Abstract
Introduction:
Anemia is a major public health problem amongst elderly population in India. Anemia in old age further worsens the age-related decline in functional ability, mobility, fatigue, bone density, and skeletal muscle mass. There is lack of evidence on the prevalence and risk factors of anemia among elderly population. Hence, this study was undertaken.
Methodology:
A community-based cross-sectional study was conducted during the year 2015–2016 in District Nainital, Uttarakhand state, India. A total of 958 subjects were selected from 30 clusters (villages) identified using population proportional to size methodology. Information on sociodemographic profile, nutritional status, body mass index, and dietary intake was obtained. Blood sample was collected from each subject on the filter paper for estimation of hemoglobin (Hb) level using cyanmethemoglobin method.
Results:
We found that 92.1% of the elderly subjects were anemic. Moderate and severe anemia was found to be significantly higher among female subjects, unemployed, illiterates, subjects using smoke-producing fuel, subjects belonging to lower socioeconomic status, malnourished and underweight subjects, subjects with self-reported hyperacidity, and subjects who had not utilized health facility and had lower iron and vitamin C intake when compared with subjects with mild anemia and normal hemoglobin levels.
Conclusion:
High prevalence of anemia exists amongst elderly subjects living at high-altitude region of rural Uttarakhand State, India. There is a need to educate the elderly population about the importance of adequate intake of foods rich in iron and vitamin C to reduce the prevalence of anemia among them.
Keywords: Anemia, elderly, geriatric, iron deficiency, old age
Introduction
Anemia is characterized by low hemoglobin (Hb) concentration and insufficient oxygen-carrying capacity to meet the body's physiological needs. It is a major public health problem among elderly in India.[1,2,3,4,5,6,7,8,9,10,11,12] A recent systematic review has reported that the global prevalence of anemia among elderly population was 17% (3%–50%).[13] The World Health Organization (WHO) has reported that globally 23.9% (164 million) of people (≥60) years residing in low-income and middle-income countries had anemia.[14]
Approximately one-third of anemia cases in older adults are attributed to nutritional deficiencies such as iron, folate, and/or vitamin B12 deficiencies.[15] Iron deficiency alone accounts for nearly half of the nutrient deficiency-related anemia cases. Whereas folate and vitamin B12 deficiencies account for 14% of all anemic elderly population.[15] About one-third causes of anemia in older people include chronic infection and inflammation, blood loss through gastrointestinal lesions, and chronic kidney disease.[15,16]
Anemia among elderly population has adverse health consequences such as fatigue, weakness, and shortness of breath leading to lowered functional ability and mobility, lower bone density and skeletal muscle mass, diminished cognitive function, increased frailty, increased risk of recurrent falls, increased risk of comorbid conditions and mortality.[15,17,18,19]
Earlier studies conducted in plain regions of the country have reported high prevalence of anemia among elderly in the range of 21%–96%.[1,2,3,4,5,6,7,8,9,10,11,12] There is lack of scientific evidence on the prevalence and risk factors of anemia among Indian elderly population residing in high-altitude regions of the country. Hence, this study was undertaken among elderly population living at high-altitude region of rural Uttarakhand, India.
Methodology
A community-based cross-sectional study was conducted in District Nainital, Uttarakhand state, India. A total of 958 elderly subjects (36.5% males and 63.5% females) were enrolled for the study. The mean age of males was 69.3 ± 7.4 years and of females was 67.9 ± 7.2 years. The elderly subjects were selected from 30 clusters (villages) identified using population proportional to size sampling methodology. Thirty elderly subjects in the age group of 60 years and above were selected from each cluster by house-to-house visit. The detailed sampling methodology undertaken for selection of the elderly subjects has been described in a previously published article.[20]
The following exclusion criteria were adopted: (i) subjects who were unable to comprehend the questions objectively and (ii) subjects who had auditory problems leading to nonresponse. An informed written consent was obtained from each subject after the explanation of the objectives and data collection parameters for the study. The study was approved by the ethical committee of All India Institute of Medical Sciences (AIIMS), New Delhi.
Data collection parameters
Sociodemographic profile
A pretested structured questionnaire was administered to obtain identification data and sociodemographic profile such as gender, age, educational qualification, present occupation, family monthly income, and financial dependency. Socioeconomic status (SES) was calculated using Kuppuswamy classification (2014).[21]
Body mass index
The height and weight of the elderly subjects were measured using standard procedures. The body mass index (BMI) was calculated using the following formula: BMI (kg/m2) = weight (kg)/height (m2). The BMI (kg/m2) was classified as <18.5 (underweight), 18.5–24.9 (normal), 25–29.9 (overweight and pre-obese), and ≥30 (obese) as per WHO classification.[22]
Dietary assessment
The dietary intake of nutrients was assessed among one-fourth of the elderly subjects (n = 248) using one-day 24-h dietary recall method. The following steps were undertaken: (i) information regarding the meal pattern and the food items (cooked and uncooked) consumed by the subject was recorded; (ii) for each cooked food item consumed, the raw ingredients used for the preparation were recorded; (iii) equivalent quantities of raw ingredients used for preparation of each food item were weighed using a SECA kitchen scale and recorded; (iv) total volume of each cooked food item was recorded using standard cups; (v) the quantity of each food item consumed by the indexed subject was assessed using standard cups/spoons/chapatti models. The cups were used to aid the respondent recall the quantities consumed by the individual subject; (vi) from step (iv) and (v), the amount of raw ingredients in grams for each food item consumed by the indexed subject was calculated; and (vii) nutritive value of the raw foods consumed was determined using the Food Composition Table from Nutritive Value of Indian foods.[23] The person responsible for cooking the food was interviewed for assessing the dietary intake of the indexed subject. The dietary intake of macronutrients (energy, protein, fat, carbohydrate), micronutrients (zinc, iron, calcium, magnesium, thiamine, riboflavin, niacin, vitamin C, folic acid), and trace elements (copper, manganese, molybdenum, chromium) by the elderly subjects was compared with the recommended dietary allowance (RDA) for Indians given by the Indian Council of Medical Research (ICMR).[24]
Hemoglobin estimation
Hb was assessed using cyanmethemoglobin methodology.[25] Anemia was defined as Hb level of <13.0 g/dL in males and <12.0 g/dL in females, respectively.
Collection of dried blood spot (DBS): Whatman filter paper number 3 was labeled with unique ID, date, and center code. Each subject was requested to clean with soap water and rub his or her hands against each other to stimulate blood flow. Third (middle) or fourth (ring) finger of each subject was selected for the finger prick for each subject.
The finger tip of each subject was wiped with alcohol swab completely and was allowed to air dry.
Subject's hand was gently kneaded from palm toward finger tip. The finger tip was pricked using lancet device with gauze No. 18/19 for deep prick. A full drop of blood was allowed to form on finger. The first drop of blood was wiped off using a sterile swab.
The second large drop of blood was collected and used for estimation of Hb level for identification of anemia among the elderly population.
Drying of blood spots: Filter papers were kept at room temperature for 4 h to allow the blood spot to air dry completely.
The drying process was considered to be completed when the blood spots had a uniformly dark brownish color and no red areas were visible.
Storage and transportation of DBS: The following steps were taken for the storage and transportation of DBS filter paper:
Each DBS filter paper was sealed in an autoseal plastic pouch to protect from dust and moisture after drying out. A total of 10 small pouches containing DBS filter paper were transferred into a larger zip lock pouch. A desiccant was kept inside each zip lock bag before zipping it. The sealed filter papers were repacked into icebox containing ice packs and transported to the field laboratory and then to the central laboratory AIIMS, New Delhi. Filter papers were stored at 4°C prior to analysis.
The standard procedure for estimation of Hb by cyanmethemoglobin method was used.
Sample size
Assuming the prevalence of anemia to be 50%,[7] the desired sample size using the formula 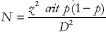 (where z is the standard normal variate corresponding to 5% level with 50% prevalence rate), 95% confidence level, 5% relative precision, design effect of 2, and 15% nonresponse, the total sample size derived at was 883 and rounded up equivalent to 900 after considering. However, we included 958 elderly subjects in the study.
(where z is the standard normal variate corresponding to 5% level with 50% prevalence rate), 95% confidence level, 5% relative precision, design effect of 2, and 15% nonresponse, the total sample size derived at was 883 and rounded up equivalent to 900 after considering. However, we included 958 elderly subjects in the study.
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 20.0 was used for conducting the statistical analysis of the data (IBM SPSS statistics for windows, version 20; IBM Corp, Armonk, NY, USA). Chi-square test was applied with 95% confidence interval (CI) to assess the association of various parameters with anemia among elderly population. The quantitative variables (Hb, 24-h dietary recall data, and BMI) were treated to yield frequencies, means, and standard deviations. Student's t-distribution was used for comparison of sociodemographic profile, dietary parameters between normal, mild, moderate, and severe anemic groups. All the data were tested at 5% level of significance. To identify possible risk factors associated with anemia, univariate linear regression analysis was performed with each factor.
Multivariate logistic regression analysis was conducted on the factors that were found to be associated with anemia to identify the independent determinants of anemia among the elderly population. Adjusted odds ratios (AORs) with 95% CIs were calculated. A P value of less than 0.05 was considered as statistically significant.
Results
The mean Hb level was 10.9 ± 1.9 g/dL among males and 9.9 ± 1.5 g/dL among females. We found that 92.1% of the elderly subjects were anemic. According to the grade of anemia, the prevalence of mild anemia was 26.6%, moderate anemia was 59.3%, and 6.2% of the elderly subjects were severely anemic [Figure 1].
Figure 1.
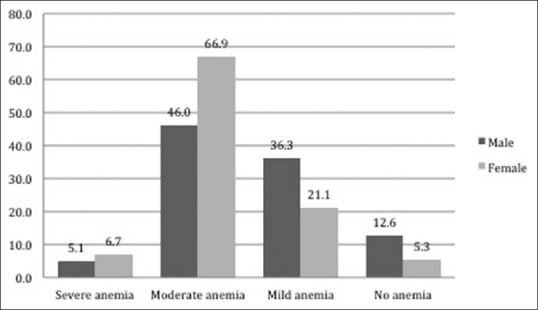
Distribution of elderly subjects according to severity of anemia
For the purpose of assessing the risk factors of anemia, data of the subjects with no anemia and mild anemia (males: Hb ≥13–11.0 g/dL, females: Hb ≥12–11.0 g/dL) were combined and compared with subjects with moderate and severe anemia (males and females: Hb ≤10.9 g/dL) on the basis of physiological outcomes of anemia during elderly.
Risk factors associated with anemia
Moderate and severe anemia was found to be significantly higher among (i) female subjects, (ii) single/divorced and separated subjects, (iii) unemployed, (iv) illiterates, (v) subjects using smoke producing fuel (all P < 0.001), (vi) subjects belonging to lower SES (P < 0.05), (vii) malnourished subjects, (viii) underweight subjects, (ix) subjects with self-reported hyperacidity, and (x) subjects who have never used any health facility (all P < 0.01) when compared with subjects with no anemia and mild anemia [Table 1].
Table 1.
Sociodemographic characteristics according to anemia status of geriatric subjects
| Parameters | Normal and mild anemia (n=331) (%) | Moderate and severe anemia (n=627) (%) | P |
|---|---|---|---|
| Age (years) | |||
| 60-70 | 198 (34.7) | 372 (65.3) | 0.941 |
| 70-80 | 95 (33.8) | 186 (66.2) | |
| ≥80 | 38 (35.5) | 69 (64.5) | |
| Gender | |||
| Male | 171 (48.9) | 179 (51.1) | <0.001† |
| Female | 160 (26.3) | 448 (73.7) | |
| Community | |||
| Others | 270 (34.8) | 505 (65.2) | 0.700 |
| SC/ST/OBC | 61 (33.3) | 122 (66.7) | |
| Religion | |||
| Christian/Muslim/Sikh | 8 (50.0) | 8 (50.0) | 0.190 |
| Hindu/Jain | 323 (34.3) | 619 (65.7) | |
| Marital status | |||
| Married | 222 (39.1) | 345 (60.9) | <0.001† |
| Single/divorced/separated | 109 (27.9) | 282 (72.1) | |
| Occupation | |||
| Skilled | 106 (46.5) | 122 (53.5) | <0.001† |
| Unskilled worker | 74 (34.3) | 142 (65.7) | |
| Unemployed | 151 (29.4) | 363 (70.6) | |
| Education | |||
| High school certificate and above | 71 (53.8) | 61 (46.2) | <0.001† |
| Middle school certificate | 34 (36.6) | 59 (63.4) | |
| Primary school certificate | 81 (34.8) | 152 (65.2) | |
| Illiterate | 145 (29.0) | 355 (71.0) | |
| Income (INR) | |||
| 13,874 and above | 50 (38.1) | 81 (61.8) | 0.131 |
| 9,249-13,873 | 28 (37.8) | 46 (62.2) | |
| 5547-9248 | 50 (35.2) | 92 (64.8) | |
| 1866-5546 | 116 (29.8) | 273 (70.2) | |
| ≤1865 | 87 (39.2) | 135 (60.8) | |
| SES | |||
| Lower SES | 220 (32.0) | 468 (68.0) | 0.024§ |
| Middle SES | 103 (40.7) | 150 (59.3) | |
| Upper SES | 8 (47.1) | 9 (52.9) | |
| Household fuel | |||
| Smokeless fuel (electricity/LPG) | 206 (40.5) | 302 (59.5) | <0.001† |
| Smoke-producing fuel (wood/coal) | 125 (27.8) | 325 (72.2) | |
| Dietary pattern | |||
| Nonvegetarian | 142 (32.7) | 292 (67.3) | 0.517 |
| Eggetarian | 17 (38.6) | 27 (61.4) | |
| Vegetarian | 172 (35.8) | 308 (64.2) | |
| Body mass index | |||
| ormal | 165 (35.0) | 306 (65.0) | 0.001‡ |
| Underweight | 63 (25.5) | 184 (74.5) | |
| Overweight/obese | 93 (42.5) | 126 (57.5) | |
| Mini-nutritional assessment | |||
| Normal nutritional status | 93 (44.3) | 117 (55.7) | 0.003§ |
| At risk of malnutrition | 189 (31.9) | 403 (68.1) | |
| Malnourished | 40 (29.8) | 94 (70.2) | |
| Presence of self-reported hyperacidity | |||
| No | 257 (37.7) | 424 (62.3) | 0.001‡ |
| Yes | 74 (26.7) | 203 (73.3) | |
| Utilization of healthcare facilities | |||
| Regular visit to the healthcare facility (at least once in a month) | 107 (38.6) | 170 (61.4) | 0.001‡ |
| Irregular visit to the healthcare facility (once in a year) | 216 (34.8) | 405 (65.2) | |
| Never visited healthcare facility | 8 (13.3) | 52 (86.7) | |
SES: socioeconomic status, †Signifies P<0.001, ‡Signifies P<0.01, §Signifies P<0.05
Dietary assessment
The mean dietary intake of nutrients, different food groups and their frequency of intake by elderly subjects stratified by anemia status is presented in Tables 2-4.
Table 2.
Mean dietary intake of nutrients among geriatric subjects according to anemia status
| Dietary intake | Male | P | Female | P | Percentage RDA (%) | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Normal and mild anemia (n=54) | Moderate and severe anemia (n=49) | Normal and mild anemia (n=38) | Moderate and severe anemia (n=101) | Normal and mild anemia (n=92) | Moderate and severe anemia (n=150) | ||||
| Nutrient | |||||||||
| Energy (kcal) | 1548.3±426.0 | 1462.7±391.2 | 0.292 | 1308.1±59.3 | 1370.8±36.4 | 0.369 | 81.2 | 84.9 | 0.220 |
| Protein (g) | 53.3±16.7 | 47.3±15.9 | 0.065 | 43.9±2.2 | 45.3±1.4 | 0.590 | 85.2 | 81.0 | 0.225 |
| Fat (g) | 46.4±15.4 | 45.7±16.7 | 0.834 | 40.3±1.9 | 44.6±1.4 | 0.096 | 192.2 | 208.6 | 0.066 |
| Zinc (mg) | 6.3±0.3 | 5.3±0.2 | 0.023§ | 5.1±1.8 | 5.2±1.9 | 0.739 | 51.7 | 49.2 | 0.288 |
| Calcium (mg) | 663.6 (548.8-981.3) | 716.4 (458.9-1055.5) | 0.911 | 659.5 (396.4-976.3) | 717.8 (411.2-942.5) | 0.936 | 112.9 | 105.1 | 0.316 |
| Thiamine (mg) | 1.5±0.1 | 1.3±0.1 | 0.070 | 1.2±0.3 | 1.2±0.4 | 0.831 | 121.7 | 114.3 | 0.145 |
| Riboflavin (mg) | 1.2±0.1 | 1.1±0.1 | 0.177 | 0.9±0.4 | 1.0±0.4 | 0.318 | 87.9 | 88.9 | 0.829 |
| Niacin (mg) | 11.4±0.6 | 10.0±0.5 | 0.065 | 9.5±3.3 | 9.3±3.0 | 0.770 | 74.7 | 72.0 | 0.421 |
| Iron (mg) | 15.8 (12.5-30.1) | 12.9 (9.4-16.2) | 0.003§ | 13.7 (9.8-27.0) | 12.1 (9.8-18.4) | 0.261 | 118.5 | 82.5 | <0.001† |
| Vitamin C (mg) | 72.75 (31.9-106.3) | 39.6 (28.9-82.8) | 0.137 | 63.9 (28.4-86.7) | 40.8 (23.2-67.1) | 0.119 | 197.5 | 142.3 | 0.005‡ |
| Folic acid (μg) | 179.5 (144.9-306.1) | 141.2 (96.8-209.6) | 0.023§ | 115.5 (91.7-177.2) | 142.3 (109.7-199.8) | 0.082 | 93.2 | 82.6 | 0.106 |
| Magnesium (mg) | 387.7±19.7 | 342.0±22.5 | 0.128 | 304.9±102.3 | 317.4±162.4 | 0.659 | 107.6 | 101.5 | 0.320 |
| Copper (mg) | 1.8±0.1 | 1.5±0.1 | 0.040§ | 1.5±0.9 | 1.4±0.6 | 0.386 | 84.3 | 73.8 | 0.013§ |
| Manganese | 5.5±0.3 | 4.4±0.3 | 0.011§ | 4.3±1.5 | 4.1±1.4 | 0.469 | 249.0 | 209.9 | 0.001‡ |
| Chromium (mg) | 0.03±0.0 | 0.03±0.0 | 0.877 | 0.03±0.0 | 0.03±0.0 | 0.095 | 66.1 | 58.0 | 0.107 |
| Food groups | |||||||||
| Cereals (g) | 200 (170-262) | 180 (160-230) | 0.133 | 187.5 (130-235) | 175 (130-205) | 0.653 | 82.5 | 78.3 | 0.280 |
| Pulses and legumes (g) | 40 (15-60) | 40 (15-55) | 0.549 | 32.5 (0-55) | 40 (20-55) | 0.399 | 65.5 | 56.0 | 0.035§ |
| Leafy vegetables (g) | 112.1±150.9 0 (0-230) | 63.7±124.8 0 (0-100) | 0.058 | 77.8±123.1 0 (0-120) | 62.4±98.1 0 (0-120) | 0.472 | 72.1 | 63.5 | 0.145 |
| Roots and tubers (g) | 60 (0-100) | 80 (60-140) | 0.036§ | 85 (30-120) | 80 (30-110) | 0.883 | |||
| Other vegetables (g) | 50.2±85.6 0 (0-65) | 44.4±70.7 0 (0-70) | 0.880 | 34.6±51.3 0 (0-60) | 42.4±53.3 25 (0-80) | 0.269 | |||
| Total vegetables (g) | 225 (110-320) | 150 (120-270) | 0.184 | 190 (100-260) | 160 (100-240) | 0.689 | |||
| Fruit (g) | 29.9±50.2 0 (0-40) | 35.1±60.3 0 (0-50) | 0.365 | 47.8±154.3 0 (0-15) | 30.5±66.5 0 (0-40) | 0.480 | 18.6 | 16.0 | 0.629 |
| Meat and poultry (g) | 40 (40-80) | 45 (40-80) | 0.897 | 40 (40-40) | 40 (40-90) | 0.527 | 80.9 | 147.8 | 0.120 |
| Milk and milk products (g) | 307.5 (180-440) | 300 (200-510) | 0.513 | 280 (150-450) | 290 (160-435) | 0.705 | 108.2 | 113.7 | 0.536 |
RDA: recommended dietary allowance, †Signifies P<0.001, ‡Signifies P<0.01, §Signifies P<0.05
Table 4.
Distribution of frequency of food group consumption among geriatric subjects over the past 1 year (n=242)
| Food groups | Normal and mild anemia (n=92) | Moderate and severe anemia (n=150) | P | ||||
|---|---|---|---|---|---|---|---|
| Regular (5-7 days a week)* | Irregular (1-4 days a week)* | Occasionally (less than once a week or never)* | Regular (5-7 days a week)* | Irregular (1-4 days a week)* | Occasionally (less than once a week or never)* | ||
| Cereals | 90 (97.8) | 2 | 0 | 150 (100) | 0 | 0 | 0.070 |
| Pulses and legumes | 61 (66.3) | 27 (29.3) | 4 | 91 (60.7) | 55 (36.7) | 4 | 0.432 |
| Vegetables | |||||||
| i) Leafy vegetables | 18 (19.6) | 66 (71.7) | 8 (8.7) | 23 (15.3) | 113 (75.3) | 14 (9.3) | 0.695 |
| ii) Roots and tubers | 58 (63.0) | 28 (30.4) | 6 (6.5) | 110 (73.3) | 35 (23.3) | 5 (3.3) | 0.197 |
| iii) Other vegetables | 24 (26.1) | 56 (60.9) | 12 (13.0) | 39 (26.0) | 94 (62.7) | 17 (11.3) | 0.919 |
| Fruits | 13 (14.1) | 54 (58.7) | 25 (27.2 | 26 (17.3) | 80 (53.3) | 44 (29.3) | 0.686 |
| Meat and poultry | 1 | 24 (26.1) | 67 (72.8) | 7 (4.7) | 40 (26.7) | 103 (68.7) | 0.308 |
| Milk and milk products | 61 (66.3) | 6 (6.5) | 25 (27.2) | 82 (54.7) | 25 (16.7) | 43 (28.7) | 0.047§ |
Figures in parenthesis denote percentages, §Signifies P<0.05
The RDA for intake of energy, protein, zinc, calcium, iron (in subjects with moderate and severe anemia), folic acid, niacin, riboflavin, copper, and chromium was not met among elderly subjects [Table 2]. Subjects with moderate and severe anemia had a significantly lower nutrient intake adequacy for iron (P < 0.001), vitamin C, copper, and manganese (all P < 0.01) when compared with subjects with no anemia and mild anemia [Table 2]. In addition, intake of pulses and legumes was found to be significantly lower among subjects with moderate and severe anemia when compared with subjects with no anemia and mild anemia [Table 2].
This study reported that the percentage of subjects who had adequate nutrient RDA (≥100%) for iron (22.7% vs. 37.0%, P < 0.01) and vitamin C (51.3% vs. 68.5%, P < 0.05) was lower amongst subjects with moderate and severe anemia when compared with subjects with no anemia and mild anemia [Table 3]. Approximately 29% of the subjects with moderate and severe anemia consumed less than 50% of the RDA for dietary iron. Inadequate consumption of vitamin C (50-99%) was found amongst 31.3% of the subjects with moderate and severe anemia.
Table 3.
Distribution of geriatric subjects according to anemia and nutrient and food group adequacy (n=242)
| Dietary intake | Normal and mild anemia (n=92) | Moderate and severe anemia (n=150) | P | ||||
|---|---|---|---|---|---|---|---|
| <50% RDA | 50%-99% RDA | ≥100% RDA | <50% RDA | 50%-99% RDA | ≥100% RDA | ||
| Nutrients | |||||||
| Energy (kcal) | 5 (5.4) | 71 (77.2) | 16 (17.4) | 11 (7.3) | 101 (67.3) | 38 (25.3) | 0.259 |
| Protein (g) | 5 (5.4) | 64 (69.6) | 23 (25.0) | 15 (10.0) | 104 (69.3) | 31 (20.7) | 0.383 |
| Fat (g) | 1 | 1 | 90 (97.8) | 0 | 7 (4.7) | 143 (95.3) | 0.144 |
| Zinc (mg) | 46 (50.0) | 44 (47.8) | 2 | 81 (54.0) | 67 (44.7) | 2 | 0.763 |
| Calcium (mg) | 11 (12.0) | 34 (37.0) | 47 (51.1) | 27 (18.0) | 51 (34.0) | 72 (48.0) | 0.454 |
| Thiamine (mg) | 0 | 24 (26.1) | 68 (73.9) | 4 (2.7) | 52 (34.7) | 94 (62.7) | 0.088 |
| Riboflavin (mg) | 12 (13.0) | 49 (53.3) | 31 (33.7) | 22 (14.7) | 75 (50.0) | 53 (35.3) | 0.874 |
| Niacin (mg) | 16 (17.4) | 61 (66.3) | 15 (16.3) | 26 (17.3) | 109 (72.7) | 15 (10.0) | 0.340 |
| Iron (mg) | 11 (12.0) | 47 (51.1) | 34 (37.0) | 44 (29.3) | 72 (48.0) | 34 (22.7) | 0.003‡ |
| Vitamin C (mg) | 15 (16.3) | 14 (15.2) | 63 (68.5) | 26 (17.3) | 47 (31.3) | 77 (51.3) | 0.012§ |
| Folic acid (μg) | 18 (19.6) | 48 (52.2) | 26 (28.3) | 34 (22.7) | 77 (51.3) | 39 (26.0) | 0.830 |
| Magnesium (mg) | 4 (4.3) | 37 (40.2) | 51 (55.4) | 13 (8.7) | 65 (43.3) | 72 (48.0) | 0.322 |
| Copper (mg) | 14 (15.2) | 54 (58.7) | 24 (26.1) | 32 (21.3) | 94 (62.7) | 24 (16.0) | 0.123 |
| Manganese | 1 | 3 | 88 (95.6) | 2 | 11 (7.3) | 137 (91.3) | 0.411 |
| Chromium (mg) | 44 (47.8) | 34 (37.0) | 14 (15.2) | 72 (48.0) | 65 (43.3) | 13 (8.7) | 0.252 |
| Food groups | |||||||
| Cereals (g) | 13 (14.1) | 57 (62.0) | 22 (23.9) | 24 (16.0) | 93 (62.0) | 33 (22.0) | 0.895 |
| Pulses and legumes (g) | 23 (35.9) | 33 (51.6) | 8 (12.5) | 51 (44.7) | 55 (48.2) | 8 (7.0) | 0.329 |
| Total vegetables (g) | 32 (34.8) | 38 (41.3) | 22 (23.9) | 66 (44.0) | 58 (38.7) | 26 (17.3) | 0.281 |
| Leafy vegetables | |||||||
| Roots and tubers | |||||||
| Other vegetables | |||||||
| Fruit (g) | 79 (85.9) | 12 (13.0) | 1 | 137 (91.3) | 10 (6.7) | 3 | 0.220 |
| Meat and poultry (g) | 6 (50.0) | 0 | 6 (50.0) | 2 (22.2) | 0 | 7 (77.8) | 0.195 |
| Milk and milk products (g) | 15 (16.5) | 30 (33.0) | 46 (50.5) | 26 (17.3) | 47 (31.3) | 77 (51.3) | 0.962 |
Figures in parenthesis denote percentages. ‡Signifies P<0.01, §Signifies P
Univariate and multivariate analyses
The risk factors for anemia among elderly subjects identified by multivariate logistic regression analysis were found to be (i) female gender, (ii) inadequate dietary intake of iron and vitamin C, and (iii) irregular visit to the healthcare facility [Table 5].
Table 5.
Univariate and multivariate logistic regression analyses of various risk factors of normal and mild anemia vs moderate and severe anemia among geriatric subjects (n=981)
| Parameters | Normal and mild anemia vs moderate and severe anemia | |||
|---|---|---|---|---|
| Unadjusted odds ratio (95% CI) | P | Adjusted odds ratio (95% CI) | P | |
| Age (years) | ||||
| 60-70 | 1.0 | - | ||
| 70-80 | 1.04 (0.77-1.41) | 0.789 | ||
| ≥80 | 0.97 (0.63-1.49) | 0.877 | ||
| Gender | ||||
| Male | 1.0 | - | 1.0 | - |
| Female | 2.67 (2.03-3.53) | <0.001 | 3.52 (1.83-6.75) | <0.001† |
| Community | ||||
| Others | 1.0 | - | ||
| SC/ST/OBC | 1.06 (0.76-1.50) | 0.700 | ||
| Education | ||||
| High school certificate and above | 1.0 | - | ||
| Middle school certificate | 2.02 (1.17-3.48) | 0.011 | ||
| Primary school certificate | 2.18 (1.41-3.38) | <0.001 | ||
| Illiterate | 2.85 (1.92-4.22) | <0.001 | ||
| Occupation | ||||
| Skilled | 1.0 | - | ||
| Unskilled worker | 1.67 (1.14-2.44) | 0.009 | ||
| Unemployed | 2.09 (1.51-2.88) | <0.001 | ||
| Income (INR) | ||||
| 13,874 and above | 1.0 | - | ||
| 9,249-13,873 | 1.01 (0.56-1.82) | 0.963 | ||
| 5547-9248 | 1.13 (0.69-1.86) | 0.613 | ||
| 1866-5546 | 0.96 (0.61-1.51) | 0.077 | ||
| ≤1865 | 1.41 (0.96-2.20) | 0.849 | ||
| SES | ||||
| Lower SES | 1.0 | - | ||
| Middle SES | 0.68 (0.51-0.92) | 0.013 | ||
| Upper SES | 0.53 (0.20-1.39) | 0.196 | ||
| Marital status | ||||
| Married | 1.0 | - | ||
| Single/divorced/separated | 1.66 (1.26-2.20) | <0.001 | ||
| Dietary pattern | ||||
| Nonvegetarian | 1.0 | - | ||
| Eggetarian | 0.77 (0.41-1.46) | 0.428 | ||
| Vegetarian | 0.87 (0.66-1.14) | 0.322 | ||
| Loss of appetite | ||||
| No | 1.0 | - | ||
| Yes | 1.19 (0.87-1.64) | 0.280 | ||
| Presence of self-reported fatigue | ||||
| No | 1.0 | - | ||
| Yes | 1.30 (0.96-1.76) | 0.089 | ||
| BMI | ||||
| Normal | 1.0 | - | 1.0 | - |
| Underweight | 1.57 (1.12-2.22) | 0.009 | 2.07 (0.96-4.48) | 0.064 |
| Overweight/obese | 0.73 (0.53-1.01) | 0.061 | 0.6 (0.28-1.32) | 0.207 |
| MNA | ||||
| Normal nutritional status | 1.0 | - | ||
| At risk of malnutrition | 1.69 (1.23-2.34) | 0.001 | ||
| Malnourished | 1.87 (1.18-2.96) | 0.008 | ||
| Utilization of healthcare facilities | ||||
| Regular visit to the healthcare facility (at least once in a month) | 1.0 | - | 1.0 | - |
| Irregular visit to the healthcare facility (once in a year) | 1.18 (0.88-1.58) | 0.268 | 3.15 (1.59-6.28) | 0.001‡ |
| Never visited healthcare facility | 4.09 (1.87-8.95) | <0.001 | 15.94 (1.77-143.7) | 0.014§ |
| Presence of self-reported hyperacidity | ||||
| No | 1.0 | - | ||
| Yes | 1.66 (1.22-2.26) | 0.001 | ||
| Household fuel | ||||
| Smokeless fuel | 1.0 | - | ||
| Smoke-producing fuel | 1.77 (1.35-2.33) | <0.001 | ||
| Adequacy of iron intake | ||||
| ≥100% RDA | 1.0 | - | 1.0 | - |
| 50%-99% RDA | 1.53 (0.84-2.79) | 0.164 | 1.26 (0.58-2.72) | 0.554 |
| <50% RDA | 4.0 (1.77-9.03) | 0.001 | 3.43 (1.20-9.77) | 0.021§ |
| Adequacy of vitamin C intake | ||||
| ≥100% RDA | 1.0 | - | 1.0 | - |
| 50%-99% RDA | 2.75 (1.39-5.44) | 0.004 | 2.50 (1.07-5.85) | 0.035§ |
| <50% RDA | 1.42 (0.69-2.91) | 0.340 | 0.86 (0.34-2.22 | 0.771 |
SES: socioeconomic status; CI: confidence interval; BMI: body mass index; MNA: mini-nutritional assessment; RDA: recommended dietary allowance. †Signifies P<0.001, ‡Signifies P<0.01, §Signifies P<0.05
The risk for developing moderate and severe anemia was found to be 3.5 times higher the 95% CI: 1.8–6.7, P < 0.001) among females when compared with males.
The odds of developing moderate and severe anemia were 3.4 (95% CI: 1.2–9.7, P < 0.05) times higher in subjects whose iron intake was less than 50% of the RDA and 2.5 (95% CI: 1.1–5.8, P < 0.05) times higher risk in subjects who did not meet the RDA for vitamin C (50%–99%) when compared with subjects with no anemia and mild anemia.
The risk for developing moderate and severe anemia was found to be higher among subjects who visited to the healthcare facility irregularly [(i) AOR for irregular visited healthcare facility (once a year) =3.1; 95% CI: 1.6–6.3, P < 0.01 and (ii) AOR for never visited healthcare facility = 15.9, P < 0.05].
Discussion
This study reported a high prevalence of anemia (92.1%) among elderly subjects residing in high-altitude region of rural Uttarakhand, India. Earlier studies conducted in plain regions of Puducherry (96%),[12] Haryana (88.7%,[5] 47.8%[1]), Karnataka (68.5%),[10] Maharashtra (67.1%),[9] Kerala (76%),[3] Delhi (57.8%),[8] Assam (45.5%),[7] Maharashtra (27.8%),[6] and Andhra Pradesh (20.6%)[11] have also reported high prevalence of anemia among elderly population based on Hb estimation. Similar findings of high burden of severe anemia of more than 6% was also reported in another study conducted in Uttarakhand among elderly population.[2]
Female elderly population had a 3.5 times higher risk for developing moderate and severe anemia possibly due to the lower dietary adequacy of hematopoietic nutrients such as iron, folic acid, copper, and vitamin C among them as reported in the previous publication.[26] In concordance with this finding, earlier studies have also reported higher prevalence of anemia among elderly women.[5,7,10]
Dietary intake and adequacy of nutrients such as iron, folic acid, vitamin C, zinc, copper, and manganese was found to be significantly lower among subjects with moderate and severe anemia when compared with subjects with normal and mild anemia status. In addition to these nutrients, lower intake of energy and protein, suggesting overall lower quantity of food consumption, may have also resulted in higher prevalence of anemia among underweight (P = 0.001) and malnourished (P = 0.003) elderly subjects.
Inadequate intake of iron and vitamin C was particularly found to be significant contributing factors in the causation of anemia among elderly subjects in this study. Subjects whose dietary iron intake was less than 50% of the RDA had 3.5 times higher risk of developing moderate and severe anemia especially iron deficiency anemia, which is the leading cause of anemia among the elderly subjects.[15] Similar results were obtained in studies conducted in Uganda, Africa, Santiago, and Chile as higher prevalence of anemia was observed among elderly with inadequate nutrient intake.[12,27] In 1996–1997, a nationwide survey conducted in India by the National Nutrition Monitoring Bureau reported that the diets of the elderly population were deficient in essential micronutrients such as iron, vitamin A, thiamine, riboflavin, folic acid, and niacin.[28] Inadequate and infrequent dietary intake of iron-rich food groups such as pulses and legumes, meat and poultry, and green leafy vegetables may have led to the high prevalence of anemia among the study subjects [Tables 3 and 4]. The risk of anemia is further increased by low bioavailability of iron (5%–8%) due to inhibitory factors such as phytates, tannins, and oxalates present in the Indian cereal-based diets.[24]
In addition, vitamin C deficiency in the diet was found to increase the risk of developing moderate and severe anemia by 2.5 times possibly due to its contributing role in increasing the iron absorption.[24]
Moderate and severe anemia was found to be significantly higher among elderly subjects belonging to low SES, educational status, and subjects who were unemployed or involved in unskilled work. Lack of knowledge about anemia and the importance of consumption of iron-rich foods, financial dependency, and monetary constraints to purchase micronutrient-rich foods such as pulses, fruits and meat, and poultry may have increased their risk to development of anemia.[5,7]
Subjects who irregularly or never visited healthcare facility had a 3 and 16 times, respectively, higher risk for developing anemia possibly due to poor diagnosis, management, and treatment of anemia or other diseases. Hyperacidity was found to be significantly associated with anemia among geriatric subjects, possibly due to impaired absorption of vitamin B12. Ineffective treatment of hyperacidity as reported in this study may have also increased their risk to anemia.
Use of smoke-producing fuel such as wood and coal were also found to be significantly associated with anemia among elderly subjects. Studies have reported that chronic exposure to smoke from smoke-producing fuel has been reported to increase the systemic inflammation and thereby compound the effects of physiological changes and iron deficiency on anemia.[29,30] These findings adds to the current knowledge of inflammation being the second most common cause of anemia after iron deficiency anemia.[15,16,31]
This study documented that anemia is a severe public health problem (prevalence of anemia ≥40%) in the rural areas of Uttarakhand State, India. Inadequate dietary intake of nutrients such as iron, folic acid, copper, and manganese was observed among the anemic subjects. The study highlights the need for primary care physicians to undertake regular testing and provision of treatment for anemia among the elderly population. In addition, primary care physicians must educate the elderly population about the adverse effects and risk factors of anemia and the importance of adequate intake of hematopoietic nutrients such as iron, folic acid, and vitamin C. This will help in reducing the overall burden of anemia among elderly population in India.
Financial support and sponsorship
The authors thank the ICMR for providing financial support for undertaking this study. The ICMR had no role in the design, analysis, or writing of this article.
Conflict of interest
There is no conflict of interest.
Ethics of human subject participation
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the ethics committee of the AIIMS.
References
- 1.Kant S, Kumar R, Malhotra S, Kaur R, Haldar P. Prevalence and determinants of anemia among adult males in a rural area of Haryana, India. J Epidemiol Glob Health. 2019;9:128–34. doi: 10.2991/jegh.k.190513.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee D, Dhar M, Pathania M, Ravikant, Rathaur VK. Mortality pattern of elderly patients at a tertiary care hospital: A study from Sub-Himalayan region, Uttarakhand, India. J Fam Med Prim Care. 2019;8:426–31. doi: 10.4103/jfmpc.jfmpc_416_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renjini B, Raj A, Krishnendu V, Rajiv M, Divyamol S, Rakesh P. High prevalence of malnutrition and anemia among elderly at old age homes in Kerala, India. J Med Allied Sci. 2019;1:1. [Google Scholar]
- 4.Bhasin A, Rao MY. Characteristics of anemia in elderly: A hospital based study in South India. Indian J Hematol Blood Transfus. 2011;27:26–32. doi: 10.1007/s12288-011-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur M, Gk K. Burden of anaemia in rural and urban Jat women in Haryana State, India. Mal J Nutr. 2009;15:175–84. [PubMed] [Google Scholar]
- 6.Malhotra VM, Kabra PR, Bhayya S, Malhotra R. Prevalence and correlates of anemia among elderly population of rural Nalgonda: A cross-sectional analytic study. Public Heal Rev Int J Public Heal Res. 2016;3:166–71. [Google Scholar]
- 7.Agarwalla R, Saikia A, Parashar M, Pathak R, Islam F. Assessment of prevalence of anemia in and its correlates among community-dwelling elderly of Assam, India: A cross-sectional study. Int J Nutr Pharmacol Neurol Dis. 2016;6:23. [Google Scholar]
- 8.Gonmei Z, Dwivedi S, Toteja GS, Singh K, Vikram NK, Bansal PG. Anemia and vitamin B12 deficiency in elderly. Asian J Pharm Clin Res. 2018;11:402. [Google Scholar]
- 9.Soni PN, Jawale RB, Soni SP, Pn S, Adv IJ, May M. Study of anemia in geriatric population: A hospital based study in Marathwada region, Maharashtra, India. Int J Adv Med. 2016;3:197–9. [Google Scholar]
- 10.Shrivastava SR, Hippargi SB, Ambali AP, Yelikar BR. Patterns of anemia in geriatric age Group. JKIMSU. 2013;2:77–81. [Google Scholar]
- 11.Vadakattu SS, Ponday LR, Nimmathota A, Nagalla B, Kondru DS, Undrajavarapu P, et al. Prevalence of nutritional anemia and hyperhomocysteinemia in urban elderly. Indian J Clin Biochem. 2019;34:330–5. doi: 10.1007/s12291-018-0756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudarshan BP, Chethan TK. A study to assess the prevalence of anemia and activities of daily living among elderly population residing in a South Indian rural community. Int J Community Med Public Health. 2016;3:437–41. [Google Scholar]
- 13.Gaskell H, Derry S, Andrew Moore R, McQuay HJ. Prevalence of anaemia in older persons: Systematic review. BMC Geriatr. 2008;8:1–8. doi: 10.1186/1471-2318-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. Worldwide Prevalence of Anaemia. WHO Report. 2005:51. [Google Scholar]
- 15.Patel KV, Guralnik JM. Epidemiology of anemia in older adults. Blood Disord Elder. 2007;45:11–20. [Google Scholar]
- 16.Mugisha JO, Baisley K, Asiki G, Seeley J, Kuper H. Prevalence, types, risk factors and clinical correlates of anaemia in older people in a rural Ugandan population. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0078394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steensma DP, Tefferi A. Anemia in the elderly: How should we define it, when does it matter, and what can be done? Mayo Clin Proc. 2007;82:958–66. doi: 10.4065/82.8.958. [DOI] [PubMed] [Google Scholar]
- 18.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119:327–34. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Chernetsky A, Sofer O, Rafael C, Ben-Israel J. Prevalence and etiology of anemia in an institutionalized geriatric population. Harefuah. 2002;141:591–4. 667. [PubMed] [Google Scholar]
- 20.Gupta A, Kapil U, Khandelwal R, Khenduja P, Sareen N, Pandey RM, et al. Prevalence and risk factors of underweight, overweight and obesity among a geriatric population living in a high-altitude region of rural Uttarakhand, India. Public Health Nutr. 2018;21:1904–11. doi: 10.1017/S1368980018000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar B, Dudala S, Rao A. Kuppuswamy's socio-economic status scale – A revision of economic parameter for 2012. Int J Res Dev Health. 2013;1:2–4. [Google Scholar]
- 22.WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 23.National Institute of Nutrition; Indian Council of Medical Research. Indian Food Composition Tables. 2017 [Google Scholar]
- 24.National Institute of Nutrition. Nutrient Requirements and Recommended Dietary Allowances for Indians. Indian Council of Medical Research. 2010 [Google Scholar]
- 25.Cook JD. Washington, DC, USA: Nutrition Foundation; 1985. International Nutritional Anemia Consultative Group. Measurements of iron status : A report of the International Nutritional Anemia Consultative Group (INACG) p. 78. [Google Scholar]
- 26.Gupta A, Khenduja P, Pandey RM, Sati HC, Sofi NY, Kapil U. Dietary intake of minerals, vitamins, and trace elements among geriatric population in India. Biol Trace Elem Res. 2017;180:28–38. doi: 10.1007/s12011-017-0972-8. [DOI] [PubMed] [Google Scholar]
- 27.Olivares M, Hertrampf E, Capurro M, Wegner D. Prevalence of anemia in elderly subjects living at home: Role of micronutrient deficiency and inflammation. Eur J Clin Nutr. 2000;54:834–9. doi: 10.1038/sj.ejcn.1601099. [DOI] [PubMed] [Google Scholar]
- 28.National Institute of Nutrition; Indian Council of Medical Research. National Nutrition Monitoring Bureau: Prevalence of Micronutrient Deficiencies: NNMB Technical Report No. 22. 2003 [Google Scholar]
- 29.Kyu HH, Georgiades K, Boyle MH. Biofuel smoke and child anemia in 29 developing countries: A multilevel analysis. Ann Epidemiol. 2010;20:811–7. doi: 10.1016/j.annepidem.2010.07.096. [DOI] [PubMed] [Google Scholar]
- 30.Page CM, Patel A, Hibberd PL. Does smoke from biomass fuel contribute to anemia in pregnant women in Nagpur, India? A cross-sectional study. PLoS One. 2015;10:e0127890. doi: 10.1371/journal.pone.0127890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. The Global Prevalence of Anaemia in 2011. 2015 [Google Scholar]


