Abstract
Human nocardiosis is primarily an opportunistic infection affecting immunocompromised patients, however, one-third of them are immunocompetent. CNS involvement is less commonly reported and associated with a grave prognosis. The majority of these patients are organ transplant recipients on immune suppressants. In the recent past, association of Nocardia asiatica with brain abscess has been reported in a few cases. We are reporting a case of isolated cerebellar abscess caused by N. asiatica in an immune-compromised adult with a review of relevant literature. A 53-year-old male presented with complaints of headache and vomiting for 14 days. There was no previous history of any comorbid illness. During presentation, he was having gait ataxia and radiology showed the right-sided cerebellar multiple lesions. Further hematological investigations revealed the patient to be HIV positive. The abscess was tapped and the pus culture showed Nocardia species. Antibiotics were started as per sensitivity and the patient did well at 3-month follow-up. Though rare, Nocardia should be kept as a differential in brain abscess patients. Owing to the different antimicrobial sensitivity patterns among Nocardia species, both appropriate speciation and susceptibility testing of uncommon species such as N. asiatica are required for their successful treatment.
Keywords: Cerebellar abscess, MALDI-TOF MS, Norcardia asiatica, opportunistic infection
Case Report
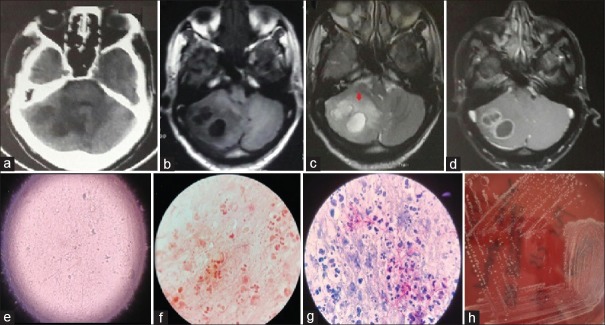
A 53-year-old male, previously healthy, presented with complaints of suboccipital headache and gait ataxia for 15 days. A clinical examination showed some positive cerebellar signs. Magnetic resonance imaging revealed two irregular ring-enhancing cystic lesions in the right cerebellar hemisphere suggestive of an abscess [Figure 1a-d]. The patient underwent right suboccipital craniotomy with abscess drainage. The wet mount of the intraoperative pus revealed filamentous structures [Figure 1e] and gram stain revealed weakly Gram-positive beaded bacilli with extensive filamentous branching in the background of polymorphonuclear leukocytes (suggestive of Nocardia) [Figure 1f]. A modified Ziehl Neelsen (ZN) staining of brain abscess material using 1% sulfuric acid revealed thin, filamentous, branching weakly acid-fast bacteria in the background of many polymorphonuclear leukocytes, also suggestive of Nocardia [Figure 1g]. With these findings suggestive of Nocardia, the patient was started empirically on Trimethoprim/sulfamethoxazole (80/400 mg OD). During the length of the hospital stay, the patient was diagnosed as HIV positive with a CD4 count of 103 cells/μl (6.22%). After 3 days of aerobic incubation at 37°C, the sample grew buff-colored, dry, cerebriform colonies on blood agar [Figure 1h]. The colonies were identified as N. asiatica by MALDI-TOF MS analysis (Microflex LT Biotyper instrument Bruker Daltonics, Bremen, Germany) (score 2.0) and subsequently antimicrobial sensitivity testing was performed according to CLSI guidelines. The strain was sensitive to all first-line drugs (amikacin, amoxicillin, amoxicillin/clavulanic acid, ampicillin, ceftriaxone, ciprofloxacin, erythromycin, imipenem, linezolid, minocycline, and trimethoprim/sulfamethoxazole).
Figure 1.
(a) Computed tomography image showing two hypodense lesions in the right cerebellum. (b-d) Magnetic resonance image showing right cerebellar lesions hypointense on T1W (b), hyperintense on T2W (c), and peripheral enhancement on contrast (d). Note perilesional edema on T2W (red arrow). (e) microscopic morphology of wet mount (f) filamentous, branching, Grampositive bacilli in abscess aspirates, (g) modified acidfast bacilli stain of abscess pus, (h) colony morphology on a blood agar plate
A PCR of 16S rRNA (605 bp) gene from sample and culture was also performed followed by Sanger sequencing of the amplified products.[1] The 16sRNA sequence was submitted in GenBank (accession numbers SUB6396545 seq1 MN538987). These sequences were compared with the sequence data of type strains deposited in GenBank using NCBI BLAST and identified as N. asiatica.
A chest X-ray was performed to look for any pulmonary lesions and had no significant findings. The patient recovered well and subsequently was initiated on antiretroviral therapy (ART).
Discussion
Nocardia is a Gram-positive bacteria with beaded, fine right-angled branching filaments (<1 μm diameter) that are usually weakly acid-fast. The clinical presentation can range from cutaneous manifestations to isolated abscesses and rarely to disseminated forms. Infection occurs mostly in immunocompromised individuals by inhalational route or direct inoculation through the skin.[2] Nocardia brain abscess accounts for only 2% of all brain abscesses with most common predisposing factors as corticosteroid use (54% of the patients) and the organ transplantation (25%).[2,3] The HIV positive population as a risk group is uncommon.[4,5] The clinical course is typically gradual and insidious over months, making early diagnosis and identification difficult leading to a poor outcome in CNS nocardiosis.[6]
Most of the extrapulmonary manifestations in nocardiosis are accompanied by a pulmonary focus subsequently disseminating to other sites. Only 20% of extrapulmonary lesions are unaccompanied by a primary lung lesion as seen in the case.[6] The present case highlights an uncommon presentation with cerebellar involvement.[7] The CNS nocardiosis typically presents as multiple brain abscesses involving the parenchyma, with nonspecific radiological features often misdiagnosed as metastases. The disease is known to masquerade various other infectious conditions like CNS tuberculosis, toxoplasmosis, and aspergillosis with its variable clinical presentation and absence of any specific signs or symptoms to guide the diagnosis. Thus, definitive diagnosis mandates invasive techniques for abscess drainage with specimen culture. Nocardiosis is one of those scenarios where the role of microscopy may be invaluable for timely diagnosis and initiation of treatment. Alerting the treating clinician when nocardiosis is first suspected based on the routine Gram stain and modified ZN staining is necessary to prevent the associated mortality.
In aerobic cultures, Nocardia species colonies usually require 5-21 days to grow. Rapid identification of culture isolates by MALDI-TOF MS aids in the diagnosis. PCR is another important diagnostic tool. Currently, PCR and sequencing of both 16S rRNA (605 bp) and hsp65 (440 bp) regions are recommended to improve species identification. However, in the present case, sequencing was unable to differentiate between closely related species. The species-level identification was possible by MALDI-TOF MS based on the manufacturer's database.
Apart from preliminary information based on the microscopy, it is also important to have an accurate species identification. Nocardia taxonomy has been linked to specific patterns of antimicrobial susceptibility (six drug pattern types) established by Wallace et al.[6] Although Trimethoprim/sulfamethoxazole is a recommended drug for most Nocardia species, certain species have a variation in their susceptibility profiles, with N. otitidiscaviarum commonly being resistant to Trimethoprim/sulfamethoxazole, while N. nova and N. farcinica are occasionally resistant.[8] Empiric treatment with Trimethoprim/sulfamethoxazole, imipenem/meropenem, ceftriaxone or amikacin is recommended. Therefore, identification of the species level has an important bearing on the course of treatment and patient outcomes. 16S rRNA is the most commonly used method, however, Rudramurthy et al., have reported certain ambiguities in the ability of 16S rRNA sequence to accurately identify species on NCBI blast mostly due to inaccurate sequences submitted as Nocardia species to GenBank.[9,10]
The epidemiology of Nocardia infections is gradually evolving; the most frequently reported species include N. farcinica, N. cyriacigeorgica, N. brasiliensis, N. otitidiscaviarum, N. amamiensis, and N. pneumoniae. Only six cases of brain abscess have been reported worldwide and this is the first report of an isolated cerebellar lesion [Table 1][15]. In three of these cases, corticosteroid use was an important predisposing factor. The first report of N. asiatica was in 2004, isolated from patients in Japan and Thailand.[11] Since then, N. asiatica has been most commonly been implicated in pulmonary infections and skin infections.[12] N. asiatica has also been implicated in cases of the olecranon bursitis and anterior mediastinal mass.[5,13]
Table 1.
Epidemiological and clinical characteristics of patient
| Author | Age (years) and sex | Brain abscess site | Other site involvement | Underlying disease | Corticosteroid use |
|---|---|---|---|---|---|
| Wakui et al.[11] (Japan) | 75 M | Single cerebral brain abscess | - | - | No |
| Ryu et al.[14] (Japan) | 44 F | Not reported | - | Guillain-Barré syndrome | No |
| El Herte et al.[5] (Lebanon) | 49 M | Multiple cerebral brain abscess | Anterior Mediastinum | Myasthenia Gravis, Malignant thymoma | Prednisolone |
| Uneda et al.[2] (Japan) | 65 M | Multiple cerebral brain abscess | Lungs | Autoimmune hemolytic anemia | Prednisolone |
| Ji Hun Jeong, et al.[15] (Korea) | 51 M | Multiple cerebral brain abscess | - | Systemic Lupus Erythematosus | Prednisolone |
| Azevedo, et al.[13] (Brazil) | 50F | Cerebral abscess | Mediastinal mass | HIV positive | No |
| Present case (India) | 53 M | Isolated cerebellar brain abscess | - | HIV positive | No |
In summary, Nocardia is an important pathogen with a wide spectrum of presentations. The present case is unique due to the absence of any accompanying pulmonary manifestations and the absence of any known predisposing factors at the time of presentation. The patient was subsequently diagnosed as HIV positive during his hospital stay after the Nocardia infection etiology had been confirmed. Definitive diagnosis requires isolation of the organism from sample. The species level identification is important as many species exhibit varied resistance to commonly used drugs.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We have hidden the identity and details of the patient and have taken written consent for reproducing the CECT images.
References
- 1.Rudramurthy SM, Honnavar P, Kaur H, Samanta P, Ray P, Ghosh A, et al. Molecular identification of clinical Nocardia isolates from India. J Med Microbiol. 2015;64:1216–25. doi: 10.1099/jmm.0.000143. [DOI] [PubMed] [Google Scholar]
- 2.Uneda A, Suzuki K, Okubo S, Hirashita K, Yunoki M, Yoshino K. Brain abscess caused by Nocardia asiatica. Surg Neurol Int. 2016;7:74. doi: 10.4103/2152-7806.186509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaman BL, Beaman L. Nocardia species: Host-parasite relationships. Clin Microbiol Rev. 1994;7:213–64. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostou T, Arvanitis M, Kourkoumpetis TK, Desalermos A, Carneiro HA, Mylonakis E. Nocardiosis of the central nervous system. Medicine (Baltimore) 2014;93:19–32. doi: 10.1097/MD.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Herte RI, Kanj SS, Araj GF, Chami H, Gharzuddine W. First report of Nocardia asiatica presenting as an anterior mediastinal mass in a patient with myasthenia gravis: A case report and review of the literature. Case Rep Infect Dis. 2012;2012:325767. doi: 10.1155/2012/325767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ. Clinical and laboratory features of the Nocardia spp.based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259–82. doi: 10.1128/CMR.19.2.259-282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual-Gallego M, Alonso-Lera P, Arribi A, Barcia J, Marco J. Nocardia farcinica abscess of the cerebellum in an immunocompetent patient: A case report and review of the literature. Asian J Neurosurg. 2016;11:454. doi: 10.4103/1793-5482.145179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao P, Zhang X, Du P, Li G, Li L, Li Z. Susceptibility profiles of Nocardia spp.to antimicrobial and antituberculotic agents detected by a microplate Alamar blue assay. Sci Rep. 2017;7:43660. doi: 10.1038/srep43660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel JB, Wallace RJ, Brown-Elliott BA, Taylor T, Imperatrice C, Leonard DGB, et al. Sequence-based identification of aerobic actinomycetes. J Clin Microbiol. 2004;42:2530–40. doi: 10.1128/JCM.42.6.2530-2540.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellmann A, Cloud JL, Andrees S, Blackwood K, Carroll KC, Kabani A, et al. Evaluation of RIDOM, MicroSeq, and GenBank services in the molecular identification of Nocardia species. Int J Med Microbiol. 2003;293:359–70. doi: 10.1078/1438-4221-00271. [DOI] [PubMed] [Google Scholar]
- 11.Wakui D, Ito H, Ikeda R, Yoshida Y, Furuya Y, Tanaka K, et al. A complicated case of Nocardia brain abscess for differential diagnosis. No Shinkei Geka. Neurol Surg. 2008;36:1011–6. [PubMed] [Google Scholar]
- 12.Suemori K, Miyamoto H, Murakami S, Yamazaki H, Ishizaki J, Matsumoto T, et al. Pulmonary nocardiosis due to Nocardia asiatica in a patient with ANCA-associated vasculitis. Kansenshogaku Zasshi. 2015;89:470–5. doi: 10.11150/kansenshogakuzasshi.89.470. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy F, de Azevedo SF, Dutra V, Dutra Souto FJ. Cerebral and mediastinal abscesses caused by Nocardia asiatica in an hiv-infected patient. Rev Soc Bras Med Trop. 2019;52 doi: 10.1590/0037-8682-0485-2018. Uberaba 2019 Epub June 06, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Ryu A, Kawahara R, Mori K, Tamura M, Nakano K, Yoshioka A. A case of brain abscess caused by Nocardia asiatica. J Jpn Soc Clin Microbiol. 2019;19:163–70. [Google Scholar]
- 15.Jeong JH, Moon SM, Park PW, Ahn JY, Kim KH, Seo JY, et al. Multiple brain abscesses caused by Nocardia asiatica in a patient with systemic lupus erythematosus: The first case report and literature review. Ann Lab Med. 2017;37:459–61. doi: 10.3343/alm.2017.37.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]