Abstract
Lectins are natural bioactive ubiquitous proteins or glycoproteins of non-immune response that bind reversibly to glycans of glycoproteins, glycolipids and polysaccharides possessing at least one non-catalytic domain causing agglutination. Some of them consist of several carbohydrate-binding domains which endow them with the properties of cell agglutination or precipitation of glycoconjugates. Lectins are rampant in nature from plants, animals and microorganisms. Among microorganisms, algae are the potent source of lectins with unique properties specifically from red algae. The demand of peculiar and neoteric biologically active substances has intensified the developments on isolation and biomedical applications of new algal lectins. Comprehensively, algal lectins are used in biomedical research for antiviral, antinociceptive, anti-inflammatory, anti-tumor activities, etc. and in pharmaceutics for the fabrication of cost-effective protein expression systems and nutraceutics. In this review, an attempt has been made to collate the information on various biomedical applications of algal lectins.
Keywords: Anti-HIV, antinociceptive, biomedical applications, cytokines, glycoproteins
Introduction
Lectins are proteins/glycoproteins of non-immune origin that bind non-covalently and reversibly to aposing cells bearing specific sugars culminating their agglutination (Singh et al., 2011a). Stillmark (1888) enunciated first lectin (then called hemagglutinin) from seeds of Ricinus communis. After that thousands of lectins have been isolated from different sources including plant seeds and roots, bacteria, algae, fungi, body fluid of invertebrates, lower vertebrates and mammalian cell membranes (Singh et al., 1999). They are type cast with respect to carbohydrate-binding specificity, molecular structure, biochemical and biomedical properties. Among microbes, occurrence of lectins has been widely reported from algae and mushrooms (Singh et al., 2010).
Algae are amidst the most diverse organisms in the plant kingdom. They are photosynthetic, mainly aquatic organisms, devoid of vascular tissues, true roots, stems, leaves and possess simple reproductive structures. According to the latest system of classification based on ultra structure of the plastid, algae are classified into four groups which are further subdivided into eight divisions (Lee, 1999): group 1 – prokaryotic algae containing division (1) Cyanophyta; group 2 – eucaryotic algae containing divisions (2) Glaucophyta, (3) Rhodophyta, (4) Chlorophyta; group 3 – eucaryotic algae containing divisions (5) Euglenophyta and (6) Dinophyta; group 4 – eucaryotic algae containing division (7) Heterokontophyta and (8) Pyrmnesiophyta.
The presence of agglutinins in marine algae was firstly reported by Boyd et al. (1966). Later on, lectins have been reported from a large number of algae. Algal lectins are generally referred to as phycolectins (Matsubara et al., 1996; Rogers et al., 1977) and they differ from plant lectins in a variety of physico-chemical characteristics. In general, marine algal lectins are monomeric, low molecular weight proteins, exhibiting high content of acidic amino acids, with isoelectric point (pI) in the range of 4–6, do not require metal ions for their biological activities and most of them show specificity for glycoproteins than monosaccharides (Hori et al., 1990; Rogers & Hori, 1993; Shiomi et al., 1981). Based on the binding properties to glycoproteins, algal lectins are categorized into three major categories, complex type N-glycan specific lectins, high mannose (HM) type N-glycan specific lectins and lectins with specificity to both the above types of N-glycans (Hori et al., 1990). Mannose binding lectins are considered essential as they interplay with cell-surface glycoconjugates. Due to their small size and presence of disulphide linkages, algal lectins are antigenic and highly stable. Furthermore, the peculiar small structure of algal lectins makes them more expedient for use as specific molecular diagnostic probes against the cell surface carbohydrates and in drug targeting (Nascimento et al., 2006). Recently, algal lectins have received greater attention due to their robust oligosaccharide-binding specificity (Okuyama et al., 2009).
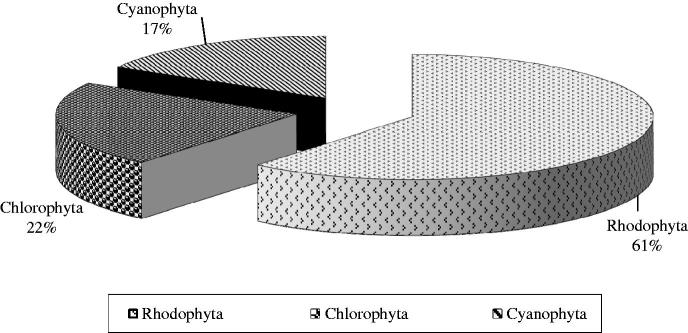
Lectins are the most versatile group of proteins used in biological and biomedical research. They posses enormous potential as they play a major role in cell–cell recognition (Singh et al., 2011b) and are widely used in drug delivery (Singh et al., 1999). Algal lectins have various biomedical properties such as anti-tumor, anti-HIV, anti-inflammatory, anti-fungal, anti-microbial, etc. (Nascimento et al., 2012; Swamy, 2011; Teixeira et al., 2012). The occurrence of biomedically important lectins among various divisions of algae is represented in Figure 1.
Figure 1.
Distribution of biomedically important lectins in algae.
Biological action spectrum of biomedically important algal lectins
Lectins have the property of adherence to sugars on cell-membranes, thereby reforming the physiology of membrane which leads to agglutination and other biochemical changes in cells (Neves et al., 2007). Algal lectins have been detected using animal erythrocytes as well as human blood type erythrocytes. The susceptibility of erythrocytes to certain lectins increases upon mild treatment with proteolytic enzymes which leads to exposure of cryptic residues present on erythrocytes surface (Sharon & Lis, 1972). The biological action spectrum of biomedically important algal lectins is summarized in Table 1.
Table 1. Biological action spectrum of biomedically important algal lectins.
| Hemagglutination activity |
Reference(s) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Algae | Animal erythrocytes |
Human erythrocytes |
|||||||||||||
| Sheep | Rabbit | Chicken | Rat | Horse | Goat | Pig | Cow | Mouse | A | B | AB | O | |||
| Blue-green algae | |||||||||||||||
| Chlorella sp.I | − | ND | ND | ND | ND | ND | − | − | ND | + | + | ND | + | Chu et al. (2007) | |
| Chlorella sp. 21 | + | ND | ND | ND | ND | ND | + | + | ND | + | + | ND | + | Chu et al. (2007) | |
| Chlorella sp.W | − | ND | ND | ND | ND | ND | + | − | ND | + | + | ND | + | Chu et al. (2007) | |
| Microcystis aeruginosa | ND | + | ND | ND | + | ND | ND | ND | ND | + | + | ND | + | Yamaguchi et al. (1998) | |
| Microcystis aeruginosa | + | + | ND | + | ND | ND | ND | ND | + | + | − | ND | + | Watanabe et al. (1987) | |
| M. viridisa | ND | + | ND | ND | + | ND | ND | ND | ND | − | − | ND | − | Yamaguchi et al. (1999) | |
| Oscillatoria agardhii | Ha | Hb | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Sato et al. (2000) | |
| Green algae | |||||||||||||||
| Boodlea coacta | ND | Hb | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Hori et al. (1986) | |
| Bryopsis hypnoides | ND | ND | Hb | ND | ND | ND | ND | ND | − | − | − | − | Hb | Niu et al. (2009) | |
| B. pennata | − | Hb | Hb | ND | ND | − | ND | Hb | ND | − | Hb | Hb | Hb | Ainouz & Sampaio (1991) | |
| B. plumosa | + | ND | ND | ND | + | ND | ND | ND | ND | − | − | ND | − | Han et al. (2010) | |
| ND | ND | ND | ND | ND | ND | ND | ND | ND | + | ND | ND | ND | Jung et al. (2010) | ||
| Caulerpa cupressoides | Hb | Hb | Hb | ND | ND | − | ND | Hb | ND | Hb | Hb | Hb | Hb | Ainouz & Sampaio (1991) | |
| − | Hd | Hc | ND | ND | ND | ND | ND | ND | Hd | Hd | ND | Hd | Freitas et al. (1997) | ||
| ND | Hb | ND | ND | ND | ND | ND | ND | ND | Hb | Hb | ND | Hb | Benevides et al. (2001) | ||
| Ulva pertusa | ND | + | − | ND | ND | ND | ND | ND | ND | − | − | ND | − | Wang et al. (2004) | |
| U. rigida | + | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Bird et al. (1993) | |
| Red algae | |||||||||||||||
| Bryothamnion seaforthii | − | Hb | Hb | ND | ND | − | ND | Hb | ND | − | − | − | − | Ainouz & Sampaio (1991) | |
| B. triquetrum | ND | Hb | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Ainouz et al. (1995) | |
| Eucheuma serra | + | Hb | ND | ND | − | ND | ND | ND | ND | ND | ND | ND | ND | Kawakubo et al. (1997) | |
| Hb | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Kawakubo et al. (1999) | ||
| Gracilaria cervicornis | − | Hb | Hb | ND | ND | − | ND | − | ND | − | − | − | − | Ainouz & Sampaio (1991) | |
| G. cornea | − | − | Hb | ND | ND | − | ND | − | ND | − | − | − | − | Ainouz & Sampaio (1991) | |
| G. ornata | ND | Hb | Hb | ND | ND | ND | ND | ND | ND | − | − | ND | − | Leite et al. (2005) | |
| G. tikvahiae | + | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Bird et al. (1993) | |
| + | + | ND | ND | ND | ND | ND | ND | ND | + | + | ND | − | Chiles & Bird (1990) | ||
| G. tikvahiae G-3 | + | + | ND | ND | ND | ND | ND | ND | ND | + | + | ND | + | Chiles & Bird (1989) | |
| G. tikvahiae McLachlan (NC) | + | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Bird et al. (1993) | |
| G. verrucosab,c | He | − | Hb | ND | ND | ND | ND | ND | ND | − | − | ND | − | Freitas et al. (1997) | |
| + | + | + | + | + | ND | + | + | ND | ND | ND | ND | ND | Kakita et al. (1999) | ||
| + | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Bird et al. (1993) | ||
| + | + | + | ND | + | ND | ND | + | ND | ND | ND | ND | ND | Shiomi et al. (1981) | ||
| G. verrucosa G-16 S | + | + | ND | ND | ND | ND | ND | ND | ND | + | + | ND | − | Chiles & Bird (1989) | |
| Hypnea cervicornis | − | Hb | − | ND | ND | − | ND | Hb | ND | − | − | − | − | Ainouz & Sampaio (1991) | |
| H. japonica | + | + | + | ND | + | ND | ND | ND | ND | + | + | ND | + | Hori et al. (1986) | |
| H. musciformis | + | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Bird et al. (1993) | |
| Hb | Hb | − | ND | ND | − | ND | Hb | ND | Hb | Hb | Hb | Hb | Ainouz & Sampaio (1991) | ||
| Kappaphycus alvarezii | Hg | Hb | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Hung et al. (2009) | |
| K. striatum | + | Hg | − | ND | ND | ND | ND | ND | ND | − | − | ND | − | Hung et al. (2011) | |
| Ptilota plumosa | ND | ND | ND | ND | ND | ND | ND | ND | ND | Hf | + | ND | Hf | Sampaio et al. (2002) | |
| P. serrata | ND | ND | ND | ND | ND | ND | ND | ND | ND | + | + | ND | + | Sampaio et al. (1999) | |
| Serraticardia maximab | Hh | Hh | Hh | ND | Hh | ND | ND | Hh | Hh | ND | ND | ND | ND | Shiomi et al. (1980) | |
| Solieria robusta | ND | Hb | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Matsubara et al. (1996) | |
| Tichocarpus crinitus | ND | + | ND | + | ND | ND | ND | ND | ND | + | + | ND | + | Molchanova et al. (2010) | |
| ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | + | Chernikov et al. (2007) | ||
+: positive haemagglutination; −: no haemagglutination; ND: haemagglutination not determined.
aHaemagglutination activity also with hen erythrocytes.
bHaemagglutination activity also with guinea pig erythrocytes.
cHaemagglutination activity also with carp erythrocytes.
dHaemagglutination activity also with goose erythrocytes.
Ha: haemagglutination activity with pronase treated erythrocytes.
Hb: haemagglutination activity with trypsin treated erythrocytes.
Hc: haemagglutination activity with bromelain treated erythrocytes.
Hd: haemagglutination activity with native, trypsin, bromelain, papain and subtilisin treated erythrocytes.
He: haemagglutination activity with bromelain and papian treated erythrocytes.
Hf: haemagglutination activity with native and papain treated erythrocytes.
Hg: haemagglutination activity with native, trypsin and papain treated erythrocytes.
Hh: haemagglutination activity with native, trypsin and protease treated erythrocytes.
Animal erythrocytes especially from sheep and rabbit have been reported to be more suitable for lectin detection in marine algae than human erythrocytes (Freitas et al., 1997). The extracts of Microcystis viridis induced agglutination in hen, rabbit and horse erythrocytes, but no agglutination has been reported with human erythrocytes (Yamaguchi et al., 1999). Hori et al. (1988) screened a plethora of marine algae for hemagglutinins. They concluded that marine algal agglutinins are most sensitive to protease treated sheep erythrocytes followed by native rabbit and sheep erythrocytes, but not to human and chicken red blood cells. Caulerpa cupressoides lectin agglutinated trypsin treated sheep, rabbit and chicken erythrocytes (Ainouz & Sampaio, 1991). Lectin activity increased significantly when rabbit red blood cells were treated with trypsin, bromelain, papain and subtilisin, but chicken erythrocytes treated with only bromelain showed agglutination (Freitas et al., 1997). Serraticardia maxima lectin has been reported to agglutinate native, trypsin and papain treated erythrocytes of horse, cow, sheep, rabbit, guinea pig, mouse and chicken. Non-treated horse erythrocytes were most agglutinated, while non-treated cow erythrocytes were least agglutinated (Shiomi et al., 1980). Lectin from Ulva rigida promoted agglutination of sheep and rabbit erythrocytes (Bird et al., 1993), whereas lectin from Ulva pertusa specifically agglutinated rabbit erythrocytes (Wang et al., 2004).
Bryothamnion seaforthii lectin has been found to agglutinate both native and trypsin treated rabbit as well as trypsin treated chicken and cow erythrocytes (Ainouz & Sampaio 1991; Ainouz et al., 1995; Vieira et al., 2004). Lectin from Bryothamnion triquetrum has been shown to agglutinate enzyme treated erythrocytes from rabbit, chicken, goat and pig (Ainouz et al., 1992). Sheep and rabbit erythrocytes were found sensitive to Gracilaria sp. lectin (Bird et al., 1993; Chiles & Bird, 1990), while extracts from Eucheuma serra agglutinated both native and trypsin treated sheep erythrocytes as well as trypsin treated rabbit erythrocytes (Kawakubo et al., 1997). Trypsin treated rabbit and chicken erythrocytes were sensitive to crude extract of red algae Gracilaria ornata, whereas no agglutination has been reported against native and trypsin treated human erythrocytes (Leite et al., 2005). The extract of Oscillatoria agardhii agglutinated both trypsin treated red blood cells of rabbit and pronase treated erythrocytes of sheep (Sato et al., 2000; Sato & Hori, 2009).
The agglutination of blood type A, B and O erythrocytes occurs due to robust binding of lectins to the N-acetyl-d-galactosamine, d-galactose and l-fucose moieties, respectively present on their surface (Khan et al., 2002). The extracts of Chlorella sp. I, Chlorella sp. 21 and Chlorella sp. W have been reported to agglutinate blood type A, B and O erythrocytes (Chu et al., 2007). When native erythrocytes were used, both the crude extract and pure lectin of Ptilota plumosa was found to be specific towards human blood group B erythrocytes. After papain treatment, only the pure lectin showed blood type B specificity, whereas the crude extract also showed low agglutination with blood group A and O erythrocytes (Sampaio et al., 2002). Human A-type specific agglutinating activity has been reported from Bryopsis plumosa (Jung et al., 2010). Native, trypsin and bromelain treated erythrocytes from mouse, chicken and humans were used to determine the blood specificity of Bryopsis hypnoides lectin. The lectin exhibited a preference for trypsin treated human blood group O and chicken erythrocytes (Niu et al., 2009). Enzyme treated erythrocytes of human ABO blood type were agglutinated by B. triquetrum (Ainouz et al., 1992).
Characteristics of biomedically important algal lectins
The binding specificity of lectins is established by the shape of binding site and the amino-acid residues to which the carbohydrate is linked. Alterations in the binding site can essentially change their specificity. Algal lectins display a considerable repertoire of carbohydrate specificities and physico-chemical characteristics. The carbohydrate specificity and characteristics profile of biomedically important algal lectins is summarized in Table 2. Most of the red algal lectins have high content of acidic and hydroxyl amino acids, but lower levels of basic amino acids. They have low molecular weight, show high affinity to glycoproteins and do not require divalent cations for their biological activity (Hori et al., 1990; Okamoto et al., 1990; Rogers & Hori, 1993; Sampaio et al., 1999).
Table 2. Characteristics of biomedically important algal lectins.
| Algae | Inhibitory sugars/Glycoproteins* | Lectin characteristics | Reference(s) |
|---|---|---|---|
| Blue-green algae | |||
| Microcystis aeruginosa | N-acetyl-d-galactosamine | Monomer, Mr 57 kDa, pI 6.4, rich in Asx & Arg, carbohydrate content 7.8% | Yamaguchi et al. (1998) |
| M. viridis | Yeast mannan, oligomannosides such as Man9GlcNAc2 | Homodimer in solution, 113 amino acid residues, Mr 13 kDa, pH stability 5–8 | Yamaguchi et al. (1999); Ziolkowska & Wlodawer (2006) |
| Nostoc ellipsosporum | Man9GlcNAc2 | Monomer, Mr 11 kDa, 101 amino acid residues | Boyd et al. (1997); Ziolkowska & Wlodawer (2006) |
| Oscillatoria agardhii | High-mannose (HM)-type N-glycans | Mr 13.9 kDa, belongs to new lectin family NIES-204 | Sato et al. (2007) |
| Scytonema varium | Man8GlcNAc2/Man9GlcNAc2, α (1–2), α (1–6)Man | Monomeric, Mr 9.7 kDa, 95 amino acid residues | Li et al. (2008); Ziolkowska & Wlodawer (2006) |
| Green algae | |||
| Boodlea coacta | High-mannose N-glycans | Mr 13.8 kDa | Sato et al. (2011b) |
| Bryopsis hypnoides | N-acetyl galactosamine, N-acetyl glucosamine, bovine submaxillary mucin | Mr 27 kDa, pI∼5–6, pH stability 4–10, hemagglutination activity independent of divalent cations | Niu et al. (2009) |
| B. plumosa | d-mannose, α-methyl-d-mannose, l-fucose N-acetyl-d-galactosamine, N-acetyl-d-glucosamine | Monomer, Mr 17 kDa, pI 7.3, hemagglutination activity independent of divalent cations, thermal stability upto 70 °C for 30 min | Han et al. (2010) |
| Caulerpa cupressoides | Raffinose, lactose, galactose and fructose, derivatives of galactose, porcine stomach mucin | Homodimer, Mr 44.7 kDa, carbohydrate content 11.05% | Freitas et al. (1997); Vanderlei et al. (2010) |
| Ulva pertusa | N-acetyl-d-glucosamine, bovine thyroglobulin | Mr 23 kDa, pH stability 6–8, thermal stability upto 70 °C for 30 min, hemagglutination activity dependent on divalent cations, carbohydrate content 1.2% | Wang et al. (2004) |
| Red algae | |||
| Amansia multifida | Avidin | Monomer, Mr 26.9 kDa, carbohydrate content 2.9% | Neves et al. (2007) |
| Bryothamnion seaforthii | Feutin, avidin, mucin | Monomeric, Mr 4.5 kDa, hemagglutination activity independent of divalent cations, thermal stability upto 90 °C for 30 min | Ainouz et al. (1995) |
| B. triquetrum | Feutin, avidin, mucin | Monomeric, Mr 3.5 kDa, 91 amino acid residues, hemagglutination activity independent of divalent cations, thermal stability upto 90 °C for 30 min | Ainouz et al. (1995); Calvete et al. (2000) |
| Eucheuma serra | Yeast mannan, IgM (mouse), tyroglobulin, high-mannose (HM)-type N-glycans | Monomeric, Mr 29 kDa, pH stability 2.5–10.5, pI 4.95, thermal stability upto 60 °C for 1 h, no carbohydrate content | Kawakubo et al. (1997) |
| Gracilaria cornea | Feutin, porcine stomach mucin | Monomeric, Mr 60 kDa, pI 4.3, hemagglutination activity independent of divalent cations, thermal stability upto 40 °C for 20 min, carbohydrate content 52.5% | Lima et al. (2005) |
| G. ornata | Porcine stomach mucin, lactotransferrin, asialofetuin, bovine & porcine thyroglobulins | Monomeric, Mr 17 kDa, pI 5.4, rich in Asx, Glx, Ser, Glu, Ala, Cys, thermal stability upto 50 °C for 60 min, carbohydrate content 2.9% | Leite et al. (2005) |
| G. tikvahiae | N-acetylneuraminic acid, glycoconjugates containing N-acetylneuraminic acid | Mr 29.7 kDa, hemagglutination activity independent of divalent cations | Chiles & Bird (1990) |
| G. verrucosa | No inhibition activity with simple sugars | Tetramer, Mr 41 kDa, subunit Mr 12 kDa & Mr 10.5 kDa, pH 4–12, pI 4.8, hemagglutination activity independent of divalent cations, thermal stability upto 40 °C for 30 min, rich in Gly & hydroxyl amino acids | Shiomi et al. (1981) |
| Griffithsia sp. | Glucose, mannose, N-acetylglucosamine | Dimeric, Mr 12.7 kDa, 121 amino acid residues | Mori et al. (2005); Ziolkowska & Wlodawer (2006) |
| Hypnea cervicornis | N-acetyl-d-galactosamine, bovine submaxillary mucin, desialylated ovine submaxillary mucin, porcine stomach mucin, asialofetuin | Mr 9.1 kDa | Nagano et al. (2005) |
| H. japonica | Complex N-glycans (transferrin, fetuin, α-acid glycoprotein), O-glycans (feutin & mucin), asialofeutin, asialomucin, glycopeptides prepared from asialofeutin, core (α1-6) fucosylated glycans | Small-sized isolectins, Mr 9.1 kDa (hypnin-1 & hypnin-2) | Hori et al. (2000); Okuyama et al. (2009) |
| H. musciformis | Porcine stomach mucin, bovine submaxillary mucin, desialylated ovine submaxillary Mucin | Mr 9.3 kDa | Nagano et al. (2005) |
| Kappaphycus alvarezii | Porcine thyroglobulin, bovine thyroglobulin, asialo-porcine thyroglobulin, asialo bovine thyroglobulin, yeast mannan | Monomeric, Mr 28 kDa, hemagglutination activity independent of divalent cations, pH stability 3–10, thermal stability upto 50 °C for 30 min, no carbohydrate content | Hung et al. (2009) |
| K. striatum | Glycoproteins bearing high-mannose-type N-glycans, porcine and bovine thyroglobulins, their asialo-derivatives and yeast mannan | Monomeric, Mr 28 kDa, pH stability 3–10, thermal stability upto 60 °C for 30 min, hemagglutination activity independent of divalent cations | Hung et al. (2011) |
| Pterocladiella capillacea | Avidin, porcine stomach mucin | Monomeric, Mr 5.8 kDa, hemagglutination activity independent of divalent cations, pH stability 7–10, thermal stability upto 60 °C for 30 min | Oliveira et al. (2002) |
| Ptilota filicina | p-nitrophenyl-N-acetyl-α-and-β-d-galactosaminide, porcine stomach mucin, bovine submaxillary gland mucin, asialo bovine mucin | Homotrimer, Mr 56.9 kDa, rich in acidic & hydroxyl amino acids, hemagglutination activity dependent on divalent cations, thermal stability upto 50 °C for 30 min | Sampaio et al. (1998) |
| P. plumosa | Galactose, glucose and their derivatives with p-nitrophenyl-α-d-galactoside | Homotrimer, Mr 52.5 kDa, rich in acidic amino acids. | Sampaio et al. (2002) |
| P. serrata | o-nitrophenyl-N-acetyl-α-d-galactoside, p-nitrophenyl-N-acetyl-β-d-galactoside, lactose, porcine stomach mucin, asialo bovine mucin and asialofetuin | Homotrimer, Mr 55.4 kDa, rich in acidic & hydroxyl amino acids. | Sampaio et al. (1999) |
| Serraticardia maxima | No inhibition activity with simple sugars | Mr 25 kDa, pH stability 4–10, hemagglutination activity independent of divalent cations | Shiomi et al. (1980) |
| Solieria filiformis | Mannan, avidin, ovalbumin, egg white | Mr 29 kDa | Benevides et al. (1996) |
| Tichocarpus crinitus | Porcine stomach mucin (type VII), feutin | Monomeric, Mr 41 kDa, pI 4.93, rich in acidic amino acids, hemagglutination activity independent of divalent cations, carbohydrate content 6.9% | Molchanova et al. (2010) |
*Only most specific are enlisted.
Lectin (OAA) from Oscillatoria agardhii belongs to a new lectin family NIES-204 and arrayed high binding specificity for high-mannose N-glycans and gp120 (envelope protein of HIV) in picomolar range (Sato & Hori, 2009). Lectin (MVL) isolated from M. viridis shown inhibition activity with yeast mannan, whereas lectin (SVN) from Scytonema varium shown the specificity for Man8GlcNAc2/Man9GlcNAc2 (Li et al., 2008; Yamaguchi et al., 1999). Cyanovirin-N (CV-N) lectin of 11 kDa from Nostoc ellipsosporum showed specificity towards Man9GlcNAc2 (Ziolkowska & Wlodawer, 2006). The structural integrity of CV-N lectin has been reported to be highly resistant to degradation upon treatment with detergents, organic solvents, freezing and heating up to 100 °C (Boyd et al., 1997).
Green algae lectin isolated from Bryopsis hypnoides shown specificity for N-acetyl glucosamine, N-acetyl galactosamine and bovine submaxillary mucin and was of 27 kDa having pI 5–6 (Niu et al., 2009). The lectin was stable in a pH range of 4–8 and does not require metal ions for hemagglutination activity. The lectin from U. pertusa exhibited carbohydrate content of 1.2% with molecular weight of 23 kDa, thermal stability up to 70 °C for 30 min and carbohydrate specificities for N-acetyl-d-glucosamine and bovine thyroglobulin (Wang et al., 2004). C. cupressoides lectin (CcL) displayed specificity for simple sugars like raffinose, lactose, galactose and fructose, derivatives of galactose and porcine stomach mucin. The molecular weight of the lectin was 44.7 kDa and it had carbohydrate content of 11.05% (Benevides et al., 2001; Freitas et al., 1997).
The three isolectins isolated from Kappaphycus alvarezii revealed their monomeric nature, having molecular weight of 28 kDa and moreover, displayed affinity for glycoproteins bearing high-mannose N-glycans (Hung et al., 2011). Lectin KAA-2 shared physico-chemical properties with ESA-2 lectin from E. serra (Sato et al., 2011a). Small-sized (9 kDa) isolectins (hypnin A1, A2, A3) from Hypnea japonica belonged to a new lectin family and showed no affinity for monosaccharides. These isolectins have been reported to bind only to core (α1-6) fucosylated N-gycans which makes them a valuable tool for cancer diagnosis and quality control of medicinal antibodies (Okuyama et al., 2009). Amansia lectin of 26.9 kDa isolated from Amansia multifida contained 2.9% neutral carbohydrates and showed specificity for avidin (Costa et al., 1999; Neves et al., 2007). Tichocarpus crinitus lectin (TCL) is an acidic glycoprotein with pI 4.93, containing 6.9% carbohydrate content and its amino acid content revealed the presence of aspartic acid and glutamic acid residues (Molchanova et al., 2010). Thermostable fetuin, avidin and mucin specific lectins have been reported from B. seaforthii and B. triquetrum with molecular weight of 4.5 kDa and 3.5 kDa, respectively (Ainouz et al., 1995). The sugar inhibition studies on lectins having molecular weight 41 kDa and 25 kDa purified from S. maxima and Gracilaria verrucosa, respectively, revealed that both are not inhibited by simple sugars (Shiomi et al., 1980; 1981).
Biomedical applications of algal lectins
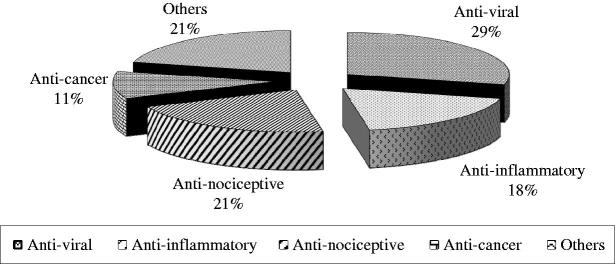
Several bioactivities have been attributed to algal lectins which include anti-inflammatory, anti-adhesion, anti-HIV, antinociceptive, antibiotic, mitogenic and human platelet aggregation inhibition activities (Harnedy & FitzGerald, 2011). The ability of these lectins to stimulate lymphocytes as well as other cells has made them important tools for experiments and diagnostics. Biomedical potential of various algal lectins is depicted in Figure 2. The most abeyant biomedical applications of algal lectins are summarized in Table 3.
Figure 2.
Biomedical applications of algal lectins.
Table 3. Biomedical applications of algal lectins.
| Algae | Lectin designated | Biomedical application(s) | Reference(s) | |
|---|---|---|---|---|
| Blue-green algae | ||||
| Microcystis viridis | MVL | Antiviral activity (EC50 = 30 mM, IC50 30 nM). | Bewley et al. (2004) | |
| Nostoc ellipsosporum | Cyanovirin-N (CV-N) | Anti-HIV activity in vitro (IC50 = 1.8 nM) (EC50 = 0.1 nM). Antiviral activity against Ebola virus (EC50 = 100 nM). Antiviral activity against Influenza A and B virus (EC50 = 0.004–0.5 μg/ml). | Barrientos et al. (2003); Boyd et al. (1997); O’Keefe et al. (2003) | |
| Oscillatoria agardhii | OAA | Inhibits HIV replication in MT-4 cells (EC50 = 44.5 nM). | Sato et al. (2007) | |
| Scytonema varium | SVN | Neutralizes both laboratory-adapted strains and primary isolates of HIV1 activity (EC50 = 0.3 & IC50 = 20.1). | Alexandre et al. (2010) | |
| Green algae | ||||
| Boodlea coacta | BCA | Anti-HIV activity in vitro in MT-4 cells (EC50 = 8.2 nM) & Anti-influenza activity inhibiting replication of influenza virus in MDCK cells | Sato et al. (2011b) | |
| Bryopsis hypnoides | – | Mediates protoplast regeneration. | Niu et al. (2009) | |
| B. plumosa | Bryohealin | Wound-healing properties. | Jung et al. (2010) | |
| Caulerpa cupressoides | CcL | Antinociceptive & anti-inflammatory effect. | Vanderlei et al. (2010) | |
| Ulva rigida | – | Stimulated mitogenesis in murine splenocytes | Bird et al. (1993) | |
| Brown algae | ||||
| Laminaria diabolica | Diabolin | Causes the development of fertilization envelope around unfertilized eggs of sea urchin (Hemicentrotus pulcherrimus). | Smit (2004) | |
| Red algae | ||||
| Amansia multifida | LEC | Antinociceptive properties. | Neves et al. (2007) | |
| Amansin | Stimulated dose dependent proliferation of human PBMC (peripheral blood mononuclear cells). Induces interferon (IFN-γ) production and neutrophil migration in vivo & in vitro. | Lima et al. (1998); Neves et al. (2001) | ||
| Bryothamnion seaforthii | BSL | Differentiate human colon carcinoma cell variants. | Pinto et al. (2009) | |
| BSL | Antinociceptive activity. | Vieira et al. (2004); Viana et al. (2002) | ||
| BSL | Block adherence of Streptococci to acquired pellicle in vitro. | Teixeira et al. (2007) | ||
| B. triquetrum | BTL | Differentiate human colon carcinoma cell variants | Pinto et al. (2009) | |
| BTL | Vasorelaxant effect. | Lima et al. (2004) | ||
| BTL | Antinociceptive activity. | Viana et al. (2002) | ||
| Eucheuma serra | ESA | Cytotoxic against cancer cell lines. ESA-immobilized lipid vesicles effectively bind to cancer cell lines. | Sugahara et al. (2001) | |
| ESA | ESA-immobilized onto span80 vesicles shows anti-tumor activity in vitro and in vivo | Omokawa et al. (2010) | ||
| Gracilaria cornea | GCL | Acaricidal activity. | Lima et al. (2005) | |
| G. verrucosa HBOI strain G-16 S | – | Mitogenic for murine splenocytes. | Bird et al. (1993) | |
| G. tikvahiae HBOI strain G-3 | – | Mitogenic for human lymphocytes & murine splenocytes. | Bird et al. (1993) | |
| G. tikvahiae HBOI strain G-5 | – | Mitogenic for murine splenocytes. | Bird et al. (1993) | |
| Griffithsia sp. | Griffithsin (GRFT) | Inhibit HIV-1 virus (IC50 = 0.4 nM). | Alexandre et al. (2010) | |
| GRFT | Potent antiviral activity against T- &-M- tropic HIV-1 (EC50 = 0.043–0.63). Inhibitor of coronavirus. | Mori et al. (2005); Ziolkowska et al. (2006) | ||
| Hypnea cervicornis | HCA | Bactericidal activities. | Siddqiui et al. (1993) | |
| HCA | Anti-inflammatory activity & antinociceptive effects. | Bitencourt et al. (2008) | ||
| HCA | Anti-hypernociceptive effect | Figueiredo et al. (2010) | ||
| H. japonica | Hypnin A | Toxicity to tumor cells. Inhibition of normal embryonic development of marine invertebrates. Specific binding to fucosylated N-glycans making it valuable tool for cancer diagnosis. | Okuyama et al. (2009) | |
| Inhibits ADP-induced platelet aggregation. | Matsubara et al. (1996) | |||
| Kappaphycus alvarezii | KAA-2 | Inhibits influenza virus infection. | Sato et al. (2011a) | |
| Phaeodactylum tricornutum | – | Anti-inflammatory, analgesic & free radical scavenging activity | Guzman et al. (2001) | |
| Solieria filiformis | – | Stimulates the growth of Gram +ve species Bacillus cereus & inhibited the growth of Gram –ve species (Serratia marcescens, Salmonella typhi, Klebsiella pneumonia, Pseudomonas aeruginosa, Enterobacter aerogenes, Proteus sp). | Holanda et al. (2005) | |
| Tichocarpus crinitus | TCL | Stimulate synthesis of pro-inflammatory cytokinies TNF-α, IFN-γ, IL-6 by human whole-blood cells. | Molchanova et al. (2010) | |
–: Lectin not designated.
Antinociceptive
A wide variety of mephitic stimuli are known to bring about powerful inhibition of pain sensation at a remote region of body; nociceptors are sensitized by tissue injury and inflammation. Kurihara et al. (2003) reported that primary nociceptor which is known as hyperalgesia in humans and nociception in animal models, which is common for all inflammatory pain types. Currently, opioids and non-steroidal anti-inflammatory drugs are used as analgesic. But due to their side-effects and low potency there is a need for an alternative. Therefore, the hunt for new compounds for controlling pain and inflammation with low side effects has switched to marine algae. Specific binding of lectins with carbohydrates acts an integral part of host defense system. This has opened up a new component of the immune system with both fundamental and practical implications (Ahmadiani et al., 1998; Vanderlei et al., 2010).
Antinociceptive effect of lectins from marine alga A. multifida. B. seaforthii and B. triquetrum has been reported both at central and peripheral levels of the nervous system (Neves et al., 2007; Viana et al., 2002). A. multifida lecin (Amansin/LEC) has also been indicated as a potential analgesic drug (Neves et al., 2007). Agglutinin from Hypnea cervicornis (HCA) showed antinociceptive activity via interaction of the lectin carbohydrate-binding site. Lectin HCA was able to reduce writhings which suggests inhibition of the release of mediators in response to acetic acid. But formalin-induced nociception suggested that inflammatory pain is mainly responsible for antinociceptive effect; however, the hot plate test postulated peripheral acting mechanism of antinociception (Bitencourt et al., 2008). Significant antinociceptive effects have also been demonstrated by Chlorella stigmatophora and Phaeodactylum tricornutum lectins which reduce neutrophil migration to peritoneal cavity (Guzman et al., 2001). Lectin from green algae C. cupressoides produces antinociceptive and anti-inflammatory response in models of nociception in mice and inflammation in rats which attributes peripheral antinociception action against the release of mediators in response to acetic acid (Vanderlei et al., 2010).
Anti-inflammatory
Inflammation is a body’s defense reaction caused by injury or damage, which is characterized by rubor (redness), tumor (swelling), calor (heat) and dolar (pain). The first phase of inflammation and edema is marked by the release of histamine and serotonin, second phase involves the release of cytokines followed by prostaglandins (Vanderlei et al., 2010). HCA lectin isolated from H. cervicornis induces anti-inflammatory effects in models of paw edema and peritonitis which is elicited by a reduction in leukocyte migration to the peritoneal cavity of the animals. Thus, the anti-inflammatory effects occur via competition of mucins of cell glycoproteins with selectins which results in neutrophil reduction and blockade of leukocyte adhesion to the endothelium (Neves et al., 2007). Anti-inflammatory effects have also been demonstrated by lectins from C. cupressoides, C. stigmatophora, P. tricornutum and A. multifida which results in neutrophil migration to peritoneal cavity and carrageenan-induced paw-edema of rats (Guzman et al., 2001; Neves et al., 2007; Vanderlei et al., 2010).
Anti-cancer
Lectins in oncology can be used as diagnostic probes and biological response modifiers. Due to their small size and several disulphide bridges, marine algal lectins possess greater stability and specificity for complex carbohydrates and glycoconjugates. Therefore, they are thought to induce immunogenicity. Many algal lectins are reported to possess anti-tumor activity against human cancer cells (Karasaki et al., 2001; Timoshenko et al., 2001; Wang et al., 2000). Tumor-specific “active targeting” is often practiced which is achieved by immobilizing tumor-specific ligands (antibodies, peptides or saccharides) onto drug-carrier systems (Forssen & Wills, 1998; Peer et al., 2007; Trochilin, 2005). E. serra agglutinin (ESA) induced cell-death of colon cancer Colo201 cells and cervix cancer HeLa cells (Sugahara et al., 2001). ESA lectin had strong mitogenic activity against human and mouse lymphocytes due to affinity for glycoproteins bearing high-mannose type N-glycans. When immobilized onto span 80 (sorbitan monooleate) vesicles, ESA drastically decreased the viability of Colo201cancer cells in vitro, whereas normal cells remained unaffected. The vesicles also manifested inhibition of cancer cell growth in nude mice and diminished tumor growth after intravenous administration to nude mice having an implanted Colo201 tumor (Kawakubo et al., 1997).
Lectins BSL and BTL from B. seaforthii and B. triquetrum, respectively, were capable of differentiating human colon carcinoma cell variants with respect to their cell membrane glycoreceptors and could be used for structural modifications of cell membrane glycoconjugates in cancer cell systems (Pinto et al., 2009). The binding of both lectins to carcinoma cells results in internalization which could be used for drug delivery.
Antiviral
Lectins derived from marine algae are a rich source of antiviral products (Triveleka et al., 2003). Antiviral activity depends on the ability to bind mannose-containing oligosaccharides present on surface of viral envelope glycoproteins. Lectins from cyanobacteria and other marine macro-algae are specific for high-mannose which makes them promising candidates for the prevention of transmission of various enveloped viruses such as human immunodeficiency virus (HIV), influenza virus, hepatitis C virus (HCV), Ebola virus and severe acute respiratory syndrome coronavirus (SARS-CoV) (Ziolkowska & Wlodawer, 2006). The specific interaction of algal lectins with target glycans on virus surfaces suppresses virus infection (Balzarini, 2007).
Boodlea coacta lectin (BCA) has been reported to be the first HIV- and influenza virus-inhibiting protein from green algae. BCA revealed potent antiviral activity against most of the influenza virus strains tested by binding to the envelope HA (a trimeric glycoprotein expressed on influenza virus membrane) including a clinical isolate of pandemic H1N1-2009 virus (Sato et al., 2011b). Lectin isolated from K. alvarezii (KAA-2) exhibited strong antiviral activity against broad range of influenza virus strains including Swine-origin H1N1 influenza virus; regardless of the virus subtype and strain. Inhibition of influenza virus propagation occurred due to the blocking of viral entry into the host cell by binding to HM glycans on the surface envelope glycoprotein HA. This clearly indicates that KAA-2 completely inhibits yeast mannan bearing HM glycans and binds strongly to HA via HM glycans. The strain-independent inhibition by KAA-2 might be more effective than antibody-based medicines that are more prone to antigenic shift/drift. KAA-2 can be used as novel antiviral agent (Sato et al., 2011a).
In a recent groundbreaking study, griffithsin from Griffithsia sp. has been reported to be a potent inhibitor of the life-cycle of the coronavirus which is responsible for SARS. The antiviral potency of griffithsin is due to presence of multiple, sugar binding sites that provide robust attachment points for complex carbohydrate molecules present on viral envelopes. Such broad antiviral activity of this lectin makes it a promising candidate for the development of a novel antiviral agent (Ziolkowska & Wlodawer, 2006). Lectin CV-N showed an inclusive variety of antiviral activity for influenza viruses (A and B), Ebola virus, human herpes virus 6, HCV and measles virus (Barrientos et al., 2003; Dey et al., 2000; Helle et al., 2006; O’Keefe et al., 2003).
Algal proteins with antiviral activities have now “emerged” in the anti-HIV battlefield displaying immense dormant applications as topical agents (Feizi et al., 2011). Most of the research on anti-HIV activity of marine algae has converged upon red and brown macroalgae (Schaeffer & Krylov, 2000). High-mannose binding nature of algal lectins makes them expedient candidates for inhibiting HIV (Botos & Woldawer, 2005). They interact with glycans and cells of the host, thus disturbing proteins of viral envelope and cells of the host. A number of lectins isolated from red algae exhibit inhibitory activity against HIV. Griffithsin/GRFT isolated from Griffithsia sp. is a completely novel protein with no homology to any of the proteins listed in BLAST database. GRFT displays potent antiviral activity against both laboratory-adapted strains and primary isolates of HIV-1 (Alexandre et al., 2010; Charan et al., 2000; Giomarelli et al., 2006; Ziolkowska & Wlodawer, 2006) at subnanomolar concentrations (IC50 = 0.4 nm and EC50 = 0.043–0.63) which inhibits cell fusion and cell-to-cell HIV transmission (Emau et al., 2007) in contrast to several other monosaccharide-specific lectins from same structural family. GRFT is not only the strongest HIV inhibitor manifesting broad spectrum activity against various clades of HIV, but also acts as an initiation point for the design of smaller peptide-based antiviral minilectins which can be directed against high-mannose sugars (Micewicz et al., 2010). CV-N lectin purified from N. ellipsosporum shares no similarity with other protein sequences which are deposited so far in public protein databases. CV-N is a potential inhibitor of both laboratory adapted and clinically isolated strains of HIV-1, HIV-2 and simian immunodeficiency virus (Bewley et al., 1998; Dey et al., 2000). Furthermore, CV-N prevents in vitro fusion and transmission of HIV-1 between infected and non-infected cells (Boyd et al., 1997). CV-N is highly resistant to physico-chemical denaturations which are caused by various denaturants, detergents, organic solvents, multiple freeze thaw cycles and heat up to 100 °C with no loss of antiviral activity. These characteristics further boost its potential as an anti-HIV microbicide (Bewley et al., 1998; Boyd et al., 1997). GRFT, CV-N and SVN are mannose specific lectins found interacting with mannose-rich glycans present on the viral envelope and blocking HIV-1 entry in vitro. This supports their potential as microbicides or topical virucide to prevent sexual transmission of HIV and AIDS (Alexandre et al., 2010; Mori et al., 2005).
The envelope glycoprotein of HIV (gp120) is among the most heavily glycosylated proteins known so far. Up to 50% of this 120-kDa glycoprotein is contributed by N-linked carbohydrates. In particular, HIV gp120 contains 20–29 N-glycosylation sites depending on the nature of the viral isolate and the type of virus clade. Highly dense carbohydrate shield on gp120 has been found to be responsible for its low antigenicity and low immunogenicity. It also protects the virus against the immune system (Balzarini et al., 2005). Envelope glycoprotein gp120 and gp 41 of HIV-1 forms a trimer complex that mediates virus entry into target cells through receptor binding events. As demonstrated in studies, gp120 is composed of variable region (V1–V5) and constant regions (C1–C5). V3 loop acts as the major determinant of viral entry. Carbohydrate moieties are affirmed to act as shields for gp120 which is highly glycosylated. Thus, carbohydrate-binding agents including CV-N and griffithsin inhibit HIV-1 infection (Hu et al., 2007).
Miscellaneous applications
Algae are promising organisms to furnish novel biochemically active compounds which are of potential importance to pharmaceutical sector and general public health. Lectin from A. multifida (Amansin) possesses the ability to induce interferon (INF-γ) production, neutrophil migration and is also a powerful stimulant of quiescent peripheral blood lymphocytes which can induce blast transformation heading for mitosis in cells in vitro. Low molecular weights of algal lectins play a vital role in the study of neutrophil migration as this prevents steric problems (Neves et al., 2001). Lectin bryohealin from B. plumosa has the potential of wound-healing (Jung et al., 2010). Similarly, lectin diabolin isolated from Laminaria diabolica initiates the development of a fertilization envelope around unfertilized eggs of sea urchin Hemicentrotus pulcherrimus which prevents its cleavage (Smit, 2004).
TCL stimulates the synthesis of pro-inflammatory cytokines TNF-α, INF-γ and interleukin-6 by human whole blood cells (Molchanova et al., 2010). TCL has also been reported to be a potent mitogen for human lymphocytes. The bacteriostatic and stimulatory effects on the growth of several Gram negative bacteria (Serratia marcescens. Salmonella typhi, Klebsiella pneumonia. Pseudomonas aeruginosa. Enterobacter aerogenes. Proteus sp) and Gram positive bacteria (Bacillus cereus) have been reported from Solieria filiformis lectin (Holanda et al., 2005).
H. japonica lectin (Hypnin A) inhibits human platelet aggregation induced by ADP or collagen in a dose-dependent manner (Matsubara et al., 1996). This lectin exhibits potent mitogenic activity against both lymphocytes from mouse and human. It also induces toxicity to tumor cells by inhibition of embryonic development of marine invertebrates (Hori et al., 2000; Matsubara et al., 1996). Gracilaria cornea lectin through multimechanistic approach showed acaricidal and antifeedant activity against Anagasta kuehniella (flour moth), which may be important for controlling pests from a new natural source (Lima et al., 2005). Interestingly, algal lectins are also used in antiadhesion trials. Lectins BTL and BSL have been shown to block adhesion of Streptococci to their mucin receptors in acquired pellicle via competition mechanism (Teixeira et al., 2007). These lectins interfere both with bacterial adhesion and aggregation. Thus, antiadhesion lectin therapy is a promising solution to the problems of caries. Lectins are widely used in lectinosorbent assays which characterize cell-binding patterns (Smit, 2004).
Future perspectives
Algae studied for lectins comprise only a negligible expanse of the total number of algal species and, therefore, a comprehensive province remains to be scrutinized. Lectins isolated from marine resources are highly diversified not only in terms of structure, but also functional aspects including specific and unique carbohydrate specificities. The research upshot concerns the evolution of powerful tools for the study of cancer, HIV and other diseases. The ultimate goal is to develop emphatic microbicides that not only stymie the transmission of cell-free viruses but also the transmission of donar-HIV infected T-cells and guards against other STDs (Huskens & Schols, 2012). The sugar binding specificity of lectins towards glycoconjugates has made them captivating proteins. This property enables them to fabricate useful tools for various therapeutic applications including cancer diagnosis and prognosis, pathological markers of diseases, glycan profiling, cell-communication, bioadhesion and for controlling a variety of infections.
Significant research on algal lectins during past few decades has accelerated the understanding of molecular-mechanism entangling adherence and recognition. The specific coupling and greater pH stability of algal lectins showed reversible linkage of algal lectins to drug enhancing penetration of drugs which can be used for targeting drugs to tumor tissue and for oral drug delivery. A number of lacunae still persist which need to be filled. Even though an invigorative role of many lectins has been evident, further pharmacokinetic studies need to be endeavored before their introduction as clinical tools. Distinct sources should be traversed to confine avant-grade lectins with dormant dupable properties. Sanguinely, further groundwork is required to endow in vivo succor of these algal lectins equivalent to their in vitro effects and can be carried forward for the development of oral drug delivery systems, mucoadhesive formulations, lectinosorbent assays and development of efficient, safe and affordable microbicides. In case of anti-HIV drugs what is now needed is to determine precisely the distinctive features among numerous lectins that confer antiviral activity. Thus, it would be possible to engineer proteins with multiple binding sites recognizing different motifs for use as anti-HIV drugs with enhanced potencies (Feizi et al., 2011). The author realizes that the need of the hour is to characterize and overcome the shortcomings in purification of algal lectins for exploring immense empire of algal lectins for biomedical applications.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmadiani A, Fereidoni M, Semnanian S, et al. (1998). Antinociceptive and anti-inflammatory effects of Sambucus ebulus rhizome extracts in rats. J Ethnopharmacol 61:229–35 [DOI] [PubMed] [Google Scholar]
- Ainouz IL, Sampaio AH, Freitas ALP, et al. (1992). Agglutination of enzyme treated erythrocytes by Brazilian marine algae. Bot Mar 35:475–9 [Google Scholar]
- Ainouz IL, Sampaio AH, Freitas ALP, et al. (1995). Comparative study on hemagglutinins from the red algae Bryothamnion seaforthii and Bryothamnion triquetrum. R Bras Fisiol Veg 7:15–9 [Google Scholar]
- Ainouz L, Sampaio H (1991). Screening of Brazilian marine algae for hemagglutinins. Bot Mar 34:211–4 [Google Scholar]
- Alexandre KB, Gray ES, Lambson BE, et al. (2010). Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 sensitivity to the lectins, griffithsin, cynovirin-N and scytovirin. Virology 402:187–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J. (2007). Targeting the glycans of glycoproteins: A novel paradigm for antiviral therapy. Nat Rev Microbiol 5:583–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J, Laethem KV, Hatse S, et al. (2005). Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV gp120. J Biol Chem 280:41005–14 [DOI] [PubMed] [Google Scholar]
- Barrientos LG, O’Keefe BR, Bray M, et al. (2003). Cyanovirin-N binds to the several glycoprotein gp (1, 2) and inhibits infectivity of Ebola virus. Antivir Res 58:47–56 [DOI] [PubMed] [Google Scholar]
- Benevides NBM, Leite AM, Freitas ALP, et al. (1996). Atividade hemaglutinante na alga vermelha Solieria filiformis. Revista Brasileira de Fisiologia Vegetal 8:117–122 [Google Scholar]
- Benevides NBM, Holanda ML, Melo FR, et al. (2001). Purification and partial characterization of the lectin from the marine green alga Caulerpa cupressoides (Vahl) C. Agardh. Bot Mar 44:17–22 [Google Scholar]
- Bewley CA, Cai M, Ray S, et al. (2004). New carbohydrate specificity and HIV-1 fusion blocking activity of the cyanobacterial protein MVL. J Mol Biol 339:901–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley CA, Gustafson KR, Boyd MR, et al. (1998). Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nature 15:571–8 [DOI] [PubMed] [Google Scholar]
- Bird KT, Chiles TC, Longley RE, et al. (1993). Agglutinins from marine macroalgae of the south eastern United States. J Appl Phycol 5:213–8 [Google Scholar]
- Bitencourt F, da S, Figueiredo JG, et al. (2008). Antinociceptive and anti-inflammatory effects of a mucin-binding agglutinin isolated from the red marine alga Hypnea cervicornis. Naunyn-Schmiedeberg’s. Arch Pharmacol 377:139–48 [DOI] [PubMed] [Google Scholar]
- Botos I, Wlodawer A (2005). Proteins that binds high-mannose sugars of the envelope. Prog Biophys Mol Biol 88:233–82 [DOI] [PubMed] [Google Scholar]
- Boyd MR, Gustafson KR, McMahon JB, et al. (1997). Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob Agents Chemother 41:1521–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WC, Almodovar LR, Boyd LG (1966). Agglutinins in marine algae for human erythrocytes. Transfusion 6:82–3 [Google Scholar]
- Calvete JJ, Costa FHF, Saker-Sampio S, et al. (2000). The amino acid sequence of the agglutinin isolated from the red marine algae Bryothamnion triquetrum defines a novel lectin structure. Cell Mol Life Sci 57:343–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan RD, Munro MH, O’Keefe BR, et al. (2000). Isolation and characterization of Myrianthus holstii lectin, a potent HIV-1 inhibitory protein from the plant Myrianthus holstii. J Nat Prod 63:1170–4 [DOI] [PubMed] [Google Scholar]
- Chernikov OV, Chikalovets IV, Molchanova VI, et al. (2007). Algae of Peter the Great Bay of the Sea of Japan as a source of lectins. Russ J Mar Biol 33:329–32 [Google Scholar]
- Chiles TC, Bird KT (1989). A comparative study of animal erythrocyte agglutinins from marine algae. Comp Biochem Physiol 94:107–11 [DOI] [PubMed] [Google Scholar]
- Chiles TC, Bird KT (1990). Gracilaria tikvahiae agglutinin. Partial purification and preliminary characterization of its carbohydrate specificity. Carbohydr Res 207:319–26 [DOI] [PubMed] [Google Scholar]
- Chu CY, Huang R, Lin LP (2007). Analysis of the agglutinating activity from unicellular algae. J Appl Phycol 19:401–8 [Google Scholar]
- Costa FHF, Sampaio AH, Neves SA, et al. (1999). Purification and characterization of a lectin from the red marine alga Amansia multifida. Physiol Mol Biol Plants 5:53–61 [Google Scholar]
- Dey B, Lerner DL, Lusso P, et al. (2000). Multiple antiviral activities of cyanovirin-N: Blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J Virol 74:4562–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emau P, Tina B, O’feeke BK, et al. (2007). Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. J Med Primatol 36:244–53 [DOI] [PubMed] [Google Scholar]
- Feizi T, Liu Y, Palma AS (2011). Bacterial, fungal and algal lectins: Combants in tug of war against HIV. Structure 19:1035–7 [DOI] [PubMed] [Google Scholar]
- Figueiredo JG, Bitencourt FS, Cunha TH, et al. (2010). Agglutinin isolated from the red marine alga Hypnea cervicornis J. Agardh reduces inflammatory hypernociception: Involvement of nitric oxide. Pharmacol Biochem Behav 96:371–7 [DOI] [PubMed] [Google Scholar]
- Forssen E, Wills M (1998). Ligand-targeted liposomes. Adv Drug Deliv Rev 29:249–71 [DOI] [PubMed] [Google Scholar]
- Freitas ALP, Teixeira DIA, Costa FHF, et al. (1997). A new survey of Brazilian marine algae for agglutinins. J Appl Phycol 9:495–501 [Google Scholar]
- Giomarelli B, Schumacher KM, Taylor TE, et al. (2006). Recombinant production of anti-HIV protein, griffithsin, by auto-induction in a fermentor culture. Protein Express Purif 47:194–202 [DOI] [PubMed] [Google Scholar]
- Guzman S, Gato A, Calleja JM (2001). Antiinflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res 15:224–30 [DOI] [PubMed] [Google Scholar]
- Han JW, Jung MG, Kim MJ, et al. (2010). Purification and characterization of a D-mannose specific lectin from the green marine alga, Bryopsis plumosa. Phycol Res 58:143–50 [Google Scholar]
- Harnedy PA, FitzGerald RJ (2011). Bioactive proteins, peptides and amino acids from macroalgae. J Phycol 47:218–32 [DOI] [PubMed] [Google Scholar]
- Helle F, Wychowski C, Vu-Dac N, et al. (2006). Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J Biol Chem 281:25177–83 [DOI] [PubMed] [Google Scholar]
- Holanda ML, Melo VMM, Silva LMCM, et al. (2005). Differential activity of a lectin from Solieria filiformis against human pathogenic bacteria. Braz J Med Biol Res 38:1769–73 [DOI] [PubMed] [Google Scholar]
- Hori K, Miyazawa K, Ito K (1990). Some common properties of lectins from marine algae. Hydrobiologia 204–205:561–6 [Google Scholar]
- Hori K, Miyazawa K, Fusetani N, et al. (1986). Hypnins, low molecular weight peptidic agglutinins from a marine red alga, Hypnea japonica. Biochim Biophys Acta 873:228–36 [Google Scholar]
- Hori K, Matsubara K, Miyazawa K (2000). Primary structures of two hemagglutinins from the marine red alga, Hypnea japonica. Biochim Biophys Acta 1474:226–36 [DOI] [PubMed] [Google Scholar]
- Hori K, Oiwa K, Miyazawa, Ito K (1988). Evidence for wide distribution of agglutinins in marine algae. Bot Mar 31:133–8 [Google Scholar]
- Hu Q, Mahmood N, Shattock RJ (2007). High-mannose-specific deglycosylation of HIV-1 gp120 induced by resistance to cyanovirin-N and the impact on antibody neutralization. Virology 368:145–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LD, Hori K, Nang HQ, et al. (2009). Seasonal changes in growth rate, carrageenan yield and lectin content in red alga Kappaphycus alvarezii cultivated in Camrah Bay, Vietnam. J Appl Phycol 21:265–72 [Google Scholar]
- Hung LD, Sato Y, Hori K (2011). High-mannose N-glycan-specific lectin from the red alga Kappaphycus striatum (Carrageenophyte). Phytochemistry 72:855–61 [DOI] [PubMed] [Google Scholar]
- Huskens D, Schols D (2012). Algal lectins as potential HIV microbicide candidates. Mar Drugs 10:1476–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MG, Lee KP, Choi HG, et al. (2010). Characterization of carbohydrate combining sites of Bryohealin, an algal lectin from Bryopsis plumosa. J Appl Phycol 22:793–802 [Google Scholar]
- Kakita H, Fukuoka S, Obika H, Kamishima H (1999). Isolation and characterisation of a fourth hemagglutinin from the red alga, Gracilaria verrucosa, from Japan. J Appl Phycol 11:49–56 [Google Scholar]
- Karasaki Y, Tsukamoto S, Mizusaki K, et al. (2001). A garlic lectin exerted an antitumor activity and induced apoptosis in human tumor cells. Food Res Int 34:7–13 [Google Scholar]
- Kawakubo A, Makino H, Ohnishi J, et al. (1997). The marine alga Eucheuma serra J. Agardh, a high yielding source of two isolectins. J Appl Phycol 9:331–8 [Google Scholar]
- Kawakubo A, Makino H, Ohnishi J, et al. (1999). Occurrence of highly yielded lectins homologous within genus Eucheuma. J Appl Phycol 11:149–56 [Google Scholar]
- Khan F, Khan RH, Sherwani A, et al. (2002). Lectins as markers for blood grouping. Med Sci Monit 8:RA293–300 [PubMed] [Google Scholar]
- Kurihara T, Nonaka T, Tanabe T (2003). Acetic-acid conditioning stimulus induces long-lasting antinociceptive of somatic inflammatory pain. Pharmacol Biochem Behav 74:841–9 [DOI] [PubMed] [Google Scholar]
- Lee RB. (1999). Phycology. Cambridge, UK: Cambridge University Press [Google Scholar]
- Leite YFMM, Silva LMCM, Amorim RCNA, et al. (2005). Purification of a lectin from the marine red alga Gracilaria ornate and its effect on the development of the cowpea weevil Callosobruchus maculates (Coleoptera: Bruchidae). Biochim Biophys Acta 1724:137–45 [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang X, Chen G, et al. (2008). Algal lectins for potential prevention of HIV transmission. Curr Med Chem 15:1096–104 [DOI] [PubMed] [Google Scholar]
- Lima AF, Criddle DN, Souza EP, et al. (2004). Red marine alga Bryothamnion triquetrum lectin induces endothelium dependent relaxation of the rat aorta via release of nitric oxide. J Pharma Pharmacol 56:1415–21 [DOI] [PubMed] [Google Scholar]
- Lima HC, Costa FHF, Sampaio AH, et al. (1998). Induction and inhibition of human lymphocyte transformation by the lectin from red marine alga Amansia multifida. J Appl Phycol 10:153–62 [Google Scholar]
- Lima MEP, Carneiro ME, Nascimento AE, et al. (2005). Purification of a lectin from the marine red alga Gracilaria cornea and its effects on the cattle tick Boophilus microplus (Acari: Ixodidae). J Agric Food Chem 53:6414–9 [DOI] [PubMed] [Google Scholar]
- Matsubara K, Sumi H, Hori K (1996). Platelet aggregation is inhibited by phycolectins. Experientia 52:540–3 [DOI] [PubMed] [Google Scholar]
- Micewicz ED, Cole AL, Jung CL, et al. (2010). Grifonin-1: A small HIV-1 entry inhibitor derived from the algal lectin, Griffithsin. PLoS One 5:e14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchanova V, Chernikov O, Chikalovets I, Lukyanov P (2010). Purification and partial characterization of the lectin from the marine alga Tichocarpus crinitus (Gmelin) Rupr. (Rhodophyta). Bot Mar 53:69–78 [Google Scholar]
- Mori T, O’Keefe BR, Sowder RC, et al. (2005). Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia spp. J Biol Chem 280:9345–53 [DOI] [PubMed] [Google Scholar]
- Nagano CS, Debray H, Nascimento KS, et al. (2005). HCA and HML isolated from red marine algae Hypnea cervicornis and Hypnea musciformis define a novel lectin family. Prot Sci 14:2167–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento KS, Cunha AI, Nascimento KS, et al. (2012). An overview of lectins purification stratergies. J Mol Recognit 25:527–41 [DOI] [PubMed] [Google Scholar]
- Nascimento KS, Nagano CS, Nunes EV, et al. (2006). Isolation and characterization of a new agglutinin from the red marine alga Hypnea cervicornis J. Agardh. Biochem Cell Biol 84:49–54 [DOI] [PubMed] [Google Scholar]
- Neves SA, Dias-Baruffi M, Freitas ALP, Roque-Barreira MC (2001). Neutrophil migration induced in vivo and in vitro by marine algal lectins. Inflamm Res 50:486–90 [DOI] [PubMed] [Google Scholar]
- Neves SA, Freitas ALP, Souza BWS, et al. (2007). Antinociceptive properties in mice of a lectin isolated from the marine alga Amansia multifida Lamouroux. Braz J Med Biol Res 40:127–34 [DOI] [PubMed] [Google Scholar]
- Niu J, Wang G, Lu F, et al. (2009). Characterization of a new lectin involved in the protoplast regeneration of Bryopsis hypnoides. Chin J Oceanol 27:502–12 [Google Scholar]
- O’Keefe BR, Smee DF, Turpin JA, et al. (2003). Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother 47:2518–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto R, Hori K, Miyazawa K, Ito K (1990). Isolation and characterization of a new hemagglutinin from red alga Gracilaria bursa-pastoris. Experientia 46:975–7 [DOI] [PubMed] [Google Scholar]
- Okuyama S, Nakamura-Tsuruta S, Tateno H, et al. (2009). Strict binding specificity of small-sized lectins from the red marine alga Hypnea japonica for core (α-1, 6) fucosylated N-glycans. Biosci Biotechnol Biochem 73:912–20 [DOI] [PubMed] [Google Scholar]
- Oliveira SRM, Nascimento AE, Maria EP, et al. (2002). Purification and characterisation of a lectin from the red marine alga Pterocladiella capillacea (S.G. Gmel.) Santel. & Hommers. Revista Brasil Bot 25:397–403 [Google Scholar]
- Omokawa Y, Miyazaki T, Walde P, et al. (2010). In vitro and in vivo anti-tumor effects of novel Span 80 vescicles containing immobilized Eucheuma serra agglutinin. Int J Pharma 389:157–67 [DOI] [PubMed] [Google Scholar]
- Peer D, Karp JM, Hong S, et al. (2007). Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2:751–60 [DOI] [PubMed] [Google Scholar]
- Pinto VPT, Debray H, Dus D, et al. (2009). Lectins from the red marine algal Species Bryothamnion seaforthii and Bryothamnion triquetrum as tools to differentiate human colon carcinoma cells. Adv Pharmacol Sci [Epub ahead of print]. doi: 10.1155/2009/862162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ, Blunden G, Evans PR (1977). Ptilota plumosa, a new source of blood group B specific lectin. Med Lab Sci 34:193–200 [PubMed] [Google Scholar]
- Rogers DJ, Hori K (1993). Marine algal lectins: New developments. Hydrobiologia 260/261:589–93 [Google Scholar]
- Sampaio AH, Rogers DJ, Barwell CJ, et al. (1999). A new isolation procedure and further characterisation of the lectin from the red marine alga Ptilota serrata. J Appl Phycol 10:539–46 [Google Scholar]
- Sampaio AH, Rogers DJ, Barwell CJ, et al. (2002). New affinity procedure for the isolation and further characterization of the blood group B specific lectin from the red marine alga Ptilota plumosa. J Appl Phycol 14:489–95 [Google Scholar]
- Sampaio AH, Rogers DJ, Barwell CJ (1998). A galactose-specific lectin from the red alga Ptilota filicina. Phytochemistry 48:765–69 [DOI] [PubMed] [Google Scholar]
- Sato T, Hori K (2009). Cloning, expression and characterization of a novel anti-HIV lectin from the cultured cyanobacterium, Oscillatoria agardhii. Fish Sci 75:743–53 [Google Scholar]
- Sato Y, Hirayama M, Morimoto K, et al. (2011b). High mannose-binding lectin with preference for the cluster of α 1–2 mannose from green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J Biol Chem 286:19446–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Morimoto K, Hirayama M, Hori K (2011a). High mannose-specific lectin (KAA-2) from the red alga Kappaphycus alvarezii potently inhibits influenza virus infection in a strain-independent manner. Biochem Biophys Res Commun 405:291–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Murakami M, Miyazawa K, Hori K (2000). Purification and characterization of novel lectin from a fresh water cyanobacterium, Oscillatoria agardhii. Comp Biochem Physiol B Biochem Mol Biol 125:169–77 [DOI] [PubMed] [Google Scholar]
- Sato Y, Okuyama S, Hori K (2007). Primary structure and carbohydrate binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium Oscillatoria agardhii. J Biol Chem 282:11021–9 [DOI] [PubMed] [Google Scholar]
- Schaeffer DJ, Krylov VS (2000). Anti-HIV activity of exracts and compounds from algae and cyanobacteria. Ecotoxicol Environ Saf 45:208–27 [DOI] [PubMed] [Google Scholar]
- Sharon N, Lis H (1972). Lectin: Cell agglutinating and sugar-specific proteins. Science 177:949–59 [DOI] [PubMed] [Google Scholar]
- Shiomi K, Yamanaka H, Kikuchi T (1981). Purification and physiochemical properties of a hemagglutinin (GVA-1) in the red alga Gracilaria verrucosa. Bull J Soc Sci Fish 47:1078–84 [Google Scholar]
- Shiomi K, Yamanaka H, Takeaki K (1980). Biochemical properties of hemagglutinins in the red alga Serraticardia maxima. Bull J Soc Sci Fish 46:1369–73 [Google Scholar]
- Siddqiui S, Shyum SBN, Usmanghani K, Shameel M (1993). Antibacterial activity and fatty acid composition of the extract from Hypnea musciformis (Gigartinales, Rhodophyta). Pak J Pharm Sci 6:45–51 [PubMed] [Google Scholar]
- Singh RS, Bhari R, Kaur HP (2010). Mushroom lectins: Current status and future perspectives. Crit Rev Biotechnol 30:90–126 [DOI] [PubMed] [Google Scholar]
- Singh RS, Bhari R, Kaur HP (2011a). Current trends of lectins from microfungi. Crit Rev Biotechnol 31:193–210 [DOI] [PubMed] [Google Scholar]
- Singh RS, Bhari R, Kaur HP (2011b). Characteristics of yeast lectins and their role in cell-cell interaction. Biotechnol Adv 29:726–31 [DOI] [PubMed] [Google Scholar]
- Singh RS, Tiwary AK, Kennedy JF (1999). Lectins: Sources, activities and applications. Crit Rev Biotechnol 19:145–78 [Google Scholar]
- Smit AJ. (2004). Medicinal and pharmaceutical uses of seaweed natural products: A review. J Appl Phycol 16:245–62 [Google Scholar]
- Stillmark H. (1888). Uber Rizin, ein giftiges Ferment aus dem Samen von Ricinus communis L.und einigen anderen Euphorbiaceen. Germany: University of Dorpat, Schnakenburg [Google Scholar]
- Sugahara T, Ohama Y, Fukuda A, et al. (2001). The cytotoxic effect of Eucheuma serra agglutinin (ESA) on cancer cells and its applications to molecular probe for drug delivery system using lipid vesicles. Cytotechnology 36:93–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy MLA. (2011). Marine algal sources for treating bacterial diseases. Adv Food Nutr Res 64:71–84 [DOI] [PubMed] [Google Scholar]
- Teixeira EH, Arruda FVS, Nascimento KS, et al. (2012). Biological applications of plants and algae lectins: An overview. In: Chang CF, ed. Carbohydrates-comprehensive studies on glycobiology and glycotechnology. Croatia: In Tech, 533--58 [Google Scholar]
- Teixeira EH, Napimoga MH, Carneiro VA, et al. (2007). In vitro inhibition of oral streptococci binding to the acquired pellicle by algal lectins. J Appl Microbiol 103:1001–6 [DOI] [PubMed] [Google Scholar]
- Timoshenko AV, Lan Y, Gabius HJ, Lala PK (2001). Immunotherapy of C3H/HeJ mammary adenocarcinoma with interleukin-2 mistletoe lectin or their combination: Effects on tumor growth capillary leakage and nitric oxide (NO) production. Eur J Cancer 37:1910–20 [DOI] [PubMed] [Google Scholar]
- Triveleka LA, Vagias C, Roussis V (2003). Natural products with anti-HIV activity from marine organism. Curr Top Med 3:1512–35 [DOI] [PubMed] [Google Scholar]
- Trochilin VP. (2005). Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–60 [DOI] [PubMed] [Google Scholar]
- Vanderlei ESO, Patoilo KKNR, Lima NA, et al. (2010). Antinociceptive and anti-inflamatory activities of lectin from the marine green alga Caulerpa cupressoides. Int Immunopharmacol 10:1113–8 [DOI] [PubMed] [Google Scholar]
- Viana GSB, Freitas ALP, Lima MML, et al. (2002). Antinociceptive activity of sulfated carbohydrates from the algae Bryothamnion seafortii (Turner) Kutz. and B. triquetrum (S.G. Gmel) M. Howe. Braz J Med Biol Res 35:713–22 [DOI] [PubMed] [Google Scholar]
- Vieira LAP, Freitas ALP, Feitosa JPA, et al. (2004). The alga Bryothamnion seafortii contains carbohydrate with antinociceptive activity. Braz J Med Biol Res 37:1071–9 [DOI] [PubMed] [Google Scholar]
- Wang H, Gao J, Ng TB (2000). A new lectin with highly potent antihepatoma and antisarcoma activities from oyster mushrooms Pleurotus ostreatus. Biochem Biophys Res Commun 275:810–6 [DOI] [PubMed] [Google Scholar]
- Wang S, Zhong FD, Zhang YJ, et al. (2004). Molecular characterization of a new lectin from the marine alga Ulva pertusa. Acta Biochim Biophys Sin 36:111–7 [DOI] [PubMed] [Google Scholar]
- Watanabe MF, Ozawa Y, Oishi S (1987). Hemagglutination activity of Microcystis aeruginusa (cyanobacterium). Nippon Suisan Gakkaishi 53:1643–6 [Google Scholar]
- Yamaguchi M, Jimbo M, Sakai R, et al. (1998). Purification and characterization of Microcystis aeruginosa (freshwater cyanobacterium) lectin. Comp Biochem Physiol B 119:593–7 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Oqawa T, Muramoto K, et al. (1999). Isolation and characterization of a mannan-binding lectin from fresh water cyanobacteria (blue-green algae) Microcystis viridis. Biochem Biophys Res Commun 265:703–8 [DOI] [PubMed] [Google Scholar]
- Ziolkowska NE, Keefe BR, Mori T, et al. (2006). Domain-Swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure 14:1127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziółkowska NE, Wlodawer A (2006). Structural studies of algal lectins with anti-HIV activity. Acta Biochem Pol 53:617–26 [PubMed] [Google Scholar]