Abstract
Large amounts of airborne microorganisms are emitted from livestock production. These emitted microorganisms may associate with dust, and are suspected to pose a risk of airborne infection to humans in vicinity and to animals on other farms. However, the extent to which airborne transmission may play a role in the epidemic, and how dust acts as a carrier of microorganisms in the transmission processes is unknown. The authors present the current knowledge of the entire process of airborne transmission of microorganisms—from suspension and transportation until deposition and infection—and their relation to dust. The sampling and the mitigation techniques of airborne microorganisms and dust in livestock production systems are introduced as well.
KEY WORDS: agriculture, airborne transmission, dust, livestock, microorganisms, production
1. INTRODUCTION
Pathogenic microorganisms may occur in high concentrations in the air inside the livestock houses. Along with ventilation, they can be emitted to the ambient environment and pose airborne infection risk to healthy animals on other farms and to humans living in the vicinity (Seedorf et al., 1998). The extent to which pathogenic microorganisms are transmitted to nearby recipients and cause disease spreading through the airborne route remains largely unclear. Actually, airborne transmission only has been considered as a possible route in historical disease outbreaks in livestock production when the outbreaks could not be attributed to other known routes such as direct contact transmission or fecal-oral transmission (Elbers et al., 2001; Gloster et al., 2003). Attempts to link pathogen transmission (between farms) to prevalent wind directions—an apparent epidemiological proof of airborne transmission—have not been always successful, and the transmission is assumed to only be favored at picky atmospheric conditions (Gloster et al., 2003; Mikkelsen et al., 2003). All these facts induce doubts on the importance of the role of airborne transmission in disease outbreaks; however, no one can conclusively exclude this transmission route due to its potentially extensive and intensive impacts if truly involved in epidemics.
Given the lack of knowledge on airborne transmission it is important to stimulate relevant research in order to better understand what role it can play. Here we define airborne transmission in livestock production as “an entire transmission process that involves pathogenic microorganisms releasing from the infected animal's excrement or secretion to air, transporting air, inhaled by a healthy animal, and eventually infecting the recipient.” The airborne transmission of certain pathogenic microorganisms from animal to animal has been demonstrated in lab-scale experiments in which healthy animals separated physically but not aerially from infected animals became infected (Berthelot-Herault et al., 2001; Brockmeier and Lager, 2002). Furthermore, some microorganisms collected kilometers away from the source farm were found to be capable of infecting healthy animals intramuscularly or intratracheally (Otake et al., 2010). However, there is still uncertainty, because of the incomplete knowledge about the entire process of airborne transmission of microorganisms, from generation and transportation through inhalation and finally to infection (Stark, 1999).
Dust probably plays a role as the carrier of the microorganisms in the air. In 1987, the importance of the relationship between airborne microorganisms and dust from livestock production systems was reviewed by Muller and Wieser (1987). The authors separately described the indoor properties (source, concentration, and constitute) of airborne microorganisms and dust, and the dispersion in ambient air outdoors. Since then, much research has been done on specific processes involved in the transmission of the airborne microorganisms and dust. However, we lack an integrated overview of and insight into all the processes involved in the airborne transmission of microorganisms in association with dust.
The objective of this article is to review current knowledge on airborne microorganisms from production systems for typical livestock species (swine, poultry and cattle), and their relation to dust. Specifically, in section 2, we identify the sources, species, size distributions, and concentrations of airborne microorganisms and dust from livestock production systems, as well as the factors affecting their concentrations. In section 3, the physical and biological decay of airborne microorganisms and dust during transmission is described. In section 4, the deposition of airborne microorganisms and dust in respiratory tracts, and the infective dose of pathogenic microorganisms to animals are introduced. In section 5, the strategies and techniques for sampling microorganisms and dust in livestock production systems are proposed. In section 6, the mitigation techniques are described.
2. AIRBORNE MICROORGANISMS AND DUST IN LIVESTOCK PRODUCTION SYSTEMS
2.1. Identification the Sources of Airborne Microorganisms and Dust
Identifying the source of microorganisms and dust in livestock production systems helps to elucidate how airborne transmission is generated, and ultimately can help to develop and implement strategies that prevent such transmission from beginning (Bull et al., 2006; Cambra-Lopez, 2010). Sources of dust in livestock production systems have been identified and assessed qualitatively and quantitatively (Aarnink et al., 1999; Donham and Gustafson, 1982). It is generally accepted that all dust sources are also sources of airborne microorganisms because these source materials somehow contain certain microbial species that may be generated together with dust. However, the source identification of airborne microorganisms has not yet been extensively investigated, and it is thought to be more complicated than the source identification of dust in at least two ways. The first way is associated with the complexity of microbial species in a source. A source material always contains a microbial flora composed of many different microbial species. The second way is associated with the dynamic viability of microorganisms in the generation process (Milne et al., 1989); microorganisms may either decay or multiply in the source material. Thus, the source identification for microorganisms should also be dynamic.
2.1.1. Source of Airborne Microorganisms
Animals shed microorganisms mainly by means of fecal excretion, which may contain large amount of microorganisms (Letellier et al., 1999; Pell, 1997). Consider two common zoonotic bacterial species, Salmonella and Escherichia, both have been found in feces: Salmonella at a concentration of 2–7 log CFU g−1 feces (Gray and Fedorka-Cray, 2001; Himathongkham et al., 1999) and E. coli at 2–6 log CFU g−1 feces (McGee et al., 2001; Omisakin et al., 2003). Feces are also an important pathway for virus shedding from infected animals (Fouchier et al., 2003). A list of viral species that may be excreted by cattle was proposed by Pell (1997), including infectious bovine rhinotracheitis and Foot-and-mouth disease (FMD) virus. Many other viruses have been recovered from feces of other animal species, such as avian influenza A virus (Webster et al., 1978) and Newcastle disease virus (Spradbrow et al., 1988) in poultry, and swine fever virus (Van Oirschot, 1979), hepatitis E virus (de Deus et al., 2007), and porcine reproductive and respiratory syndrome virus (PRRSV) (Yoon et al., 1993) in pigs. The microorganisms in feces can become airborne when dried fecal particles are disturbed by air flow or animal activity. Some studies have managed to identify feces as the source of airborne microorganisms. A study by Duan et al. (2009) found the airborne E. coli strains inside and downwind from the pig houses were closely associated with those isolated from pig feces. Water content binds particles in feces and prevents their suspension, so, the microorganisms in dry feces that have low water content become airborne more easily than microorganisms in fresh feces. The water content of fresh feces (or manure, when urine is not separated) is the range of 60–90% (Derikx et al., 1994), depending on the animal species. Under typical livestock housing environmental conditions, it may take hours or days to dry the feces to a water content less than 20%—the water content of airborne dust in livestock production systems (Aarnink et al., 1999; Zhao et al., 2013). This means the microorganisms must undergo a latent period between the moment they are excreted in the feces and the moment they become airborne.
During inhalation and exhalation the surface of the mucus in the respiratory tract is destabilized through an interplay between surface tension and viscous forces (Edwards et al., 2004). This can result in mucus's microorganisms to become airborne and expelled from the body via breathing, coughing, and sneezing. This source of airborne microorganisms is widely accepted in the human model of disease transmission, and pathogenic microorganisms have been frequently recovered from the exhaled aerosols (Fabian et al., 2008; Weber and Stilianakis, 2008). Only a few studies have been carried out to directly detect the microorganisms in exhaled air of animals. By sampling exhaled air in masks placed over the heads of infected pigs, Cho et al. (2006) recovered PRRSV and Hermann et al. (2008) recovered Mycoplasma hyopneumoniae and Bordetella bronchiseptica. In contrast, Hermann et al. (2008) failed in recovering PRRSV, Porcine circovirus 2, swine influenza virus, and Porcine respiratory coronavirus in the exhaled air of infected pigs, although they were found in the oral and nasal swabs. Similarly, Zhao et al. (2013) could not recover infectious brusal disease virus in the exhaled air of infected broilers. These results indicate that some microorganisms in animal respiratory tracts might not readily become suspended in the air or be expelled out of the body, which implies that this is perhaps not an major source of airborne microorganisms as compared to animal feces (Zhao et al., 2013). However, the reason the microorganisms may not be detected could be because the quantities of exhaled microorganisms were below the detection limit of the sampling devices. Therefore, animal respiratory tracts can only be excluded as a source of airborne microorganisms until it has been incontrovertibly established that every single microorganism is detectable.
Organic materials such as feed may serve as carriers for variety of microorganisms. The microorganisms may originate from the soil and are transferred to standing crops by wind, rain, mechanical agitation, or insects (Maciorowski et al., 2007). Both nonpathogenic and pathogenic microorganisms have been recovered from feed; analysis has revealed concentrations of Gram-negative bacteria in feed as high as 5 log CFU g−1 (Hofacre et al., 2001). These microorganisms can be disseminated together with feed particles during feeding (Andersson et al., 1999; Chang et al., 2001); the extent of dissemination depends greatly on how the feed is given (e.g., dry vs. wet feed delivery and powder vs. pellet feed delivery; Pearson and Sharples, 1995).
Litter is a mixture of bedding materials (e.g., wood shavings, chopped straw, sawdust, and rice hulls) animal feces, dander, and feed (Torok et al., 2009). The provision of litter in livestock production systems may improve animal welfare by increasing the incidence of natural behaviors (Appleby and Hughes, 1991), which, however, may result in more microorganisms being present in the air than in housing systems without litter (Madelin and Wathes, 1989; Vucemilo et al., 2007). The microorganisms arrive in litter during the harvesting and processing of the bedding material, and through animal excretion and secretion. The concentrations of aerobic bacteria in poultry litter range from 3 to 9 log CFU g−1 (Lu et al., 2003; Martin et al., 1998). Most of the bacteria in the poultry litter are Gram-positive. Gram-negative bacteria and mold account for a small fraction of the total microbial count, but due to the high concentration of the total microorganisms, their numbers can still be high in some cases (Martin et al., 1998). Surprisingly, some pathogenic bacteria that are commonly recovered from animal feces (e.g., E. coli, Salmonella, and Campylobacter) are not always detectable in litter (Lu et al., 2003). The reason could be the less favorable microenvironment for microbial survival in the feces-bedding mixture than in feces alone. Microorganisms, including Globicatella sulfidofaciens, Corynebacterium ammoniagenes, Corynebacterium urealyticum, Clostridium aminovalericum, Arthrobacter sp., and Denitrobacter permanens, which may be involved in degradation of wood and cycling of nitrogen and sulfur have been identified in poultry litter (Lu et al., 2003).
Other possible sources of airborne microorganisms in livestock houses include animal skin (Baird-Parker, 1962; Gailiunas and Cottral, 1966; Kloos et al., 1976) and animal products (e.g., broken eggs, spoiled milk (de Reu et al., 2008; Donaldson et al., 1983; Doyle, 1984; Doyle and Roman, 1982), farm workers and visitors (Newell and Fearnley, 2003; Nishiguchi et al., 2007), and ambient air (Gloster et al., 2003; Martin et al., 1996).
2.1.2. Sources of Dust in Animal Houses
Sources of airborne dust include feed, animal skin and feather debris, feces, litter, microorganisms, pollen, and insect parts (Aarnink et al., 1999; Donham et al., 1986). The contribution of these sources to airborne dust varies, depending on the animal species and the housing system. Heber et al. (1988a) reported that the main source of airborne dust in pig houses was feed, which is consistent with the findings of Donham et al. (1986) and Aarnink et al. (1999). Muller and Wieser (1987) found that 55–68% of the airborne dust in floor layer systems with litter originated from the bedding materials in litter, while 80–90% of the airborne dust in layer systems with battery system originated from feedstuff (Table 1). In floor systems with wood shavings as litter for three-week old broilers, Aarnink et al. (1999) found that airborne dust mainly (>10%) originated from down feathers and urine components. The contribution of feed to the airborne dust largely depends on its composition and how it has been processed (Pearson and Sharples, 1995M; e.g., crumbles or pellets). The contribution of feces is probably related to the housing system (e.g., with or without litter [straw bedding vs. liquid manure]). Table 1 lists the main sources of dust and gives an estimation of their contributions.
Table 1. Sources of airborne dust in animal houses.
| Animal | Housing type | Source | Contribution | Reference |
|---|---|---|---|---|
| Layers | Floor housing with litter | Bedding material in litter | 55–68% | (Muller and Wieser, |
| Feathers | 2–12% | 1987) | ||
| Excrement | 2–8% | |||
| Layers | Battery housing | Feed | 80–90% | (Muller and Wieser, 1987) |
| Feathers | 4–12% | |||
| Excrement | 2–8% | |||
| Broilers | Floor housing with litter | Feathers | >10% | (Aarnink et al., 1999) |
| Crystalline dust | >10% | |||
| Feed, microorganisms | <1% | |||
| Rearing pigs | Partially slatted floors | Feed | >10% | (Aarnink et al., 1999) |
| Skin particles | >10% | |||
| Feces, crystalline dust | 1–3% |
2.2. Species of Airborne Microorganisms
A large fraction of the airborne microorganisms in livestock production systems are bacteria, of which the most dominant are Gram-positive bacteria, accounting for approximately 90% of the bacterial flora (Zucker et al., 2000). The most common species of these Gram-positive bacteria are Staphylococcus, Streptococcus, and Enterococci (Clark et al., 1983; Hartung, 1992; Matkovic et al., 2007). The Gram-negative bacteria account for only a small fraction of airborne bacteria (Zucker et al., 2000). Bakutis et al. (2004) reported that in terms of the total bacterial count, the proportion of Gram-negative bacteria was approximately 10% in cattle houses, 4.9% in pig houses, and 2.6% in poultry houses. Zucker et al. (2000) found that the airborne Gram-negative bacteria in pig and cattle houses were aerobic and include Enterobacteriaceae, Pseudomonadaceae, and Neisseriaceae; no culturable obligate anaerobic Gram-negative bacteria were isolated. Possible reasons for the smaller proportion of airborne Gram-negative bacteria in livestock production systems are that their excretion by animals is less than their counterparts and these bacteria are more vulnerable to environmental stress such as oxidation, radiation, and dehydration, probably because of their thinner cell walls (Pal et al., 2007; Theunissen et al., 1993). The proportion of fungi, molds, and yeasts in the airborne microbial flora in animal houses is low (Hartung, 1992; Lee et al., 2006). The most frequently reported fungi in poultry, pig, and dairy houses are Aspergillus sp., Alternaria sp., Cladosporium sp., Penicillium sp., Fusarium sp., Scopulariopsis sp., and yeast (Chang et al., 2001; Cormier et al., 1990; Martin et al., 1996; Matkovic et al., 2007; Vittal and Rasool, 1995; Wilson et al., 2002).
2.3. Size Distribution of Airborne Microorganisms and Dust
The size of an airborne particle determines its transportation, sedimentation, and resuspension, as well as its deposition in the respiratory tracts of recipients. Investigations of the size distribution of microorganisms and dust in livestock production systems may provide a useful overview of their quantitative importance, indicate the health risk for human and animals, and facilitate the establishment and evaluation of control techniques.
According to interests by different scientific sectors, sizes of airborne particles are differently categorized. For concerns in occupational health, particle sizes are categorized into three categories: inhalable (<100 μm), thoracic (<10 μm), and respirable (<4 μm or 5 μm; Cambra-Lopez et al., 2010; Curtis et al., 1975; Madelin and Wathes, 1989; Zhang, 2004). In environmental science, recent research is increasingly focusing on dust with aerodynamic diameter smaller than 10 μm (PM10) and 2.5 μm (PM2.5). The size distribution of airborne dust has been expressed either in mass or in counts.
Zhao et al. (2011a) found that in three pig houses about 73–95% of the airborne bacteria were in the nonrespirable range (Figure 1). A similar result was reported by Curtis et al. (1975): nonrespirable bacteria accounted for approximately 78–89% of the airborne bacteria in pig houses. The size distribution of microorganisms in poultry houses depends on the type of housing system. In broiler rooms with wood-shaving litter, most of the bacteria were nonrespirable in the full life cycle of broilers (eight weeks); a similar distribution pattern was found in broiler rooms with raised netting floor only after the birds were older than six weeks. When the birds were two to five weeks old, the proportions of airborne respirable and nonrespirable bacteria were similar (Madelin and Wathes, 1989).
Figure 1. Size distribution of airborne bacteria in the exhaust air from three commercial fattening pig houses measured with an Andersen Six Stage viable bioaerosol sampler (Zhao et al., 2011a).
Heber et al. (1988a) reported that nonrespirable particles (>4 μm) in pig houses accounted for more than 80% in mass, but less than 30% in terms of count. Expressed as percentage of total dust, Lai et al. (2010) found the mass of PM10 was 30–54% in pig houses, 41–69% in poultry houses, and 36% in cattle houses. In all three types of house, PM10 count was more than 99% of the total dust. The equivalent figures for PM2.5 mass were 1–3% in pig houses, 2–8% in poultry houses (2009), and 5% in cattle houses. For PM25 counts the figures were 90–99% in pig houses, 88–92% in poultry houses and 99% in cattle houses. The difference in the mass and numeric size distribution is caused by the fact that small dust particles contribute little to mass.
That more microorganisms and less dust particles are found in the nonrespirable range indicates that a nonrespirable dust particle is more likely to be loaded with microorganisms than a respirable one. This is a reasonable hypothesis, because the larger a particle is, the greater the chance it may contain microorganisms. Nowadays, the size distribution of airborne microorganisms is normally determined with the Andersen stage impactor (Andersen, 1958; Zhao et al., 2011a). This sampler actually gives the counts of particles (in seven different size ranges) that contain microorganisms. In some cases, for instance a biosecurity assessment for occupational health, it is more important to quantify the individual microorganism in the collected particles rather than the microbial-containing particles themselves, because the former will give a better idea of the risk of infection. For this purpose, predetachment of microorganisms from dust particles (Zhao et al., 2011b) or visually microbiological counting techniques (Yamaguchi et al., 2012) may be required.
2.4. Concentrations of Airborne Microorganism and Dust
Concentrations of airborne microorganisms and dust in livestock production systems have been investigated in previous studies (Kim et al., 2008; Radon et al., 2002; Zhao et al., 2011a). Due to the huge spatial and temporal variations in microorganism and dust concentrations and the difference in sampling techniques used, it is difficult to compare the data between studies. So far, the studies by Seedorf et al. (1998) and Takai et al. (1998) still provide the most representative concentration data on microorganisms and dust in livestock production systems, and sampling methods were detailed in the publications by Phillips et al. (1998) and Wathes et al. (1998). These data are summarized in Table 2, together with the PM10 and PM2.5 concentration measured by Lai et al. (2010). For microorganisms, the highest concentrations of airborne bacteria and fungi were found in broiler houses. The concentrations found in layer, pig and cattle houses were lower than those in broiler houses, but still much higher than those in ambient air (Wang et al., 2010). For dust, the highest dust concentrations were found in poultry houses, and the lowest dust concentrations were in cattle houses.
Table 2. Concentrations of airborne microorganisms and dust in livestock production systems.
| Bacteria[a] | Fungi[a] | Inhalable Dust[b] | Respirable Dust[b] | PM10[c] | PM2.5[c] | |
|---|---|---|---|---|---|---|
| Animal |
log CFU m−3 |
log CFU m−3 |
mg m−3 |
mg m−3 |
mg m−3 |
mg m−3 |
| Broiler | 6.4 | 4–5 | 3.8–10.4 | 0.42–1.14 | 0.9–2.4 | 0.04–0.09 |
| Layer | 4–5 | 3–4 | 1.0–8.8 | 0.03–1.26 | 5.9–6.1 | 0.25–0.29 |
| Pig | 5.1 | 3.7 | 0.6–5.1 | 0.09–0.46 | 0.2–2.0 | 0.01–0.07 |
| Cattle | 4.3 | 3.8 | 0.1–1.2 | 0.03–0.17 | 0.1 | 0.01 |
2.5. Factors Affecting Concentrations of Airborne Microorganisms and Dust in Livestock Houses
The concentrations of airborne microorganisms and dust in animal houses are affected by animal, housing system and management. In this section, these factors are discussed separately, but one should realize that these factors always interactively affect the concentration because they are intercorrelated. For instance, animal activity is associated with animal age, weight, and light schedule, and ventilation rate is affected by outdoor and set-point temperature, humidity, and animal species and age.
2.5.1. Animal
The animal factor can be further detailed into subfactors such as age, weight, activity, and stocking density. The concentrations of airborne microorganisms and dust generally increase concomitantly with animal age and weight (Hinz and Linke, 1998; Predicala et al., 2001; Yoder and Van Wicklen, 1988). However, an inverse relationship has also been found. Madelin and Wathes (1989) found a decrease of microorganisms and dust concentrations in the late fattening period of broilers. A similar result was reported by Saleh et al. (2005). The decrease in concentration of microorganisms and dust is probably because the older broilers occupied all the floor space, which limited their activity.
In general, higher concentrations of bacteria, fungi, and dust are measured when the animals are more active, as can be inferred from the finding that their concentrations were higher in day time than at night (Seedorf et al., 1998; Takai et al., 1998). Image and infrared technology allows animal activity to be automatically detected (Gloster et al., 2007; Pedersen and Pedersen, 1995). Using an infrared detector, Haeussermann et al. (2007) demonstrated that the indoor concentrations of PM10 were associated with pig activity. A similar study by Heber et al. (2006) showed that both total dust and PM10 were correlated with the pig activity. However, Gloster et al. (2007) failed to establish the correlation between the concentration of airborne FMD virus and pig activity quantified by taking sequential pictures. The reason is not clear, but the authors explained that the virus production appears to be more closely associated with other factors (e.g., physiological symptoms; Gloster et al., 2007). Hardly any other information is available on the relation between quantified animal activity and concentrations of microorganisms.
2.5.2. Housing System
Compared to a cage system, an aviary system contained higher concentrations of microorganisms (de Reu et al., 2005) and dust (Appleby and Hughes, 1991). This is because in an aviary system, laying hens have more scopes for moving horizontally and vertically and perform dust bathing behavior in the litter. Housing systems with bedded floors caused more air quality problems, although such housing systems are generally thought to be more beneficial for animal welfare (Kim et al., 2008; Madelin and Wathes, 1989; Quarles et al., 1970). The type of bedding material also affects the concentration of microorganisms in the air. For instance, in broiler houses, straw bedding released less bacteria in the air than wood shavings did (Banhazi et al., 2008a). The authors argued that this was probably because wood shavings provided a better microenvironment for bacteria viability and multiplication.
Comparisons of dust concentrations between natural and mechanical ventilation systems showed that with mechanical ventilation, less respirable (Phillips, 1986) and total dust was found in pig houses (Chiba et al., 1985) and there was less total dust in turkey houses (Janni and Redig, 1986). By contrast, concentrations of total bacteria and fungi were lower in naturally ventilated pig houses without bedding materials (deep-pit manure system with slats, and manure removal system by scraper) than in mechanically ventilated houses. The contradictory results found for the effect of type of ventilation (natural or mechanical) on microorganisms and dust are not fully understood, but it seems likely that the situations (including the management) of the ventilation systems vary between the different studies, making the data less comparable.
2.5.3. Management
Feed management may play an important role in dust concentration in livestock production systems. Previous studies have shown that dust concentrations were reduced by giving pelleted feed rather than powdered feed, wet feed rather than dry feed, and coated feed rather than uncoated feed (Clark and McQuitty, 1988; Pearson and Sharples, 1995; Zeitler et al., 1987). The effect of feeding management on airborne microorganism concentration has not been extensively studied.
Maintaining good hygiene in livestock production systems may help to improve the air quality with respect to microorganisms and dust. For instance, cleaning (e.g., removing litter, scrubbing surfaces, and disinfecting the house) between two production circles may reduce both airborne microorganisms and dust (Banhazi et al., 2008a). Investigations of the relation between hygiene and air quality have found they are not always positively correlated. Duchaine et al. (2000) reported that a housing system that appeared cleaner contained more airborne bacteria than one that appeared dirtier. The probable explanation is that houses with more settled dust on the surfaces are more readily ranked as dirtier, but dust accumulated on surfaces is not an appropriate indicator of the concentration of bacteria in the air.
Ventilation management (e.g., adjusting the rate at which indoor air is exchanged with outdoor air by mechanical ventilation systems) is to control the temperature and other aerial variables such as humidity and gas concentrations inside livestock houses. Previous studies have shown that lower concentrations of microorganisms and dust can be achieved by increasing the ventilation rate in livestock production systems (Duchaine et al., 2000; Hinz and Linke, 1998; Kim et al., 2007b). However, a nonsignificant correlation between these variables has also been reported in livestock production systems (Banhazi et al., 2008b; Seedorf et al., 1998). The probable reason for these contradictory findings is that ventilation affects the concentration of microorganisms and dust in two ways: by exhausting airborne microorganisms and dust to outdoors through air exchange, thereby reducing their indoor concentrations, and by producing airflow turbulence above surfaces, agitating the particles and causing them to suspend in the air, thus compromising the removal effect. Smaller airborne particles seem to be more effectively removed from livestock houses by ventilation than bigger particles (Kuehn, 1988) because they are readily transported in the air streams.
A high relative humidity in the air reduced the dust concentration in livestock production systems (Guarino et al., 1999). In humid environments, the dust particles bind to the surface and are not easily suspended, and those in the air aggregate and to settle faster (Heber et al., 1988b; Takai et al., 1998). In contrast, humidity seems not to affect the concentrations of total and Gram-negative bacteria (Attwood et al., 1987; Banhazi et al., 2008b; Nicks et al., 1993). A high humidity favors survival and/or multiplication of dehydration-sensitive microbial species in the sources (de Rezende et al., 2001), which might trade off the physical settlement of airborne microorganisms. Experimental validation of this hypothesis is of importance, because it may strengthen the understanding of the interaction between airborne microorganism concentration and air humidity. This will help to formulate effective air humidification strategies for reducing both, dust and microorganisms, instead of achieving one by compromising the other. This information will also be valuable for understanding the bioenvironment in livestock houses where air humidification (e.g., water spray and evaporation cooling pad) is used to ameliorate animal heat stress in summer time (Brown-Brandl et al., 2010; Tao and Xin, 2003).
Indoor temperature management is well regulated for the poultry and pig industries, to optimize productivity. Al Homidan et al. (1997) reported that the total dust in broiler rooms increased when the temperature was set 2°C above the recommended level. The correlation between dust concentration and temperature reverses from positive to negative when the temperature is extremely high, apparently because animal activity decreases at high temperature and thus fewer particles from surfaces are disturbed and suspended (Donkoh, 1989; Guarino et al., 1999; Wylie et al., 2001). As well, a high temperature triggers a series of events that may affect the dust concentrations in livestock production systems, such as increasing the ventilation rate and activating wet cooling systems (Simmons and Lott, 1996). Although some researchers (Banhazi et al., 2008a) have suggested there is a relation between the concentration of microorganisms and temperature, little information is available so far.
3. DECAY OF MICROORGANISMS AND DUST IN THE AIR
The decay of microorganisms and dust is a parameter that cannot be ignored in empirical and theoretical models of airborne transmission (Lighthart and Frisch, 1976; Yu et al., 2004); it may help when assessing health impacts from exposure. The term “decay” encompasses both physical and biological means. Physical decay is the physical elimination of a particle from the air by means of a series of processes such as gravitational sedimentation, impaction and electrostatic precipitation; biological decay is the loss of biological activity of an airborne microorganism owing to loss of enzyme activities or denaturing of membrane phospholipids, proteins or nucleic acids (Cox, 1989). For biological decay, the situation may be more complicated in some cases when the microorganisms go into a viable but nonculturable (McKay, 1992; Oliver, 2010; Trevors, 2011) or dormancy state (Locey, 2010). It is clear that physical decay applies to the elimination of dust particles, whereas both physical and biological decay apply to airborne microorganisms.
3.1. Physical Decay
Mechanisms affecting physical decay of particles are listed in Table 3. In reality, these mechanisms may have collective effect on a particle, but the dominant mechanism varies mainly with particle size (e.g., decay of larger particles is prone to drive by gravimetric sedimentation whereas decay of smaller particles is mainly determined by diffusion (Abadie et al., 2001; He et al., 2005). Physical decay can be quantified by the rate of deposition (deposition rate loss coefficient or deposition velocity; Deshpande et al., 2009). Lai (2002) reviewed the deposition rate for particles ranging from 0.01 to 10 μm and found it had a U-shaped pattern. The lowest deposition rate was found for particles ranging from 0.1 to 1 μm because particles in this range were less affected by either sedimentation or diffusion than their counterparts do. Besides particle size, deposition rate is affected by other factors such as room furnishing and air speed. Thatcher et al. (2002) found increasing surface area (provide more furniture) and air speed (by means of elevating ventilation rate) increased the deposition rate of particles (0.5–10 μm) in an experimental room.
Table 3. Mechanisms associating physical decay of microorganisms and dust.
| Mechanism | Definition[a] | Reference |
|---|---|---|
| Advection | The mean transport of a particle by the mean motion of the atmosphere, and occurs when the spatial gradient is nonzero and the particle is transported along the mean wind | (Baldocchi et al., 1988) |
| Brownian diffusion | The process of mass transfer of particles brought about by a random molecular motion (Brownian motion) and associated with a concentration gradient | (Vaithiyalingam et al., 2002) |
| Thermophoresis | The motion of a particle under the influence of a temperature gradient | (Langer and Holcombe, 1999) |
| Gravitational sedimentation | The separation of dispersed particles from gaseous phase under action of gravity | (Wunsch, 1994) |
| Impaction | The deposition of particles due to their momentum causing them to deviate from airflow streamlines and impacting at bifurcations | (Katz et al., 2001) |
| Electrostatic precipitation | The use of an electrostatic field for precipitating or removing charged particles from a gas flow in which the particles are carried | (Shen and Pereira, 1979) |
[a]Some definitions in this table that were originally for gases or molecules in nonaerial environments have been modified to make them suitable for describing the associating mechanisms of microorganisms and dust in the air.
3.2. Biological Decay
The biological decay of airborne microorganisms has been expressed in different ways (e.g., decay rate [or death rate], survival, or half-life). The decay rate is the decrease in concentration of viable microorganisms over time. A proportionality constant (k) indicates the extent of decay rate, and is shown in equation 1, where Co is the initial concentration of airborne microorganisms, Ct is the concentration of microorganisms at time t after initial (Phillips et al., 1964). The survival represents the percentage of viable microorganisms left at a certain moment vis-à-vis the initial microbial count (Wu, 2009). The half-life, t1/2, is the time taken for the concentration of viable microorganisms in the air to decrease by half (see equation 2). Previous studies showed that the biological decay of airborne microorganisms was species-dependent and was determined by many external factors, such as humidity, oxygen concentration, temperature, ozone concentration, radiation (UV, γ-ray, X-ray), air ions, and air pollutants (CO, SO2, and NOx; Benbough, 1971; Lighthart, 1973).
| (1) |
| (2) |
3.3. Environmental Factors Affecting Biological Decay
For long distance airborne transmission between farms, microorganisms may be exposed to unfavorable environmental conditions that induce death or dormancy of microorganisms (Lennon and Jones, 2011; Locey, 2010; Wu et al., 2012). In this section we present an introduction of several environmental factors and their working mechanisms on biological decay of microorganisms.
The effect of humidity on the biological decay of airborne microorganisms has been investigated since the 1950s. In the early studies, the measure most used for humidity was relative humidity (RH; the ratio of the actual water vapor pressure of the air to the water vapor pressure of saturated air at a certain temperature). The results of these studies showed that different microorganisms were prone to decay either at low RH (Lighthart, 1973), at median RH (Wright et al., 1968b), or at higher RH (Songer, 1967; Theunissen et al., 1993). More recently, a few studies have used absolute humidity (AH; the actual water content of the air) as another measure of humidity. For instance, Shaman and Kohn (2009) reported that the survival of airborne influenza virus was more significantly constrained by AH than by RH. The authors argued that RH is a meaningful physical quantity and for certain organisms may affect biological response; however, the AH can be of greater biological significance for many organisms. Some studies reported significant effects of other measures of humidity, such as evaporation potential (EP; the difference between actual water vapor content in the air and the water vapor content in saturated air at the same temperature), on microbial survival (Zhao et al., 2012). Although a bunch of studies have been carried out, it is not yet fully understood how humidity influences microbial decay.
Temperature profoundly affects the biological decay of airborne microorganisms. In general, the higher the ambient temperature is, the faster the microorganisms decay. For instance, the decay rate of Flavobacterium sp. is 0.007 log min−1 at –2 to 24°C, but increases to 0.017 log min−1 at 29 to 49°C (Ehrlich et al., 1970a). A faster decay at higher temperature has also been reported for other microbial species, such as E. coli, S. marcescens (Ehrlich et al., 1970b), and Newcastle disease virus (Kournikakis et al., 1988). Prescott et al. (2005) have stated that high temperature may damage microorganisms either by denaturing the enzymes, transport carriers, and other proteins, or by melting and disintegrating the lipid bilayer, or both.
The ambient environment is full of various types of radiation, including UV (10–400 nm) and visible light (400–750 nm), which may inactivate the microorganisms. The wavelength of UV between 10 nm and 120 nm has the highest energy, but this type of UV is blocked by normal dioxygen in air and cannot reach the ground. The rest of UV radiation, from low to high energy, is categorized as UV-A (320–400 nm), UV-B (280–320 nm), and UV-C (100–280 nm), of which UV-C is considered to be the most germicidal. The UV-C wavelength between 250–270 nm can be effectively absorbed by microbial genetic material, and is the UV wavelength most lethal to microorganisms (Keyser et al., 2008). The germicidal effect of UV-C light on bacteria and viruses is primarily due to the formation of pyrimidine (thymine and cytosine) dimers that inhibit the replication and function of genetic material (Giese and Darby, 2000; Prescott et al., 2005). Bacteria decay more readily under UV radiation than RNA viruses (Harris et al., 1987; Hijnen et al., 2006). The probable reason for this is that the thymine of bacterial DNA is more vulnerable to dimerization induced by UV than the uracil of viral RNA. The mechanism whereby UV-A and UV-B (also called near-UV) inactivates microorganisms is suspected to be the breaks in strands of genetic materials that are induced by the UV itself and toxic tryptophan photoproducts (Prescott et al., 2005). Visible light may also damage microorganisms. Microbial pigments become excited when they absorb light energy, and are transferred to O2, generating singlet oxygen (1O2), which is a highly reactive oxidant (Valduga et al., 1993). As a self-protecting mechanism, some microorganisms may possess carotenoids that can absorb the excitation energy and reduce the formation of singlet oxygen, thus preventing cells from being damaged by light radiation (McCambridge and McMeekin, 1981).
Oxygen may accept electrons forming other toxic derivatives such as superoxide radical, hydrogen peroxide, and hydroxyl radical, which may easily destroy the cellular constituents. Many aerobic microorganisms contain enzymes such as superoxide dismutase and catalase, which protect the cell against oxidation by the derivatives; all the strictly anaerobic microorganisms lack these enzymes (Prescott et al., 2005). The effect of oxygen level on decay of airborne Serratia marcescens 8UK and E. coli B was studied by Cox (1989), who reported that oxygen was toxic for these microorganisms only when RH was lower than 70%. The toxicity increased with oxygen concentrations up to 30%; higher concentrations produced no additional toxicity. This finding was generally in agreement with other similar studies (Benbough, 1967; Hess, 1965). Viruses seem to be less sensitive to oxygen than bacteria. The decay of airborne viruses that included Semliki Forest virus, Langat virus, T7 coliphage, Poliovirus, and Encephalomyocarditis virus was no different whether they were aerosolized in the air or in nitrogen (Benbough, 1971; de Jong et al., 1975).
Bacteria exposed to ozone (O3) may be inactivated due to damage to the cell surface (Giese and Christensen, 1954; Scott and Lesher, 1963) and destruction of the intracellular enzymes, protein and genetic material (Barron, 1954; Ingram and Haines, 1949; Kim et al., 1999). Ozone may damage the viral nucleic acids of viruses and alter the polypeptide chains of the viral protein coat (de Mik and de Groot, 1977; Kim et al., 1999; Roy et al., 1981). Investigations of the ozone effect on decay of airborne microorganisms showed that fungi seemed more resistant to ozone than bacteria; Gram-positive bacteria were more resistant than Gram-negative ones (Heindel et al., 1993; Kowalski et al., 1998). Ozone alone can be toxic to airborne microorganisms; however, its toxicity is enhanced when ozone reacts with compounds in the ambient air, known as open air factors (OAF). Studies have shown that mixture of ozone with olefins (de Mik et al., 1977; Druett and Packman, 1972) and ozone with negative air ions (Fan et al., 2002) are more toxic to airborne microorganisms than ozone alone.
The dust particles to which microorganisms adhere may protect them from biological decay. When microorganisms are carried by the dust, they may suffer less radiation and exposure to toxic gas, and less fluctuation in micro-climate (Milling et al., 2005). It has been found that individual bacteria are effectively inactivated by ozone; however, when these bacteria were covered with a coating of organic matter, as a bio aerosols, ozone in permissible concentration had no effect (Elford and van den Ende, 1942). As well as providing physical protection, the composition of dust particles might give bio-chemical support to microorganisms. In order to metabolize, microorganisms require carbon, oxygen, nitrogen, phosphorus, sulfur, and other elements (Maus et al., 2001). Compounds containing these elements are abundant in airborne dust from livestock production systems (Aarnink et al., 1999; Muller and Wieser, 1987). An interesting hypothesis is that microbial decay in particles differs, depending on the composition of the particles. Actually, in some laboratory experiments, it appears that decay of microorganisms differs as they are aerosolized from liquid suspensions with different chemical compounds. (Benbough, 1971; Hess, 1965).
3.4. Studies on Biological Decay
There have been extensive studies on the biological decay of microorganisms at different temperature and RH levels, using aerosol experiments. In these experiments, microorganisms were aerosolized in airspace that was sampled at certain intervals. The biological decay was indicated by the amounts of collected microorganisms at different sampling moments. The results of selected studies are summarized in Tables 4 and 5. The selection of references follows the procedures and screening criteria as below: first, all references related to biological decay of any species of airborne microorganisms were searched in literature databases (Web of Science, Scopus, Google Scholar); second, only references in which biological decay was investigated by excluding confounding effect of physical decay (e.g., using inert tracers or labeled microorganisms) were selected; third, due to the fact that some microorganisms showed a biphase biological decay (with a fast initial decay rate in the first few seconds or minutes after aerosolization, followed by a slow secondary decay) and the airborne transmission concerned is long distance and time (Brankston et al., 2007; Cox, 1971; Songer, 1967; Webb, 1959), only the secondary biological decay is presented in the tables. The tables show the highest biological decay at the least favorable temperature/RH, and the lowest biological decay at the most favorable temperature/RH. The values of biological decay have been presented in three ways: decay rate, survival and half-life. By reviewing the results in Tables 4 and 5, it proofs that the biological decay largely varies between the microbial species and is profoundly affected by temperature and RH.
Table 4. Biological decay of bacteria under different relative humidity (RH) and temperature (Temp).
| Bacteria | RH levels involved (%) | Temp levels involved (°C) | H/L[a] | Extreme RH for decay (%) | Extreme Temp for decay (°C) | Decay | Survival [b] | Half-life | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Chlamydia pneumoniae | 5, 50, 95 | 8.5, 15, 25, 35 | H | 50 | 35 | — | 0% (1.5) | — | (Theunissen et al., 1993) |
| L | 95 | 15, 25 | — | >10%* (5.5) | — | ||||
| 32–87 | 15, 30 | H | <50% | 30 | — | — | 3 min | (Wathes et al., 1986) | |
| L | High RH | 15 | — | — | 83 min | ||||
| E. coli (lyophilized) | 20*–100* | 26.5 | H | 50*, 90*–100* | 26.5 | — | 0.9*–2*% (30) | — | (Cox, 1970) |
| L | 20* | 26.5 | — | 20*–30*% (30) | — | ||||
| Flavobacterium | 25, 45, 65, 85, 99 | 24 | H | 99 | 24 | 2.6% min−1 | — | — | (Ehrlich et al., 1970a) |
| L | 85 | 24 | 1.3% min−1 | — | — | ||||
| 85, 100 | –40,–18, –2, 24, 29, 38, 49 | H | 85 | 49 | <0.01% min−1 | — | — | (Ehrlich et al., 1970a) | |
| L | 100 | −40,–18 | 4.7% min−1 | — | — | ||||
| Legionella pneumophila strain 74/81 | 30, 60, 90 | 20 | H | 60 | 20 | - | <1% (120) | — | (Dennis and Lee, 1988) |
| L | 90 | 20 | — | >10*% (120) | — | ||||
| Mycoplasma laidlawii | 10, 25, 40, 50, 60, 75, 90 | 27 | H | 40 | 27 | — | <1% (300) | — | (Wright et al., 1968b) |
| L | 10, 90 | 27 | — | 10*–100*% (60) | — | ||||
| Mycoplasma gallisepticum | 10, 25, 40, 50, 60, 75, 90 | 27 | H | 50, 60 | 27 | — | 0% (300) | — | (Wright et al., 1968b) |
| L | 10 | 27 | — | 100%* (60) | — | ||||
| Mycoplasma pneumoniae | 10, 25, 30, 40, 50, 60, 75, 80, 90 | 27 | H | 60, 80 | 27 | — | 0% (240) | — | (Wright et al., 1968a) |
| L | 10 | 27 | — | 50% (240) | — | ||||
| Pasteurella multocida | 28, 40, 59, 79 | 22.6 | H | 79 | 22.6 | — | 2.1% (45) | — | (Thomson et al., 1992) |
| L | 40 | 22.6 | — | 8.9% (45) | — | ||||
| Pasteurella tularensis | 0*–90* | 26.8 | H | 50* | — | — | 0.2*–0.3*% (15) | — | (Cox and Goldberg, 1972) |
| L | 90* | — | — | 100%* (15) | — | ||||
| 20*–95* | — | H | 55 | — | — | 0.2*–1*% (15) | — | (Cox, 1971) | |
| L | 95* | — | — | >50%* (15) | — | ||||
| Pasteurella tularensis (lyophilized) | 0*–90* | 26.8 | H | 81 | — | — | 0.4*–0.6*% (15) | — | (Cox and Goldberg, 1972) |
| L | 0* | — | — | >10*% (15) | — | ||||
| 20*–95* | — | H | 75 | — | — | 0.01*−0.02*% (15) | — | (Cox, 1971) | |
| L | 20* | — | — | 10*–20*% (15) | — | ||||
| Pseudomonas fluorescens | 23, 39, 60, 79 | — | H | 79 | — | — | 0.01*–0.1*% (60) | — | (Handley and Webster, 1993) |
| L | 39, 60 | — | — | 1*–3.2*% (60) | — | ||||
| Rhizobium meliloti | 30, 50, 70, 95 | 20 | H | 30 | 20 | — | 0.1*–1*% (300) | — | (Won and Ross, 1969) |
| L | 50, 70, 95 | 20 | — | >10*% (300) | — | ||||
| Sarcina lutea | 1/3, 23/27, 45/52, 73/76, 88/96 | 15 | H | 1/3 | 15 | 0.346 log% h−1 | — | — | (Lighthart, 1973) |
| L | 45/52 | 15 | 0.001 log% h−1 | — | — | ||||
| Serratia marcescens | 40, 97 | 25 | H | 40 | 25 | — | 2% (32) | — | (Hess, 1965) |
| L | 97 | — | — | 100% (32) | — | ||||
| 45/52, 73/76, 88/96 | 15 | H | 45/52 | 15 | 0.368 log% h−1 | — | — | (Lighthart, 1973) | |
| L | 73.0/75.5 | 15 | 0.057 log% h−1 | — | — | ||||
| Staphylococcus no. 1600 | 39, 75 | — | H | 75 | — | 0.0189 log min−1 | — | — | (Strasters and Winkler, 1966) |
| L | 39 | — | 0.0037 log min−1 | — | — | ||||
| Streptococcal L-Forms | 20, 40, 60, 80 | 27 | H | 40 | 27 | — | 0% (30) | — | (Stewart and Wright, 1970) |
| L | 20 | 27 | — | >10*% (240) | — |
*Estimated readings from figures. [a]H = highest biological decay of airborne microorganisms (i.e., worst survival and shortest half-life time). L = lowest biological decay of airborne microorganisms (i.e., optimal survival and longest half-life time). [b] In brackets is the time span (in minutes) between which survival rate corresponds to.
Table 5. Biological decay of viruses under different relative humidity (RH) and temperature (Temp).
| Virus | RH levels involved (%) | Temp levels involved (°C) | H/L[a] | Extreme RH for decay (%) | Extreme Temp for decay (°C) | Decay | Survival[c] | Half-life | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Bacteriaphage S-13 | 20, 50, 80 | 21 | H | 50 | 21 | — | 0.1*–1*% (120) | — | (Dubovi and Akers, 1970) |
| L | 80 | 21 | — | >10*% (120) | — | ||||
| Bacteriaphage MS-2 | 20, 50, 80 | 21 | H | 50 | 21 | — | 0.01*–0.1*% (120) | — | (Dubovi and Akers, 1970) |
| L | 20 | 21 | — | 1*–10*% (120) | — | ||||
| Bovine parainfluenza type 3 | 30, 90 | 6, 32 | H | 90 | 32 | — | 0% (180) | — | (Elazhary and Derbyshire, 1979) |
| L | 90 | 6 | — | 1.6–4.0% (180) | — | ||||
| Newcastle disease virus | 10, 35, 90 | 23 | H | 35 | 23 | — | 0.1*–10*% (90) | — | (Songer, 1967) |
| L | 10 | 23 | — | >10*% (90) | — | ||||
| Infectious bovine rhinotracheitis virus | 10, 35, 90 | 23 | H | 35 | 23 | — | 0.1*–10*% (90) | — | (Songer, 1967) |
| L | 90 | 23 | — | >10*% (90) | — | ||||
| Vesicular stomatitis virus | 10, 35, 90 | 23 | H | 35 | 23 | — | 1*–10*% (90) | — | (Songer, 1967) |
| L | 10 | 23 | — | >10*% (90) | — | ||||
| E. coli B T3 bacteriophage | 10, 35, 90 | 23 | H | 35 | 23 | — | 0% (90) | — | (Songer, 1967) |
| L | 90 | 23 | — | >10*% (90) | — | ||||
| Bovine rotavirus UK | 30, 50, 80 | 20 | H | 80 | 20 | — | — | 3 h | (Ijaz et al., 1994) |
| L | 50 | 20 | — | — | 18 h | ||||
| Mouse rotavirus | 30, 50, 80 | 20 | H | 80 | 20 | — | — | 2 h | (Ijaz et al., 1994) |
| L | 50 | 20 | — | — | 24 h | ||||
| Poliovirus type 1 Sarbin | 30, 50, 80 | 20 | H | 30, 50 | 20 | — | — | n.r. | (Ijaz et al., 1985b) |
| L | 80 | 20 | — | — | 9 hs | ||||
| Human corona virus 229E | 30, 50, 80 | 6, 20 | H | 80 | 20 | — | — | 3.3 h | (Ijaz et al., 1985a) |
| L | 50 | 6 | — | — | 102.5 h | ||||
| Human rotavirus | 30, 50, 80 | 6, 20 | H | 80 | 6 | — | — | 1.7 h | (Ijaz et al., 1985c) |
| L | 50 | 6 | — | — | 57.4 h | ||||
| Bovine rotavirus | 20, 50, 80 | 10, 20, 30 | H | 50 | 30 | 2.39 log h−1 | — | — | (Moe and Harper, 1983) |
| L | 90 | 10 | 0.03 log h−1 | — | — | ||||
| E. coli B T3 coliphage | 8, 30, 50, 80, 95 | 21 | H | 8 | 21 | — | 0% (240) | — | (Hatch and Warren, 1969) |
| L | 95 | 21 | — | >10*% (240) | — | ||||
| Pasteurella pestis Bacteriophage | 20, 40, 50, 60, 72, 95 | 21 | H | 40, 50, 60 | 21 | — | 0.1*–10*% (240) | — | (Hatch and Warren, 1969) |
| L | 20, 72, 95 | 21 | — | >10*% (240) | — | ||||
| Encephalomyoca-rditis Virus | 5*–90* | 10, 20, 30, 37 | H | 10*–20* | 37 | 0.001*–0.01*% (30–35) | — | (de Jong et al., 1975) | |
| L | 80*–90* | 20 | 100*% (30–35) | — | |||||
| Foot and Mouth disease virus O1 BFS 1860 | 20, 30, 40, 50, 60, 70 | 19–22 | H | 20 | 19–22 | — | 0.01*–0.1*% (5) | — | (Barlow and Donaldson, 1973) |
| L | 50, 60, 70 | 19–22 | — | 0.01*–11*% (5) | — | ||||
| 55, 70 | 18–23 | H | 55 | 18–23 | n.c. | — | — | (Donaldson, 1972) | |
| L | 70 | 18–23 | 3.15 log h−1 | — | — | ||||
| Foot and Mouth disease virus O2 Brescia | 55, 70 | 18–23 | H | 55 | 18–23 | n.c. | — | — | (Donaldson, 1972) |
| L | 70 | 18–23 | 2.60 log h−1 | — | — | ||||
| Foot and Mouth disease virus O1 Lombardy | 55, 70 | 18–23 | H | 55 | 18–23 | n.c. | — | — | (Donaldson, 1972) |
| L | 70 | 18–23 | 2.38 log h−1 | — | — | ||||
| Foot and Mouth disease virus C Noville | 55, 70 | 18–23 | H | 55 | 18–23 | 2.90 log h−1 | — | — | (Donaldson, 1972) |
| L | 70 | 18–23 | 1.88 log h−1 | — | — | ||||
| Foot and Mouth disease virus A5 Eystrup | 55, 70 | 18–23 | H | 55 | 18–23 | 2.60 log h−1 | — | — | (Donaldson, 1972) |
| L | 70 | 18–23 | 1.78 log h−1 | — | — | ||||
| Foot and Mouth disease virus C Lebanon | 55, 70 | 18–23 | H | 55 | 18–23 | 2.40 log h−1 | — | — | (Donaldson, 1972) |
| L | 70 | 18–23 | 1.43 log h−1 | — | — | ||||
| Foot and Mouth disease virus A22 Iraq | 55, 70 | 18–23 | H | 55 | 18–23 | 3.28 log h−1 | — | — | (Donaldson, 1972) |
| L | 70 | 18–23 | 1.25 log h−1 | — | — | ||||
| Foot and Mouth disease virus O1 Pacheco | 55, 70 | 18–23 | H | 55 | 18–23 | 2.05 log h−1 | — | — | (Donaldson, 1972) |
| L | 70 | 18–23 | 1.06 log h−1 | — | — | ||||
| Influenza virus | 20/25, 34/36, 49/51, 64/65, 81/82 | 7.0/8.0, 20.5/24.0, 32.0 | H | 81 | 32.0 | — | 0% (240) | — | (Harper, 1961) |
| L | 23/25 | 7.0/8.0 | — | 61% (1380) | — | ||||
| Vaccinia virus | 17/20, 48/51, 80/84 | 10.5/11.5, 21.0/23.0, 31.5/33.5 | H | 80/83 | 31.5/33.5 | — | 0% (1380) | — | (Harper, 1961) |
| L | 20 | 10.5/11.5 | — | 66% (1380) | — | ||||
| Venezuelan equine encephalomyelitis virus | 19/23, 48/50, 81/86 | 9.0/9.5, 21.0/23.0, 32.0/ 33.0 | H | 81/85 | 32.0/33.0 | — | 0% (360) | — | (Harper, 1961) |
| L | 19 | 9.0/9.5 | — | 26% (1380) | — | ||||
| Poliomyelitis virus | 18/23, 35/36, 49/51, 64/65, 80/81 | 20.5/23.5 | H | 49/51 | 20.5/23.5 | — | 0% (360) | — | (Harper, 1961) |
| L | 80/81 | 20.5/23.5 | — | 85% (1380) | — | ||||
| 20, 80 | — | H | 20 | — | — | 2.5% (60) | — | (Benbough, 1971) | |
| L | 80 | — | — | 53% (60) | — | ||||
| Japanese B Encephalitis Virus | 30, 55, 80 | 24 | H | 80 | 24 | — | — | 28 min | (Larson et al., 1980) |
| L | 30 | 24 | — | — | 62 min | ||||
| Langat virus | 20, 80 | — | H | 80 | — | — | 10% (60) | — | (Benbough, 1971) |
| L | 20 | — | — | 52% (60) | — | ||||
| Semliki Forest virus | 20, 80 | — | H | 80 | — | — | 51% (60) | — | (Benbough, 1971) |
| L | 20 | — | — | 67% (60) | — | ||||
| E. coli B T7 coliphage | 20, 80 | — | H | 20 | — | — | 0.05% (60) | — | (Benbough, 1971) |
| L | 80 | — | — | 57% (60) | — | ||||
| Lassa virus Josiah | 30, 55, 80 | 24, 32, 38 | H | 80 | 32 | 6.9% min−1 | 0.3% (60) | 10.1 min | (Stephenson et al., 1984) |
| L | 30 | 24 | 1.3% min−1 | 16.9% (60) | 54.6 min | ||||
| Pseudorabies virus | 55, 85 | 4, 22 | H | 85 | 22 | — | — | 17.4 min | (Schoenbaum et al., 1990) |
| L | 55 | 4 | — | — | 43.6 min | ||||
| Newcastle disease virus | 20/30, 50, 80 | 10, 15, 20, 25, 30 | H | 80 | 25, 30 | — | 8% (360) | — | (Kournikakis et al., 1988) |
| L | 20/30 | 10 | — | 56% (360) | — | ||||
| Porcine reproductive and respiratory syndrome virus | 5–90 | 5–41 | H | 63.8 | 30 | — | — | 3.3 min | (Hermann et al., 2007) |
| L | 17.1 | 5 | — | — | 192.7 min | ||||
| Psittacosis agent | 30, 50, 80 | 26.7 | H | 80 | 26.7 | 6.73% min−1 | — | — | (Mayhew and Hahon, 1970) |
| L | 30 | 26.7 | 0.64% min−1 | — | — | ||||
| Reovirus type 1 Lang | 25/35, 45/55, 65/75, 85/95 | 21/24 | H | 65/75, 25/35 | 21/24 | 3.2–3.3% min−1 | — | — | (Adams et al., 1982) |
| L | 85/95 | 21/24 | 1.5–2.5% min−1 | — | — | ||||
| Yellow fever virus | 30, 50, 80 | 26.7 | H | 50 | 26.7 | 7.04% min−1 | — | — | (Mayhew and Hahon, 1970) |
| L | 30 | 26.7 | 3.26% min−1 | — | — | ||||
| Variola virus | 30, 50, 80 | 26.7 | H | 30 | 26.7 | 0.86% min−1 | — | — | (Mayhew and Hahon, 1970) |
| L | 80 | 26.7 | 0.56% min−1 | — | — | ||||
| Respiratory Syncytial Virus | 20, 30, 40, 50, 60, 70, 80, 90 | 20.5 | H | 80 | 20.5 | 1.49 log h−1 | — | — | (Rechsteiner and Winkler, 1969) |
| L | 20 | 20.5 | 0.47 log h−1 | — | — | ||||
| Rift Valley fever virus ZH-501 | 30, 55, 80 | 24 | H | 80 | 24 | 10.1% min−1 | — | 6.9 min | (Brown et al., 1982) |
| L | 30 | 24 | 0.9% min−1 | — | 77.0 min | ||||
| Rift Valley fever virus SA-51 | 30, 55, 80 | 24 | H | 80 | 24 | 6.1% min−1 | — | 11.4 min | (Brown et al., 1982) |
| L | 30 | 24 | 1.3% min−1 | — | 53.3 min | ||||
| Rotavirus SA11 | 25, 50, 80 | 20 | H | 80 | 20 | — | — | <2 h | (Sattar et al., 1984) |
| L | 50 | 20 | — | — | 40 h | ||||
| St. Louis encephalitis (SLE) virus | 29, 46, 60, 80 | 21 | H | 80 | 21 | — | 14[b]% (360) | — | (Rabey et al., 1969) |
| L | 29 | 21 | — | 79[b]% (360) | — | ||||
| Venezuelan equine encephalomyelitis virus | 30, 60 | 22 | H | 60 | 22 | — | 0.006–77.3% (60) | — | (Berendt and Dorsey, 1971) |
| L | 30 | 22 | — | 0.02–88.7% (60) | — | ||||
| Rhinovirus-14 | 30, 50, 80 | 20 | H | 30, 50 | 20 | — | <0.25% (15) | — | (Karim et al., 1985) |
| L | 80 | 20 | — | 30% (1440) | 13.7 hs |
*Estimated readings from the figures. n.c. = not calculated due to no infectious virus was recovered. [a]H = highest biological decay of airborne microorganisms (i.e., worst survival and shortest half-life time); L = lowest biological decay of airborne microorganisms (i.e., optimal survival and longest half-life time). [b]Calculated by dividing the amount of virus collected in the last air sample (360 min) by that in the first air sample (15 min). [c]In brackets is the time span (in minutes) between which survival rate corresponds to.
Most of the previous studies have used wet aerosolization in which microbial suspensions were aerosolized. Wet aerosolization does mimic the fate of microorganisms expelled from animal respiratory tracts in wet aerosols. However, after they have been generated, the large wet aerosols containing microorganisms settle on surfaces and the small ones shrink into dry nuclei; both processes are very fast, taking only seconds to minutes (Kincaid and Longley, 1989; Sun and Ji, 2007; Wells, 1934). Therefore, the microorganisms in wet aerosols can only be transported over short distances and induce infections in limited areas (Brankston et al., 2007). For microorganisms released from dry sources such as feces and litter, which may be more closely associated with long distance transmission, dry aerosolization is recommended. Dry aerosolization may give a picture of the biological decay of microorganisms that differs from the decay in wet aerosolization because the microorganisms may suffer either dehydration stress at low ambient RH or rehydration at high ambient RH (Cox, 1971).
4. DEPOSITION AND INFECTIVE DOSE
4.1. Particle Deposition in Respiratory Tract of Humans and Animals
According to Heyder et al. (1986), the probability of deposition will be different for each particle even if all the particles in the air inhaled in one breath are identical, because the inhaled air with particles penetrates the respiratory tract to different depths where it remains for different periods of time, and because of the stochastic nature of particle transport. Therefore, particle deposition (in the respiratory tract) refers to the “mean probability” of an inspired particle being collected on airway surfaces. Particle deposition in the respiratory tract depends on particle characteristics (e.g., size, shape, density) and breathing pattern (e.g., nasal/oral breath, respiratory flow rate, cycle period), and is commonly expressed as a function of particle size.
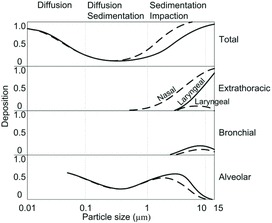
Particle deposition in the human respiratory tract has been well documented (Brown et al., 2002; James et al., 1991; Lippmann et al., 1980). In principle, the deposition of particles is governed by the mechanisms of diffusion for particles <0.1 μm, or by diffusion and sedimentation for 0.1–1 μm particles, or by sedimentation and impaction for particles >1 μm (Heyder, 2004). On the basis of previous experimental studies, Heyder et al. (1986) developed a semiempirical deposition model for particles ranging from 0.005 μm to 15 μm. The model-simulated deposition pattern for slow inspiration over a long period for both oral and nasal breathing is shown in Figure 2. The deposition has been shown for three regions of the respiratory tract—extra-thoracic, bronchial, and alveolar—based on how far down the tract particles may be deposited. In addition, total deposition is given (the sum of the deposition in the three regions). It can be seen that the total deposition is least for particles of 0.1–1 μm, and that the deposition increases as the size of small particles (<0.1 μm) decreases, and the size of the larger particles (>1 μm) increases. Particles larger than 10 μm are mainly deposited in the extrathoracic region, and cannot penetrate the alveolar region during slow and fast oral and nasal inspiration.
Figure 2. Deposition of unit density spherical particles in human respiratory tract at a mean flow rate of 250 cm3 s−1 and a breathing cycle period of 8 s. Solid curves show the deposition curve for steady oral breathing, and dashed curves for steady nasal breathing. Deposition = 1 indicates all particles deposit, and deposition = 0 means no particle deposit at a certain region. Adapted from Heyder et al. (1986).
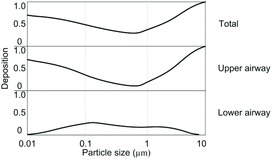
The deposition in the human respiratory tract cannot be extrapolated to livestock because of the discrepancy in morphology of human and animal respiratory systems (Corbanie et al., 2006). Particle deposition in guinea pig head, trachea, and lungs was investigated by Harper and Morton (1953). They found an increasing regional deposition in guinea pig head for larger particles: 35.7% of the 1 μm particles were deposited in the guinea pig head region, compared with 98.1% of the 10 μm particles. The reverse was true for lung deposition: 55.2% of 1 μm particles and 0.6% of 10 μm were deposited in this region. The particle size most deposited in the trachea was 2.5 μm. A model of particle deposition in the guinea pig respiratory tract was established by Schreider and Hutchens (1979), who found that 99% of unit density particles of 10 μm or more could be deposited in the nasopharyngeal-tracheobronchial region (Figure 3). The lowest deposition was 10% for a particle size of 0.8 μm. About 17% of particles ranging from 0.08 to 4 μm could be deposited in the pulmonary region. However, this model was compromised by several assumptions that were made by the authors (e.g., laminar airflow in the respiratory tract, equal expansion of all lobes and alveoli, and complete mixing of the particles in the alveoli.
Figure 3. Deposition of unit density spherical particles in the respiratory tract of guinea pig at a tidal volume of 4.44 cm3 and a respiratory rate of 60 breaths min−1. NP-TB: nasopharyngeal-tracheobronchial region. P = pulmonary region. Adapted from Schreider and Hutchens (1979).
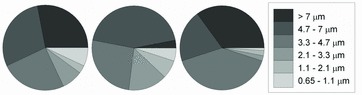
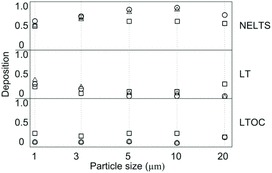
Hayter and Besch (1974) investigated the regional deposition of five particle sizes (0.091, 0.176, 0.312, 1.1, and 3.7 to 7 μm) in chicken. The particles deposited in the head and anterior trachea were in the 3.7–7 μm size range, those deposited in the lung and posterior air sacs were 1.1 μm size, and those deposited in the caudal regions of the birds were in the size classes 0.091 and 0.176 μm. Those of size 0.312 μm were deposited mainly in upper airways. These early data of particle deposition in chicken airways are suspected to be compromised by the use of anesthetized chickens, because anesthesia alters the animals’ breathing pattern. Corbanie et al. (2006) investigated the deposition of particles in a wider range (1, 3, 5, 10, and 20 μm) in unanaesthetized chickens of three ages. Unlike the definition of deposition that was proposed by Heyder et al. (1986), Corbanie et al. (2006) defined deposition as the percentage of particles deposited in a particular region of the respiratory tract among those in the entire tract. They found that particles larger than 5 μm were too large to be deposited in the lungs and air sacs in 2- and 4-week-old chickens, as low percentages of particles were recovered in these regions (Figure 4). For 1-day-old chicks, however, the particle deposition in lungs and air sacs was independent of particle size and even particles of 20 μm were deposited in the lower airway, possibly due to the chicks having a different breathing pattern than older chickens.
Figure 4. Deposition of fluorescent particles in the respiratory tract of 1-day-old (□), 2-week-old (○), and 4-week-old (Δ) broilers. NELTS = nose, eyes, larynx, trachea and syrinx. LT = lungs and thoracic air sacs. LTOC = lower beak, tongue, esophagus and crop. Adapted from Corbanie et al. (2006).
The deposition pattern of monodispersed particles (3.3 μm) in calf airways was studied by Jones et al. (1987). They found those particles were preferentially deposited in the trachea and major bronchi.
4.2. Infective Dose
Inhalation of pathogens may result in infection of recipients. Infection is likely to fit a single-hit model, which means that one pathogenic microorganism may trigger an infection in a recipient. From this point of view, the infective dose (ID; or occurrence of infection) is therefore related to the probability that a recipient becomes infected after taking in a certain dose of pathogenic microorganisms, following a Poisson distribution. The ID of pathogenic microorganisms to human and animals has generally been expressed in two ways. In one way, ID is expressed as the number of infected recipients out of a population after a dose of microorganisms has been administered. The other way is to determine the microbial concentrations required to infect 50% of a population (ID50).
Table 6 lists the ID of several pathogenic microorganisms. The ID of the same microorganism varies, depending on the recipient animal species. For instance, a lower ID of FMD virus is needed to infect sheep and cattle than to infect a pigs (Alexandersen and Donaldson, 2002; Donaldson et al., 1987; Gibson and Donaldson, 1986). It can be also seen that a certain dose of a microorganism is not always capable of infecting all recipients, probably because of a difference in the resistance of individual recipients (due to e.g., age, breed; Roy, 1980). Furthermore, the route by which the microorganisms are administered may also be responsible for variation in ID (Cafruny and Hovinen, 1988; Zimmerman et al., 1993). In previous studies, the administration routes were either nasally or orally, or via aerosols, and these reflect different deposition situations and regions for microorganisms in the respiratory tract. Nasal administration simulates infection because larger microbial particles are deposited in the upper airways, the oral ministration may simulate oral breathing, and the aerosol administration simulates infection because smaller microbial particles are deposited in deeper airways. It was reported that the microorganisms had preferential sites for multiplication and infection because of the complex vulnerability of regions in respiratory tracts (Cafruny and Hovinen, 1988; Druett et al., 1953; Druett et al., 1956). This being so, infection occurs more readily when the microorganisms are administered to the more vulnerable region. Baskerville (1981) summarized the preferred infection regions of some microorganisms to animals by categorizing nose, pharynx, and tonsils as the upper respiratory tract, and the trachea, bronchi and bronchioles, and alveoli as the lower respiratory tract (Table 7).
Table 6. Infective dose (ID) of some pathogenic microorganisms.
| Microorganism | ID | Recipient | Administration | Infected/Total (or% infected) | Reference |
|---|---|---|---|---|---|
| Campylobacter jejuni | 90 CFU | 3-day chickens | Orally | 9/10 | (Ruiz-Palacios et al., 1981) |
| E. coli (O157:H7) | 6000 CFU | 3-month pigs | — | 6/8 | (Cornick and Helgerson, 2004) |
| E. coli (O157:H7) | <300 CFU | 10-week calves | Orally | 2/17 | (Besser et al., 2001) |
| E. coli (O157:H7) | 107 CFU | 3-year steer | Stomach tube | 2/5 | (Cray and Moon, 1995) |
| Salmonella typhimurium | 1000 CFU | 10–14-day pigs | Intranasal | 1/5 | (Loynachan and Harris, 2005) |
| Salmonella enteritides | <10 CFU | >52-week molted layers | Orally | 50% | (Holt, 1993) |
| Salmonella enteritides | 6500–56000 CFU | >52-week unmolted layers | Orally | 50% | (Holt, 1993) |
| FMD virus (strain O1 Lausanne) | 1700 TCID50 | 20–30 kg pigs | Aerosol | 5/8 | (Alexandersen and Donaldson, 2002) |
| FMD virus (strain O1 BFS 1860) | 13–398 TCID50 | 43–166 kg calves | Aerosol | 10/12 | (Donaldson et al., 1987) |
| FMD virus (strain O1 BFS 1860) | 10–50 TCID50 | 26–82 kg sheep | Aerosol | 7/12 | (Gibson and Donaldson, 1986) |
| FMD virus (strain SAT 2 SAR 3/79) | 25–251 TCID50 | 118–150 kg calves | Aerosol | 11/15 | (Donaldson et al., 1987) |
| PRRSV | 10 TCID50 | 4–5-week pigs | Intranasal | 2/3 | (Yoon et al., 1999) |
| Porcine rotavirus | 1 PFU | 2-hr piglets | Pharynx | 2/2 | (Graham et al., 1987) |
| Encephalomyocarditis virus | 108.8 TCID50 | 4–6-week pigs | Intranasal | 2/5 | (Zimmerman et al., 1993) |
| Influenza A/Texas/91 (H1N1) virus | 100000 TCID50 | 18–33-year-old humans | Intronasal | 24/33 | (Hayden et al., 1996) |
| Influenza A2/Bethesda/10/63 | 1–5 TCID50 | 21–40-year-old man | Aerosol | 4/14 | (Alford et al., 1966) |
| Rotavirus | 0.9 FFU | 18–45-year-old man | Orally | 1/7 | (Ward et al., 1986) |
| Salmonella newport | 150000 CFU | Human | Orally | 1/6 | (McCullough and Eisele, 1951) |
| Salmonella derby | 1.5×107 CFU | Human | Orally | 3/6 | (McCullough and Eisele, 1951) |
| Salmonella bareilly | 13000 CFU | Human | Orally | 1/6 | (McCullough and Eisele, 1951) |
CFU = colony forming unit; TCID50 = 50% tissue culture infective dose; PFU = plaque forming unit; FFU = focus forming unit.
Table 7. Initial infection region of microorganisms (Baskerville, 1981).
| Microorganism | Animal | Infection region |
|---|---|---|
| Bordetella bronchiseptica | Pig | URT |
| Haemophilus spp. | Pig | URT |
| Pasteurella spp. | Pig | URT |
| Cattle | URT | |
| Sheep | URT | |
| Mycoplasma | Poultry | URT |
| Pig | URT | |
| Cattle | URT | |
| Bovine herpesvirus-1 | Cattle | URT |
| Parainfluenza-3 | Cattle | URT |
| Sheep | URT | |
| Infectious laryngo-tracheitis virus | Poultry | URT |
| Infectious bronchitis virus | Poultry | URT |
| Aujeszky's disease virus | Pig | URT |
| Aspergillus fumigatus and other fungi | Poultry | LRT |
| Respiratory syncytial virus | Cattle | LRT |
| Adenoviruses | Cattle | LRT |
Note. URT = upper respiratory tract (including nose, pharynx, and tonsil); LRT = lower respiratory tract (including trachea, bronchi and bronchioles, and alveoli).
5. SAMPLING AIRBORNE MICROORGANISMS AND DUST IN LIVESTOCK HOUSES
Sampling protocols for dust in ambient air have been legislated by the U.S. Environmental Protection Agency (2006) and the European Committee for Standardization (European Commission, 1998, 2005). Nowadays, the official sampling protocols focus increasingly on small particles (e.g., PM10 and PM2.5) because these have the potential to be suspended for longer time, transported for longer distance, and deposited in the lower respiratory tract, thus are more hazardous to human and animal health. To ensure unbiased sampling, these protocols specify many details, such as sampling duration, type of sampler, and sample handling.
The protocols for ambient air may not be directly applicable for dust sampling in livestock production systems where the dust concentrations are much higher than those in ambient air. In addition, to date there is no gold standard for sampling airborne microorganisms. Given that the sampling of microorganisms and dust in livestock production systems is increasingly being performed for assessing the biosecurity of air environments and for evaluating mitigation techniques, the sampling protocol must be well designed in order to assure reliable data. Subsequently, sampling strategies and samplers for airborne microorganisms and dust are highlighted, taking into consideration their sampling in livestock production systems.
5.1. Sampling Strategy
Isokinetic sampling is the ideal sampling method, because it has been devised to sample the true numbers of particles in the air. Such sampling can be achieved if the sampler inlet is in alignment with and facing the direction of air flow and if the air velocity within the sampler is the same as the ambient air velocity (Zhang, 2004). However, true isokinetic sampling is impossible in practice due to variations in the surrounding air flow pattern (air direction and velocity) and the limitations of some samplers (Liu and Pui, 1981). As a concession, the current legislation aims to reduce the sampling bias in nonisokinetic samplings by stipulating the range of conditions under which the samplings may be performed.
The sampling location should be chosen bearing in mind the research purpose. When human health is of concern, sampling should be carried out near the human breathing zone. One option is to fit a portable sampler on a worker's body at a height of 150–170 cm above floor level and within a radius of 30 cm around the mouth (Aarnink et al., 2011b; Ouellette et al., 1999). There are difficulties in doing the same with animals, therefore stationary samplers in their breathing zones are recommended. The recommended level of the breathing zone is 30–40 cm above floor level for pigs, 10–25 cm for poultry, and shoulder height for cattle (Kim et al., 2007a; Topisirovic, 2003; Zhao et al., 2011d). For growing animals these figures should be adjusted according to the animal height at a certain age. When emissions of microorganisms and dust are of interest, the best sampling location is in or near the air outlet. Care should be taken not to place the samplers at a location where the air speed is too high, because the efficiency of the sampler (the ratio of the aerial pollutant concentration calculated from the air samples to the true concentration in the air) may drift far from 100% (Grinshpun et al., 1994; Hofschreuder et al., 2007).
For ambient air, the daily and annual thresholds for PM10 have been set at 50 and 40 μg m−3, respectively (European Commission, 1999); the annual threshold for PM2.5 has been set at 25 μg m−3 (European Commission, 2008). To assess the dust concentration, the sampling period is generally 24 hr. The sampling duration for ambient air may also be applied when sampling dust in animal houses to obtain daily mean concentrations. For studies collecting information on dust fluctuations during the day, continuous samplings for short periods should be performed. This kind of sampling can be achieved by interval sampling or sampling with real-time optical samplers.
Due to the lack of a standard, the sampling duration for airborne microorganisms in different studies varies, but is normally less than 1 hr. The duration is determined by taking account of the estimated concentrations of microorganisms and the characteristics of the sampler in use. The sampling duration can be set at a short period (a few minutes) when the total microorganism is abundant in livestock houses. When the microorganism of interest is sporadically present, the period should be set long enough to collect enough microorganisms for further quantification analysis. Some samplers have not been designed to be used for long sampling duration. For instance, severe evaporation of liquid medium in All Glass Impinger (AGI-30) occurs when the sampling period is long and this may affect its sampling efficiency. The recommended maximum duration of the sampling period for the AGI-30 is 30 min. Impactors may easily become overloaded when samples are taken in livestock houses (Thorne et al., 1992), therefore, the sampling duration is limited to minutes or even to seconds. Filtration method would not encounter the problems (evaporation of sampling liquid and overloading) of impingers and impactors; however, long sampling duration by filtration may not benefit in microorganism collection because of the biological decay of microorganisms owing to dehydration (Griffin et al., 2011; Wang et al., 2001). In order to collect detectable amounts of microorganisms and to get representative samples over a longer period, some high-volume and durable samplers that can be continuously operated over hours have been developed and applied, including OMNI-3000 and high-volume impinger (Griffin et al., 2011; Kesavan and Schepers, 2006).
Other aspects in sampling and detection strategies include practical and economic considerations. The portability of the instrument and its ancillaries (e.g., weight and dimensions) are factors that should be considered. A high-quality pump is required for dust filtration sampling, and it should be able to provide a constant airflow when ambient temperature changes or when pressure differences increases because of dust accumulation on the filter. The culture-dependent techniques for microorganism numeration are time consuming and particularly expensive; culture-independent techniques such as quantitative PCR provide possibilities for rapid and accurate analysis which is critical for biological emergency (Postollec et al., 2011; Ståhl et al., 2011), but its incapability to distinguish between viable and dead microorganisms needs to be considered (Yáñez et al., 2011).
5.2. Samplers for Microorganisms
Current samplers for airborne microorganisms are generally based on one of three main principles (i.e., impaction, impingement, or filtration; Table 8). These different sampling principles have their advantages and disadvantages. Samplers using the impaction principle (e.g., Andersen Six Stage Impactor) can be used to distinguish the microorganisms according to their sizes (Andersen, 1958). In addition, the bacteria impacted on agar plates may be directly incubated for viable counts. However, its susceptibility to overloading limits its sampling in livestock houses to short periods, which may result in nonrepresentative samples. The problem of overloading can be overcome by using samplers with the impingement principle, because the liquid samples may be decimally diluted and analyzed after sampling. A disadvantage of this sampler is that it may not be able to sample for a long time due to evaporation of the collection liquid (Lin et al., 1997). Filtration is a user-friendly method in a practical situation, but not suitable for sampling microorganisms that are vulnerable to dehydration stress.
Table 8. Common samplers for airborne microorganisms.
| Sampling principle | Collection medium | Example samplers |
|---|---|---|
| Impaction | Agar plate | Andersen One/Two/Six Stage Impactor (Andersen Instruments Incorporated, Atlanta, GA, USA) |
| Casella Slit Sampler (Casella Ltd, Bedford, England) | ||
| Surface Air Systems (Cherwell Laboratories, Bicester, England) | ||
| Burkard air sampler (Burkard Manufacturing Co. Ltd., Rickman Worth, England) | ||
| Impingement | Liquid medium | AGI-30 (Ace Glass, Vineland, USA) |
| Multistage May Liquid Impinger (AW Dixon, Beckenham, Kent, England) | ||
| Filtration | Filter | Sartorius MD8 AirPort/AirScan with gelatin or polytetrafluoroethylene filter (Sartorius, Göttingen, Germany) |
| Button Personal Inhalable Sampler (SKC, Inc., Pennsylvania, USA) |
A sampler with known efficiency is a prerequisite for a reliable evaluation of the microbial concentration. The efficiency of a bioaerosol sampler includes physical and biological efficiency. The physical efficiency describes how well nonviable airborne particles are aspirated by the device's inlet and transported to the collection medium, referred to as inlet sampling efficiency, and how well the bioaerosol sampler retains these particles in its medium, referred to as collection efficiency (Griffiths and Stewart, 1999; Nevalainen et al., 1992). For particles in the range from 1 to 10 μm and an airflow rate between 0 and 500 cm s−1, the inlet sampling efficiency of the Andersen Six Stage Impactor is 90–150% when the opening faces in direction of the air flow and 8–100% when it is oriented perpendicularly to the horizontal aerosol flow (Grinshpun et al., 1994). The inlet efficiency of the AGI-30 for particles of 1 μm is close to 100%, but it is reduced to 70–90% for 5 μm particles and to 20–30% for 10 μm particles (Grinshpun et al., 1994). When the 50% collection efficiency of the Andersen Six Stage Impactor and AGI-30 was investigated it was found that the Andersen Six Stage Impactor has 50% collection efficiency for 6.6–7.0 μm particles at the first stage and 0.57–0.65 μm particles for the last stage (Andersen, 1958; Nevalainen et al., 1992). The 50% collection efficiency of AGI-30 was for particles of 0.31 μm (Nevalainen et al., 1992).
Filters vary in their physical efficiency. Some are highly effective. By measuring the particle concentration upstream and downstream of filters with an optical particle counter, polytetrafluoroethylene (PTFE) filters and gelatin filters were confirmed to collect more than 93% of particles, even down to 80 nm (Burton et al., 2007).
If the particles are living organisms, they may be inactivated during sampling due to impaction stress (Stewart et al., 1995), impingement stress (Shipe et al., 1959; Tyler and Shipe, 1959; Tyler et al., 1959), and/or dehydration stress (Li et al., 1999). Therefore, in order to indicate how well a bioaerosol sampler maintains the microbial viability and prevents cell damage during sampling, the concept of biological efficiency has been introduced (Griffiths and Stewart, 1999).
In some studies, the efficiency has been evaluated by comparing samplers side by side in an environment with unknown microbial concentration (Engelhart et al., 2007; Henningson et al., 1982; Thorne et al., 1992). This method easily ranks the performance of different samplers; however, it neither reveals whether the amount of microorganisms collected in the samples accurately represents the microorganism content of the air nor distinguishes between the physical and biological efficiency. Other studies separately investigated the physical and biological efficiency of bioaerosol samplers in aerosol experiments, in which a known amount of microorganisms together with an indicator (either labeled microorganisms, or inert tracer compound) were nebulized in an isolator. The physical efficiency can be determined by comparing the amount of tracer collected by a bioaerosol sampler with that collected by the reference sampler (a sampler that has a high physical efficiency); the biological efficiency is subsequently indicated by the change in the ratio of microorganisms/indicator. Using this method, Zhao et al. (2011b; 2011c) reported the efficiency of the Andersen Six Stage Impactor, AGI-30, OMNI-3000, and gelatin filter in sampling Enterococcus faecalis, E. coli, Campylobacter jejuni, Mycoplasma synoviae, and Gumboro vaccine virus.
5.3. Samplers for Dust
European reference samplers use the filtration principle to collect dust from the air (European Commission, 1998; European Commission, 2005); while in US, a list of reference and equivalent samplers, including tapered element oscillating microbalance (TEOM), filter, and optical sampler, have been proposed by the U.S. Environmental Protction Agency (1997).
For sampling dust in certain size fractions (e.g., PM10 and PM2.5), a preseparator for separating the coarse dust from the target dust particle sizes has to be installed in front of the filter/sensor/dust collector. Sampling systems with pre-separators using the impaction principle have been legislated as reference methods for measuring dust in ambient air in the United States (U.S. Environmental Protction Agency, 1997) and European countries (European Commission, 1998, 2005). These systems show steep collection curves for sampling fine dust in ambient air where dust load is low (Kenny et al., 2000). However, the preseparator with impaction principle may become overloaded when sampling in dusty livestock production systems, thereby resulting in overestimated concentrations of fine dust (Zhao et al., 2009). In contrast, a preseparator with cyclone principle has been found to be less vulnerable to overloading in dusty environments than preseparators based on impaction (Zhao et al., 2009).
Optical dust samplers are now commercially available. These dust samplers can monitor the real-time dust concentrations, and no further process is needed after sampling (unlike the gravimetric method, in which filters must be weighed). Moreover, some optical samplers may separately record the concentrations of dust in different size ranges. However, the optical samplers have limitations in humid environments, because it cannot distinguish between droplets and dry dust. Another limitation of using optical samplers lies to the difficulty in converting count to mass concentrations without knowing the true density of the particles in concern.
6. MITIGATION TECHNIQUES FOR AIRBORNE MICROORGANISMS AND DUST
Many techniques have been applied in practice to reduce the concentrations and emissions of airborne microorganisms and dust in the livestock industry. They vary in their utility, but can be grouped into two main principles. The first principle, to control particles at source, includes techniques such as feed coating and oil spraying, which has been stated to be “the most effective means” of controlling airborne particles in the space (Pearson and Sharples, 1995). The second principle is air purification. Ionization (electrostatic) and air scrubbers are examples.
6.1. Control at Source
The fatty substances often added to feed to increase metabolizable energy content may reduce the airborne microorganisms and dust in animal houses (Pearson and Sharples, 1995). Gore et al. (1986) reported that concentrations of airborne bacteria were reduced by 27%, and settled dust by 45–47%, when 5% soybean oil was added to the pig diet. This result is consistent with the study by Welford et al. (1992), who found a 31% reduction of inhalable dust with 2% oil addition to the feed. Other substances, such as tallow, lecithin and lignin have also been used as effective feed additives for the purpose of particle control in animal houses (Dawson, 1990; Pearson and Sharples, 1995).
Spraying techniques reduce the particle concentrations mainly by coating the surfaces, thereby, preventing particles from being suspended or resuspended from their sources (Takai, 2007). Kim et al. (2006) reported that spraying 60 ml m−2 of tap water, salt water, treated manure, microbial additive, soybean oil, artificial spice, and essential oil may all reduce particles in pig houses. These authors found an average reduction of 53% for airborne bacteria, 51% for fungi, and 30% for total dust. The substance for spraying found to be the most effective additive for reducing dust was soybean oil. In broiler rooms, Aarnink et al. (2009) reported that PM10 reduction increased linearly from 55 to 85% when daily rapeseed oil application rates increased from 6 to 24 ml m−2. The PM2.5 reduction was not related to application rate and was about 80%. In aviary systems for layers, a 34% reduction for PM10 and 50% reduction for PM2.5 were achieved by daily spraying with 20 ml m−2 (Aarnink et al., 2009). Although a high oil application rate achieves high PM10 reduction in broiler rooms, it may adversely affect animal health (Aarnink et al., 2011b). When spraying 24 ml m−2 daily, there was a tendency for statistically higher footpad lesion in broilers. Aarnink et al. (2009) recommended limiting oil application to 16 ml m−2.
6.2. Air Purification
The ionization (also referred to as electrostatic) technique produces negative ions in the air, which causes airborne particles to become negatively charged. The negatively charged particles are attracted to earthed or positively charged surfaces. Previous studies have shown promising reduction effects on dust in animal houses using ionization techniques, total dust was reduced by 13–61% in poultry houses (Lyngtveit and Eduard, 1997; Mitchell et al., 2000; Mitchell et al., 2004; Richardson et al., 2003), and by 45–58% in pig houses (Rosentrater, 2003; Tanaka and Zhang, 1996). Cambra-Lopez et al. (2009) reported that the technique produced average reductions of 36% for PM10 and 10% for PM2.5. The disparity is probably caused by the different charging mechanisms to particles. The small particles (<0.1 μm) are charged by the thermal charging mechanism, which is proportional to the diameter; large particles (>0.5 μm) are charged by field charging mechanism, which is proportional to the square of the diameter (Bundy, 1984). Ionization has the potential to prevent airborne transmission of microorganisms (Gast et al., 1999; Holt et al., 1999), however, no reduction of airborne bacteria, fungi and mold was found in broiler houses by this technique (Cambra-Lopez et al., 2009). Knowledge of ionization on reduction of airborne microorganisms in animal houses is still limited and needs to be augmented.
End-of-pipe techniques, such as dedusters, air filters, and scrubbers have been installed at air outlets of animal houses to minimize emissions of air pollutants. Zhang et al. (2001) found a deduster removed 90% total dust from the air exhausted from a pig house. Because dedusters are based on the centrifuge principle, they are more effective at removing larger particles than smaller ones. The deduster studied by Zhang et al. (2001) had a removal efficiency of 90% for particles larger than 10 μm, 77% for 7 μm particles, and 50% for 4 μm particles. The low removal efficiency for small particles was assumed to be particle re-entrainment due to high air turbulence. Acid and biological air scrubbers were originally developed to reduce ammonia and odor emissions, and they also appear to be effective in reducing particle emissions. An acid scrubber that uses sulfuric acid may achieve a 70% reduction of total bacteria (Aarnink et al., 2011a). In a lab-scale experiment, Aarnink et al. (2011a) found that E. faecalis and Gumboro virus could be reduced by 100% when per-acetic acid was used as the circulation solution in a scrubber. The biological scrubbers are not consistent in reducing microorganisms (Seedorf and Hartung, 1999), probably because the microorganisms for digesting odorous compounds can also be emitted to the ambient air. The removal of total dust by biological scrubbers was found to be 22–96% (Seedorf and Hartung, 1999).
Combined techniques have been applied in practice: for instance, oil spraying combined with feed coating (Takai and Pedersen, 2000) and multi-stage air scrubbers (Zhao et al., 2011a). These techniques are more consistent and effective in reducing emissions of airborne microorganisms and dust, as well as other gaseous pollutants (Ogink and Bosma, 2007; Zhao et al., 2008). A disadvantage of combined scrubbing techniques is the relatively high energy use and the complexity for use on practical farms. Therefore, further research is needed to develop energy-saving and simple-to-operate combined scrubbing techniques.
7. CONCLUSIONS
The most important source of airborne microorganisms is the animals themselves by means of excretion. The sources of dust include excrement, litter, feed, skin, and feathers.
Airborne microorganisms in animal houses are mostly bacteria, with Gram-positive bacteria predominating; fungi account for only a small proportion of microorganisms.
Concentrations of both microorganisms and dust are high in animal houses. They are affected by animal type, housing system, management, and environmental factors. Because these factors play an interrelated role on the concentrations of microorganisms and dust, integrated research on the effects is required.
The microorganisms transmitted in the air suffer physical and biological decay. The physical decay largely depends on their size, and the biological decay is mainly determined by environmental factors, such as humidity, temperature, radiation, and toxic gases. In airborne transmission, microorganisms may be carried by dust particles that may protect microorganisms from biological decay. Knowledge of the role of dust in the transportation of microorganisms is still lacking, and needs to be expanded.
Microorganisms are deposited on different regions in the respiratory tract, mainly depending on their size. They have different preferred infection regions in the respiratory tract. The amount of microorganisms needed to induce an infection varies with microorganism species and animal species.
Reference methods for microorganism and dust sampling in animal houses need to be legislated. These methods should be resistant to highly microbial and dusty environments, and the efficiency of the sampling devices needs to be investigated.
Different techniques have been applied to reduce airborne microorganisms and dust in and from animal houses. Combining several abatement techniques may achieve higher and more consistent reduction. Energy-saving and simple-to-operate combined techniques are of interest for further development.
REFERENCES
- Aarnink A. J. A., Landman W.J. M., Melse R.W., Zhao Y., Ploegaert J.P. M., and Huynh T.T. T. (2011a). Scrubber capabilities to remove airborne microorganisms and other aerial pollutants from exhaust air of animal houses. Transactions of the ASABE, 54, 1921–1930. [Google Scholar]
- Aarnink A.J. A., Mosquera J., Winkel A., Cambra-Lopez M., Van Harn J., Buisonje F.E. D., Van Hattum T., and Ogink N.W. M. (2009). Options for dust reduction from poultry houses. Proc. CIGR International Symposium on ‘Agricultural Technologies in a Changing Climate’, Brisbane, Australia.
- Aarnink A.J. A., Roelofs R.F. M. M., Ellen H., and Gunnink H. (1999). Dust sources in animal houses . Proc. Proceedings on Dust Control in Animal Production Facilities, Horsens, Denmark.
- Aarnink A.J. A., van Harn J., van Hattum T.G., Zhao Y., and Ogink N.W. M. (2011b). Dust reduction in broiler houses by spraying rapeseed oil. Transactions of the ASABE, 54, 1479–1489. [Google Scholar]
- Abadie M., Limam K., and Allard F. (2001). Indoor particle pollution: effect of wall textures on particle deposition. Building and Environment, 36, 821–827. [Google Scholar]
- Adams D.J., Spendlove J.C., Spendlove R.S., and Barnett B.B. (1982). Aerosol stability of infectious and potentially infectious reovirus particles. Applied and Environmental Microbiology, 44, 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Homidan A., Robertson J.F., and Petchey A.M. (1997). Effect of temperature, litter and light intensity on ammonia and dust production and broiler performance. British Poultry Science, 38, S5–S17. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., and Donaldson A.I. (2002). Further studies to quantify the dose of natural aerosols of foot-and-mouth disease virus for pigs. Epidemiology and Infection, 128, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford R.H., Kasel J.A., Gerone P.J., and Knight V. (1966). Human influenza resulting from aerosol inhalation. Proceedings of the Society for Experimental Biology and Medicine, 122, 800–804. [DOI] [PubMed] [Google Scholar]
- Andersen A.A. (1958). New sampler for the collection, sizing, and enumeration of viable airborne particles. Journal of Bacteriology, 76, 471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A.M., Weiss N., Rainey F., and Salkinoja Salonen M.S. (1999). Dust borne bacteria in animal sheds, schools and children's day care centres. Journal of Applied Microbiology, 86, 622–634. [DOI] [PubMed] [Google Scholar]
- Appleby M.C., and Hughes B.O. (1991). Welfare of laying hens in cages and alternative systems: Environmental, physical and behavioural aspects. World's Poultry Science Journal, 47, 109–128. [Google Scholar]
- Attwood P., Brouwer R., Ruigewaard P., Versloot P., De Wit R., Heederik D., and Boleij J.S. M. (1987). A study of the relationship between airborne contaminants and environmental factors in dutch swine confinement buildings. American Industrial Hygiene Association Journal, 48, 745–751. [DOI] [PubMed] [Google Scholar]
- Baird-Parker A.C. (1962). The occurrence and enumeration, according to a new classification, of micrococci and staphylococci in bacon and on human and pig skin. Journal of Applied Microbiology, 25, 352–361. [Google Scholar]
- Bakutis B., Monstviliene E., and Januskeviciene G. (2004). Analyses of airborne contamination with bacteria, endotoxins and dust in livestock barns and poultry houses. Acta Veterinaria Brno, 73, 283–289. [Google Scholar]
- Baldocchi D.D., Hincks B.B., and Meyers T.P. (1988). Measuring biosphere-atmosphere exchanges of biologically related gases with micrometeorological methods. Ecology, 69, 1331–1340. [Google Scholar]
- Banhazi T.M., Seedorf J., Laffrique M., and Rutley D.L. (2008a). Identification of the risk factors for high airborne particle concentrations in broiler buildings using statistical modelling. Biosystems Engineering, 101, 100–110. [Google Scholar]
- Banhazi T.M., Seedorf J., Rutley D.L., and Pitchford W.S. (2008b). Identification of risk factors for sub-optimal housing conditions in Australian piggeries: Part 2. Airborne pollutants. Journal of Agricultural Safety and Health, 14, 21–39. [DOI] [PubMed] [Google Scholar]
- Barlow D.F., and Donaldson A.I. (1973). Comparison of the aerosol stabilities of foot-and-mouth disease virus suspended in cell culture fluid or natural fluids. Journal of General Virology, 20, 311–318. [DOI] [PubMed] [Google Scholar]
- Barron E.S. G. (1954). The role of free radicals and oxygen in reactions produced by ionizing radiations. Radiation Research, 1, 109–124. [PubMed] [Google Scholar]
- Baskerville A. (1981). Mechanisms of infection in the respiratory tract. New Zealand Veterinary Journal, 29, 235–238. [DOI] [PubMed] [Google Scholar]
- Benbough J.E. (1967). Death mechanisms in airborne Escherichia coli. Microbiology, 47, 325–333. [DOI] [PubMed] [Google Scholar]
- Benbough J.E. (1971). Some factors affecting the survival of airborne viruses. Journal of General Virology, 10, 209–220. [DOI] [PubMed] [Google Scholar]
- Berendt R.F., and Dorsey E.L. (1971). Effect of simulated solar radiation and sodium fluorescein on the recovery of Venezuelan equine encephalomyelitis virus from aerosols. Applied and Environmental Microbiology, 21, 447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot-Herault F., Gottschalk M., Labbe A., Cariolet R., and Kobisch M. (2001). Experimental airborne transmission of Streptococcus suis capsular type 2 in pigs. Veterinary Microbiology, 82, 69–80. [DOI] [PubMed] [Google Scholar]
- Besser T.E., Richards B.L., Rice D.H., and Hancock D.D. (2001). Escherichia coli O157:H7 infection of calves: Infectious dose and direct contact transmission. Epidemiology and Infection, 127, 555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankston G., Gitterman L., Hirji Z., Lemieux C., and Gardam M. (2007). Transmission of influenza A in human beings. Lancet Infectious Diseases, 7, 257–265. [DOI] [PubMed] [Google Scholar]
- Brockmeier S.L., and Lager K.M. (2002). Experimental airborne transmission of porcine reproductive and respiratory syndrome virus and Bordetella bronchiseptica. Veterinary Microbiology, 89, 267–275. [DOI] [PubMed] [Google Scholar]
- Brown J.L., Dominik J.W., and Larson E.W. (1982). Airborne survival of Rift Valley fever virus. Army Medical Research Institute of Infectious Diseases, Maryland, USA. [Google Scholar]
- Brown J.S., Zeman K.L., and Bennett W.D. (2002). Ultrafine particle deposition and clearance in the healthy and obstructed lung. American Journal of Respiratory and Critical Care Medicine, 166, 1240–1247. [DOI] [PubMed] [Google Scholar]
- Brown-Brandl T., Eigenberg R., and Nienaber J. (2010). Water spray cooling during handling of feedlot cattle. International Journal of Biometeorology, 54, 609–616. [DOI] [PubMed] [Google Scholar]
- Bull S.A., Allen V.M., Domingue G., Jorgensen F., Frost J.A., Ure R., Whyte R., Tinker D., Corry J.E. L., Gillard-King J., and Humphrey T.J. (2006). Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Applied and Environmental Microbiology, 72, 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy D.S. (1984). Rate of dust decay as affected by relative humidity, ionization and air movement. Transactions of the ASAE, 27, 865–870. [Google Scholar]
- Burton N.C., Grinshpun S.A., and Reponen T. (2007). Physical collection efficiency of filter materials for bacteria and viruses. Annals of Occupational Hygiene, 51, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafruny W.A., and Hovinen D.E. (1988). The relationship between route of infection and minimum infectious dose: Studies with lactate dehydrogenase-elevating virus. Journal of Virological Methods, 20, 265–268. [DOI] [PubMed] [Google Scholar]
- Cambra-Lopez M. (2010). Control of particulate matter emissions from poultry and pig houses. PhD, Universidad Politécnica de Valencia, Valencia, Spain. [Google Scholar]
- Cambra-Lopez M., Aarnink A.J. A., Zhao Y., Calvet S., and Torres A.G. (2010). Airborne particulate matter from livestock production systems: A review of an air pollution problem. Environmental Pollution, 158, 1–17. [DOI] [PubMed] [Google Scholar]
- Cambra-Lopez M., Winkel A., Van Harn J., Ogink N.W. M., and Aarnink A. (2009). Ionization for reducing particulate matter emissions from poultry houses. Transactions of the ASABE, 52, 1757–1771. [Google Scholar]
- Chang C.W., Chung H., Huang C.F., and Su H.J. J. (2001). Exposure of workers to airborne microorganisms in open-air swine houses. Applied and Environmental Microbiology, 67, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba L.I., Peo E.R., Jr., Lewis A.J., Brumm M.C., Fritschen R.D., and Crenshaw J.B. (1985). Effect of dietary fat on pig performance and dust levels in modified open-front and environmentally regulated confinement buildings. Journal of Animal Science, 61, 763–781. [DOI] [PubMed] [Google Scholar]
- Cho J.G., Dee S.A., Deen J., Trincado C., Fano E., Jiang Y., Faaberg K., Murtaugh M.P., Guedes A., and Collins J.E. (2006). The impact of animal age, bacterial coinfection, and isolate pathogenicity on the shedding of Porcine reproductive and respiratory syndrome virus in aerosols from experimentally infected pigs. Canadian Journal of Veterinary Research, 70, 297–301. [PMC free article] [PubMed] [Google Scholar]
- Clark P.C., and McQuitty J.B. (1988). Air quality in farrowing barns. Canadian Agricultural Engineering, 30, 173–178. [Google Scholar]
- Clark S., Rylander R., and Larsson L. (1983). Airborne bacteria, endotoxin and fungi in dust in poultry and swine confinement buildings. American Industrial Hygiene Association Journal, 44, 537–541. [DOI] [PubMed] [Google Scholar]
- Corbanie E.A., Matthijs M.G., van Eck J.H., Remon J.P., Landman W.J., and Vervaet C. (2006). Deposition of differently sized airborne microspheres in the respiratory tract of chickens. Avian Pathology, 35, 475–485. [DOI] [PubMed] [Google Scholar]
- Cormier Y., Tremblay G., Meriaux A., Brochu G., and Lavoie J. (1990). Airborne microbial contents in two types of swine confinement buildings in Quebec. American Industrial Hygiene Association Journal, 51, 304–309. [DOI] [PubMed] [Google Scholar]
- Cornick N.A., and Helgerson A.F. (2004). Transmission and infectious dose of Escherichia coli O157:H7 in swine. Applied and Environmental Microbiology, 70, 5331–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.S. (1970). Aerosol survival of Escherichia coli B disseminated from the dry state. Applied and Environmental Microbiology, 19, 604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.S. (1971). Aerosol survival of Pasteurella tularensis disseminated from the wet and dry states. Applied and Environmental Microbiology, 21, 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.S. (1989). Airborne bacteria and viruses. Science Progress, 73, 469–499. [PubMed] [Google Scholar]
- Cox C.S., and Goldberg L.J. (1972). Aerosol survival of Pasteurella tularensis and the influence of relative humidity. Applied and Environmental Microbiology, 23, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cray W.C., and Moon H.W. (1995). Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Applied and Environmental Microbiology, 61, 1586–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S.E., Drummond J.G., Grunloh D.J., Lynch P.B., and Jensen A.H. (1975). Relative and qualitative aspects of aerial bacteria and dust in swine houses. Journal of Animal Science, 41, 1512–1520. [DOI] [PubMed] [Google Scholar]
- Dawson J.R. (1990). Minimizing dust in livestock buildings: Possible alternatives to mechanical separation. Journal of Agricultural Engineering Research, 47, 235–248. [Google Scholar]
- de Deus N., Seminati C., Pina S., Mateu E., Martín M., and Segales J. (2007). Detection of hepatitis E virus in liver, mesenteric lymph node, serum, bile and faeces of naturally infected pigs affected by different pathological conditions. Veterinary Microbiology, 119, 105–114. [DOI] [PubMed] [Google Scholar]
- de Jong J.C., Harmsen M., and Trouwborst T. (1975). Factors in the inactivation of Encephalomyocarditis virus in aerosols. Infection and Immunity, 12, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mik G., and de Groot I. (1977). Mechanisms of inactivation of Bacteriophage phiX174 and its DNA in aerosols by ozone and ozonized cyclohexene. The Journal of Hygiene, 78, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mik G., de Groot I., and Gerbrandy J.L. F. (1977). Survival of aerosolized bacteriophage X174 in air containing ozone–olefin mixtures. Epidemiology and Infection, 78, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reu K., Grijspeerdt K., Heyndrickx M., Zoons J., de Baere K., Uyttendaele M., Debevere J., and Herman L. (2005). Bacterial eggshell contamination in conventional cages, furnished cages and aviary housing systems for laying hens. British Poultry Science, 46, 149–155. [DOI] [PubMed] [Google Scholar]
- de Reu K., Messens W., Heyndrickx M., Rodenburg T.B., Uyttendaele M., and Herman L. (2008). Bacterial contamination of table eggs and the influence of housing systems. World's Poultry Science Journal, 64, 5–19. [Google Scholar]
- de Rezende C.L., Mallinson E.T., Tablante N.L., Morales R., Park A., Carr L.E., and Joseph S.W. (2001). Effect of dry litter and airflow in reducing Salmonella and Escherichia coli populations in the broiler production environment. Journal of Applied Poultry Research, 10, 245–251. [Google Scholar]
- Dennis P.J., and Lee J.V. (1988). Differences in aerosol survival between pathogenic and non-pathogenic strains of Legionella pneumophila serogroup 1. Journal of Applied Microbiology, 65, 135–141. [DOI] [PubMed] [Google Scholar]
- Derikx P.J. L., Willers H.C., and ten Have P.J. W. (1994). Effect of pH on the behaviour of volatile compounds in organic manures during dry-matter determination. Bioresource Technology, 49, 41–45. [Google Scholar]
- Deshpande B.K., Frey H.C., Cao Y., and Liu X. (2009). Modeling of the penetration of ambient PM2.5 to indoor residential microenvironment. Proc. 102nd Annual Conference and Exhibition, Air & Waste Management Association, Detroit, USA.
- Donaldson A.I. (1972). The influence of relative humidity on the aerosol stability of different strains of foot-and-mouth disease virus suspended in saliva. Journal of General Virology, 15, 25–33. [DOI] [PubMed] [Google Scholar]
- Donaldson A.I., Gibson C.F., Oliver R., Hamblin C., and Kitching R.P. (1987). Infection of cattle by airborne foot-and-mouth disease virus: Minimal doses with O1 and SAT 2 strains. Research in Veterinary Science, 43, 339–346. [PubMed] [Google Scholar]
- Donaldson A.I., Sellers R.F., and Lacey J. (1983). Quantitative data on airborne foot-and-mouth disease virus: Its production, carriage and deposition [and discussion]. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 302, 529–534. [Google Scholar]
- Donham K.J., and Gustafson K.E. (1982). Human occupational hazards from swine confinement. Annals of the American Conference of Governmental Industrial Hygienists, 2, 137–142. [Google Scholar]
- Donham K.J., Scallon L.J., Popendorf W., Treuhaft M.W., and Roberts R.C. (1986). Characterization of dusts collected from swine confinement buildings. American Industrial Hygiene Association Journal, 47, 404–410. [DOI] [PubMed] [Google Scholar]
- Donkoh A. (1989). Ambient temperature: A factor affecting performance and physiological response of broiler chickens. International Journal of Biometeorology, 33, 259–265. [DOI] [PubMed] [Google Scholar]
- Doyle M.P. (1984). Association of Campylobacter jejuni with laying hens and eggs. Applied and Environmental Microbiology, 47, 533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M.P., and Roman D.J. (1982). Prevalence and survival of Campylobacter jejuni in unpasteurized milk. Applied and Environmental Microbiology, 44, 1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druett H.A., Henderson D.W., Packman L., and Peacock S. (1953). Studies on respiratory infection. I. The influence of particle size on respiratory infection with anthrax spores. The Journal of Hygiene, 51, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druett H.A., and Packman L.P. (1972). The germicidal properties of ozone—olefin mixtures. Journal of Applied Microbiology, 35, 323–329. [DOI] [PubMed] [Google Scholar]
- Druett H.A., Robinson J.M., Henderson D.W., Packman L., and Peacock S. (1956). Studies on respiratory infection. II. The influence of aerosol particle size on infection of the guinea-pig with Pasteurella pestis. The Journal of Hygiene, 54, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E.J., and Akers T.G. (1970). Airborne stability of tailless bacterial viruses S-13 and MS-2. Applied and Environmental Microbiology, 19, 624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine C., Grimard Y., and Cormier Y. (2000). Influence of building maintenance, environmental factors, and seasons on airborne contaminants of swine confinement buildings. American Industrial Hygiene Association Journal, 61, 56–63. [PubMed] [Google Scholar]
- Edwards D.A., Man J.C., Brand P., Katstra J.P., Sommerer K., Stone H.A., Nardell E., and Scheuch G. (2004). Inhaling to mitigate exhaled bioaerosols. Proceedings of the National Academy of Sciences of the United States of America, 101, 17383–17388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich R., Miller S., and Walker R.L. (1970a). Effects of atmospheric humidity and temperature on the survival of airborne Flavobacterium. Applied Microbiology, 20, 884–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich R., Miller S., and Walker R.L. (1970b). Relationship between atmospheric temperature and survival of airborne bacteria. Applied and Environmental Microbiology, 19, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazhary M.A., and Derbyshire J.B. (1979). Aerosol stability of bovine parainfluenza type 3 virus. Canadian Journal of Comparative Medicine, 43, 295–304. [PMC free article] [PubMed] [Google Scholar]
- Elbers A.R. W., Stegeman J.A., and De Jong M.C. M. (2001). Factors associated with the introduction of classical swine fever virus into pig herds in the central area of the 1997/98 epidemic in the Netherlands. The Veterinary record, 149, 377–382. [DOI] [PubMed] [Google Scholar]
- Elford W.J., and van den Ende J. (1942). An investigation of the merits of ozone as an aerial disinfectant. Epidemiology and Infection, 42, 240–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart S., Glasmacher A., Simon A., and Exner M. (2007). Air sampling of Aspergillus fumigatus and other thermotolerant fungi: Comparative performance of the Sartorius MD8 airport and the Merck MAS-100 portable bioaerosol sampler. International Journal of Hygiene and Environmental Health, 210, 733–739. [DOI] [PubMed] [Google Scholar]
- European Commission (1998). Air quality: Determination of the PM10 fraction of suspended particulate matter: Reference method and field test procedure to demonstrate reference equivalence of measurement methods. European Committee for Standardization, Brussels. Belgium [Google Scholar]
- European Commission (1999). Relating to limit values for sulphur dioxide, nitrogen dioxide and oxides of nitrogen, particulate matter and lead in ambient air (The first daughter directive). Council Directive 1999/30/EC. Official Journal of the European Communities, L163/41. [Google Scholar]
- European Commission (2005). Ambient air quality-Standard gravimetric measurement method for the determination of the PM2.5 mass fraction of suspended particulate matter. European Committee for Standardization, Brussels, Belgium. [Google Scholar]
- European Commission (2008). The European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe. [Google Scholar]
- Fabian P., McDevitt J.J., DeHaan W.H., Fung R.O. P., Cowling B.J., Chan K.H., Leung G.M., and Milton D.K. (2008). Influenza virus in human exhaled breath: an observational study. PLoS One, 3, e2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Song J., Hildebrand P.D., and Forney C.F. (2002). Interaction of ozone and negative air ions to control microorganisms. Journal of Applied Microbiology, 93, 144–148. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A. M., Osterhaus A.D. M. E., and Brown I.H. (2003). Animal influenza virus surveillance. Vaccine, 21, 1754–1757. [DOI] [PubMed] [Google Scholar]
- Gailiunas P., and Cottral G.E. (1966). Presence and persistence of foot-and-mouth disease virus in bovine skin. Journal of Bacteriology, 91, 2333–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast R.K., Mitchell B.W., and Holt P.S. (1999). Application of negative air ionization for reducing experimental airborne transmission of Salmonella enteritidis to chicks. Poultry Science, 78, 57–61. [DOI] [PubMed] [Google Scholar]
- Gibson C.F., and Donaldson A.I. (1986). Exposure of sheep to natural aerosols of foot-and-mouth disease virus. Research in Veterinary Science, 41, 45–49. [PubMed] [Google Scholar]
- Giese A.C., and Christensen E. (1954). Effects of ozone on organisms. Physiological Zoology, 27, 101–115. [Google Scholar]
- Giese N., and Darby J. (2000). Sensitivity of microorganisms to different wavelengths of UV light: implications on modeling of medium pressure UV systems. Water Research, 34, 4007–4013. [Google Scholar]
- Gloster J., Champion H.J., Sorensen J.H., Mikkelsen T., Ryall D.B., Astrup P., Alexandersen S., and Donaldson A.I. (2003). Airborne transmission of foot-and-mouth disease virus from Burnside Farm, Heddonon-the-Wall, Northumberland, during the 2001 epidemic in the United Kingdom. Veterinary Record, 152, 525–533. [DOI] [PubMed] [Google Scholar]
- Gloster J., Williams P., Doel C., Esteves I., Coe H., and Valarcher J. (2007). Foot-and-mouth disease: Quantification and size distribution of airborne particles emitted by healthy and infected pigs. The Veterinary Journal, 174, 42–53. [DOI] [PubMed] [Google Scholar]
- Gore A.M., Kornegay E.T., and Veit H.P. (1986). The effects of soybean oil on nursery air quality and performance of weanling pigs. Journal of Animal Science, 63, 1–7.3733583 [Google Scholar]
- Graham D.Y., Dufour G.R., and Estes M.K. (1987). Minimal infective dose of rotavirus. Archives of virology, 92, 261–271. [DOI] [PubMed] [Google Scholar]
- Gray J.T., and Fedorka-Cray P.J. (2001). Survival and infectivity of Salmonella choleraesuis in swine feces. Journal of Food Protection, 64, 945–949. [DOI] [PubMed] [Google Scholar]
- Griffin D., Gonzalez C., Teigell N., Petrosky T., Northup D., and Lyles M. (2011). Observations on the use of membrane filtration and liquid impingement to collect airborne microorganisms in various atmospheric environments. Aerobiologia, 27, 25–35. [Google Scholar]
- Griffiths W.D., and Stewart I.W. (1999). Performance of bioaerosol samplers used by the UK biotechnology industry. Journal of Aerosol Science, 30, 1029–1040. [Google Scholar]
- Grinshpun S.A., Chang C.W., Nevalainen A., and Willeke K. (1994). Inlet characteristics of bioaerosol samplers. Journal of Aerosol Science, 25, 1503–1522. [Google Scholar]
- Guarino M., Caroli A., and Navarotto P. (1999). Dust concentration and mortality distribution in an enclosed laying house. Transactions of the ASAE, 42, 1127–1133. [Google Scholar]
- Haeussermann A., Gotz M., and Hartung E. (2007). Particulate emissions from deep-bedded growing-finishing pigs . Proc. Dustconf 2007—How to improve air quality, Maastricht, the Netherlands.
- Handley B.A., and Webster A.J. F. (1993). Some factors affecting airborne survival of Pseudomonas fluorescens indoors. Journal of Applied Microbiology, 75, 35–42. [DOI] [PubMed] [Google Scholar]
- Harper G.J. (1961). Airborne micro-organisms: Survival tests with four viruses. The Journal of Hygiene, 59, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper G.J., and Morton J.D. (1953). The respiratory retention of bacterial aerosols: experiments with radioactive spores. The Journal of Hygiene, 51, 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G.D., Adams V.D., Sorensen D.L., and Curtis M.S. (1987). Ultraviolet inactivation of selected bacteria and viruses with photoreactivation of the bacteria. Water Research, 21, 687–692. [Google Scholar]
- Hartung J. (1992). Emissions of airborne substances from stalls of domestic animals. Pneumologie, 46, 196–202. [PubMed] [Google Scholar]
- Hatch M.T., and Warren J.C. (1969). Enhanced recovery of airborne T3 coliphage and Pasteurella pestis bacteriophage by means of a presampling humidification technique. Applied and Environmental Microbiology, 17, 685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F.G., Treanor J.J., Betts R.F., Lobo M., Esinhart J.D., and Hussey E.K. (1996). Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA, 275, 295–299. [PubMed] [Google Scholar]
- Hayter R.B., and Besch E.L. (1974). Airborne-particle deposition in the respiratory tract of chickens. Poultry Science, 53, 1507–1511. [DOI] [PubMed] [Google Scholar]
- He C., Morawska L., and Gilbert D. (2005). Particle deposition rates in residential houses. Atmospheric Environment, 39, 3891–3899. [Google Scholar]
- Heber A.J., Lim T., Ni J.Q., Tao P., Schmidt A.M., Koziel J.A., Hoff S.J., Jacobson L.D., Zhang Y.H., and Baughman G.B. (2006). Quality-assured measurements of animal building emissions : Particulate matter concentrations. Journal of the Air & Waste Management Association, 56, 1642–1648. [DOI] [PubMed] [Google Scholar]
- Heber A.J., Stroik M., Faubion J.M., and Willard L.H. (1988a). Size distribution and identification of aerial dust particles in swine finishing buildings. Transactions of the ASAE, 31, 882–887. [Google Scholar]
- Heber A.J., Stroik M., Nelssen J.L., and Nichols D.A. (1988b). Influence of environmental factors on concentrations and inorganic content of aerial dust in swine finishing buildings. Transactions of the ASAE, 31, 875–881. [Google Scholar]
- Heindel T.H., Streib R., and Botzenhart K. (1993). Effect of ozone on airborne microorganisms. International Journal of Hygiene and Environmental Health, 194, 464–480. [PubMed] [Google Scholar]
- Henningson E., Roffey R., and Bovallius A. (1982). A comparative study of apparatus for sampling airborne microorganisms. Grana, 20, 155–159. [Google Scholar]
- Hermann J.R., Brockmeier S.L., Yoon K.J., and Zimmerman J.J. (2008). Detection of respiratory pathogens in air samples from acutely infected pigs. Canadian Journal of Veterinary Research, 72, 367–370. [PMC free article] [PubMed] [Google Scholar]
- Hermann J.R., Hoff S., Munoz-Zanzi C., Yoon K.J., Burkhardt A.C., and Zimmerman J.J. (2007). Effect of temperature and relative humidity on the stability of infectious porcine reproductive and respiratory syndrome virus in aerosols. Veterinary Research, 38, 81–93. [DOI] [PubMed] [Google Scholar]
- Hess G.E. (1965). Effects of oxygen on aerosolized Serratia marcescens. Applied and Environmental Microbiology, 13, 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyder J. (2004). Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. The Proceedings of the American Thoracic Society, 1, 315–320. [DOI] [PubMed] [Google Scholar]
- Heyder J., Gebhart J., Rudolf G., Schiller C.F., and Stahlofen W. (1986). Deposition of particles in the human respiratory tract in the size range 0, 005–15 μm. Journal of aerosol science, 17, 811–825. [Google Scholar]
- Hijnen W.A. M., Beerendonk E.F., and Medema G.J. (2006). Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: A review. Water Research, 40, 3–22. [DOI] [PubMed] [Google Scholar]
- Himathongkham S., Bahari S., Riemann H., and Cliver D. (1999). Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiology Letters, 178, 251–257. [DOI] [PubMed] [Google Scholar]
- Hinz T., and Linke S. (1998). A comprehensive experimental study of aerial pollutants in and emissions from livestock buildings. Part 2: Results. Journal of Agricultural Engineering Research, 70, 119–129. [Google Scholar]
- Hofacre C.L., White D.G., Maurer J.J., Morales C., Lobsinger C., and Hudson C. (2001). Characterization of antibiotic-resistant bacteria in rendered animal products. Avian Diseases, 45, 953–961. [PubMed] [Google Scholar]
- Hofschreuder P., Aarnink A.J. A., Zhao Y., and Ogink N.W. M. (2007). Measurement protocol for determining fine dust emission factors of animal housing systems. Proc. Proceedings of Dustconf 2007, How to Improve Air Quality, Maastricht, the Netherlands.
- Holt P.S. (1993). Effect of induced molting on the susceptibility of white leghorn hens to a Salmonella enteritidis infection. Avian Diseases, 37, 412–417. [PubMed] [Google Scholar]
- Holt P.S., Mitchell B.W., Seo K.H., and Gast R.K. (1999). Use of negative air ionization for reducing airborne levels of Salmonella Enterica serovar enteritidis in a room containing infected caged layers. Journal of Applied Poultry Research, 8, 440–446. [Google Scholar]
- Ijaz M.K., Brunner A.H., Sattar S.A., Nair R.C., and Johnson-Lussenburg C.M. (1985a). Survival characteristics of airborne human Coronavirus 229E. Journal of General Virology, 66, 2743–2748. [DOI] [PubMed] [Google Scholar]
- Ijaz M.K., Sattar S.A., Alkarmi T., Dar F.K., Bhatti A.R., and Elhag K.M. (1994). Studies on the survival of aerosolized bovine rotavirus (UK) and a murine rotavirus. Comparative Immunology, Microbiology and Infectious Diseases, 17, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz M.K., Sattar S.A., Johnson-Lussenburg C.M., and Springthorpe V.S. (1985b). Comparison of the airborne survival of calf rotavirus and poliovirus type 1 (Sabin) aerosolized as a mixture. Applied and Environmental Microbiology, 49, 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz M.K., Sattar S.A., Johnson-Lussenburg C.M., Springthorpe V.S., and Nair R.C. (1985c). Effect of relative humidity, atmospheric temperature, and suspending medium on the airborne survival of human Rotavirus. Canadian Journal of Microbiology, 31, 681–685. [DOI] [PubMed] [Google Scholar]
- Ingram M., and Haines R.B. (1949). Inhibition of bacterial growth by pure ozone in the presence of nutrients. Journal of Hygiene, 47, 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A.C., Stahlhofen W., Rudolf G., Egan M.J., Nixon W., Gehr P., and Briant J.K. (1991). The respiratory tract deposition model proposed by the ICRP Task Group. Radiation Protection Dosimetry, 38, 159–165. [Google Scholar]
- Janni K.A., and Redig P.T. (1986). Factors affecting the occurrence of aspergillosis in turkeys. ASAE Paper No. 86-4533, ASAE, St. Joseph, Michigan . [Google Scholar]
- Jones C.D., and Bull J.R. (1987). Deposition and clearance of radiolabelled monodisperse polystyrene spheres in the calf lung. Research in Veterinary Science, 42, 82–91. [PubMed] [Google Scholar]
- Karim Y.G., Ijaz M.K., Sattar S.A., and Johnson-Lussenburg C.M. (1985). Effect of relative humidity on the airborne survival of Rhinovirus-14. Canadian Journal of Microbiology, 31, 1058–1061. [DOI] [PubMed] [Google Scholar]
- Katz I.M., Schroeter J.D., and Martonen T.B. (2001). Factors affecting the deposition of aerosolized insulin. Diabetes Technology & Therapeutics, 3, 387–397. [DOI] [PubMed] [Google Scholar]
- Kenny L.C., Gussman R., and Meyer M. (2000). Development of a sharp-cut cyclone for ambient aerosol monitoring applications. Aerosol Science and Technology, 32, 338–358. [Google Scholar]
- Kesavan J.S., and Schepers D.R. (2006). Characteristics and sampling efficiencies of OMNI 3000 aerosol samplers. Edgewood Chemical Biological Center, Maryland. [Google Scholar]
- Keyser M., Műller I.A., Cilliers F.P., Nel W., and Gouws P.A. (2008). Ultraviolet radiation as a non-thermal treatment for the inactivation of microorganisms in fruit juice. Innovative Food Science & Emerging Technologies, 9, 348–354. [Google Scholar]
- Kim J.G., Yousef A.E., and Dave S. (1999). Application of ozone for enhancing the microbiological safety and quality of foods: A review. Journal of Food Protection, 62, 1071–1087. [DOI] [PubMed] [Google Scholar]
- Kim K., Ko H.J., Choi H.L., Kim H.T., Kim Y.S., Roh Y.M., Lee C.M., and Kim C.N. (2007a). Farmer and pig exposure to aerial contaminants in a pig confinement building. Transactions of the ASABE, 50, 993–998. [Google Scholar]
- Kim K.Y., Ko H.J., Kim H.T., and Kim C.N. (2006). Effect of spraying biological additives for reduction of dust and bioaerosol in a confinement swine house. Annals of Agricultural and Environmental Medicine, 13, 133–138. [PubMed] [Google Scholar]
- Kim K.Y., Ko H.J., Kim H.T., Kim C.N., and Kim Y.S. (2008). Assessment of airborne bacteria and fungi in pig buildings in Korea. Biosystems Engineering, 99, 565–572. [Google Scholar]
- Kim K.Y., Ko H.J., Kim H.T., Kim Y.S., Roh Y.M., and Kim C.N. (2007b). Effect of ventilation rate on gradient of aerial contaminants in the confinement pig building. Environmental Research, 103, 352–357. [DOI] [PubMed] [Google Scholar]
- Kincaid D.C., and Longley T.S. (1989). A water droplet evaporation and temperature model. Transactions of the ASAE, 32, 457–463. [Google Scholar]
- Kloos W.E., Zimmerman R.J., and Smith R.F. (1976). Preliminary studies on the characterization and distribution of Staphylococcus and Micrococcus species on animal skin. Applied and Environmental Microbiology, 31, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kournikakis B., Netolitzky D., and Fildes J. (1988). Effects of temperature and relative humidity on the survival of Newcastle disease virus aerosols in the rotating drum. Defence Research and Development Canada, Alberta, Canada. [Google Scholar]
- Kowalski W.J., Bahnfleth W.P., and Whittam T.S. (1998). Bactericidal effects of high airborne ozone concentrations on Escherichia coli and Staphylococcus aureus. Ozone: Science & Engineering, 20, 205–221. [Google Scholar]
- Kuehn T.H. (1988). Predicting air flow patterns and particle contamination in clean rooms. Journal of Aerosol Science, 19, 1405–1408. [Google Scholar]
- Lai A.C. K. (2002). Particle deposition indoors: A review. Indoor Air, 12, 211–214. [DOI] [PubMed] [Google Scholar]
- Lai H.T. L., Aarnink A.J. A., Cambra-Lopez M., Huynh T.T. T., Parmentier H.K., and Groot Koerkamp P.W. G. (2010). Size distribution of airborne particles in animal houses. Biosystems Engineering. [Google Scholar]
- Langer D., and Holcombe J.A. (1999). Thermophoretic collection and analysis of submicrometer Ag particles emitted from a graphite tube-type electrothermal vaporizer. Analytical Chemistry, 71, 582–588. [DOI] [PubMed] [Google Scholar]
- Larson E.W., Dominik J.W., and Slone T.W. (1980). Aerosol stability and respiratory infectivity of Japanese B encephalitis virus. Infection and Immunity, 30, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.A., Adhikari A., Grinshpun S.A., McKay R., Shukla R., and Reponen T. (2006). Personal exposure to airborne dust and microorganisms in agricultural environments. Journal of Occupational and Environmental Hygiene, 3, 118–130. [DOI] [PubMed] [Google Scholar]
- Lennon J.T., and Jones S.E. (2011). Microbial seed banks: The ecological and evolutionary implications of dormancy. Nature Reviews Microbiology, 9, 119–130. [DOI] [PubMed] [Google Scholar]
- Letellier A., Messier S., Paré J., Ménard J., and Quessy S. (1999). Distribution of Salmonella in swine herds in Québec. Veterinary Microbiology, 67, 299–306. [DOI] [PubMed] [Google Scholar]
- Li C.S., Hao M.L., Lin W.H., Chang C.W., and Wang C.S. (1999). Evaluation of microbial samplers for bacterial microorganisms. Aerosol Science and Technology, 30, 100–108. [Google Scholar]
- Lighthart B. (1973). Survival of airborne bacteria in a high urban concentration of carbon monoxide. Applied and Environmental Microbiology, 25, 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthart B., and Frisch A.S. (1976). Estimation of viable airborne microbes downwind from a point source. Applied and Environmental Microbiology, 31, 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X.J., Willeke K., Ulevicius V., and Grinshpun S.A. (1997). Effect of sampling time on the collection efficiency of all-glass impingers. American Industrial Hygiene Association Journal, 58, 480–488. [Google Scholar]
- Lippmann M., Yeates D.B., and Albert R.E. (1980). Deposition, retention, and clearance of inhaled particles. British Medical Journal, 37, 337–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.Y. H., and Pui D.Y. H. (1981). Aerosol sampling inlets and inhalable particles. Atmospheric Environment, 15, 589–600. [Google Scholar]
- Locey K.J. (2010). Synthesizing traditional biogeography with microbial ecology: The importance of dormancy. Journal of Biogeography, 37, 1835–1841. [Google Scholar]
- Loynachan A.T., and Harris D.L. (2005). Dose determination for acute Salmonella infection in pigs. Applied and Environmental Microbiology, 71, 2753–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Sanchez S., Hofacre C., Maurer J.J., Harmon B.G., and Lee M.D. (2003). Evaluation of broiler litter with reference to the microbial composition as assessed by using 16S rRNA and functional gene markers. Applied and Environmental Microbiology, 69, 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngtveit T., and Eduard W. (1997). Reduction of dust exposure by negative air ionisation. Annals of Agricultural and Environmental Medicine, 4, 229–232. [Google Scholar]
- Maciorowski K.G., Herrera P., Jones F.T., Pillai S.D., and Ricke S.C. (2007). Effects on poultry and livestock of feed contamination with bacteria and fungi. Animal Feed Science and Technology, 133, 109–136. [Google Scholar]
- Madelin T.M., and Wathes C.M. (1989). Air hygiene in a broiler house: Comparison of deep litter with raised netting floors. British Poultry Science, 30, 23–37. [DOI] [PubMed] [Google Scholar]
- Martin S.A., McCann M.A., and Waltman W.D. (1998). Microbiological survey of Georgia poultry litter. Journal of Applied Poultry Research, 7, 90–98. [Google Scholar]
- Martin W.T., Zhang Y.H., Willson P., Archer T.P., Kinahan C., and Barber E.M. (1996). Bacterial and fungal flora of dust deposits in a pig building. Occupational and Environmental Medicine, 53, 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkovic K., Vucemilo M., Vinkovic B., Seol B., Pavicic Z., and Matkovic S. (2007). Qualitative structure of airborne bacteria and fungi in dairy barn and nearby environment. Czech Journal of Animal Science, 52, 249–254. [Google Scholar]
- Maus R., Goppelsröder A., and Umhauer H. (2001). Survival of bacterial and mold spores in air filter media. Atmospheric Environment, 35, 105–113. [Google Scholar]
- Mayhew C.J., and Hahon N. (1970). Assessment of aerosol mixtures of different viruses. Applied and Environmental Microbiology, 20, 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J., and McMeekin T.A. (1981). Effect of solar radiation and predacious microorganisms on survival of fecal and other bacteria. Applied and Environmental Microbiology, 41, 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough N.B., and Eisele C.W. (1951). Experimental human salmonellosis: III. Pathogenicity of strains of Salmonella newport, Salmonella derby, and Salmonella bareilly obtained from spray-dried whole egg. The Journal of Infectious Diseases, 89, 209–213. [DOI] [PubMed] [Google Scholar]
- McGee P., Bolton D.J., Sheridan J.J., Earley B., and Leonard N. (2001). The survival of Escherichia coli O157:H7 in slurry from cattle fed different diets. Letters in Applied Microbiology, 32, 152–155. [DOI] [PubMed] [Google Scholar]
- McKay A.M. (1992). Viable but non-culturable forms of potentially pathogenic bacteria in water. Letters in Applied Microbiology, 14, 129–135. [Google Scholar]
- Mikkelsen T., Alexandersen S., Astrup P., Champion H.J., Donaldson A.I., Dunkerley F.N., Gloster J., S rensen J.H., and Thykier-Nielsen S. (2003). Investigation of airborne foot-and-mouth disease virus transmission during low-wind conditions in the early phase of the UK 2001 epidemic. Atmospheric Chemistry and Physics Discussions, 3, 2101–2110. [Google Scholar]
- Milling A., Kehr R., Wulf A., and Smalla K. (2005). Survival of bacteria on wood and plastic particles: Dependence on wood species and environmental conditions. Holzforschung, 59, 72–81. [Google Scholar]
- Milne A., Theodorou M.K., Jordan M.G. C., King-Spooner C., and Trinci A.P. J. (1989). Survival of anaerobic fungi in feces, in saliva, and in pure culture. Experimental Mycology, 13, 27–37. [Google Scholar]
- Mitchell B.W., Holt P.S., and Seo K.H. (2000). Reducing dust in a caged layer room: an electrostatic space charge system. Journal of Applied Poultry Research, 9, 292–296. [Google Scholar]
- Mitchell B.W., Richardson L.J., Wilson J.L., and Hofacre C.L. (2004). Application of an electrostatic space charge system for dust, ammonia, and pathogen reduction in a broiler breeder house. Applied Engineering in Agriculture, 20, 87–93. [Google Scholar]
- Moe K., and Harper G.J. (1983). The effect of relative humidity and temperature on the survival of bovine rotavirus in aerosol. Archives of Virology, 76, 211–216. [DOI] [PubMed] [Google Scholar]
- Muller W., and Wieser P. (1987). Dust and microbial emissions from animal production. In Strauch D. (Ed.), Animal production and environmental health (pp. 47–89). Elsevier, Amsterdam, the Netherlands. [Google Scholar]
- Nevalainen A., Pastuzska J., Liebhaber F., and Willeke K. (1992). Performance of bioaerosol samplers: collection characteristics and sampler design considerations. Atmospheric Environment, 26A, 531–540. [Google Scholar]
- Newell D.G., and Fearnley C. (2003). Sources of Campylobacter colonization in broiler chickens. Applied and Environmental Microbiology, 69, 4343–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicks B., Canart B., and Vandenheede M. (1993). Temperature, air humidity and air pollution levels in farrowing or weaner pig houses. Pig News and Information, 14, 77N–78N. [Google Scholar]
- Nishiguchi A., Kobayashi S., Yamamoto T., Ouchi Y., Sugizaki T., and Tsutsui T. (2007). Risk factors for the introduction of avian influenza virus into commercial layer chicken farms during the outbreaks caused by a low-pathogenic H5N2 virus in Japan in 2005. Zoonoses and Public Health, 54, 337–343. [DOI] [PubMed] [Google Scholar]
- Ogink N.W. M., and Bosma B.J. J. (2007). Multi-phase air scrubbers for the combined abatement of ammonia, odor and particulate matter emissions . International Symposium on Air Quality and Waste Management for Agriculture, Broomfield, Colorado.
- Oliver J.D. (2010). Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiology Reviews, 34, 415–425. [DOI] [PubMed] [Google Scholar]
- Omisakin F., MacRae M., Ogden I.D., and Strachan N.J. C. (2003). Concentration and prevalence of Escherichia coli O157 in cattle feces at slaughter. Applied and Environmental Microbiology, 69, 2444–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake S., Dee S., Corzo C., Oliveira S., and Deen J. (2010). Long distance airborne transport of infectious PRRSV and Mycoplasma hyopneumoniae from a swine population infected with multiple viral variants. Veterinary Microbiology, 145, 198–208. [DOI] [PubMed] [Google Scholar]
- Ouellette C.A., Feddes J.J. R., Wenger I.I., and Barber E.M. (1999). A portable environmental monitoring system to assess barn worker indoor air exposure. Journal of Agricultural Safety and Health, 5, 383–394. [DOI] [PubMed] [Google Scholar]
- Pal A., Pehkonen S.O., Yu L.E., and Ray M.B. (2007). Photocatalytic inactivation of Gram-positive and Gram-negative bacteria using fluorescent light. Journal of Photochemistry and Photobiology A: Chemistry, 186, 335–341. [Google Scholar]
- Pearson C.C., and Sharples T.J. (1995). Airborne dust concentrations in livestock buildings and the effect of feed. Journal of Agricultural Engineering Research, 60, 145–154. [Google Scholar]
- Pedersen S., and Pedersen C.B. (1995). Animal activity measured by infrared detectors. Journal of Agricultural Engineering Research, 61, 239–246. [Google Scholar]
- Pell A.N. (1997). Manure and microbes: Public and animal health problem? Journal of Dairy Science, 80, 2673–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G., Harris G.J., and Jones M.W. (1964). Effect of air ions on bacterial aerosols. International Journal of Biometeorology, 8, 27–37. [DOI] [PubMed] [Google Scholar]
- Phillips P.A. (1986). Dust levels in mechanically versus naturally ventilated hog barns. ASAE Paper No. 86-4041, ASAE, St. Joseph, Michigan. [Google Scholar]
- Phillips V.R., Holden M.R., Sneath R.W., Short J.L., White R.P., Hartung J., Seedorf J., Schroder M., Linkert K.H., Pedersen S., Takai H., Johnsen J.O., Koerkamp P., Uenk G.H., Scholtens R., Metz J.H. M., and Wathes C.M. (1998). The development of robust methods for measuring concentrations and emission rates of gaseous and particulate air pollutants in livestock buildings. Journal of Agricultural Engineering Research, 70, 11–24. [Google Scholar]
- Postollec F., Falentin H., Pavan S., Combrisson J., and Sohier D. (2011). Recent advances in quantitative PCR (qPCR) applications in food microbiology. Food Microbiology, 28, 848–861. [DOI] [PubMed] [Google Scholar]
- Predicala B.Z., Maghirang R.G., Jerez S.B., Urban J.E., and Goodband R.D. (2001). Dust and bioaerosol concentrations in two swine-finishing buildings in Kansas. Transactions of the ASAE, 44, 1291–1298. [Google Scholar]
- Prescott L.M., Harley J.P., and Klein D.A. (2005). Microbiology (6th ed.). McGraw-Hill, New York. [Google Scholar]
- Quarles C.L., Gentry R.F., and Bressler G.O. (1970). Bacterial contamination in poultry houses and its relationship to egg hatchability. Poultry Science, 49, 60–66. [Google Scholar]
- Rabey F., Janssen R.J., and Kelley L.M. (1969). Stability of St. Louis encephalitis virus in the airborne state. Applied and Environmental Microbiology, 18, 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radon K., Danuser B., Iversen M., Monso E., Weber C., Hartung J., Donham K.J., Palmgren U., and Nowak D. (2002). Air contaminants in different European farming environments. Annals of Agricultural and Environmental Medicine, 9, 41–48. [PubMed] [Google Scholar]
- Rechsteiner J., and Winkler K.C. (1969). Inactivation of respiratory syncytial virus in aerosol. Journal of General Virology, 5, 405–410. [Google Scholar]
- Richardson L.J., Mitchell B.W., Wilson J.L., and Hofacre C.L. (2003). Effect of an electrostatic space charge system on airborne dust and subsequent potential transmission of microorganisms to broiler breeder pullets by airborne dust. Avian Diseases, 47, 128–133. [DOI] [PubMed] [Google Scholar]
- Rosentrater K. (2003). Performance of an electrostatic dust collection system in swine facilities. CIGR Ejournal, 5, 1–10. [Google Scholar]
- Roy D., Wong P.K., Engelbrecht R.S., and Chian E.S. (1981). Mechanism of enteroviral inactivation by ozone. Applied and Environmental Microbiology, 41, 718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy J.H. B. (1980). Factors affecting susceptibility of calves to disease. Journal of Dairy Science, 63, 650–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios G.M., Escamilla E., and Torres N. (1981). Experimental Campylobacter diarrhea in chickens. Infection and Immunity, 34, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M., Seedorf J., and Hartung J. (2005). Influence of animal age and season on bio-aerosol concentrations in a broiler house. Proc. ISAH 2005, Warsaw, Poland. [Google Scholar]
- Sattar S.A., Ijaz M.K., Johnson-Lussenburg C.M., and Springthorpe V.S. (1984). Effect of relative humidity on the airborne survival of Rotavirus SA11. Applied and Environmental Microbiology, 47, 879–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum M.A., Zimmerman J.J., Beran G.W., and Murphy D.P. (1990). Survival of Pseudorabies virus in aerosol. American Journal of Veterinary Research, 51, 331–333. [PubMed] [Google Scholar]
- Schreider J.P., and Hutchens J.O. (1979). Particle deposition in the guinea pig respiratory tract. Journal of Aerosol Science, 10, 599–607. [Google Scholar]
- Scott D.B. M., and Lesher E.C. (1963). Effect of ozone on survival and permeability of Escherichia coli. Journal of Bacteriology, 85, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedorf J., and Hartung J. (1999). Reduction efficiencies of a biofilter and a bioscrubber for bioaerosols from two different piggeries. Berliner und Munchener Tierarztliche Wochenschrift, 112, 444–447. [PubMed] [Google Scholar]
- Seedorf J., Hartung J., Schroder M., Linkert K.H., Phillips V.R., Holden M.R., Sneath R.W., Short J.L., White R.P., Pedersen S., Takai H., Johnsen J.O., Metz J.H. M., Groot Koerkamp P.W. G., Uenk G.H., and Wathes C.M. (1998). Concentrations and emissions of airborne endotoxins and microorganisms in livestock buildings in Northern Europe. Journal of Agricultural Engineering Research, 70, 97–109. [Google Scholar]
- Shaman J., and Kohn M. (2009). Absolute humidity modulates influenza survival, transmission, and seasonality. Proceedings of the National Academy of Sciences, 106, 3243–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T.T., and Pereira N.C. (1979). Electrostatic precipitation. In Wang L.K., and Pereira N.C. (Eds.), Handbook of environmental engineering, volume 1: Air and noise pollution control (pp.). Humana Press Inc, Clifton, NJ. [Google Scholar]
- Shipe E.L., Tyler M.E., and Chapman D.N. (1959). Bacterial aerosol samplers. II. Development and evaluation of the Shipe sampler. Applied Microbiology, 7, 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J.D., and Lott B.D. (1996). Evaporative cooling performance resulting from changes in water temperature. Applied Engineering in Agriculture, 12, 497–500. [Google Scholar]
- Songer J.R. (1967). Influence of relative humidity on the survival of some airborne viruses. Applied and Environmental Microbiology, 15, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradbrow P.B., Samuel J.L., and Ibrahim A.L. (1988). Serological response of chickens to oral vaccination with newcastle disease virus. Veterinary Microbiology, 16, 255–262. [DOI] [PubMed] [Google Scholar]
- Ståhl M., Kokotovic, Hjulsager B., Breum C.K., Ø. S., and Angen Ø. (2011). The use of quantitative PCR for identification and quantification of Brachyspira pilosicoli, Lawsonia intracellularis and Escherichia coli fimbrial types F4 and F18 in pig feces. Veterinary Microbiology, 151, 307–314. [DOI] [PubMed] [Google Scholar]
- Stark K.D. C. (1999). The role of infectious aerosols in disease transmission pigs. The Veterinary Journal, 158, 164–181. [DOI] [PubMed] [Google Scholar]
- Stephenson E.H., Larson E.W., and Dominik J.W. (1984). Effect of environmental factors on aerosol-induced Lassa virus infection. Journal of Medical Virology, 14, 295–303. [DOI] [PubMed] [Google Scholar]
- Stewart R.H., and Wright D.N. (1970). Recovery of airborne Streptococcal L-Forms at various relative humidities. Applied and Environmental Microbiology, 19, 865–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S.L., Grinshpun S.A., Willeke K., Terzieva S., Ulevicius V., and Donnelly J. (1995). Effect of impact stress on microbial recovery on an agar surface. Applied and Environmental Microbiology, 61, 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasters K.C., and Winkler K.C. (1966). Viability of hospital Staphylococci in air. Microbiology and Molecular Biology Reviews, 30, 674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., and Ji J. (2007). Transport of droplet expelled by coughing in ventilated rooms. Indoor and Built Environment, 16, 493–504. [Google Scholar]
- Takai H. (2007). Factors influencing dust reduction efficiency of spraying of oil-water mixtures in pig buildings . Proc. Dustconf 2007: How to Improve Air Quality, Maastricht, the Netherlands.
- Takai H., and Pedersen S. (2000). A comparison study of different dust control methods in pig buildings. Applied Engineering in Agriculture, 16, 269–277. [Google Scholar]
- Takai H., Pedersen S., Johnsen J.O., Metz J.H. M., Koerkamp P., Uenk G.H., Phillips V.R., Holden M.R., Sneath R.W., Short J.L., White R.P., Hartung J., Seedorf J., Schroder M., Linkert K.H., and Wathes C.M. (1998). Concentrations and emissions of airborne dust in livestock buildings in Northern Europe. Journal of Agricultural Engineering Research, 70, 59–77. [Google Scholar]
- Tanaka A., and Zhang Y. (1996). Dust settling efficiency and electrostatic effect of a negative ionization system. Journal of Agricultural Safety and Health, 2, 39–47. [Google Scholar]
- Tao X., and Xin H. (2003). Surface wetting and its optimization to cool broiler chickens. Transactions of the ASABE, 46, 483–490. [Google Scholar]
- Thatcher T.L., Lai A.C. K., Moreno-Jackson R., Sextro R.G., and Nazaroff W.W. (2002). Effects of room furnishings and air speed on particle deposition rates indoors. Atmospheric Environment, 36, 1811–1819. [Google Scholar]
- Theunissen H.J., Lemmens-den Toom N.A., Burggraaf A., Stolz E., and Michel M.F. (1993). Influence of temperature and relative humidity on the survival of Chlamydia pneumoniae in aerosols. Applied and Environmental Microbiology, 59, 2589–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson C.M., Chanter N., and Wathes C.M. (1992). Survival of toxigenic Pasteurella multocida in aerosols and aqueous liquids. Applied and Environmental Microbiology, 58, 932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne P.S., Kiekhaefer M.S., Whitten P., and Donham K.J. (1992). Comparison of bioaerosol sampling methods in barns housing swine. Applied and Environmental Microbiology, 58, 2543–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topisirovic G. (2003). Influence of different ventilation systems on inhalable and respirable dust particles concentrations distribution in weaning and finishing fattening pig houses. Journal of Agricultural Sciences, 48, 187–204. [Google Scholar]
- Torok V.A., Hughes R.J., Ophel-Keller K., Ali M., and MacAlpine R. (2009). Influence of different litter materials on cecal microbiota colonization in broiler chickens. Poultry Science, 88, 2474–2481. [DOI] [PubMed] [Google Scholar]
- Trevors J.T. (2011). Viable but non-culturable (VBNC) bacteria: Gene expression in planktonic and biofilm cells. Journal of Microbiological Methods, 86, 266–273. [DOI] [PubMed] [Google Scholar]
- Tyler M.E., and Shipe E.L. (1959). Bacterial aerosol samplers. I. Development and evaluation of the All-Glass Impinger. Applied Microbiology, 7, 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler M.E., Shipe E.L., and Painter R.B. (1959). Bacterial aerosol samplers. III. Comparison of biological and physical effects in liquid impinger samplers. Applied Microbiology, 7, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (1997a). Ambient air monitoring reference and equivalent methods. U.S. EPA, Washington, DC . [Google Scholar]
- U.S. Environmental Protection Agency (1997b). Ambient air monitoring reference and equivalent methods. U.S. EPA, Washington, DC . [Google Scholar]
- U.S. Environmental Protection Agency (2006). National ambient air quality standards for particulate matter; final rule. U.S. EPA, Washington, DC . [Google Scholar]
- Vaithiyalingam S., Nutan M., Reddy I., and Khan M. (2002). Preparation and characterization of a customized cellulose acetate butyrate dispersion for controlled drug delivery. Journal of Pharmaceutical Sciences, 91, 1512–1522. [DOI] [PubMed] [Google Scholar]
- Valduga G., Bertoloni G., Reddi E., and Jori G. (1993). Effect of extracellularly generated singlet oxygen on Gram-positive and Gram-negative bacteria. Journal of Photochemistry and Photobiology B: Biology, 21, 81–86. [DOI] [PubMed] [Google Scholar]
- Van Oirschot J.T. (1979). Experimental production of congenital persistent swine fever infections: I. Clinical, pathological and virological observations. Veterinary Microbiology, 4, 117–132. [Google Scholar]
- Vittal B., and Rasool S. (1995). Enumeration of airborne molds in some indoor environments of Madras city (India) by cultural and non-cultural volumetric samplers. Aerobiologia, 11, 201–204. [Google Scholar]
- Vucemilo M., Matkovic K., Vinkovic B., Jaksic S., Granic K., and Mas M. (2007). The effect of animal age on air pollutant concentration in a broiler house. Czech Journal of Animal Science, 52, 170–174. [Google Scholar]
- Wang C.C., Fang G.C., and Kuo C.H. (2010). Bioaerosols as contributors to poor air quality in Taichung City, Taiwan. Environmental Monitoring and Assessment, 166, 1–9. [DOI] [PubMed] [Google Scholar]
- Wang Z., Reponen T., Grinshpun S.A., Gorny R.L., and Willeke K. (2001). Effect of sampling time and air humidity on the bioefficiency of filter samplers for bioaerosol collection. Journal of Aerosol Science, 32, 661–674. [Google Scholar]
- Ward R.L., Bernstein D.I., Young E.C., Sherwood J.R., Knowlton D.R., and Schiff G.M. (1986). Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. The Journal of Infectious Diseases, 154, 871–880. [DOI] [PubMed] [Google Scholar]
- Wathes C.M., Howard K., and Webster A.J. F. (1986). The survival of Escherichia coli in an aerosol at air temperatures of 15 and 30 C and a range of humidities. The Journal of Hygiene, 97, 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathes C.M., Phillips V.R., Holden M.R., Sneath R.W., Short J.L., White R.P., Hartung J., Seedorf J., Schroder M., Linkert K.H., Pedersen S., Takai H., Johnsen J.O., Koerkamp P., Uenk G.H., Metz J.H. M., Hinz T., Caspary V., and Linke S. (1998). Emissions of aerial pollutants in livestock buildings in Northern Europe: Overview of a multinational project. Journal of Agricultural Engineering Research, 70, 3–9. [Google Scholar]
- Webb S.J. (1959). Factors affecting the viability of air-borne bacteria. I. Bacteria aerosolized from distilled water. Canadian Journal of Microbiology, 5, 649–669. [Google Scholar]
- Weber T.P., and Stilianakis N.I. (2008). Inactivation of influenza A viruses in the environment and modes of transmission: A critical review. Journal of Infection, 57, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R.G., Yakhno M., Hinshaw V.S., Bean W.J., and Copal M.K. (1978). Intestinal influenza: Replication and characterization of influenza viruses in ducks. Virology, 84, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford R.A., Feddes J.J. R., and Barber E.M. (1992). Pig building dustiness as affected by canola oil in the feed. Canadian Agricultural Engineering, 34, 365–365. [Google Scholar]
- Wells W.F. (1934). On air-borne infection: Study II. Droplets and droplet nuclei. American Journal of Epidemiology, 20, 611–618. [Google Scholar]
- Wilson S.C., Morrow-Tesch J., Straus D.C., Cooley J.D., Wong W.C., Mitlohner F.M., and McGlone J.J. (2002). Airborne microbial flora in a cattle feedlot. Applied and Environmental Microbiology, 68, 3238–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won W.D., and Ross H. (1969). Reaction of airborne Rhizobium meliloti to some environmental factors. Applied and Environmental Microbiology, 18, 555–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D.N., Bailey G.D., and Hatch M.T. (1968a). Role of relative humidity in the survival of airborne Mycoplasma pneumoniae. Journal of Bacteriology, 96, 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D.N., Bailey G.D., and Hatch M.T. (1968b). Survival of airborne Mycoplasma as affected by relative humidity. Journal of Bacteriology, 95, 251–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.S. (2009). Antibacterial and static dissipating composites of poly (butylene adipate-co-terephthalate) and multi-walled carbon nanotubes. Carbon, 47, 3091–3098. [Google Scholar]
- Wu Y., Chan C., Chew G., Shih P., Lee C., and Chao H. (2012). Meteorological factors and ambient bacterial levels in a subtropical urban environment. International Journal of Biometeorology, doi: 10.1007/s00484-011-0514-6. [DOI] [PubMed] [Google Scholar]
- Wunsch O. (1994). Oscillating sedimentation of spheres in viscoplastic fluids. Rheologica Acta, 33, 292–302. [Google Scholar]
- Wylie L.M., Robertson G.W., MacLeod M.G., and Hocking P.M. (2001). Effects of ambient temperature and restricted feeding on the growth of feathers in growing turkeys. British Poultry Science, 42, 449–455. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Ichijo T., Sakotani A., Baba T., and Nasu M. (2012). Global dispersion of bacterial cells on Asian dust. Scientific Reports, 2, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez M.A., Nocker A., Soria-Soria E., Múrtula R., Martínez L., and Catalán V. (2011). Quantification of viable Legionella pneumophila cells using propidium monoazide combined with quantitative PCR. Journal of Microbiological Methods, 85, 124–130. [DOI] [PubMed] [Google Scholar]
- Yoder M.F., and Van Wicklen G.L. (1988). Respirable aerosol generation by broiler chicken. Transactions of the ASAE, 31, 1510–1517. [Google Scholar]
- Yoon I.J., Joo H., Christianson W.T., Morrison R.B., and Dial G.D. (1993). Persistent and contact infection in nursery pigs experimentally infected with porcine reproductive and respiratory syndrome (PRRS) virus. Journal of Swine Health and Production, 1, 5–8. [Google Scholar]
- Yoon K.J., Zimmerman J.J., Chang C.C., Cancel-Tirado S., Harmon K.M., and MaGinley M.J. (1999). Effect of challenge dose and route on porcine reproductive and respiratory syndrome virus (PRRSV) infection in young swine. Veterinary Research, 30, 629–638. [PubMed] [Google Scholar]
- Yu I.T. S., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H. W., Leung D.Y. C., and Ho T. (2004). Evidence of airborne transmission of the severe acute respiratory syndrome virus. The New England Journal of Medicine, 350, 1731–1739. [DOI] [PubMed] [Google Scholar]
- Zeitler M.H., Konig M., and Groth W. (1987). Effect of the type of feed (meal, pellets or fluid) and season on the concentration and particle size of dust particles in pig houses. Deutsche Tierarztliche Wochenschrift, 94, 420–424. [PubMed] [Google Scholar]
- Zhang Y., Polakow J.A., Wang X., Riskowski G.L., Sun Y., and Ford S.E. (2001). An aerodynamic deduster to reduce dust and gas emissions from ventilated livestock facilities. Proc. Livestock Environment VI: Proceedings of the 6th International Symposium, Kentucky, USA.
- Zhang Y.H. (2004). Indoor air quality engineering. CRC Press, Boca Raton, Florida . [Google Scholar]
- Zhao Y., Aarnink A.J. A., Cambra-Lopez M., and Fabri T. (2013). Viral shedding and emission of airborne infectious bursal disease virus from a broiler room. British Poultry Science, 54, 87–95. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Aarnink A.J. A., de Jong M.C. M., Ogink N.W. M., and Groot Koerkamp P.W. G. (2011a). Effectiveness of multi-stage scrubbers in reducing emissions of air pollutants from pig houses. Transactions of the ASABE, 54, 285–293. [Google Scholar]
- Zhao Y., Aarnink A.J. A., Dijkman R., Teun F., de Jong M.C. M., and Groot Koerkamp P.W. G. (2012). Effect of temperature, relative humidity, absolute humidity and evaporation potential on survival of airborne Gumboro vaccine virus. Applied and Environmental Microbiology, 78, 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Aarnink A.J. A., Doornenbal P., Huynh T.T. T., Groot Koerkamp P.W. G., de Jong M.C. M., and Landman W.J. (2011b). Investigation of the efficiencies of bioaerosol samplers for collecting aerosolized bacteria using a fluorescent tracer. I: Effects of non-sampling processes on bacterial culturability. Aerosol Science and Technology, 45, 423–431. [Google Scholar]
- Zhao Y., Aarnink A.J. A., Doornenbal P., Huynh T.T. T., Groot Koerkamp P.W. G., Landman W.J., and de Jong M.C. M. (2011c). Investigation of the efficiencies of bioaerosol samplers for collecting aerosolized bacteria using a fluorescent tracer. II: Sampling efficiency, and half-life time. Aerosol Science and Technology, 45, 432–442. [Google Scholar]
- Zhao Y., Aarnink A.J. A., Groot Koerkamp P.W. G., Hagenaars T.J., Katsma W.E. A., and de Jong M.C. M. (2011d). Detection of airborne Campylobacter with three bioaerosol samplers for alarming bacteria transmission in broilers. Biological Engineering Transactions, 3, 177–186. [Google Scholar]
- Zhao Y., Aarnink A.J. A., Groot Koerkamp P.W. G., Ogink N.W. M., and De Jong M.C. M. (2008). Removal efficiency of dust and bacteria by multi-stageair scrubbers in a pig house . Proc. The Eighth International Symposium of Livestock Environment, Iguassu Falls, Brasil.
- Zhao Y., Aarnink A.J. A., Hofschreuder P., and Groot Koerkamp P.W. G. (2009). Evaluation of an impaction and a cyclone pre-separator for sampling high PM10 and PM2.5 concentrations in livestock houses. Journal of Aerosol Science, 40, 868–878. [Google Scholar]
- Zimmerman J.J., Schwartz K., Hill H.T., Meetz M.C., Simonson R., and Carlson J.H. (1993). Influence of dose and route on transmission of encephalomyocarditis virus to swine. Journal of Veterinary Diagnostic Investigation, 5, 317–321. [DOI] [PubMed] [Google Scholar]
- Zucker B.A., Trojan S., and Muller W. (2000). Airborne Gram-negative bacterial flora in animal houses. Journal of Veterinary Medicine Series B, 47, 37–46. [DOI] [PubMed] [Google Scholar]


