Abstract
Background:
Hepatic encephalopathy (HE) is an established clinical manifestation in chronic liver disease (CLD). It is associated with various factors including gastrointestinal bleed, constipation, and dyselectrolemia. Recently 25-hydroxyvitamin D (25-OHD) deficiency has been identified as one of the factors associated with the development of HE. The current study was aimed to assess the level of 25-OHD in patients with CLD and hepatic encephalopathy and the relationship between 25-OHD deficiency and hepatic encephalopathy.
Materials and Methods:
This cross-sectional study included 100 subjects of either sex between 18 and 60 years of age, diagnosed as CLD on the basis of ultrasonography with hepatic encephalopathy and 50 age, sex-matched CLD subjects without encephalopathy. Hemogram, hepatic and renal functions, serum electrolytes, coagulation profile, and serum 25-hydroxyvitamin D levels were recorded.
Results:
The baseline variables were matched for age, sex, hepatic and kidney function, and coagulation profiles. The hemoglobin (P = 0.002) and platelet count (P = 0.0003) were significantly lower in subjects with HE. The mean level of 25-OHD was significantly lower in subjects with HE as compared to the control group (25.62 ± 21.94 nmol/L vs 37.44 ± 18.61 nmol/L, P < 0.001). The mean 25-OHD level was 30.64 ± 21.64 nmol/L in grade 1 HE, 12.03 ± 11.05 nmol/L in grade 3 with P < 0.0001, and 18.8 ± 16.88 nmol/L in grade 4 with P < 0.0001 when compared to grade 1. Moderate and severe deficiency of 25-OHD level was significantly associated with higher grades of HE, i.e. grades 3 and 4 (P < 0.0001). There was a significant negative correlation between 25-OHD levels and worsening grades of hepatic encephalopathy (person's correlation coefficient r = -0.354; P = 0.0003).
Conclusion:
In this cohort of North Indian population, serum 25-OHD level was significantly lower in patients with CLD and HE. The levels of 25-OHD showed a significant negative correlation with hepatic encephalopathy.
Keywords: Chronic liver disease, encephalopathy, Vitamin D
Introduction
Patients with chronic liver disease (CLD) manifest various nutritional abnormalities which are associated with progression and severity of liver disease. The manifestation is believed to be due to alteration in absorption of nutrients and altered metabolism as well as alteration in synthetic function of liver. Increasing severity and progression of disease is also associated with worsening of these alterations.[1] A manifestation of progression of disease manifests in the form of hepatic encephalopathy (HE) which is described as a complex amalgam of neurological symptoms which include impairment of cognitive and motor functions. It is seen in up to 55% of chronic liver disease patients.[2] Pathogenesis of HE is multifactorial, and its exact mechanism has not been clearly understood yet. For decades the pathogenesis of HE has been believed to be related to high levels of ammonia, because of impaired detoxification in the liver, as a result of liver cirrhosis/failure or due to portosystemic shunts in patients with cirrhosis. The manifestation of hepatic encephalopathy is associated with various factors including upper gastrointestinal bleed, electrolyte imbalance, and spontaneous bacterial peritonitis.[3,4,5,6] One of the various factors which has been identified recently to be associated with hepatic encephalopathy is 25-hydroxyvitamin D deficiency.[7]
25-hydroxyvitamin D (25-OHD) is a group of fat-soluble Seco-steroids associated with regulation of calcium, magnesium, and phosphate metabolism and absorption. Apart from these activities, it is also associated with various other biological effects with roles suggested as an antiinflammatory and immunomodulatory agent.[8] Its role has been demonstrated to be associated with various neurological deficits like loss of cognitive function, dementia, and Alzheimer's disease.[9,10] 25-OHD deficiency is associated with a number of psychiatric conditions such as autistic spectrum disorder and data indicating 25-OHD deficiency in early life affects neuronal differentiation, axonal connectivity, and brain structure and function. Studies have shown that a low level of 25-hydroxyvitamin D level was associated with greater risk of cognitive impairment in older as well as younger adults by stepwise linear regression and Pearson's correlation.[11]
25-hydroxyvitamin D also regulates cell proliferation and differentiation, and has immunomodulatory, antiinflammatory, and antifibrotic properties. These effects are relevant in the pathogenesis and treatment of many causes of chronic liver disease.[12,13] 25-OHD deficiency is frequently present in chronic liver disease and may predict nonresponse to antiviral therapy in chronic hepatitis C. Small studies suggest that 25-OHD supplementation improves sustained viral response rates, while 1α-hydroxylase polymorphisms and 25-OHD -binding protein are also implicated in therapeutic outcomes.[14] 25-OHD deficiency also closely relates to the severity of nonalcoholic fatty liver disease (NAFLD) and is implicated in the pathogenesis of insulin resistance, a key factor in the development of NAFLD.[15,16]
Few studies have also demonstrated worsening of 25-OHD deficiency to be associated with progression of disease. Patients with hepatic encephalopathy have been shown to have significantly lower vitamin D levels than nonencephalopathic patients.[17] This correlation suggests that vitamin D deficiency may have an unrecognized role in the development of HE. However, there are very few studies which have examined the role of 25-OHD deficiency in hepatic encephalopathy. Thus, this study was planned to assess the levels of 25-OHD in patients with hepatic encephalopathy and to find its correlation with various grades of HE.
Materials and Methods
The primary objective of the study was to assess the level of 25-hydroxyvitamin D in patients with hepatic encephalopathy in cirrhosis of liver. The secondary objective of the study was to assess the correlation between 25-hydroxyvitamin D level and grades of hepatic encephalopathy (HE) in cirrhosis of liver.
The study was conducted in the Department of Medicine at a teaching tertiary care institute in North India. For the study, persons of either sex between 18 and 60 years of age and diagnosed as a case of chronic liver disease on the basis of ultrasonography with hepatic encephalopathy diagnosed based on West haven criteria were included.[1] Age and sex-matched individuals with chronic liver disease without hepatic encephalopathy constituted the control group.
Patients with chronic kidney disease, acute fulminant hepatic failure, recent onset cerebrovascular accidents, hepatocellular carcinoma, sepsis, and other causes of neurological deficits surgery were excluded.
The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000 and approved by the institutional review board, and informed consent was obtained from all study subjects.
Based on the previous studies, the sample size was calculated with 80% power and a 5% level of significance. We enrolled 100 patients in the study group and 50 in the control group.
A detailed record of clinical history and physical examination was done with special attention in the relevance of liver disease for all the enrolled subjects fulfilling the inclusion and exclusion criteria. All enrolled subjects underwent investigations for complete hemogram, liver function test, kidney function test, serum electrolytes, coagulation profile, and serum 25-hydroxyvitamin D levels. 25-hydroxyvitamin D level was measured by solid-phase ELISA, CALBIOTECH 25(OH) vitamin D ELISA. Results were expressed in nmol/L. Whole blood samples were collected by venipuncture and allowed to clot prior to processing.
25-hydroxyvitamin D level was defined as sufficient (>75 nmol/L), insufficient (50–75 nmol/L), mild deficiency (25–50 nmol/L), moderate deficiency (12.5-25 nmol/L), and severe deficiency (<12.5 nmol/L).[18]
Statistical analysis categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD and median. The normality of data was tested by the Kolmogorov-Smirnov test. Quantitative variables were compared using the unpaired t-test. Qualitative variables were compared using the Chi-square test. A correlation was assessed using Pearson's correlation coefficient. A P value of < 0. 05 was considered statistically significant. All analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
On the assessment of baseline variables, [Table 1] the age (years) of the enrolled subjects was comparable in the study and control groups (which included both covert and overt HE) (46.77 ± 13.76 vs 43.64 ± 13.89; P = 0.54). The control group had slightly higher hemoglobin levels (g%) (8.64 ± 1.76 vs 7076 ± 1.6; P = 0.002). The mean platelet levels (per cu mm) also showed higher numbers in control group (97846 ± 38379.99 vs 88622 ± 91194.23; P = 0.0003). The total leucocyte count, serum bilirubin, SGOT and SGPT, serum creatinine, PT, and INR were comparable in both groups. There were significantly higher levels of ALP (IU/L) in the study group (160.85 ± 48.65 vs 112.78 ± 38.65; P = 0.0001). There were significant differences in the electrolyte's levels in the study group as compared to the control group. Serum sodium levels (mEq/L) were 136.95 ± 5.1 in study against 134.6 ± 4.92 with P = 0.017. Similarly, serum potassium levels (mEq/L) were higher in study group (4.2 ± 0.77 vs 3.88 ± 0.65; P = 0.001). The levels of serum 25-OHD (nmol/L) were significantly lower in the study group (25.62 ± 21.94 vs 37.44 ± 18.61; P < 0.0001). The differences in the various baseline parameters between the study and the control group highlight the fact that there are variable factors associated with the development of hepatic encephalopathy.
Table 1.
Baseline variable in the study and control groups
| Baseline Variables | Study Group (n=100) | Control Group (n=50) | P |
|---|---|---|---|
| Age (years) | 46.77±13.76 | 43.64±13.89 | 0.054 |
| Hb (g%) | 7.76±1.6 | 8.64±1.76 | 0.002 |
| TLC (per cu mm) | 6462.4±3486.72 | 7114.8±3797.35 | 0.255 |
| Platelet (per cu mm) | 88622±91194.23 | 97846±38379.94 | 0.0003 |
| Bilirubin (mg/dl) | 3.6±2.32 | 3.86±3.77 | 0.06 |
| SGOT (IU/L) | 84.33±70.4 | 93.82±71.42 | 0.063 |
| SGPT (IU/L) | 73.98±49.73 | 75.66±37.48 | 0.172 |
| ALP (IU/L) | 160.85±48.65 | 112.78±38.65 | <0.0001 |
| Creatinine (mg/dl) | 1.76±0.75 | 1.55±0.61 | 0.158 |
| Sodium (mg/dl) | 136.95±5.12 | 134.6±4.92 | 0.017 |
| Potassium (mEq/L) | 4.2±0.77 | 3.88±0.65 | 0.001 |
| PT | 16.47±2.94 | 15.72±2.41 | 0.108 |
| INR | 1.7±0.49 | 1.81±0.45 | 0.096 |
| 25-Hydroxyvitamin D Level (nmol/L) | 25.62±21.94 | 37.44±18.61 | <0.0001 |
Data expressed in Mean±SD unless specified, n: number, Hb: Hemoglobin, TLC: Total leucocyte count, SGOT: Serum glutamic-oxaloacetic transaminase, SGPT: Serum glutamic-pyruvic transaminase, ALP: Alkaline phosphatase, PT: Prothrombin time, INR: international normalized ratio
Alcohol consumption was found to be the most common cause for chronic live disease (62% in study vs 58% in control). Viral hepatitis and unknown causes were responsible for CLD in remaining subjects. [Table 2]
Table 2.
Etiology of chronic liver disease in control and study groups
| Control n (%) | Study n (%) | P | |
|---|---|---|---|
| Etiology | |||
| Alcoholic cirrhosis | 29 (58) | 62 (62) | 0.939 |
| Hepatitis B virus | 09 (18) | 17 (17) | |
| Hepatitis C virus | 06 (12) | 09 (09) | |
| Unknown | 06 (12) | 12 (12) | |
n: number, HE: hepatic encephalopathy
Majority of the patients in the study group had overt hepatic encephalopathy (grade2,3,4 HE) seen in 80% of the patients. Covert HE (grade 1) comprised of the remaining 20% in the study group. [Table 3]
Table 3.
Various grades of hepatic encephalopathy in study group
| HE grades in study group | n (%) |
|---|---|
| 0 | 0 (0) |
| 1 | 20 (20) |
| 2 | 30 (30) |
| 3 | 20 (20) |
| 4 | 30 (30) |
n: number, HE: hepatic encephalopathy
Serum 25-OHD levels were found to be sufficient only in 4% in study and 2% in control groups. Severe deficiency was seen in 38% in study group as compared to only 6% in the control group [Table 4]. The mean levels of serum 25-hydroxyvitamin D in various grades of hepatic encephalopathy showed significant difference. Whereas the level was 30.64 ± 21.64 in grade 1 HE, it was as low as 12.03 ± 11.05 in grade 3 HE and 18.8 ± 16.88 in grade 4 with a P < 0.0001 [Table 5, Figure 1].
Table 4.
Serum 25-hydroxyvitamin D levels in study and control groups
| 25-Hydroxyvitamin D Level (nmol/L) | Control n (%) | Study n (%) | P |
|---|---|---|---|
| Severe deficiency | 03 (06) | 38 (38) | <0.0001 |
| Moderate deficiency | 11 (22) | 26 (26) | <0.0001 |
| Mild deficiency | 20 (40) | 17 (17) | <0.0001 |
| Insufficiency | 15 (30) | 15 (15) | <0.0001 |
| Sufficient | 01 (02) | 04 (04) | 0.121 |
n: number, HE: hepatic encephalopathy
Table 5.
Serum 25-hydroxyvitamin D levels in various grades of hepatic encephalopathy in study group
| 25-Hydroxyvitamin D Level (nmol/L) | HE grade 1 | HE grade 2 | HE grade 3 | HE grade 4 | P |
|---|---|---|---|---|---|
| Mean±SD | 30.64±21.64 | 38.16±24.83 | 12.03±11.05 | 18.8±16.88 | <.0001 |
| Median | 21.7 | 36.5 | 9.65 | 12.45 | |
| Min-Max | 5.6-78 | 3.49-105 | 3-52.5 | 3.97-66.4 | |
| Interquartile Range | 13-53 | 15.200-56 | 5.700-12.850 | 8-24 |
HE: Hepatic encephalopathy, SD: Standard deviation
Figure 1.
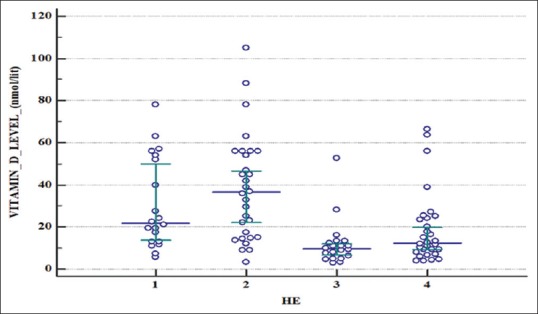
Whisker plot showing 25-hydroxyvitamin D levels in various grades of Hepatic encephalopathy
Correlation between serum 25-OHD level and hepatic encephalopathy was assessed by Pearson's correlation coefficient and a negative correlation was found. A lower level of serum 25-OHD was associated with increasing presence of hepatic encephalopathy (r = -0.354; P = 0.003) [Figure 2].
Figure 2.
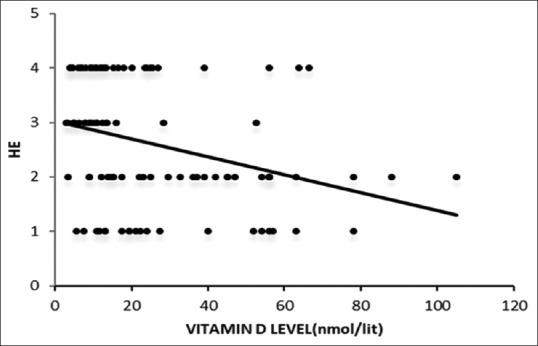
Shows correlation of serum 25-hydroxyvitamin D levels with hepatic encephalopathy (HE)
Discussion
The current study was aimed to study the levels of 25-hydroxyvitamin D in various grades of hepatic encephalopathy and compare it with a group of subjects with chronic liver disease without hepatic encephalopathy.
The mean age of the enrolled subjects in the study group was found to be 46.77 ± 13.76 years. The age of presentation is lower than those enrolled in an Australian cohort by Vidot et al. in which the mean age was 53 ± 8 years.[7] The lower age of presentation may be due to differences in the genetic, racial makeup as well tolerability and alcohol metabolism.
Males constituted the majority of the enrolled subjects which was 80% in the study group. The figure is similar to those enrolled in other studies, in which males constituted about 78% of the enrolled subjects.[7]
There was significant difference noted in the etiology of chronic liver disease in our study as compared to other studies. Whereas alcohol intake was associated with CLD in 62% in our study, viral hepatitis was found to be the major cause of CLD in the Australian cohort, which constituted almost 55% cases.[7] The difference may again be attributed to differences in the genetic, racial make-up as well tolerability and alcohol metabolism.
The mean level of serum 25-hydroxyvitamin D was found to be 25.62 ± 21.94 nmol/l in our study in comparison to 36 ± 15 nmol/l in the study in Australian Cohort.[7] The lower levels in our study may be due to racial difference, the difference in body structure, poor nutrition as well as genetic make of patients. However, the levels were significantly higher that the levels in a German cohort, in which the level of 25-hydroxyvitamin D was found to be 9.2 nmol/L.[17] 96% of the enrolled subjects in the study group were found to have some deficiency of 25-OHD in our study. The figure is similar to other studies by Vidot et al. where the figure was almost 98% and 92.4% in the study by Arteh et al. but in contrast to a figure of 61% in the study by Kubesch et al.[7,17,19] Moderate deficiency of 25-OHD level was found in 26% case in our study. This finding is in accordance with various other studies.[7] The percentage of subjects with severe deficiency of 25-OHD was slightly higher in our study and was seen in 38% of cases. The similar figure was 29.5% in the study by Arteh et al.[19]
Moderate and severe deficiency of 25-OHD was significantly associated with higher grades of hepatic encephalopathy, i.e. grade 3 and 4 with a P < 0.0001. The finding is similar to the Australian study where moderate deficiency was found in 49 Patients. 36 had grade 1-2 HE compared with 13 who did not have HE (P < 0.0001). There was significant correlation between severity of Vitamin D deficiency and severity of liver disease (P < 0.0001) and significant correlation between severity of liver disease and the presence of HE (P < 0.0001). Individuals are more likely to have Vitamin D deficiency if they had HE compared to patients without HE (P < 0.0001).[7]
The study also assessed the correlation of 25-OHD levels with various grades of hepatic encephalopathy. Our results showed a strong negative correlation between 25-OHD levels and worsening grades of hepatic encephalopathy (person's correlation coefficient r = -0.354; P = 0.0003). A similar result was observed in a study assessing the correlation between the 25-hydroxyvitamin D levels and Model for end-stage liver disease with P < 0.0001. A similar finding was demonstrated by Kubesch et al. on-25-hydroxyvitamin D deficiency and its association with hepatic decompensation and inflammation in patients with liver cirrhosis also concluded that 25-hydroxyvitamin D levels were inversely associated with liver cirrhosis-related systemic inflammation and the risk of hepatic decompensation.[17]
Although there seems a significant association between 25-OHD levels and hepatic encephalopathy, it still remains unclear whether there is a threshold level of 25-OHD beyond which there is a steady reduction in the cognitive performance of the subjects with CLD and hepatic encephalopathy. In addition, it does not prove the causal association of 25-OHD deficiency with HE. To demonstrate a causative relationship of 25-OHD with HE, it is necessary to examine the effects of 25-OHD supplementation on encephalopathy syndrome in patients with CLD.[20]
The current study is able to demonstrate significantly lower levels of 25-hydroxyvitamin D in patients with CLD and hepatic encephalopathy. The study also demonstrates a significant negative correlation between the levels of 25-OHD and presence of hepatic encephalopathy in CLD patients. The high prevalence of 25-OHD deficiency in patients with chronic liver disease can easily be addressed with necessary dietary modifications as well as through its supplementation. It paves the way for interventional studies on the effect of supplementation of 25-OHD in various grades of hepatic encephalopathy. Since the diagnostic facility for Vitamin D deficiency is widely available now and the primary contact of these individuals are with the primary care physicians, the role of primary care physicians becomes more important to identify such modifiable deficiencies which can be easily corrected if identified early.
The current study had certain limitations. Although the study showed a significant negative correlation between 25-OHD deficiency and HE, a causal effect can not be attributed. We did not use the severity scores of chronic liver disease to assess the relation between the severity of CLD with 25-OHD levels. A follow-up was also not done to assess for associated mortality. Cause effect and benefit of 25-OHD supplementation in HE after adjusting for various other causative factors may be undertaken in a larger group.
Conclusion
25-hydroxyvitamin D deficiency is very common in patients with chronic liver disease and there is a significant negative correlation between its level and worsening grades of hepatic encephalopathy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–35. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 2.Kalaitzakis E, Bjornsson E. Hepatic encephalopathy in patients with liver cirrhosis: Is there a role of malnutrition? World J Gastroenterol. 2008;14:3438–9. doi: 10.3748/wjg.14.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rai R, Saraswat VA, Dhiman RK. Gut microbiota: Its role in hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S29–36. doi: 10.1016/j.jceh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–13. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 5.Guevara M, Baccaro ME, Ríos J, Martín-Llahí M, Uriz J, Ruiz del Arbol L, et al. Risk factors for hepatic encephalopathy in patients with cirrhosis and refractory ascites: Relevance of serum sodium concentration. Liver Int. 2010;30:1137–42. doi: 10.1111/j.1478-3231.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 6.Bémeur C, Butterworth RF. Nutrition in the management of cirrhosis and its neurological complications. J Clin Exp Hepatol. 2014;4:141–50. doi: 10.1016/j.jceh.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidot H, Potter A, Cheng R, Allman-Farinelli M, Shackel N. Serum 25-hydroxyvitamin D deficiency and hepatic encephalopathy in chronic liver disease. World J Hepatol. 2017;9:510–8. doi: 10.4254/wjh.v9.i10.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borella E, Nesher G, Israeli E, Shoenfeld Y. Vitamin D: A new anti-infective agent? Ann N Y Acad Sci. 2014;1317:76–83. doi: 10.1111/nyas.12321. [DOI] [PubMed] [Google Scholar]
- 9.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: Preventing “D”ecline? Mol Aspects Med. 2008;29:415–22. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 11.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Iruzubieta P, Terán Á, Crespo J, Fábrega E. Vitamin D deficiency in chronic liver disease. World J Hepatol. 2014;6:901–15. doi: 10.4254/wjh.v6.i12.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trépo E, Ouziel R, Pradat P, Momozawa Y, Quertinmont E, Gervy C, et al. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J Hepatol. 2013;59:344–50. doi: 10.1016/j.jhep.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Jin CN, Chen JD, Sheng JF. Vitamin D deficiency in hepatitis C virus infection: What is old. what is new? Eur J Gastroenterol Hepatol. 2018;30:741–6. doi: 10.1097/MEG.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 15.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517–24. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Hariri M, Zohdi S. Effect of vitamin D on non-alcoholic fatty liver disease: A systematic review of randomized controlled clinical trials. Int J Prev Med. 2019;10:14. doi: 10.4103/ijpvm.IJPVM_499_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubesch A, Quenstedt L, Saleh M, Rüschenbaum S, Schwarzkopf K, Martinez Y, et al. Vitamin D deficiency is associated with hepatic decompensation and inflammation in patients with liver cirrhosis: A prospective cohort study. PLoS One. 2018;13:e0207162. doi: 10.1371/journal.pone.0207162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–8. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 20.Jha AK, Jha SK, Kumar A, Dayal VM, Jha SK. Effect of replenishment of Vitamin D on survival in patients with decompensated liver cirrhosis: A prospective study. World J Gastrointest Pathophysiol. 2017;8:133. doi: 10.4291/wjgp.v8.i3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]


