Abstract
Background:
According to the GBD report published in 2016, the burden of cancer in Kerala is 135.3/100,000 population in contrast to the national average of 100/100,000 population. Cancer is a complex disease that requires broad engagement of various departments and organizations to implement a community based health promotion strategy.
Objective:
To estimate the prevalence of diagnosed cancers, warning signs and selected risk factors of cancer in Niranam Panchayath of Pathanamthitta district, Kerala.
Methodology:
A total of 13,736 population was covered by door to door survey using a structured questionnaire. The questionnaire collected information on the sociodemographic variables of the residents, source of water supply, warning signs of cancer and details of diagnosed cancer cases.
Results:
The mean age of the population was 39.7 ± 21 years. The prevalence of diagnosed cases of cancer in our study was 652/100,000 population. Most common type of cancer identified was Breast cancer (37.3%). The prevalence of any warning sign among the study population was 400/100,000 population. Breast lump was the common warning sign identified. Increasing age, female gender and occupational status were the factors found to be significantly associated with cancer.
Recommendations:
Community based health education to increase awareness, screening for cancers and breast self-examination in the community could help in early diagnosis and prevention at primary level. Scientific study to assess the risk factors of cancers using case control design could be done in this population along with soil and water sample testing for carcinogens.
Keywords: Cancer, community-based burden, Kerala, risk factors public-private partnership, warning signs
Introduction
Cancer is the second leading cause of death globally after cardiovascular diseases. The prevalence of cancer was conventionally much more evident in developed nations but, in recent years, it has increased substantially in developing countries as well.[1,2] The estimates from the Global Burden of Disease (GBD) suggest that about 70% of all cancer deaths are now concentrated among low- and middle-income countries.[2] Cancer registration in India was initiated in 1964 and expanded since 1982, through initiation of the National Cancer Registry Program (NCRP) by the Indian Council of Medical Research. NCRP currently has 26 population-based registries and seven hospital-based registries. Yet, Indian cancer registries, mostly in urban areas, cover less than 15% of the population.[3] The India state-level disease burden initiative is a collaboration with the GBDs, Injuries, and risk factors study (GBD) to produce subnational disease burden estimates for India. This initiative recently reported the variable health transition across the states of India from 1990 to 2016 based on analysis done as part of GBD 2016. It shows that Kerala has the highest cancer incidence in the country. The incidence of cancer in Kerala is 135.3/100,000 population in contrast to the national average of 100/100,000 population. There is an alarming rise in the cancer incidence in Kerala from 1990 (74/100,000 population) to 2016 (135.3/100,000 population).[2,4]
The health care delivery environment in India has distinctive challenges. Inadequate infrastructure and a constrained health care delivery process further intensify the complexity. Public-private partnership or PPP in the context of the health sector is an instrument for improving the health of the population.[5] Cancer is a complex disease that requires the broad engagement of various departments and organizations to develop a patient-centered delivery system. In this scenario, the PPP model can be used to accelerate the diffusion to the communities for health promotion.[6] A study was done in Kerala to assess the feasibility and to explore the challenges of a district-wide door-to-door breast cancer screening program in Kannur district. The study recommended that community participation with the engagement of the health system and local self-government are required for implementing a comprehensive cancer screening strategy.[7] This again reinforces the importance of a multi-sectoral and integrated approach for cancer prevention and care in the community.[6,7] Traditionally, oncologists in tertiary care hospitals provided the majority of cancer screening, treatment and follow-up for patients with cancer. Cancer care is mainly looked upon only as a tertiary level of care but the fact that primary care has an important role to play in cancer care including awareness generation, screening, diagnosis, and community-based follow-up and rehabilitation are seldom recognized. Workforce shortages in oncology especially in a country like India with reduced sustainability of a specialist-based model of care and increasing need underline the importance of providing facilities at the primary care level in the community. Enhancing the capacity of primary care providers to deliver cancer care and facilitating their collaboration with a secondary and tertiary level of care are strategies that could address this problem.[8,9,10,11]
A meeting was called by the district administration to discuss this perceived problem of an increasing number of cancer cases in some areas of Pathanamthitta district as reported by lay leaders in the community. It was decided to verify this public concern and perception regarding the increasing prevalence of cancer cases in this area. The present study was conducted by the department of community medicine in collaboration with the district administration and the district health services with the following objectives.
Objectives
To estimate the prevalence of diagnosed cancers among residents in Niranam Panchayath of Pathanamthitta district, Kerala
To find out the prevalence of self-reported warning signs/symptoms of cancer among the residents in Niranam Panchayath
To find out the association between cancer and selected risk factors (age, gender, source of drinking water, and occupation).
Methodology
The study was conducted in Niranam Panchayath in Thiruvalla, Pathanamthitta district. It lies in the western part of Thiruvalla, identified as the upper Kuttanad region. There are around 3,800 families living in Niranam Panchayath. All residents of Niranam Panchayath were included in the study. All houses which were locked after three visits were excluded from the survey. Data regarding cancer was collected using a structured questionnaire that was developed after discussing with experts in the field from medical colleges in Thiruvalla and the district administration. The questionnaire collected information on the sociodemographic variables of the residents, source of water supply, warning signs of cancer, and details of diagnosed cancer cases. The data was collected by trained field workers through the door-to-door survey in 3 months (August–October 2019). Ethical approval was obtained for the study from the Institutional Ethical Committee and written informed consent was taken from the participants. The data were entered in Microsoft Excel and analyzed using Epi Info software. Descriptive measures were calculated. Suitable statistical tests like Pearson's Chi-square test and Fisher's exact test were used as appropriate. (All tests were 2-sided). A P value <0.05 was considered to be statistically significant.
Results
Section 1—Sociodemographic details
Total houses included in the study were 3,765 and the total population covered was 13,736. The mean age of the population was 39.7 ± 21 years. Sociodemographic details of the population are shown in Table 1. (We have mentioned the data available for each variable (n) as there was missing data). The majority of the population belonged to the middle age category of 40–59 years. Nearly 21% of the population were elderly people. The population was distributed in 13 wards of Niranam. Wards 2 and 7 constituted the maximum number of population among the 13 wards. More than 90% of the population were permanent residents of that area. Gender distribution in the population was almost equal, with 51.3% females and 48.7% males. Major drinking water source among the population was own well (67%) followed by piped water supply (29.2%). The majority of the population were not working (unemployed/retired/housewife/students). The major occupation in the population included manual laborers, farming, and office jobs.
Table 1.
Distribution of study population based on sociodemographic details
| Variables | Categories | Number (Percentage) |
|---|---|---|
| Gender (n=13,627) | Males | 6632 (48.7%) |
| Females | 6995 (51.3%) | |
| Age groups (n=13,572) | ≤5 years | 641 (4.7%) |
| 5-18 years | 1983 (14.6%) | |
| 18-40 years | 3977 (29.3%) | |
| 40-60 years | 4023 (29.6%) | |
| ≥60 years | 2948 (21.7%) | |
| Source of drinking water (n=12,289) | Common well | 309 (2.5%) |
| Own well | 8233 (67%) | |
| Pipe | 3586 (29.2%) | |
| Water tanker | 52 (0.4%) | |
| Others | 109 (0.9%) | |
| Residential status (n=13,497) | Yes | 12,497 (92.6%) |
| No | 1000 (7.4%) | |
| Occupation (n=10,807) | Under-five/students | 3015 (27.9%) |
| Office job | 513 (4.7%) | |
| Currently not working | 4103 (38%) | |
| Agriculture/livestock | 153 (1.4%) | |
| Daily wage laborer | 1491 (13.8%) | |
| Others | 1532 (14.2%) | |
| Ward Distribution (n=13,567) | Ward 1 | 1012 (7.5%) |
| Ward 2 | 1240 (9.1%) | |
| Ward 3 | 1161 (8.6%) | |
| Ward 4 | 1197 (8.8%) | |
| Ward 5 | 874 (6.4%) | |
| Ward 6 | 956 (7%) | |
| Ward 7 | 1265 (9.3%) | |
| Ward 8 | 1199 (8.8%) | |
| Ward 9 | 994 (7.3%) | |
| Ward 10 | 1098 (8.1%) | |
| Ward 11 | 876 (6.5%) | |
| Ward 12 | 959 (7.1%) | |
| Ward 13 | 736 (5.4%) |
Section II—Details regarding cancer
The prevalence of individuals with diagnosed cancer among the study population was 0.652% or 652/100,000 population (89 cases/13,647 (population with data on cancer status available). The prevalence of cancer was higher (≥1000/100,000) in wards 8 (1100/100,000), 11 (1300/100,000), 12 (1000/100,000), and 13 (1000/100,000) compared to other wards details in Figure 1. The prevalence of cancer among females was 0.8% or 800/100,000 population. The cancer prevalence was found to be highest in the age group ≥60 years details in Table 2.
Figure 1.
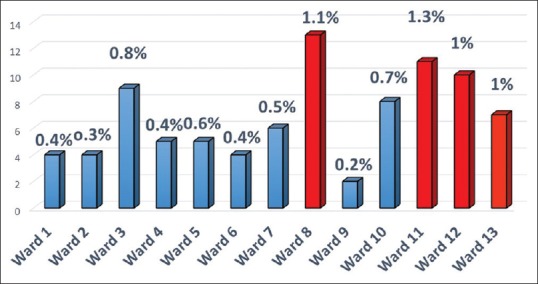
Ward wise prevalence of cancer in the study population
Table 2.
Age and gender-wise prevalence of cancer in the study population
| Variables | Categories | Prevalence |
|---|---|---|
| Gender | Males | 500/100,000 |
| Females | 800/100,000 | |
| Age groups | ≤5 yrs | 156/100,000 |
| 6-18 years | 0 | |
| 19-39 years | 227/100,000 | |
| 40-59 years | 600/100,000 | |
| ≥60 years | 1900/100,000 |
The majority of the cases had breast cancer (37.3%) followed by the upper gastrointestinal tract (GIT) (19.3%) and genitourinary (18.1%) [Table 3]. The prevalence of warning signs of cancer was 0.4% or 400/100,000 population in those who were not diagnosed with cancer. Breast lump was the most common warning sign among those populations who did not have diagnosed cancers (0.28% or 280/100,000 population). Other warning signs identified were nonhealing oral ulcers (two people), postmenopausal bleeding (two people), and nipple discharge (one person).
Table 3.
Leading five cancer sites in the study population (n=83)
| Site | Number (%) |
|---|---|
| Breast | 31 (37.3%) |
| Throat | 8 (9.6%) |
| Oral | 6 (7.2%) |
| Uterus | 6 (7.2%) |
| Prostate | 5 (6%) |
Section III—Factors associated with cancer
The association of cancer with selected risk factors such as age, gender, source of the drinking water source, and occupational status were studied. Increasing age, female gender, occupational status (agriculture/livestock) were observed to be significant risk factors for cancer in this population. No significant association was found between cancer and the source of drinking water [Table 4].
Table 4.
Factors associated with cancer
| Variables | Cancer | Total | Chi-square, P | ||
|---|---|---|---|---|---|
| No | Yes | ||||
| Gender n=13,582 | Male | 6573 (99.5%) | 33 (0.5%) | 6606 | Chi-square=4.79 P=0.029 |
| Female | 6920 (99.2%) | 56 (0.8%) | 6976 | ||
| Age group n=13,528 | ≤5 yrs | 637 (99.7%) | 1 (0.2%) | 638 | Fishers exact=93 P<0.0001 |
| 6-18 yrs | 1977 (100%) | 0 | 1977 | ||
| 19-39 yrs. | 3951 (99.8%) | 9 (0.2%) | 3960 | ||
| 40-59 yrs | 3987 (99.4%) | 24 (0.6%) | 4011 | ||
| ≥60 yrs. | 2887 (98.1%) | 55 (1.9%) | 2942 | ||
| Occupation n=10,782 | Under-five/student | 3012 (99.9%) | 1 (0.01%) | 3013 | Fisher's exact=68.7 P<0.0001 |
| Office job | 512 (100%) | 0 | 512 | ||
| Not working | 4029 (98.6%) | 57 (1.4%) | 4086 | ||
| Agriculture/livestock | 151 (98.7%) | 2 (1.3%) | 153 | ||
| Daily wage laborer | 1484 (99.7%) | 5 (0.3%) | 1489 | ||
| Others | 1526 (99.8%) | 3 (0.2%) | 1529 | ||
| Water source n=12,225 | Common well | 302 (99%) | 3 (1%) | 305 | Fisher's exact=2.87 P=0.493 |
| Own well | 8144 (99.4%) | 46 (0.6%) | 8190 | ||
| Pipe | 3543 (99.3%) | 26 (0.7%) | 3569 | ||
| Water tanker | 52 (100%) | 0 | 52 | ||
| Others | 108 (99.1%) | 1 (0.9%) | 109 | ||
Discussion
Our study estimated the prevalence of diagnosed cancer, warning signs and selected risk factors among residents in a selected Panchayath of Pathanamthitta district, Kerala with a population of approximately 14,000. The study was done in the PPP model in collaboration with the district administration and district health services. The mean age of the population was 39.7 ± 21 years and gender distribution was almost equal. More than one-third of the population was not currently working (unemployed/retired/housewife, etc). The proportion of the elderly was more than 20%. This was expected, as Kerala state has the highest proportion of elderly people in the country and the Pathanamthitta district has the highest proportion of elderly in the state.[12] We compared the data with a nearby Panchayath area and the data was comparable with 23% of the elderly population and equal gender distribution. Own well was the major source of drinking water in the population, followed by piped water supply.
The prevalence of diagnosed cancer in the population was 652/100,000. According to a study published in PLOS One in 2014, the cancer prevalence is estimated to be 83 per 100,000 persons with a greater prevalence reported in the urban population (110 per 100,000 persons). The age-standardized prevalence rates mentioned are 97/100,000 (India), 83/100,000 (rural India) and 130/100,000 (urban India).[4] The prevalence reported in our study population is almost seven times higher compared to the overall prevalence in India. Among all the states in India, Kerala state shows the highest incidence of cancer according to GLOBOCAN report 2016. The pattern of cancer incidence across years was published in the report and it was shown that there is an alarming rise in the cancer incidence in Kerala from 1990 (74/100,000 pop) to 2016 (135.3/100,000 pop).[2] Part of this could be explained by the increasing life expectancy or better surveillance system in Kerala.[13,14] However, some areas in Kerala show higher prevalence and no large scale population-based study has been done in those areas to compare the data. The high prevalence of cancer in this area underscores the need to develop a primary palliative care system in the community. Several research studies have emphasized the importance of the primary care physician being an indispensable element in the continuum of palliative care provision in the community. Collaborative care between palliative care specialist and primary care/family physician will ensure a seamless transition to community-based cancer care.[11] The most common cancer identified among females in this study was breast cancer which is similar to other studies done in India.[2,7] There are very few community-based studies done to identify the prevalence and risk factors of cancer in Kerala. Most of the studies are hospital-based. According to a study done (unpublished data, personal information) by Alleppey Medical College in 2010, the prevalence of diagnosed cases in the community was 450/100,000 population. Alleppey is one of the areas in Kerala where a high prevalence of cancer is reported. The prevalence in our study is higher compared to the Alleppey study.
Breast lump was the most common warning sign (39 people) identified among the population. A similar study done in Kannur to screen for breast cancer in the population of 1.5 lakhs identified 21 cases of a breast lump in screening. The proportion of people with breast lump identified in our population was much higher compared to that study which again underscores the need for large scale community-based screening in this district.[7] Increasing age, female gender and occupational status (agriculture and livestock) were observed to be significant risk factors for cancer in this population. In an article published in PLOS One in 2014, the burden of cancer among the elderly cohort (70+) was shown to be significantly higher at 385 per 100,000 people in India. Cancer was more prevalent among females (96/100,000 population). The prevalence among the elderly population and females was 1900/100,000 population 800/100,000 population respectively and it was higher compared to the national prevalence.[4] The variation across different occupational groups has not much explored in other studies. The prevalence was found to be higher in the not- working category and agriculture/livestock category. The higher proportion of elderly people in that category could explain the higher prevalence. Several studies abroad have pointed out the association between agriculture occupation and cancer. A study from the USA has shown that exposure to several organophosphate insecticides was associated with elevated cancer risk. A similar study from Canada among agricultural workers showed an increased prevalence of cancer due to various factors such as pesticide use, increased sun exposure etc., We could not find many studies from a developing country setting for adequate comparisons.[15,16,17,18,19] The source of drinking water was not significantly associated with cancer prevalence in this population.
Strength of the study
This study was done in collaboration with district administration and health services as a PPP model which is the biggest strength of the study. The study was community-based, covering nearly 14,000 population. This is one of the very few studies that looked into the community-based screening of warning signs of cancer in addition to prevalence and risk factors, in Kerala.
Limitations
As we had to cover a large population, only selected risk factors were studied. The data lacked specificity as the data was not collected by medical professionals but by trained ASHA workers/field workers. Data on period prevalence and mortality were not assessed as only point prevalence was taken. The data on warning signs was based on self-report and not by clinical examination. Even though the prevalence of cancer was high the number of cancer cases was small to study statistical significance in a subanalysis.
Conclusion
The prevalence of diagnosed cases of cancer in our study was 652/100,000 population. The most common type of cancer identified was breast cancer. The prevalence of any warning sign among the study population was 400/100,000 population. Breast lump was the common warning sign identified. Increasing age, female gender, and occupational status were the factors found to be significantly associated with cancer.
Recommendations
Community-based awareness generation and screening for cancers should be planned at the primary level with special emphasis on breast cancer which is common in this study population. In addition to this, breast self-examination should be encouraged and taught among the females in the community for early detection and prevention. A scientific study to assess the risk factors of cancers (especially breast cancer) using case-control design could be done in this population. Area inspection and mapping of cases to be done to identify common risk factors among cases (geographical location, pesticide use, etc.), and soil and water samples should be tested for carcinogens.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflict of interest
There is no conflict of interest.
References
- 1.Nagai H, Kim YH. Cancer prevention from the perspective of global cancer burden patterns. J Thorac Dis. 2017;9:448–51. doi: 10.21037/jtd.2017.02.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators IS-LDBIC. The burden of cancers and their variations across the states of India: The GBD study 1990-2016. Lancet Oncol. 2018;19:1289–306. doi: 10.1016/S1470-2045(18)30447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee S, Chattopadhyay A, Senapati SN, Samanta DR, Elliott L, Loomis D, et al. Cancer registration in India-Current scenario and future perspectives. Asian Pac J Cancer Prev. 2016;17:3687–96. [PubMed] [Google Scholar]
- 4.Rajpal S, Kumar A, Joe W. Economic burden of cancer in India: Evidence from cross-sectional nationally representative household survey, 2014. PLoS One. 2018;13:e0193320. doi: 10.1371/journal.pone.0193320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramachandran, Ramakrishnan, Public Private Partnership (PPP) in Indian Health Care. 2012. [Last accessed on 2019 Oct 17]. Available from: https://ssrn.com/abstract=2186897 or http://dx.doi.org/10.2139/ssrn.2186897 .
- 6.Holden DJ, Reiter K, O’Brien D, Dalton K. The strategic case for establishing public-private partnerships in cancer care. Health Res Policy Syst. 2015;13:44. doi: 10.1186/s12961-015-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parambil NA, Philip S, Tripathy JP, Philip PM, Duraisamy K, Balasubramanian S. Community engaged breast cancer screening program in Kannur District, Kerala, India: A ray of hope for early diagnosis and treatment. Indian J Cancer. 2019;56:222–7. doi: 10.4103/ijc.IJC_397_18. [DOI] [PubMed] [Google Scholar]
- 8.Tomasone JR, Vukmirovic M, Brouwers MC, Grunfeld E, Urquhart R, O’Brien MA, et al. Challenges and insights in implementing coordinated care between oncology and primary care providers: A Canadian perspective. Curr Oncol. 2017;24:120–3. doi: 10.3747/co.24.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams E, Boulton M, Rose P, Lund S, Richardson A, Wilson S, et al. Views of cancer care reviews in primary care: A qualitative study. Br J Gen Pract. 2011;61:173–82. doi: 10.3399/bjgp11X567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patil AD, Salvi NR, Shahina B, Pimple AS, Mishra AG, Chauhan LS, et al. Perspectives of primary healthcare providers on implementing cancer screening services in tribal block of Maharashtra, India. South Asian J Cancer. 2019;8:145–9. doi: 10.4103/sajc.sajc_290_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atreya S, Jeba J, Pease N, Thyle A, Murray S, Barnard A, et al. Primary palliative care competency framework for primary care and family physicians in India-Collaborative work by Indian association of palliative care and academy of family physicians of India. J Family Med Prim Care. 2019;8:2563–7. doi: 10.4103/jfmpc.jfmpc_451_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanitha V, Noushad A. Migration impact on left-behind elderly's labour participation in Kerala. Indian J Econ Dev. 2018;6:1–6. [Google Scholar]
- 13.Thomas MB, James KS. Changes in mortality and human longevity in Kerala: Are they leading to the advanced stage? Glob Health Action. 2014;7:22938. doi: 10.3402/gha.v7.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathur P, Mehrotra R, Fitzmaurice C, Dhillon PK, Nandakumar A, Dandona L, et al. Cancer trends and burden in India-Authors’ response. Lancet Oncol. 2018;19:e664. doi: 10.1016/S1470-2045(18)30857-X. [DOI] [PubMed] [Google Scholar]
- 15.Engel LS, Werder E, Satagopan J, Blair A, Hoppin JA, Koutros S, et al. Insecticide use and breast cancer risk among farmers’ Wives in the agricultural health study. Environ Health Perspect. 2017;125:097002. doi: 10.1289/EHP1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis LM, Lerro CC, Friesen MC, Andreotti G, Koutros S, Sandler DP, et al. A prospective study of cancer risk among Agricultural Health Study farm spouses associated with personal use of organochlorine insecticides. Environ Health. 2017;16:95. doi: 10.1186/s12940-017-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kachuri L, Harris MA, MacLeod JS, Tjepkema M, Peters PA, Demers PA. Cancer risks in a population-based study of 70,570 agricultural workers: Results from the Canadian census health and Environment cohort (CanCHEC) BMC Cancer. 2017;17:343. doi: 10.1186/s12885-017-3346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Maele-Fabry G, Willems J. Occupation related pesticide exposure and cancer of the prostate: A meta-analysis. Occup Environ Med. 2003;60:634–42. doi: 10.1136/oem.60.9.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutros S, Alavanja MC, Lubin JH, Sandler DP, Hoppin JA, Lynch CF, et al. An update of cancer incidence in the agricultural health study. J Occup Environ Med. 2010;52:1098–105. doi: 10.1097/JOM.0b013e3181f72b7c. [DOI] [PMC free article] [PubMed] [Google Scholar]


