Abstract
Introduction:
Liver biopsy is considered as the gold standard for diagnosis of chronic liver disease, yet liver biopsy is an invasive method that may be associated with complications. Therefore, non-invasive methods are needed to diagnose fibrosis. This study was conducted to compare liver stiffness measured by Shear-wave Elastography (SWE) to fibrosis in liver biopsy.
Method and Materials:
In this prospective study, 176 adult patients with chronic liver disease of different etiologies were included. All patients were evaluated using SWE and a liver biopsy. The diagnostic accuracy of SWE was evaluated using receiver operating characteristics (ROC) plots based on the degree of fibrosis (METAVIR score). SPSS software version 19 was used for statistical analysis and P < 0.05 considered significant.
Results:
There was a significant correlation between liver stiffness and fibrosis stage (ρ=0.939; P < 0.0001). The ROC curve AUC were 0.871, 0.895 and 0.937 for fibrosis stages F2, F3 and F4 respectively. The cutoff values were 8.6 kPa for F2, 10.7 kPa for F3, and 13.8 kPa for F4, with sensitivity and specificity of 81.76% and 77.01%, 90.20% and 78.40%, 89.53% and 94.38% respectively.
Conclusion:
The results of this study showed that liver SWE is an effective non-invasive method for assessing liver fibrosis in patients with chronic liver disease of different etiologies.
Keywords: Fibrosis stage, liver biopsy, liver stiffness, shear wave elastography
Introduction
Liver disease is one of the most common diseases in the world. Viral liver diseases are affecting about 500 million people in the world, and up to one million deaths occur annually from cirrhosis and hepatocellular carcinoma.[1] The prognosis and treatment outcome for these patients are related to liver fibrosis stage, especially in patients with hepatitis C.[1] Advanced stages of liver disease often result in fibrosis, which is characterized by the excessive accumulation of extracellular matrix proteins (ECM).[2,3] The normal hepatic structure was disorganized by fibrous scar, and the more fibrosis develops, resulting in the more hepatocytes damage, portal hypertension and impaired liver function, and ultimately liver failure and hepatocellular carcinoma.[4,5] Liver fibrosis is a response to chronic hepatic injury resulting from viral infections, excessive alcohol consumption, non-alcoholic steatohepatitis (NASH), viral hepatitis B (HBV) and C (HCV), autoimmune hepatitis, non-alcoholic fatty liver disease (NAFLD) and cholestatic liver disease. The condition is a reversible process; however, in developed stages, it can lead to cirrhosis and hepatocellular carcinoma.[6,7,8,9,10]
Chronic liver disease is a major public health problem, causing significant morbidity and mortality around the world. The timely diagnosis and determination of the fibrosis stage is necessary for the management and treatment of patients with chronic liver disease.[7,8,9] the liver biopsy and histological studies are the best way to diagnose chronic liver disease.[11,12,13] The process is an invasive diagnostic technique which carries a significant limitations, such as the high risk nature, high cost, complications (including bleeding, pneumothorax, hemothorax, and death), and low acceptability.[7,8,9] the rate of mortality following liver biopsy is 1 out of 10,000 in some studies.[7] In addition, liver biopsy may be limited by the size of the specimen obtained as well as sampling, intraobserver, and interobserver variability.[13] Also, in patients with advanced liver fibrosis, ascites, as well as coagulation disorders are present, which are two of the contraindications for Liver biopsy.[12] Therefore, noninvasive methods are essential for evaluating clinical fibrosis, and more recently researches has focused on the evaluation of non-invasive methods for the assessment of liver fibrosis.[11] The best replacement for biopsy in assessing liver fibrosis is the measurement of liver stiffness by elastography.[9] Ultrasound and MR are used for elasticity imaging.[14] These methods are capable of evaluating the differences in soft tissue elastic properties during mechanical stress.[1] Elastography is one of the latest technological advances in ultrasound that measures resilient and tissue consistency especially in soft tissues.[14] SWE is a technique based on the production of shear waves by tissue displacement induced by ultrasound beam or external pressure.[1] In this method, tissue properties are determined indirectly based on the shear wave speed).[15] This study aimed to compare the liver stiffness measured by SWE with liver biopsy results to determine liver Fibrosis.
Method and Materials
This is a prospective, analytical, epidemiological study that was conducted in patients with impaired hepatic enzymes referring to Golestan and Imam Khomeini hospitals in Ahvaz in 2018. After obtaining the ethics code (IR.AJUMS.REC.1397.179) from the ethics committee of Jundishapur University of Medical Sciences in Ahvaz, eligible patients were enrolled based on inclusion and exclusion criteria. All subjects were informed about the study protocol before entering the study, and written informed consent was obtained from each of them. All tests and us done for patients were paid by researchers and patients were not paid for participating in the study.
In this study, patients with impaired hepatic enzymes were subjected to biopsy and elastography to determine the severity of fibrosis and cirrhosis. The demographic and clinical characteristics of patients including age, gender, height, weight, BMI, underlying disease (diabetes, hypertension, ischemic heart disease) and the results of liver function tests including ALT, AST, AlkP, total and direct bilirubin and serological tests including ANA and ceruloplasmin were documented using a structured checklist designed to collect case information. All patients were also evaluated for viral markers including hepatitis B and C and HIV infection. The results for liver size, fatty liver, and its grade were also recorded. Finally, the results of biopsy and SWE ultrasound of each patient were compared using statistical methods.
Liver ultrasound guided biopsy was performed using a 17-gauge needle. The needle was placed in the middle line of the 9th and 11th Intercostal space and the minimum acceptable sample length was 15 mm. All samples were fixed using formalin and stored in paraffin. Standard histological staining techniques (Hematoxylin and eosin and Trichrome Reticulin) were used to analyze the pathology of the liver samples which, were examined by experienced pathologists who were unaware of the results of the liver imaging.
All patients who underwent liver biopsy during the study were recalled and SWE ultrasound were done by an experienced radiologist to determine the degree of stiffness of the liver. For this purpose, the French supersonic SWE ultrasound was used. The elastography was conducted with the manufacturer's instructions and standard principles. The elastography on the right lobe of the liver was carried out using the M-probe placed on the intercostal space (Trans-Thoracic view) and patients were in the dorsal decubitus position with full abduction of the right arm. By choosing a region of interest (ROI) of 15 mm in each SWE image, the mean and standard deviation of elasticity were shown within the ROI. For each patient, 5 values were calculated and the average of these values was recorded as the result of the liver stiffness in kilopascal units (kPa).
Two-dimensional SWE can be performed with one probe in all patients, independent of body weight, as the region of interest can be positioned manually at different depths in the liver. Ascites is not a limitation for ARFI US methods, enabling its performance in decompensated liver cirrhosis for prognostic reasons. The risk of overestimating liver stiffness values has been reported, with other confounding factors including alanine aminotransferase flares, congestive heart failure, excessive alcohol intake, and acute viral hepatitis.[16] Therefore, the patients with cofounding factors were re-evaluated.
The Ishak Score was used for grading fibrosis as follows[17]:
0 = No fibrosis
1 = Expansion of some portal areas with or without septa
2 = Expansion of most portal areas with or without septa
3 = Expansion of most portal areas with portal-portal bridging
4 = Expansion of most portal areas with portal-portal and portal-central bridging
5 = Bridging with occasional nodules
6 = Cirrhosis.
The stages of fibrosis were determined from 0 to 4 according to the METAVIR classification system[18]:
F0 = no fibrosis
F1 = portal fibrosis without septa
F2 = portal fibrosis and few septa
F3 = portal fibrosis with multiple septa and without cirrhosis
F4 = cirrhosis.xs
Inclusion criteria
Age over 18 years
Written consent to participate in the study
Elevated liver enzymes.
Exclusion criteria
Biopsy samples smaller than 15 mm
Hepatic transplant patients in last 6 months
Coagulation disorders and risk of bleeding following biopsy
Patients with biopsy results did not meet the required quality criteria.
Statistical analyses
SPSS software (SPSS Inc., Chicago, IL, USA) version 19 was used for statistical analysis. In order to compare the prediction of liver fibrosis grades with SWE, ROC analysis was performed and the AUC for the different stages of liver fibrosis was calculated. The Spearman correlation coefficient and Wilcoxon Signed Ranks test were used to analyze the relationship between the degrees of fibrosis in two methods. The significance level in the tests was considered to be P < 0.05.
Also, the accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of SWE in detecting liver fibrosis were calculated.
Results
In this study, 176 patients aged 18 to 84 (median = 36 years) participated. The basic characteristics of the subjects studied are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the subjects
| Variable | category | |
|---|---|---|
| Age (years) | 37.44±15.59 (18-84) | |
| Gender | Female | 113 (64.2) |
| Male | 63 (35.8) | |
| Co-Morbidity | Diabetes Mellitus | 54 (30.68) |
| Hypertension | 42 (23.9) | |
| Ischemic heart disease | 15 (8.52) | |
| Liver Function Test (LFT) | ALT (IU/L) | 130.09±179.03 (15-1600) |
| AST (IU/L) | 99.89±152.45 (15-1436) | |
| AlkP | 408.37±230.77 (149-1660) | |
| Billirubin (total) (mg/dL) | 1.85±2.74 (0.50 - 18.50) | |
| Billirubin (direct) (mg/dL) | 0.80±1.78 (0.06 - 10.20) | |
| Viral marker | HCVab (positive) | 5 (2.84) |
| HBsAg (positive) | 34 (19.32) | |
| HIVab (positive) | 6 (3.40) | |
| serology | ANA (positive) | 34 (19.32) |
| Ceruloplasmin | Positive: 3 (1.70) | |
| Decreased: 11 (6.25) | ||
| Span (Positive) | 42 (23.86) | |
| Fatty Liver | No | 60 (34.10) |
| Grade 1 | 39 (22.16) | |
| Grade 2 | 44 (25.00) | |
| Grade 3 | 33 (18.75) |
The mean of liver stiffness measured by elastography in patients was 10.57 ± 4.77 (range: 5.80 ± 31.00 kPa). There was a significant difference in the level of stiffness of the liver in different degrees of fibrosis based on the Ishak score system (P = 0.0001) Figure 1.
Figure 1.
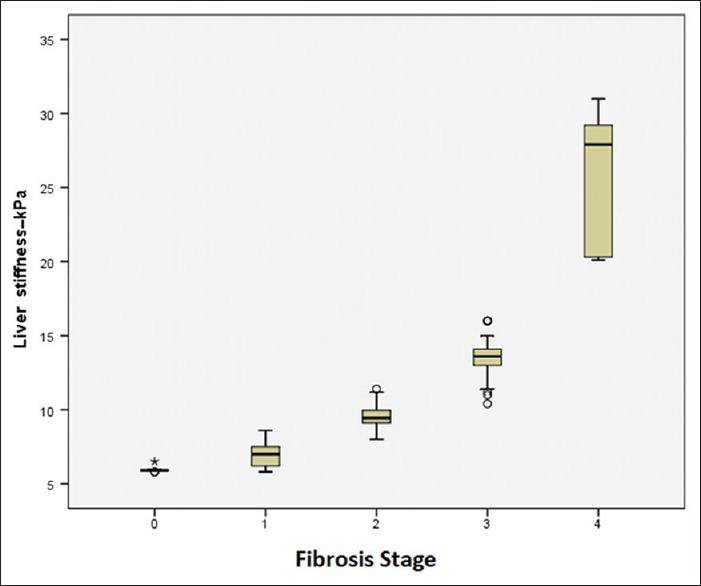
Liver stiffness in SWE based on degree of fibrosis
- Numbers are presented as mean ± standard deviation (range) or N (%).
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; AlkP: Alkaline phosphatase
The mean elasticity based on the METAVIR system in patients with F0 fibrosis was 5.95 ± 0.19 kPa, in the F1 fibrosis was 6.90 ± 0.76 KPa, F2 was 9.63 ± 0.74, at stage F3 was 13.62 ± 1.32 and in the F4 stage, it was 26.23 ± 4.34 kPa. Which showed a significant difference in the level of liver stiffness in different degrees of fibrosis (P = 0.0001).
The top and bottom lines of each box represent the first and third quartiles (25th and 75th percentiles). The middle line of each box are median and the lines of the upper and lower boxes are 5th and 95th percentiles.
The relationship between liver stiffness measured by SWE with different variables in degrees of fibrosis (based on METAVIR system) showed that there was a direct and significant correlation between liver stiffness with ALT (P = 0.009) And AST (P = 0.0001), Hypertension (P = 0.002), HBV (P = 0.030), fatty liver (P = 0.041) and fatty liver grade (P = 0.003) was observed.
In addition, assessment of the accuracy of elastography in determining of different degrees of liver fibrosis showed that there is a significant relationship between the SWE and liver biopsy (P = 0.0001). The ROC curve AUC were 0.871, 0.895 and 0.937 for fibrosis stages F2, F3 and F4 respectively. The cutoff values were 8.6 kPa for F2, 10.7 kPa for F3, and 13.8 kPa for F4, with sensitivity and specificity of 88.76% and 77.01%, 90.20% and 78.40%, 89.53% and 94.38%, respectively [Table 2].
Table 2.
Diagnostic value of SWE for detecting different degrees of liver fibrosis
| Parameter | Cut-off (kPa) | AUC | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|
| ≥ F2 | 8.8 | 0.871 | 88.76% | 77.01% | 82.95% | 79.80% | 87.01% |
| ≥ F3 | 11.5 | 0.895 | 90.20% | 78.40% | 84.66% | 82.18% | 88.00% |
| F4 | 18.1 | 0.937 | 89.53% | 94.38% | 92.00% | 93.90% | 90.32% |
Discussion
Prognosis and management of chronic liver diseases, including viral hepatitis B and C, are highly dependent on liver fibrosis,[1] therefore evaluating the degree of fibrosis is an important part of managing the patients with chronic liver disease.[19] Although, liver biopsy is the gold standard for liver fibrosis, the invasive nature and rare but high risk side effects of liver biopsy such as bleeding, pneumothorax, hemothorax, and death, increases the need of non-invasive test to evaluate liver fibrosis.[9,20] Over the past decade, ultrasound techniques have been widely developed and available to estimate the stage of liver fibrosis. These non-invasive methods are able to evaluate differences in the soft tissue elastic properties by inducing mechanical stress and examining the changes of tissues. The basis of SWE is the production of shear waves by tissue displacement induced by ultrasound beam or external pressure.[1] The aim of this study was to investigate the diagnostic accuracy of SWE as a non-invasive method to predict liver fibrosis by liver stiffness in patients with liver disease with different etiologies, compared to liver biopsy as a gold standard.
In the present study, the mean liver stiffness measured by SWE in patients with different stages of hepatic fibrosis (METAVIR score) was significantly different. The mean liver stiffness in patients with advanced fibrosis was higher than those with early stages of fibrosis. Therefore, SWE is an effective predictor for different degrees of liver fibrosis. In the study of Moustafa et al.[14] the mean liver stiffness measured by SWE in patients with different stages of liver fibrosis was significantly higher than healthy controls. Also, the stiffness of liver in patients with advanced fibrosis was significantly different from the early stage of fibrosis. Cassinotto et al.[8] also showed that liver stiffness measured by SWE is significantly related to the fibrosis stage. Another study by Guibal et al.[21] also showed that SWE is a non-invasive, accurate and repeatable method for assessing liver fibrosis, especially for the diagnosis of significant fibrosis in patients with various etiologies of liver fibrosis. In addition, the liver stiffness assessed by SWE had a direct and significant relationship with liver biopsy using the METAVIR system.
SWE is a new ultrasound technique in which the radiation force is produced by ultrasonic beam for induction of mechanical vibration. Reliability and repeatability of SWE is not operator dependent to produce appropriate vibration in tissue. Using the ultrasound tracking techniques and the Young's modulus formula, quantitative information can be expressed as an elasticity index in kilopascal units. Therefore, SWE can directly measure tissue stiffness and can be considered as a diagnostic measure for liver fibrosis.[22]
In our study, the ROC curve AUC were 0.871, 0.895, and 0.937 and sensitivity and specificity were 81.76% and 77.01%, 90.20% and 78.40%, 89.53% and 94.38% for fibrosis stages F2, F3, and F4, respectively. The most diagnostic accuracy was related to more advanced fibrosis (F3 and F4). These findings are consistent with other studies. For instance, Ferrailli et al.[23] and Verlinden et al.[24] and Zeng et al.[25] also had the highest level of diagnostic accuracy in stage F3 and higher of liver fibrosis. Furthermore meta-analysis findings by Li et al.[22] which showed that SWE had a high diagnostic accuracy in F3 (90% susceptibility and 81%) and F4 (sensitivity 87% and 88%) and the accuracy of the F2 stage was also relatively high (85% sensitivity and 81% specificity). The cutoff values for each stage of liver fibrosis had both sensitivity and specificity higher than 80%. Moustafa et al.[14] also showed that SWE had a high accuracy in the diagnosis of F3 (90% sensitivity and 81%) and F4 (sensitivity 87% and 88%) stages and moderate accuracy in F2 stage (85% sensitivity and 81% specificity). In addition, Guibal et al.[21] showed the sensitivity and specificity of SWE at different stages were 85.1% and 82.7% (F2), 88.9% and 90.3% (F3), 93.3% and 3.98% (F4). The ROC curve AUC in the F2, F3, and F4 stages was reported to be 0.904, 0.958 and 0.988 respectively. Also, there was a significant relationship between the percentage of fibrosis and liver stiffness measured by SWE. All these results suggest that SWE has a high diagnostic value for assessing liver fibrosis.
The findings of the present study are also consistent with the results of Ferraioli et al., Which showed that AUC of ROC for diagnosing mild fibrosis (F0-F1) and significant fibrosis (F2≤) were 0.84 and 0.92, respectively.[26] Bavu et al., showed that the ROC curve AUC of SWE for diagnosis of F2, F3 and F4 was 0.95, 0.96, and 0.97, respectively.[27] Another study by Ferraioli et al., in patients with HCV showed that the SWE AUC for F2, F3, and F4 stages (based on METAVIR scores) was 0.92, 0.98, and 0.94, respectively.[23] Cassinotto et al.[8] reported the good diagnostic power of supersonic SWE for the stages of fibrosis and AUC was between 0.86 and 0.89. It was also shown that the diagnostic accuracy of supersonic SWE for fibrosis stages are higher than ARFI and fibroscan methods.
Application and accuracy of the SWE for diagnosis of fibrosis stages defines by its cutoff. Until now, no single cutoff values have been provided for the detection of different stages of liver fibrosis, and various studies have reported different cutoff values.[23,25,26,27,28,29,30] The optimal cutoff in our study for the distinction between F0 and F1 had low sensitivity, specificity, and accuracy; hence, like other studies, we put F0 and F1 in same group together. In the present study, SWE performance for assessing fibrosis thresholds (METAVIR score) was similar to previous studies in patients with different etiologies,[8] or specific etiology of fibrosis, including HCV or HIV infections.[23,29] The mean cutoffs of SWE for F2, F3 and F4 was 8.8 kPa, 11.5 and 18.1 kPa, respectively. Guibal et al.[21] also reported similar cut-offs. These results are close to some studies, Verlinden et al.[24] reported cutoffs of 8.5, 10.4, and 11.3 for liver stiffness by SWE at stage F2, F3, and F4 respectively and Bavu et al.[27] showed that the SWE ROC CURVE AUC for diagnosis of F2, F3 were 9.12, 10.08, and 13.3 kPa, respectively. The cutoff obtained in our study for each stage of fibrosis in the SWE method was higher than some studies. In other studies, the cutoffs were between 0.8 to 7.1 kPa for significant fibrosis (F2≤), 7.9 to 8.9 kPa for severe fibrosis (F3≤), and 10.1 to 10.7 kPa for cirrhosis (F4).[8,23,29] The difference in device type and ultrasound technique and operator experience can be the reason for the difference in results. Also, the variability of the METAVIR score system may be the reason of these differences, especially for significant and severe fibrosis.[31] We also believe that METAVIR score may be associated with a higher cut-off rate for cirrhosis. Although each stage of fibrosis is associated with well-defined stage of fibrosis, but cirrhosis includes all stages of fibrosis without any upper limit. Therefore, the optimal cut-off for cirrhosis is likely to be very dependent to the study population, which goes up with increasing cirrhosis in the subjects, and when the subjects with severe cirrhosis are excluded, the liver's stiffness decreases As shown in the study by Ferrailli et al.[23] the mean stiffness in the cirrhosis phase was 15.6 kPa, however it was 25.8 kPa in study by Guibal et al.[21] and 13.8 kPa in our study.
The normal liver tissue contains 5.5 mg/g of collagen, while the cirrhotic liver contains more than 30 mg/g of collagen. Semi-quantitative histological scores, such as the METAVIR score, are not able to accurately estimate liver fibrosis. Therefore, a quantitative evaluation of liver fibrosis along with liver biopsy can reduce the variability of results and misclassification due to a semi-quantitative histologic staging.[21]
Although liver stiffness is used as a predictor of liver fibrosis, some of the confounder factors affecting the results of liver stiffness measurements have been reported. In this regard, controversial results have also been reported in some studies.[32,33,34]
The results of this study showed a direct and significant relationship between liver stiffness in different stages of fibrosis with ALT and AST levels. The highest levels of AST and ALT were seen in patients with F3 fibrosis and lowest levels were those with F0. These results are consistent with the findings of the study by Alempijevic et al.[9] In addition Kelleher et al. showed that AST > 60 IU/L had a significant relationship with hepatic fibrosis.[35] Verlinden et al.[24] reported a significant relationship between liver stiffness and AST levels. Moreover, in other studies, there was a significant relationship between AST and ALT levels with liver stiffness.[36,37] In contrast, two studies using SWE showed no association between AST and ALT and histological analysis with liver stiffness.[23,25]
Finally, although the present study evaluated the SWE diagnostic function at various stages of liver fibrosis in a relatively small population, the population included patients with different etiologies of liver fibrosis. Also, since the liver transplant has an effect on liver stiffness during the early weeks after transplantation.[38] we excluded patients who had liver transplant in less than 6 months.
Conclusion
The results of this study showed that the level of liver stiffness measured by SWE has a positive and significant correlation with the stage of liver fibrosis evaluated by biopsy. Therefore, liver SWE is an effective non-invasive method for assessing liver fibrosis, especially in patients with advanced stages (F3 and F4) of chronic liver disease of different etiologies. Therefore, SWE can be an alternative tool for liver biopsy in high risk patients and can be routinely used in the clinical setting for screening liver fibrosis in the general population during normal ultrasonography.
However, epidemiological studies on the use and performance of SWE in liver fibrosis are necessary. Further studies are needed to evaluate the ways in which liver stiffness and biological tests can be used together to improve the diagnostic performance of fibrosis.
Limitations
This study has some limitations that should be considered in future studies.
- In this study liver biopsy was used as a gold standard for evaluation of liver fibrosis. The diagnostic performance of biopsy is usually limited by some variables, including the sample, as well as the interobserver and intraobserver variations. To reduce these limitations, we exclude all patients with biopsy specimens of non-specific qualitative criteria. In addition, biopsies less than 15 mm were excluded from the study.
- The population studied was heterogeneous, and the number of patients with viral hepatitis B and C was low. Therefore, future studies should be carried out on populations with a specific etiology.
- Although the SWE has the ability to replicate interobserver and intraobserver, as reported in previous studies,[23,26,29] this ability was not investigated in our study.
- Low sample size and not using of other non-invasive modalities such as MR elastography, two-dimensional SWE and fibrosis serum markers (for example, APRI, ASPRI and FIB-4) were other limitations of this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ferraioli G, Parekh P, Levitov AB, Filice C. Shear wave elastography for evaluation of liver fibrosis. J Ultrasound Med. 2014;33:197–203. doi: 10.7863/ultra.33.2.197. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J, Zhai F, Cheng J, He Q, Luo J, Yang X, et al. Evaluating the significance of viscoelasticity in diagnosing early-stage liver fibrosis with transient elastography. PloS One. 2017;12:e0170073. doi: 10.1371/journal.pone.0170073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernatik T, Strobel D, Hahn EG, Becker D. Doppler measurements: A surrogate marker of liver fibrosis? Eur J Gastroenterol Hepatol. 2002;14:383–7. doi: 10.1097/00042737-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, et al. Elastography assessment of liver fibrosis: Society of radiologists in ultrasound consensus conference statement. Radiology. 2015;276:845–61. doi: 10.1148/radiol.2015150619. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy P, Wagner M, Castera L, Hong CW, Johnson CL, Sirlin CB, et al. Quantitative elastography methods in liver disease: Current evidence and future directions. Radiology. 2018;286:738–63. doi: 10.1148/radiol.2018170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Katwyk S, Coyle D, Cooper C, Pussegoda K, Cameron C, Skidmore B, et al. Transient elastography for the diagnosis of liver fibrosis: A systematic review of economic evaluations. Liver Int. 2017;37:851–61. doi: 10.1111/liv.13260. [DOI] [PubMed] [Google Scholar]
- 7.Hashemi SA, Alavian SM, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Caspian J Intern Med. 2016;7:242–52. [PMC free article] [PubMed] [Google Scholar]
- 8.Cassinotto C, Boursier J, de Ledinghen V, Lebigot J, Lapuyade B, Cales P, et al. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology (Baltimore, Md) 2016;63:1817–27. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 9.Alempijevic T, Zec S, Nikolic V, Veljkovic A, Stojanovic Z, Matovic V, et al. Doppler ultrasonography combined with transient elastography improves the non-invasive assessment of fibrosis in patients with chronic liver diseases. Med Ultrason. 2017;19:7–15. doi: 10.11152/mu-921. [DOI] [PubMed] [Google Scholar]
- 10.Aube C, Winkfield B, Oberti F, Vuillemin E, Rousselet MC, Caron C, et al. New Doppler ultrasound signs improve the non-invasive diagnosis of cirrhosis or severe liver fibrosis. Eur J Gastroenterol Hepatol. 2004;16:743–51. doi: 10.1097/01.meg.0000108357.41221.e5. [DOI] [PubMed] [Google Scholar]
- 11.Lutz HH, Schroeter B, Kroy DC, Neumann U, Trautwein C, Tischendorf JJ. Doppler ultrasound and transient elastography in liver transplant patients for noninvasive evaluation of liver fibrosis in comparison with histology: A prospective observational study. Dig Dis Sci. 2015;60:2825–31. doi: 10.1007/s10620-015-3682-0. [DOI] [PubMed] [Google Scholar]
- 12.Lutz HH, Gassler N, Tischendorf FW, Trautwein C, Tischendorf JJ. Doppler ultrasound of hepatic blood flow for noninvasive evaluation of liver fibrosis compared with liver biopsy and transient elastography. Dig Dis Sci. 2012;57:2222–30. doi: 10.1007/s10620-012-2153-0. [DOI] [PubMed] [Google Scholar]
- 13.Jeong WK, Lim HK, Lee HK, Jo JM, Kim Y. Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography (Seoul, Korea) 2014;33:149–60. doi: 10.14366/usg.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moustafa EF, Makhlouf N, Hassany SM, Helmy A, Nasr A, Othman M, et al. Non-invasive assessment of liver fibrosis in patients with hepatitis C: Shear wave elastography and colour Doppler velocity profile technique versus liver biopsy. Arab J Gastroenterol. 2017;18:6–12. doi: 10.1016/j.ajg.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: Principles and techniques. Diagn Interv Imaging. 2013;94:487–95. doi: 10.1016/j.diii.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, Huang S, Teng H, Wang P, Wu M, Zhou X, et al. Diagnostic accuracy of point shear wave elastography and transient elastography for staging hepatic fibrosis in patients with non-alcoholic fatty liver disease: A meta-analysis. BMJ Open. 2018;8:e021787. doi: 10.1136/bmjopen-2018-021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 18.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 19.Papastergiou V, Tsochatzis E, Burroughs AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol. 2012;25:218–31. [PMC free article] [PubMed] [Google Scholar]
- 20.Duarte-Rojo A, Altamirano JT, Feld JJ. Noninvasive markers of fibrosis: Key concepts for improving accuracy in daily clinical practice. Ann Hepatology. 2012;11:426–39. [PubMed] [Google Scholar]
- 21.Guibal A, Renosi G, Rode A, Scoazec JY, Guillaud O, Chardon L, et al. Shear wave elastography: An accurate technique to stage liver fibrosis in chronic liver diseases. Diagn Interv Imaging. 2016;97:91–9. doi: 10.1016/j.diii.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Zhang C, Li J, Huo H, Song D. Diagnostic accuracy of real-time shear wave elastography for staging of liver fibrosis: A meta-analysis. Med Sci. 2016;22:1349–59. doi: 10.12659/MSM.895662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: A pilot study. Hepatology. 2012;56:2125–33. doi: 10.1002/hep.25936. [DOI] [PubMed] [Google Scholar]
- 24.Verlinden W, Bourgeois S, Gigase P, Thienpont C, Vonghia L, Vanwolleghem T, et al. Liver fibrosis evaluation using real-time shear wave elastography in hepatitis C-monoinfected and human immunodeficiency virus/hepatitis C-coinfected patients. J Ultrasound Medicine. 2016;35:1299–308. doi: 10.7863/ultra.15.08066. [DOI] [PubMed] [Google Scholar]
- 25.Zeng J, Liu GJ, Huang ZP, Zheng J, Wu T, Zheng RQ, et al. Diagnostic accuracy of two-dimensional shear wave elastography for the non-invasive staging of hepatic fibrosis in chronic hepatitis B: A cohort study with internal validation. Eur Radiol. 2014;24:2572–81. doi: 10.1007/s00330-014-3292-9. [DOI] [PubMed] [Google Scholar]
- 26.Ferraioli G, Tinelli C, Zicchetti M, Above E, Poma G, Di Gregorio M, et al. Reproducibility of real-time shear wave elastography in the evaluation of liver elasticity. Eur J Radiol. 2012;81:3102–6. doi: 10.1016/j.ejrad.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: A clinical study on 113 hepatitis C virus patients. Ultrasound Med BIol. 2011;37:1361–73. doi: 10.1016/j.ultrasmedbio.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Poynard T, Munteanu M, Luckina E, Perazzo H, Ngo Y, Royer L, et al. Liver fibrosis evaluation using real-time shear wave elastography: Applicability and diagnostic performance using methods without a gold standard. J Hepatol. 2013;58:928–35. doi: 10.1016/j.jhep.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: Comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910–8. doi: 10.1148/radiol.13130128. [DOI] [PubMed] [Google Scholar]
- 30.Jeong JY, Kim TY, Sohn JH, Kim Y, Jeong WK, Oh Y-H, et al. Real time shear wave elastography in chronic liver diseases: Accuracy for predicting liver fibrosis, in comparison with serum markers. World J Gastroenterol. 2014;20:13920–9. doi: 10.3748/wjg.v20.i38.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dohan A, Guerrache Y, Boudiaf M, Gavini JP, Kaci R, Soyer P. Transjugular liver biopsy: Indications, technique and results. Diagn Interv Imaging. 2014;95:11–5. doi: 10.1016/j.diii.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Cho HJ, Seo YS, Lee KG, Hyun JJ, An H, Keum B, et al. Serum aminotransferase levels instead of etiology affects the accuracy of transient elastography in chronic viral hepatitis patients. J Gastroenterol Hepatol. 2011;26:492–500. doi: 10.1111/j.1440-1746.2010.06419.x. [DOI] [PubMed] [Google Scholar]
- 33.Fung J, Lai CL, Cheng C, Wu R, Wong DK, Yuen MF. Mild-to-moderate elevation of alanine aminotransferase increases liver stiffness measurement by transient elastography in patients with chronic hepatitis B. Am J Gastroenterol. 2011;106:492–6. doi: 10.1038/ajg.2010.463. [DOI] [PubMed] [Google Scholar]
- 34.Iijima H, Tada T, Kumada T, Kobayashi N, Yoshida M, Aoki T, et al. Comparison of liver stiffness assessment by transient elastography and shear wave elastography using six ultrasound devices. Hepatol Res. 2019;49:676–86. doi: 10.1111/hepr.13319. [DOI] [PubMed] [Google Scholar]
- 35.Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: The SHASTA index. J Hepatol. 2005;43:78–84. doi: 10.1016/j.jhep.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 37.Cales P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konate A, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–81. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- 38.Yoon JH, Lee JY, Woo HS, Yu MH, Lee ES, Joo I, et al. Shear wave elastography in the evaluation of rejection or recurrent hepatitis after liver transplantation. Eur Rad. 2013;23:1729–37. doi: 10.1007/s00330-012-2748-z. [DOI] [PubMed] [Google Scholar]


