Abstract
Aim/Objectives:
This study was aimed to detect extended-spectrum beta-lactamase (ESBL) producing Pseudomonas species isolated from various clinical samples by phenotypic methods with their susceptibility testing.
Materials and Methods:
Hundred Pseudomonas isolates were taken from various clinical samples of patients attending outpatient department (OPD) and inpatient department (IPD). Antimicrobial susceptibility test and ESBL detection were assessed using CLSI guidelines on Mueller Hinton agar.
Results:
Out of 100 Pseudomonas isolates, 46 isolates were from female and 54 were from male patients. More cases of pseudomonal infection were in the age group between 46 and 60 years (34%), and 59% of Pseudomonas species were isolated from patients belongs to urban areas and the rest 41% were from rural. The isolates collected from OPD were 61% and rest 39% from IPD. Pseudomonas species showed maximum resistance to cephalosporin group of antibiotics and showed least resistance to imipenem, and showed 100% susceptibility to colistin. ESBL production was detected in 42% of total isolates.
Conclusion:
The present study highlights that the Pseudomonas species remains an important cause of nosocomial infections. ESBL producing Pseudomonas species continue to be an important organism causing life-threatening infections. Multidrug resistance was seen in most of the strains. Resistance is developing even to combination of ceftazidime clavulanic acid. Resistance is developing to last resort of antibiotic, i.e. imipenem also. This gives the alarming signal for the future, making the therapeutic options more difficult. Strict infection control measures are to be taken to contain this so-called water and soil organisms as Pseudomonas.
Keywords: Antimicrobial, ß-lactamases (ESBLs), pseudomonal infection, susceptibility test
Introduction
The worldwide emergence of multidrug-resistant bacterial strains in hospitals and community continues to be a problem of due scientific concern, especially infections caused by Pseudomonas species and Pseudomonas aeruginosa in particular.[1] Pseudomonas spp. are one of the most common gram-negative pathogens associated with infections and show a high level of intrinsic resistance to antimicrobial drugs and an ability to become even more drug-resistant.[2,3] These characteristics are caused by selective pressure of mutations in chromosomal genes that lead to production of extended-spectrum beta-lactamase (ESBL) and AmpC hyperexpression, repression or inactivation of oprD, and overexpression of efflux pumps.[3] In addition, Pseudomonas spp. are able to acquire other drug-resistant determinants by horizontal transfer of mobile genetic elements coding for class B carbapenemases (also called metallo-β-lactamases [MBLs]).[4] Pseudomonas spp. may also acquire resistance to antibiotics due to permeability barrier of the cell surface in the form of biofilm production. Biofilm-producing organisms are far more resistant to antimicrobial agents than organisms which do not. In some extreme cases, the concentrations of antimicrobials required to achieve bactericidal activity against adherent organisms can be three- to four-fold higher than for those bacteria which do not produce biofilm, depending on the species and drug combination.[5] The versatility and ability of Pseudomonas spp. to combine different resistance mechanisms have led to emergence of strains that are resistant to multiple antimicrobial drugs, which severely limits therapeutic options for treating infections.[6] This emphasizes the need for the detection of isolates that produce these enzymes to avoid therapeutic failures and nosocomial outbreaks.
Pseudomonas Aeruginosa
Pseudomonas aeruginosa is an important conditioned pathogen, which is known to cause nosocomial septicemia and burn infections, which are very difficult to treat particularly in the burn wards.[7,8] Extended spectrum beta-lactamases (ESBLs)-producing P. aeruginosa pose a serious threat as a healthcare-associated infections.[9] Since the discovery of ESBLs in 1983, their prevalence has been reported threateningly in many regions of the world and now comprises over three hundred variants.[10]
When inappropriate antimicrobial therapy is used to treat infections caused by ESBL-producing bacteria, failure in the clinical treatment will occur frequently.[11] P. aeruginosa infrequently found as part of the human microflora in healthy individuals is a gram-negative, non-glucose fermenter rod. It is the primary cause of ventilator-associated pneumonia in the intensive care unit.[12,13] In recent years, nosocomial infections caused by P. aeruginosa have been recognized as an acute problem in hospitals due to its intrinsic resistance to many antibiotic classes and its capacity to acquire practical resistance to all effective antibiotics.[14] Presently, genetic techniques supported by phenotypic tests enabled us to be informed of a detailed characteristic of strains isolated from clinical and environmental wards.[15] To determine the genetic relationship between clinical and environmental isolates of P. aeruginosa, there are some techniques such as restriction endonucleases analysis, multilocus enzyme electrophoresis, biotyping, pulsefield gel electrophoresis, and ribotyping. Among them, the PCR-ribotyping is an efficient technique used during last 10 years and based on the amplification of spacer regions or intervening sequences between 16S and 23S rDNA genes.[16] There are some previous studies in Iran about the role of P. aeruginosa in nosocomial infection; Khorvash et al. study indicates that there are good tools to detect P. aeruginosa nosocomial infection by procalcitonin (PCT) and C-reactive protein (CRP)[17] and Japoni et al. study showed that there are some difficulty in the treatment of multi-drug resistant (MDR) P. aeruginosa.[18]
Extended Spectrum Beta-Lactamase (Esbl)
ESBL are relatively group of plasmid-mediated enzymes. The first ESBL, an oxyimino beta-lactamase, was described in 1983 in Frankfurt, Germany.[19] These are enzymes that mediate resistance to extended-spectrum cephalosporins, carbapenums, and monobactums but do not affect cephamycins (cefoxitin and cefotetan) and carbapenems (meropenem or imipenem).[20] The overall prevalence of beta-lactamases and in particular the ESBL is rising with increased usage of higher generation cephalosporins and is presenting a major challenge.[21]
Types of extended-spectrum beta-lactamase production
TEM beta-lactamases (class A)
TEM-1 is the most commonly encountered beta-lactamase in Gram-negative bacteria. Up to 90% of ampicillin resistance in E. coli is due to the production of TEM-1.[22] The amino acid substitutions responsible for the ESBL phenotype cluster around the active site of the enzyme and change its configuration, allowing access to oxyimino-beta-lactam substrates. Based upon different combinations of changes, currently 140 TEM-type enzymes have been described. TEM-10, TEM-12, and TEM-26 are among the most common in the US.[23,24,25]
SHV beta-lactamases (class A)
SHV-1 shares 68% of its amino acids with TEM-1 and has a similar overall structure. The SHV-1 beta-lactamase is most commonly found in K. pneumoniae and is responsible for up to 20% of the plasmid-mediated ampicillin resistance in this species. ESBLs in this family also have amino acid changes around the active site, most commonly at positions 238 or 238 and 240. More than 60 SHV varieties are known. SHV-5 and SHV-12 are among the most common.[23]
CTX-M beta-lactamases (class A)
CTX-M-14, CTX-M-3, and CTX-M-2 are the most widespread. CTX-M-15 is currently (2006) the most widespread type in E. coli in the UK and is widely prevalent in the community.[26] An example of beta-lactamase CTX-M-15, along with ISEcp1, has been found to have recently transposed onto the chromosome of Klebsiella pneumoniae ATCC BAA-2146.[27]
OXA beta-lactamases (class D) were long recognized as a less common but also plasmid-mediated beta-lactamase variety that could hydrolyze oxacillin and related anti-staphylococcal penicillins. Other plasmid-mediated ESBLs, such as PER, VEB, GES, and IBC beta-lactamases, have been described but are uncommon and have been found mainly in P. aeruginosa and at a limited number of geographic sites.
Antibiotic resistance
Pseudomonas is a gram negative bacteria so are naturally resistant to penicillin and beta-lactam antibiotics, and mostly sensitive to piperacillin, imipenem, ticarcillin, or ciprofloxacin.[28] P. aeruginosa is increasingly recognized as an emerging opportunistic pathogen of clinical relevance. One of its most worrying characteristics is its low antibiotic susceptibility.[29] Some recent studies have shown that phenotypic resistance associated to biofilm formation or to the emergence of small-colony-variants may be important in the response of P. aeruginosa populations to antibiotic treatment.[30]
Aim and Objectives
Aim: To study the ESBL production among clinical isolates of Pseudomonas spp. by phenotypic methods.
Objectives:
To isolate Pseudomonas spp. from various clinical samples
To identify the antimicrobial susceptibility of Pseudomonas spp.
To detect ESBL productions by Pseudomonas spp.
Materials and Method
This study is a prospective study conducted in the Department of Microbiology on 100 Pseudomonas spp. isolated from various clinical samples of OPD and IPD. Sample showing growth of Pseudomonas spp. was inclusion criterion and cases other than Pseudomonas spp. were exclusion criteria. Ethical clearance was taken from ethical committee of Institute.
Inoculation of samples
All isolates were routinely cultured on MacConkey's and blood agar plates as shown in Figures 1 and 2. These plates were routinely incubated at 37°C aerobically and after overnight incubation, they were checked for bacterial growth. The organisms were identified as per standard laboratory methods of identification.[31]
Figure 1.

Pseudomonas on blood agar
Figure 2.

Pseudomonas on MacConkey agar
Organism identified were confirmed by putting up biochemical test. Antibiotic sensitivity test was done by Kirby Bauer disc diffusion method on Muller Hinton agar (MHA) according to the National Committee for the clinical Laboratory Standard.
Detail of sample collection and sample processing are given in Annexure 2 and Annexure 3, respectively.
Colony morphology
The colonies were studied for following characters:
Pigment production: The presence of the blue phenazine pigment pyocynin, pyorubrin, and pyoverdin was absolute confirmation of organism. Pigment diffuses into the medium. Pyoverdin (fluorescein), pyorubrin, and pyomelanin were also produced by Pseudomonas spp. Some stains were non-pigmented.
Size and shape: large 2–3 mm in diameter, irregularly round.
Surface: moist, smooth.
Structure: irregularly round.
Edges: irregular.
Contour: flat, spreading.
Consistency: sometimes mucoid.
Opacity: translucent
Iridescence: many strain exhibit a moth-eaten type of colonial lysis with a metallic sheen known as iridescence.
Hemolysis: often the presence of hemolysis around the colonies.
Emulsifiability : emulsifiable in normal saline
Characteristic Smell: grape-like odor of amino-acetophenone produced from tryptophan.
Morphology and staining characters of non-lactose fermenting colonies on MacConkey's agar were studied by gram's staining method as shown in Figure 3. The identification of the isolated bacteria was confirmed as being Pseudomonas species by studying their motility (hanging drop method or by growing them in semisolid agar medium), pigment production, odor, and by subjecting them to various biochemical tests as shown in Figure 4a and b.
Figure 3.

Microscopic view of Pseudomonas
Figure 4.

(a) catalase positive Pseudomonas. (b) oxidase positive test for Pseudomonas
Biochemical confirmation test
Oxidase : positive (+)
Catalase: positive (+)
Citrate utilization test : citrate utilized (positive)
Nitrate reduction test : positive (+)
Gelatin liquefaction test,: positive (+)
Motility : motile
Oxidative/fermentative medium: oxidative
Triple sugar iron agar : K/K
Antibiotic susceptibility testing
Antibiotic susceptibility testing was done for all the Pseudomonas spp. isolates under the standard CLSI guidelines for the following antimicrobials[32]:
Piperacilin
Ciproflaxacin
Gentamycin
Amikacin
Tobramycin
Cotrimoxazole
Cefoperazone
Cefepime
Ceftazidime
Ceftazidieme + Clavulanic acid
Meropenem
Imipenem
Colistin.
ESBL detection
ESBL detection was done by phenotypic test, i.e. combined disc diffusion method recommended by CLSI.
Common initial steps.[32]
Four to five colonies of the test strain were transferred to 1 ml of normal saline to match turbidity to 0.5 McFarland standard.
Using this inoculum, lawn culture was made on cation-balanced MHA plate with a sterile cotton swab.
Excess broth was expressed by rotating the swab against the inner side of the suspension tube.
Inoculum was allowed to dry for 15 min before putting the antibiotic disc.
Phenotypic confirmation test for ESBL was done by ceftazidime and ceftazidime + clavulanic acid on MHA according to CLSI guideline as shown in Figures 5 and 6.[31]
Figure 5.
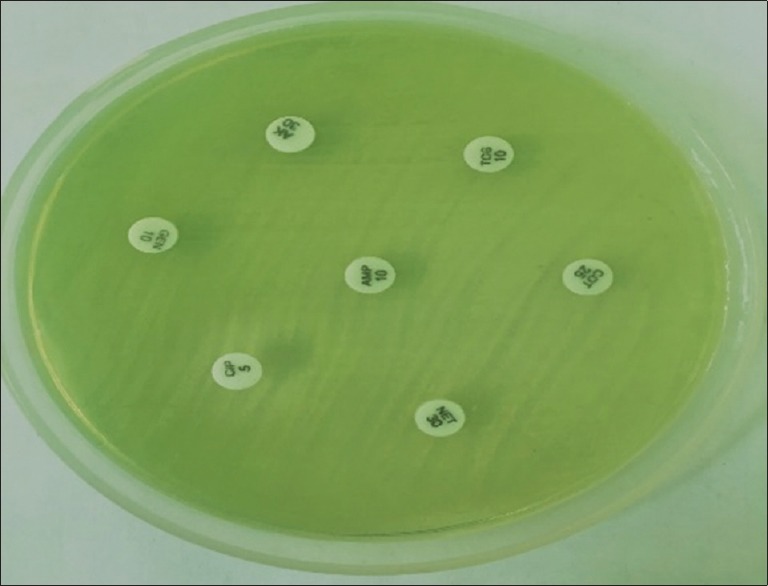
Pseudomonas on MHA along with antimicrobial discs
Figure 6.

Positive double disk diffusion test for Pseudomonas with ceftazidime and cefta + zidime with clavulanic acid
Results
Our study shows Pseudomonas infection in 54% and 46% in male and female respectively as shown in Table 1 and Graph 1 and 39% of cases were indoor whereas 61% were outdoor patients, as shown in Tables 2 and Graph 2 and infection was predominant in age group 46-60 as shown in Table 3 and Graph 3. Pseudomonal infection was more in urban population than in rural with maximum rate of Pseudomonal isolated from pus sample as shown in Tables 4, 5 and Graphs 4, 5. Table 6 and Graph 6 demonstrates the antibiotic resistance pattern of Pseudomonas spp. 88%, 80% resistance was encountered by ceftazidime, cefepime followed by cortimoxazole, and piperacillin showing 61% and, 61% respectively. The antibiotics showing least resistance i.e were gentamycin, meropenem and imepenem, the resistance being 30%, 17% and 15% and colistin is 0% resistance. The rate of ESBL positive samples out of total 100 samples studied 42% were showing ESBL positive Pseudomonas while 58% showed non ESBL producing Pseudomonas species as shown in Table 7 and Graph 7. Table 8 and Graph 8 shows distribution of ESBL positive sample in OPD(20%) and IPD(22%). Table 9 and Graph 9 shows gender-wise distribution of Pseudomonas from various clinical isolation. Antibiotics resistance pattern of Pseudomonas showed high resistance with Cefoperazone, Cefepime and Ceftaziime and Impipenem and Meropenem showed higher antibacterial activities in Cepalosporine and Carbapenem group of antibiotics respectively as in Tables 10, 11 and Graphs 10, 11. Out of 100 samples studies, 84% were pigment production while 16% showed non-pigment producing Psedomonas as in Table 12 and Graph 12.
Table 1.
Demographic profile among all patients with Pseudomonas infection; 54% of patients were male, whereas 46% patients were female
| Pseudomonas species isolated | No. of samples (100) |
|---|---|
| Male | 54%(54) |
| Female | 46%(46) |
Graph 1.

Pseudomonas infection was predominant in male compared to female
Table 2.
Distribution of Pseudomonas isolation from OPD/IPD of hospital; 39% of cases were indoor, whereas 61% were outdoor patients
| Samples from different departments (OPD/IPD) | No. of culture positive Pseudomonas samples |
|---|---|
| OPD | 61%(61) |
| IPD | 39%(39) |
Graph 2.

Rate of culture positive Pseudomonas samples from OPD and IPD
Table 3.
Pseudomonas infection was predominant in the age group of 46-60
| Different age groups with Pseudomonas infection | No. of Pseudomonas (out of 100) |
|---|---|
| 0-15 | 05 |
| 16-30 | 25 |
| 31-45 | 29 |
| 46-60 | 34 |
| >60 | 07 |
Graph 3.

Pseudomonas infection was predominant in the age group of 46–60 years (34)
Table 4.
Pseudomonas infection was predominant in people residing in the urban areas (59%) as appose to those in rural areas (41%)
| Isolation of Pseudomonas from residential status | No. of Pseudomonas spp. |
|---|---|
| Urban | 59% (59) |
| Rural | 41% (41) |
Table 5.
Rate of Pseudomonas spp. from various clinical samples
| Specimen Type | No. of Pseudomonas stains (n=100) | |
|---|---|---|
| 1 | Sputum | 30 |
| 2 | Pus | 50 |
| 3 | CSF | 02 |
| 4 | Other body fluid | 05 |
| 5 | Urine | 10 |
| 6 | Blood | 03 |
The maximum rate of Pseudomonas isolated from pus-50, sputum-30, urine-10, cerebral spinal fluid-02, other body fluid-05, blood-03
Graph 4.

Residential status of patients from urban and rural
Graph 5.

Pseudomonas species isolation from various clinical samples
Table 6.
Antibiotic resistance patterns of Pseudomonas spp; 88% and 80% resistances were encountered by ceftazidime and cefepime, followed by cortimoxazole and piperacillin showing 61% and 61%, respectively. The antibiotics showing least resistance were gentamycin, meropenem, and imepenem, the resistance being 30%, 17%, and 15%, respectively, and colistin showed 0% resistance
| Name of antibiotic | % of antibiotic resistant Pseudomonas stains |
|---|---|
| Piperacilin | 61% |
| Ciproflaxacin | 46% |
| Gentamycin | 30% |
| Amikacin | 38% |
| Tobramycin | 38% |
| Cotrimoxazole | 61% |
| Cefoperazone | 71% |
| Cefepime | 80% |
| Ceftazidime | 88% |
| Ceftazidieme + Clavulanic acid | 30% |
| Meropenem | 17% |
| Imipenem | 15% |
| Colistin | 0% |
Graph 6.

Antibiotic resistance patterns of Psedomonas species
Table 7.
Rate of ESBL positive samples out of total 100 samples studied; 42% were showing ESBL positive Pseudomonas, whereas 58% showed non-ESBL producing Pseudomonas species
| Total cases studied | No. of ESBL positive samples | No. of non-ESBL producing samples |
|---|---|---|
| 100 | 42 42% | 58 58% |
Graph 7.

Rate of ESBL Positive samples
Table 8.
Distribution of ESBL producing Pseudomonas spp. from OPD and IPD
| Department of hospital (OPD/IPD) | No. of ESBL positive sample |
|---|---|
| OPD | 20 |
| IPD | 22 |
Graph 8.

Distribution of ESBL producing Pseudomonas isolated from OPD/IPD of hospital
Table 9.
Gender-wise distribution of Pseudomonas spp. from various clinical isolations
| Age group (years) | Total no. of cases | % of total case | Samples from sputum | Pus | Urine | Blood | CSF | Other body fluid | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | |||
| 0-15 | 05 | 5% | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| 16-30 | 25 | 25% | 4 | 5 | 5 | 9 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 31-45 | 29 | 29% | 4 | 4 | 10 | 9 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 46-60 | 34 | 34% | 4 | 5 | 9 | 8 | 3 | 2 | 1 | 0 | 1 | 0 | 1 | 0 |
| >60 | 7 | 7% | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 2 | 2 |
| Total | 100 | 100% | 15 | 15 | 24 | 26 | 4 | 6 | 3 | 1 | 2 | 0 | 1 | 2 |
Graph 9.

Gender-wise distribution of Pseudomonas from various clinical isolations
Table 10.
Antibiotic resistance patterns of Pseudomonas against cephalosporin group of antibiotics. cefoperazone, cefepime, and ceftazidime showed high antibiotic resistance, whereas ceftaziime with combination of clavulanic acid showed less resistance
| Cephaloosporin group of antibiotics | Resistant | Intermediate | Sensitive |
|---|---|---|---|
| Cefoperazone | 71 | 2 | 27 |
| Cefepime | 80 | 2 | 18 |
| Ceftazidime | 88 | 0 | 14 |
| Ceftazidime + Ca | 30 | 0 | 70 |
Table 11.
Antibiotic resistance patterns of Pseudomonas against carbapenem group of antibiotics. Imipenem and meropenem demonstrated significantly higher antibacterial activity
| Carbapenem group of antibiotics | Resistant | Intermediate | Sensitive |
|---|---|---|---|
| Imipenem | 15 | 2 | 83 |
| Meropenem | 17 | 11 | 72 |
Graph 10.

Antibiotic resistance patterns of Pseudomonas against cephalosporin group of antibiotics
Graph 11.

Antibiotic resistance patterns of Pseudomonas against carbapenem group of antibiotics
Table 12.
Rate of pigment and non-pigment producing Pseudomonas spp. Out of total 100 samples studied, 84% were pigment-producing, whereas 16% showed non-pigment producing Pseudomonas
| No. of total cases studied | No. of pigment-producing Pseudomonas | No. of non-pigment producing Pseudomonas |
|---|---|---|
| 100 | 84 (84%) | 16 (16%) |
Graph 12.

Rate of pigment-producing and non-pigment producing Pseudomonas among 100 case studied
Discussion
In recent times, emergence of antibiotic resistance has threatened the effectiveness of many antibiotic agents and it is recognized as a public health threat. P. aeruginosa which has particular propensity for drug resistance has been reported to be associated with increased mortality and morbidity.[33] The incidence of Pseudomonas was higher in males than in females particularly after the age of 50 years.[34]
Present study was conducted on 100 Pseudomonas isolation from various clinical sample. Out of which, 54 % of isolation was done from male and 46 were from female patients [Table 1 and Graph 1] and this was in concordance with the study conducted by Uslan DZ, Crane SJ et al.[33] During the study period, 100 Pseudomonas isolates were recovered from a variety of specimens collected at the microbiology department. Out of 100 isolates 61% were from in patient department (IPD), while 39% of cases were from outpatient department (OPD) of the hospital [Table 2 and Graph 2] while in the study of Basak et al[35] 81.6% P. aeruginosa strains were isolated from IPD.
In our study age-wise distribution of clinical isolates showed that Pseudomonas was common in the age group between 46-60 years. On comparison, we found that little difference in results in studies of Khan et al. (2008)[34] and Rashid et al. (2007).[36] It has been observed that age plays an important role in the patient's susceptibility to Pseudomonas infection i.e. maximum number of patient's were in age group of 46-60 years followed by 31-45 years and 16-30 years. This reason could be attributed to the facts that this age group (46-60) goes out of home and were at utmost risk to acquire an infection [Table 3 and Graph 3]. Etiology of Pseudomonas infection depends on various demographic characteristics that include the place of study (rural/urban). An area-wise distribution of Pseudomonas isolated was also analyzed. In this study, isolation of Pseudomonas was high in patients from urban areas 59% rather than in patients from rural areas 41% [Table 4 and Graph 4]. This may be because in rural areas people are less exposed to environmental problems that's way bacteria get less resistant. But in urban areas people are more exposed to environmental problems (air pollution, water pollution etc).
The distribution of specimens of Pseudomonas may vary with each hospital as each hospital facility has a different environment associated with it. More than 90% of the Pseudomonas isolates were obtained from pus, sputum, urine, and tracheal aspirates. Similar results had been obtained in different studies in India reported by Chander A et al., Mohanasoundaram and Arora et al.[37,38,39] In this present study, the maximum number of Pseudomonas was isolated from pus was 50% (out of 100 samples), sputum 30%, urine 10%, and other body fluid 05% [Table 5 and Graph 5].
Pseudomonas spp. is inherently resistant to many antimicrobial agents, thus posing a great challenge in nosocomial infection. In the present study, antimicrobial susceptibility patterns of Pseudomonas from various clinical samples were analyzed for culture and sensitivity test. And 88% and 80% resistances were shown to ceftazidime and cefoperazone, [Table 6 and Graph 6]; however, least resistance, i.e. maximum sensitivity, was shown to gentamycin, meropenem, and imipenem which was also illustrated by Okesola et al.[40] and Fam et al.[41] Tavajjohi et al.[42] and Roychaudhury et al.[41] found resistance to imipenem and gentamycin in comparison to our study. Such increase in resistance could be due to the fact that Roychaudhury et al.[43] included only patients who were on ventilator and taken several antibiotics before and, thus, developed resistance. There are only fewer studies reported in the literature regarding the etiology and antimicrobial susceptibility patterns of the Pseudomonas infections from particular geographical region.
In the present study of 100 samples, 42% cases were ESBL positive Pseudomonal bacteria, where as 58% showed growth of non-ESBL producing Psedomonas [ [Table 7 and Graph 7] In Woodfort[44] et al study in teritiary care hospital in China, 63.5% Pseudomonas were ESBL positive. In Yu et al[45] study, 59% of isolates were ESBL positive and all isolates were susceptible to imipenem. Bajpai et al[46] demonstrated a higher rate of culture positive isolation being 48% and Chen et al[47] demonstrated a lower rate, i.e. 21%, this variation rate could be due to the regional variation.
In this study, ESBL positive Pseudomonas infection in IPD patients were 22% in comparison to OPD patients i.e. 20% [Table 8 and Graph 8]. It may be due to healthcare issues in hospitalized patients. A similar observation was made by Anupurba S et al.,[48] who reported the isolation of P. aeruginosa to be more common in IPD patients 42% compared to that in the OPD cases 26.57%. They expressed their view that the duration of hospital stay was directly proportional to a higher prevalence of the infection because the rate of isolation of the organism was higher in IPD patients than in OPD patients.
P. aeruginosa is inherently resistant to many antimicrobial agents, mainly due to the synergy between multidrug efflux system or a type 1 AmpC β-lactamase and low outer membrane permeability. The age and sex wise distribution of patients diagnosed with ESBL positive Pseudomonas infection followed the natural epidemiological pattern.[49] Out of 100 sample 42% was ESBL positive Pseudomonas and 58% was non ESBL producing Pseudomonas. The maximum non ESBL producing Pseudomonas were isolated from females [Table 9 and Graph 9] and in case of urine samples female has more number of ESBL positive Pseudomonas.
The incidence of UTI by Pseudomonas is greater in women than man, which may be either due to anatomical predisposition or urolithial mucosal adherence to mucopolysaccharide lining or other host factors.[49,50] The dose, as well as the incidence of toxicity, was subsequently reduced with semisynthetic penicillins like ticarcillin, which makes it the preferred ureidopenicillin against Pseudomonas infections. Our results are in corroboration with the one reported by other workers, SV Chitnis et al[51] so much so that the overall resistance to various generations of cephalosporin was high on account of the production of ESBL by the bacteria involved P. Mathur et al[52] in percent study Ceftazidime was 88% resistant, 0% intermediate, and 14% sensitive (maximum resistance) and ceftazidime + clavulanic acid 30% resistance, 0% intermediate, and 70% sensitive (maximum sensitive) [Table 10 and Graph 10].
In our study, notable resistance to Pseudomonas was observed against carbapenems. The resistance to carbapenems, especially in Pseudomonas, results from reduced levels of drug accumulation or increased expression of pump efflux.[53,54] The resistance may also be due to the production of ESBL or MBL, which can be chromosomally encoded or plasmid-mediated.[55] The carbapenem hydrolyzing enzyme carbapenamase may be class B-extended spectrum β-lactamases or class D-oxacillanases or class A-clavulanic acid inhibitory enzymes.[56] In percent study, imipenem is more sensitive to Pseudomonas; it was 15% resistance, 2% intermediate, and 83% of sensitive (maximum sensitive to carbapenem group of antibiotics) and for meropenem it was 17% resistance, 11% intermediate, and 72% sensitive. [Table 11 and Graph 11] Imipenem and meropenem were also found to be the most effective antibiotics against the ESBL-producing P. aeruginosa isolates in the study of Shaikh et al.[57]
In the present was showed number of pigment producing Pseudomonas is more in comparison to non-pigment producing Pseudomonas. The pigment producing Pseudomonas is 84% in comparison to non-pigment producing which were 16% [Table 12 and Graph 12]. Finlayson EA et al[58] stated that antibiotic resistance might not be associated with the pigment producing Pseudomonas. However, pigment production appeared to be more significantly associated with multidrug-resistant, presence of virulence-associated gene, and expression of certain virulence factors, most notably elastase, protease, siderophore, and DNase activity. Since pigment production is easy to determine, this might to be a good starting point to identify the virulence status of an isolate. Resistant to different antibiotics (amoxicillin/clavulinic, sulphamethaxzole/trimethoprim, doxycycline and ceftazidime) determined by disc diffusion method was also seen in the study of Abbas et al. in 266 urine samples and concluded that the resistance rates in P. aeruginosa were higher than global values.[59]
Conclusion
To conclude, the present study highlights that the Pseudomonas species remains an important cause of nosocomial infections. ESBL producing Pseudomonas species continue to be an important organism causing life-threatening infections. Multidrug resistance was seen in most of the stains and even to combination of ceftazidime clavulanic acid the resistance was seen. Resistance is developing to imipenem also. This gives the alarming signal for the future making the therapeutic options more difficult. Strict infection control measures are to be taken to contain this so-called water and soil organisms as Pseudomonas. This article can bring the drugs of choice to clinicians in the treatment and primary care of patient with Pseudomonas infection and help to avoid excessive and injudicious use of extended-spectrum cephalosporins and carbapenems in every hospital. Urgent work is required to develop quicker, cost-effective, and reliable diagnostic tools as well as new effective therapies and proper antibiotic policies should be formulated for the effective defenses against this organism.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dundar DM. In-vitro efficacy of synergistic antibiotic combinations in multidrug resistant Pseudomonas aeruginosa strains. Yonsei Med J. 2010;15:111–6. doi: 10.3349/ymj.2010.51.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nseir S, Di Pompeo C, Brisson H, Dewavrin F, Tissier S, Diarra M, et al. Intensive care unit-acquired Stenotrophomonas maltophilia: Incidence, risk factors, and outcome. Critical Care. 2006;10:R143. doi: 10.1186/cc5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: Risk factors and clinical impact. Antimicrob Agents Chemother. 2006;50:43–8. doi: 10.1128/AAC.50.1.43-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: Our worst nightmare? Clin Infect Dis. 2002;34:634–40. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 5.Dunne WM Jr. Bacterial adhesion: Seen any good biofilms lately? Clin Microbiol Rev. 2002;15:155–66. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obritsch MD, Fish DN, MacLaren R, Jung R. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob Agents Chemother. 2004;48:4606–10. doi: 10.1128/AAC.48.12.4606-4610.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao B, Whang H, Sun H, Zhu Y, Chen M. Risk factors and clinical outcomes of nosocomial multi-drug-resistant Pseudomonas aeruginosa infections. J Hosp Infect. 2004;57:112–8. doi: 10.1016/j.jhin.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Goosssens H. Susceptibility of multi-drug-resistant Pseudomonas aeruginosa in intensive care units: Results from the European MYSTIC study group. Clin Microbiol Infect. 2003;9:980–3. doi: 10.1046/j.1469-0691.2003.00690.x. [DOI] [PubMed] [Google Scholar]
- 9.Rossolini GM, Mantengoli E. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin Microbiol Infect. 2005;11(Suppl 4):17–32. doi: 10.1111/j.1469-0691.2005.01161.x. [DOI] [PubMed] [Google Scholar]
- 10.Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-β-lactamases: The quiet before the storm. Clin Microbiol Rev. 2002;18:306–25. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Jelsbak L, Marvig RL, Damkiær S, Workman CT, Rau MH, et al. Evolutionary dynamics of bacteria in a human host environment. Proc Natl Acad Sci U S A. 2011;108:7481–6. doi: 10.1073/pnas.1018249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nseir S, Ader F, Lubret R, Marquette CH. Pathophysiology of airway colonization in critically ill COPD patient. Curr Drug Targets. 2011;12:514–20. doi: 10.2174/138945011794751537. [DOI] [PubMed] [Google Scholar]
- 13.Fuentefria DB, Ferreira AE, Corção G. Antibiotic-resistant Pseudomonas aeruginosa from hospital wastewater and superficial water? Are they genetically related. J Environ Manage. 2011;92:250–5. doi: 10.1016/j.jenvman.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Czekajło-Kołodziej U, Giedrys-Kalemba S, Medrala D. Phenotypic and genotypic characteristics of Pseudomonas aeruginosa strains isolated from hospitals in the north–west region of Poland. Pol J Microbiol. 2006;55:103–12. [PubMed] [Google Scholar]
- 15.Wolska K, Szweda P. Comparative evaluation of PCR ribotyping and ERIC PCR for determining the diversity of clinical Pseudomonas aeruginosa isolates. Pol J Microbiol. 2008;57:157–63. [PubMed] [Google Scholar]
- 16.Khorvash F, Abdi F, Dialami K, Mehrabi A. Can serum procalcitonin and C-reactive protein as nosocomial infection markers in hospitalized patients without localizing signs? J Res Med Sci. 2011;16:1280–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Japoni A, Farshad S, Alborzi A. Pseudomonas aeruginosa: Burn Infection, Treatment and Antibacterial Resistance. Iran Red Crescent Med J. 2009;11:244–53. [Google Scholar]
- 18.Knothe H, Shah P, Kremery V, Antal M. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983;11:315–7. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby GA, Carreras I. Activities of beta-lactam antibiotics against Escherichia coli strains producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1990;34:858–62. doi: 10.1128/aac.34.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falagas ME, Karageorgopoulos DE. Extended-spectrum β-lactamase-producing organisms. Journal of Hospital infection. 2009;73:345–54. doi: 10.1016/j.jhin.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Cooksey R, Swenson J, Clark N, Gay E, Thornsberry C. Patterns and mechanisms of beta-lactam resistance among isolates of Escherichia coli from hospitals in the United States. Antimicrob Agents Chemother. 1990;34:739–45. doi: 10.1128/aac.34.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paterson DL, Hujer KM, Hujer AM, Yeiser B, Bonomo MD, Rice LB, et al. Extended-spectrum beta-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: Dominance and widespread prevalence of SHV- and CTX-M-type beta-lactamases. Antimicrob Agents Chemother. 2003;47:3554–60. doi: 10.1128/AAC.47.11.3554-3560.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradford PA. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–51. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med. 2005;352:380–91. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- 25.Woodford N, Ward E, Kaufmann ME, Turton J, Pearson A, Harry S, et al. Molecular Characterisation of Escherichia coli isolates producing CTX-M-15 Extended-spectrum beta-lactamases (ESBL) in United Kingdom. Health Protection Agency. 2006:11–19. [Google Scholar]
- 26.Hudson CM, Bent ZW, Meagher RJ, Williams KP. Resistance determinants and mobile genetic elements of an NDM-1-encoding Klebsiella pneumoniae strain. PLoS One. 2014;9:e99209. doi: 10.1371/journal.pone.0099209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Eldere J. Multicentre surveillance of Pseudomonas aeruginosa susceptibility patterns in nosocomial infections. J Antimicrob Chemother. 2003;51:347–52. doi: 10.1093/jac/dkg102. [DOI] [PubMed] [Google Scholar]
- 28.Poole K. Efflux-mediated multiresistance in Gram-negative bacteria. Clin Microbiol Infect. 2004;10:12–26. doi: 10.1111/j.1469-0691.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 29.Cornelis P. Pseudomonas: Genomics and Molecular Biology. 1st ed. Caister Academic Press; 2008. ISBN 1-904455-19-0. [Google Scholar]
- 30.Collee JG, Marr W. Practical Book of Medical Microbiology by Mackie & McCartney. 14th ed. Ectinburn: Churchill Livingstone; 1996. pp. 85–8. 380. 106-8. [Google Scholar]
- 31.Clinical and laboratory standard institute. Performance standards for antimicrobial susceptibility testing 23rd informational supplement CLSI document M100-s23. Clinical and Laboratory Standards Institute Wayne, PA; 2013. [Google Scholar]
- 32.Hirsch EB, Tam VH. Impact of multidrugresistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res. 2010;10:441–51. doi: 10.1586/erp.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uslan DZ, Crane SJ, Steckelberg JM, Cockerill FR, 3rd, St Sauver JL, Wilson WR, et al. Age- and sex-associated trends in bloodstream infection: A population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–9. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 34.Khan JA, Iqbal Z, Rahman SU, Farzana K, Khan A. Prevalence and resistance pattern of Pseudomonas aeruginosa against various antibiotics. Pak J Pharm Sci. 2008;21:311–5. [PubMed] [Google Scholar]
- 35.Basak S, Attal RO, Rajurkar M. Sudhakar C, editor. Pseudomonas aeruginosa and newer β-lactamases: An emerging resistance threat. Infection Control–Updates. 2012:181–98. InTech ISBN: 978-953-51-0055-3, InTech. [Google Scholar]
- 36.Rashid A, Chowdhury A, Rahman Sufi HZ, Begum SA, Muazzam N. Infections by Pseudomonas and antibiotic resistance pattern of the isolates from Dhaka Medical college Hospital, Bangladesh. J Med Microbio. 2007;1:48–51. [Google Scholar]
- 37.Anil C, Shahid RM. Antimicrobial susceptibility patterns of pseudomonas aeruginosa clinical isolates at a tertiary care hospital in Kathmandu, Nepal. Asian J Pharm Clin Res. 2013;6:235–8. [Google Scholar]
- 38.Arora D, Jindal N, Kumar R, Romit Emerging antibiotic resistance in Pseudomonas aeruginosa. Int J Pharm Sci. 2011;3:82–4. [Google Scholar]
- 39.Mohanasoundaram KM. The antibiotic resistance pattern in the clinical isolates of Pseudomonas aeruginosa in a tertairy care hospital; 2008–2010 (A 3 year study) J Clin Diagn Res. 2011;5:491–4. [Google Scholar]
- 40.Okesola AO, Ige OM. Trends in bacterial pathogens of lower respiratory tract infection. Indian J Chest Dis Allied Sci. 2008;50:206–7. [PubMed] [Google Scholar]
- 41.Fam N, Diab M, Helmi H, El-Defrawy I. Phenotypic Detection of Metallo-β-Lactamases and Extended Spectrum β-Lactamases Among Gram Negative Bacterial Clinical Isolates. Egyptian J Medical Microbiol. 2006;15:719–29. [Google Scholar]
- 42.Tavajjohi Z, Moniri R. Detection of ESBL and MDR in Pseudomonas aeruginosa infection in tertiary-care teaching hospital. Iran J Clin Infect Dis. 2011;6:18–23. [Google Scholar]
- 43.Roychaudhury S, ghosh S, chaturvedi AN, Debnath A. Antibiotic sensitivity pattern of bacterial isolates of ventilated patients from the intensive care unit of a tertiary care hospital in rural India. J Chem Pharma Res. 2014;6:1500–3. [Google Scholar]
- 44.Woodford N, Zhang J, Kaufmann ME, Yarde S, Tomas MM, Faris C, et al. Detection of Pseudomonas aeruginosa isolates producing VEB-type extended-spectrum beta-lactamases in the United Kingdom. J Antimicrob Chemother. 2008;62:1265–8. doi: 10.1093/jac/dkn400. [DOI] [PubMed] [Google Scholar]
- 45.Yu YS, Qu TT, Zhou JY, Wang J, Li HY, Walsh TR. Integron containing the VIM-2 metallo-β-lactamase gene among imipenem-resistant Pseudomonas aeruginosa strains from different Chinese hospitals. J Clin Microbiol. 2006;44:4242–5. doi: 10.1128/JCM.01558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bajpai T, Pandey M, Varma M, Bhatambare GS. Prevalence of extended spectrum betalactamase producing uropathogens and their antibiotic resistance profile in patients visiting a tertiary care hospital in central India: Implications on empiric therapy. Indian Journal of Pathology and Microbiology. 2014;57:407–12. doi: 10.4103/0377-4929.138733. [DOI] [PubMed] [Google Scholar]
- 47.Chen Z, Niu H, Chen G, Li M, Li M, Zhou Y. Prevalence of ESBL producing Pseudomonas aeruginosa isolates from different wards in a Chinese teaching hospital. Int J Clin Exp Med. 2015;8:19400–5. [PMC free article] [PubMed] [Google Scholar]
- 48.Anupurba S, Battacharjee A, Garg A, Ranjansen M. The antimicrobial susceptibility of Psuedomonas aeruginosa isolated from wound infections. Indian J Dermatol. 2006;51:286–8. [Google Scholar]
- 49.Tambekar DH, Dhanorkar DV, Gulhane SR, Khandelwal VK, Dudhane MN. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotech. 2006;5:1562–5. [Google Scholar]
- 50.Ronald AR, Pattulo MS. The natural history of urinary infection in adults. Med Clin North Am. 1991;75:299–312. doi: 10.1016/s0025-7125(16)30455-2. [DOI] [PubMed] [Google Scholar]
- 51.Chitnis SV, Chitnis V, Sharma N, Chitnis DS. Current status of drug resistance among gram negative bacilli isolated from admitted cases in a tertiary care centre. J Assoc Physicians India. 2003;51:28–32. [PubMed] [Google Scholar]
- 52.Mathur P, Kapil A, Das B, Dhawan B. Prevalence of extended spectrum β-lactamase producing gram negative bacteria in a tertiary care hospital. Indian J Med Res. 2002;115:153–7. [PubMed] [Google Scholar]
- 53.Gupta E, Mohanty S, Sood S, Dhawan B, Das BK, Kapil A. Emerging resistance to Carbapenems in a tertiary care hospital in North India. Indian J Med Res. 2006;124:95–8. [PubMed] [Google Scholar]
- 54.Kurokawa H, Yagi T, Shibata N, Shibayama K, Arakawa Y. Worldwide proliferation of Carbapenem resistant gram negative bacteria. Lancet. 1999;354:955. doi: 10.1016/S0140-6736(05)75707-X. [DOI] [PubMed] [Google Scholar]
- 55.Navneeth BV, Sridaran D, Sahay D, Belwadi MR. A preliminary study on metallo β- lactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian J Med Res. 2002;116:264–7. [PubMed] [Google Scholar]
- 56.Yu YS, Yang Q, Xu XW, Kong HS, Xu GY, Zhong BY. Typing and characterization of Carbapenem resistant Acinobacter calcoaceticus-baumannii complex in a Chinese hospital. J Med Microbiol. 2004;53:653–6. doi: 10.1099/jmm.0.05513-0. [DOI] [PubMed] [Google Scholar]
- 57.Shaikh S, Fatima J, Shakil S, Rizvi SMD, Kamal MA. Prevalence of multidrug resistant and extended spectrum beta-lactamase producing Pseudomonas aeruginosa in a tertiary care hospital. Saudi J Biol Sci. 2015;22:62–4. doi: 10.1016/j.sjbs.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finlayson EA, Brown PD. Comparison of antibiotic resistance and virulence factors in pigmented and non-pigmented Pseudomonas aeruginosa. West Indian Med J. 2011;2011;60:24–32. [PubMed] [Google Scholar]
- 59.Abbas HA, El-Ganiny AM, Kamel HA. Phenotypic and genotypic detection of antibiotic resistance of Pseudomonas aeruginosa isolated from urinary tract infections. African Health Sciences. 2018;18:11–21. doi: 10.4314/ahs.v18i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]


