Abstract
The worldwide prevalence of herpes simplex virus (HSV) and the shortage of efficient vaccines and novel therapeutic strategies against HSV are widely global concerns. The abundance on the virion and the major stimulus for the virus-neutralizing antibodies makes gD a predominant candidate for cure of HSV infection. In this study, we generated a monoclonal antibody (mAb), termed m27f, targeting to glycoprotein D (gD) of HSV-2, which also has cross-reactivity against HSV-1 gD. It has a high level of neutralizing activity against both HSV-1 and HSV-2, and binds to a highly conserved region (residues 292–297) within the pro-fusion domain of gD. It can effectively block HSV cell-to-cell spread in vitro. The pre- or post-attachment neutralization assay and syncytium formation inhibition assay revealed that m27f neutralizes HSV at the post-binding stage. Moreover, therapeutic administration of m27f completely prevented infection-related mortality of mice challenged with a lethal dose of HSV-2. Our newly identified epitope for the neutralizing antibody would facilitate studies of gD-based HSV entry or vaccine design, and m27f itself demonstrated a high potential for adaptation as a protective or therapeutic drug against HSV.
Keywords: Herpes simplex virus, Glycoprotein D, Monoclonal antibody, Neutralization
Highlights
-
•
We generated a novel neutralizing mAb m27f whose epitope located in the pro-fusion domain of gD.
-
•
The protective efficiency of m27f was evaluated in vitro and in vivo.
-
•
m27f neutralizes HSV at the post-binding stage.
1. Introduction
Herpes simplex virus (HSV) is a prevalent worldwide human pathogen that infects epithelial cells before it establishes latency in trigeminal or sacral nerve root ganglia (Steiner and Benninger, 2013), causing mucocutaneous lesions, keratitis, and encephalitis (Dropulic and Cohen, 2012). There are two serotypes of HSV, HSV-1 and HSV-2, also known as human herpesvirus 1 and 2 (HHV-1 and HHV-2). The most common clinical sign of HSV-1 is herpes labialis, as well as ocular diseases, including conjunctivitis and keratitis (Everett, 2014). HSV-2 is the main cause of recurrent genital herpes, which is one of the most predominant sexually transmitted diseases, and HSV-2 infection of newborns during delivery is usually accompanied by a high mortality rate (Cleary et al., 2005, Domeika et al., 2010, Overall, 1994). Moreover, epidemiological and biological studies have revealed that HSV-2 dramatically increases the risk of HIV infection (Barnabas and Celum, 2012, Freeman et al., 2006, Sartori et al., 2011) and may also act in conjunction with human papillomavirus (HPV) infection to increase the risk of invasive cervical carcinoma (Smith et al., 2002, Zhao et al., 2012). According to World Health Organization (WHO) statistics, in 2012, there were 417 million people aged 15–49 years living with HSV-2 infection worldwide (Looker et al., 2015), and the newly infected population is estimated to be 23 million per year (Looker et al., 2008). Therefore, the development of vaccines and novel therapeutic strategies against HSV has become very urgent.
Both initial entry of the virion into cells and subsequent lateral spread of HSV require the interaction of four viral glycoproteins, gB, gD, gH and gL, with at least one of the cell receptors, either herpesvirus entry mediator (HVEM) or nectin-1. Among these viral glycoproteins, gD acts as the receptor-binding glycoprotein, and gH, gL and gB are required for membrane fusion execution. The gD ectodomain is organized into two structurally and functionally differentiated regions. The N terminus (residues 1–260) carries the receptor binding sites, and the C terminus functions as the pro-fusion domain (residues 260–310), which is required for triggering virus-cell fusion. In nature, the C terminus of the gD ectodomain binds to its N-terminal region and masks the receptor-binding site, resulting in a closed conformation (Cocchi et al., 2004, Fusco et al., 2005). After binding to a cell surface receptor, either HVEM or nectin-1, gD undergoes conformational changes to adopt an open conformation, which is a key step in activating the core fusion machinery of gH/gL and gB. gD is the most abundantly expressed glycoprotein on the virion, and it induces both humoral and cellular immunity and is the major stimulus of virus-neutralizing antibodies together with gB (Bender et al., 2007, Eisenberg et al., 2012, Fotouhi et al., 2008, Nicola et al., 1998, Whitbeck et al., 1999). Therefore, gD has been the predominant candidate for HSV vaccine or novel therapeutic strategies (Awasthi and Friedman, 2014, Shin and Iwasaki, 2013).
Monoclonal antibodies (mAbs) represent a useful tool for the study of the structure and function of target proteins, and virus-host interactions, as well as for the development of promising therapeutic agents. Anti-gD mAbs have been arranged into groups that recognize distinct discontinuous native and continuous denatured epitopes. Most mAbs that have virus-neutralizing activity recognize discontinuous epitopes. Residues 1 to 23, 264 to 279, and 284 to 301 of the extracellular domain are major continuous antigenic determinants on gD (Isola et al., 1989). mAbs recognizing epitopes located in these continuous determinant regions have no or low virus-neutralizing ability, except for the mAb 1D3, which can neutralize viral infectivity by blocking gD-receptor interaction (Cairns et al., 2014).
Our current investigation found a mAb, m27f that recognizes a new continuous epitope (residues 292 to 297) within the pro-fusion domain of HSV and possesses a high level of virus-neutralizing activity. It showed a high degree of neutralizing activity against both HSV-1 and HSV-2, completely abrogated viral cell-to-cell spread, and inhibited syncytium formation in vitro. In addition, it also exhibited highly therapeutic effects in a HSV-2 infected mouse model, implying its high potential for adaptation as a protective or therapeutic interventions.
2. Materials and methods
2.1. Cells and viruses
Cell lines used in the study are African green monkey kidney (Vero) cell line (ATCC, No. CCL-81) and Baby Hamster Kidney (BHK) cell line (ATCC, No. CCL-10). Cells were cultured in Dulbecco's modified Eagle medium (DMEM) (Thermo Fisher Scientific) with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific). HSV-1 F strain and HSV-2 333 strain were kindly provided by Microorganisms and Viruses Culture Collection Center, Wuhan Institute of Virology, CAS (serial number: IVCAS6.0182, IVCAS6.0183). Viruses were propagated in Vero cells and cell lysate stocks were prepared as previously described (Morrison and Knipe, 1996). Virus titers were determined in Vero cells (Navarro et al., 1992).
2.2. Mice
Female BALB/c mice, six-weeks of age, were purchased from the Hubei Provincial Center for Disease Control and Prevention (Wuhan, China) and maintained under specific pathogen-free conditions at the Central Animal Laboratory of Wuhan Institute of Virology, Chinese Academy of Sciences (WIV, CAS. License number: SYXK2014-0034). All animal experiments were approved by the Institutional Animal Ethical Committee of WIV, CAS (Approval: WIVA23201403). All procedures were carried out under pentobarbital sodium (Sigma) anesthesia and all efforts were made to minimize any suffering and the number of animals used in the study.
2.3. Generation of mAbs
MAbs against HSV-2 gD protein were prepared as described previously (Evan et al., 1985). In brief, the nucleotide sequence encoding the ectodomain (residues 1 to 318) plus the natural signal sequence of HSV-2 gD protein, gD1-318t, was cloned into the pET-32a vector (Novagen) and expressed in Escherichia coli BL21. The protein was purified under denaturing conditions in the presence of 8M urea by using a Ni-nitrilotriacetic acid (NTA) column and then dialyzed (Pirestani et al., 2014). The quantity of purified protein was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). BALB/c mice (n = 2) were immunized with 60 μg of purified gD1-318t in complete Freund's adjuvant (Sigma). After 2, 4, and 6 weeks, the immunizations was repeated with gD1-318t conjugate in incomplete Freund's adjuvant (Sigma). Fusion with SP2/0 myeloma cells was carried out by using standard methodology (de StGroth and Scheidegger, 1980). Hybridomas culture supernatant was screened by enzyme-linked immunosorbent assay (ELISA) and Western blot analysis. Totally 25 strains of positive hybridoma cells were obtained. The mAbs were purified by protein A-Sepharose™ (Sigma) according to the manufacturer's instructions.
2.4. Western blot analysis
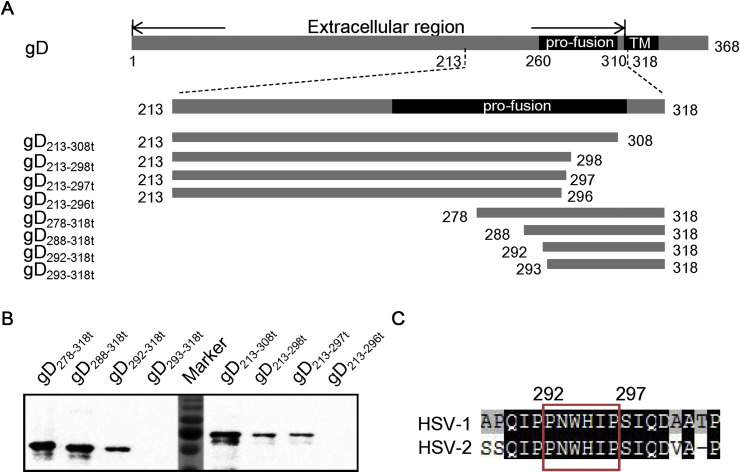
For epitope mapping, the ectodomain of HSV-2 gD protein was dissected into seven truncated fragments, gD1-55t, gD35-95t, gD73-151t, gD125-194t, gD170-233t, gD213-278t and gD254-318t, with any two adjacent fragments sharing an overlap of 20–26 amino acids (Fig. 1 A). Amino acids 213 to 318 of the gD protein were further truncated into eight fragments, gD213-308t, gD213-298t, gD213-297t, gD213-296t, gD278-318t, gD288-318t, gD292-318t and gD293-318t (Fig. 3A). The nucleotides encoding the above fragments were PCR-amplified using the primer pairs listed in Table S1, cloned into pMAL-C2x (NEB) and then expressed in E. coli DH10β. For characterization of recognizing speciality, Vero cells infected with HSV-1 or HSV-2 at an multiplicity of infection (MOI) of 5 pfu per cell were harvested at 48 h post infection (p.i.) and rinsed with PBS. The protein samples were separated by 10% SDS-PAGE, and transferred onto a polyvinylidene fluoride (PVDF) membrane (Merck Millipore, Massachusetts, USA) by a Trans-Blot apparatus (Bio-Rad). The Western blot analyses were performed with primary antibodies generated from above-mentioned screening positive hybridoma cells which against HSV-2 gD protein: m27f and 21C11. Pre-immune mouse serum and β-actin (Sigma) antibodies were used as controls. After washing, the blots were incubated with horseradish peroxidase-conjugated goat anti-mouse antibody. The immunoreactive signals were visualized with SuperSignal West Pico Chemiluminescent Substrate (Fermentas) and the MicroChemi Bio-imaging System (DNR, USA).
Fig. 1.
Mapping the recognition region of the mAbs against HSV-2 gD protein. (A) Schematic diagram of gD protein and truncation strategy. The structure of full-length gD protein is shown at the top; the truncated gD proteins used for the sequential mapping experiment are shown below. Numbers indicate amino acid positions. The domain (pro-fusion) was defined according to Fusco et al. (2005). (B) Recognition region mapping of mAbs m27f and 21C11. The truncated gD proteins expressed by pMAL-C2x vector were presented as antigens. m27f and 21C11 were used as the primary antibodies respectively for Western blot analysis.
Fig. 3.
Detailed epitope mapping of m27f. (A) Schematic diagram of the gD protein and accurate truncation strategy. The structure of the full-length gD protein is shown at the top; the truncated gD proteins used for the sequential mapping experiment are shown below. Numbers indicate amino acid positions. The domain (pro-fusion) was defined according to Fusco et al. (2005). (B) Epitope mapping of m27f. The truncated gD proteins expressed by pMAL-C2x vector were presented as antigens. MAb m27f was used as the primary antibody for Western blot analysis. (C) Alignment analysis. Residues 292 to 297 are highly conserved in both HSV-1 and HSV-2. Sequence alignment was performed with DNAMAN V6.
2.5. Immunofluorescence assay (IFA)
Vero monolayers were infected with HSV-1 or HSV-2 at an MOI of 5 pfu per cell. At 48 h p.i., the cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.05% Triton X-100, and washed three times with phosphate-buffered saline (PBS). To characterize the recognizing speciality of m27f and 21C11, we immunostained cells with them respectively for 2 h at 37 °C. Pre-immune mouse serum and mouse Immunoglobulin G (Beyotime) were used as controls. All the antibodies were diluted 1:100 in blocking solution (5% BSA in PBS). After being washed for three times with PBS, the cells were incubated with the secondary antibody, fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Abcam, Hangzhou, China). The cells were observed by fluorescence microscopy.
2.6. Virus neutralization assay
The HSV neutralization assay was based on a previously described protocol (Bag et al., 2013). In brief, Vero cell were seeded into 12 well plates (Costar) and incubated overnight to achieve confluent monolayers. m27f and 21C11 were serially diluted in DMEM supplemented with 2% FBS and then combined with HSV-1 or HSV-2 (100 pfu/well) respectively (final concentration of m27f or 21C11 for HSV-1 ranged from 240 μg/ml to 0, and final concentration of m27f or 21C11 for HSV-2 ranged from 120 μg/ml to 0). Pre-immune mouse serum was used as negative control. The virus-antibody mixtures were incubated for 1 h at 37 °C with gentle rocking. After 1 h incubation, the mixtures were inoculated over Vero cell monolayers in plates at 37 °C for 2 h. After 2 h adsorption the cells were covered with overlay media with 1% methylcellulose to form plaques. The plaques developed after 72 h of incubation were fixed and visualized by staining with crystal violet. Plaques were counted and the percentage inhibition was determined relative to the negative control. The effective concentration of mAbs that inhibited the number of viral plaques was interpolated from the dose-response curves. Virus neutralization assays were repeated at least twice and reported as number of plaques relative to control ± SEM.
The pre- or post-attachment neutralization assays were performed as described with modifications (Krawczyk et al., 2011). Briefly, virus-antibody mixture was incubated for 1 h at 4 °C before inoculating over the Vero cells (pre-attachment), while prechilled (4 °C for 15 min) Vero cells were infected with HSV-1 or HSV-2 (100 pfu/well) at 4 °C for 1 h to allow virus adsorption before serial dilutions of antibodies were added (post-attachment). Inoculated Vero cells were then incubated for 2 h at 37 °C. Neutralization efficacy was determined after 72 h as described above for the standard neutralization assay.
2.7. Cell-to-cell spread assay
Cell-to-cell spread assay was based on a previously described protocol (Krawczyk et al., 2011). BHK cells were grown on glass coverslips and incubated overnight to achieve confluent monolayers. Cells were infected with HSV-1 or HSV-2 at an MOI of 0.01 pfu per cell. After 2 h of adsorption at 37 °C, the virus inoculum was removed and cells were washed twice with PBS and then incubated in DMEM containing 2% FBS in the presence of antibodies, m27f (500 μg/ml) and 21C11 (500 μg/ml). Mouse IgG (500 μg/ml), or medium alone was used as control. To visualize the viral spread by immunofluorescence, after 48 h p.i., cells were fixed with 4% paraformaldehyde, permeabilized with 0.05% Triton X-100, and washed three times with PBS. Cells were incubated with mouse polyclonal antibody against gD as primary antibody and FITC-conjugated goat anti-mouse IgG as secondary antibody. Immunofluorescence images were observed by fluorescence microscopy. To quantify the cell-to-cell spread, the number of infected cells with GFP fluorescence per total cells was calculated in nine randomly chosen areas, representing ∼3000 cells for each group. The percentage infection was determined relative to control. The experiment was repeated twice.
2.8. Syncytium formation inhibition assay
Syncytium formation inhibition assay was performed as described with modifications (Navarro et al., 1992). In brief, monolayers of Vero cells grown in 24-well plates were infected with HSV-1 or HSV-2 at an MOI of 5 pfu per cell. After 2 h of adsorption at 37 °C, the virus inoculum was removed and cells were washed with PBS twice then incubated in DMEM containing 2% FBS in the presence of antibodies, m27f (500 μg/ml) and 21C11 (500 μg/ml), mouse IgG (500 μg/ml), or medium alone as a control. After 48 h p.i., cells were fixed and then stained with hematoxylin-eosin (HE) staining. The inhibition of syncytium formation was examined by light microscopy. To quantify the inhibition of syntytium formation, the numbers of nuclei in syncytia were counted in randomly chosen area, and the percentage inhibition was determined relative to no antibody treatment group. Only syncytia containing 3 or more nuclei were analyzed. Three independent experiments were calculated.
2.9. Mouse protection experiments
Mouse intravaginal HSV-2 challenge was established as previously described (Cena-Diez et al., 2016). Six-weeks-old female BALB/c mice were rendered susceptible to infection by subcutaneous injection with 2.5 mg of medoxyprogesterone acetate (Sigma) 5 days prior to challenge (Parr et al., 1994). Mice were randomly assigned to five groups of 7 or 8 each. Mice were anesthetized with pentobarbital sodium and intravaginally challenged with 1 × 106 pfu/20 μl of HSV-2. The infected mice were passively immunized by intravenous injection of 1, 3, 7 and 15 mg/kg of m27f at 24, 48, and 72 h after intravaginal HSV-2 challenge. PBS group was set as control. Mice in each group were examined daily for symptoms of genital disease, body weight over 16 days, as well as mortality for 30 days. Disease score was graded from 0 to 4: no apparent infection scored 0; genital erythema scored 1; moderate genital infection scored 2; purulent genital ulceration, hair loss and generally poor condition scored 3; severe ulceration of genital and surrounding tissue, and hind limb paralysis scored 4. Mice that developing 20% loss in body weight, debilitating symptoms of disease or paralysis were sacrificed and considered to have died the following day in all survival analyses. Surviving mice were sacrificed at the indicated times after infection.
2.10. Statistical analyses
Data are presented as means ± standard error of the mean (SEM). Sequence alignment was performed with DNAMAN V6. Statistical analysis was performed using GraphPad Prism 5. Significant differences were analyzed by Log-rank (Mantel-Cox) test and Gehan-Breslow-Wilcoxon test. P-values were calculated, and statistical significance is reported as highly significant using *(p < 0.05), ** (p < 0.01), or *** (p < 0.001).
3. Results
3.1. Generation of HSV-2 gD-specific mAbs
To develop functional monoclonal antibody against HSV-2 gD, we immunized BALB/c mice with purified extracellular domain of gD protein. After the primary screening of hybridomas by ELISA, Western blot analysis and Immunofluorescence assay (IFA), a total of 25 strains of positive hybridoma cells were obtained (Table S2). Epitope mapping of these mAbs was determined using the expressed truncated gD proteins, including gD1-55t, gD35-95t, gD73-151t, gD125-194t, gD170-233t, gD213-278t and gD254-318t, with an overlap of 20–26 amino acids between each pair of adjacent fragments (Fig. 1A), by Western blot analysis (data not shown). Among these 25 mAbs, m27f and another 14 mAbs recognized truncated gD254-318t, which is located in the C-terminus (residues 260–310) of the gD ectodomain (Fig. 1B). In contrast, 21C11 (Fig. 1B) and the remaining 9 mAbs recognized both N-terminal fragments, gD1-55t and gD35-95t, suggesting that they recognized the overlap region (residues 35–55) of the two fragments. Therefore, two kinds of mAb, as represented by m27f and 21C11, recognizing different epitopes of HSV-2 gD, were chosen for further characterization.
3.2. Characterization of mAbs m27f and 21C11
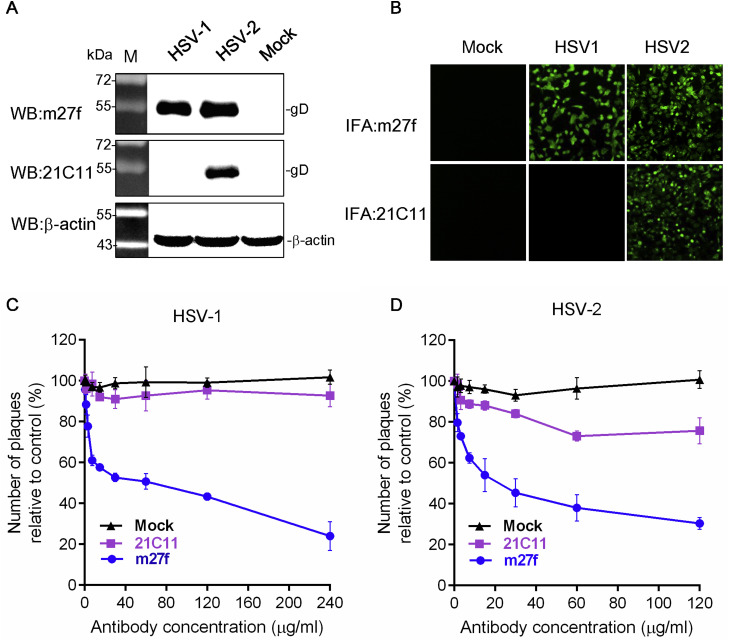
To characterize the specific recognition ability of m27f and 21C11, we first tested their ability to recognize denatured and native forms of HSV-1 gD and HSV-2 gD by Western blot assay and IFA. Western blot analysis revealed that m27f reacted with the expected gD band in the sample of both HSV-1 and HSV-2 infected Vero cells, while 21C11 only recognized gD in the HSV-2 infected Vero cells sample, but not the HSV-1 infected sample (Fig. 2 A). The IFA results demonstrated that m27f detected native forms of gD in the cells infected with either HSV-1 or HSV-2, while 21C11 only detected native gD in the cells infected with HSV-2 (Fig. 2B). These results suggested that m27f could recognize the denatured and native forms of gD of both HSV-1 and HSV-2, while 21C11 could only recognize the denatured and native forms of gD of HSV-2.
Fig. 2.
Characterization of mAbs m27f and 21C11. (A) Western blot analysis. Vero cell monolayers were infected with HSV-1 or HSV-2 at an MOI of 5 pfu per cell. At 48 h p.i., cells were harvested for Western blot analysis using m27f or 21C11 as the primary antibody. β-actin antibodies were used as control. (B) IFA. Cells were infected with HSV-1 or HSV-2 at an MOI of 5 pfu per cell. At 48 h p.i., cells were fixed and subjected to IFA using m27f or 21C11 as the primary antibody and FITC-conjugated goat anti-mouse IgG as the secondary antibody. (original magnification, ×100) (C) and (D) Virus neutralization assay. HSV-1 or HSV-2 (100 pfu/well) was incubated with indicated serially diluted antibodies m27f, 21C11 or pre-immune serum. At 72 h p.i., cells were stained and the number of plaques counted. The percentage inhibition was determined relative to the negative control. Representative data (mean ± SEM) from three independent experiments are shown.
To further confirm whether m27f and 21C11 could neutralize HSV infection, virus neutralization assays were performed. Either HSV-1 or HSV-2 (100 pfu/well) were pre-incubated with serially diluted mAbs, m27f and 21C11, before infection into Vero monolayer cells. Pre-immune mouse serum was used as negative control. Neutralization assay demonstrated that m27f neutralized both HSV-1 and HSV-2 in a dose-dependent manner, with about ∼60% HSV-1 infection and about ∼70% HSV-2 infection being inhibited at an antibody concentration of 120 μg/ml. In contrast, 21C11 demonstrated no neutralizing activity of HSV-1 and low neutralizing activity of HSV-2 infection groups (only about 20% virus infection being inhibited at a concentration of 120 μg/ml) (Fig. 2C and D). These results suggested that m27f but not 21C11, is a neutralizing mAb against HSV-1 and HSV-2 infection.
3.3. Epitope mapping of m27f
As shown above, m27f had a high level of neutralization activity against both HSV-1 and HSV-2, and m27f recognized a linear epitope, gD254-318t, which is located in the C-terminus of the gD ectodomain. To identity accurately the epitope recognized by m27f, we constructed a series of further truncations of the C-terminal region (residues 213 to 318) of HSV-2 gD protein (Fig. 3 A). By Western blot analysis, gD213-297t but not gD213-296t that could be recognized by m27f, indicating that P297 is the last C-terminal amino acid critical for m27f binding (Fig. 3B). Similarly, P292 is the first N-terminal amino acid critical for m27f binding. On this basis, 292PNWHIP297 was considered to be the precise epitope for m27f binding. Sequence alignment showed that these residues are highly conserved among HSV-1 and HSV-2 (Fig. 3C). These results explain why m27f recognized the gD protein of both HSV-1 and HSV-2.
3.4. m27f blocks HSV cell-to-cell spread
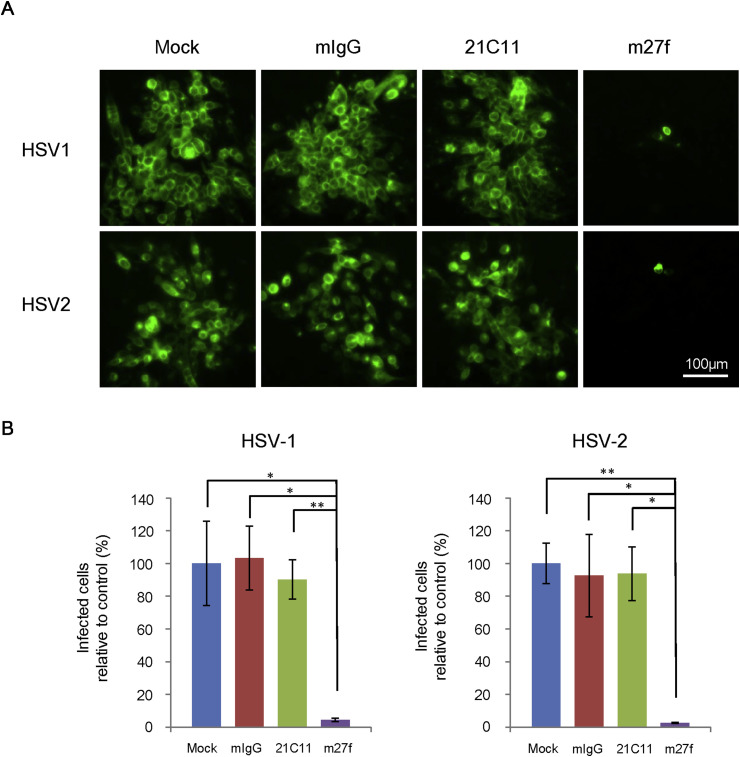
Direct transmission between adjacent cells (cell-to-cell spread) is an efficient route for HSV to bypass the host immune system. Not all neutralizing mAbs can prevent cell-to-cell spread of herpesviruses (Co et al., 1991, Hooks et al., 1976). Therefore, blocking of cell-to-cell spread is considered to be an important aspect in evaluating the in vivo protective efficiency of neutralizing antibodies. We herein analyzed the ability of m27f to inhibit cell-to-cell spread of HSV-1 and HSV-2 in vitro. Confluent BHK cell monolayers were initially incubated with either HSV-1 or HSV-2 at an MOI of 0.01 pfu per cell. To prevent viral spread through viral particles moving across the cell culture supernatant (cell-free spread), BHK cell monolayers were treated with an excess of m27f or 21C11, and treatment with mouse IgG or culture medium alone was performed as controls. After 48 h p.i., the transmission of virus was detected by immunostaining with mouse polyclonal antibody against gD. As shown in Fig. 4 A, m27f completely inhibited cell-to-cell spread of both HSV-1 and HSV-2, as evidenced by observing the limited fluorescence caused by virus infection. In contrast, neither 21C11 nor mouse IgG could sufficiently inhibit transmission of HSV-1 or HSV-2, and extensive virus spread on BHK cell monolayer was observed by immunostaining. When the infected cells were quantified, the results clearly showed that m27f is able to inhibit cell-to-cell transmission in HSV-infected cells (Fig. 4B).
Fig. 4.
m27f blocks cell-to-cell spread of HSV-1 and HSV-2. (A) Monolayers of BHK cells were infected HSV-1 or HSV-2 at an MOI of 0.01 pfu per cell. Cell monolayers were treated with an excess of antibodies, m27f and 21C11 (500 μg/ml each). Mouse IgG (500 μg/ml) or culture medium alone was performed as controls. At 48 h p.i., cells were fixed and observed by fluorescence microscopy. Bar represents 100 μm. The experiments were repeated at least twice. (B) The number of infected cell with GFP fluorescence per total cells was calculated in randomly chosen areas. The percentage infection was determined relative to control. The experiment was repeated twice. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.5. m27f neutralizes HSV-1 and HSV-2 infection at the post-binding stage
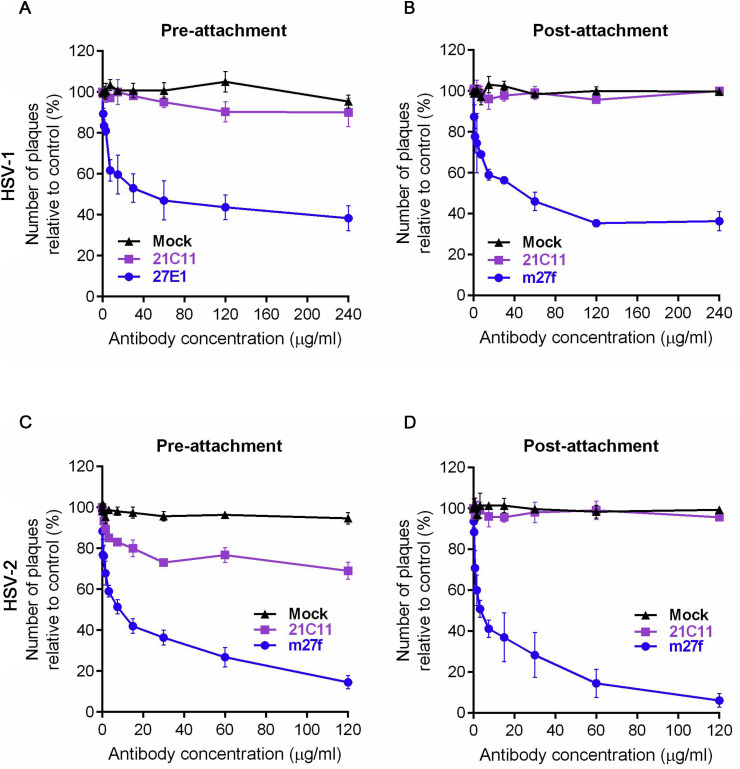
To further characterize the mode of action of m27f, we analyzed whether this mAb could inhibit receptor binding. We compared the neutralization efficacy of m27f when the antibody was added before (pre-attachment) or after (post-attachment) HSV virions interacted with Vero cells. 21C11 was chosen as a negative control. By pre- or post-attachment assays, when m27f was added before virus-host interaction (pre-attachment), about 60% HSV-1 infection was inhibited at a concentration of 120 μg/ml (Fig. 5 A), about 65% HSV-1 infection was inhibited at a concentration of 120 μg/ml when m27f was added after HSV-1 virions interacted with Vero cells (post-attachment) (Fig. 5B). Almost equal neutralization efficacy of m27f was observed, irrespective of whether the antibody was added before or after HSV-1 virions interacted with Vero cells. In contrast, the negative control, 21C11, had no neutralizing ability either before or after virions were incubated with cells. The HSV-2 infection group showed a similar result. About 80% HSV-2 infection was inhibited in the pre-attachment treatment (Fig. 5C) and about 90% HSV-2 infection was inhibited in the post-attachment treatment with an antibody concentration of 120 μg/ml of m27f (Fig. 5D). In contrast, 21C11 had low (20% HSV-2 infection being inhibited) or no neutralizing ability in the pre-attachment or post-attachment treatments, respectively (Fig. 5C and D). The existing data indicated that m27f does not interfere with the virus binding process, m27f therefore neutralizes HSV entry at the post-binding stage.
Fig. 5.
m27f neutralizes HSV-1 and HSV-2 at the post-binding stage. Comparison of neutralization efficacy of m27f when serial dilutions of antibodies were added before (A and C, pre-attachment) or after (B and D, post-attachment) HSV virions interacted with Vero cells. 21C11 was used as a control. Neutralization efficacies were determined after 72 h as described above for the standard neutralization assay. The percent inhibition was determined relative to the negative control. Representative data (mean ± SEM) from at least 3 independent experiments performed are shown.
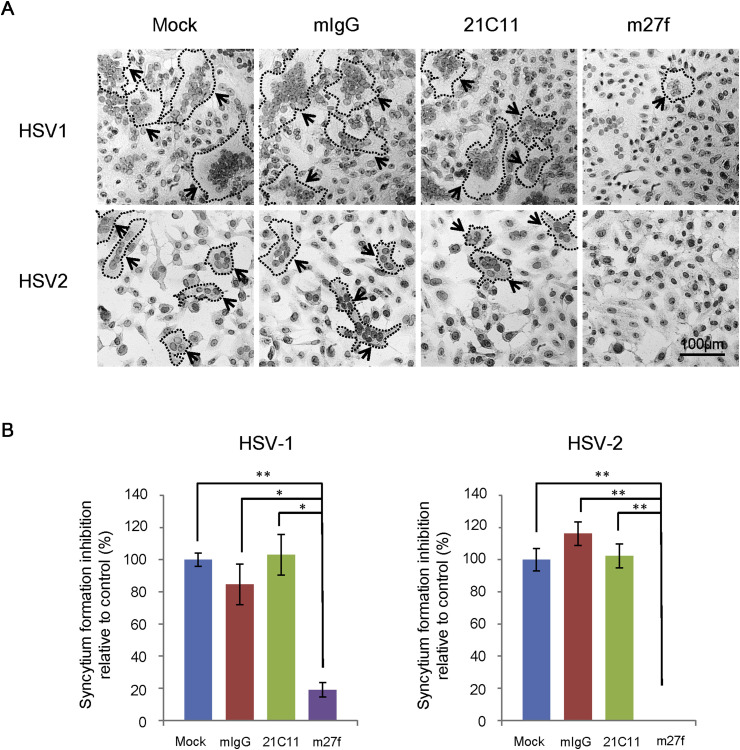
3.6. m27f inhibits syncytium formation of HSV-1 and HSV-2
After binding of gD to a cell surface receptor, it undergoes a conformational change, which is also a key event for triggering later steps leading to fusion. Since m27f might neutralize HSV entry at the post-binding stage, we asked whether m27f could inhibit the virus-induced membrane fusion process. Monolayers of Vero cells were infected with HSV-1 or HSV-2 at an MOI of 5 pfu per cell. After 2 h of adsorption at 37 °C, the virus inoculum was removed and cells were washed twice with PBS, the cells were then incubated with an excess of m27f, 21C11, mouse IgG, or medium alone as a control. After 48 h, syncytium formation was observed by staining with HE staining. As shown in Fig. 6 A, in both HSV-1 and HSV-2 infection groups, obvious syncytia with multi-nuclear cells were observed when infected cells were treated with pre-immune serum, mouse IgG or 21C11. However, when cells were incubated with m27f, syncytium formation was almost completely inhibited. The syncytium formation inhibition of Vero cells with different treatment was quantified in Fig. 6B. This result suggested that m27f indeed inhibited the HSV-induced membrane fusion.
Fig. 6.
m27f inhibits syncytium formation of HSV-1 and HSV-2. (A) Vero cells were infected with HSV-1 or HSV-2 at an MOI of 5 pfu per cell and then incubated in DMEM containing 2% FBS in the presence of antibodies, m27f (500 μg/ml) and 21C11 (500 μg/ml). Mouse IgG (500 μg/ml), or medium alone were used as control. After 48 h, cells were fixed and then stained with HE staining. The inhibition of syncytium formation was observed by light microscopy. Bar represents 100 μm. The experiments were repeated at least twice. (B) Syncytium formation of Vero cells with different treatment as shown in panel A was quantified by counting the number of nuclei in syncytia of randomly chosen area. The percentage inhibition was determined relative to no antibody treatment group. Only syncytia containing 3 or more nuclei were analyzed. Data were representative of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001.
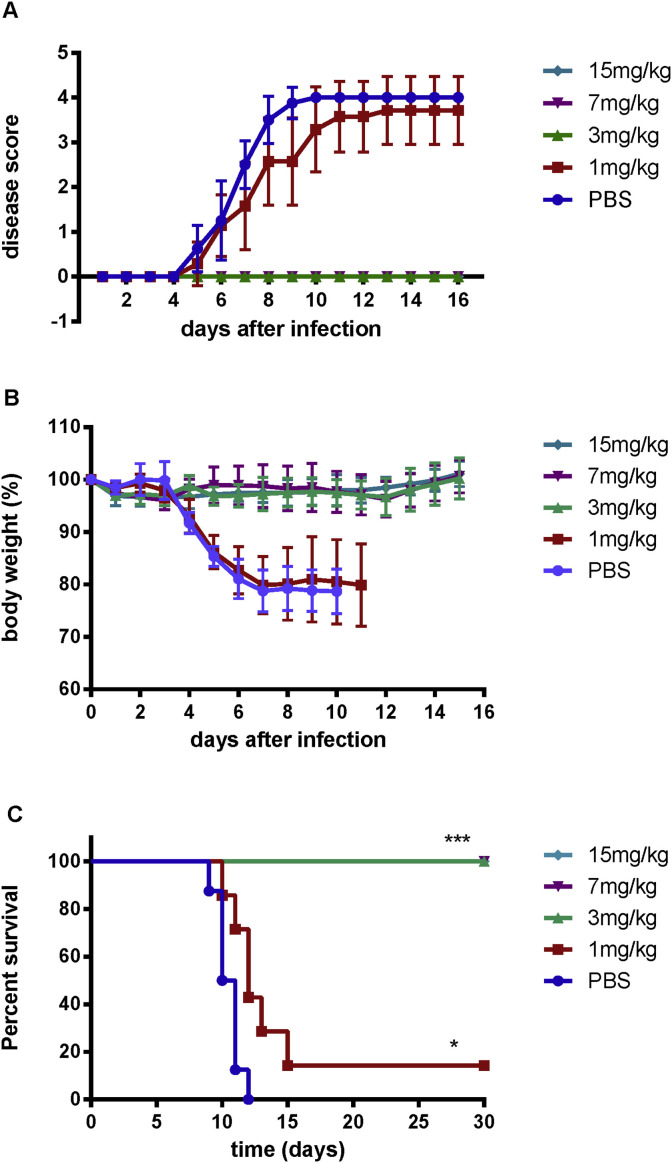
3.7. m27f protects mice from lethal HSV-2 infection
To investigate the protective efficacy of m27f in vivo, we exploited a lethal female BALB/c mice model for HSV infection. To assess the therapeutic efficiency of m27f, mice were randomly assigned to five groups of 7 or 8 each. In PBS-treated group, intravaginal infection of HSV-2 (1 × 106 pfu/20 μl) of six-weeks-old female BALB/c mice resulted in rapid progressive disease (Fig. 7 A) and steady decrease of body weight from day 4 post infection (Fig. 7B), with a median survival time of 10 days (Fig. 7C). The experimental groups of BALB/c mice were treated intravenously with 1, 3, 7, or 15 mg/kg of antibody at 24, 48 and 72 h after intravaginal HSV-2 challenge. As shown in Fig. 7, mice receiving 1 mg/kg of m27f were slightly protected against lethal infection by HSV-2, the body weight of this group decreased as fast as the PBS-treated group, with a median survival time of 2 days longer than those of control mice treated with PBS. However, Mice treated with 3 mg/kg or higher concentrations of m27f were completely protected from lethal HSV-2 infection (Fig. 7C). No symptom of HSV-2 infection (Fig. 7A) or loss of body weight was observed among these groups (Fig. 7B).
Fig. 7.
m27f protects mice from lethal HSV-2 infection. (A) Symptoms of genital disease in each group was scored daily as described in the text for 16 days after intravaginally HSV-2 challenge. Data was shown as the mean values of mice in each group. (B) Mice were weighed daily and body weight are expressed as a percentage of the value prior to treatment. Data represent mean ± standard error. (n = 8 animals per group for PBS; n = 7 for all other groups) (C) Survival of mice in each group monitored for indicated days. (n = 8 animals per group for PBS; n = 7 for all other groups). Data were analyzed by Log-rank (Mantel-Cox) test and Gehan-Breslow-Wilcoxon test. * p < 0.05, *** p < 0.001.
4. Discussion
Glycoprotein D (gD) is the most abundant glycoprotein on the virion and the major stimulus for virus-neutralizing antibodies of HSV. For both vaccine design and novel therapeutic strategies, it is important to study epitopes on gD that stimulate virus-neutralizing antibodies. According to the properties of a panel of mAbs to gD and gD mutants, which were used to define the relationship between gD structure and function, anti-gD mAbs have been previously arranged into groups that recognize distinct discontinuous native and continuous denatured epitopes. Most mAbs that recognize discontinuous native epitopes have virus-neutralizing activity. For example, MC23 (which recognizes residues 213 and 216), DL11 (which recognizes residues 132 and 140), MC5 (which recognizes residues 75–79) and MC2 (which recognizes residue 246) have been reported previously to be neutralizing mAbs that recognize discontinuous conformational epitopes. (Krummenacher et al., 1998, Lazear et al., 2012, Muggeridge et al., 1990, Nicola et al., 1998, Whitbeck et al., 1999). There are also a number of reported mAbs that recognize continuous denatured epitopes of HSV gD. Among these mAbs, most have no or low virus-neutralizing ability, except for the mAb 1D3 (which recognizes residues 10 to 20), which can neutralize viral infectivity by blocking gD-receptor interaction (Cairns et al., 2014). In this study, we found a novel mAb, m27f, to be another virus-neutralizing mAb whose epitope is located within a continuous antigenic determinant.
Epitope mapping assays indicated that m27f recognized conserved residues 292 to 297 which located in the C-terminal pro-fusion domain (residues 260–310) of gD (Fig. 1, Fig. 3). Sequences alignment analysis showed that this epitope is highly conserved among a broad range of type-1 and type-2 HSV strains (data not shown). This may also explain why the recognition specificity of m27f was type-common. It is likely that m27f can be efficacious among divergent isolates yet the breadth of m27f neutralizing activity against a panel of HSV-1 and -2 isolates in vitro need to be performed. In addition, the resistance evaluation of m27f and Alanine scanning of 292–297 should also be carried out in the future.
The recognition specificity of m27f was type-common, and m27f had a high level of HSV virion neutralizing activity (Fig. 2). The epitopes of three reported type-common mAbs, BD78 (which recognizes residues 262 to 272), DL6 (which recognizes residues 272 to 279), and BD66 (which recognizes residues 280 to316), were continuous and also located in the C-terminal pro-fusion domain of gD. However, they had no neutralization activity against both HSV-1 strain KOS and HSV-2 strain 333 (Isola et al., 1989, Lazear et al., 2012). Previously, Hooks and his colleagues found HSV-1, Human and murine CMV and vaccinia could spread in the presence of neutralizing antibodies generated in rabbits (Hooks et al., 1976). Co et al. humanized two murine monoclonal antibodies against HSV gB and gD, which had been shown to neutralizing HSV-1 in vitro, however, neither humanized and murine anti-gD mAb was able to protect against viral spread. While humanized and murine anti-gB mAb protected cells from viral spread (Co et al., 1991). Our research showed that m27f is able to inhibit cell-to-cell spread of HSV-1 and HSV-2 in vitro (Fig. 4), which is an efficient way for HSV to bypass the host immune system through the direct transmission adjacent cells. To our knowledge, m27f is the first epitope-mapped neutralizing mAb against HSV gD reported to be capable of inhibiting cell-to-cell spread of HSV-1 and HSV-2.
Antibodies against entry glycoproteins have the ability to neutralize virions via interfering with receptor binding or subsequent fusion events. For example, antibodies targeting both stalk (S2) and globular (S1) subunits of the spike protein of severe acute respiratory syndrome (SARS) coronavirus were able to abolish the binding between S protein and its cellular receptors. MAbs neutralize HIV by inhibiting the binding of gp120 to the CD4 receptor (Wibmer et al., 2015, Zeng et al., 2006). The above-mentioned neutralizing mAbs MC23 and DL11 neutralize virus by blocking binding of gD to both receptors, HVEM and nectin-1. In addition, there are many reports of mAbs blocking virus entry at the post-receptor-binding step. For instance, neutralizing mAbs specific for the C-terminal domain of the murine leukemia virus (MuLV) surface (SU) envelope protein subunit interfere with a post-attachment event necessary for membrane fusion (Burkhart et al., 2003). MAb 44D11 against the F2 subunit of the Helicoverpa armigera nucleopolyhedrovirus F protein neutralizes virus entry by inhibiting membrane fusion (Zou et al., 2016). Our data from pre-attachment and post-attachment neutralization assays indicated that m27f does not interfere with the virus binding process; instead, it might neutralize HSV entry at the post-binding stage (Fig. 5). By observing HSV-induced membrane fusion following treatment with m27f, we determined that m27f could almost completely inhibit syncytium formation (Fig. 6), which further confirms that m27f could inhibit the membrane fusion event rather than the virus binding process. Consistent with current findings, earlier studies (Cocchi et al., 2004, Fusco et al., 2005) established that the pro-fusion domain (residues 260–310) is required for viral infectivity and fusion but not for receptor binding and the substitutions of some Pro residues in PFD (amino acids 288, 289, 291, and 292) affected steps in entry and fusion. However, the detailed mechanism of how m27f inhibits the HSV fusion process needs further study.
Based on the high level of HSV virion neutralizing activity, we also investigated whether application of m27f could confer protection in vivo by exploiting a lethal HSV-2 intravaginally infected female BALB/c mouse model to evaluate the potency of m27f as therapeutic alternative to counteract HSV infection. Our data demonstrated that mAb m27f conferred protection in a dose-dependent manner and that 3 mg/kg or more of m27f provided full protection (Fig. 7). Consistently, intracutaneously injection human recombinant neutralizing mAb HSV8 specific for gD (type-common and its epitope located in residues 234 to 275) can protect mice effectively when administered systemically (Sanna et al., 1996, Zeitlin et al., 1996). Passive immunization of immunocompetent animals with gD specific mAb HD1 (a type-common neutralizing mAb, and its discontinuous epitope located in residues 1 to 234) postexposure at appropriate times demonstrated protection against 3 HSV-1 strains (HTZ, MP, and a mutant strain mP) and 1 HSV-2 strain G -induced neurological disease (Dix et al., 1981, Pereira et al., 1980). Furthermore, intravaginally administered murine anti-gB mAb post HSV-1 infection conferred full protection in an immunodeficient non-obese diabetic (NOD)/severe combined immunodeficiency (SCID) mouse model (Krawczyk et al., 2011). These results encourage further potency of neutralizing mAbs for clinical therapy against severe HSV diseases.
In conclusion, our results have demonstrated for the first time that mAb m27f targeting a new continuous epitope (residues 292 to 297) within the pro-fusion domain has a high level of virus-neutralizing activity. These finding will enrich the HSV glycoprotein D-specific neutralizing antibodies and will facilitate the development of vaccine design or novel therapeutic strategies.
Acknowledgments
We thank members of our laboratory for discussions and the Core Facility and Technical Support of Wuhan Institute of Virology for technical assistance. This work was supported by grants from the National Science Foundation of China (no. 81501430 and 31621061); the National Key Research and Development Program (2016YFE0113500) from the Ministry of Science and Technology of China; and the European Union's Horizon 2020 EVAg project (no. 653316).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.antiviral.2017.10.013.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Awasthi S., Friedman H.M. Status of prophylactic and therapeutic genital herpes vaccines. Curr. Opin. Virol. 2014;6:6–12. doi: 10.1016/j.coviro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Bag P., Ojha D., Mukherjee H., Halder U.C., Mondal S., Chandra N.S., Nandi S., Sharon A., Sarkar M.C., Chakrabarti S., Chattopadhyay D. An indole alkaloid from a tribal folklore inhibits immediate early event in HSV-2 infected cells with therapeutic efficacy in vaginally infected mice. PloS One. 2013;8 doi: 10.1371/journal.pone.0077937. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Barnabas R.V., Celum C. Infectious co-factors in HIV-1 transmission herpes simplex virus type-2 and HIV-1: new insights and interventions. Curr. HIV Res. 2012;10:228–237. doi: 10.2174/157016212800618156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender F.C., Samanta M., Heldwein E.E., de Leon M.P., Bilman E., Lou H., Whitbeck J.C., Eisenberg R.J., Cohen G.H. Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions. J. Virol. 2007;81:3827–3841. doi: 10.1128/JVI.02710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart M.D., Kayman S.C., He Y., Pinter A. Distinct mechanisms of neutralization by monoclonal antibodies specific for sites in the N-terminal or C-terminal domain of murine leukemia virus SU. J. Virol. 2003;77:3993–4003. doi: 10.1128/JVI.77.7.3993-4003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns T.M., Huang Z.Y., Whitbeck J.C., Ponce de Leon M., Lou H., Wald A., Krummenacher C., Eisenberg R.J., Cohen G.H. Dissection of the antibody response against herpes simplex virus glycoproteins in naturally infected humans. J. Virol. 2014;88:12612–12622. doi: 10.1128/JVI.01930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cena-Diez R., Vacas-Cordoba E., Garcia-Broncano P., de la Mata F.J., Gomez R., Maly M., Munoz-Fernandez M.A. Prevention of vaginal and rectal herpes simplex virus type 2 transmission in mice: mechanism of antiviral action. Int. J. Nanomed. 2016;11:2147–2162. doi: 10.2147/IJN.S95301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary K.L., Pare E., Stamilio D., Macones G.A. Type-specific screening for asymptomatic herpes infection in pregnancy: a decision analysis. BJOG Int. J. Obstet. Gynaecol. 2005;112:731–736. doi: 10.1111/j.1471-0528.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Co M.S., Deschamps M., Whitley R.J., Queen C. Humanized antibodies for antiviral therapy. Proc. Natl. Acad. Sci. U. S. A. 1991;88:2869–2873. doi: 10.1073/pnas.88.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F., Fusco D., Menotti L., Gianni T., Eisenberg R.J., Cohen G.H., Campadelli-Fiume G. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7445–7450. doi: 10.1073/pnas.0401883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de StGroth S.F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J. Immunol. Methods. 1980;35:1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]
- Dix R.D., Pereira L., Baringer J.R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect. Immun. 1981;34:192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeika M., Bashmakova M., Savicheva A., Kolomiec N., Sokolovskiy E., Hallen A., Unemo M., Ballard R.C., Eastern European Network for, S., Reproductive, H Guidelines for the laboratory diagnosis of genital herpes in eastern European countries. Euro Surveill. Bull. Eur. les Mal. Transm. Eur. Commun. Disease Bull. 2010;15 doi: 10.2807/ese.15.44.19703-en. [DOI] [PubMed] [Google Scholar]
- Dropulic L.K., Cohen J.I. The challenge of developing a herpes simplex virus 2 vaccine. Expert Rev. Vaccines. 2012;11:1429–1440. doi: 10.1586/erv.12.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R.J., Atanasiu D., Cairns T.M., Gallagher J.R., Krummenacher C., Cohen G.H. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G.I., Lewis G.K., Ramsay G., Bishop J.M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D. HSV-1 biology and life cycle. Methods Mol. Biol. 2014;1144:1–17. doi: 10.1007/978-1-4939-0428-0_1. [DOI] [PubMed] [Google Scholar]
- Fotouhi F., Soleimanjahi H., Roostaee M.H., Dalimi Asl A. Expression of the herpes simplex virus type 2 glycoprotein D in baculovirus expression system and evaluation of its immunogenicity in Guinea pigs. Iran. Biomed. J. 2008;12:59–66. [PubMed] [Google Scholar]
- Freeman E.E., Weiss H.A., Glynn J.R., Cross P.L., Whitworth J.A., Hayes R.J. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- Fusco D., Forghieri C., Campadelli-Fiume G. The pro-fusion domain of herpes simplex virus glycoprotein D (gD) interacts with the gD N terminus and is displaced by soluble forms of viral receptors. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9323–9328. doi: 10.1073/pnas.0503907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks J.J., Burns W., Hayashi K., Geis S., Notkins A.L. Viral spread in the presence of neutralizing antibody: mechanisms of persistence in foamy virus infection. Infect. Immun. 1976;14:1172–1178. doi: 10.1128/iai.14.5.1172-1178.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola V.J., Eisenberg R.J., Siebert G.R., Heilman C.J., Wilcox W.C., Cohen G.H. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 1989;63:2325–2334. doi: 10.1128/jvi.63.5.2325-2334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk A., Krauss J., Eis-Hubinger A.M., Daumer M.P., Schwarzenbacher R., Dittmer U., Schneweis K.E., Jager D., Roggendorf M., Arndt M.A. Impact of valency of a glycoprotein B-specific monoclonal antibody on neutralization of herpes simplex virus. J. Virol. 2011;85:1793–1803. doi: 10.1128/JVI.01924-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C., Nicola A.V., Whitbeck J.C., Lou H., Hou W., Lambris J.D., Geraghty R.J., Spear P.G., Cohen G.H., Eisenberg R.J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear E., Whitbeck J.C., Ponce-de-Leon M., Cairns T.M., Willis S.H., Zuo Y., Krummenacher C., Cohen G.H., Eisenberg R.J. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J. Virol. 2012;86:1563–1576. doi: 10.1128/JVI.06480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker K.J., Garnett G.P., Schmid G.P. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. World Health Organ. 2008;86:805–812. doi: 10.2471/BLT.07.046128. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker K.J., Magaret A.S., Turner K.M., Vickerman P., Gottlieb S.L., Newman L.M. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PloS One. 2015;10 doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L.A., Knipe D.M. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology. 1996;220:402–413. doi: 10.1006/viro.1996.0328. [DOI] [PubMed] [Google Scholar]
- Muggeridge M.I., Wilcox W.C., Cohen G.H., Eisenberg R.J. Identification of a site on herpes simplex virus type 1 glycoprotein D that is essential for infectivity. J. Virol. 1990;64:3617–3626. doi: 10.1128/jvi.64.8.3617-3626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro D., Paz P., Pereira L. Domains of herpes simplex virus I glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology. 1992;186:99–112. doi: 10.1016/0042-6822(92)90064-v. [DOI] [PubMed] [Google Scholar]
- Nicola A.V., Ponce de Leon M., Xu R., Hou W., Whitbeck J.C., Krummenacher C., Montgomery R.I., Spear P.G., Eisenberg R.J., Cohen G.H. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J. Virol. 1998;72:3595–3601. doi: 10.1128/jvi.72.5.3595-3601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J.C., Jr. Herpes simplex virus infection of the fetus and newborn. Pediatr. Ann. 1994;23:131–136. doi: 10.3928/0090-4481-19940301-06. [DOI] [PubMed] [Google Scholar]
- Parr M.B., Kepple L., McDermott M.R., Drew M.D., Bozzola J.J., Parr E.L. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Investig. J. Tech. Methods Pathol. 1994;70:369–380. [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J.R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect. Immun. 1980;29:724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirestani M., Dalimi A., Sarvi S., Khoramabadi N. Evaluation of immunogenicity of novel isoform of EG95 (EG95-5G1) from Echinococcus granulosus in BALB/C mice. Iran. J. Parasitol. 2014;9:491–502. [PMC free article] [PubMed] [Google Scholar]
- Sanna P.P., De Logu A., Williamson R.A., Hom Y.L., Straus S.E., Bloom F.E., Burton D.R. Protection of nude mice by passive immunization with a type-common human recombinant monoclonal antibody against HSV. Virology. 1996;215:101–106. doi: 10.1006/viro.1996.0011. [DOI] [PubMed] [Google Scholar]
- Sartori E., Calistri A., Salata C., Del Vecchio C., Palu G., Parolin C. Herpes simplex virus type 2 infection increases human immunodeficiency virus type 1 entry into human primary macrophages. Virol. J. 2011;8:166. doi: 10.1186/1743-422X-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Iwasaki A. Generating protective immunity against genital herpes. Trends Immunol. 2013;34:487–494. doi: 10.1016/j.it.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.S., Herrero R., Bosetti C., Munoz N., Bosch F.X., Eluf-Neto J., Castellsague X., Meijer C.J., Van den Brule A.J., Franceschi S., Ashley R., International Agency for Research on Cancer Multicentric Cervical Cancer Study, G Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J. Natl. Cancer Inst. 2002;94:1604–1613. doi: 10.1093/jnci/94.21.1604. [DOI] [PubMed] [Google Scholar]
- Steiner I., Benninger F. Update on herpes virus infections of the nervous system. Curr. Neurol. Neurosci. Rep. 2013;13:414. doi: 10.1007/s11910-013-0414-8. [DOI] [PubMed] [Google Scholar]
- Whitbeck J.C., Muggeridge M.I., Rux A.H., Hou W., Krummenacher C., Lou H., van Geelen A., Eisenberg R.J., Cohen G.H. The major neutralizing antigenic site on herpes simplex virus glycoprotein D overlaps a receptor-binding domain. J. Virol. 1999;73:9879–9890. doi: 10.1128/jvi.73.12.9879-9890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibmer C.K., Moore P.L., Morris L. HIV broadly neutralizing antibody targets. Curr. Opin. HIV AIDS. 2015;10:135–143. doi: 10.1097/COH.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin L., Whaley K.J., Sanna P.P., Moench T.R., Bastidas R., De Logu A., Williamson R.A., Burton D.R., Cone R.A. Topically applied human recombinant monoclonal IgG1 antibody and its Fab and F(ab')2 fragments protect mice from vaginal transmission of HSV-2. Virology. 1996;225:213–215. doi: 10.1006/viro.1996.0589. [DOI] [PubMed] [Google Scholar]
- Zeng F., Hon C.C., Yip C.W., Law K.M., Yeung Y.S., Chan K.H., Malik Peiris J.S., Leung F.C. Quantitative comparison of the efficiency of antibodies against S1 and S2 subunit of SARS coronavirus spike protein in virus neutralization and blocking of receptor binding: implications for the functional roles of S2 subunit. FEBS Lett. 2006;580:5612–5620. doi: 10.1016/j.febslet.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Cao X., Tang J., Zhou L., Gao Y., Wang J., Zheng Y., Yin S., Wang Y. A novel multiplex real-time PCR assay for the detection and quantification of HPV16/18 and HSV1/2 in cervical cancer screening. Mol. Cell. Probes. 2012;26:66–72. doi: 10.1016/j.mcp.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Zou Z., Liu J., Wang Z., Deng F., Wang H., Hu Z., Wang M., Zhang T. Characterization of two monoclonal antibodies, 38F10 and 44D11, against the major envelope fusion protein of Helicoverpa armigera nucleopolyhedrovirus. Virol. Sin. 2016;31:490–499. doi: 10.1007/s12250-016-3831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.