Abstract
Cell culture-based vaccine technology is a flexible and convenient approach for vaccine production that requires adaptation of the vaccine strains to the new cells. Driven by the motivation to develop a broadly permissive cell line for infection with a wide range of viruses, we identified a set of the most relevant host receptors involved in viral attachment and entry. This identification was done through a review of different viral entry pathways and host cell lines, and in the context of the Baltimore classification of viruses. In addition, we indicated the potential technical problems and proposed some solutions regarding how to modify the host cell genome in order to meet industrial requirements for mass production of antiviral vaccines. Our work contributes to a finer understanding of the importance of breaking the host–virus recognition specificities for the possibility of creating a cell line feasible for the production of vaccines against a broad spectrum of viruses.
Keywords: Virus, attachment factor, entry receptor, entry pathway, vaccine production, susceptible cell line
Introduction
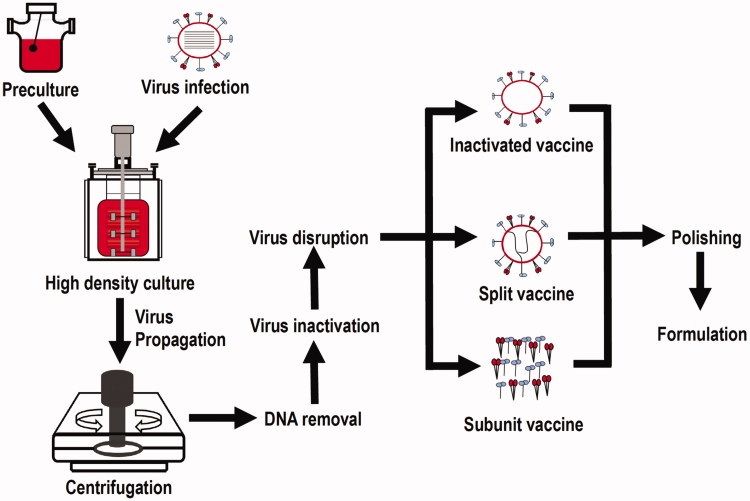
Vaccination is the best and most widely used approach to control many infectious diseases (Perdue et al. 2011). In the early 20th century, vaccines were mostly produced in animal tissues, such as nervous tissues extracted from rabbits or goats, or obtained from blood serums of infected animals (Aubrit et al. 2015). Cell-based vaccine manufacturing, which uses cultured cells of mammalian origin as the host to produce viruses, is a highly flexible approach for viral vaccine production (Montomoli et al. 2012), and has obtained increasing acceptance from regulatory agencies and led to major advances in viral vaccine development. During cell-based vaccine manufacturing, candidate vaccine viruses are grown in mammalian tissue culture of cells with a finite lifespan followed by virus extraction from cells in the liquid culture, purification, and test or modification for specific vaccine production (Vlecken et al. 2013) (Figure 1). Among the four major types of vaccines, i.e. live-attenuated vaccines, inactivated vaccines, subunit/recombinant/polysaccharide/conjugate vaccines, toxoid vaccines, the first three types are suitable to be manufactured using the cell-based approach. Several animal cells have been used for vaccine production for years, such as African green monkey kidney (Vero) cells that were used to produce vaccines against polio and rabies, Madin Darby canine kidney (MDCK) cells that were employed to manufacture influenza vaccines, and chicken embryo fibroblasts (CEFs) that were used to produce vaccines against measles, mumps, rabies and tick-borne encephalitis (Genzel 2015). As one successful example, IMOVAX® Polio is a sterile suspension of types 1–3 of inactivated poliomyelitis vaccine and prepared from poliovirus cultured in Vero cells, which has been widely used to prevent poliomyelitis for both primary immunization and boosters through stimulating antibody production in the body. Health intervention using polio vaccine was proved to be cost-effective in the United States (Thompson and Tebbens 2006), Kano and Nigeria (Qadar 2014). Besides cost-effectiveness, the use of cell lines in vaccine production is advantageous in its fast speed and stable vaccine qualities produced. Additionally, cell-based approaches allow for multiple viral vaccines being produced in the same production platforms in a more sterile environment (Audsley and Tannock 2008; Perdue et al. 2011). However, cell-based vaccines require adaptation of the vaccine strains to the new cells (Genzel 2015). For instance, selection pressure was used to adapt MDCK cells to grow in suspension to produce influenza viruses, and the generated MDCK suspension cells were used by Novartis Vaccines to produce the first licenced cell-based influenza vaccine (Gregersen et al. 2011). This adaption sometimes proves to be a difficult task with variable outcomes, resulting in the limited established cell lines for vaccine production despite the intense efforts on novel cell line design for vaccine production (Genzel 2015). Over the past few years, enhancing virus replication in hosts with a goal of reducing vaccine manufacturing costs has been attempted. For example, van der Sanden et al. found that knocking down multiple genes, alone or in pairwise combination, increased viral titres more than 20-fold and over 50-fold, respectively, in poliovirus (van der Sanden et al. 2016). On the other hand, vaccines against different viruses need to be produced using different cell lines, due to the limited tropism of the viruses. This specificity is one of the reasons why the technical cost of the development of a novel cell line for vaccine production remains high. Further, some viruses such as cytomegalovirus (CMV) and Epstein–Barr virus (EBV) impose great challenges to human health but still lack effective vaccines available on the market (Morello et al. 2007; Sokal et al. 2007), and viruses such as hepatitis B virus (HBV) are difficult to culture by nature that needs a robust culture model to be established (Zeisel et al. 2015). Thus, there is an urgent need to break the specificity between host and viruses with the potential of constructing a cell line that can be infected by a broad range of viruses.
Figure 1.
Schematic illustration on cell-based vaccine production.
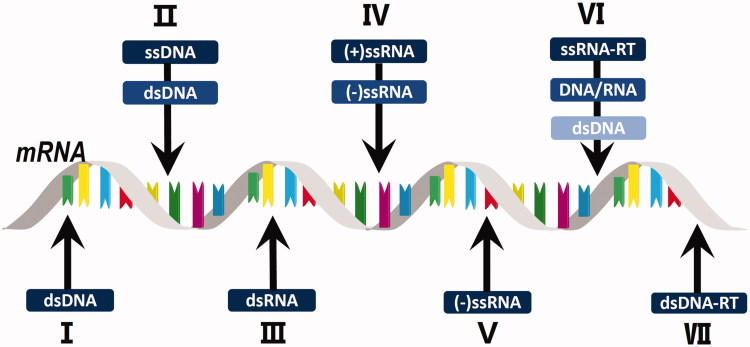
A few cell lines have already been used for the production of multiple viruses. For example, Vero cells were shown to be highly susceptible to infection with a multitude of different viruses and have been used to produce vaccines against many viral diseases such as Japanese encephalitis, West Nile encephalitis, dengue fever, Ross River disease, chikungunya, severe acute respiratory syndrome (SARS), smallpox and influenza (Barrett et al. 2009). Many factors are known to affect susceptibility of the cells to viral invasion. For example, host antiviral restriction factors induce cellular resistance against a number of viral pathogens (Kluge et al. 2015), and capsid proteins of small non-enveloped DNA viruses play an important role in intracellular membrane perturbation in the early stages of viral infection (Bilkova et al. 2014). Therefore, identifying the key determinants influencing the susceptibility of cells to different viruses, as classified by the Baltimore system, is of the utmost importance. Baltimore classification is a virus classification system that categorizes viruses into families depending on their type of genome and method of replication. Specifically, this system groups viruses into seven classes: double-stranded DNA viruses (dsDNA), single-stranded DNA virus (ssDNA), double-stranded RNA viruses (dsRNA), positive single-stranded RNA viruses (+ssRNA), negative single-stranded RNA viruses (−ssRNA), positive single-stranded retro RNA viruses (ssRNA-RT) and double-stranded DNA retro viruses (dsDNA-RT) (Figure 2).
Figure 2.
Baltimore classification of viruses and their nucleic acid form prior to mRNA transcription.
Here we review the viral entry pathways and host receptors mediating viral invasion, with the aim of deciphering the essential factors orchestrating the differential use of cellular receptors involved in the infection of diverse types of viruses. We aimed to identify the minimally required receptor panel for the possibility of constructing cells that can be infected by a broad range of viruses through categorizing virus entry mechanisms and summarizing receptors mediating the entry process according to Baltimore subtyping, and used typical or well-known example viruses to illustrate each summarized entry pathway. We did not differentiate animal or human viruses in this review as it is common to use animal cells for human vaccine production such as the use of MDCK cells for influenza vaccine production, and the use of chicken embryonic fibroblasts (CEFs) for producing vaccines against measles, mumps, rabies and tick-borne encephalitis (Genzel 2015); and the establishment of cell lines feasible for multiple virus production is not limited to human vaccines but also applies to animal vaccines. This study seeks to further our understanding of the mechanisms behind the virus–host interactions, and will arm researchers with the molecular tools for establishing a potentially omnipotent cell line with a broad host tropism or permissivity. This cell line will help us develop a cost- and time-effective vaccine manufacturing platform.
Virus entry pathways
Viruses can be classified into diverse categories depending on their chemical and physical characteristics, such as nucleic acid form, envelope presence, replication mode, host organism and disease type they cause. Virions generally enter host cells through endocytosis, fusion or direct penetration, with endocytosis being the dominant entry mechanism. As endocytosis is prevalent among various types of viruses, fusion and direct penetration are specific to enveloped (possessing an enclosing envelope) and naked (consisting only of a nucleocapsid) viruses, respectively. Viral entry pathways or mechanisms can be cell-type specific, which are primarily determined by host receptors (Karasneh and Shukla 2011). Or, viruses may adopt distinct modes when entering cells of the same type in the presence of environmental perturbations such as trypsin (Suzuki et al. 1985).
There are two types of cellular endocytosis: phagocytosis and pinocytosis (Lakadamyali et al. 2004). While phagocytosis is typically employed for large particles and bacteria digestion, pinocytosis is responsible for cellular intake of fluids, macromolecules and small pathogens (Lakadamyali et al. 2004). Viruses largely utilize pinocytosis to assist endocytosis-mediated cell entry, which could be classified into three subtypes: clathrin-mediated endocytosis, caveolin-mediated endocytosis and non-clathrin non-caveolin-mediated endocytosis, according to the vesicles used (Mercer et al. 2010). Clathrin-mediated endocytosis is one of the best characterized types of endocytosis for virus entry. During this pathway, ligands enter cells via clathrin-coated pits (CCPs), these pits are internalized to form clathrin-coated vesicles followed by uncoating and subsequent delivery to the early endosomes where they are exposed to an acidic environment (Johnson and Vogt 2010). Internalized cargoes and membrane proteins are typically progressed to the late endosomes and lysosomes for degradation (Vale-Costa and Amorim 2016). In the latter case, the luminal pH is rapidly reduced from 6.5 to 5.5 (Zhu 2014). Caveolin-mediated endocytosis occurs via caveolae, caveolin-associated membrane invaginations, which are pinched off to form endocytic vesicles followed by fusion with caveolin-containing membrane compartments (caveosomes) (Lakadamyali et al. 2004). This process may or may not involve the recognition of host receptors, but depends on the presence of lipid rafts and cholesterol during virus entry. Once the virus is internalized, it migrates to caveolin positive vesicles that are trafficked to the perinuclear region and fused with caveosomes (Johnson and Vogt 2010). There is controversy over the nature of the “caveosome” once it was found to be an artefact of fixing/staining procedures (Parton and Howes 2010; Engel et al. 2011). The number of non-clathrin, non-caveolin-mediated endocytosis mechanisms and molecular identities involved in this process are not completely resolved due to the lack of proper biomarkers. This process requires a receptor-mediated conformational transition for efficient cargo internalization (Damm et al. 2005; Grassart et al. 2010). Alternatively, endocytosis independent of clathrin and caveolin was proposed to be similar to phagocytosis, which transports cargoes using vesicles such as phagosomes (Suzuki et al. 1985).
Enveloped viruses can penetrate cells without disrupting the cellular membrane by fusing its envelope with the host cell at the plasma membrane (namely “fusion”) (Zhang et al. 2010) or utilizing endocytosis (Husain and Moss 2005). Naked viruses (non-enveloped), on the other hand, need a transient permeabilization (or disruption) of the cellular membrane to permit their translocation into the cytoplasm, namely “direct penetration” (Payne and Norrby 1978). Fusion is pH dependent (from within an intracellular vesicle) or pH independent (at the plasma membrane). Direct penetration requires sequential conformational alterations of the viral capsid induced by proteolytic enzymes such as trypsin, low pH, activation by cellular motors or membrane pore formation. In some cases, viruses can utilize multiple entry pathways.
Clathrin-mediated endocytosis—an example of an enveloped RNA virus
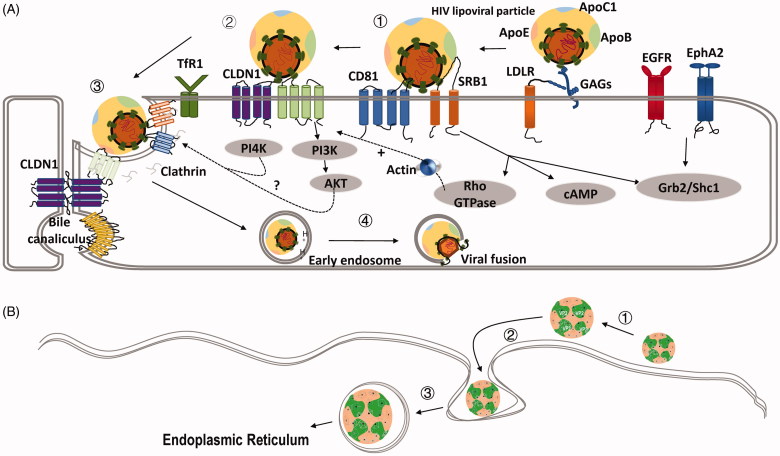
Hepatitis C virus (HCV) is an enveloped positive single-stranded RNA virus (group IV in the Baltimore Classification), which is comprised of a nucleocapsid surrounded by a host-derived membrane containing the E1 and E2 HCV glycoproteins (Gastaminza et al. 2010). HCV uses clathrin-mediated endocytosis, which consists of three steps: virus attachment, receptor-mediated endocytosis and endosomal fusion. After initial attachment of HCV to the host cell surface mediated by heparan sulphate (HS) chains from heparan sulphate proteoglycans (HSPG), more specifically syndecan-1 (SDC1) and SDC4, and low-density lipoprotein (LDL) receptor (LDLR), the virus interacts with: (a) co-receptors such as scavenger receptor class B type I (SRBI), CD81, claudin-1 (CLDN1), occludin (OCLN), (b) entry factors such as EGFR (Hu et al. 2018), ephrin receptor A2 (EphA2) (Zhang et al. 2018), transferrin receptor 1 (TfR1) and (c) cholesterol transporter Niemann-Pick C1-like 1 (NPC1L1), which collaboratively complete endocytosis (Figure 3(A)). At the final step, the fusion process is triggered in a receptor-independent but pH-dependent fashion and is dependent on the lipid composition of the target cell membrane (Haid et al. 2009; Shi et al. 2013; Lefèvre et al. 2014). Specifically, CD81 and CLDN1 naturally form a complex under the regulation of EGFR and the GTPase H-Ras; HCV interacts with the CD81/CLDN1 complex followed by interactions with OCLN that ultimately lead to its internalization through clathrin-mediated endocytosis (Banse et al. 2018). The critical role of tight junctions is emphasized by the indispensability of both tight junction proteins CLDN1 and OCLN.
Figure 3.
Schematic representations of clathrin-mediated endocytosis and caveolin-mediated endocytosis. (A) HCV entry process illustrating the clathrin-mediated endocytosis. ①HCV binds GAGs (HS from syndecan-1 and syndecan-4) and LDLR, which have high affinity for the ApoE (apolipoprotein E). SRBI (scavenger receptor B type I) plays an important role in both binding and post-binding steps of viral entry through interaction with virion-associated lipoprotein or HCV E2. ②Binding of SRBI to HCV particles allows exposure of CD81 binding sites on HCV E2 and transfer of the virus particles to CD81. ③The virion is primed by the low-pH fusion activity of CD81 and CLDN1 and translocates to the tight junctions in order to be endocytosed. Viral internalization is dependent on clathrin-mediated endocytosis. Junction protein occludin (OCLN) can contribute to this process. TfR1, EGFR, and EphA2 (ephrin receptor A2) play a role in HCV infection at the level of glycoprotein-mediated entry, acts after CD81, and possibly are involved in the HCV particle internalization. PI3K-AKT and PI4K pathways are engaged in the late step of HCV entry. However, the molecular mechanisms need to be investigated. ④Following internalization, HCV fusion occurs in the early endosomes. Low pH environment and virion-associated cholesterol are required for the fusion process. NPC1L1 may play a role in this process via cholesterol transport. After fusion between the viral envelope and an endosomal membrane, the viral genome is released into the cytosol and replication takes place. Reprinted with permission from Zhu (2014). (B) SV40 entry process illustrating caveolin-mediated endocytosis. ①SV40 binding to the host cell is codirected by the capsid and VP2. ② The bound virus traverses the membrane and enters a caveolae. ③ The virus is endocytosed and transported in the caveolae-coated vesicles to endoplasmic reticulum.
Caveolin-mediated endocytosis—an example of a naked DNA virus
Simian vacuolating virus 40 (SV40), a polyomavirus found in both monkeys and humans, consists of a naked icosahedral virion with a dsDNA genome. SV40 encodes three late structural proteins, VP1, VP2, and VP3 (Daniels et al. 2006). While VP2 and VP3 function in the translocation of viral DNA across the endoplasmic reticulum (ER) membrane, VP1 functions as a viral attachment protein binding to GM1 (the receptor for SV40) and regulates the functionalities of VP2 and VP3 during viral assembly and penetration by controlling their solubility and membrane integration (Daniels et al. 2006).
SV40 uses caveolin-mediated endocytosis (Figure 3(B)) and non-clathrin non-caveolin-mediated endocytosis to enter host cells. While SV40 exploits the transmembrane proteins such as integrins to enter cells via the non-clathrin non-caveolin-mediated pathway (Stergiou et al. 2013), it binds to GM1 during caveolin-mediated endocytosis (Boulant et al. 2015). Over 50 kinases have been identified to regulate SV40 entry and its early infection steps (Pelkmans et al. 2005). Nonetheless, lipid rafts and cholesterols are required for virus entry, and an acidic condition is needed for triggering the disassembly of capsid and viral genome release (Kosukegawa et al. 1996). Thus, SV40 first binds to the host cell surface and diffuses along the membrane until it reaches a caveolae. Next, the virions are endocytosed into caveolin-1-coated vesicles, which later converge into a caveosomes followed by the budding of caveolin-1-devoid vesicles that deliver the virus to ER.
Non-clathrin non-caveolin-mediated endocytosis and fusion—an example of an enveloped DNA virus
Herpes simplex viruses (HSVs) are enveloped dsDNA viruses, consisting of two family members, i.e. HSV-1 and HSV-2. HSV-1 commonly causes “cold sores” and HSV-2 is often referred to as “genital herpes.” These viruses have a broad spectrum of cell hosts (Spear and Longnecker 2003) due to the use of multiple alternative receptors and entry pathways, such as non-clathrin non-caveolin-mediated endocytosis and pH-independent fusion with the plasma membrane of host cells (Gianni et al. 2013; Albecka et al. 2016).
The non-clathrin non-caveolin-mediated endocytosis pathway shares many features with professional phagocytosis that probably requires actin rearrangement, dynamin assembly, RhoA GTPase and tyrosine kinase involvement (Kirkham and Parton 2005). In brief, HSPG-mediated HSV adsorption occurs on the cell surface followed by phagocytosis-like uptake and phagosome-mediated transportation. The presence of nectin-1 (the cell adhesion molecule, which is an HSV receptor) or herpes virus entry mediator (HVEM) in those vesicles enables virions to fuse their envelopes with the vesicular membrane and release the nucleocapsid into the cytoplasm for further replication (Clement et al. 2006).
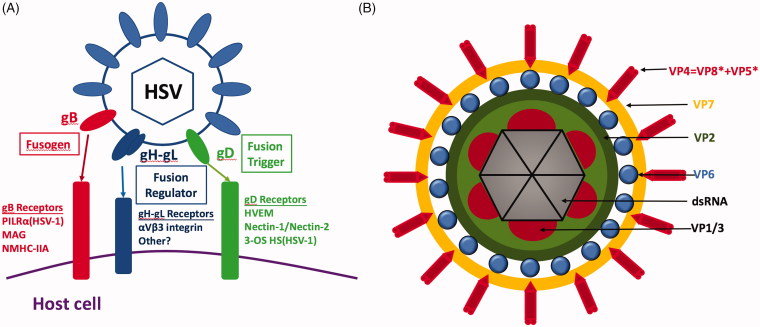
The HSV-cell fusion is pH independent and is facilitated by at least five viral glycoproteins, namely gB, gC, gD, gH, and gL (Figure 4(A)). The infection is initiated by the interaction of gC and/or gB with cellular HSPG that captures HSV and attach it to host cell surface, and internalized by interactions between viral gD and the mediating entry receptors. This interaction allows tight anchoring of viruses to the plasma membrane of the host cells, and consequential conformational changes of the gD that triggers virus–cell membranes fusion (Karasneh and Shukla 2011).
Figure 4.
Schematic representations of the structures of representative viruses that employ diverse entry pathways: non-clathrin, non-caveolin-mediated endocytosis and direct penetration. (A) HSV-1 glycoproteins and their identified cell receptors required for viral entry. The gB receptors are known to be crucial for virus attachment and membrane fusion (Fusogen), gD receptors are reported to trigger the fusion process (Fusion Trigger) and gH-gL receptors are known to regulate the fusion process with the mechanism less understood (Fusion Regulator). Reprinted with permission from Karasneh and Shukla (2011). (B) Structure of rotavirus. The three concentric capsid protein layers are the VP7 layer with VP4 spikes, the VP6 layer and the VP2 layer. The dsRNA segments are packed inside and associated to the RNA polymerase complexes VP1 and VP3.
The gB receptors work collaboratively with HSPG in the attachment stage (Karasneh and Shukla 2011) and play important roles during membrane fusion. The existence of three cellular receptors, the paired immunoglobulin-like type 2 receptor-α (PILRα), myelin-associated glycoprotein (MAG), and non-muscle myosin heavy chain IIA (NMHC-IIA), of viral gB has been widely accepted. While the expressions of PILRα and MAG (which share 5–12% homology) are limited to certain cell types (Suenaga et al. 2010), NMHC-IIA is ubiquitously expressed in numerous human tissues and cell lines (Vicente-Manzanares et al. 2009). The gD receptors are indispensable for triggering virus entry into the cells. There are three classes of gD receptors which belong to structurally unrelated molecular families. HVEM (Herpesvirus Entry Mediator) is the first identified gD receptor, which belongs to the tumour necrosis factor receptor family, and is expressed in a variety of cell types. The second class of receptors includes nectin-1 and -2. While both nectins are broadly expressed in a wide range of human tissues and cell lines, nectin-1 is expressed on all tested cell lines sensitive to HSV-1 and HSV-2 invasion (Akhtar and Shukla 2009), and nectin-2 is a weak receptor for HSV-2 (Lopez et al. 2000). The third class refers to 3-O-Sulphated heparan sulphate proteoglycan (3-OS HS), a highly sulphated form of HS that recognizes HSV-1 but not HSV-2. The 3-OS HS has a lower cell surface expression than nectin-1. The interactions between gH and gL receptors regulate the fusion process. Thus, the gH-gL receptor, the integrin αvβ3, the gB receptor and PILRα collectively determine the entry pathway of HSV (Karasneh and Shukla 2011).
Direct penetration—an example of a naked RNA virus
Rotaviruses (RVs) are naked particles composed of three protein layers and the core containing the double-stranded RNA genome and enzymes of the replication complex. The concentric capsid protein layers are termed VP7, VP6, and VP2 (from the outer to inner layer). The VP4 spike protein is buried within the VP7 glycoprotein, which together comprises the outer capsid of the complete RV particle (Figure 4(B)).
Three modes have been proposed for RV infection: direct penetration of the plasma membrane (Liprandi et al. 1997), clathrin-mediated endocytosis (Chemello et al. 2002), and non-clathrin non-caveolin-mediated endocytosis (Sanchez-San Martin et al. 2004). Sialic acid (SA) receptors function at the initial attachment step, then integrins such as α2β1, α4β1, αxβ2, αvβ3 take over at a post-attachment step (Graham et al. 2003), and hsc70 joins the mediation of RVs binding process (Johnson and Vogt 2010). As outlined above, transient permeabilization of the plasma membrane is needed for some naked viruses. In the case of RVs, this step is primarily mediated by the interaction between virus external proteins (VP4 and VP7) and the host receptors, as described above.
Trypsin plays an important role in direct penetration of RVs. Previous studies have shown that trypsin-untreated RV particles could successfully enter cells via the endocytic pathway. However, this event did not result in the release of viral genome into the cytoplasm and viral replication. Thus, trypsin-mediated cleavage is required for efficient RV infection (Suzuki et al. 1985). In particular, trypsin cleaves VP4 into VP8* and VP5*, which mediates the binding of SA-dependent and SA-independent viral strains to cells. Next, cellular integrins α2β1, α4β1, and hsc70 interact with VP5*, and integrins αxβ2 and αvβ3 interact with protein VP7 (Graham et al. 2003) in the subsequent entry processes. Although the exact molecular mechanism of the RVs–host interaction is not completely understood, the current model proposes the interaction between VP4 and host cellular membrane, during which the trypsin-mediated cleavage releases VP8* from VP5* and exposes the hydrophobic domain of VP5*, leading to membrane disruption and viral penetration (Arias et al. 2015). The trypsin-mediated cleavage ultimately helps to dissolve the double-shelled viral capsid and cellular membrane. It was shown in vivo that VP4 is stable but non-infectious in the uncleaved state that is advantageous to resist environmental degradation until it infects a susceptible host, and becomes unstable but infectious once cleaved in the lumen of the host’s gastrointestinal tract by trypsin (Ludert et al. 1996).
Host receptors
Initiation of infection is mediated by viral attachment and entry receptors, which are essential factors determining the entry pathway and cellular tropism of a given virus. Host receptors capture viral particles and mediate the penetration of viral genome into the cell where the intracellular infective cycle of viruses initiate (Casasnovas 2013). For example, the positive single strand naked RNA viruses, picornavirus and echovirus 1 (both belonging to the Picornaviridae family), use clathrin- and caveolin-mediated endocytosis to enter cells, respectively. This process is mediated by their interaction with α2β1 integrin (VLA-2) present on host lymphocytes (Johnson and Vogt 2010).
We summarized the current knowledge about the associations between host receptors and virus nucleic acid types for some randomly selected, well-known viruses categorized by the Baltimore system and according to the reported evidences (Table 1). These receptors are grouped as attachment factors (HSPG, SA), entry receptors (integrin, CD46, CD150, Nectin 4, TfR1, LDLR) according to the viral entry steps in which they are primarily involved in. Overall, viruses are attracted by attachment factors to cell surface where entry factors take over to mediate the viral internalization process.
Table 1.
Receptor usage by representative viruses for each Baltimore class (or group).
| Baltimore subtype | Viral nucleic acid form | Virus | Envelope | Entry pathways | Attachment factors | Entry receptors |
|---|---|---|---|---|---|---|
| I: dsDNA | dsDNA | HSV | Yes | NCNC, F (Karasneh and Shukla 2011) | HS (Karasneh and Shukla 2011) | Integrin, HVEM, Nectin1/2 (Karasneh and Shukla 2011; Gianni et al. 2013) |
| HPV | No | CME, CaE, NCNC (Shafti-Keramat et al. 2003; Schelhaas et al. 2012) | HS (Shafti-Keramat et al. 2003) | Integrin, GFR, CD63, CD151 (Raff et al. 2013; Aksoy et al. 2014) | ||
| II: +ssDNA | dsDNA | CPV | No | CME (Vihinen-Ranta et al. 1998) | SA (Lofling et al. 2013) | TfR (Lofling et al. 2013; Lee et al. 2019) |
| PCV | No | CME, NCNC (Misinzo 2005; Misinzo et al. 2009) | HS,SA (Misinzo et al. 2006; Nauwynck et al. 2012) | TfR (Misinzo 2005) | ||
| III: dsRNA | dsRNA | RV | No | CME, NCNC, DF (Liprandi et al. 1997; Chemello et al. 2002; Sanchez-San Martin et al. 2004) | SA, HBGA, GMx (Ruiz et al. 2009; Hu et al. 2012) | Integrin, HSC70 (Zarate et al. 2003; Ruiz et al. 2009) |
| BTV | No | CME (Forzan et al. 2007) | HS, SA (Mecham and McHolland 2010; Zhang et al. 2010) | Integrin, TfR, CD63 (Forzan et al. 2007) | ||
| IV: +ssRNA | −ssRNA | BVDV | Yes | CME (Mathapati et al. 2010) | HS (Maurer et al. 2004) | LDLR, CD46 (Agnello et al. 1999; Maurer et al. 2004) |
| HRV | No | DF (Kolatkar et al. 1999) | LDLR, ICAM1 (Kolatkar et al. 1999) | |||
| V: -ssRNA | −ssRNA | MV | Yes | F (Schneider-Schaulies et al. 2001) | SA (Talekar et al. 2013) | Nectin4, CD150, CD46 (Schneider-Schaulies et al. 2001; Delpeut et al. 2014) |
| CDV | Yes | F (Lamb et al. 2006) | HS (Fujita et al. 2007) | Nectin4, CD150 (von Messling et al. 2005; Pratakpiriya et al. 2012) | ||
| VI: +ssRNA-RT | dsDNA | HIV | Yes | F (Saphire et al. 2001) | HS, CD4 (Saphire et al. 2001) | Integrin (Monini et al. 2012) |
| HTLV-1 | Yes | F (Ghez et al. 2006) | HS (Lambert et al. 2009) | GLUT1 (Ghez et al. 2006) | ||
| VII: dsDNA-RT | dsRNA | HBV | Yes | CME, CaE (Macovei et al. 2010; Huang et al. 2012) | HS (Glebe and Bremer 2013) | ASGPR, NTCP, P80, HSC70, HSC60 (Ryu et al. 2000; Zhang et al. 2011; Glebe and Bremer 2013) |
| DHBV | Yes | CaE, NCNC (Köck et al. 1996; Macovei et al. 2010) | P170 (Li et al. 1996) | P120 (Li et al. 1996) |
“Viral nucleic acid form” refers to the form of viral nucleic acids before genetic information passaging. Entry pathways are annotated using different abbreviations, i.e. CME: clathrin‐mediated endocytosis; CaE: caveolin‐mediated endocytosis; DF: direct penetration; F: fusion; NCNC: non‐clathrin, non‐caveolin mediated endocytosis. Abbreviations of some principle receptors are: CD: cluster of differentiation; CCR5: C‐C chemokine receptor type 5; CXCR4: C‐X‐C chemokine receptor type 4; HBGA: histo‐bloodgroup antigen; GFR: growth factor receptor; GMx: ganglioside x; P170 or DCPD: duck carboxypeptidase D; GLUT1: glucose transporter 1; HVEM: herpesvirus entry mediator, belonging to tumour necrosis factor receptor family and a regulator of immune responses); ICAM1: intercellular adhesion molecule 1; NTCP: sodium taurocholate co‐transporting polypeptide; P120: P protein of duck glycine decarboxylase. References corresponding to the information source of virus entry pathways and host mayor receptors are listed. Two randomly selected viruses from each Baltimore subtype are included here. BTV: bluetongue virus; BVDV: bovine viral diarrhoea virus; CDV: canine distemper virus; CPV: canine parvovirus; DHBV: duck hepatitis B virus; HIV: human immunodeficiency virus; HBV: hepatitis B virus; HSV: herpes simplex virus; HPV: human papillomavirus; HRV: human rhinovirus; MV: measles virus; PCV: porcine circovirus.
Attachment factors
Attachment factors capture viruses and attach them to host cells that initiate the entry process, but do not necessarily trigger conformational changes in viral envelope proteins (Schnierle 2019). Attachment factors are typical not virus-specific and can be used by various types of viruses. Their presence enables virus entry, infection, replication and spread.
Glycosaminoglycan (GAG)
Glycosaminoglycans (GAGs) are long unbranched polysaccharides consisting of a repeating disaccharide unit, which contains heparan sulphate (HS), chondroitin sulphate (CS) and keratan sulphate (KS). These GAGs differ in their disaccharide repeating units, which are the building units for the polysaccharides. While most cell surface proteoglycans contain HS (the most common form of GAGs), many are hybrid structures comprising of both HS and CS such as PCV2 that utilises HS and CS-B receptors for attachment (Misinzo et al. 2006), and a few contains exclusively CS (Rostand and Esko 1997).
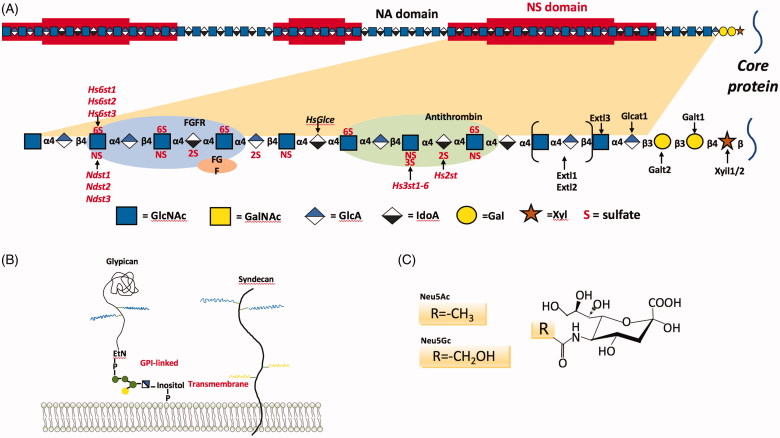
HS has two forms: the linear polysaccharide and the proteoglycan form in which two or three HS chains are attached in close proximity to the cell surface or to the extracellular matrix (ECM) proteins (namely heparan sulphate proteoglycans, HSPG). Both forms of HS are abundantly expressed on the surface of almost all cell types. HS is decorated with negatively charged sulphate groups that enable it to attract positively charged viral glycoproteins. It has been widely accepted that HS is an attachment receptor for many viruses, and accumulating evidence suggests that HS has an important role in enhancing viral infection. A wide spectrum of viruses is attracted by HS including porcine circovirus (PCV) (Nauwynck et al. 2012) and bluetongue virus (BTV) (Mecham and McHolland 2010).
The core proteins carrying HS chains are contributed to the attachment process (Bacsa et al. 2011) (Figure 5(A)). HSPGs are glycoproteins containing one or more covalently attached HS chains (65) (Figure 5(B)). The best-characterized HSPGs expressed on cell surface are categorized into three groups: syndecan, glypican and beta-glycan family proteins. While beta-glycans bear HS chains at some proportion or under certain conditions (namely part-time proteoglycans), each syndecan and glypican harbours HS chains as a fixed feature. There are at least four syndecans and six glypicans (Pomin and Mulloy 2018). Glypicans commonly co-exist with syndecans, with the latter being the most frequently reported HSPGs functioning as attachment receptors during viral entry. HSPGs, particularly syndecans, have been reported to mediate the entry of many viruses including HSV (Bacsa et al. 2011), adenovirus (ADV) (Dechecchi et al. 2000), adeno-associated virus (AAV) (Johnson and Vogt 2010), human papillomavirus (HPV) (Shafti-Keramat et al. 2003), canine parvovirus (CPV) (Lofling et al. 2013), human immunodeficiency virus (HIV) (Saphire et al. 2001), human T-lymphotropic virus-1 (HTLV-1) (Lambert et al. 2009) and HBV (Glebe and Bremer 2013).
Figure 5.
Schematic representations of the structures of two major attachment receptors. (A) Schematic depiction on details of HS. HSPG is composed of HS and core proteins, with the HS structure being shown in the upper panel and a fragment amplified in the lower panel for details. HS biosynthesis is initiated by the attachment of xylose to specific serine residues in the HSPG core proteins followed by the formation of a linkage tetrasaccharide, glucuronic acid-galactose-galactose-xylose (GlcA-Gal-Gal-Xyl). Extl3 attaches the first N-acetyl-D-glucosamine (GlcNAc) residue and an enzyme complex composed of Ext1 and Ext2, alternately adds GlcA and GlcNAc to the nascent chain. The chains simultaneously undergo a series of processing reactions that begin by the removal of the acetyl groups from clusters of GlcNAc residues and substitution of the free amino groups with sulfate, catalyzed by one or more N-deacetylase-N-sulfotransferases (Ndst). The C5 epimerase (HsGlce) epimerizes the D-glucuronic acids immediately adjacent to N-sulfoglucosamine units to L-iduronic acid (IdoA). A series of O-sulfotransferases can then add sulfate. As shown in the top of the figure in red shading, the modifications occur in clusters of variable length (N-sulfated or NS domains), which are interspersed by unmodified domains (N-acetylated or NA domains). The modified domains make up binding sites for protein ligands as depicted for antithrombin, FGF and FGF receptor. (B) Schematic depiction on the global structure of two types of typical HSPGs. Syndecans are transmembrane proteins that bear HS chains distal from the plasma membrane. Some syndecans also contain ChS chains, which, by homology to syndecan-1, are located close to the membrane. Glypicans are covalently linked to a phosphatidyl inositol in the outer leaflet of the plasma membrane (GPI-linked). The HS chains are located on a likely extended protein domain near the plasma membrane. The glypican ectodomains are presumably compact and globular proteins as featured by 14 conserved cysteine residues. (C) Structure of the two common forms of SA, Neu5Ac, and Neu5Gc. (D) Structure of SA in two linkage conformations, α2,3-SA, α2,6-SA. Reprinted with permission from de Graaf and Fouchier (2014) and Byrd-Leotis et al. (2017).
Sialic acid (SA)
Sialic acid refers to the N- or O-substituted derivatives of neuraminic acid, which is a monosaccharide with a nine-carbon backbone. SA is among the most diverse sugars found on the glycan chains of mammalian cell surfaces. The predominant form of SAs are N-acetylneuraminic acid (Neu5Ac or NANA) and N-glycolylneuraminic acid (Neu5Gc) (Varki and Varki 2007) (Figure 5(C)). Neu5Ac is the primary SA form in mammalian cells (Varki et al. 2009). Neu5Gc not only exists in mammals (e.g. pigs, monkeys), but also in some bird species (except chickens (Byrd-Leotis et al. 2017)). Besides, gangliosides (used by a number of SA-binding viruses) are present in most mammalian tissues, with the highest concentration being found in brain grey matter (Kolter 2012).
SAs stabilize the plasma membrane (Varki 2007), modulate the interactions of cells with the environment including pathogens such as Middle East respiratory syndrome coronavirus (MERS-CoV) (Li et al. 2017), and can act as biological masks or pathogen decoys that prevent the recognition by viruses (Matrosovich and Klenk 2003). Therefore, it is not surprising that numerous viruses utilize SA as attachment determinants. Binding activity of SA largely depends on the negatively charged carboxylate group at C-1 carbon atom, which is typically ionized at physiological pH (Varki et al. 2009).
The roles played by SA in attaching all influenza A virus (IAV) strains have been extensively studied (Byrd-Leotis et al. 2017). IAVs recognize two types of SA depending on the position where they link to galactose, i.e. α2,3-SA and α2,6-SA (Figure 5(D)). While avian IAV preferentially recognizes α2,3-SA terminating glycans as receptors (Byrd-Leotis et al. 2017), human IAV recognizes α2,6-SA (Rogers et al. 1983). In this process, haemagglutinin (HA) mediates viral entry through binding to the cellular receptors and facilitates fusion of the virion membrane with the endosomal membrane (Byrd-Leotis et al. 2017). Conversely, neuraminidase (NA) can cleave off the SAs from the cellular membrane and viral glycoproteins, facilitating the egress of nascent virions (Byrd-Leotis et al. 2017; Du et al. 2019). HA and NA glycoproteins collectively determine the optimal level of HA affinity and NA enzymatic cleavage to allow for productive viral infection (Byrd-Leotis et al. 2017). Similar attachment mechanisms are revealed in RV (Ruiz et al. 2009), CPV (Lofling et al. 2013), feline panleukopenia virus (FPV) (Lofling et al. 2013) and PCV (Nauwynck et al. 2012).
Entry receptors
Internalization receptors are the primary functional members during the virion internalization process, whose absence may eventually lead to the failure of virus entry. Receptors of several cellular processes, including endocytic and signalling pathways, are typically hijacked by viruses to mediate, trigger or enhance their internalization. Examples include receptors of the immune response pathway such as integrin, CD150 and HVEM, receptors of the nutrient transportation such as that of iron (transferrin receptor, TfR), lipids (low density lipoprotein receptor, LDLR), glucose (glucose transporter 1, GLUT1) and sodium taurocholate (sodium taurocholate cotransporting polypeptide, NTCP) or receptors of growth and cell adhesion such as growth factor receptor (GFR), nectin cell adhesion molecule 4 (Nectin4) and intercellular cell adhesion molecule 1 (ICAM1). By comparing typical receptors adopted by viruses of different Baltimore subtypes, we identified five receptors that are commonly used to mediate virus entry (Table 1) which are discussed in detail below.
Integrins
Integrins are a large family of transmembrane glycoproteins found in a variety of organisms ranging from sponges, corals, nematodes, echinoderms to mammals (Srichai and Zent 2010). These molecules are heterodimeric receptors consisting of different combinations between 18 α and 8 β subunits, which form at least 24 integrins (Hussein et al. 2015) (Figure 6(A)). Each integrin subunit consists of three domains, the extracellular, transmembrane and cytoplasmic domains. The extracellular domain is the largest part, ranging from 80 to 150 kDa (Srichai and Zent 2010). The transmembrane domain, a single-spanning structure comprising of 25–29 amino acids, is α-helical coiled coil structure and exists as homo- or heterodimer. Except for β4, which contains over 1000 amino acids in its cytoplasmic domain, the cytoplasmic domain is a short unstructured domain comprising of 10–70 amino acids (Srichai and Zent 2010). Integrins can shift between high- and low- binding affinity states to transduce intracellular signals. In the inactive state, its extracellular domain is in a bent conformation and cannot bind to the ligands. Triggered by intracellular signals, integrins undergo conformational changes to expose the external ligand-binding site for efficient signal transduction from outside to inside (Schneider-Schaulies et al. 2001). Although the binding of some integrins requires an activation through alterations in the intracellular domain (inside-out signalling), other integrins can bind their ligands in the inactive state. On the other hand, ligands binding to the extracellular domain leads to subsequent transmission of cellular signals from outside-in (Arana et al. 2008). These functions make integrins important players in many critical signalling events including virus entry (Hussein et al. 2015). During cell invasion, viruses physically interact with integrins, many of which are localized to and associated with membrane rafts (Srichai and Zent 2010).
Figure 6.
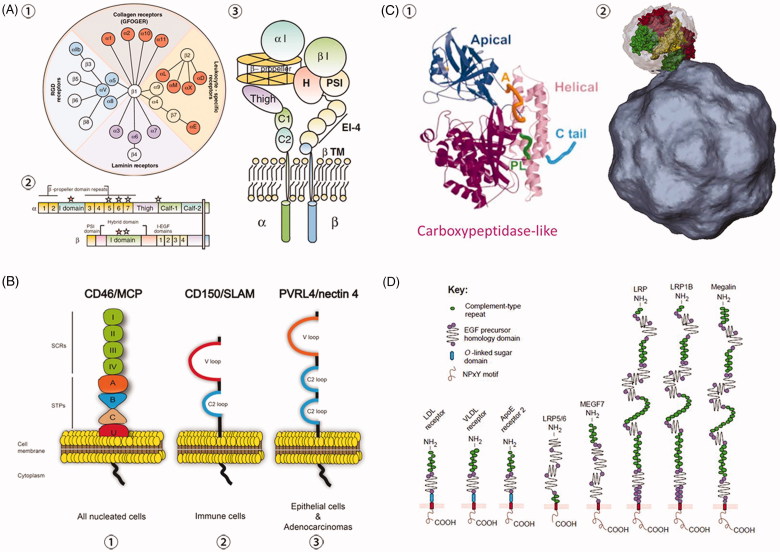
Schematic representations of the structures of four primary internalization receptors. (A) Illustration of integrin pairing and structure. ① Integrin family member and pairing in vertebrates, as well as their primary functions. ② Integrin primary structure with prototypical αI-domain-containing integrin heterodimer as an example. Half of α integrin subunits contain the αI-domain, and all integrins contain a βI domain in the β subunit. ‘Stars’ show divalent cation-binding sites. ③ Integrin tertiary structure with prototypical αI-domain-containing integrin heterodimer as an example. Reprinted with permission from Barczyk et al. (2010). (B) Structure of receptors CD46, CD150 (SLAM) and Nectin4 (PVRL4). CD46, CD150 and Nectin4 are all transmembrane receptors sharing similar structures, and are expressed in all nucleated cells, immune cells and epithelial cells, respectively. ① CD46 structure consists of four short consensus repeats (SCRs I, II, III, IV), a serine/threonine/proline region (STPs A, B, C), a sequence of unknown significance (U), a transmembrane sequence and a cytoplasmic domain. ② CD150 contains a variable (V) domain and a constant (C2) Ig-like repeat in its extracellular domain. CD150 is the universal immune receptor for all morbilliviruses. ③ Nectin4 extracellular domain is composed of a variable domain and two C2 domains. To date, Nectin4 was shown to serve as an epithelial receptor for MV, CDV, and PPRV. Reprinted with permission from Delpeut et al. (2014). (C) Structure of TfR monomer and dimer. ① TfR monomer. A (orange) shows the apical loop (residues 312 to 328); PL (green) shows the carboxypeptidase-like loop (residues 469 to 476); C tail (blue) shows the C-terminal tail (residues 750 to 760). Reprinted with modification and permission from Bennett et al. (2000). ② One of the two apical domains (green) of a TfR dimer is shown interacting with the virus surface (grey). The other two domains shown are helical domains and carboxypeptidase-like domains of the surface-rendered TfR molecule are yellow and red, respectively. Reprinted with permission from Hafenstein et al. (2007). (D) Structure of LDLR family members in mammalian cells. All members share common motifs, including a single membrane anchor, complement-type repeats (ligand binding domains) and EGF precursor homology domains (required for acid-dependent release of ligands in endosomes). NPxY designates the four-amino-acid motif Asp-Pro-x-Tyr, which is proposed by some studies to mediate the clustering of the receptors into coated pits. O-linked sugar domains are found in some but not all LDLR species, which are considered to function as a hydrophilic spacer keeping lipophilic ligands away from the lipid bilayer of the plasma membrane. LRP refers to ‘LDL-receptor-related protein’; MEGF7 is short for ‘multiple EGF-repeat-containing protein 7’. Reprinted with permission from Nykjaer and Willnow (2002).
The roles of integrins in serving as the internalization receptors have been reported for a variety of viruses. For instance, echovirus and integrin α2β1 (Johnson and Vogt 2010), RV and α2β1, α4β1, αxβ2, αvβ3 (Graham et al. 2003), HSV and integrins αvβ6, αvβ8 (Gianni et al. 2013), Kaposi’s sarcoma-associated herpesvirus (KSHV) and integrins αvβ3, αvβ5, α3β1, α9β1 (Walker et al. 2014), HIV-1 and integrins αvβ3, αvβ5, α5β1, α4β7 (Monini et al. 2012), foot-and-mouth disease virus (FMDV) and integrins αvβ3, αvβ6, αvβ1, αvβ8, α5β1 (Lawrence et al. 2013), as well as SV40 and integrin α2β1 (Stergiou et al. 2013). Several viruses such as AVs and herpesviruses (HVs) harbour integrin-recognition motifs (displayed on the viral envelope or on the capsid), with RGD (Arg-Gly-Asp) being the minimal peptide sequence for integrin binding. Over half of the known integrins recognize this motif and virus internalization is mediated in a RGD-dependent manner (Hussein et al. 2015). The non-RGD-binding integrins could also effectively promote virus entry, during which integrin binding facilitates adhesion, cytoskeleton rearrangement, integrin activation and enhances intracellular signalling (Hussein et al. 2015).
Transferrin receptor (TfR)
The natural function of TfR is assisting iron uptake into mammal or human cells through endo- and exocytosis of the iron-binding protein, transferrin (Tf). This pathway is used by certain viruses for cell invasion (Wang et al. 2006; Radoshitzky et al. 2007). So far, two types of human TfRs were identified, TfR1 and TfR2. The TfR1 has a high affinity to serum Tf. Unlike the ubiquitously expressed TfR1, TfR2 has a 25 to 30 fold lower affinity, and it is specific to certain cell types. Thus, TfR largely refers to TfR1.
Human TfR is a homodimer type II transmembrane protein composed of an NH3-terminal cytoplasmic region (residues 1–67), transmembrane domain (residues 68–88), and a large extracellular portion (residues 89 to 760). While the cytoplasmic domain harbours the internalization motif YTRF (Tyr-Thr-Arg-Phe), the extracellular part contains a binding site of Tf (Green et al. 2002) (Figure 6(C)). TfR dimer has a globular, extracellular structure protruding approximately 30 Å from the cell surface. The TfR monomer contains three distinct domains, organized in a butterfly-like shape in the form of dimers. Each monomer has a carboxypeptidase-like, an apical, and a helical domain, with the apical domain, distal from the membrane-binding region, being the most critical for virus binding (Bennett et al. 2000).
CPV and FPV bind to TfR via the apical domain (Palermo et al. 2003). TfR is also used by other viruses for cell infection, including PCV (Misinzo 2005), BTV (Forzan et al. 2007), various New World arenaviruses (Radoshitzky et al. 2007), and mouse mammary tumour virus (MMTV) (Wang et al. 2006). While the arenaviruses’ binding site is mapped to the apical domain of the Tf receptor, including the conserved tyrosine 211 in the human TfR1 sequence (Radoshitzky et al. 2008), the mouse mammary tumour virus (MMTV) binds the TfR through distinct domains (i.e. apical and protease-like domains) (Wang et al. 2006). Though these viruses utilizing TfR for viral entry do not necessarily share the same mechanism of action, what we focus on here is the commonalities on receptor usage shared by viruses for viral entry. The underlying mechanisms and the subsequent replication steps are out of the scope of this review.
Low-density lipoprotein receptor (LDLR)
Low-density lipoprotein receptor mediates the endocytosis of cholesterol-rich low-density lipoproteins (LDL) in all nucleated cells (Leren 2014). LDLRs are clustered in clathrin-coated pits, which are pinched off from the surface to form coated endocytic vesicles transporting LDL into the cell (Leren 2014). Viruses such as HCV, bovine viral diarrhoea virus (BVDV), human rhinovirus (HRV) take advantage of this process to facilitate their internalization to host cells (Agnello et al. 1999; Kolatkar et al. 1999).
Members of the LDLR family have been found in a variety of species ranging from roundworms to vertebrates. Mammals express nine LDLRs, which share common motifs required for endocytosis (Figure 6(D)). These LDLRs are membrane glycoproteins consisting of the extracellular domain, mono-transmembrane domain and cytoplasmic tail (Nykjaer and Willnow 2002). The extracellular domains of LDLRs comprise clusters of complement-type repeats required for ligand binding and epidermal growth factor precursor homology domains that are essential for the pH-dependent ligand release in endosomes (Nykjaer and Willnow 2002). The cytoplasmic domain harbours an NPxY (Asp-Pro-x-Tyr) motif, which is critical during virus internalization via clathrin-coated pits. Other studies demonstrated a dominant role of the YXXL (Tyr-x-x-Hydrophobic) motif during the endocytosis of extracellular cargo (Li et al. 2000). The natural ligands of LDLRs include lipoproteins containing apolipoprotein B-100 (ApoB-100) or apolipoprotein E (ApoE), LDL or very low-density lipoproteins (VLDL) respectively. The LDLR has been proposed as an entry factor for several viruses. For example, HCV virions interact with lipoprotein and apolipoproteins to form complexes termed lipoviroparticles. Studies have shown that lipoviroparticles are recruited to the cell membrane via interactions with LDLR (Agnello et al. 1999). In the case of HCV, the role of the LDLR-mediated uptake of viral particles is complex. It seems that the pathway does not lead to an effective viral infection (Albecka et al. 2012), and LDLR redundantly participates in the entry of lipoviroparticles (Yamamoto et al. 2016) that is important for optimal viral genome replication.
Nectin4
Nectin4, also known as PVRL4 (poliovirus receptor-related 4), belongs to a family of cell adhesion molecules that is abundantly expressed in epithelial tissues, including mouth, upper respiratory tract and stomach. Nectin4 plays key roles in the formation of cadherin-based adherens junctions, cell movement, proliferation, differentiation and in viruses spread from immune to epithelial cells (Frenzke et al. 2013) and survival.
Nectin4 is a mono-transmembrane structure consisting of one variable domain (V loop) and two constant domains (C2 loop) in the extracellular space and a cytoplasmic tail (Figure 6(B)). Similar to CD150/SLAM and CD46, Nectin4 is a receptor mediating the entry of measles virus. Nectin4 binds measles virus (MV) through its V loop during the internalization process (Noyce et al. 2011). Moreover, Nectin4 facilitates viral entry into host cells of other members of the Morbillivirus genus including, canine distemper virus (CDV) and peste des petits ruminants virus (PPRV) (Delpeut et al. 2014).
CD150
CD150, also named signalling lymphocytic activation molecule (SLAM), is a glycosylated transmembrane protein that is constitutively expressed on immature thymocytes, activated T and B lymphocytes, memory cells and dendritic cells (Cocks et al. 1995).
CD150 contains two highly glycosylated immunoglobulin domains and is placed into the CD2 family based on its structural features (Ono et al. 2001). Like other CD2 family members, CD150 comprises an N-terminal membrane-distal V domain and a membrane-proximal C2 domain, followed by the trans-membrane segment and cytoplasmic tail; and the V domain of CD150 is essential for its binding with MV during the entry process (Ono et al. 2001) (Figure 6(B)). Canine CD150 is also a cellular receptor of CDV, another negative single-stranded RNA virus (von Messling et al. 2005).
CD46
Cluster of differentiation 46 (CD46), also known as membrane cofactor protein (MCP), is ubiquitously expressed on most human nucleated cells. This protein belongs to regulators of complement activation (RCA) protein family, which are type I transmembrane proteins composed of short consensus repeats (SCRs). CD46 consists of an extracellular domain, a mono-transmembrane domain, and a cytoplasmic tail, with the extracellular portion comprised of four short consensus repeats (SCRs I, II, III, and IV), a Ser-Thr-Pro region (STPs A, B, C) and a sequence of unknown function (U) (Figure 6(B)).
CD46 mediates the entry of MV into the cells (Schneider-Schaulies et al. 2001); however, only vaccine or laboratory-adapted strains of MV use CD46 as the entry receptor (Delpeut et al. 2014). MV binds SCR-I and SCR-II, which are critical for virus internalization. Moreover, attachment of viral particles is enhanced by SCR III and IV (Devaux et al. 1997). Besides MV, CD46-mediated virus internalization has been reported for several unrelated viruses such as ADV (Segerman et al. 2003) and BVDV (Schelp et al. 2000).
Opportunities and challenges
Shared biological features of viruses provide opportunities for developing cells susceptible to a broad range of viruses
Host receptors are key factors and targets for the recognition and binding of viruses, and they contribute to the host range and pathogenicity of the incoming viruses. Viruses sharing the same entry pathways may recognize distinct receptors during infection. Furthermore, receptors belonging to the same family may participate in different entry pathways. These seemingly random events do have principles to follow, suggesting opportunities towards the construction of cells capable of producing vaccines against a broad spectrum of viruses.
The success of the early steps of viral infection is determined by the interactions between viruses and cell attachment factors and internalization receptors. Polysaccharides, such as HS, or saccharide derivatives, such as SA, are frequently displayed on cell surfaces and are required for efficient virus attachment and entry into cells (Table 1). These protruding saccharide chains are conventionally attached to proteins forming proteoglycans, such as HSPG and SA proteoglycan. Proteoglycans are abundantly present in the ECMof virtually all mammalian cells. They have a fiber-like structures that render ECM elastic and adhesive (Esko et al. 2009). In addition to the sticky extracellular environment fostered, partially and primarily by proteoglycans, the polyanionic nature of HS and SA attached to them considerably enhances their ability to interact with positively charged viral glycoproteins. Glycosylation affects protein folding, modulates protein thermostability and is critical for virus attachment and internalization. For instance, JC virus (JCV) infection of host cells depends on its interactions with cell surface asparagine (N)-linked SAs and the serotonin 5-hydroxytryptamine2A receptor (5-HT2AR) (Maginnis et al. 2010). However, saccharides are not feasible targets for the modulation of host cells, since glycosylation differs among species, requires additional enzymes and involves many technical problems. Glycosylation refers to the addition of a carbohydrate or glycan to a noncarbohydrate structure that can occur in almost all surface components including proteins and lipids. Glycosylation has many different forms such as N- and O-glycosylation, glycosylphosphatidylinositol glycosylation and lipid glycosylation, with the amount, distribution and type varying across species (Cagno et al. 2019). These characteristics substantially restrict glycosylation in being considered as targets for surface modulation. Moreover, although binding to saccharides is widely used by viruses for their attachment to the host cell, it has been shown that binding to saccharides is dispensable for infection in cell culture for numerous viruses (e.g. coronaviruses). For example, HSPG blockage results in the failure of SARS virus entry even in the presence of the internalization factor ACE2 (Lang et al. 2011). The SA alone enables the infection of influenza (Byrd-Leotis et al. 2017) and paramyxoviruses (Villar and Barroso 2006). Nonetheless, the SA binding was shown to be dispensable for transmissible gastroenteritis virus (TGEV) infection in cell culture (Schwegmann-Weßels et al. 2011).
Viruses classified within the same Baltimore group can use the same receptors for cell infection, which is in contrast with our canonical understanding that identical entry receptors are utilized by viruses from the same taxonomic family (e.g. TfR is utilized by members of the Parvoviridae family such as FPV and CPV during infection (Lofling et al. 2013; Lee et al. 2019). Take TfR as an example, the PCV, which belongs to the Circoviridae family, shares the same Baltimore group II with the CPV that also uses TfR as the receptor (Misinzo 2005). This generalization applies to viruses of many other families, resulting in several representative (common) internalization receptors corresponding to viruses of all Baltimore groups. In brief, integrins are frequently used by double-stranded DNA (group I: dsDNA) and RNA viruses (group III: dsRNA); TfR is frequently recruited during the entry process of the single-stranded DNA viruses (group II: ssDNA); LDLRs commonly mediate the entry process of the positive sense single-stranded RNA viruses (group IV: +ssRNA); CD150 and Nectin4 are likely to facilitate the internalization of negative sense single-stranded RNA viruses (group V: -ssRNA) (von Messling et al. 2005; Pratakpiriya et al. 2012). We did not find any marked preference on receptor usage for the internalization of reverse transcribing viruses including both positive-sense single-stranded RNA retroviruses (group VI: ssRNA-RT) and partially double-stranded DNA hepadnaviruses (group VII: dsDNA-RT), which are excluded from the following discussions. Though the aforementioned associations are not absolute, it is reasonable to propose development of a semi-universal cell culture for the production of vaccines against most of viruses via expressing integrins, TfR, LDLR, and Nectin4/CD150 on the cell surface. To this end, we have identified the four critical receptors comprising the minimum panel for cells to be sensitive to a broad spectrum of viruses, namely preferred receptor panel (PRP). This hypothesis, once tested true, will aid in our efforts towards the establishment of novel cell system for cost-efficient vaccine production that is adaptable to new viral strains or emerging viruses. However, we are aware that viruses presenting totally different types of genome (classified into different Baltimore subtypes) may share the same organ tropism and entry factors such as in the case of HBV and HCV that both target liver and use EGFR (Lupberger et al. 2011; Iwamoto et al. 2019) and NTCP (Yan et al. 2012; Verrier et al. 2016) for viral entry, and those belonging to the same Baltimore group may use different receptors for cell entry such as the differential use of HVEM, Nectin 1/2, GFR, CD63, CD151 in mediating the entry of type I viruses besides integrin (Table 1).
It is worth mentioning that we propose the inclusion of these four types of receptors in PRP for producing cells potentially susceptible to a broad range of viruses, but do not exclude the possibility of other receptors being required in cell optimization given the diversified molecular features of different initial cell lines. For instance, surface immunoglobulin (Ig) receptor mediates the entry of HBV to naïve B cells (Lazdina et al. 2001), the phosphatidylserine (PtdSer) receptor HAVCR1 is responsible for Hepatitis A virus (HAV) infection in the immune system (Manangeeswaran et al. 2012), and 2019-nCoV (novel coronavirus) that had recently led to the novel pneumonia outbreak in China (Huang et al. 2020) and SARS-CoV primarily use ACE2 for cell entry (Zamoto et al. 2006; Wu 2020). The reason we consider integrins, TfR, LDLR and Nectin4/CD150 as minimally required is because of their high frequencies in being needed during the invasion processes of various types of viruses as classified by the Baltimore system and the broad spectrum of virus families they cover, and other receptors can be considered alone or together with the PRP on an as-needed basis for cell line optimization to mediate the entry of a particular virus or alike. We did not include attachment receptors in PRP despite their high prevalence during virus entry as PRP, being a minimum receptor panel for cell optimization, includes the most representative and indispensable receptors that only those mediating the actual entry process meet this criterion. In addition, we did not claim that Nectin4 and CD150 are interchangeable; but rather, because of their similar frequency patterns in contributing to the entry of type V viruses, we would assume using one of them might be sufficient (which however should be experimentally tested). This obviously does not exclude the possibility that Nectin4 and CD150 interact and are both needed during virus entry.
The potential success of establishing an omnipotent cell line may also resolve several problems faced by vaccine production. There are several prevalent viruses that have no vaccine available such as CMV and EBV. Human CMV causes serious problems in immunocompromised or immunologically immature hosts; though many candidate vaccines including live attenuated CMV have been developed, none passed through the phase 3 clinical trial (Morello et al. 2007). Thus, the development of a vaccine to prevent human CMV infection has been assigned with the top priority by the US Institute of Medicine (Sung and Schleiss 2010). EBV is associated with a wide spectrum of cancers such as Hodgkin lymphoma, non-Hodgkin lymphoma, Burkitt lymphoma, T cell lymphoma, nasopharyngeal carcinoma, smooth muscle tumours and HIV (Cohen et al. 2011). Thus, developing vaccines against EBV provides substantial benefits to human public health. However, no attempt for EBV vaccine development has achieved desirable outcome. For instance, vaccines against EBV glycoprotein gp350 (a common strategy for EBV vaccine development) failed to protect against EBV infection but did reduce the incidence of infectious mononucleosis (IM) by 78% (Sokal et al. 2007). On the other hand, establishing cells infectible by a broad range of viruses provides opportunities for producing vaccines against viruses that lack robust culture models. For example, primary cultures of human hepatocytes (PHH) are the primary in vitro model for HBV infection which, however, do not expand in culture, and have limited life span and supply (Zeisel et al. 2015). HepaRG cells can differentiate into hepatocyte-like cells following a DMSO-mediated differentiation process, which are susceptible to HBV infection and support HBV cccDNA production. However, the differentiation process is time-consuming that may affect experiment reproducibility and reduce the infection efficiency. It was demonstrated that NTCP, a specific receptor mediating HBV infection, can increase cells’ susceptibility to HBV, and hepatoma cell lines overexpressing NTCP could sensitize cells to HBV infection (Somiya et al. 2016). This opens the opportunity of establishing a cell line susceptible to HBV infection that is easy to culture by nature.
Technical obstacles impose challenges for constructing cells that can be infected by a broad range of viruses
Though the four proposed representative internalization receptors offer us an exciting potential for constructing cells that can be used for multiple vaccine production, there are some technical obstacles to overcome. The excessive number of family members of certain receptors imposes additional challenges in receptor selection. Integrins and LDLR are not single molecules but harbour multiple family members. Thus, selection of the appropriate family member of integrins and LDLR for the PRP is a critical and challenging task. Genetic modulation to enable expression of multiple receptors on the cell membrane with correct folding and modification imposes further concerns. One could, of course, modify genetically the cell line by introducing the receptors of the PRP sequentially (one by one) or use one (all-in-one) expression plasmid to introduce them as a whole. However, the former approach is time-consuming and challenged by the limited number of available selection markers especially when the construction of a plethora of receptors (e.g. separate expression of multiple domains of one receptor) was necessary; the latter approach is sometimes not feasible if the sequences encoding the receptors were too large (plasmid stability issues) that imposes a big challenge to cell transfection. Synthetic biology may offer great help here by redesigning the profile of cellular receptors that incorporate all the necessary biological parts (minimally required parts of the PRR) and assembling them into an integrate signalling transduction system.
Further challenges in the understanding of the mechanisms of viral entry and internalization are related to functionalities of receptors and host sensitivity. While viruses belonging to groups I, II, III use dsDNA form for mRNA transcription, those belonging to groups IV and V use—ssRNA (Figure 6). Interestingly, among the four representative internalization factors, integrin (groups I and III viruses) and TfR (group II viruses) function as dimers, LDLR (group IV viruses) and Nectin4/CD150 (group V viruses) are monomers. This observation suggests that there is an association between virus replication mechanisms and signal transduction mediated by internalization receptors. Moreover, it indicates the importance of keeping these receptors as either dimers or monomers, respectively, in order to ensure their functionality for effective viral entry.
The type I interferon signalling plays a central role in host Defence that induces a plethora of antiviral proteins fight against viral invasions, which is activated on recognition of viral components via pattern recognition receptors (PRRs) following the expression of multiple IFN-stimulated genes (Li et al. 2006; Chew et al. 2009). PRRs play differential roles on viral identification, i.e. Toll-like receptors (TLRs) such as TLR3/7/8/9 recognize pathogen-associated molecular patterns, RIG-I-like receptors (RLRs) including RIG-I and MDA5 detect RNA structures, DNA sensors such as cGAS, IFI16, DDX41, DAI and a few DNA damage response proteins identify cytoplasmic DNA (O’Neill and Bowie 2010; Unterholzner 2013; Hornung 2014; Muller et al. 2019). Viruses have established various strategies to evade host antiviral responses. Take HSV-1 as the example, its tegument protein kinase US3 could reduce TLR3 expression to dampen TLR3-mediated antiviral response (Peri et al. 2008); its tegument protein UL36 could block IFN-beta production via deubiquitinating TRAF3 (a crucial component of the RLR-mediated pathway) (Wang et al. 2013); and by somewhat unknown mechanism HSV-1 infection could affect the stability and functionality of STING, an important adaptor for type I IFN induction by cytosolic DNA (Kalamvoki and Roizman 2014). Antiviral restriction factors, such as APOBEC3G, SAMHD1, Tetherin/BST-2 and TRIM5α are important components of the first line of host defense (reviewed in Kluge et al. 2015; Colomer-Lluch et al. 2018). Generally, they are interferon-inducible proteins that block viral replication and propagation. These molecular mechanisms, developed by viruses to target the host defense system, can be utilized to artificially block the host antiviral responses through expressing, for example, the inhibitors of PRR signalling or by knocking down the expression of the antiviral restriction factors. Recently, one such attempt was made through a genome-wide screening of the host genes important for poliovirus (PV), IAV and RV replication, followed by small interfering RNAs (siRNA) knock-down of the selected identified genes (Murray et al. 2017). Using a panel of 12 different viruses, it was shown that inhibition of the selected host genes allowed for overcoming antiviral defenses and increasing host cells’ (Vero and Hep-2) permissiveness and viral replication. Thus, it might be possible to create a universal or semi-universal cell line. One strategy to achieve this goal could be a combination of two approaches—modification of the pro- and anti-viral genes together with the expression of the panel of receptors preferred by various viruses. However, artificially establishing a favourable cellular environment for viral invasion is not an easy task as it has to enable concomitant expression of multiple suppressors against various cellular defense pathways under precise time and spatial control without disrupting cell homeostasis. An alternative and, perhaps, more practical strategy, which we propose here, is to select cell lines with low and well-characterized innate immune profile as the starting material for developing a cell model that is susceptible to a broad range of viruses and genetically modify them with the PRP.
A growing number of evidence suggested that host- and virus-specific interactions of viral molecules with the host innate immune system are determinants of virus–host range and virulence (Rothenburg and Brennan 2019). Thus, different species-specific cell types would be necessary in cases of narrow host range such as Nectin4 that is naturally expressed on bovine cells and does not interact with viruses recognizing murine Nectin4, and that variola virus causes solely human smallpox (Haller et al. 2014). To overcome these obstacles, we can preferentially select the starting cell line (such as Vero cells, which does not produce interferon) and design an alternative receptor expression system.
Host sensitivity may be affected by a number of factors, including various types of stress conditions presented to the cells. For example, excessive oxidative stress may compromise the functionality of receptors on host cell surface leading to reduced viral penetration ability (Beck et al. 2006). Other types of stress related to subjecting host cells to electric fields may lead to wider channels (pores) in the membrane thereby facilitating intracellular virus penetration. In many real situations, stress affecting cells is multi-modal (reactive radicals, heat, electric fields, ions, etc.), and sources of multi-modal stress such as atmospheric pressure, low-temperature plasmas (Dai et al. 2018) are of interest for further exploration of fundamental virus–host interaction mechanisms. Intensive experimental and computational studies are needed to clarify the many interesting effects arised from diverse opportunities for modulating functionalities of receptors and host cell sensitivity.
It is worth mentioning that receptors mediating the entry of certain viruses may differ between models in vivo and in vitro. For example, CD46 enables efficient infection of vaccine- or laboratory-adapted strains of measles virus (MV), but does not support in vivo invasion of many MV strains (Blixenkrone-Møller et al. 1998). To construct cells for multiple vaccine production, it does not matter whether the receptor functions in vivo as long as it enables efficient infection of cultured cells by the given virus.
In addition, PRP may not be appropriate to serve as the primary choice under urgent situations such as in the cases of SARS and NCP (novel coronavirus pneumonia) where ACE2 is the known entry receptor of these pathogens (Zamoto et al. 2006; Wu 2020) and vaccine production optimization is not the primary task during disease epidemics. However, members from PRP may be considered if the host receptor was unknown under urgent situations.
The successful establishment of cell lines feasible for viral vaccine production requires critical assessment of their biosafety, competitiveness and fulfilling of any regulatory requirements as discussed in Genzel (2015).
Lastly, we would like to mention that establishing a cell line that can be infected by a broad range of viruses not only requires sufficient knowledge and good design of surface receptors, but also needs to ensure rapid virus replication so that they could effectively complete their life cycles in cells. The latter is not the topic of this review, but is addressed in another paper of us (Dai et al. 2019).
Conclusion
Viruses use one or multiple entry pathways for cell infection that require host receptor(s). This demonstrates the indispensable role of receptors during the host–virus recognition process, and suggests the potential for establishing an omnipotent cell line for vaccine production against a wide spectrum of viruses without species limitation. By identifying the shared features of the attachment and entry processes of viruses classified within the same Baltimore subtype, discussing the possibility and potential technical obstacles towards the development of an omnipotent cell line susceptible to a broad range of viruses, we delineated the future directions in this particular research regime.
Summarizing points
In this article, we reviewed virus entry pathways and host receptors mediating the virus entry process.
We propose two attachments and four internalization receptors indispensable for the entry of most viruses.
In this paper, we discussed the opportunities and challenges for establishing an omnipotent cell line for vaccine production via genetic modulation.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant number 81972789], the National Science and Technology Major Project [grant number 2018ZX10302205-004-002], the Six Talent Peaks Project in Jiangsu Province [grant number SWYY-128], Technology Development Funding of Wuxi [Grant No. WX18IVJN017], and the Natural Sciences and Engineering Research Council of Canada Discovery Grant [grant number RPGIN-2017-04897].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
XFD selected the topic, initiated the project, conceptualized the ideas, and drafted the manuscript. XFD and XZ prepared the figures and tables. LA introduced critical references and revised the manuscript. All authors contributed to literature search, analysis, interpretation and manuscript preparation. KO coordinated the collaboration among authors. All authors have critically reviewed, discussed, and approved all the content of the manuscript for publication. The authors would like to thank Mackenzie Waltke for proofreading this manuscript.
References
- Agnello V, Abel G, Elfahal M, Knight GB, Zhang Q-X. 1999. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 96(22):12766–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar J, Shukla D. 2009. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 276(24):7228–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy P, Abban CY, Kiyashka E, Qiang W, Meneses PI. 2014. HPV16 infection of HaCaTs is dependent on beta4 integrin, and alpha6 integrin processing. Virology. 449:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albecka A, Belouzard S, de Beeck AO, Descamps V, Goueslain L, Bertrand-Michel J, Tercé F, Duverlie G, Rouillé Y, Dubuisson J, et al. 2012. Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology. 55(4):998–1007. [DOI] [PubMed] [Google Scholar]
- Albecka A, Laine RF, Janssen AFJ, Kaminski CF, Crump CM. 2016. HSV-1 glycoproteins are delivered to virus assembly sites through dynamin-dependent endocytosis. Traffic. 17(1):21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana E, Harwood NE, Batista FD. 2008. Regulation of integrin activation through the B-cell receptor. J Cell Sci. 121(14):2279–2286. [DOI] [PubMed] [Google Scholar]
- Arias CF, Silva-Ayala D, Lopez S. 2015. Rotavirus entry: a deep journey into the cell with several exits. J Virol. 89(2):890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrit F, Perugi F, Léon A, Guéhenneux F, Champion-Arnaud P, Lahmar M, Schwamborn K. 2015. Cell substrates for the production of viral vaccines. Vaccine. 33(44):5905–5912. [DOI] [PubMed] [Google Scholar]
- Audsley JM, Tannock GA. 2008. Cell-based influenza vaccines: progress to date. Drugs. 68(11):1483–1491. [DOI] [PubMed] [Google Scholar]
- Bacsa S, Karasneh G, Dosa S, Liu J, Valyi-Nagy T, Shukla D. 2011. Syndecan-1 and syndecan-2 play key roles in herpes simplex virus type-1 infection. J Gen Virol. 92(4):733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banse P, Moeller R, Bruening J, Lasswitz L, Kahl S, Khan A, Marcotrigiano J, Pietschmann T, Gerold G. 2018. CD81 receptor regions outside the large extracellular loop determine hepatitis C virus entry into hepatoma cells. Viruses. 10(4):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullberg D. 2010. Integrins. Cell Tissue Res. 339(1):269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett PN, Mundt W, Kistner O, Howard MK. 2009. Vero cell platform in vaccine production: moving towards cell culture-based viral vaccines. Expert Rev Vaccines. 8(5):607–618. [DOI] [PubMed] [Google Scholar]
- Beck MA, Handy J, Levander OA. 2006. The role of oxidative stress in viral infections. Ann N Y Acad Sci. 917(1):906–912. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Lebron JA, Bjorkman PJ. 2000. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature. 403(6765):46–53. [DOI] [PubMed] [Google Scholar]
- Bilkova E, Forstova J, Abrahamyan L. 2014. Coat as a dagger: the use of capsid proteins to perforate membranes during non-enveloped DNA viruses trafficking. Viruses. 6(7):2899–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixenkrone-Møller M, Bernard A, Bencsik A, Sixt N, Diamond LE, Logan JS, Wild TF. 1998. Role of CD46 in measles virus infection in CD46 transgenic mice. Virology. 249(2):238–248. [DOI] [PubMed] [Google Scholar]
- Boulant S, Stanifer M, Lozach PY. 2015. Dynamics of virus-receptor interactions in virus binding, signaling, and endocytosis. Viruses. 7(6):2794–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd-Leotis L, Cummings RD, Steinhauer DA. 2017. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int J Mol Sci. 18(7):E1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagno V, Tseligka ED, Jones ST, Tapparel C. 2019. Heparan sulfate proteoglycans and viral attachment: true receptors or adaptation bias? Viruses. 11(7):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casasnovas JM. 2013. Virus-receptor interactions and receptor-mediated virus entry into host cells. Subcell Biochem. 68:441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemello ME, Aristimuno OC, Michelangeli F, Ruiz M-C. 2002. Requirement for vacuolar H+ -ATPase activity and Ca2+ gradient during entry of rotavirus into MA104 cells. J Virol. 76(24):13083–13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew T, Taylor KE, Mossman KL. 2009. Innate and adaptive immune responses to herpes simplex virus. Viruses. 1(3):979–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BYJT, Shukla D. 2006. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 174(7):1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks BG, Chang C-CJ, Carballido JM, Yssel H, de Vries JE, Aversa G. 1995. A novel receptor involved in T-cell activation. Nature. 376(6537):260–263. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Fauci AS, Varmus H, Nabel GJ. 2011. Epstein-Barr virus: an important vaccine target for cancer prevention. Sci Transl Med. 3(107):107fs7–107fs7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer-Lluch M, Ruiz A, Moris A, Prado JG. 2018. Restriction factors: from intrinsic viral restriction to shaping cellular immunity against HIV-1. Front Immunol. 9(2876):2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Bazaka K, Richard DJ, Thompson ERW, Ostrikov KK. 2018. The emerging role of gas plasma in oncotherapy. Trends Biotechnol. 36(11):1183–1198. [DOI] [PubMed] [Google Scholar]
- Dai X, Hakizimana O, Zhang X, Kaushik AC, Zhang J. 2019. Orchestrated efforts on host network hijacking: processes governing virus replication. Virulence. 11(1):183–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm E-M, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A. 2005. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J Cell Biol. 168(3):477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R, Rusan NM, Wadsworth P, Hebert DN. 2006. SV40 VP2 and VP3 insertion into ER membranes is controlled by the capsid protein VP1: implications for DNA translocation out of the ER. Mol Cell. 24(6):955–966. [DOI] [PubMed] [Google Scholar]
- de Graaf M, Fouchier RA. 2014. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. Embo J. 33(8):823–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechecchi MC, Tamanini A, Bonizzato A, Cabrini G. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology. 268(2):382–390. [DOI] [PubMed] [Google Scholar]
- Delpeut S, Noyce RS, Richardson CD. 2014. The tumor-associated marker, PVRL4 (nectin-4), is the epithelial receptor for morbilliviruses. Viruses. 6(6):2268–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P, Buchholz CJ, Schneider U, Escoffier C, Cattaneo R, Gerlier D. 1997. CD46 short consensus repeats III and IV enhance measles virus binding but impair soluble hemagglutinin binding. J Virol. 71(5):4157–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Guo H, Nijman VS, Doedt J, van der Vries E, van der Lee J, Li Z, Boons G-J, van Kuppeveld FJM, de Vries E, et al. 2019. The 2nd sialic acid-binding site of influenza A virus neuraminidase is an important determinant of the hemagglutinin-neuraminidase-receptor balance. PLOS Pathog. 15(6):e1007860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S, Heger T, Mancini R, Herzog F, Kartenbeck J, Hayer A, Helenius A. 2011. Role of endosomes in simian virus 40 entry and infection. J Virol. 85(9):4198–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko JD, Kimata K, Lindahl U. 2009. Proteoglycans and sulfated glycosaminoglycans In Varki A, editors. Essentials of glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- Forzan M, Marsh M, Roy P. 2007. Bluetongue virus entry into cells. J Virol. 81(9):4819–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzke M, Sawatsky B, Wong XX, Delpeut S, Mateo M, Cattaneo R, von Messling V. 2013. Nectin-4-dependent measles virus spread to the cynomolgus monkey tracheal epithelium: role of infected immune cells infiltrating the lamina propria. J Virol. 87(5):2526–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Miura R, Yoneda M, Shimizu F, Sato H, Muto Y, Endo Y, Tsukiyama-Kohara K, Kai C. 2007. Host range and receptor utilization of canine distemper virus analyzed by recombinant viruses: involvement of heparin-like molecule in CDV infection. Virology. 359(2):324–335. [DOI] [PubMed] [Google Scholar]
- Gastaminza P, Dryden KA, Boyd B, Wood MR, Law M, Yeager M, Chisari FV. 2010. Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture. J Virol. 84(21):10999–11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzel Y. 2015. Designing cell lines for viral vaccine production: where do we stand? Biotechnol J. 10(5):728–740. [DOI] [PubMed] [Google Scholar]
- Ghez D, Lepelletier Y, Lambert S, Fourneau J-M, Blot V, Janvier S, Arnulf B, van Endert PM, Heveker N, Pique C, et al. 2006. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J Virol. 80(14):6844–6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni T, Salvioli S, Chesnokova LS, Hutt-Fletcher LM, Campadelli-Fiume G. 2013. Alphavbeta6- and alphavbeta8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLOS Pathog. 9(12):e1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebe D, Bremer CM. 2013. The molecular virology of hepatitis B virus. Semin Liver Dis. 33(2):103–112. [DOI] [PubMed] [Google Scholar]
- Graham KL, Halasz P, Tan Y, Hewish MJ, Takada Y, Mackow ER, Robinson MK, Coulson BS. 2003. Integrin-using rotaviruses bind alpha2beta1 integrin alpha2 I domain via VP4 DGE sequence and recognize alphaXbeta2 and alphaVbeta3 by using VP7 during cell entry. J Virol. 77(18):9969–9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassart A, Meas-Yedid V, Dufour A, Olivo-Marin J-C, Dautry-Varsat A, Sauvonnet N. 2010. Pak1 phosphorylation enhances cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic. 11(8):1079–1091. [DOI] [PubMed] [Google Scholar]
- Green F, O'Hare T, Blackwell A, Enns CA. 2002. Association of human transferrin receptor with GABARAP. FEBS Lett. 518(1–3):101–106. [DOI] [PubMed] [Google Scholar]
- Gregersen J-P, Schmitt H-J, Trusheim H, Bröker M. 2011. Safety of MDCK cell culture-based influenza vaccines. Future Microbiol. 6(2):143–152. [DOI] [PubMed] [Google Scholar]
- Hafenstein S, Palermo LM, Kostyuchenko VA, Xiao C, Morais MC, Nelson CDS, Bowman VD, Battisti AJ, Chipman PR, Parrish CR, et al. 2007. Asymmetric binding of transferrin receptor to parvovirus capsids. Proc Natl Acad Sci USA. 104(16):6585–6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haid S, Pietschmann T, Pecheur EI. 2009. Low pH-dependent hepatitis C virus membrane fusion depends on E2 integrity, target lipid composition, and density of virus particles. J Biol Chem. 284(26):17657–17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller SL, Peng C, McFadden G, Rothenburg S. 2014. Poxviruses and the evolution of host range and virulence. Infect Genet Evol. 21:15–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V. 2014. SnapShot: nucleic acid immune sensors, part 1. Immunity. 41(5):868–868.e1. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H-C, Chen C-C, Chang W-C, Tao M-H, Huang C. 2012. Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. J Virol. 86(17):9443–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Crawford SE, Czako R, Cortes-Penfield NW, Smith DF, Le Pendu J, Estes MK, Prasad BVV. 2012. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature. 485(7397):256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Moss B. 2005. Role of receptor-mediated endocytosis in the formation of vaccinia virus extracellular enveloped particles. J Virol. 79(7):4080–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein HAM, Walker LR, Abdel-Raouf UM, Desouky SA, Montasser AKM, Akula SM. 2015. Beyond RGD: virus interactions with integrins. Arch Virol. 160(11):2669–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhang S, Shen Y, Yang Q. 2018. Epidermal growth factor receptor is a co-factor for transmissible gastroenteritis virus entry. Virology. 521:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M, Saso W, Sugiyama R, Ishii K, Ohki M, Nagamori S, Suzuki R, Aizaki H, Ryo A, Yun J-H, et al. 2019. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc Natl Acad Sci USA. 116(17):8487–8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Vogt PK. 2010. Cell entry by non-enveloped viruses. Curr Top Microbiol Immunol. 343:v–vii. [PubMed] [Google Scholar]
- Kalamvoki M, Roizman B. 2014. HSV-1 degrades, stabilizes, requires, or is stung by STING depending on ICP0, the US3 protein kinase, and cell derivation. Proc Natl Acad Sci USA. 111(5):E611–E617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasneh GA, Shukla D. 2011. Herpes simplex virus infects most cell types in vitro: clues to its success. Virol J. 8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham M, Parton RG. 2005. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 1746(3):349–363. [DOI] [PubMed] [Google Scholar]
- Kluge SF, Sauter D, Kirchhoff F. 2015. SnapShot: antiviral restriction factors. Cell. 163(3):774–774.e1. [DOI] [PubMed] [Google Scholar]
- Köck J, Borst EM, Schlicht HJ. 1996. Uptake of duck hepatitis B virus into hepatocytes occurs by endocytosis but does not require passage of the virus through an acidic intracellular compartment. J Virol. 70(9):5827–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolatkar PR, Bella J, Olson NH, Bator CM, Baker TS, Rossmann MG. 1999. Structural studies of two rhinovirus serotypes complexed with fragments of their cellular receptor. Embo J. 18(22):6249–6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter T. 2012. Ganglioside biochemistry. ISRN Biochem. 2012:506160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosukegawa A, Arisaka F, Takayama M, Yajima H, Kaidow A, Handa H. 1996. Purification and characterization of virus-like particles and pentamers produced by the expression of SV40 capsid proteins in insect cells. Biochim Biophys Acta. 1290(1):37–45. [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X. 2004. Endocytosis of influenza viruses. Microbes Infect. 6(10):929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA, Paterson RG, Jardetzky TS. 2006. Paramyxovirus membrane fusion: lessons from the F and HN atomic structures. Virology. 344(1):30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Bouttier M, Vassy R, Seigneuret M, Petrow-Sadowski C, Janvier S, Heveker N, Ruscetti FW, Perret G, Jones KS, et al. 2009. HTLV-1 uses HSPG and neuropilin-1 for entry by molecular mimicry of VEGF165. Blood. 113(21):5176–5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J, Yang N, Deng J, Liu K, Yang P, Zhang G, Jiang C. 2011. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLOS One. 6(8):e23710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P, LaRocco M, Baxt B, Rieder E. 2013. Examination of soluble integrin resistant mutants of foot-and-mouth disease virus. Virol J. 10(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdina U, Cao T, Steinbergs J, Alheim M, Pumpens P, Peterson DL, Milich DR, Leroux-Roels G, Sallberg M. 2001. Molecular basis for the interaction of the hepatitis B virus core antigen with the surface immunoglobulin receptor on naive B cells. J Virol. 75(14):6367–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Callaway HM, Cifuente JO, Bator CM, Parrish CR, Hafenstein SL. 2019. Transferrin receptor binds virus capsid with dynamic motion. Proc Natl Acad Sci USA. 116(41):20462–20471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre M, Felmlee DJ, Parnot M, Baumert TF, Schuster C. 2014. Syndecan 4 is involved in mediating HCV entry through interaction with lipoviral particle-associated Apolipoprotein E. PLOS One. 9(4):e95550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leren TP. 2014. Sorting an LDL receptor with bound PCSK9 to intracellular degradation. Atherosclerosis. 237(1):76–81. [DOI] [PubMed] [Google Scholar]
- Li W, Hulswit RJG, Widjaja I, Raj VS, McBride R, Peng W, Widagdo W, Tortorici MA, van Dieren B, Lang Y, et al. 2017. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc Natl Acad Sci USA. 114(40):E8508–E8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Paz Marzolo M, van Kerkhof P, Strous GJ, Bu G. 2000. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 275(22):17187–17194. [DOI] [PubMed] [Google Scholar]
- Liprandi F, Moros Z, Gerder M, Ludert JE, Pujol FH, Ruiz MC, Michelangeli F, Charpilienne A, Cohen J. 1997. Productive penetration of rotavirus in cultured cells induces coentry of the translation inhibitor alpha-sarcin. Virology. 237(2):430–438. [DOI] [PubMed] [Google Scholar]
- Li JS, Tong SP, Wands JR. 1996. Characterization of a 120-Kilodalton pre-S-binding protein as a candidate duck hepatitis B virus receptor. J Virol. 70(9):6029–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang J, Kumar A, Zheng M, Atherton SS, Yu F-SX. 2006. Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines, interferons and TLR7 in human corneal epithelial cells. Immunology. 117(2):167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofling J, Lyi SM, Parrish CR, Varki A. 2013. Canine and feline parvoviruses preferentially recognize the non-human cell surface sialic acid N-glycolyl neuraminic acid. Virology. 440(1):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Cocchi F, Menotti L, Avitabile E, Dubreuil P, Campadelli-Fiume G. 2000. Nectin2alpha (PRR2alpha or HveB) and nectin2delta are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J Virol. 74(3):1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludert JE, Krishnaney AA, Burns JW, Vo PT, Greenberg HB. 1996. Cleavage of rotavirus VP4 in vivo. J Gen Virol. 77(3):391–395. [DOI] [PubMed] [Google Scholar]
- Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, et al. 2011. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 17(5):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macovei A, Radulescu C, Lazar C, Petrescu S, Durantel D, Dwek RA, Zitzmann N, Nichita NB. 2010. Hepatitis B virus requires intact caveolin-1 function for productive infection in HepaRG cells. J Virol. 84(1):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maginnis MS, Haley SA, Gee GV, Atwood WJ. 2010. Role of N-linked glycosylation of the 5-HT2A receptor in JC virus infection. J Virol. 84(19):9677–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manangeeswaran M, Jacques J, Tami C, Konduru K, Amharref N, Perrella O, Casasnovas JM, Umetsu DT, DeKruyff RH, Freeman GJ, et al. 2012. Binding of hepatitis A virus to its cellular receptor 1 inhibits T-regulatory cell functions in humans. Gastroenterology. 142(7):1516–1525 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathapati BS, Mishra N, Rajukumar K, Nema RK, Behera SP, Dubey SC. 2010. Entry of bovine viral diarrhea virus into ovine cells occurs through clathrin-dependent endocytosis and low pH-dependent fusion. In Vitro Celldevbiol-Animal. 46(5):403–407. [DOI] [PubMed] [Google Scholar]
- Matrosovich M, Klenk HD. 2003. Natural and synthetic sialic acid-containing inhibitors of influenza virus receptor binding. Rev Med Virol. 13(2):85–97. [DOI] [PubMed] [Google Scholar]
- Maurer K, Krey T, Moennig V, Thiel H-J, Rumenapf T. 2004. CD46 is a cellular receptor for bovine viral diarrhea virus. J Virol. 78(4):1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecham JO, McHolland LE. 2010. Measurement of bluetongue virus binding to a mammalian cell surface receptor by an in situ immune fluorescent staining technique. J Virol Methods. 165(1):112–115. [DOI] [PubMed] [Google Scholar]
- Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu Rev Biochem. 79(1):803–833. [DOI] [PubMed] [Google Scholar]
- Misinzo G. 2005. Binding and entry characteristics of porcine circovirus 2 in cells of the porcine monocytic line 3D4/31. J Gen Virol. 86(7):2057–2068. [DOI] [PubMed] [Google Scholar]
- Misinzo G, Delputte PL, Lefebvre DJ, Nauwynck HJ. 2009. Porcine circovirus 2 infection of epithelial cells is clathrin-, caveolae- and dynamin-independent, actin and Rho-GTPase-mediated, and enhanced by cholesterol depletion. Virus Res. 139(1):1–9. [DOI] [PubMed] [Google Scholar]
- Misinzo G, Delputte PL, Meerts P, Lefebvre DJ, Nauwynck HJ. 2006. Porcine circovirus 2 uses heparan sulfate and chondroitin sulfate B glycosaminoglycans as receptors for its attachment to host cells. J Virol. 80(7):3487–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monini P, Cafaro A, Srivastava IK, Moretti S, Sharma VA, Andreini C, Chiozzini C, Ferrantelli F, Cossut MRP, Tripiciano A, et al. 2012. HIV-1 tat promotes integrin-mediated HIV transmission to dendritic cells by binding Env spikes and competes neutralization by anti-HIV antibodies. PLOS One. 7(11):e48781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montomoli E, Khadang B, Piccirella S, Trombetta C, Mennitto E, Manini I, Stanzani V, Lapini G. 2012. Cell culture-derived influenza vaccines from Vero cells: a new horizon for vac-cine production. Expert Rev Vaccines. 11(5):587–594. [DOI] [PubMed] [Google Scholar]
- Morello CS, Kelley LA, Munks MW, Hill AB, Spector DH. 2007. DNA immunization using highly conserved murine cytomegalovirus genes encoding homologs of human cytomegalovirus UL54 (DNA polymerase) and UL105 (helicase) elicits strong CD8 T-cell responses and is protective against systemic challenge. J Virol. 81(14):7766–7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Lauster D, Wildenauer HHK, Herrmann A, Block S. 2019. Mobility-based quantification of multivalent virus-receptor interactions: new insights into influenza A virus binding mode. Nano Lett. 19(3):1875–1882. [DOI] [PubMed] [Google Scholar]
- Murray J, Todd KV, Bakre A, Orr-Burks N, Jones L, Wu W, Tripp RA. 2017. A universal mammalian vaccine cell line substrate. PLOS One. 12(11):e0188333–e0188333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauwynck HJ, Sanchez R, Meerts P, Lefebvre DJ, Saha D, Huang L, Misinzo G. 2012. Cell tropism and entry of porcine circovirus 2. Virus Res. 164(1–2):43–45. [DOI] [PubMed] [Google Scholar]
- Noyce RS, Bondre DG, Ha MN, Lin L-T, Sisson G, Tsao M-S, Richardson CD. 2011. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLOS Pathog. 7(8):e1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Willnow TE. 2002. The low-density lipoprotein receptor gene family: a cellular Swiss army knife? Trends Cell Biol. 12(6):273–280. [DOI] [PubMed] [Google Scholar]
- O’Neill LAJ, Bowie AG. 2010. Sensing and signaling in antiviral innate immunity. Curr Biol. 20(7):R328–R333. [DOI] [PubMed] [Google Scholar]
- Ono N, Tatsuo H, Tanaka K, Minagawa H, Yanagi Y. 2001. V domain of human SLAM (CDw150) is essential for its function as a measles virus receptor. J Virol. 75(4):1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo LM, Hueffer K, Parrish CR. 2003. Residues in the apical domain of the feline and canine transferrin receptors control host-specific binding and cell infection of canine and feline parvoviruses. J Virol. 77(16):8915–8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Howes MT. 2010. Revisiting caveolin trafficking: the end of the caveosome. J Cell Biol. 191(3):439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne LG, Norrby E. 1978. Adsorption and penetration of enveloped and naked vaccinia virus particles. J Virol. 27(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. 2005. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 436(7047):78–86. [DOI] [PubMed] [Google Scholar]
- Perdue ML, Arnold F, Li S, Donabedian A, Cioce V, Warf T, Huebner R. 2011. The future of cell culture-based influenza vaccine production. Expert Rev Vaccines. 10(8):1183–1194. [DOI] [PubMed] [Google Scholar]
- Peri P, Mattila RK, Kantola H, Broberg E, Karttunen HS, Waris M, Vuorinen T, Hukkanen V. 2008. Herpes simplex virus type 1 Us3 gene deletion influences toll-like receptor responses in cultured monocytic cells. Virol J. 5(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomin VH, Mulloy B. 2018. Glycosaminoglycans and proteoglycans. Pharmaceuticals. 11(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratakpiriya W, Seki F, Otsuki N, Sakai K, Fukuhara H, Katamoto H, Hirai T, Maenaka K, Techangamsuwan S, Lan NT, et al. 2012. Nectin4 is an epithelial cell receptor for canine distemper virus and involved in neurovirulence. J Virol. 86(18):10207–10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadar SMZ. 2014. Polio cost effectiveness in Nigeria; a lesson to be learnt. Am J Pharm Sci. 2(5B):4–7. [Google Scholar]
- Radoshitzky SR, Abraham J, Spiropoulou CF, Kuhn JH, Nguyen D, Li W, Nagel J, Schmidt PJ, Nunberg JH, Andrews NC, et al. 2007. Transferrin receptor 1 is a cellular receptor for new world haemorrhagic fever arenaviruses. Nature. 446(7131):92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoshitzky SR, Kuhn JH, Spiropoulou CF, Albarino CG, Nguyen DP, Salazar-Bravo J, Dorfman T, Lee AS, Wang E, Ross SR, et al. 2008. Receptor determinants of zoonotic transmission of New World hemorrhagic fever arenaviruses. Proc Natl Acad Sci USA. 105(7):2664–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff AB, Woodham AW, Raff LM, Skeate JG, Yan L, Da Silva DM, Schelhaas M, Kast WM. 2013. The evolving field of human papillomavirus receptor research: a review of binding and entry. J Virol. 87(11):6062–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GN, Pritchett TJ, Lane JL, Paulson JC. 1983. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology. 131(2):394–408. [DOI] [PubMed] [Google Scholar]
- Rostand KS, Esko JD. 1997. Microbial adherence to and invasion through proteoglycans. Infect Immunol. 65(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenburg S, Brennan G. 2019. Species-specific host-virus interactions: implications for viral host range and virulence. Trends Microbiol. 28(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MC, Leon T, Diaz Y, Michelangeli F. 2009. Molecular biology of rotavirus entry and replication. Sci World J. 9:1476–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu CJ, Cho D-Y, Gripon P, Kim HS, Guguen-Guillouzo C, Hong HJ. 2000. An 80-kilodalton protein that binds to the pre-S1 domain of hepatitis B virus. J Virol. 74(1):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-San Martin C, Lopez T, Arias CF, Lopez S. 2004. Characterization of rotavirus cell entry. J Virol. 78(5):2310–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphire ACS, Bobardt MD, Zhang Z, David G, Gallay PA. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol. 75(19):9187–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelhaas M, Shah B, Holzer M, Blattmann P, Kühling L, Day PM, Schiller JT, Helenius A. 2012. Entry of human papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis. PLOS Pathog. 8(4):e1002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelp C, Greiser-Wilke I, Moennig V. 2000. An actin-binding protein is involved in pestivirus entry into bovine cells. Virus Res. 68(1):1–5. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies J, Meulen V, Schneider-Schaulies S. 2001. Measles virus interactions with cellular receptors: consequences for viral pathogenesis. J Neurovirol. 7(5):391–399. [DOI] [PubMed] [Google Scholar]
- Schnierle BS. 2019. Cellular attachment and entry factors for chikungunya virus. Viruses. 11(11):1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwegmann-Weßels C, Bauer S, Winter C, Enjuanes L, Laude H, Herrler G. 2011. The sialic acid binding activity of the S protein facilitates infection by porcine transmissible gastroenteritis coronavirus. Virol J. 8(1):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G, Arnberg N. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol. 77(17):9183–9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. 2003. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 77(24):13125–13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Jiang J, Luo G. 2013. Syndecan-1 serves as the major receptor for attachment of hepatitis C virus to the surfaces of hepatocytes. J Virol. 87(12):6866–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Léonard P, Moreels A, Haumont M, Bollen A, Smets F, Denis M, et al. 2007. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis. 196(12):1749–1753. [DOI] [PubMed] [Google Scholar]
- Somiya M, Liu Q, Yoshimoto N, Iijima M, Tatematsu K, Nakai T, Okajima T, Kuroki K, Ueda K, Kuroda S, et al. 2016. Cellular uptake of hepatitis B virus envelope L particles is independent of sodium taurocholate cotransporting polypeptide, but dependent on heparan sulfate proteoglycan. Virology. 497:23–32. [DOI] [PubMed] [Google Scholar]
- Spear PG, Longnecker R. 2003. Herpesvirus entry: an update. J Virol. 77(19):10179–10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srichai MB, Zent R. 2010. Integrin structure and function In: Zent R, Pozzi A, editors. Cell extracellular matrix interaction in cancer. New York (NY): Springer Science + Business Media; p. 19–41. [Google Scholar]
- Stergiou L, Bauer M, Mair W, Bausch-Fluck D, Drayman N, Wollscheid B, Oppenheim A, Pelkmans L. 2013. Integrin-mediated signaling induced by simian virus 40 leads to transient uncoupling of cortical actin and the plasma membrane. PLOS One. 8(2):e55799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. 2010. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci USA. 107(2):866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Schleiss MR. 2010. Update on the current status of cytomegalovirus vaccines. Expert Rev Vaccines. 9(11):1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kitaoka S, Konno T, Sato T, Ishida N. 1985. Two modes of human rotavirus entry into MA 104 cells. Arch Virol. 85(1–2):25–34. [DOI] [PubMed] [Google Scholar]
- Talekar A, Moscona A, Porotto M. 2013. Measles virus fusion machinery activated by sialic acid binding globular domain. J Virol. 87(24):13619–13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, Tebbens RJ. 2006. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal. 26(6):1423–1440. [DOI] [PubMed] [Google Scholar]
- Unterholzner L. 2013. The interferon response to intracellular DNA: why so many receptors? Immunobiology. 218(11):1312–1321. [DOI] [PubMed] [Google Scholar]
- Vale-Costa S, Amorim MJ. 2016. Recycling endosomes and viral infection. Viruses. 8(3):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sanden SMG, Wu W, Dybdahl-Sissoko N, Weldon WC, Brooks P, O'Donnell J, Jones LP, Brown C, Tompkins SM, Oberste MS, et al. 2016. Engineering enhanced vaccine cell lines to eradicate vaccine-preventable diseases: the polio end game. J Virol. 90(4):1694–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. 2007. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 446(7139):1023–1029. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH. et al. 2009. Sialic acids In: Varki A, editor. Essentials of glycobiology. New York (NY): Cold Spring Harbor Laboratory Press; http://www.ncbi.nlm.nih.gov/books/NBK1920/. [PubMed] [Google Scholar]
- Varki NM, Varki A. 2007. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest. 87(9):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier ER, Colpitts CC, Bach C, Heydmann L, Zona L, Xiao F, Thumann C, Crouchet E, Gaudin R, Sureau C, et al. 2016. Solute carrier NTCP regulates innate antiviral immune responses targeting hepatitis C virus infection of hepatocytes. Cell Rep. 17(5):1357–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. 2009. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 10(11):778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihinen-Ranta M, Kalela A, Makinen P, Kakkola L, Marjomaki V, Vuento M. 1998. Intracellular route of canine parvovirus entry. J Virol. 72(1):802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar E, Barroso IM. 2006. Role of sialic acid-containing molecules in paramyxovirus entry into the host cell: a minireview. Glycoconj J. 23(1–2):5–17. [DOI] [PubMed] [Google Scholar]
- Vlecken DHW, Pelgrim RPM, Ruminski S, Bakker WAM, van der Pol LA. 2013. Comparison of initial feasibility of host cell lines for viral vaccine production. J Virol Methods. 193(1):28–41. [DOI] [PubMed] [Google Scholar]
- von Messling V, Oezguen N, Zheng Q, Vongpunsawad S, Braun W, Cattaneo R. 2005. Nearby clusters of hemagglutinin residues sustain SLAM-dependent canine distemper virus entry in peripheral blood mononuclear cells. J Virol. 79(9):5857–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LR, Hussein HA, Akula SM. 2014. Disintegrin-like domain of glycoprotein B regulates Kaposi’s sarcoma-associated herpesvirus infection of cells. J Gen Virol. 95(8):1770–1782. [DOI] [PubMed] [Google Scholar]
- Wang E, Albritton L, Ross SR. 2006. Identification of the segments of the mouse transferrin receptor 1 required for mouse mammary tumor virus infection. J Biol Chem. 281(15):10243–10249. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang K, Li J, Zheng C. 2013. Herpes simplex virus 1 ubiquitin-specific protease UL36 inhibits beta interferon production by deubiquitinating TRAF3. J Virol. 87(21):11851–11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. 2020. Compensation of ACE2 function for possible clinical management of 2019-nCoV-induced acute lung injury. Virol Sin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Fukuhara T, Ono C, Uemura K, Kawachi Y, Shiokawa M, Mori H, Wada M, Shima R, Okamoto T, et al. 2016. Lipoprotein receptors redundantly participate in entry of hepatitis C virus. PLOS Pathog. 12(5):e1005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, et al. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 3:e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamoto A, Taguchi F, Fukushi S, Morikawa S, Yamada YK. 2006. Identification of ferret ACE2 and its receptor function for SARS-coronavirus. Adv Exp Med Biol. 581:519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate S, Cuadras MA, Espinosa R, Romero P, Juarez KO, Camacho-Nuez M, Arias CF, Lopez S. 2003. Interaction of rotaviruses with Hsc70 during cell entry is mediated by VP5. J Virol. 77(13):7254–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel MB, Lucifora J, Mason WS, Sureau C, Beck J, Levrero M, Kann M, Knolle PA, Benkirane M, Durantel D, et al. 2015. Towards an HBV cure: state-of-the-art and unresolved questions–report of the ANRS workshop on HBV cure. Gut. 64(8):1314–1326. [DOI] [PubMed] [Google Scholar]
- Zhang X, Boyce M, Bhattacharya B, Zhang X, Schein S, Roy P, Zhou ZH. 2010. Bluetongue virus coat protein VP2 contains sialic acid-binding domains, and VP5 resembles enveloped virus fusion proteins. Proc Natl Acad Sci USA. 107(14):6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lin S-M, Chen T-Y, Liu M, Ye F, Chen Y-R, Shi L, He Y-L, Wu L-X, Zheng S-Q, et al. 2011. Asialoglycoprotein receptor interacts with the preS1 domain of hepatitis B virus in vivo and in vitro. Arch Virol. 156(4):637–645. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li Y, Wang HB, Zhang A, Chen ML, Fang ZX, Dong XD, Li SB, Du Y, Xiong D, et al. 2018. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat Microbiol. 3(2):1–8. [DOI] [PubMed] [Google Scholar]
- Zhu YZ. 2014. How hepatitis C virus invades hepatocytes: the mystery of viral entry. World J Gastroentrol. 20(13):3457–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]