buceta E., Flores A., Quesada E., Balzarini J., Noppen S., Liekens S., Schols D., Neyts J., Leyssen P., Mirabelli C., Camarasa M.J., San-Felix A. Structure-activity relationship studies on a Trp dendrimer with dual activities against HIV and enterovirus A71. Modifications on the amino acid. Antivir. Res. 2017;139:32–40. doi: 10.1016/j.antiviral.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Marty F.M., Ljungman P., Chemaly R.F., Maertens J., Dadwal S.S., Duarte R.F., Haider S., Ullmann A.J., Katayama Y., Brown J., Mullane K.M., Boeckh M., Blumberg E.A., Einsele H., Snydman D.R., Kanda Y., DiNubile M.J., Teal V.L., Wan H., Murata Y., Kartsonis N.A., Leavitt R.Y., Badshah C. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N. Engl. J. Med. 2017;377:2433–2444. doi: 10.1056/NEJMoa1706640. [DOI] [PubMed] [Google Scholar]
- Mehellou Y., Rattan H.S., Balzarini J. The ProTide prodrug technology: from the concept to the clinic. J. Med. Chem. 2018;61:2211–2226. doi: 10.1021/acs.jmedchem.7b00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier C. Nucleoside diphosphate and triphosphate prodrugs - an unsolvable task? Antivir. Chem. Chemother. 2017;25:69–82. doi: 10.1177/2040206617738656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier C., Balzarini J. Application of the cycloSal-prodrug approach for improving the biological potential of phosphorylated biomolecules. Antivir. Res. 2006;71:282–292. doi: 10.1016/j.antiviral.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Miller E.H., Obernosterer G., Raaben M., Herbert A.S., Deffieu M.S., Krishnan A., Ndungo E., Sandesara R.G., Carette J.E., Kuehne A.I., Ruthel G., Pfeffer S.R., Dye J.M., Whelan S.P., Brummelkamp T.R., Chandran K. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 2012;31:1947–1960. doi: 10.1038/emboj.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiknine-Djian E., Weisblum Y., Panet A., Wong H.N., Haynes R.K., Wolf D.G. The artemisinin derivative artemisone is a potent inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 2018;62:13. doi: 10.1128/AAC.00288-18. e00288-00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls E., Badia R., Torres-Torronteras J., Ruiz A., Permanyer M., Riveira-Muñoz E., Clotet B., Marti R., Ballana E., Esté J.A. Palbociclib, a selective inhibitor of cyclin-dependent kinase4/6, blocks HIV-1 reverse transcription through the control of sterile [alpha] motif and HD domain-containing protein-1 activity. AIDS. 2014;28:2213–2222. doi: 10.1097/QAD.0000000000000399. [DOI] [PubMed] [Google Scholar]
- Pujantell M., Franco S., Galván-Femenía I., Badia R., Castellví M., Garcia-Vidal E., Clotet B., de Cid R., Tural C., Martínez M.A., Riveira-Muñoz E., Esté J.A., Ballana E. ADAR1 affects HCV infection by modulating innate immune response. Antivir. Res. 2018;156:116–127. doi: 10.1016/j.antiviral.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Pujantell M., Riveira-Muñoz E., Badia R., Castellví M., Garcia-Vidal E., Sirera G., Puig T., Ramirez C., Clotet B., Esté J.A., Ballana E. RNA editing by ADAR1 regulates innate and antiviral immune functions in primary macrophages. Sci. Rep. 2017;7:13339. doi: 10.1038/s41598-017-13580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaturro P., Cortese M., Chatel-Chaix L., Fischl W., Bartenschlager R. Dengue virus non-structural protein 1 modulates infectious particle production via interaction with the structural proteins. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M.Y., Resnick I.B., Chou S., Neumann A.U., Lurain N.S., Stamminger T., Caplan O., Saleh N., Efferth T., Marschall M., Wolf D.G. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin. Infect. Dis. 2008;46:1455–1457. doi: 10.1086/587106. [DOI] [PubMed] [Google Scholar]
- Slusarczyk M., Serpi M., Pertusati F. Phosphoramidates and phosphonamidates (ProTides) with antiviral activity. Antivir. Chem. Chemother. 2018;26 doi: 10.1177/2040206618775243. 2040206618775243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staring J., van den Hengel L.G., Raaben M., Blomen V.A., Carette J.E., Brummelkamp T.R. KREMEN1 is a host entry receptor for a major group of enteroviruses. Cell Host Microbe. 2018;23:636–643. doi: 10.1016/j.chom.2018.03.019. e635. [DOI] [PubMed] [Google Scholar]
- Staring J., von Castelmur E., Blomen V.A., van den Hengel L.G., Brockmann M., Baggen J., Thibaut H.J., Nieuwenhuis J., Janssen H., van Kuppeveld F.J., Perrakis A., Carette J.E., Brummelkamp T.R. PLA2G16 represents a switch between entry and clearance of Picornaviridae. Nature. 2017;541:412–416. doi: 10.1038/nature21032. [DOI] [PubMed] [Google Scholar]
- Suchard M.A., Lemey P., Baele G., Ayres D.L., Drummond A.J., Rambaut A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018;4:vey016. doi: 10.1093/ve/vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Chu C.K., Boudinot F.D. Development and optimization of anti-HIV nucleoside analogs and prodrugs: a review of their cellular pharmacology, structure-activity relationships and pharmacokinetics. Adv. Drug Deliv. Rev. 1999;39:117–151. doi: 10.1016/s0169-409x(99)00023-x. [DOI] [PubMed] [Google Scholar]
- Vere Hodge R.A. vol. 100. 2013. pp. 276–285. (Meeting Report: 26th International Conference on Antiviral Research). Antiviral Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere Hodge R.A. vol. 111. 2014. pp. 143–153. (Meeting Report: 27th International Conference on Antiviral Research, in Raleigh, NC, USA). Antiviral Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere Hodge R.A. vol. 123. 2015. pp. 172–187. (Meeting Report: 28th International Conference on Antiviral Research in Rome, Italy). Antiviral Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere Hodge R.A. vol. 137. 2017. pp. 23–40. (Meeting Report: 29th International Conference on Antiviral Research in La Jolla, CA, USA). Antiviral Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D.M., Mutai N., Heath C.J., Vulule J.M., Mutuku F.M., Ndenga B.A., LaBeaud A.D. Unrecognized dengue virus infections in children, Western Kenya, 2014-2015. Emerg. Infect. Dis. 2017;23:1915–1917. doi: 10.3201/eid2311.170807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Bardelli M., Espinosa D.A., Pedotti M., Ng T.S., Bianchi S., Simonelli L., Lim E.X.Y., Foglierini M., Zatta F., Jaconi S., Beltramello M., Cameroni E., Fibriansah G., Shi J., Barca T., Pagani I., Rubio A., Broccoli V., Vicenzi E., Graham V., Pullan S., Dowall S., Hewson R., Jurt S., Zerbe O., Stettler K., Lanzavecchia A., Sallusto F., Cavalli A., Harris E., Lok S.M., Varani L., Corti D. A human Bi-specific antibody against Zika virus with high therapeutic potential. Cell. 2017;171:229–241 e215. doi: 10.1016/j.cell.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinschenk L., Schols D., Balzarini J., Meier C. Nucleoside diphosphate prodrugs: nonsymmetric DiPPro-nucleotides. J. Med. Chem. 2015;58:6114–6130. doi: 10.1021/acs.jmedchem.5b00737. [DOI] [PubMed] [Google Scholar]
- Worobey M., Watts T.D., McKay R.A., Suchard M.A., Granade T., Teuwen D.E., Koblin B.A., Heneine W., Lemey P., Jaffe H.W. 1970s and 'Patient 0' HIV-1 genomes illuminate early HIV/AIDS history in North America. Nature. 2016;539:98–101. doi: 10.1038/nature19827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Swanson M., Talla A., Graham D., Strizki J., Gorman D., Barnard R.J., Blair W., Sogaard O.S., Tolstrup M., Ostergaard L., Rasmussen T.A., Sekaly R.P., Archin N.M., Margolis D.M., Hazuda D.J., Howell B.J. HDAC inhibition induces HIV-1 protein and enables immune-based clearance following latency reversal. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92901. [DOI] [PMC free article] [PubMed] [Google Scholar]