Abstract
Highly pathogenic human coronaviruses associated with a severe respiratory syndrome, including Middle East respiratory syndrome coronavirus (MERS-CoV), have recently emerged. The MERS-CoV epidemic started in 2012 and is still ongoing, with a mortality rate of approximately 35%. No vaccine is available against MERS-CoV and therapeutic options for MERS-CoV infections are limited to palliative and supportive care. A search for specific antiviral treatments is urgently needed. Coronaviruses are enveloped viruses, with the spike proteins present on their surface responsible for virus entry into the target cell. Lectins are attractive anti-coronavirus candidates because of the highly glycosylated nature of the spike protein. We tested the antiviral effect of griffithsin (GRFT), a lectin isolated from the red marine alga Griffithsia sp. against MERS-CoV infection. Our results demonstrate that while displaying no significant cytotoxicity, griffithsin is a potent inhibitor of MERS-CoV infection. Griffithsin also inhibits entry into host cells of particles pseudotyped with the MERS-CoV spike protein, suggesting that griffithsin inhibits spike protein function during entry. Spike proteins have a dual function during entry, they mediate binding to the host cell surface and also the fusion of the viral envelope with host cell membrane. Time course experiments show that griffithsin inhibits MERS-CoV infection at the binding step. In conclusion, we identify griffithsin as a potent inhibitor of MERS-CoV infection at the entry step.
Keywords: Antiviral, Lectin, MERS-CoV, Virus entry
Highlights
-
•
We analyze the anti-MERS-CoV potential of the lectin griffithsin.
-
•
Griffithsin inhibits MERS-CoV infection at the entry step.
-
•
Griffithsin inhibits binding of MERS-CoV to the cell surface potentially by interacting with spike protein glycans.
1. Introduction
Coronaviruses belong to the Coronaviridae family. They are enveloped viruses with a large single stranded, positive-sense RNA genome and infect both humans and animals. Until 2003, human coronaviruses were responsible for only mild respiratory diseases, mainly common colds. This changed with the emergence of a highly pathogenic coronavirus associated with a severe respiratory syndrome, namely the severe acute respiratory syndrome coronavirus (SARS-CoV). In 2012, a new deadly human coronavirus emerged in the Arabian Peninsula, Middle East respiratory syndrome coronavirus (MERS-CoV) responsible for severe pneumonia that can be associated with additional clinical symptoms such as vomiting, diarrhea or renal failure (Alhogbani, 2016, Zaki et al., 2012). The MERS-CoV epidemic is still ongoing, and so far more than 1700 laboratory-confirmed cases have been diagnosed with a mortality rate of approximately 35%. There are neither clinically approved antivirals nor vaccines available against MERS-CoV and therapeutic options for MERS-CoV infections are limited to palliative and supportive care.
The MERS-CoV spike protein is a main determinant of virus entry into host cells as it mediates both binding to the DPP4 (dipeptidyl peptidase 4) receptor and fusion of the viral envelope with host cell membrane (Millet and Whittaker, 2014, Raj et al., 2013). It is a type I fusion protein and is highly glycosylated with 19 predicted N-glycosylation sites. One option to block MERS-CoV infection is to take advantage of the highly glycosylated nature of its spike protein by using lectins in order to inhibit MERS-CoV entry and thus virus propagation. Griffithsin (GRFT) is a 121 amino acid long lectin that was isolated from the red marine alga Griffithsia sp. Griffithsin has been shown to have antiviral activity against HIV-1 within the picomolar range (Mori et al., 2005). Characterization of griffithsin structure has demonstrated that it is a domain-swapped homodimer with three carbohydrate binding domains on each monomer that bind to terminal mannose residues linked on HIV envelope protein gp120 N-glycans (Ziółkowska et al., 2006). Griffithsin has also been identified as a potent inhibitor of hepatitis C virus (HCV) and SARS-CoV infection in vitro and in vivo with minimal toxicity (Meuleman et al., 2011, O'Keefe et al., 2010). Therefore, we evaluated the anti-MERS-CoV activity of griffithsin.
2. Materials and methods
2.1. Cells and virus
HEK-293T (ATCC), Huh-7 (Nakabayashi et al., 1982), MRC-5 (ATCC), and Vero-81 (ATCC) cells were grown at 37 °C 5% CO2 in DMEM (Corning) supplemented with 10% fetal bovine serum (ThermoFisher), 10 mM HEPES (Corning), 100 IU/mL penicillin and 100 μg/mL streptomycin (Corning).
MERS-CoV strain EMC/2012 was generously donated by Ralph Baric (UNC Chapel Hill) and Bart Haagmans (Utrecht University). The virus was propagated in Vero-81 cells, with viral titrations performed using TCID50 assays. All experiments involving authentic MERS-CoV were performed in a biosafety level 3 facility (Animal Health Diagnostic Center, Cornell University).
2.2. Reagents
PBS (Corning) was used as negative control in all experiments. Mannonanose-di-(N-acetyl-D-glucosamine) (Man-9 Glycan), was purchased from Sigma Aldrich. CellTiter-Glo luminescent cell viability assay reagents and Luciferase assay kit were purchased from Promega.
2.3. Cell viability assay
2.5 × 104 Huh-7, MRC-5, and Vero-81 cells were seeded in 96-well plates and incubated for 16 h at 37 °C 5% CO2 incubator. The cells were then treated with increasing concentrations of griffithsin, from 0 (PBS) up to 2 μg/mL, a range used in subsequent experiments. The treated cells were incubated at 37 °C 5% CO2 for 2 h. The medium was replaced with fresh growth medium without griffithsin and cells were incubated for another 7 h at 37 °C 5% CO2. To test the effect of griffithsin for longer incubation times, the cells were left treated with either 0 or 1 μg/mL griffithsin for 24 h or 48 h. The cells were then lysed and viability assayed using the CellTiter-Glo kit according to manufacturer's guidelines. Luminescence (relative luminescence units - RLU) readings were performed using a GloMax 20/20 luminometer (Promega). The percent cell viability was calculated by the following equation:
The assays were carried out using triplicate wells, and data corresponds to the average of three independent experiments.
2.4. Dose-response effect of griffithsin on MERS-CoV infection
1.25–1.5 × 105 Huh-7, MRC-5, and Vero-81 cells were seeded in wells of 8-well chamber slides (Ibidi) and incubated at 37 °C 5% CO2 for 16 h. Increasing concentrations of griffithsin (0–2 μg/mL) were added to MERS-CoV strain EMC/2012 viral inoculums that were used to infect cells at a multiplicity of infection (m.o.i.) of 10. Non-infected cells and PBS-treated virus inoculum were used as negative controls. The infected cells were incubated at 37 °C 5% CO2 for 2 h, after which the inoculum was removed, followed by PBS washing to remove unbound virions and addition of cell culture medium. The infection was left to proceed at 37 °C 5% CO2 for an additional 7 h. The wells of chamber slides were then processed for immunofluorescence assay as described in 2.8. Immunofluorescence microscopy assays.
2.5. Effect of griffithsin on MERS-CoV production
3 × 105 Huh-7 and MRC-5 cells were seeded in 24-well plate wells and incubated at 37 °C 5% CO2 for 16 h. Cells were infected with virus inoculum that was untreated or treated with 1 μg/mL griffithsin. The infected cells were incubated at 37 °C 5% CO2 for 2 h, after which the inoculum was removed, followed by PBS washing to remove unbound virions and addition of cell culture medium containing or not 1 μg/mL griffithsin. Cells were left to incubate at 37 °C 5% CO2. At 24 h and 48 h post infection time points, cell culture supernatants were harvested and stored at −80 °C. Cell titrations were performed using a TCID50 assay on Vero-81 cells. Results are expressed as log10 TCID50/mL and represent averages of two independent experiments.
2.6. Effect of griffithsin activity on MERS-CoV pseudotyped virion entry
Particles pseudotyped with either MERS-CoV-S envelope proteins (MERSpp), genotype 2a HCV envelope proteins (HCVpp), or the G envelope glycoprotein of vesicular stomatitis virus (VSV-Gpp) were produced as previously described (Belouzard et al., 2009, Op De Beeck et al., 2004). Pseudotyped virions were used to inoculate Huh-7 cells in 48-well plates for 3 h in the presence of griffithsin. The inoculum was removed and cells were further incubated with culture medium for 45 h. Cells were lysed and luciferase activity was detected by using a Luciferase Assay kit (Promega) and light emission measured by using a Tristar LB 941 luminometer (Berthold Technologies). For competition experiments with Man-9, griffithsin was preincubated with Man-9 at different concentrations for 3 h at 37 °C before being added to MERSpp during the 3 h inoculation step of Huh-7 cells. The inoculum was removed and luciferase activity quantified 45 h post inoculation as described above.
2.7. Effect of griffithsin activity on MERS-CoV entry steps
1.5 × 105 Huh-7 cells were seeded in wells of 8-well chamber slides (Ibidi) and incubated at 37 °C 5% CO2 for 16 h. Virus infection was divided into four steps: 1) inoculation and attachment of virus for 1 h at 4 °C, 2) PBS wash to remove unbound virus, followed by further binding of bound virus for 1 h at 4 °C, 3) internalization for 1 h at 37 °C, and 4) infection for 8 h at 37 °C. In all conditions, cells were infected with MERS-CoV strain EMC/2012 at an m.o.i. of 10, with griffithsin (1 μg/mL) added or not at different steps during virus entry. In experimental condition a, griffithsin was not present at any step. In condition b, griffithsin was only present during initial attachment of virus on cells. In condition c, griffithsin was added only during the further binding step. In condition d, griffithsin was present only during internalization. Finally, in condition e, griffithsin was present during initial attachment, further binding and internalization steps (1–3), but not during infection. After MERS-CoV infection was left to proceed for 8 h at 37 °C, the wells of the chamber slides were processed for immunofluorescence assay as described in 2.8. Immunofluorescence microscopy assays.
2.8. Immunofluorescence microscopy assays
Immunofluorescence were performed as previously described (Millet and Whittaker, 2014).
For quantifications of each condition, 5 fields were randomly acquired. For each field, the total number of cells (DAPI stain) and number of infected cells (spike labeling) were counted using automatic cell counting software (Imaris 7.6.5). The results of quantification are expressed in percent-infected cells. The experiment was performed using duplicate wells, and data corresponds to the average of three independent experiments.
2.9. Interaction of griffithsin with MERS-CoV spike protein
Purified V5-tagged soluble MERS-CoV spike proteins were incubated in 1% Triton X-100 in PBS in presence or absence of 2 μg/ml of griffithsin for 2 h at 4 °C followed by addition of polyclonal anti-griffithsin antibodies and a 2 h incubation. The protein-antibodies complexes were precipitated with protein A sepharose beads pre-incubated with 5% BSA to avoid nonspecific binding. Proteins were separated by 8% SDS-PAGE and were transferred to nitrocellulose membrane (Amersham). Spike proteins were immunodetected with an anti-V5 antibody (ThermoFisher).
2.10. Statistical analyses
Analysis of experimental data, calculations of standard deviations (s.d.) and standard error of the mean (s.e.m.), and graph plotting were performed using GraphPad Prism 6.0. Statistical analyses were performed using one-way ANOVA test (dose-responses for cell viability and viral infectivity) and two-tailed Student's t-test (viral titer assays, competition with Man9, and binding assay). For p-value significance, the following convention was used: not significant (n.s.) p > 0.05, significant (*) p ≤ 0.05, highly significant (**) p ≤ 0.01, very highly significant (***) p ≤ 0.001.
3. Results
3.1. Dose-dependent inhibition of MERS-CoV infection by griffithsin
We assessed the effect of griffithsin on cell viability and tested its potential cytotoxicity by treating Huh-7, MRC-5 and Vero-81 cells with a range of griffithsin concentrations that was used in subsequent experiments, 0–2 μg/mL (Fig. 1 A), or with 1 μg/mL for longer treatment exposure times (Fig. 1B). Griffithsin does not decrease cell viability at the doses and times tested, results that are in agreement with a previous investigation (Nixon et al., 2013). To determine the inhibitory activity of griffithsin on MERS-CoV infectivity, we have carried out dose-response infection experiments in three different cell lines (Fig. 1C and D). Huh-7, MRC-5, and Vero-81 cells were infected with strain EMC/2012 of MERS-CoV at an m.o.i. of 10 in presence of increasing concentrations of griffithsin (0–2 μg/mL). The infected cells were fixed at an early infection time point (9 h) and assayed by immunofluorescence microscopy analysis (Fig. 1C) followed by quantification of the percentage of infected cells for each concentration (Fig. 1D). Griffithsin significantly decreased infectivity by MERS-CoV, with a clear, dose-dependent inhibitory effect in all cell lines tested (Fig. 1C and D). Even at the most diluted concentration of 0.125 μg/mL tested, the inhibitory activity of griffithsin on MERS-CoV infection was significant, with a minimal drop of 44.7% in infectivity for Huh-7 cells and a maximal drop of 63.2% for Vero-81 cells compared to the condition without the lectin. At 2 μg/mL, griffithsin inhibited viral infectivity by more than 90% in all cell lines tested. Because we have performed our assay using high m.o.i. and a short infection time, these results show the strong inhibitory activity of griffithsin on early steps of the MERS-CoV viral cycle.
Fig. 1.
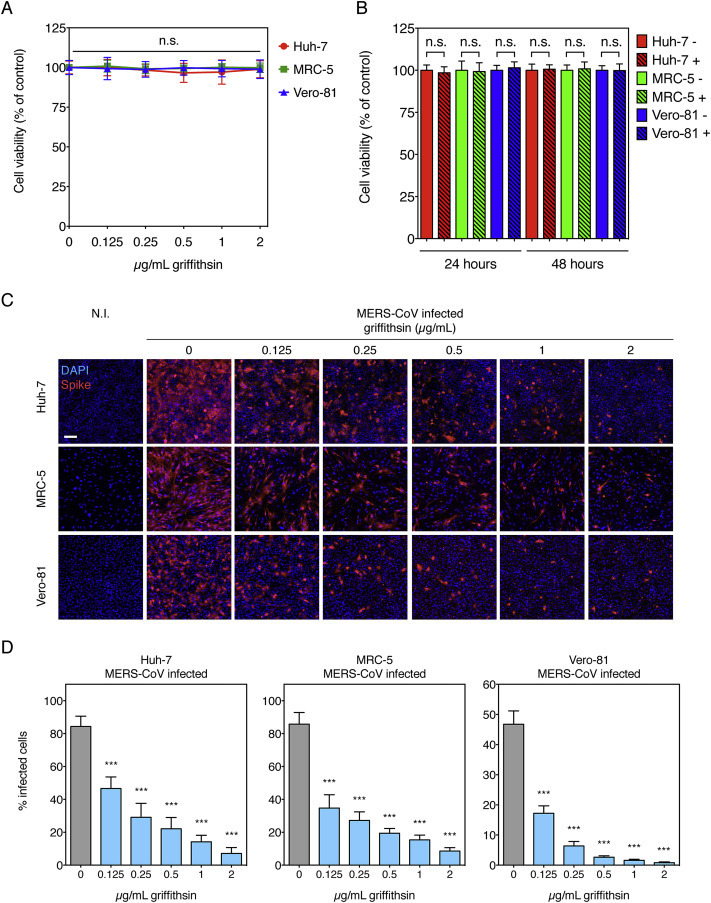
Effect of griffithsin on cell viability and MERS-CoV infectivity. Griffithsin cell viability dose-response (A) and effect after extended incubation times (B). Huh-7, MRC-5, and Vero-81 cells were treated with increasing concentrations of griffithsin (A) or with 0 μg/mL (−) or 1 μg/mL (+) of the lectin (B). For (A), after 2 h of incubation, supernatants were replaced with medium without griffithsin and cells were incubated at 37 °C for 7 h. In (B), cells were left treated with griffithsin for 24 h or 48 h. Cell viability was measured using a luminescence-based ATP quantitation assay. Results are expressed as average of cell viability (% of 0 μg/mL control), with error bars representing SD from the average of three independent experiments. Data were statistically analyzed using one-way ANOVA test (A) or using a two-tailed Student t-test (B), with the following convention for p-value significance: not significant (n.s.), p > 0.05. (C) Dose-response analysis of griffithsin on MERS-CoV infection. Immunofluorescence assay of MERS-CoV-infected Huh-7, MRC-5, and Vero-81 cells in presence of increasing concentrations of griffithsin. Cells were infected with MERS-CoV strain EMC/2012 at an m.o.i. of 10, in presence of increasing amounts of griffithsin for 2 h at 37 °C. PBS was used for the non-treated control condition. Cells were washed with PBS and the inoculum replaced with cell growth medium. The cells were incubated at 37 °C 5% CO2 for an additional 7 h. Cells were then fixed, immunolabeled for MERS-CoV S, and stained for nuclei (DAPI). N.I.: non infected control. (D) Quantification of MERS-CoV-positive cells in presence of increasing concentration of griffithsin. For each condition, five 10 × objective fields were randomly acquired and analyzed for total number of cells (DAPI nuclei stain) and S-positive cells (infected cells). Results are expressed as percentage infected cells, with error bars representing SD from the average of three independent experiments. Data were statistically analyzed using one-way ANOVA test with the following convention for p-value significance: very highly significant (***), p ≤ 0.001.
3.2. Inhibition of MERS-CoV production by griffithsin
We investigated the effect of griffithsin on MERS-CoV virus titers (Fig. 2 ). Huh-7 and MRC-5 cells were infected with MERS-CoV in presence or not of 1 μg/mL griffithsin. At 24 h and 48 h post infection, cell supernatants were harvested and analyzed for virus titers by performing a TCID50 assay (Fig. 2). For both cell lines, griffithsin significantly decreased virus titers at both 24 and 48 h time points, confirming the inhibitory role of the lectin on MERS-CoV infection.
Fig. 2.
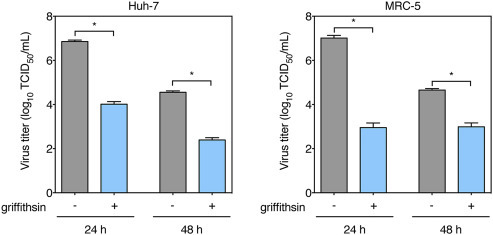
Effect of griffithsin on MERS-CoV virus titers. Huh-7 and MRC-5 cells were seeded in wells of 24-well plates and infected with MERS-CoV in presence or not of 1 μg/mL griffithsin. After 2 h of incubation, the inoculum was removed, and cells were incubated in presence or absence of 1 μg/mL of griffithsin. At 24 h and 48 h post infection, supernatants were harvested. TCID50 assays were performed on the collected supernatants using Vero-81 cells. Results are expressed as average of log10 TCID50/mL, with error bars representing SD from the average of two independent experiments. Data were statistically analyzed using two-tailed Student t-test with the following convention for p-value significance: significant (*), p ≤ 0.05.
3.3. Dose-dependent inhibition of MERS-CoV pseudotyped virion entry by griffithsin
Griffithsin has been shown to inhibit cellular entry of numerous viruses including SARS-CoV. To confirm the results obtained with MERS-CoV and determine the activity of this molecule on entry mediated by MERS-CoV spike protein, MERS-CoV pseudotyped virions (MERSpp) were produced and used to infect Huh-7 cells. A dose-dependent inhibition of MERSpp infection was observed in the presence of griffithsin (Fig. 3 A) suggesting that this compound is able to inhibit MERS-CoV entry. As expected, griffithsin was also able to inhibit HCVpp infection and had no effect on VSV-Gpp infection as previously described (Meuleman et al., 2011). Griffithsin is known to interact with N-linked high mannose oligosaccharides. The antiviral effect of this compound was shown to be due to the interactions of griffithsin with the mannoses linked to the viral envelope protein (Moulaei et al., 2010). To confirm that the inhibition observed is specific of griffithsin activity, a competition assay using N-linked high mannose oligosaccharides mannonose-di-(N-acetyl-D-glucosamine) (Man-9 Glycan) was performed. As shown in Fig. 3B, incubation of MERSpp with griffithsin preincubated first with Man-9 Glycan significantly restores the infectivity of pseudotyped particles which suggests that griffithsin interacts with mannoses of MERS-CoV S envelope and impairs its functions during entry. To further confirm that griffithsin interacts with MERS-CoV spike protein, we performed a co-immunoprecipitation assay. Soluble MERS-CoV spike proteins were incubated in presence or absence of griffithsin. The presence of spike proteins complexed with griffithsin was analyzed by Western blot after immunoprecipitation with a polyclonal anti-griffithsin antibody (Fig. 3C). Western blot analysis revealed that spike protein could be coimmunoprecipitated only in presence of griffithsin.
Fig. 3.
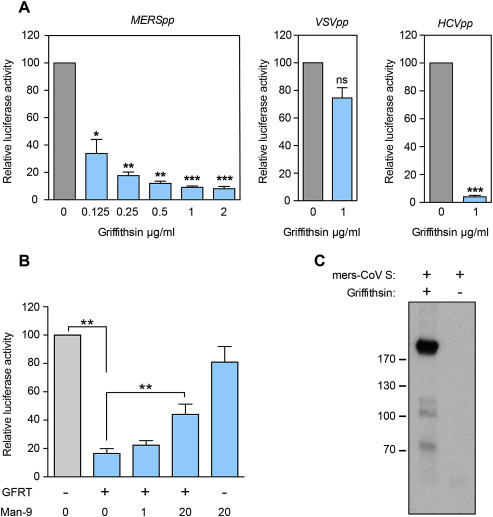
Dose-dependent inhibition of MERS-coV pseudotyped virion infection by griffithsin and competition with Man-9. (A) Huh-7 cells were inoculated with MERSpp in the presence of griffithsin at given concentrations. In parallel, Huh-7 cells were inoculated with HCVpp or VSV-Gpp in the presence of griffithsin at 1 μg/mL. Intracellular firefly luciferase signal was measured 48 h post infection and normalized to untreated cultures. (B) Griffithsin (0.5 μg/mL) and Man-9 at the indicated concentrations were pre-incubated for 3 h at 37 °C before being added to the MERSpp for infection of Huh-7. Intracellular firefly luciferase signal was measured as described above. Experiment was performed three times in duplicate or triplicate. Error bars represent SEM. Data were statistically analyzed using two-tailed Student's t-test comparing results with non-treated condition with the following convention for p-value significance: not significant (n.s.), significant (*) p > 0.05; highly significant (**) p ≤ 0.01, very highly significant (***), p ≤ 0.001. (C) Soluble MERS-CoV spike protein was incubated in presence or absence of 2 μg/mL griffithsin. Then griffithsin was immunoprecipitated and MERS-CoV spike protein was detected in Western blot.
3.4. Griffithsin acts on initial stages of infection
To better define which stage in the virus life cycle griffithsin acts on, we performed an infection assay using authentic MERS-CoV with griffithsin present at different times during viral entry steps (Fig. 4 A). In this experiment, viral entry steps were divided into attachment at 4 °C, further binding at 4 °C, internalization at 37 °C. In the control conditions, griffithsin was either not present during entry steps (a) or present during all entry steps (e). In the latter condition, griffithsin had a significant inhibitory effect on infectivity compared to control condition (a), a result which recapitulates well the dose-response results (Fig. 4B and C). We do note however that the infectivity in condition (a) (31.7%) is lower than observed in the non-treated control of the dose response experiments (84.3%). We attribute this drop to differences in the infection assays used, such as shorter inoculum contact time and multiple PBS washes. In condition (b), where griffithsin is only present during the first attachment step, a significant decrease in infectivity was measured (Fig. 4C). Such significant inhibition was not observed for conditions (c), and (d), where griffithsin was present only at later steps in entry (further binding without inoculum, and internalization, respectively). Taken together, these data reveal that griffithsin acts on very early stages of virus entry, probably by directly interacting with the glycosylated part of MERS-CoV spike protein and inhibiting its binding function.
Fig. 4.
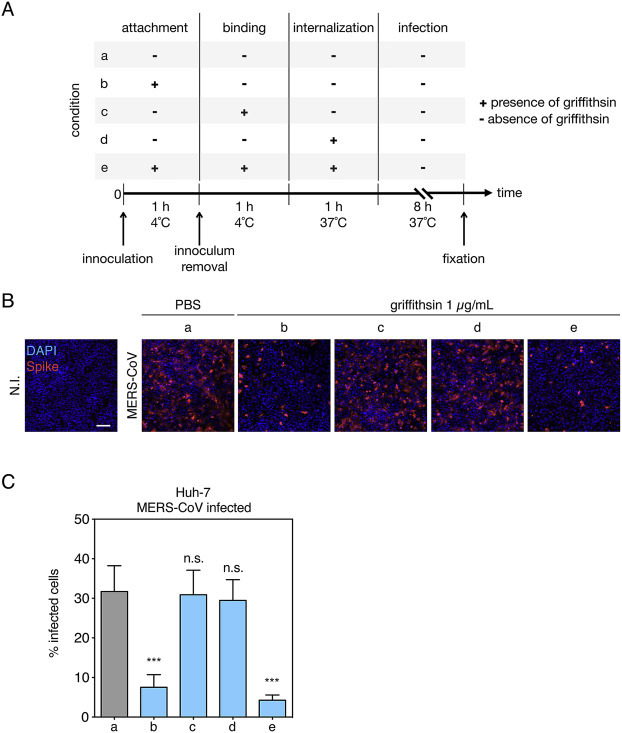
Inhibitory activity of griffithsin at early stages of MERS-CoV infection. (A) Schematic of experimental time course. Infection of Huh-7 cells was divided into four steps: the initial viral inoculation (attachment) step was performed at 4 °C for 1 h in presence (conditions b and e) or absence (PBS in a, c, and d) of 1 μg/mL griffithsin, using an m.o.i. of 10. During the second step, the viral inoculum was removed and cells were washed three times with cold PBS and kept at 4 °C for 1 h, enabling further binding of virus to cell surfaces, in presence (c and e) or absence (a, b, and d) of 1 μg/mL griffithsin. For the third step, the cells were placed at 37 °C for 1 h allowing for internalization of virions to occur, in presence (d and e) or absence (a, b, and c) of 1 μg/mL griffithsin. The infection was left to proceed in a last 8 h step at 37 °C in absence griffithsin. (B) Immunofluorescence assay of MERS-CoV-infected Huh-7 cells with griffithsin present at different stages of infection. Cells were infected and treated as described in (A) then fixed, immunolabeled and analyzed as described previously in Fig. 2. (C) Quantification of MERS-CoV-positive Huh-7 cells for each condition tested. Cells were analyzed as described in Fig. 2. Results are expressed as percentage infected cells, with error bars representing SD from the average of three independent experiments. Data were statistically analyzed using two-tailed Student's t-test comparing results with non-treated condition (a) with the following convention for p-value significance: not significant (n.s.), p > 0.05; very highly significant (***), p ≤ 0.001.
4. Discussion
MERS-CoV emerged in 2012 in Saudi Arabia causing severe pneumonia less than 10 years after the SARS-CoV emergence in China. In the case of MERS-CoV, person-to-person transmission has occurred, however transmission is not sustained, with a basic reproduction number below 1. MERS-CoV is a zoonotic virus, and camels and bats are its most likely reservoir. SARS-CoV-like viruses have been isolated in bats and it is likely that bats are the reservoir of a SARS-CoV ancestor. Considering the increasing number and diversity of coronaviruses identified/isolated in bats and the proven propensity of coronaviruses to cross the species barrier and infect new hosts, it is likely that new coronaviruses causing severe diseases in humans may emerge in the future. Currently, there are no drugs with proven efficacy to treat MERS-CoV or any other coronavirus infection. Treatments are limited to supportive care for respiratory and circulatory functions and preventive care to protect renal, hepatic and neurological functions and to prevent secondary infection. As a consequence, new therapies are urgently needed. Virus features conserved among the coronavirus family are attractive targets for the development of antiviral therapy to fight current and future emerging coronavirus infections.
Coronavirus spike proteins are the main determinant of virus entry and tropism (Belouzard et al., 2012). These glycoproteins are variable in sequence and length but share the same organization with two domains S1 and S2. The S1 domain is responsible for receptor binding and attachment of the virus to the host cell surface whereas S2 contains the fusion machinery. A common feature of coronavirus spike proteins is their high glycosylation with 19–39 potential glycosylation sites, coronavirus spike proteins being exclusively N-glycosylated. Therefore, the carbohydrate-binding proteins lectins are attractive anti-coronavirus candidates. Griffithsin is a lectin that was isolated from the red marine alga Griffithsia sp. and has been shown to inhibit HIV, HCV, and SARS-CoV within the nanomolar range.
We demonstrated that while griffithsin has minimal to no effect on cell viability, it is a potent inhibitor of MERS-CoV infectivity and production in vitro. By using particles pseudo-typed with the MERS-CoV spike protein, we have shown that griffithsin acts during MERS-CoV entry. Among the 3 viral proteins anchored in the MERS-CoV envelope, in addition to the spike protein, only the M protein harbors a potential N-glycosylation site in its short N-terminal domain. For MHV, it has been shown that the M protein may be an additional target for lectins and enhance its antiviral effects. Griffithsin inhibits MERS-CoV pseudotyped-particles to the same extent than MERS-CoV infection, suggesting that the spike protein is the main target of griffithsin for its antiviral effect. Indeed, our results indicate a direct interaction of griffithsin with MERS-CoV spike protein. For HIV, griffithsin binds to the terminal mannoses of the N-glycans of gp120, this binding slightly interferes with CD4 binding and likely prevents conformational rearrangement required during entry (Mori et al., 2005). For SARS-CoV, it has been shown that griffithsin does not affect ACE2 binding and it has been suggested that griffithsin acts at a post-binding step during entry (O'Keefe et al., 2010). Other plant lectins, Galanthus nivalis agglutinin (GNA), Hippeasttrum hybrid lectin (HHA) and Urtica dioica agglutinin (UDA) have also been shown to prevent MHV entry at a post-binding step probably by interfering with the conformational rearrangements of the spike protein that drive the fusion of the viral envelope with the host cell membrane (van der Meer et al., 2007). For MERS-CoV, our results show that griffithsin inhibits infection with a different mechanism. In fact, we found that griffithsin interferes with infection as early as during the attachment step, likely by preventing interaction between spike and DPP4, but is not able to prevent MERS-CoV infection when added at a post-binding step. Indeed, a similar mechanism of receptor binding interference has already been observed for HCV (Meuleman et al., 2011). Crystal structures of MERS-CoV receptor binding domain (RBD, residues 367 to 606) have been resolved in combination with DPP4 or in its absence. The MERS-CoV RBD contains a core domain with an accessory subdomain lying on the edge of the core structure (Chen et al., 2013, Lu et al., 2013). Both subdomains contain N-linked glycans on N210 in the core domain and on N487 in the accessory domain. The contributions of these sugar moieties to the virus-receptor interaction are unknown, but one can imagine that any involvement of one of these N-glycans in receptor binding could be impaired by griffithsin. In conclusion, griffithsin has a low cytotoxicity, likely interacts with any coronavirus spike proteins because of their highly glycosylated nature and is able to hamper coronavirus spike protein functions. Griffithsin should be considered as an interesting drug candidate to develop for the treatment and/or prevention of current but also future emerging coronavirus infections.
Acknowledgments
This work was supported by the French National Agency for Research (ANR, ANR-14-CE15-0008) (KS, JD, and SB). Work in the Whittaker lab is supported by the National Institutes of Health Grant R21 AI111085. We are grateful to Sophana Ung for his assistance in the illustrations. We also thank Susan Daniel and the Whittaker lab for helpful discussions.
References
- Alhogbani T. Acute myocarditis associated with novel middle east respiratory syndrome coronavirus. Ann. Saudi Med. 2016:860–864. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. PNAS. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Rajashankar K.R., Yang Y., Agnihothram S.S., Liu C., Lin Y.-L., Baric R.S., Li F. Crystal structure of the receptor-binding domain from newly emerged middle east respiratory syndrome coronavirus. J. Virol. 2013;87:10777–10783. doi: 10.1128/JVI.01756-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J., Zhang B., Shi Y., Yan J., Gao G.F. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuleman P., Albecka A., Belouzard S., Vercauteren K., Verhoye L., Wychowski C., Leroux-Roels G., Palmer K.E., Dubuisson J. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob. Agents Chemother. 2011;55:5159–5167. doi: 10.1128/AAC.00633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell entry of middle east respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. PNAS. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., O'Keefe B.R., Sowder R.C., Bringans S., Gardella R., Berg S., Cochran P., Turpin J.A., Buckheit R.W., McMahon J.B., Boyd M.R. Isolation and characterization of Griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- Moulaei T., Shenoy S.R., Giomarelli B., Thomas C., McMahon J.B., Dauter Z., O'Keefe B.R., Wlodawer A. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between multivalent binding to carbohydrates and anti-HIV activity. Structure. 2010;18:1104–1115. doi: 10.1016/j.str.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- Nixon B., Stefanidou M., Mesquita P.M.M., Fakioglu E., Segarra T., Rohan L., Halford W., Palmer K.E., Herold B.C. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 2013;87:6257–6269. doi: 10.1128/JVI.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K.S., McMahon J.B., Palmer K.E., Barnett B.W., Meyerholz D.K., Wohlford-Lenane C.L., McCray P.B. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein Griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op De Beeck A., Voisset C., Bartosch B., Ciczora Y., Cocquerel L., Keck Z., Foung S., Cosset F.-L., Dubuisson J. Characterization of functional hepatitis C virus envelope glycoproteins. J. Virol. 2004;78:2994–3002. doi: 10.1128/JVI.78.6.2994-3002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H.W., Müller M.A., Dijkman R., Muth D., Demmers J.A.A., Zaki A., Fouchier R.A.M., Thiel V., Drosten C., Rottier P.J.M., Osterhaus A.D.M.E., Bosch B.J., Haagmans B.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer F.J.U.M., de Haan C.A.M., Schuurman N.M.P., Haijema B.J., Verheije M.H., Bosch B.J., Balzarini J., Egberink H.F. The carbohydrate-binding plant lectins and the non-peptidic antibiotic pradimicin A target the glycans of the coronavirus envelope glycoproteins. J. Antimicrob. Chemother. 2007;60:741–749. doi: 10.1093/jac/dkm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Ziółkowska N.E., O'Keefe B.R., Mori T., Zhu C., Giomarelli B., Vojdani F., Palmer K.E., McMahon J.B., Wlodawer A. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure. 2006;14:1127–1135. doi: 10.1016/j.str.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


