ABSTRACT
Aims: To determine the seroprevalence of canine respiratory coronavirus (CRCoV) in New Zealand dogs, and to explore associations with age, sex, breed, month, and geographical region of sampling and reported presence of clinical signs suggestive of respiratory disease.
Methods: A total of 1,015 canine serum samples were randomly selected from submissions to a diagnostic laboratory between March and December 2014, and were analysed for CRCoV antibodies using a competitive ELISA. Logistic regression analysis was used to determine associations between seroprevalence of CRCoV and breed category, age, sex, sampling month, region, and reported health status of dogs.
Results: Overall, 538/1,015 (53.0%) samples were seropositive for CRCoV, with 492/921 (53.4%) positive dogs in the North Island and 46/94 (49%) in the South Island. Age of dog, sampling month, region, and presence of abnormal respiratory signs were included in the initial logistic regression model. Seroprevalence was higher in dogs aged ≥3 compared with ≤2 years (p < 0.01). The lowest seroprevalence was observed in July (30/105; 28.5%) and August (32/100; 32%), and the highest in June (74/100; 74%). Seroprevalence in dogs from Auckland was higher than in dogs from the Hawkes Bay, Manawatu, Marlborough, and Waikato regions (p < 0.05). Abnormal respiratory signs (coughing, nasal discharge, or sneezing) were reported for 28/1,015 (2.8%) dogs sampled. Seroprevalence for CRCoV tended to be higher among dogs with respiratory signs (67.9 (95% CI = 47.6–83.4)%) than dogs with no reported respiratory signs (52.6 (95% CI = 49.5–55.7)%).
Conclusions: Serological evidence of infection with CRCoV was present in more than half of the dogs tested from throughout New Zealand. Differences in CRCoV seroprevalence between regions and lack of seasonal pattern indicate that factors other than external temperatures may be important in the epidemiology of CRCoV in New Zealand.
Clinical relevance: Our data suggest that CRCoV should be included in investigations of cases of infectious canine tracheobronchitis, particularly if these occur among dogs vaccinated with current vaccines, which do not include CRCoV antigens.
KEYWORDS: Canine respiratory coronavirus, ELISA, seroprevalence, New Zealand, survey
Introduction
Canine respiratory coronavirus (CRCoV) is a large enveloped RNA virus that is classified within a species Betacoronavirus 1 in the family Coronaviridae (Erles et al. 2003). The virus was first isolated from dogs with respiratory disease in a rehoming shelter in the United Kingdom (Erles et al. 2003). On the day of admission to that shelter 30% of dogs were seropositive, and nearly all seronegative dogs seroconverted to CRCoV after a 3-week stay at the shelter, indicating high transmissibility of the virus. The presence of CRCoV antibody on entry to the shelter was associated with decreased risk of development of respiratory disease, suggesting an aetiological involvement of the virus in infectious canine tracheobronchitis (ICT). Results of several further studies in the United Kingdom and other countries also suggested that CRCoV contributes to development of respiratory disease in infected dogs (Erles and Brownlie 2005; Ellis et al. 2005; Kaneshima et al. 2006). However affected dogs were often co-infected with several respiratory pathogens, and not all dogs with serological evidence of recent CRCoV infection developed respiratory disease, making the establishment of an aetiological link between CRCoV infection and disease challenging (Erles et al. 2003; Erles and Brownlie 2005). This is similar to the situation observed for other respiratory pathogens of dogs, underscoring the fact that the aetiology of ICT is complex and factors other than exposure to a specific pathogen are likely to contribute to the outcome of infection (Erles and Brownlie 2008; Mitchell et al. 2017).
Canine respiratory coronavirus is closely related to bovine coronavirus (BCoV) and human coronavirus-OC43, but distinct from canine enteric coronavirus (CECoV), which belongs to the genus Alphacoronavirus, and is an aetiological agent of enteric disease in dogs (Erles et al. 2003; Decaro et al. 2007). Antibodies raised to CRCoV are not cross-reactive with CECoV (Decaro et al. 2007; Priestnall et al. 2007). Similarly, vaccines against CECoV do not elicit protection against CRCoV infection (Erles and Brownlie 2008). A high percentage identity was found between the amino acid sequence of the spike protein of BCoV and CRCoV (Erles et al. 2003), enabling the use of BCoV antigens for the detection of CRCoV antibody (Priestnall et al. 2006; Decaro et al. 2007; Soma et al. 2008).
There are limited data on CRCoV epidemiology in New Zealand. Based on a single cross-sectional survey of 251 dogs, Knesl et al. (2009) reported that 73 (29%) dogs were seropositive for CRCoV. In another New Zealand-based study, 47/94 (50%) dogs sampled had antibody to CRCoV (Sowman et al. 2018). Some of the dogs affected by ICT seroconverted to CRCoV between acute and convalescent sampling, suggesting that CRCoV infection was associated with the development of disease in those dogs. However there was a poor match between diseased and healthy dogs in terms of age, breed, and use, so no conclusions could be made regarding aetiological involvement of CRCoV in development of ICT (Sowman et al. 2018).
The aim of the present study was to investigate the epidemiology of CRCoV in a large sample of dogs in New Zealand, to explore the associations between seroprevalence for CRCoV and age, sex, breed, month, and geographical region of sampling, as well as the reported presence of clinical signs suggestive of respiratory disease.
Materials and methods
Sample collection
A convenience sample of canine sera was obtained from a commercial veterinary laboratory (New Zealand Veterinary Pathology Ltd., Palmerston North, NZ) on a monthly basis. Approximately 100 serum samples, representing 10% of monthly laboratory submissions, were randomly selected every month from March to December 2014. This was done by physically pulling samples out of a bag containing all monthly laboratory submissions and checking these against an Excel database containing available data for each sample. Only those samples with information on sex, age, the region of sampling, and clinical history were included in the study. Samples having incomplete information and duplicate sera having the same label numbers were excluded. In addition, 17 samples collected from racing Greyhounds that were sent directly to our laboratory were also included in the study. Four were from dogs with respiratory disease and 13 from healthy dogs. Dogs were categorised as healthy or sick based on the information provided on the submission form. Healthy dogs included those presented for procedures such as pre-anaesthetic work-up or pre-mating progesterone concentrations, while sick dogs included those presented for a variety of infectious or non-infectious diseases. The serum samples were also categorised into those that came from dogs for which no abnormal respiratory signs were listed on the submission form, and those for which at least one of the clinical signs commonly associated with respiratory disease (coughing, sneezing, or nasal discharge) was listed. Sera were stored at −20°C until assayed.
Detection of CRCoV antibody
Presence of CRCoV antibody in canine sera was determined using a commercially available competitive ELISA with BCoV antigen (BIO K 392- Monoscreen AbELISA Bovine coronavirus/Competition, Bio-X diagnostics, Rochefort, Belgium). The test was performed according to the manufacturer’s instructions using provided positive and negative controls. The results were calculated based on the optical density at 450 nm (OD450) and presented as percentage of inhibition (POI) according to the formula:
The manufacturer’s recommended cut-offs for BCoV antibody were used. Accordingly, samples with POI ≥ 20% were considered positive for CRCoV antibody.
Statistical analyses
Based on the results of the ELISA samples were categorised as seropositive or seronegative and this was the dependent variable for analyses. Independent variables included the dog-related variables of age (≤2, 3–6, 7–10, ≥11 years), sex (female/male), breed group (pet dogs, working dogs, non-descript dogs), health status (healthy/not healthy), and presence of respiratory signs (yes/no), as well as the sampling-related variables of month of sampling (March to December 2014) and geographical region (Auckland, Hawkes Bay, Manawatu, Marlborough/Canterbury, Nelson/Tasman, Northland, Otago, Taranaki, Waikato, Wellington). For some analyses, geographical regions were categorised as South Island and North Island. There were 161 dog breeds which were categorised as working dogs (Huntaway, Heading dogs, and Greyhounds), pet dogs which included all other recognised breeds, and non-descript dogs which included unspecified breeds. Associations between seroprevalence and categorical variables were initially examined using a two-tailed Pearson’s χ2 or Kruskal–Wallis tests. Those variables that were associated (p ≤ 0.20) were included in a multivariable model, which was refined by a stepwise backwards selection process retaining variables with p ≤ 0.05. The mean POI for dogs of different age categories or health status were compared using one-way ANOVA and Tukey’s test.
All statistical analyses were performed using R statistical software v 3.1.0 (R Development Core team, 2012; R Foundation for Statistical Computing, Vienna, Austria) or GraphPad Prism (version 5.03, GraphPad Software, San Diego, CA, USA).
Results
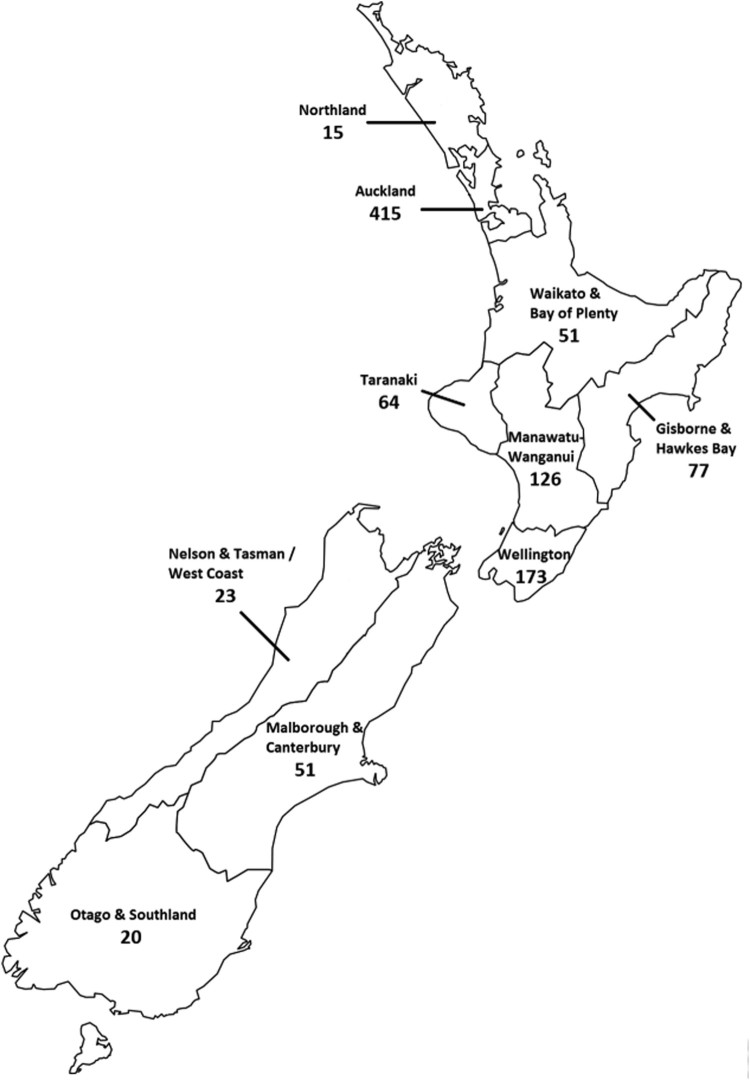
Samples were analysed from a total of 1,015 dogs. The median age of dogs was 8 years (min 1 week, max 18 years), and there were similar numbers of male and female dogs (Table 1). The origin of the samples by geographical region is shown in Figure 1: 921/1,015 (90.7%) were submitted from the North Island and 94/1,015 (9.2%) from the South Island. Overall, 538/1,015 (53.0%) samples were seropositive for CRCoV, with 492/921 (53.4%) positive dogs in the North Island and 46/94 (49%) in the South Island. The seroprevalence of CRCoV among dogs classified as healthy (69/133; 51.8 (95% CI = 43.1–60.6)%) was similar to that among dogs classified as diseased (469/882; 53.2 (95%CI = 49.8–56.5)%, p = 0.780).
Table 1. Number of serum samples randomly selected from submissions to a veterinary diagnostic laboratory between March and December 2014 that were tested for antibodies to canine respiratory coronavirus, with the number that were from female or male dogs and the median (min, max) age of dogs.
| Month | N | Female | Male | Age (years) |
|---|---|---|---|---|
| March | 100 | 55 | 45 | 6.5 (0.02,16) |
| April | 100 | 65 | 35 | 8 (0.25,16) |
| May | 105 | 55 | 50 | 8 (0.33, 15) |
| June | 100 | 71 | 29 | 8 (0.15, 16) |
| July | 105 | 50 | 55 | 8 (0.16, 18) |
| August | 100 | 47 | 53 | 8 (0.58, 16) |
| September | 104 | 54 | 50 | 7 (0.41, 15) |
| October | 100 | 51 | 49 | 9 (0.58, 15) |
| November | 101 | 55 | 46 | 7 (0.75, 15) |
| December | 100 | 54 | 46 | 8 (0.91, 16) |
| Total | 1,015 | 557 | 458 | 8 (0.02, 18) |
Figure 1.
Map of New Zealand showing the number of serum samples from each region that were randomly selected from submissions to a veterinary diagnostic laboratory between March and December 2014 and were tested for antibodies to canine respiratory coronavirus.
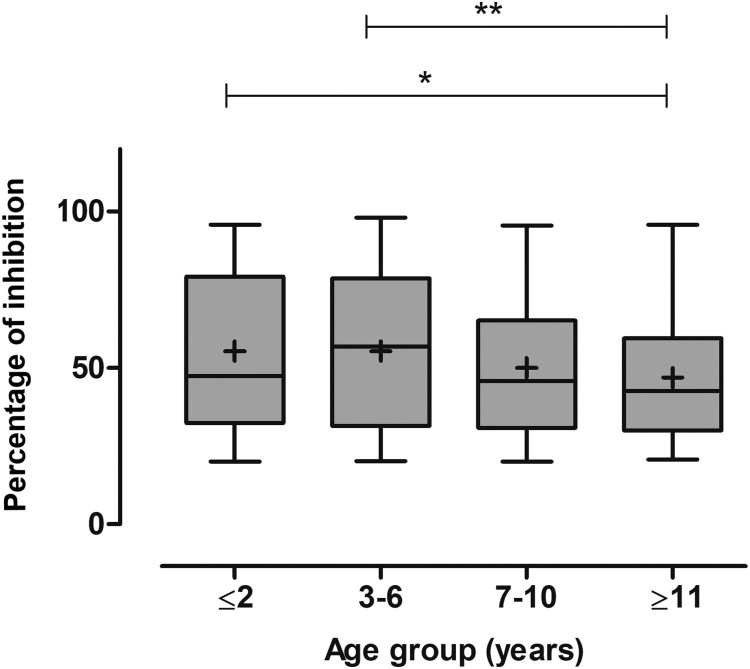
Univariate analyses revealed associations between samples seropositive for CRCoV and sampling month, age, and geographical region (p < 0.05). No statistical association was found between CRCoV seroprevalence and health status, breed, or gender of the dogs sampled (p > 0.2). Four variables satisfied the criteria for inclusion in the initial multivariable model (p ≤ 0.2), namely age, sampling month, region, and presence of abnormal respiratory signs (Table 2). Age, sampling month, and region were retained in the final model (p ≤ 0.05). Seroprevalence for CRCoV was higher in dogs aged ≥3 compared with ≤2 years (p < 0.01), but mean POI in seropositive dogs was lower in older than in younger dogs (Figure 2).
Table 2. Results of the multivariable logistic regression model for variables associated with the seroprevalence of canine respiratory coronavirus, in serum samples (n = 1,015) submitted to a veterinary diagnostic laboratory between March and December 2014 from throughout New Zealand.
| Variable | Pos/N (%)a | OR (95% CI) | P-valueb |
|---|---|---|---|
| Month of sampling | |||
| March | 63/100 (63) | Ref | |
| April | 60/100 (60) | 0.859 (0.47–1.54) | 0.612 |
| May | 58/105 (55) | 0.6733 (0.37–1.19) | 0.177 |
| June | 74/100 (74) | 1.498 (0.80–2.80) | 0.199 |
| July | 30/105 (29) | 0.218 (0.11–0.39) | <0.001 |
| August | 32/100 (32) | 0.248 (0.13–0.44) | <0.001 |
| September | 70/104 (67) | 1.158 (0.63–2.10) | 0.626 |
| October | 49/100 (49) | 0.473 (0.26–0.84) | 0.012 |
| November | 46/101 (46) | 0.450 (0.25–0.80) | 0.007 |
| December | 56/100 (56) | 0.648 (0.35–1.16) | 0.147 |
| Age of dog (years) | |||
| ≤2 | 71/169 (42) | Ref | |
| 3–6 | 132/244 (54) | 1.759 (1.15–2.69) | 0.008 |
| 7–10 | 164/303 (54) | 1.787 (1.19–2.69) | 0.005 |
| ≥11 | 171/299 (57) | 1.770 (1.18–2.67) | 0.005 |
| Region | |||
| Auckland | 243/415 (59) | Ref | |
| Hawkes Bay | 35/77 (45) | 0.634 (0.37–1.06) | 0.087 |
| Manawatu | 54/126 (43) | 0.458 (0.29–0.70) | <0.001 |
| Marlborough/Canterbury | 19/51 (37) | 0.371 (0.19–0.69) | 0.002 |
| Nelson/Tasman | 13/23 (57) | 0.836 (0.34–2.08) | 0.693 |
| Northland | 9/15 (60) | 1.026 (0.33–3.36) | 0.964 |
| Otago | 14/20 (70) | 1.597 (0.60–4.76) | 0.366 |
| Taranaki | 37/64 (58) | 0.864 (0.49–1.52) | 0.61 |
| Waikato | 22/51 (43) | 0.521 (0.27–0.97) | 0.042 |
| Wellington | 92/173 (53) | 0.775 (0.53–1.13) | 0.186 |
| Abnormal respiratory signs | |||
| No | 519/987 (52.6) | Ref | |
| Yes | 19/28 (67.9) | 2.206 (0.97–5.39) | 0.067 |
aNumber of seropositive samples/total number of samples.
bSignificance of difference compared to reference category (Ref).
Figure 2.
Box and whisker plots showing the percentage of inhibition of canine respiratory coronavirus (CRCoV) antibody, as measured by a blocking ELISA using bovine coronavirus as antigen, in serum samples from dogs that were seropositive for CRCoV and aged ≤2 (n = 71), 3–6 (n = 132), 7–10 (n = 164) or ≥11 (n = 171) years. The median is indicated by the middle line of each box, the mean by a cross, the 75th and 25th percentiles are indicated by the upper and lower edges of the boxes and minimum and maximum values are indicated by the whiskers. The significance of differences are indicated by * (p < 0.05) and ** (p < 0.01).
Abnormal respiratory signs (coughing, nasal discharge, or sneezing) were reported for only 28/1,015 (2.8%) dogs sampled. Seroprevalence for CRCoV tended to be higher among dogs with respiratory signs (67.9 (95% CI = 47.6–83.4)%) than dogs with no reported respiratory signs (52.6 (95% CI = 49.5–55.7)%). In addition, seropositive dogs with abnormal respiratory signs (n = 19) tended to have higher mean POI (60.0 (95% CI = 49.6–70.3)%) compared to all other seropositive dogs (n = 519) (50.8 (95% CI = 48.8–52.7)%, p = 0.082), but mean POI was similar to that of seropositive healthy dogs (n = 69) (55.2 (95% CI = 49.5–61.0)%, p = 0.439).
Discussion
The results of this study provided evidence that infection with CRCoV was common among dogs in New Zealand. Overall, 53% of the sera tested were positive for CRCoV antibodies. This was higher than the 29% reported previously in New Zealand (Knesl et al. 2009). Both studies used sera submitted to the same diagnostic laboratory as the source of diagnostic material. However in the 2009 study 251 samples were tested in comparison to 1,015 in the current study. Hence, the current estimation of CRCoV seroprevalence may be more accurate than the previous one. The population sampled in the 2009 study was somewhat loosely defined as representing a wide geographic area encompassing the central and lower North Island of New Zealand. Although samples in the current study originated from a wider geographical area, including the South Island, the majority of samples were from the central and lower North Island, so of similar distribution to the previous study. The difference in seroprevalence is also unlikely to be attributed to differences in the serological tests used, as the sensitivity and specificity of BCoV-based competitive ELISA for detection of CRCoV antibody was reported to be 94.4% and 96.7%, respectively, when compared to a fluorescent antibody test with CRCoV antigen that was used in the previous study (Priestnall et al. 2006).
The timing of sample collection may have contributed to the differences observed between the two studies. Unfortunately, the timing of sample collection was not provided by Knesl et al. (2009), but in the current study the lowest seroprevalences were observed in July (29%) and August (32%), which were similar to the 29% reported by Knesl et al. (2009). This may also be supported by the fact that 50% of dogs tested as part of another New Zealand-based survey were seropositive for CRCoV (Sowman et al. 2018). The number of samples tested in that study was only 93, but, as in the current study, the samples were collected over a period of several months from July 2012 to August 2013. Finally, we cannot exclude the possibility that the seroprevalence of CRCoV has increased in New Zealand since 2009.
The New Zealand data appear to be similar to the seroprevalence of CRCoV reported from other countries, including 59.1% in Canada, 54.7% in the United States of America, 36% in the United Kingdom, and 30.3% in the Republic of Ireland (Priestnall et al. 2006), as well 54% reported from a multicentre study that included 572 dogs from six European countries sampled over a period of 2 years (Mitchell et al. 2017). Similar to our results, other studies found regional differences in CRCoV seroprevalence, as can be exemplified by differences between various regions in the United States of America and in the United Kingdom. It has been suggested that the CRCoV seroprevalence may be higher in areas with a higher density of humans, and therefore presumably canine, populations (Priestnall et al. 2006). However CRCoV seroprevalence was similar in some of the less densely populated regions of the South Island and the more densely populated region of Auckland. On the other hand, regional differences were apparent, as seroprevalence in dogs from Auckland was higher in comparison to dogs from the Hawkes Bay, Manawatu, Marlborough, and Waikato regions. One possible reason for these differences is sample size and associated selection bias. In addition, population density in most parts of New Zealand, possibly with the exception of Auckland, is not uniform. Hence it may have been of interest to stratify the samples by the size of town/city, in addition to the geographical region. This was not performed as we did not have access to addresses of the submitters beyond the region classification. Overall, our data suggest that the epidemiology of CRCoV in New Zealand is similar to that observed overseas, particularly in Europe and in the United States of America.
The CRCoV seroprevalence varied between different months, but there was no clear seasonal pattern observed. The lowest CRCoV seroprevalences (22% and 17%) were detected in the winter months of July and August, but the highest seroprevalence (74%) was also observed in winter (June). This suggests that temporal differences in CRCoV seroprevalence were more likely to be related to factors other than external temperatures. These could include the increased contact between infected and non-infected dogs through activities such as training, competitions, short- or long-term kennelling or travel, but the exact nature of such interactions and their influence on the spread of CRCoV remain to be elucidated.
Similarly to results from other surveys (Priestnall et al. 2006, 2007; Knesl et al. 2009), the seroprevalence of CRCoV was higher in dogs aged ≥3 compared with ≤2 years. This is most easily explained by the increased likelihood of exposure to the virus for older dogs, which had more opportunities for contact with infectious dogs or contaminated environments than younger dogs. However we did not see a relative decline in the percentage of seropositive dogs among those older than 10 years, as reported by Priestnall et al. (2006, 2007). Those authors suggested that this could be related to the age-related fall in the efficiency of the immune response. In the current study, mean POI was lowest in seropositive dogs >10 years of age, which may support this conclusion. As it is currently unknown how long CRCoV antibodies persist in dogs, the lower POI detected in older dogs may also represent residual antibody due to past exposure as opposed to recent infection.
No statistically significant difference was observed between the seroprevalence of CRCoV in healthy and sick dogs, although seroprevalence tended to be higher in dogs with abnormal respiratory signs compared to those with no reported respiratory signs. While this is consistent with the overseas data (Erles et al. 2003; Soma et al. 2008), the number of dogs with respiratory signs in the current study was low, and thus these dogs were poorly matched to either healthy dogs or dogs with clinical problems other than respiratory disease. In addition, some dogs categorised as having abnormal respiratory signs may have been included due to non-infectious causes, as the group allocation was made based on limited clinical data provided on the submission form. Finally, the retrospective nature of the study, combined with the samples being sourced from a diagnostic laboratory, was likely to introduce a selection bias towards dogs with a variety of health problems compared with the general population. Hence field studies using similarly sized, age-matched groups of dogs with and without respiratory disease would be needed to further investigate the impact of CRCoV infection on the health status of affected dogs.
The sampled population contained 161 different dog breeds making the analysis of breed associations with seroprevalence of CRCoV impractical. Therefore breeds were categorised into broad use categories instead. This may have introduced some confounding as it is possible that some dogs of breeds that are typically used as farm working dogs may have been kept as pet dogs, while some dogs that were classified as pets based on their breeds may have been used as working dogs. None-the-less, there appeared to be no difference in seroprevalence of CRCoV among dogs from different use categories, which was consistent with results of An et al. (2010) who reported no difference in CRCoV seroprevalence between farm dogs and pet dogs. Also consistent with overseas findings (Erles and Brownlie 2005; Soma et al. 2008) was the lack of association between the sex of the dog and seroprevalence of CRCoV, indicating that sex-related activities or behaviours are unlikely to be associated with the likelihood of exposure to the virus.
In conclusion, we have shown serological evidence that more than half of the dogs tested from throughout New Zealand were infected with CRCoV at some point during their lives. Further studies into the virus-host interactions and the impact of CRCoV infection on the health status of dogs under local New Zealand conditions are warranted. The importance of CRCoV in ICT remains to be elucidated. However, considering the apparent high seroprevalence of CRCoV in New Zealand, this virus should be included in investigations of cases of ICT, particularly if these occur among dogs vaccinated with current vaccines, which do not include CRCoV antigens.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
The study was partly funded by the New Zealand Greyhound Association.
Acknowledgements
Sincere thanks to Raewynne Pearson and Adrienne French for supplying the serum samples for ELISA and to Janis Bridges for help with statistical analysis. This study was partly funded by the New Zealand Greyhound Racing Association.
References
- An D-J, Jeoung H-Y, Jeong W, Chae S, Song D-S, Oh J-S, Park B-K.. A serological survey of canine respiratory coronavirus and canine influenza virus in Korean dogs. The Journal of Veterinary Medical Science 72, 1217–9, 2010. doi: 10.1292/jvms.10-0067 [DOI] [PubMed] [Google Scholar]
- Decaro N, Desario C, Elia G, Mari V, Lucente MS, Cordioli P, Colaianni ML, Martella V, Buonavoglia C.. Serological and molecular evidence that canine respiratory coronavirus is circulating in Italy. Veterinary Microbiology 121, 225–30, 2007. doi: 10.1016/j.vetmic.2006.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JA, McLean N, Hupaelo R, Haines DM.. Detection of coronavirus in cases of tracheobronchitis in dogs: a retrospective study from 1971 to 2003. The Canadian Veterinary Journal 46, 447, 2005 [PMC free article] [PubMed] [Google Scholar]
- Erles K, Toomey C, Brooks HW, Brownlie J.. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 310, 216–23, 2003. doi: 10.1016/S0042-6822(03)00160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K, Brownlie J.. Investigation into the causes of canine infectious respiratory disease: antibody responses to canine respiratory coronavirus and canine herpesvirus in two kennelled dog populations. Archives of Virology 150, 1493–504, 2005. doi: 10.1007/s00705-005-0533-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K, Brownlie J.. Canine respiratory coronavirus: an emerging pathogen in the canine infectious respiratory disease complex. Veterinary Clinics of North America: Small Animal Practice 38, 815–25, 2008. doi: 10.1016/j.cvsm.2008.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshima T, Hohdatsu T, Satoh K, Takano T, Motokawa K, Koyama H.. The prevalence of a group 2 coronavirus in dogs in Japan. The Journal of Veterinary Medical Science 68, 21–5, 2006. doi: 10.1292/jvms.68.21 [DOI] [PubMed] [Google Scholar]
- Knesl O, Allan F, Shields S.. The seroprevalence of canine respiratory coronavirus and canine influenza virus in dogs in New Zealand. New Zealand Veterinary Journal 57, 295–8, 2009. doi: 10.1080/00480169.2009.58624 [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Cardwell JM, Leach H, Walker CA, Le Poder S, Decaro N, Rusvai M, Egberink H, Rottier P, Fernandez M.. European surveillance of emerging pathogens associated with canine infectious respiratory disease. Veterinary Microbiology 212, 31–8, 2017. doi: 10.1016/j.vetmic.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestnall SL, Brownlie J, Dubovi EJ, Erles K.. Serological prevalence of canine respiratory coronavirus. Veterinary Microbiology 115, 43–53, 2006. doi: 10.1016/j.vetmic.2006.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestnall SL, Pratelli A, Brownlie J, Erles K.. Serological prevalence of canine respiratory coronavirus in southern Italy and epidemiological relationship with canine enteric coronavirus. Journal of Veterinary Diagnostic Investigation 19, 176–80, 2007. doi: 10.1177/104063870701900206 [DOI] [PubMed] [Google Scholar]
- Soma T, Ishii H, Miyata K, Hara M.. Prevalence of antibodies to canine respiratory coronavirus in some dog populations in Japan. Veterinary Record 163, 368–9, 2008. doi: 10.1136/vr.163.12.368 [DOI] [PubMed] [Google Scholar]
- Sowman HR, Cave NJ, Dunowska M.. A survey of canine respiratory pathogens in New Zealand dogs. New Zealand Veterinary Journal 66, 236–42, 2018. doi: 10.1080/00480169.2018.1490214 [DOI] [PubMed] [Google Scholar]