Abstract
Introduction:
At present, laparoscopic surgery is a very common method, especially for the removal of the gallbladder, because pain and anxiety following surgery is a major problem in surgical operations. Various studies have demonstrated the effectiveness of gabapentin and dexmedetomidine in reducing pain intensity after surgery. The present study is aimed at examining the sedative and analgesic effects of gabapentin and dexmedetomidine in patients undergoing laparoscopic cholecystectomy.
Methods:
This was a double-blinded clinical trial involving 40 patients who were candidates for laparoscopic cholecystectomy. The patients were randomly allotted in two groups of dexmedetomidine (n = 20) and gabapentin (n = 20). Then, pain intensity based on the visual analog scale (VAS) and sedation level based on the Ramsay Sedation Scale (RSS) were measured at the curtained times. As the data were not normally distributed, the Mann–Whitney U test was used to analyze the data, and the significance level was set at 0.05.
Results:
Across the follow-up points, more reduction in pain intensity was observed in the dexmedetomidine group as compared with the gabapentin group. The available dissimilarities between these two groups in pain decrement at the recovery room and 3 h after being discharged from the recovery room were not significant (P ≥ 0.414). In addition, across all the time points, there was considerable growth in sedation in the dexmedetomidine group in comparison with the gabapentin group (P < 0.024). This finding indicated that dexmedetomidine was more effective than gabapentin in creating sedation.
Conclusion:
Compared with gabapentin, dexmedetomidine leads to more pain reduction after surgery and better sedation during and after surgery.
Keywords: Analgesic effects, dexmedetomidine, gabapentin, laparoscopic surgery, sedation
Introduction
In recent years, laparoscopic surgery has received a lot of attention because it is a relatively easy technique compared with other methods, preferred by the patients, and related to lower length of hospital stay and lower costs of hospitalization.[1] Laparoscopic surgery is accompanied by pain after surgery, therefore, reducing pain after surgery using medications with fewer side effects is important in the recovery and early ambulation of patients who receive laparoscopic surgery.[2]
On the other hand, anxiety before laparoscopic surgery has an important role in the general condition of patients after surgery. Previous studies have shown that about 13.5% of anxiety in the patients is because of the presurgery worries that may result from patients’ limited information on the process of surgery before receiving it.[3] Therefore, considerable efforts have been made by the researchers to minimize pain and worry in the patients. According to studies, the administration of sedatives is one of the methods used by the hospital personnel to reduce anxiety in the patients. However, some physicians believe that sedative drugs are associated with side effects, the most important of which are negative effects on the cardiovascular and respiratory systems (such as respiratory depression), apnea, and low blood pressure (BP). Therefore, the medical personnel uses different treatment regimens to reduce pain during and after surgery, including the use of narcotics, local anesthetics, and nonsteroidal antiinflammatory drugs. Narcotics are the main sedative drugs used to reduce pain during or after surgery, but they are associated with some side effects. Therefore, researchers are looking for alternative methods to effectively reduce pain in the patients and the use of narcotics.[4] In addition, researchers in this domain are searching for methods and tests useful in finding alternative drugs with fewer side effects. Gabapentin and dexmedetomidine are among the drugs considered to be effective in reducing pain after surgery.
Dexmedetomidine is a well-known sedative with pharmacological properties that make it less necessary to combine it with a supplement or another drug.[5,6] It creates sedative, analgesic, anxiolytic, and sympatholytic effects through activating the alpha-2 (α2) receptors in the brain and spinal cord.[7,8,9,10,11,12,13,14] Contrary to other sedatives, a patient who is administered dexmedetomidine regains consciousness by stimulation. It also leads to less respiratory depression compared with other sedatives.[7,14] Research evidence has shown that this drug protects the body (including heart, nerves, etc.) against ischemia and hypoxic injury.[7,15] Therefore, dexmedetomidine can be one of the best options for reducing pain after surgery.[7,8,9] Gabapentin was first introduced as an antiepileptic drug in 1993.[4,16,17,18] It is a chemical analog of the inhibitory neurotransmitter gamma aminobutyric acid (GABA) and also determined as adjuvant therapy for chronic pain syndromes.[4,16,19,20,21,22,23] Therefore, gabapentin is used to reduce pain in patients with different conditions, such as postherpetic neuralgia, postpoliomyelitis neuropathy, diabetic neuropathy, and reflex sympathetic dystrophy.[16,17,19,23,24] Previous studies have shown that using gabapentin as a prodrug can reduce pain[23,25] and gabapentin has selective effects on the stages of pain sensation in the central nervous system (CNS).[4,26] In addition, in more than 11 studies, gabapentin has been identified as an analgesic drug that can significantly reduce pain after surgery.[4,27] In recent years, gabapentin has been used as an anesthetic prodrug in some surgeries under general (GA) or local anesthesia (LA), as it has analgesic and sedative effects because of being a structural analog of GABA.[4] Although there is still some debate about the effects of gabapentin, it is generally accepted that it has positive effects on reducing pain after surgery and reduced use of narcotics.[4,20,23,28,29,30,31] The administration of gabapentin before surgery reduces anxiety and improves recovery after surgery.[29] Therefore, the present study aims to examine the sedative and analgesic effects of dexmedetomidine and gabapentin and determine which drug is more effective in reducing pain in the patients undergoing laparoscopic surgery.
Methods
Trial design
A double-blind randomized clinical trial (RCT) was conducted at a teaching hospital, affiliated to the Semnan University of Medical Sciences from May 19, 2016 to March 19, 2017. The study was approved by the Ethics Committee of Semnan University of Medical Science (ID: 1394.142) and registered at the Iranian Registry of Clinical Trials (IRCT2016011822794N2).
Participants
The patients who participated in this study were equally and randomly categorized into two groups of dexmedetomidine and gabapentin. The selection of participants was carried out based on the following criteria:
Aged 18–65 years
The American Society of Anesthesiologists (ASA) 1, 2
Candidate for laparoscopic gallbladder removal
Informed consent to participate in the study.
The exclusion criteria was as follows:
History of a systemic disease (high BP, diabetes, different kinds of collagen vascular disease, ischemic heart disease, ischemic renal disease, liver and digestive disorders, allergies, lack of access to patient's medical record, underlying disorders affecting consciousness [e.g. psychosis, Alzheimer disease, epilepsy, psychological disorders
Drug addiction
Alcohol abuse
Pregnancy
Convulsions
Refusing to participate in the study.
The participants were randomly allotted to the specified groups by means of random number table so that the patients who met the inclusion criteria were alternatively assigned to gabapentin or dexmedetomidine groups.
Procedure and interventions
An anesthesiologist prescribed the drugs and an anesthesia expert performed anesthesia evaluation 1 h before surgery. Gabapentin 300 mg capsules and 0.5 μg/kg and 0.6 μg/kg of dexmedetomidine were applied for intubation and maintaining anesthesia, respectively. All the patients underwent GA using the following medications:
Midazolam
Fentanyl
Thiopental
Lidocaine
Atracurium.
Anesthesia was maintained using isoflurane and Atracurium.
Outcome measure
Pain intensity after surgery was assessed based on the visual analog scale (VAS) before the induction of anesthesia and performing the surgery, 3 h after surgery, and while being discharged from the recovery room. The VAS assessed pain intensity on a 10-point scale ranging from 0 (no pain) to 10 (maximum pain experienced by the person). The level of sedation was evaluated in accordance with the Ramsay sedation scale (RSS) which include:
Anxiety, agitation, and being restless
Tranquil, oriented, and cooperative
Showing reaction to commands
Showing swift reaction to a loud auditory stimulus or light glabellar tap
A slow reaction to a loud auditory stimulus or light glabellar tap
Without any reactions to a loud auditory stimulus or light glabellar tap.
Sample size
Through considering 0.9 for 1-β value and 0.05 for α test level, the size of the sample should be consisting of 40 patients who were candidates for laparoscopic gallbladder removal under GA. The selection of participants was carried out randomly, which divided them into two equal groups of gabapentin and dexmedetomidine.
Statistical analysis
The data were analyzed using the Statistical Package for the Social Sciences (SPSS) software, V. 16. The data were presented as frequency, mean, and standard deviation (SD). Mann–Whitney U tests were used to compare the data among the study groups. The significance level was set at 0.05.
Results
Forty patients were equally divided into two groups of gabapentin 300 mg capsules and dexmedetomidine 0.5 μg/kg. Table 1 shows the age distribution of the patients in both groups. As you can see in the table, the mean age of participants was 24 ± 62 among the gabapentin group and 20 ± 61 among thedexmedetomidine group; there were not any notable differences of age between these two groups (P ≥ 0.185). There were two men (10%) and eighteen women (90%) in the gabapentin group, and six men (30%) and fourteen women (70%) in the dexmedetomidine group; this indicates a considerable difference between these two groups in the ration of women and men (P ≥ 0.021).
Table 1.
Demographic description of the sample
| Variables | n (%) | ||
|---|---|---|---|
| Age Groups | Gabapentin | 20-28.2 | 3 (15) |
| 28.3-36.4 | 6 (30) | ||
| 36.5-44.6 | 2 (10) | ||
| 44.7-52.8 | 4 (20) | ||
| 52.9-61 | 5 (25) | ||
| Dexmedetomidine | 24-31.6 | 3 (15) | |
| 31.7-39.2 | 3 (15) | ||
| 39.3-46.8 | 4 (20) | ||
| 46.9-54.4 | 5 (25) | ||
| 54.5-62 | 5 (25) | ||
| Sex | Gabapentin | Male | 2 (10) |
| Female | 18 (90) | ||
| Dexmedetomidine | Male | 6 (30) | |
| Female | 14 (70) | ||
Figure 1 presents the pain intensity distribution for both groups at the time points following surgery, based on the VAS and in a comparative manner. Based on the P value of the nonparametric Mann–Whitney U test, the observed differences between the two groups at recovery and 3 h after discharge from the recovery room are not statistically significant (P ≥ 0.414). Figure 1 also shows the pain intensity in the patients based on the VAS.
Figure 1.
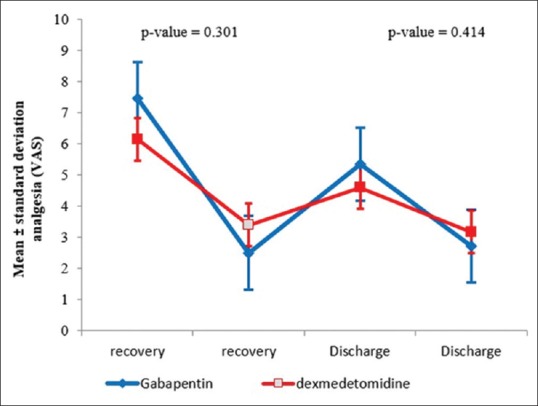
Distribution of changes in the postsurgery pain in the two groups
This means that the lowest pain intensity was observed in the dexmedetomidine group, and the smallest difference between the two groups was observed at recovery. Similarly, pain intensity 3 h after surgery was lower in the dexmedetomidine group. Consumption of antinausea medication (Metoclopramide) at recovery was higher in the gabapentin group; Metoclopramide was intravenously injected in nine patients (45%) in this group. Figure 2 shows the sedation levels based on the Ramsay Sedation Score, before anesthesia and surgery, 3 h after surgery and being discharged from the recovery room. As shown in Figure 2, the sedation means score at recovery is lower for the gabapentin group than the dexmedetomidine group. In other words, at all the follow-up time points after surgery, sedation is significantly higher in the dexmedetomidine group. In addition, the sedation level significantly decreases in both groups over time. According to the results of repeated measures ANOVA, this decrease is dependent on the time factor: as the drug loses its effectiveness over time, the sedation level also decreases. Additionally, in accordance with the P value of nonparametric Mann–Whitney U test, there is a considerable difference between these two groups in the score of sedation at recovery (P ≥ 0.024). In other words, dexmedetomidine is more effective in creating sedation than gabapentin. Moreover, in accordance with the Mann–Whitney U test results, the presence of a considerable diversity between these two groups within the score of sedation was seen 3 h after surgery (P ≥ 0.001).
Figure 2.

Changes in the sedation levels based on the Ramsay Sedation Score at the follow-up points after surgery
Discussion
Pain management after surgery is aimed at reducing the pain and discomfort in the patients with few side effects as possible and in a cost-effective way. The selection of the proper analgesia method may be influenced by the selected technique of anesthesia as much as it is affected by the place and duration of surgery. Pain control after surgery is very important for minimizing the duration of recovery and the length of hospital stay. According to the study results, there was more reduction in pain intensity at follow-up in the dexmedetomidine group than in the gabapentin group. In addition, at all the assessment points, the sedation level significantly increased in the dexmedetomidine group compared with the gabapentin group. Gabapentin applies its effects by affecting the α2δ-1 subunits from voltage-dependent calcium channels located in the dorsal root ganglia, dorsal horn neurons, and the upper parts of the spinal cord. One of the optional α2 adrenergic receptor agonists which have essential effects on the locus caeruleus is dexmedetomidine. In electroencephalography (EEG), dexmedetomidine leads to stimulations similar to natural sleep. In addition, it has sedative, anxiolytic, and analgesic effects without creating sleepiness.[32] In recent years, gabapentin has been used to reduce postsurgery pain in different kinds of surgeries, and many research studies have focused on the effects of this drug,[16,33] but dexmedetomidine seems to be one of the best alternatives to reduce postsurgery pain, because of its sedative and analgesic effects. No study has yet compared the effectiveness of these two drugs, therefore, here only the results of the studies related to this topic are presented.
Choi et al. examined the effects of dexmedetomidine, fentanyl, and remifentanil on hemodynamic stability during surgery, quality of sedation, and pain intensity after surgery. There were not any remarkable differences among these three groups in the postsurgery pain decrement, but the dexmedetomidine group was significantly different from the other two groups in terms of heart rate, BP, and sedation.[34] In another study by Sharma et al., with 100 candidates for laparoscopic cholecystectomy, the results indicated the significant effectiveness of dexmedetomidine in reducing the need for analgesic drugs after surgery (P = 0.001). They also found that postanaesthetic shivering was significantly lower in the dexmedetomidine group.[35] It should be noted that Yu et al. found a reduction in pain intensity after laparoscopic cholecystectomy and reduced the need for pethidine, because of prescribing ropivacaine together with postanaesthetic. This combination can also reduce the time of walking without walking aid and improve analgesia and quality of sleep in the first night following surgery, without increasing the side effects of surgery.[36]
In agreement with our results, Bakri et al. compared the administration of dexmedetomidine and dexamethasone before skin incision. The two groups in this study received either 1 μg/kg of dexmedetomidine or 8 mg dexamethasone. 6 h after surgery, the mean RSS was significantly higher in the dexmedetomidine group (4.2 ± 0.8).[37]
Our results are in line with those of Jung-kyn park et al. who found lower VAS scores in patients receiving dexmedetomidine one hour after surgery. They also found that the dexmedetomidine group needed fewer analgesics within 24 h after surgery (43.5 ± 18.5 mg) compared with the normal group (66.6 ± 39.6 mg).[38]
Gurbet et al. concluded that continuous intravenous dexmedetomidine infusion during the surgery of abdomen was impressive in the reduction of the severity of pain and the need for morphine consumption after surgery, without increasing the incidence of side effects. However, they did not report the sedation level in the patients who had received dexmedetomidine during surgery.[39] In line with our findings, Aho et al.[40] reported that patients undergoing laparoscopic tubal ligation, the application of dexmedetomidine could decrease the consumption of analgesic agents and the scores of pain. At a similar examination, Arian et al.[11] noted that through applying dexmedetomidine, a very significant decrease could be seen in postsurgery morphine consumption by 66%.[11]
Conclusion
It can be concluded that compared with gabapentin, dexmedetomidine consumption before surgery can lead to more reduction in the pain intensity after surgery, less need for narcotics, and more sedation in the patents, without leading to major side effects.
Ethics approval and consent to participate
The Ethics Committee of Semnan University of Medical Sciences approved the study and all of the participants signed the form of informed consent.
Author's contributions
AA and BB as the supervisors of this study played the main roles at the present study, they critically reviewed the paper and provided the final draft. A data collector, the main investigator, and the first draft provider were HZ, RB, and AM, respectively. RB and AM as advisors of this study contributed to the writing process. The statistical data were advised and analyzed by HZ. Finally, the provided manuscript was checked and approved by all authors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors of this study appreciate all of patients who participated within this study.
References
- 1.Moslemi TF, Rasouli S. Postoperative complications after ambulatory gynecologic laparoscopy: Comparison between three different techniques. Med J Tabriz Univ Med Sci. 2010;31:69–75. [Google Scholar]
- 2.Hassani V, imani F, Alimian M, Abdolalizadeh M. Comparing the analgesic effect pregabalin and gabapentin as premedication in laparoscopic procedures. JAP. 2012;2:30–7. [Google Scholar]
- 3.Nazari-Vanani R, Rahimi-Madiseh M, Drees F. Evaluation of preoperative anxiety and stress, and ways to modify it, the patients in Kashani hospital operating room in 2013. J Clin Nurs Midwifery. 2014;4:53–60. [Google Scholar]
- 4.Entezari S, Salarian S. Evaluation of gabapentine effects on anxiety and pain after ocular surgery under local anesthesia by tetracaine. Razi J Med Sci. 2010;6:7–13. [Google Scholar]
- 5.Aantaa R. Assessment of the sedative effects of dexmedetomidine, an α2-adrenoceptor agonist, with analysis of saccadic eye movements. Pharmacol Toxicol. 1991;68:394–8. doi: 10.1111/j.1600-0773.1991.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 6.Sumping ST, El-Moalem HE, Hsu YW, Young C, Somma J. Comparison of analgesic effects-dexmedetomidine against remifentanil. Anesthesiology. 2001;2001:B11–B11. [Google Scholar]
- 7.Afonso J, Reis F. Dexmedetomidine: Current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012;62:125–33. doi: 10.1016/S0034-7094(12)70110-1. [DOI] [PubMed] [Google Scholar]
- 8.Venn R, Grounds R. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: Patient and clinician perceptions. Br J Anaesth. 2001;87:684–90. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- 9.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: A review of clinical applications. Curr Opin Anesthesiol. 2008;21:457–61. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- 10.Salama AK, Abdallah NM. Multimodal analgesia with pregabalin and dexmedetomidine in morbidly obese patients undergoing laparoscopic sleeve gastrectomy: A prospective randomized double blind placebo controlled study. Egyptian J Anaesth. 2016;32:293–8. [Google Scholar]
- 11.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 12.Vaughns JD, Martin C, Nelson J, Nadler E, Quezado ZM. Dexmedetomidine as an adjuvant for perioperative pain management in adolescents undergoing bariatric surgery: An observational cohort study. J Pediatr Surg. 2017;52:1787–90. doi: 10.1016/j.jpedsurg.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Yang, YC, Meng QT, Pan X, Xia ZY, Chen XD. Dexmedetomidine produced analgesic effect via inhibition of HCN currents. Eur J Pharmacol. 2014;740:560–4. doi: 10.1016/j.ejphar.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, et al. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675–701. doi: 10.1053/j.gastro.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Panzer O, Moitra V, Sladen RN. Pharmacology of sedative-analgesic agents: Dexmedetomidine, remifentanil, ketamine, volatile anesthetics, and the role of peripheral mu antagonists. Anesthesiol Clin. 2011;29:587–605. doi: 10.1016/j.anclin.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007;104:1545–6. doi: 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed] [Google Scholar]
- 17.Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain–A systematic review of randomized controlled trials. Pain. 2006;126:91–101. doi: 10.1016/j.pain.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Rose M, Kam P. Gabapentin: Pharmacology and its use in pain management. Anaesthesia. 2002;57:451–62. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, et al. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 20.Rai AS, Khan JS, Dhaliwal J, Busse JW, Choi S, Devereaux PJ, et al. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials. J Plastic Reconstr Aesthet Surg. 2017;70:1317–28. doi: 10.1016/j.bjps.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 21.Seib RK, Paul JE. Preoperative gabapentin for postoperative analgesia: A meta-analysis. Can J Anesth. 2006;53:461. doi: 10.1007/BF03022618. [DOI] [PubMed] [Google Scholar]
- 22.Dahl JB, Mathiesen O, Møiniche S. ’ Protective premedication’: An option with gabapentin and related drugs? A review of gabapentin and pregabalin in the treatment of post-operative pain. Acta Anaesthesiol Scand. 2004;48:1130–6. doi: 10.1111/j.1399-6576.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 23.Mansour Ghanaee M, Fariba M, Soheila B, Reza ES, Maryam S, Misa NP, et al. The analgesic effects of gabapentin after total abdominal hysterectomy. Iranian J Obstet Gynecol Infertil. 2012;15:17–25. [Google Scholar]
- 24.Mao J, Chen LL. Gabapentin in pain management. Anesth Analg. 2000;91:680–7. doi: 10.1097/00000539-200009000-00034. [DOI] [PubMed] [Google Scholar]
- 25.Mathur A, Keshri U, Mehrotra S. A comparative evaluation of gabapentin and clonidine premedication on post-operative analgesia requirement following abdominal surgeries under general anesthesia. J Evol Med Dent Sci. 2014;3:9897–907. [Google Scholar]
- 26.Imani F, Rahimzadeh P, Faiz H, Nowruzina S, Shakeri A, Ghahremani M. Comparison of the post-caesarean analgesic effect of adding dexmedetomidine to paracetamol and ketorolac: A randomized clinical trial? Anesth Pain Med. 2018;8:e85311. doi: 10.5812/aapm.85311. doi: 10.5812/aapm.85311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathiesen O, Møiniche S, Dahl JB. Gabapentin and postoperative pain: A qualitative and quantitative systematic review, with focus on procedure. BMC Anesthesiol. 2007;7:6. doi: 10.1186/1471-2253-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabritius ML, Wetterslev J, Mathiesen O, Dahl JB. Dose-related beneficial and harmful effects of gabapentin in postoperative pain management-post hoc analyses from a systematic review with meta-analyses and trial sequential analyses. J Pain Res. 2017;10:2547–63. doi: 10.2147/JPR.S138519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahimzadeh P, Faiz SHR, Imani F, Derakhshan P, Amniati S. Comparative addition of dexmedetomidine and fentanyl to intrathecal bupivacaine in orthopedic procedure in lower limbs. BMC Anesthesiol. 2018;18:62. doi: 10.1186/s12871-018-0531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akhondzadeh R, Rashidi M, Gousheh M, Olapour A, Baniahmad A. The effect of adding dexmedetomidine as an adjuvant to lidocaine in forearm fracture surgeries by supraclavicular block procedure under ultrasound-guided. Anesth Pain Med. 2018;8:e74355. doi: 10.5812/aapm.74355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turan A, Memiş D, Karamanlioǧlu B, Yaǧiz R, Pamukçu Z, Yavuz E. The analgesic effects of gabapentin in monitored anesthesia care for ear-nose-throat surgery. Anesth Analg. 2004;99:375–8. doi: 10.1213/01.ANE.0000136646.11737.7B. [DOI] [PubMed] [Google Scholar]
- 32.Brull R, Macfarlane AJ, Chan VW. Spinal, Epidural, Caudal Anesthesia. 8th ed. Vol. 1. Philadelphia, PA: ELSEVIER Saunders publication; Millers Anesthesia; 2015. pp. 1709–10. [Google Scholar]
- 33.Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: A combined systematic review and meta-analysis. Anesth Analg. 2012;115:428–42. doi: 10.1213/ANE.0b013e318249d36e. [DOI] [PubMed] [Google Scholar]
- 34.Choi JW, Joo JD, Kim DW, In JH, Kwon SY, Seo K, Han D, et al. Comparison of an intraoperative infusion of dexmedetomidine, fentanyl, and remifentanil on perioperative hemodynamics, sedation quality, and postoperative pain control. J Korean Med Sci. 2016;31:1485–90. doi: 10.3346/jkms.2016.31.9.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma R, Gupta R, Choudhary R, Singh Bajwa SJ. Postoperative analgesia with intravenous paracetamol and dexmedetomidine in laparoscopic cholecystectomy surgeries: A prospective randomized comparative study. Int J Appl Basic Med Res. 2017;7:218–222. doi: 10.4103/ijabmr.IJABMR_25_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu JM, Sun H, Wu C, Dong CS, Lu Y, Zhang Y. The analgesic effect of ropivacaine combined with dexmedetomidine for incision infiltration after laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2016;26:449–54. doi: 10.1097/SLE.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 37.Bakri MH, Ismail EA, Ibrahim A. Comparison of dexmedetomidine and dexamethasone for prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Korean J Anesthesiol. 2015;68:254–60. doi: 10.4097/kjae.2015.68.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JK, Cheong SH, Lee KM, Lim SH, Lee JH, Cho K, et al. Does dexmedetomidine reduce postoperative pain after laparoscopic cholecystectomy with multimodal analgesia? Korean J Anesthesiol. 2012;63:436–40. doi: 10.4097/kjae.2012.63.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anesth. 2006;53:646. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]
- 40.Aho MS, Erkola OA, Scheinin H, Lehtinen AM, Korttila KT. Effect of intravenously administered dexmedetomidine on pain after laparoscopic tubal ligation. Anesth Analg. 1991;73:112–8. doi: 10.1213/00000539-199108000-00002. [DOI] [PubMed] [Google Scholar]


