Abstract
India is a land of spices and medicinal plants. Ayurvedic medications and methods are commonly practised in India for curing several ailments. Lepidium sativum (garden cress) is an important herb that belongs to Brassicaceae family. It is believed that the plant has its origin in Ethiopia but is now cultivated throughout the world. The plant is well-known in Ayurveda for its beneficial properties it holds. The present study describes the fracture healing property of the methanolic and aqueous extract of Lepidium sativum seeds. For the study, 21 Charles foster rats were used. They were grouped into three groups each containing seven rats: control, methanolic, and aqueous group. Rats were anesthetized using ether vapors and fractures were induced in each rat from all the three groups using hand held three-point bending technique. The broken bone fragments were then stabilized using splints. The control group was administered with normal saline, along with food and water, post-fracture. The methanolic group was administered with the methanolic extract of Lepidium sativum seeds at dose of 400 mg/kg given orally, post-fracture along with food and water. The third group received aqueous extract of the seeds in doses of 550 mg/kg orally, along with daily food and water intake for a period of 8 weeks. The results were evaluated both radiologically and biochemically. X-rays were done on day 0, 2nd week, and 4th week post-fractures to look for the callus formation and serum levels for calcium, phosphorus, and alkaline phosphatases were evaluated on 0 day, 1 week, 2nd week, 4th week, 6th week, 8th week, and 10th week post-injury. It was observed at the end of the study period that the methanolic group had significant callus formation starting at the 2nd week itself post-fracture. The serum levels of calcium, phosphorus, and alkaline phosphatases at 4th, 6th, and 8th weeks had significant P values in the methanolic group rats.
Keywords: Charles foster rats, fracture healing, lepidium sativum seeds, methanolic and aqueous extract, three- point bending method
Introduction
Ayurveda, in India is about 5,000 years old, and consists of holistic herbal medications that are currently very popular in India. It is estimated that 80% of the total Indian population uses folk medicine to treat common diseases.[1] India is a developing country with majority of population living in rural areas with basic amenities and poor healthcare facilities. The place of medicinal plants in preventing common diseases is further examined under the five core principles of the Primary Health Care (PHC) approach. Lepidium stivum is a medicinal plant and can be used as an essential drug to improve mother and child health as an abundant source of calcium and phosphorus. Global trade in herbs is over USD 100 billion per annum, India and China's medicinal plant trade is about two to five billion USD annually. Medicinal plants also fit perfectly into the modeling for priorities in PHC as proposed by McDonald and Ollerenshaw (2011).[2] It is thus need of an hour to develop medications and methods within the reach of common population.
Lepidium sativum, commonly known as garden cress, is an edible herb growing to a height of 50 cm. It is well-known to mankind by several different names as Halim, Holan, Chandrasura, etc., The plant belongs to Brassicaceae family and grows well in all types of soil and climate.[3] The plant has its origin in Egypt and South West Asia, but is now cultivated throughout the world for its seeds. It is widely used as an analgesic, anti- spasmodic, anti-diarrhoeal, galactagogue, hepatoprotective,[4] antioxidant, anti- inflammatory[5] diuretic, etc.
Fractures are common globally and its healing is a physiological process, due to the ability of the bone to regenerate. The treatment for fractures is stabilizing the broken bone fragments medically or surgically, prescribing analgesics to relieve pain and anti-inflammatory drugs to counteract inflammation. Sushruta, in Ayurveda, also describes Lepidium sativum being used in bone healing. The plant is a rich source of proteins, carbohydrates, dietary fibers and minerals such as calcium, phosphorus, potassium, zinc, etc.[5] Phytochemical study of the plant reveals components such as tannins, flavonoids, glycosides, phenols, lectin, and mucilage in it.[6,7]
The seeds of garden cress are oval, pointed at one end, reddish-brown coat, and a wing like projection on both edges. The endosperm has polygonal cells and an embryo inside. On soaking seeds in water, it becomes mucilaginous.[8,9]
Materials and Methods
1. Collection of seeds: Seeds of Ls were purchased from a local store. They were shade- dried, grinded to a fine state. Two extracts namely aqueous and methanolic were prepared from the powdered seeds.
2. Extract preparation
Methanolic extract- The extract was prepared by maceration process. 250 g of powdered seeds were dissolved in 500 ml of methanol, stored at room temperature for 3 days.
Aqueous extract- The extract was prepared using Soxhlet technique. The powdered seeds were kept in the thimble and distilled water was heated at a boiling temperature. The vapors were condensed using cold running tap water that dripped down in the flask through the extract.
3. Dose and route:
The extracts were dissolved in 5% CMC and was administered orally in the rats, using a feeding tube, from the day of fracture induction to a period of 2 months. The methanolic extract and aqueous extracts were administered at doses of 400 mg/kg and 550 mg/kg, respectively.
4. Animals-
21. Charles foster rats of either sex, weighing 150--200 g, were purchased from the animal house of BHU, Varanasi. They were kept in the Department of Pharmacology, IMS, BHU where the experiment was carried out.
The study was conducted as per CPCSEA guidelines. The approval was taken from Animal Ethical Committee vide letter no- Dean/2017/CAEC/249. The rats were kept in polypropylene cages in the animal house, with 12/12 h light and dark cycle at 27 ± 2 celsius and provided standard environmental conditions. Animal ethical permission granted vide letter no Dean/2017/CAEC/249.
5. Grouping of animals-
21 rats were divided into three groups:
Control group- receiving normal saline orally,
Methanolic group- receiving methanolic extract of the seeds orally,
Aqueous group- receiving aqueous extract of seeds orally, with adequate nutrition throughout the study period.
6. Method of fracture induction.
Vapors of ether were used to anesthetise the rat. Right tibia was identified and a closed fracture was induced using hand held three-point bending method.[10,11]
7. Evaluation by X-rays
The fracture healing was evaluated by X-rays done from the Radiology Department, Sir Sunderlal Hospital, BHU, Varanasi. They were done at 0 day, 2 weeks, and 4 weeks to look for the callus formation.
8. Blood collection
The rats were anesthetized and blood was obtained via retroorbital route, using thin capillary tubes. 5 ml of blood withdrawn from each rat was kept in small labelled test tubes. They were then centrifuged at 2,000 rpm for the serum to separate from the blood components. Serum was then used for testing using appropriate kits.
8. Biochemical analysis
For biochemical analysis, kits for calcium, phosphorus, and alkaline phosphatases were purchased from a local dealer, manufactured by TransAsia Bio-Medicals LTD, Solan, in technical collaboration with ERBA diagnostics Mannheim GmbH.
Alkaline phosphatase-
Kinetic method used for the evaluation is recommended by International Federation of clinical chemistry.
Principle- This method utilizes 4-nitrophenyl phosphate as the substrate. Under optimized conditions ALP present in the sample catalyses the reaction and forms 4 nitrophenol and phosphate.[12]
Phosphorus-
The method is a modification by Wang et al. of Daly and Ertingshausen's method.[13]
Principle- Inorganic phosphorus combines with ammonium molybdate in the presence of strong acids to form phosphomolybdate and is directly proportional to the concentration of inorganic phosphorus present.
Calcium-
Method used is a modification of O-Cresolphthalein Complexone method of Moorehead and Briggs.[14]
Principle- OCPC reacts with calcium in alkaline solution to form a purple colored complex. The intensity of the purple color is proportional to the calcium concentration.
Results and Observations
The X-ray examination was done at 0 day, 4 weeks, and 8 weeks for the callus formation.
Figure 1.
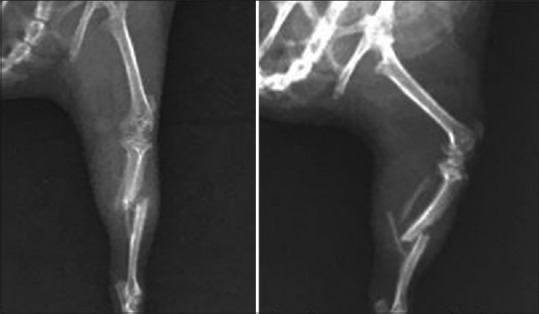
X-ray showing tibial fracture in control group on day of induction
Figure 3.
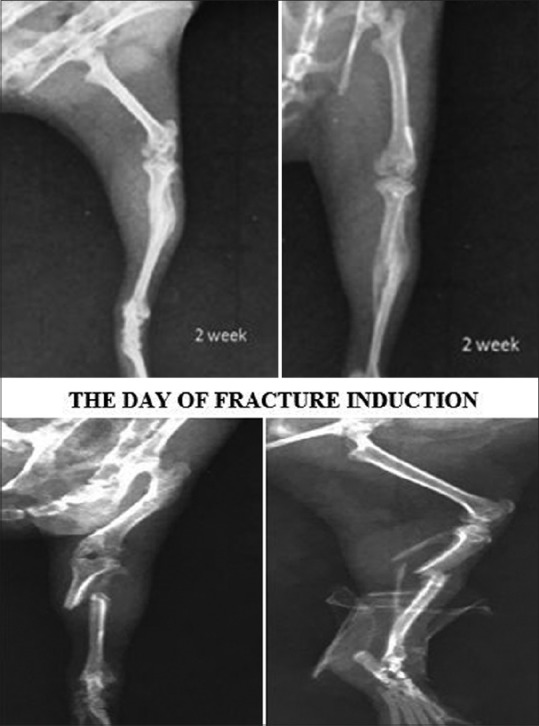
X-ray showing tibial fracture in methanolic group 14 days post fracture
On the day of fracture induction
Figure 2.
X-ray showing tibial fractures in aqueous group on the day of fracture induction and 14 days post fracture
Figure 4.
X-ray showing tibial fracture in control group after 14 days
After 2 weeks of fracture
Figure 5.
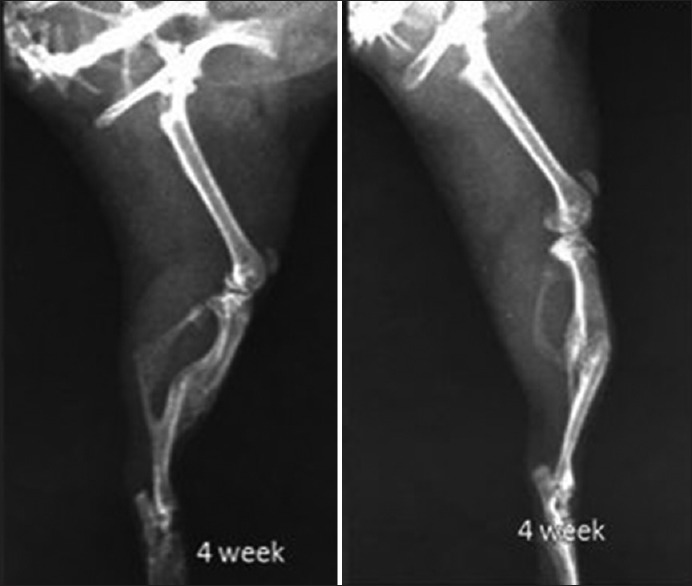
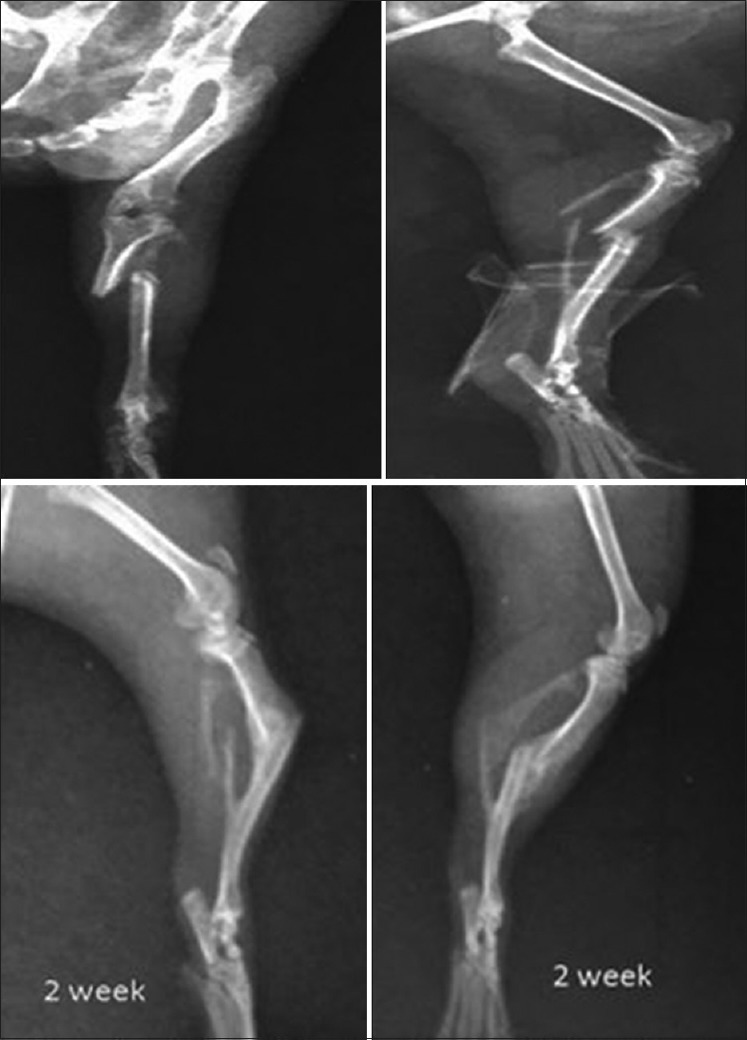
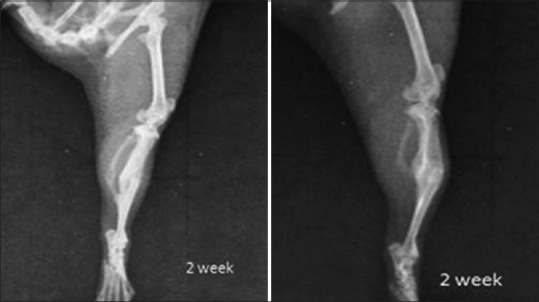
X-ray showing tibial fracture in control group 4 weeks post-fracture
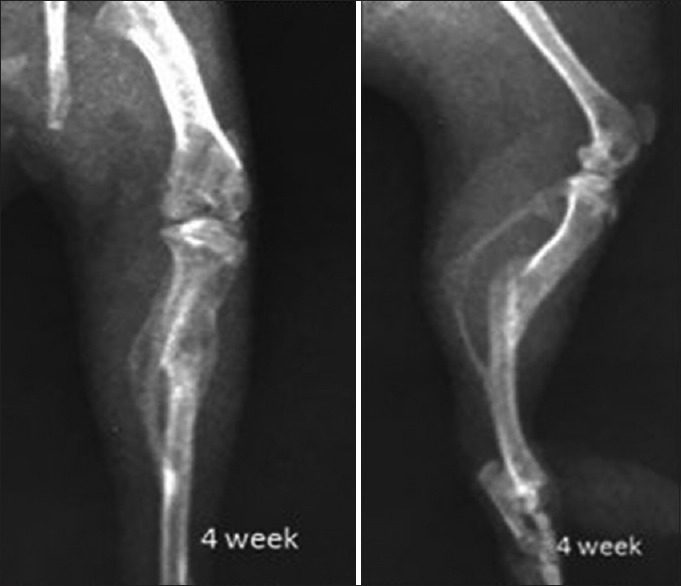
Figure 7.
X-ray showing tibial fracture in methanolic group 4 weeks post-fracture
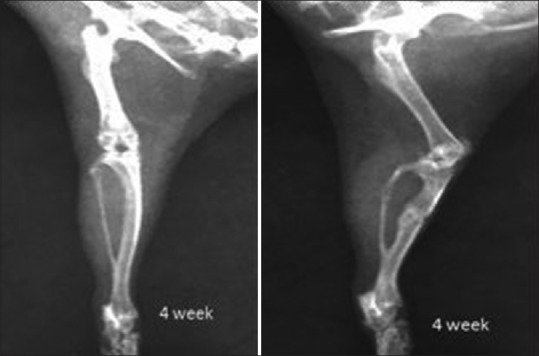
Figure 6.
X-ray showing tibial fracture in aqueous group 4 weeks post-fracture
After 4 weeks of fracture
Observation table of the biochemical parameters at each week
The values obtained are expressed as mean ± SD for all the three groups-
Discussion
The callus formation was evaluated radiologically on day of fracture induction, 2nd week, and 4th week post-fracture. It was observed in the present study that 14 days after the fracture induction, the callus in methanolic group was larger when compared with the other two groups. 4 weeks post-fracture, the callus formed in the methanolic group was significantly larger and had completely healed fragments with no visible fracture line when compared with the other two groups.
Juma[15] et al. in his study mentioned that the callus formation in rabbits for the group treated with LS extract was statistically significant with P <.001 for CM (circumference) in 6 weeks and P <.004 for CM in 12 weeks when compared with the controls respectively.
Another study by Yadav[6] et al. done for evaluating fracture healing activity of the ethanolic extract of LS on internally fixed rat's femoral osteotomy model had significant callus formation in the test group.
The results for the present study are comparable with the results of the previous studies done earlier. It has been claimed in these studies that the extract of LS had tannins, flavonoids, alkaloids, glycosides, mucilage, and other phenolic compounds that helped in callus formation. We propose in the present work the same mechanism for callus formation. It is the presence of flavonoids and tannins in the methanolic extract of Lepidium sativum seeds that has helped in bone repair.
Serum calcium levels [Graph 1]
Graph 1.
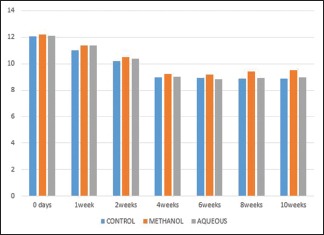
Showing serum levels of calcium at different intervals
In the present work, the mean values for serum calcium were continuously decreasing for about a month from base line values of 12.06 ± 0.57 to 8.94 ± 0.28 post-fracture. They were within normal serum concentrations in 6th, 8th, and 10th week [Table 1].
Table 1.
SERUM CALCIUM
| DURATION | CONTROL | METHANOL | AQUEOUS | F | P |
|---|---|---|---|---|---|
| 0 days | 12.06±0.57 | 12.18±0.56 | 12.11±0.54 | 0.85 | 0.918 |
| 1 week | 11.0±0.49 | 11.39±0.45 | 11.36±0.29 | 0.37 | 0.694 |
| 2 weeks | 10.21±0.47 | 10.52±0.41 | 10.39±0.30 | 0.39 | 0.659 |
| 4 weeks | 8.94±0.28 | 9.25±0.26@ | 9.0±0.16 | 3.18 | 0.05 |
| 6 weeks | 8.90±0.23 | 9.20±0.31 | 8.81±0.26 | 3.75 | 0.043 |
| 8 weeks | 8.85±0.23 | 9.40±0.19* | 8.90±0.42 | 7.07 | 0.005 |
| 10 weeks | 8.88±0.32 | 9.52±0.13# | 8.95±0.35 | 14.63 | 0.000 |
*P<0.005, #P<0.0001, @P<0.05
Struck et al.[16] in his study evaluates serum findings after an experimental fracture in dogs had reported similar decreasing trends of serum calcium during 1st week with values as 8.51 ± 0.15 postop with P < 0.000. The levels of calcium then returned to its baseline values in the 4th week of the study.
Westlin[17] et al. (1972) in his study has evaluated serum calcium levels of 50 patients after femoral fracture. He has reported the mean values of calcium in these patients as 4.50 ± 0.10 that was decreased as compared with control (4.82 ± 0.23), for about a month and then returned to normal levels in 8th, 16th, and 32nd week post-fracture.
Our results are comparable with the previous studies. It was claimed in these previous studies that this decrease in serum calcium for initial weeks was related to the changes in bone mineral metabolism that took place after the fracture. We propose the same theory of bone mineral metabolism for decreased serum calcium levels in the present study for the control group.
The group of rats treated with methanolic extract of seeds of LS had observed significantly higher values of S. Calcium at 4th (9.25 ± 0.2), 6th (9.20 ± 0.3), 8th (9.40 ± 0.19), and 10th (9.52 ± 0.13) week post-fracture with significant P < 0.05, P < 0.005, and P < 0.000 respectively, when compared with the other two groups [Table 1].
Our findings of the present study are in comparison with the study by Gokavi[18] et al. It was concluded in the study that positive effect on bone healing for LS extract was due to the rich calcium content.
The phytochemical analysis of LS seeds done by Doke[19] et al. has proved the presence of calcium in concentrations of 210.51 ± 1.08 mg/100 g of seeds, so we propose in the present study that the methanolic extract of seeds of LS has provided the calcium that had increased serum calcium levels that were required for callus formation.
Serum phosphorus levels [Graph 2]
Graph 2.
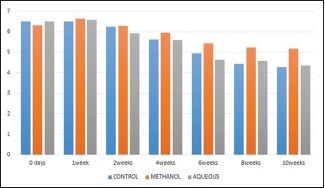
Graph showing serum levels of phosphorus at regular intervals
In this present work, mean values for serum phosphorus initially increased post-fracture, with mean values of 6.02 ± 0.58 in 2nd week and 5.62 ± 0.47 in 4th week. The values then returned to normal range in 8th and 10th week [Table 2].
Table 2.
SERUM PHOSPHORUS
| DURATION | CONTROL | METHANOL | AQUEOUS | F | P |
|---|---|---|---|---|---|
| 0 days | 6.49±1.06 | 6.33±0.86 | 6.70±0.63 | 0.30 | 0.737 |
| 1 week | 6.51±0.62 | 6.63±0.57 | 6.57±0.88 | 0.14 | 0.986 |
| 2 weeks | 6.02±0.58 | 6.04±0.45 | 5.94±0.58 | 0.180 | 0.836 |
| 4 weeks | 5.62±0.47 | 5.95±0.74* | 5.60±0.25 | 8.44 | 0.000 |
| 6 weeks | 4.94±1.78 | 5.43±0.42* | 4.65±0.29 | 12.66 | 0.000 |
| 8 weeks | 4.44±0.24 | 5.24±0.40* | 4.59±0.28 | 12.22 | 0.000 |
| 10 weeks | 4.27±0.23 | 5.17±0.55 | 4.34±0.21 | 0.948 | 0.406 |
*P<0.000
A study by Hunsberger and Ferguson[20] has demonstrated that physiologically the serum phosphorus was rising for 2 weeks post-fracture (4.73 ± 0.32) before returning to the normal values. It was claimed in the study that activated osteoblasts and cartilage forming cells in the callus secrete some active phosphatase enzyme that hydrolyse salts of phosphoric esters, thereby increasing concentrations of inorganic phosphorus in serum for a short period of time soon after the fracture. Struck[16] et al. also confirmed raised phosphorus in serum at 1st week (4.80 ± 0.88). It was proposed by him that the rise in serum phosphorus is due to increased osteoblastic activity.
Our findings for serum phosphorus in this study is comparable to the previous mentioned studies, so we propose the same mechanism of increased osteoblastic activity during initial phases of bone healing responsible for raised serum phosphorus levels during first few weeks in control group. In the present work, significant differences in mean values for serum phosphorus with values as 6.30 ± 0.45 in 2nd week, 5.95 ± 0.74 in the 4th week, 5.43 ± 0.42 in the 6th week were found, when compared with the other two groups. Soliman et al.[21] in his study on protective effects of LS against bone loss induced by steroids had significantly higher levels of phosphorus in bone (102.77 ± 4.15) for LS treated groups as compared with the osteoporotic controls with values as 88.16 ± 4.17. He measured serum phosphorus in the treated groups and they were also higher when compared with controls 1.36 ± 0.11 [Table 2].
It was claimed in this study that the fracture healing property of LS is due to the phosphorus and other phytoestrogens present in LS. It is well documented by Doke et al. in his phytochemical analysis on LS seeds that the total concentration of phosphorus in LS is about 625.81 ± 14.59 mg/100 g of seeds. Another study documenting the presence of phosphorus is by Rawal[22] et al. He has mentioned that LS is a rich source of both calcium and phosphorus with % composition of 0.36 and 0.11%, respectively.
It has been proposed in the study by Winger[23] et al. that phosphorus is essential for normal development and responsiveness to BMP that binds to mesenchymal stem cells and causes differentiation and proliferation of osteoprogenitor cells.
The results of the present study are comparable with the previous studies. Therefore, we also propose that the serum levels were raised due to increased concentration of phosphorus in LS extract that accelerated bone healing by increasing osteoblastic proliferation.
Serum alkaline phosphatase levels [Graph 3]
Graph 3.
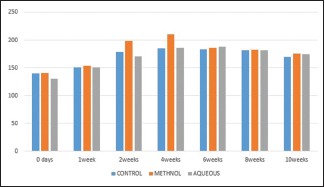
Showing serum levels of alkaline phosphatases at regular intervals
Alkaline phosphatase is a marker of bone formation. In this present work, the mean values of serum ALP observed were raised after the fracture for 4 weeks with peak values of 185.48 ± 9.82, and then returned to their normal range at 8th and 10th weeks [Table 3].
Table 3.
SERUM ALKALINE PHOSPHATASES
| DURATION | CONTROL | METHANOL | AQUEOUS | F | P |
|---|---|---|---|---|---|
| 0 day | 140.5±34.82 | 141.5±19.30 | 130.9±17.69 | 0.377 | 0.691 |
| 1 week | 151.1±16.52 | 153.2±23.19 | 150.4±15.03 | 0.043 | 0.958 |
| 2 weeks | 178.64±18.52 | 198.15±3.08* | 170.87±16.52 | 6.633 | 0.007 |
| 4 weeks | 185.48±9.82 | 210.4±6.92# | 185.8±5.95 | 23.88 | 0.000 |
| 6 weeks | 184.17±5.91 | 185.70±3.91 | 187.82±7.49 | 0.66 | 0.527 |
| 8 weeks | 180.88±6.92 | 182±11.14 | 181.58±6.99 | 0.030 | 0.970 |
| 10 weeks | 170.10±22.2 | 175.50±12.76 | 174.70±5.27 | 0.260 | 0.774 |
*P<0.005, #P<0.000
Semb[24] et al. in his study mentioned that from 4 h after fracture the levels of ALP were increasing, with values reaching its maximum in 24 h post-trauma. The values then decreased and reached its normal range after 14 days.
It was proposed in the study that enzyme levels were increased due to proliferation and increased activity of cells in the fracture site. He has also proved it in his study that this ALP was majorly derived from bones [Table 3].
Singh[25] et al. in his study has evaluated serum ALP in 95 patients of diaphyseal fracture, which were divided into three groups: group A with patients who have achieved union of bones by 6 months, group B1 who had patients achieving union in 9 months, and group B2 who have not achieved union even in 9 months. He has correlated levels of ALP in blood with clinic-radiological findings as well. He has concluded in his study that group A patients had healing occurring with values of ALP reaching 689 U/L in 3rd week and for group B healing was completed in 28.2 weeks with ALP as 500 U/L. Serum ALP levels were not significant in group B2 and remained normal for about 6 months. It was claimed in the study that increased S. ALP demonstrated healing. It was considered an excellent predictor of bone healing.
Westlin et al. performed the study for evaluation of serum ALP post-femoral neck fractures, concluded that ALP activity was doubled and remained high for about 2 months before reaching the normal level. He concluded in his study that the increase in levels of ALP was depicting increased cell differentiation and proliferation in the fracture area.[17]
We have results in the present study similar, so we propose increased cell proliferation as the cause of increased ALP levels.
Conclusions
We propose that the significant callus formation in the methanolic group was due to flavonoids, tannins, phytoestrogen, etc., present in the extract of garden cress seeds.
There were significantly increased mean values of serum calcium in the methanolic group at 4th, 6th, 8th, and 10th week post-injury. We propose that this increase in mean values was due to high amounts of calcium in the extract.
There were significantly increased levels of serum phosphorus in the methanolic group at 4th, 6th, and 8th week post-fracture. We propose that this increase in mean values was due to high content of phosphorus in the extract.
There were significantly increased levels of serum alkaline phosphatase as a result of increased osteoblastic activity in the methanolic group during 2nd and 4th week after fracture.
We conclude that Lepidium sativum is a rich source of calcium and phosphorus that accelerates bone healing and can be used as an alternative to present supplements prescribed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singh V. Medicinal plants and bone healing. Natl J Maxillofacial Surg. 2017;8:4–11. doi: 10.4103/0975-5950.208972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med. 2013;10:210–29. doi: 10.4314/ajtcam.v10i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manohar D, Viswanatha GL, Nagesh S, Jain V, Shivaprasad HN. Ethnopharmacology of lepidium sativum linn (Brassicaceae): A review. Int J Phytother Res. 2012;2:1. [Google Scholar]
- 4.Balgoon MJ. Assessment of the protective effect of lepidium sativum against aluminium induced liver and kidney effects in albino rats? Biomed Res Int. 2019;2019:4516730. doi: 10.1155/2019/4516730. doi: 10.1155/2019/4516730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alqahtani FY, Aleanizy FS, Mahmoud AZ, Farshori NN, Alfaraj R, Al-sheddi ES, et al. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J Biol Sci. 2019;26:1089–92. doi: 10.1016/j.sjbs.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav YC, Jain A, Srivastava DN, Jain A. Fracture healing activity of ethanolic extract of L. Sativum seeds in internally fixed rat's femoral osteotomy model. Int J Pharm Pharm Sci. 2011:193–7. [Google Scholar]
- 7.Kaiyrkulova A, Li J, Aisa HA. Chemical constituents of lepidium sativum seeds. Chem Nat Compd. 2019;55:736–7. [Google Scholar]
- 8.Guha M, Doke S. Garden cress (Lepidium sativum L.) seed- an important medicinal source: A review. J Nat Prod Plant Resour. 2014;1:69–80. [Google Scholar]
- 9.Prajapati VD, Maheriya PM, Jani GK, Patil PD, Patel BN. Lepidium sativum ninn: A current addition to the family of mucilage and its applications. Int J Biol Macromol. 2014;65:72–80. doi: 10.1016/j.ijbiomac.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Willingham MD, Lee KL, Stephans AL, Ye J, Silva MJ. Age related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int. 2010;86(6):470–83. doi: 10.1007/s00223-010-9359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayagara AR, Langlet A, Hambli R. On dynamic behavior of bone: Experimental and numerical study of porcine ribs subjected to impact loads in dynamic three-point bending tests. J Mech Behav Biomed Mater. 2019;98:336–47. doi: 10.1016/j.jmbbm.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Burtis CA, Ashwood ER, Bruns DE. Tietz textbook of clinical chemistry. 3rd Edition. Elseviers; 1999. p. 745. [Google Scholar]
- 13.Henry RJ, Cannon DC, Winkleman JW, editors. Clinical Chemistry Principles ans technics. 2nd edition. NY: Harper & Row; 1974. p. 722. [Google Scholar]
- 14.Moorehead WR, Briggs HC. 2 Amino 2 methyl-propanol as the alkalizing agent in an improved continuous flow cresolphthalein complexone procedure for ca in serum. Clin Chemistry. 1974;20:1458. [PubMed] [Google Scholar]
- 15.Juma AH. The effects of Lepidium sativum seeds on fracture induced healing in rabbits. Med Gen Med. 2007;9:23. [PMC free article] [PubMed] [Google Scholar]
- 16.Struck H, Dabew D, Hernández-Richter HJ, Benfer J. Klinischchemische befunde im serum bei experimentellar fraktursetzung and verchiedener be-handlung. Enzym Biol Clin. 1969;10:463. [Google Scholar]
- 17.Westlin NE, Nilson BE. The plasma concentration of alkaline phosphatase, phosphorus and calcium following femoral neck fractures. Acta Orthop Scandinav. 1972;43:504–10. doi: 10.3109/17453677208991272. [DOI] [PubMed] [Google Scholar]
- 18.Govaki SS, Malleshi NG, Guo M. Chemical composition of garden cress (Lepidium sativum) seeds and its fractions and its use of bran as a functional ingredient. Plants Foods Hum Nutr. 2004;59:105–11. doi: 10.1007/s11130-004-4308-4. [DOI] [PubMed] [Google Scholar]
- 19.Doke S, Guha M. Garden cress (Lepidium sativum Linn) seed; an important medicinal source. A review. J Nat Plant Resour. 2014;4:69–80. [Google Scholar]
- 20.Hunsberger A, Ferguson LK. Variations in phosphatase and inorganic phosphorus in serum during fracture repair. Arch Surg. 1932;24:1052–60. [Google Scholar]
- 21.Soliman G, Samad A, Nora A, Maged S, Taimmi AL, Gabar GA, Kader A. Potential protective effects of vigna radiate and lepidium sativum against bone loss induced by prednisolone acetate in male and female rats. Indo Am J Pharma. 2017:41085–94. [Google Scholar]
- 22.Rawal N. A comprehensive review of lepidium sativum linn, A traditional medicinal plant. World J Pharm Pharm Sci. 2016;5:1593–601. [Google Scholar]
- 23.Winger NA, Luderer HF, Cox MK, Sooy K, Gerstenfeld LC, Demay MB. Acute phosphate restriction leads to impaired fracture healing and resistance to BMP-2. J Bone Miner Res. 2010;25:724–33. doi: 10.1359/jbmr.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semb TH, Gudmundson CR, Westlin NE, Hallander LB. Alkaline phosphatase activity and isoenzymesin experimental fractures. Clin Chim Acta. 1971;31:375–80. doi: 10.1016/0009-8981(71)90406-2. [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Sabir A, Srivastava RN. Evaluation of serum alkaline phosphatase +*as a biomarker of healing process progression of simple diaphyseal fracture in adult/patient. Int Res J Biol Sci. 2103;2:40–3. [Google Scholar]


