Abstract
Micronutrients’ deficiency is a common phenomenon among a majority of the population residing in the low- and middle-income countries (LMICs) especially among women and children. Given the widespread prevalence of micronutrients’ deficiency in LMICs, iron-fortified foods could be of potential benefits for both the mother and the development of fetus. Present study aims to provide the evidence on the impact of iron fortification on hemoglobin (Hb) concentration during pregnancy and evaluates the specific maternal and pregnancy outcomes. We conducted systematic review by using search engines such as PubMed, Cochrane Library, Medline, EMbase, and secondary references. Meta-analyses were performed to calculate summary estimates on Hb during pregnancy, low birth weight (LBW), and preterm births. The weighted mean difference (WMD) and relative risk (RR) were calculated using random-effects models. Sources of heterogeneity were explored through meta-regression. Eight studies were included for the final analysis. The overall pooled estimate of Hb showed a significant increase in the fortification group compared with the control group [WMD = 4.45 g/L; 95% confidence interval (CI) = 2.73, 6.17 g/L; I2 = 83%, τ2 = 6.80, ρ <0.00001]. There has been a notable reduction in iron deficiency anemia (IDA) among pregnant women with substantial heterogeneity. Meta-regression suggests that the duration of feeding was positively associated with the effect size. Present review provides an evidence for the substantial benefits of iron fortification during pregnancy for reducing preterm births and risk of LBW. The safety, efficacy, and effective delivery of iron fortification need further research. Systematic review registration: PROSPERO International prospective register of systematic reviews – CRD42018116931.
Keywords: Birth outcomes, deficiency, iron fortification, meta-analysis, systematic review
Introduction
Iron deficiency is among the most common nutritional deficiencies globally,[1,2] and it affects more than 20% of the world's population, as iron imparts an important role in the formation of blood.[3,4] Iron deficiency often leads to anemia which is defined as having a blood hemoglobin (Hb) level below standard which usually results due to insufficient dietary intake of iron, poor utilization of iron from ingested food, or combination of both.[5] The incidence of iron deficiency anemia (IDA) is higher among infants, teenagers, and women in the childbearing age, in both developed countries and in low- and middle-income countries (LMICs).[6]
Nutrition status in infancy, before conception, or during pregnancy has a significant influence on the health status among pregnant women and also for fetus/newborn. Iron deficiency contributes to one of the highest prevalence of micronutrient deficiencies among pregnant women. Anemia affects approximately 41.8% of all pregnancies globally, with iron deficiency accounting for half of the cases.[7]
As per the World Health Organization (WHO) estimates, about 35%–75% (56% on average) of pregnant women in LMICs and around 20% of women from developed countries are anemic.[8] A large proportion of women in the reproductive age especially in the developing countries are exposed to the possibility of anemia during pregnancy[9,10] and projected to be at the risk of multiple micronutrients deficiencies.[11] They are prone to deficiencies such as iron, folic acid, iodine, zinc, vitamins A and D, riboflavin, B6, and B12, with the likelihood of adverse effects on childbearing mothers.[12,13,14]
Maternal IDA has adverse effects on birth outcomes including a greater risk of low birth weight (LBW) and preterm delivery.[15] Every year, more than 20 million infants are born with LBW worldwide.[16] About 3.6 million infants die during the neonatal period and two-thirds of these deaths occur in South Asia and sub-Saharan Africa.[17] More than one-third of child deaths are attributable to maternal and child under nutrition.[18] LBW (birth weight <2500 g), small-for-gestational age (SGA; whose birth weight lies below the 10th percentile for a particular gestational age), preterm birth (birth before the 37th week of gestation), stillbirth, perinatal (death from the 28th week of gestation through the first week after delivery), and neonatal mortality (death within 28 days of delivery) are notable adverse outcomes of nutritional deficiencies in pregnancy.[15,19,20]
The incidence of LBW in developing countries varies from 6% to 30%, and at least one-third of these are SGA, especially in settings with high rates of maternal under nutrition. Also, there are evidences of preterm births among them.[21,22,23,24]
The role of iron fortification in early nutrition programming for pregnant women needs to be emphasized for addressing the challenges faced due to inadequate awareness regarding the long-term probable health consequences of suboptimal iron intake during pregnancy. To overcome IDA, adequate disease control measures, dietary diversification, supplementation, and fortification in food have been adopted. Iron-fortified food is considered to be a long-term and sustainable strategy in the present scenario.[25] A systematic review and meta-analysis is essential for exploring the impact of iron-fortified foods during pregnancy and its effect on growth of fetus, to provide a basis for future research. We conducted a systematic review of trials on iron fortification by comparing with placebo-controlled trials. We also conducted a meta-regression analysis to explore sources of heterogeneity.
Methods
Search strategy and inclusion criteria
The steps in this process were conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines for meta-analysis.[24,26] We evaluated all the available literature and identified potentially relevant published trails on iron fortification during pregnancy and its effects on pregnancy outcomes, from January 1990 to December 2017. The databases searched included PubMed, Cochrane Systematic Reviews, World Health Organization Regional Databases, EMbase, Medline, ProQuest, selected databases (i.e. www.mdm.ca, www.eguidelines.co.uk, www.g-i-n.org, www.ncchta.org, www.evidance.nhs.uk, www.nice.org.uk, www.omni.ac.uk, www.shef.ac.uk, www.sign.ac.uk, and www.tripdatabase.com), and secondary references of relevant literature. We used mesh terms such as iron fortification during pregnancy and outcome, micronutrient fortification during pregnancy, food fortification on pregnancy, multiple micronutrient fortification during pregnancy, dual and triple fortification on pregnancy, and prevalence of iron fortification during pregnancy outcomes.
We applied the following inclusion criteria: (i) all prospective randomized, controlled trials (RCTs) evaluating iron alone and iron with multiple micronutrient fortification in women during pregnancy; (ii) only trials that compared an intervention group receiving iron fortification with a placebo control group; (iii) only trials that examined any of the following outcome: birth weight, LBW, preterm birth, SGA, perinatal deaths, and neonatal death; and (iv) RCTs evaluating change in Hb levels with intervention that included iron and multiple micronutrient fortification in comparison to control alone or iron alone were analyzed. We did not conduct subgroup analyses with respect to different dosages of iron fortification.
Data extraction and quality assessment
We have identified and read the title and abstracts of the studies in the web search and excluded the studies which were irrelevant. Furthermore, full text was retrieved for the included studies. Only the peer-reviewed published studies were included to avoid any publication bias. The extraction of data consisted of obtaining sample size, age, duration of intervention, levels of fortification, and measures of outcome in the intervention and control groups. The search, data extraction, and quality assessment were completed independently by two content experts according to the inclusion criteria and confirmed using recommended criteria for RCT.[27,28,29] Concealment of allocation was classified as “adequate,” “unclear,” “inadequate,” or “not used,” based on randomization, blinding, and reporting of withdrawals. Blinding was classified as “double blinding,” “single blinding,” “no blinding,” or “unclear.” In designs using two or more different intervention groups (different levels of fortification or administration regimens) and a single control group, the sample size of the control group was equally allotted to the number of intervention groups while retaining the same mean value for the change and its outcome measures. In reporting such designs, each intervention subgroup was analyzed separately. Thus, some studies contributed more than one intervention component with a single control group for the statistical analysis and resulted in a greater number of trials than the number of studies included.[27]
Statistical analysis
The major focus of the study was to look into the mean change in Hb concentration due to the consumption of iron-fortified foods. The effect size, which is the difference in means between the iron-fortified and the control groups, is referred to as the weighted mean difference (WMD) and was calculated for each included trials.[30] Similarly, the relative risk (RR) was calculated for selected trials.[31] Once an effect size was estimated for each trial, the overall effect of these results was assessed by Cochrane's Q statistic, which measures consistency among studies.[32,33] The Q test was computed under the assumption of homogeneity among the effect sizes and the statistic follows the Chi-square distribution with k-1 degrees of freedom (dfs), where k is the number of studies. Another method for quantifying the heterogeneity among the studies in a meta-analysis consists of estimating the variance (τ2) between studies. The parameter I2 quantifies the percentage of total variation in study estimates due to heterogeneity rather than sampling error.[33] The overall WMD and the RR of these results were measured for sampling error (homogeneous; τ2 = 0). A fixed-effects model was applied to obtain the pooled effect size with 95% confidence interval (CI). If heterogeneity exists (τ2 > 0) than in that case, a random-effects model is performed. The heterogeneity of results was depicted in the form of a forest plot.[27,32] Forest plot typically for each study represents a blob in the middle of the 95% CI that characterizes the RR estimates. The pooled or combined result of the WMD and RR in effect size is denoted by a diamond, with the width of 95% CIs for the combined data (Noble, Stephanie 2019). A vertical line indicates no effect and to differentiate between the trails which favor the intervention or the control group.
The forest plot also shows Cochrane's Q statistic, τ2, df, I2, Z, and ρ value. An I2 > 50% indicates a significant heterogeneity between the trials.[34] Publication bias was assessed with the funnel plot and Egger regression test. This is equivalent to a weighted, linear, ordinary least squares (OLS) regression model with standard error (SE) as a covariate.[35,36] If there are evidences of heterogeneity, a meta-regression approach is used to test the heterogeneity by relating study characteristics. The major confounders were identified, followed by a meta-analysis to estimate the net pooled effect size, after standardizing the effect of confounding variables. Statistical analyses were performed with Review Manager (RevMan) software version 5.3 and STATA 14.
Results
Trail flow
A total of 963 studies were identified, of which 880 were excluded initially as they were not fitting into our selection criteria. These excluded studies were not relevant to the purpose of the present analysis, as they were not RCTs. Further with the screening of the titles, 83 potentially relevant studies were included. Finally, 13 studies were selected for inclusion in meta-analysis. Out of the total included studies, two of each were eligible for LBW and preterm birth outcomes. Furthermore, we have evaluated the impact of intervention on major outcomes such as Hb concentrations during pregnancy, LBW, and preterm births [Figure 1].
Figure 1.
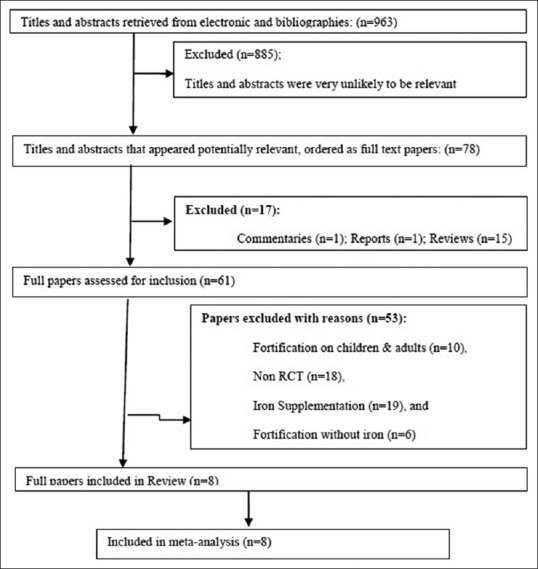
Flow diagram for inclusion in this study of randomized, controlled trials assessing the effect of iron-fortified foods
Study characteristics and data synthesis
The baseline characteristics of all included studies[36,37,38,39,40,41,42,43] such as the location, initial sample size, type of intervention, participants, duration of the study, age of the participants, levels of fortification, intervention composition, and limitations are taken into consideration for the analysis.
The results from the meta-analysis indicate that the mean change in Hb concentration was significantly higher in the group of mothers with iron fortification when compared with the control group (n = 3872; WMD = 4.45 g/L, 95% CI = 2.73, 6.17 g/L; I2 = 83%, τ2 = 6.80, ρ < 0.00001), as depicted in the forest plot [Figure 2].
Figure 2.
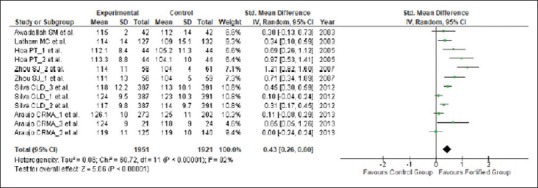
Effect of iron fortification on mean Hb concentration in comparison to no treatment or placebo control during pregnancy
Significant heterogeneity was observed for the mean Hb concentration among the included trials. All statistical tests of heterogeneity such as Q statistic (χ2 = 63.14, df = 11), which was more than df; τ2 greater than zero (τ2 = 6.80); and I2 greater than 50% (I2 = 83%), which were higher than the expected value, indicate toward heterogeneity in the included studies. We have used meta-regression analysis to detect the source of heterogeneity and indicated that the duration of the intake of fortified food was positively related to the effect size. The significant differences in the extent of improvement in Hb levels are shown in the forest plot [Figure 2] which may be due to the differences in the feeding regimens of iron-fortified foods during pregnancy. The funnel plot [Figure 3] was symmetrical, indicating that there was absence of any publication bias which was confirmed using Egger's weighted regression method (Egger test, ρ = 0.69).
Figure 3.
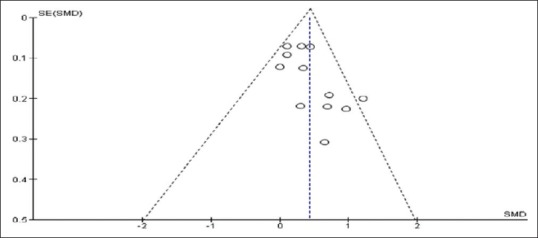
Funnel plot of the included trials that evaluated the effect of iron fortification on Hb concentration during pregnancy
There have been evidences of marginal reduction in the LWB and preterm births, as shown in Figure 4. There were instances of reductions in iron deficiency during pregnancy and in the fetus growth due to iron fortification. Although there was lack of heterogeneity among the groups in terms of LBW and preterm births, there has been a slight reduction in LBW by 18% (n = 2688; RR = 0.82; 95% CI = 0.65, 1.05; I2 0%) and in preterm births by 26% (n = 2811; RR = 0.74, 95% CI = 0.43, 1.27; I2 = 79%).
Figure 4.
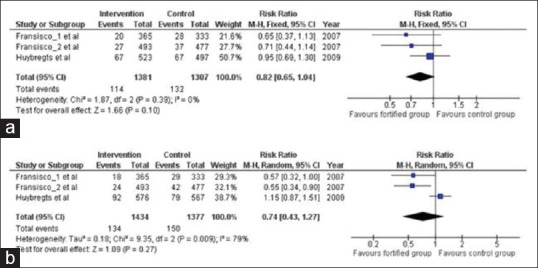
Impact of iron fortification on (a) LBW and (b) preterm birth in comparison to no intervention or placebo control
Discussion
The significance of maternal nutrition both before conception and during pregnancy is increasingly recognized among the scientific and medical community and among women not only for her own health but also for the growth of fetus and her child. Early nutritional programs during pregnancy and during lactation should be influenced by maternal nutrition requirements which are of critical concern. Increased requirement for iron, folic acid, and iodine before and during pregnancy is generally not available in well-balanced diet; rather, they can be provided by food fortification to address their requirements.
The current meta-analysis of eight studies consists of 12 trials which indicates that iron fortification was positively correlated with increased Hb concentration in intervention group when compared with the controls. However, there were evidences of heterogeneity in the study results across the trials. The intervention in terms of iron fortification varies among the trials; hence, the results should be interpreted cautiously.
We have used sequential statistical methods to verify the implementation of iron-fortified foods which improve the Hb concentration in the beneficiaries. While performing the meta-analysis, presence of any notable heterogeneity influences the results. We have used Q statistic, τ2, and I2, to test the heterogeneity and the results are shown in the form of a forest plot.[44] The results of included trials in the analysis show the mean difference in Hb concentration (4.45 g/L). It indicates that iron fortification improves the mean Hb level among the pregnant women.
Furthermore, the results indicate toward higher value of Q than the dfs. It shows the presence of heterogeneity and significant variations in the mean Hb concentration between the intervention and control groups due to the systematic underlying differences.[45,46] The value of τ2 also indicates that the variance of WMD and RR was more than zero, which confirms the presence of heterogeneity among the trials.[47,48]
Another measure of heterogeneity, the I2 statistic, which is a derivative of Q, was more than 50%, also suggests toward heterogeneity among the trials.[49,50] Due to the presence of heterogeneity among the included trials, random-effects meta-analysis was performed rather than fixed-effects meta-analysis.[51] The random-effects meta-analysis indicates a significant impact of iron fortification on Hb concentration,[27] LWB, and preterm births among the beneficiaries. It provides an evidence-based result, which suggests that iron-fortified food significantly reduces iron deficiency and anemia among pregnant women.[27,52] Furthermore, meta-analysis regression was performed to understand the net effect of iron-fortified food on Hb concentration and to explore the effect of confounders such as levels of fortification, age, and duration of intervention.[27,53] It was observed that feeding of fortification is an effective confounder.
Another important step in meta-analysis is the publication bias, which results in inflated estimates. There were evidences of heterogeneity among the trials, as few of the trials were not fit to the funnel.[54,55,56,57] Egger's regression test (Lindsay 1997) also suggests that there was absence of any publication bias in the study (ρ = 0.69). However, concerns have been raised in terms of benefits of iron supplementation and its probable side effects. Few studies have reported that there have been some adverse outcomes of the use of iron supplementation during pregnancy.[58,59,60]
Though studies have indicated that consumption of iron supplementation during pregnancy has a positive impact on maternal iron status at birth, they have not indicated toward reductions in characteristics that are correlated with maternal anemia, increased risk of preterm birth, and LBW.[15,20] In general, it is assumed that the iron status of the fetus during pregnancy is quite independent of maternal iron status, except if possibly when the infants are delivered by severely anemic women.[61,62,63]
This study emphasizes the need for primary care and nutritional awareness among the reproductive age group women regarding the short- and long-term benefits of an iron-fortified food. Low intake of iron is of significant clinical relevance among pregnant women as it results in adverse health outcomes for both the mother and the growth of the fetus. Healthcare professionals should become more proactive in supporting women during pregnancy to follow iron-balanced diet or selection of good sources of iron-fortified food. Availability of evidence-based information and target education of healthcare professionals need to be enhanced as there is lack of awareness regarding the potential benefits of iron-fortified food for women during pregnancy. Healthcare professionals also require adequate information and awareness regarding the role of iron-fortified food during pregnancy. The intake of iron-fortified food varies globally as each region has its own unique challenges and necessities. Therefore, the most appropriate feeding regimen needs to be designed according to the requirements of primary care among the reproductive age women. Similar principle is applied for public awareness programs due to cultural dissimilarities and varying levels of education.
Conclusion
Iron deficiency and IDA are prevalent among pregnant women. The extent to which iron deficiency affects maternal and neonatal health is uncertain. This study suggests that maternal IDA may be associated with adverse outcomes, including preterm birth, LBW, higher maternal mortality, and inferior neonatal health. A sizeable proportion of pregnant women are affected so as the development of fetus, due to these deficiencies especially among the LMICs such as India. There is a crucial need for formulating appropriate interventions and policies which are designed to prevent micronutrient deficiencies and in turn loss of human potential. Further research is required to explore the benefits of iron fortification on maternal and neonatal health outcomes during pregnancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lynch SR. The impact of iron fortification on nutritional anaemia. Best Pract Res Clin Haematol. 2005;18:333–46. doi: 10.1016/j.beha.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Fanzo J, Hawkes C, Udomkesmalee E, Afshin A, Allemandi L, Assery O, et al. 2018 Global Nutrition Report. 2019 [Google Scholar]
- 3.Bruner AB, Joffe A, Duggan AK, Casella JF, Brandt J. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet. 1996;348:992–6. doi: 10.1016/S0140-6736(96)02341-0. [DOI] [PubMed] [Google Scholar]
- 4.Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: The IRONOUT HF randomized clinical trial. JAMA. 2017;317:1958–66. doi: 10.1001/jama.2017.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breymann C, Dudenhausen JW. Iron Deficiency in Women. Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy. 2018:1–14. [Google Scholar]
- 6.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 7.Wright S, Earland D, Sakhuja S, Junkins A, Franklin S, Padilla L, et al. Anemia in pregnancy in Western Jamaica. Int J Womens Health. 2017;9:431–9. doi: 10.2147/IJWH.S129567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashmi AH, Solomon N, Lee SJ, Min AM, Gilder ME, Wiladphaingern J, et al. Nutrition in transition: Historical cohort analysis summarising trends in under- and over-nutrition among pregnant women in a marginalised population along the Thailand-Myanmar border from 1986 to 2016. Br J Nutr. 2019;121:1413–23. doi: 10.1017/S0007114519000758. [DOI] [PubMed] [Google Scholar]
- 9.Allen LH. Anemia and iron deficiency: Effects on pregnancy outcome. Am J Clin Nutr. 2000;71(5 Suppl):1280S–4S. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- 10.Breymann C, Milman N, Mezzacasa A, Bernard R, Dudenhausen J FER-ASAP investigators. Ferric carboxymaltose vs.oral iron in the treatment of pregnant women with iron deficiency anemia: An international, open-label, randomized controlled trial (FER-ASAP) J Perinat Med. 2017;45:443–53. doi: 10.1515/jpm-2016-0050. [DOI] [PubMed] [Google Scholar]
- 11.Kehoe SH, Dhurde V, Bhaise S, Kale R, Kumaran K, Gelli A, et al. Barriers and facilitators to fruit and vegetable consumption among rural Indian women of reproductive age. Food Nutr Bull. 2019;40:87–98. doi: 10.1177/0379572118816459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alaofè H, Burney J, Naylor R, Taren D. Prevalence of anaemia, deficiencies of iron and vitamin A and their determinants in rural women and young children: A cross-sectional study in Kalalé district of Northern Benin. Public Health Nutr. 2017;20:1203–13. doi: 10.1017/S1368980016003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Nutrition Landscape Information System (NLIS) country profile indicators: Interpretation guide. 2010 [Google Scholar]
- 14.Devakumar D, Fall CH, Sachdev HS, Margetts BM, Osmond C, Wells JC, et al. Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: A systematic review and meta-analysis. BMC Med. 2016;14:90. doi: 10.1186/s12916-016-0633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2017;4:CD004905. doi: 10.1002/14651858.CD004905.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathias CT, Mianda S, Ginindza TG. Evidence of the factors that influence the utilisation of Kangaroo Mother Care by parents with low-birth-weight infants in low-and middle-income countries (LMICs): A scoping review protocol. Syst Rev. 2018;7:55. doi: 10.1186/s13643-018-0714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellizzi S, Bassat Q, Ali MM, Sobel HL, Temmerman M. Effect of puerperal infections on early neonatal mortality: A secondary analysis of Six demographic and health surveys. PLoS One. 2017;12:e0170856. doi: 10.1371/journal.pone.0170856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y, Zou Z, Yang Z, Wang Z, Yang Y, Ma J, et al. Prevalence of excess body weight and underweight among 26 Chinese ethnic minority children and adolescents in 2014: A cross-sectional observational study. BMC Public Health. 2018;18:562. doi: 10.1186/s12889-018-5352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer MS. The epidemiology of adverse pregnancy outcomes: An overview. J Nutr. 2003;133:1592S–6S. doi: 10.1093/jn/133.5.1592S. [DOI] [PubMed] [Google Scholar]
- 20.Haider BA, Yakoob MY, Bhutta ZA. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health. 2011;11:S19. doi: 10.1186/1471-2458-11-S3-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flenady V. Keeling's Fetal and Neonatal Pathology. Springer, Cham; 2015. Epidemiology of fetal and neonatal death; pp. 141–64. [Google Scholar]
- 22.Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. Anew and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:e35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 23.Zeitlin J, Bonamy AE, Piedvache A, Cuttini M, Barros H, Van Reempts P, et al. Variation in term birthweight across European countries affects the prevalence of small for gestational age among very preterm infants. Acta Paediatr. 2017;106:1447–55. doi: 10.1111/apa.13899. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Internal Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 25.Gayer J, Smith G. Micronutrient fortification of food in Southeast Asia: Recommendations from an expert workshop. Nutrients. 2015;7:646–58. doi: 10.3390/nu7010646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Athe R, Vishnu Vardhana Rao M, Madhavan Nair K. Impact of iron-fortified foods on Hb concentration in children (<10 year): A systematic review and meta-analysis of randomized controlled trails. Public Health Nutr. 2014;17:579–86. doi: 10.1017/S1368980013000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke M, Oxman A. Cochrane Reviewers’ Handbook. Update Software. 2000 [Google Scholar]
- 28.Egger M, Davey-Smith G, Altman D, editors. Systematic Reviews in Health Care: Meta-analysis in Context. John Wiley and Sons; 2008. [Google Scholar]
- 29.Garrison MM, Christakis DA. A systematic review of treatments for infant colic. Pediatrics. 2000;106:184–90. [PubMed] [Google Scholar]
- 30.Mendu VVR, Nair KPM, Athe R. Systematic review and meta-analysis approach on vitamin A fortified foods and its effect on retinol concentration in under 10 year children. Clin Nutr ESPEN. 2019;30:126–30. doi: 10.1016/j.clnesp.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 32.Sterne JAC, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med. 1995;14:395–411. doi: 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- 36.Latham MC, Ash DM, Makola D, Tatala SR, Ndossi GD, Mehansho H. Efficacy trials of a micronutrient dietary supplement in schoolchildren and pregnant women in Tanzania. Food Nutr Bull. 2003;24(Suppl 2):S120–8. doi: 10.1177/15648265030244S209. [DOI] [PubMed] [Google Scholar]
- 37.Awadallah SM, Abu-Elteen KH, Elkarmi AZ, Qaraein SH, Salem NM, Mubarak MS. Maternal and cord blood serum levels of zinc, copper, and iron in healthy pregnant Jordanian women. J Trace Elements Exp Med. 2004;17:1–8. [Google Scholar]
- 38.Hoa PT, Khan NC, van Beusekom C, Gross R, Condo WL, Khoi HD. Milk fortified with iron or iron supplementation to improve nutritional status of pregnant women: An intervention trial from rural Vietnam. Food Nutr Bull. 2005;26:32–8. doi: 10.1177/156482650502600104. [DOI] [PubMed] [Google Scholar]
- 39.Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: Long-term follow-up of a randomized controlled trial–. Am J Clin Nutr. 2006;83:1112–7. doi: 10.1093/ajcn/83.5.1112. [DOI] [PubMed] [Google Scholar]
- 40.da Silva CL, Saunders C, Szarfarc SC, Fujimori E, da Veiga GV. Anaemia in pregnant women before and after the mandatory fortification of wheat and corn flours with iron. Public Health Nutr. 2012;15:1802–9. doi: 10.1017/S1368980012001206. [DOI] [PubMed] [Google Scholar]
- 41.Araújo CRMA, Uchimura TT, Fujimori E, Nishida FS, Veloso GBL, Szarfarc SC. Hemoglobin levels and prevalence of anemia in pregnant women assisted in primary health care services, before and after fortification of flour. Rev Brasil Epidemiol. 2013;16:535–45. doi: 10.1590/S1415-790X2013000200027. [DOI] [PubMed] [Google Scholar]
- 42.Mardones F, Urrutia M-T, Villarroel L, Rioseco A, Castillo O, Rozowski J, et al. Effects of a dairy product fortified with multiple micronutrients and omega-3 fatty acids on birth weight and gestation duration in pregnant Chilean women. Public Health Nutr. 2008;11:30–40. doi: 10.1017/S1368980007000110. [DOI] [PubMed] [Google Scholar]
- 43.Roberfroid D, Huybregts L, Lanou H, Henry MC, Meda N, Menten J, et al. Effects of maternal multiple micronutrient supplementation on fetal growth: A double-blind randomized controlled trial in rural Burkina Faso–. Am J Clin Nutr. 2008;88:1330–40. doi: 10.3945/ajcn.2008.26296. [DOI] [PubMed] [Google Scholar]
- 44.Deeks JJ, Higgins JPT, Altman DG Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions. 2019:241–84. [Google Scholar]
- 45.Qu XH, Huang XL, Xiong P, Zhu CY, Huang YL, Lu LG, et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol. 2010;16:886. doi: 10.3748/wjg.v16.i7.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jayasinghe C, Polson R, van Woerden HC, Wilson P. The effect of universal maternal antenatal iron supplementation on neurodevelopment in offspring: A systematic review and meta-analysis. BMC Pediatr. 2018;18:150. doi: 10.1186/s12887-018-1118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu E, Pimpin L, Shulkin M, Kranz S, Duggan CP, Mozaffarian D, et al. Effect of zinc supplementation on growth outcomes in children under 5 years of age. Nutrients. 2018;10:377. doi: 10.3390/nu10030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saso S, Panesar SS, Siow W, Athanasiou T. Evidence Synthesis in Healthcare. London: Springer; 2011. “Systematic review and meta-analysis in clinical practice.”; pp. 67–113. [Google Scholar]
- 49.Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;20:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 50.Anzures-Cabrera J, Higgins JP. Graphical displays for meta-analysis: An overview with suggestions for practice. Res Synth Methods. 2010;1:66–80. doi: 10.1002/jrsm.6. [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–20. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 52.Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet. 2013;382:452–77. doi: 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- 53.Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 54.Noble S. Reliability and Validity of fMRI Mapping Methods. PhD diss. Yale University; 2019. [Google Scholar]
- 55.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 56.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 57.Athe R, Mendu VVR, Krishnapillai MN. A meta-analysis combining parallel and cross-over randomized controlled trials to assess impact of iodine fortified foods on urinary iodine concentration among children. Asia Pac J Clin Nutr. 2015;24:496–503. doi: 10.6133/apjcn.2015.24.3.10. [DOI] [PubMed] [Google Scholar]
- 58.Allen LH. Pregnancy and iron deficiency: Unresolved issues. Nutr Rev. 1997;55:91–101. doi: 10.1111/j.1753-4887.1997.tb06460.x. [DOI] [PubMed] [Google Scholar]
- 59.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA International Child Development Steering Group. Child development: Risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–57. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 60.Verhoef H, Mwangi MN, Cerami C, Prentice AM. Antenatal iron supplementation and birth weight in conditions of high exposure to infectious diseases. BMC Med. 2019;17:146. doi: 10.1186/s12916-019-1375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cetin I, Bühling K, Demir C, Kortam A, Prescott SL, Yamashiro Y, et al. Impact of micronutrient status during pregnancy on early nutrition programming. Ann Nutr Metab. 2019;74:269–78. doi: 10.1159/000499698. [DOI] [PubMed] [Google Scholar]
- 62.Scholl TO, Reilly T. Anemia, iron and pregnancy outcome. J Nutr. 2000;130:443S–7S. doi: 10.1093/jn/130.2.443S. [DOI] [PubMed] [Google Scholar]
- 63.Dijkhuizen MA, Greffeille V, Roos N, Berger J, Wieringa FT. Interventions to improve micronutrient status of women of reproductive age in Southeast Asia: A narrative review on what works, what might work, and what doesn’t work. Matern Child Health J. 2019;23:18–28. doi: 10.1007/s10995-018-2637-4. [DOI] [PubMed] [Google Scholar]


