Abstract
The causative agent of severe acute respiratory syndrome (SARS) has been identified as SARS-associated coronavirus (SARS-CoV), but the prophylactic treatment of SARS-CoV is still under investigation. We constructed a recombinant adenovirus containing a truncated N-terminal fragment of the SARS-CoV Spike (S) gene (from −45 to 1469, designated Ad-SN), which encoded a truncated S protein (490 amino-acid residues, a part of 672 amino-acid S1 subunit), and investigated whether this construct could induce effective immunity against SARS-CoV in Wistar rats. Rats were immunized either subcutaneously or intranasally with Ad-SN once a week for three consecutive weeks. Our results showed that all of the immunized animals generated humoral immunity against the SARS-CoV spike protein, and the sera of immunized rats showed strong capable of protecting from SARS-CoV infection in vitro. Histopathological examination did not find evident side effects in the immunized animals. These results indicate that an adenoviral-based vaccine carrying an N-terminal fragment of the Spike gene is able to elicit strong SARS-CoV-specific humoral immune responses in rats, and may be useful for the development of a protective vaccine against SARS-CoV infection.
Abbreviations: Ad-LacZ, recombinant replication-incompetent adenoviral vector containing β-galactosidase gene; Ad-SN, recombinant replication-incompetent adenoviral vector containing the SN fragment of SARS-CoV; ELISA, enzyme-linked immuno-sorbent assay; MOI, multiplicity of infection; PBS, phosphate-buffered saline; PBST, PBS containing 0.05% Tween 20; SARS, severe acute respiratory syndrome; SARS-CoV, SARS-associated coronavirus; SN, the N-terminal fragment of the Spike gene (from −45 to 1469); SPF, specific pathogen-free; S, spike; pfu, plaque-forming unit
Keywords: Vaccine, SARS-associated coronavirus, Adenoviral vector, Spike gene, Humoral immunity
1. Introduction
Severe acute respiratory syndrome (SARS) is a life-threatening contagious disease caused by the SARS-associated coronavirus (SARS-CoV) (Ksiazek et al., 2003, Rota et al., 2003). Since the first SARS case emerged in China's Guangdong province in November 2002, the disease has affected over 8000 victims in 31 countries and devitalized near 1000 lives (Schlagenhauf and Ashraf, 2003). To date, no specific, effective medicine or vaccine has been developed against SARS (Berger et al., 2004), which is currently treated with heteropathy and/or conservative therapeutics. Although the first SARS outbreak was controlled by the mains of quarantine, WHO's reports in 2004 (http://www.wpro.who.int/sars/docs/update/update_07022004_revisedfinal.asp and http://www.wpro.who.int/sars/docs/pressreleases/pr_31012004.asp) indicated that SARS remains a constant threat to public. Efforts to develop safe, efficient SARS vaccines are under way worldwide (Marshall and Enserink, 2004). It is, thus, important to develop a safe and effective vaccine against SARS-CoV. An inactivated SARS-CoV vaccine seems to be the most facile and convenient option, but inactivated vaccines have been associated with risks of infection. For example, monkey models and children immunized with inactivated measles virus vaccine may develop severe cases of atypical measles (Polack et al., 1999, Fulginiti et al., 1967). Therefore, developing a gene-based vaccine may be a more promising choice.
The SARS-CoV genome is a single strand RNA molecule encoding four structural proteins: spike (S) glycoprotein, membrane (M) protein, envelope (E) protein and nucleocapsid (N) protein (Rota et al., 2003). S protein contains two subunits, S1 and S2. S1 is responsible for recognizing and binding to receptors on host cells, while S2 directs fusion between the viral and cell membranes (Ingallinella et al., 2004, Yang et al., 2004a). Angiotensin-converting enzyme 2 (ACE2) has recently been identified as a cellular receptor of SARS-CoV (Li et al., 2003b), and the receptor-binding region of spike was defined as amino acid residues 318–510 of the S1 subunit (Wong et al., 2004). Both soluble ACE2 receptor and mAb to S1 subunit can interfere receptor association and block SARS-CoV infection (Hofmann et al., 2004, Sui et al., 2004). Presumably, a vaccine designed to interfere with the binding between the S protein and its receptor on host cells would also prevent the spread of SARS. Buchholz et al. (2004) reported that S glycoprotein, of the structural proteins, appears to act as the only significant SARS-CoV neutralization antigen in a hamster model. A human mAb to S1 subunit was found to neutralize SARS coronavirus by blocking receptor association (Sui et al., 2004). Moreover, previous studies concerning the development of vaccines against animal coronaviruses have suggested that the S1 subunit contains neutralizing epitopes that confer protection to animals (e.g., birds and pigs) upon viral challenge (Cavanagh, 2003, Correa et al., 1990, Delmas et al., 1990, Song et al., 1998).
It has recently been reported that viral expression of the full-length S protein or S1 subunit of SARS coronavirus elicits a high titer of neutralizing antibodies in monkey models (Bukreyev et al., 2004, Gao et al., 2003). However, they almost lack safety evaluation. Weingartl et al. (2004) reported recently that immunization with modified vaccinia virus expressing full-length spike (S) protein of SARS virus was associated with enhanced hepatitis, while developed a more rapid and vigorous neutralizing antibody response. S protein was presumed that induced strong inflammatory responses in ferret liver tissue. In this study, we constructed a recombinant adenovirus expressing a shorter, truncated S1 subunit of SARS-CoV (Ad-SN), and investigated its ability to induce humoral immunity against SARS-CoV and its safety in rats. Our study demonstrated that high titer antibodies against SARS-CoV were elicited in a rat model following both subcutaneous (s.c.) and intranasal (i.n.) administration of Ad-SN, the latter of which is targeted at fostering the respiratory mucosal immunity for protection against airborne diseases such as SARS. This result indicates that our construct could be developed into a safe SARS-CoV vaccine.
2. Materials and methods
2.1. Cell lines and animals
The 293 cell line (CRL-1573) is a human embryonic kidney cell line containing nucleotides 1-4344 of adenovirus type 5 (Ad5) (Louis et al., 1997, Graham et al., 1977). The Vero-E6 cell line is an Africa green monkey cell line (CRL-1586). Both cell lines were purchased from the ATCC, and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C with 5% CO2 and saturated humidity. RPMI 1640, supplements, and serum were obtained from Life Technologies (Gibco/BRL). Wistar rats (6–8 week old) were obtained from the Experimental Animal Center, Sun Yat-sen University (Guangzhou, China), and housed in SPF (specific pathogen free) animal facilities.
2.2. Construction of recombinant adenovirus
Adeno-X™ expression system (Clontech Laboratories, Inc.), comprising adenovirus type 5 genome with a deletion in the E1 and E3 regions (ΔE1, 343–3465 bp; ΔE3, 28,756–30,561 bp), was utilized to construct a recombinant adenovirus carrying nucleotides −45 to 1469 of Spike gene of SARS-CoV (Ad-SN) by in vitro ligation (Mizuguchi and Kay, 1998, Mizuguchi and Kay, 1999), which encoded a truncated S1 subunit of SARS-CoV S protein (490 N-terminal amino-acid residues) (Fig. 1 ). Ad-LacZ, a recombinant adenoviral vector containing β-galactosidase gene, was constructed according to the same procedure and used as vector control. Both of the two viruses were propagated in 293 cells, purified by CsCl-banding and titered with the Adeno-X™ rapid titer kit (BD Biosciences, Inc.) (Eykholt et al., 2000). The prepared recombinant adenoviruses were monitored to prevent from contamination of bacterium, fungus, mycoplasma, wild type adenovirus and other viruses.
Fig. 1.
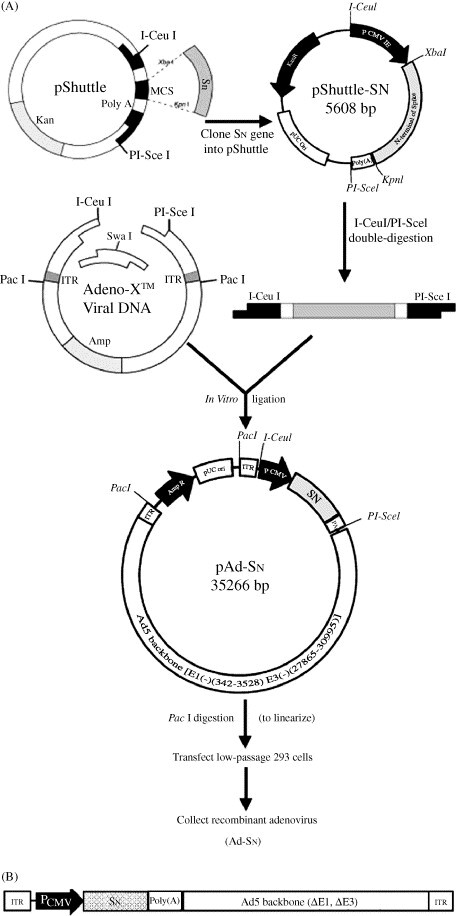
(A) Construction of recombinant adenovirus Ad-SN and (B) genomic structure of Ad-SN.
2.3. Reverse transcription-polymerase chain reaction (RT-PCT) analysis
For detecting SN expression at the mRNA level in Vero-E6 cells infected with recombinant adenoviruses, total cellular RNAs were isolated with the TRIZOL® reagent (Invitrogen), and then digested with RQ1 RNase-free DNase I (Promega) to remove residual DNA. The resulting mRNA was transcribed into cDNAs by the AMV Reverse Transcriptase System (Invitrogen) with oligo(dT)12–18, and RT-PCR was performed with SN-specific primers P1 (5′-CGAGTACATATCTGATGCC-3′) and P2 (5′-ACGCCATAGCACTTAAAGG-3′) to generate a 624 bp DNA fragment. β-actin was amplified with primers P3 (5′-CGTCTTCCCCTCCATCGTG-3′) and P4 (5′-CCCTCA TAGATGGGCACAG-3′) to produce a 417 bp fragment that was assayed as an internal control.
2.4. Western blot analysis
Cell lysates, culture supernatant or homogenized tissue samples were separated by 10% SDS-PAGE and transferred to a PVDF membrane. Blots were visualized with a Phototope-HRP Western blot detection system (New England BioLabs). Briefly, blots were blocked with 5% non-fat dry milk powder in TTBS (20 mM Tris–HCl, pH 8.0, 0.8% NaCl, 0.1% Tween-20) overnight at 4 °C, followed by a 4 h incubation at room temperature with one of the following primary antibodies: rabbit anti-spike IgG (1:200) (Abgent Cat# AP6009b), serum from a convalescent SARS patient, or sera from Ad-SN-immunized rats. Blots were rinsed with TTBS solution, and incubated with HRP-linked anti-rabbit/human/rat IgG (Santa Cruz, 1:1000) for 2 h. After rinsing, blots were incubated with LumiGLO™ (New England BioLabs) for 1 min and exposed to X-ray film.
2.5. Immunization of rats and detection of SARS-CoV-specific antibodies
Rats (n = 10 per group) were intranasally or subcutaneously immunized with Ad-SN (1 × 107 pfu/(dose·rat)), or with controls (Ad-LacZ or PBS). For intranasal immunization, viruses were dropped into the noses of rats after the animals were anaesthetized with amylobarbitone. Rats were immunized on day 0, 7 and 14. Sera were collected on day 0, 7, 14, 21 and 28. SARS-CoV-specific antibodies (IgGs) were titered by enzyme-linked immuno-sorbent assay (ELISA). Briefly, 96-well microtiter plates coated with SARS-CoV full antigens (provided by the Chinese Academy of Military Medicine Chinese Academy of Sciences, Huada Co. Ltd., Beijing) were blocked with PBS (pH 7.4) containing 1% BSA. Serial dilutions of rat sera were added to the wells and incubated at 37 °C for 30 min. Wells were rinsed five times with PBST (PBS containing 0.05% Tween 20), followed by a 20 min incubation with HRP-linked anti-rat IgG (1:5000) at 37 °C, five times of rinsing and another 10 min incubation with the substrate solution at 37 °C for in darkness. The reactions were terminated by stop solution, and optical densities (ODs) were determined using a microplate reader set at 450 nm with a wavelength correction at 630 nm. The endpoint titer was defined as the reciprocal of the highest dilution resulting in an OD ≥ 0.16 above that of the negative control. Samples were analyzed in duplicate.
2.6. Neutralizing activity analysis
The neutralizing activities of sera from immunized rats were tested in an in vitro micro-neutralization assay. Briefly, heat-inactivated rat sera were diluted two-fold and incubated with 100 TCID50 of SARS-CoV (BJ01 stain) at 37 °C for 1 h. Samples were then added into 96-well plates seeded with Vero-E6 cells (1 × 104 cells/well). The plates were incubated at 37 °C for 3–4 days, and the cytopathic effects (CPE) were observed daily. The neutralization titer was measured as the reciprocal of the highest serum dilution that completely inhibited Vero-E6 cell lysis in at least 60% of tested wells. All procedures were done at biosafety level 3.
2.7. Histopathological examination
Immunizated rats were sacrificed at day 28, various tissues (including lung, liver, colon, heart, brain, spleen, kidney, etc.) were collected, fixed with 10% PBS-buffered formalin, embedded in paraffin. Histopathological examination was performed on tissue sections stained with hematoxylin and eosin.
3. Results
3.1. Construction and characterization of the recombinant adenovirus, Ad-SN
To construct the recombinant adenoviral vector, an N-terminal fragment of the SARS-CoV Spike gene (from −45 to 1468, SN), derivated from a Guangdong patient suffering from SARS, was firstly cloned into the XbaI and KpnI sites of pShuttle. And then SN expression cassette was cloned into pAdeno-X™ by in vitro ligation at the unique restriction sites, I-CeuI and PI-SceI, to produce plasmid pAd-SN, which contained the Ad5 genome lacking the E1/E3 region, with PacI sites at each end of the Ad5 genome, and the SN expression cassette inserted into the E1 deletion site (Fig. 1). PCR analysis showed that pAd-SN contained the SN expression cassette, and endonuclease restriction digestion generated the expected fragments (data not shown). Sequencing analysis showed that the cloned SN gene has three variant bases (723G-T, 1024G-A, 1294C-T) compared with the SARS-CoV (BJ01 isolate) Spike gene, which encodes two deduced amino acid changes (342Gly-Arg, 432Pro-Ser) (GenBank accession no. AY862402).
Recombinant adenovirus Ad-SN was obtained by transfecting PacI-linearized pAd-SN DNA into 293 cells. PCR analysis showed that recombinant adenovirus Ad-SN contained the inserted SN gene, and restriction analysis showed that Ad-SN had the same digestion patterns as pAd-SN/PacI (data not shown). In addition, no contaminations of bacterium, fungus, mycoplasma, wild type adenovirus or other viruses were detected in prepared Ad-SN (data not shown).
3.2. Expression of the truncated S1 subunit of spike protein (SN)
For detecting SN in vitro expression at the transcriptional level, Vero-E6 cells were infected with Ad-SN or Ad-LacZ at the indicated multiplicities of infection (MOIs) for 48 h, and the expression of SN mRNA was tested by RT-PCR. Our results showed that SN mRNA was detected in Vero-E6 cells infected with Ad-SN, but not in cells infected with Ad-LacZ. Moreover, the expression of SN mRNA in cells infected with Ad-SN was dose-dependent (Fig. 2A).
Fig. 2.
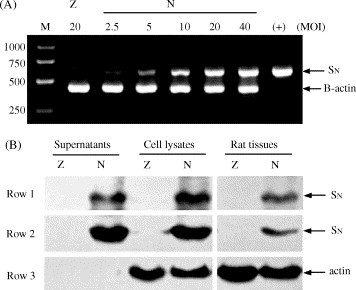
Expression of the SN fragment in vitro and in vivo (Z: Ad-LacZ; N: Ad-SN). (A) Vero-E6 cells (2 × 105 cells/well) were seeded into a 6-well plate and cultured for 16 h until cells reached 80% confluence. Cells were then infected with Ad-SN or Ad-LacZ at the indicated MOIs for 48 h. Total cellular RNAs were isolated, and SN mRNA was detected by RT-PCR amplification, with β-actin used as the internal control (SN 624 bp, β-actin 417 bp). (B) Vero-E6 cells were infected with Ad-SN or Ad-LacZ at 20 MOI for 48 h, then cells and the culture supernatants were collected. Alternatively, rats were injected subcutaneously with Ad-SN or Ad-LacZ (1 × 107 pfu/rat), after 48 h, tissues near the injection site were sampled and homogenized. Western blotting was used to detect SN protein in the infected cells, culture supernatants and rat tissues using the Phototope-HRP Western blot detection system (New England BioLabs). Serum from convalescent SARS patients (Row 1) or rabbit anti-spike IgG, SARS spike N-term D204 antibody (Row 2) were used as the primary antibodies, with an anti-actin mAb (C-2) (Santa Cruz, sc-8432) (Row 3) as the control.
For detecting expression of SN protein in vitro, Vero-E6 cells were infected with Ad-SN or Ad-LacZ at 20 MOI for 48 h, and the infected cells and their culture supernatants were collected. For detecting SN protein expression in vivo, rats were injected subcutaneously with Ad-SN or Ad-LacZ (1 × 107 pfu/rat). Fourty-eight hours later, injection locus tissues were sampled and homogenized. Western blotting was performed to detect SN protein in the various samples. A specific ∼60 kDa protein band, consistent with the predicted size of the SN protein, was clearly observed in Ad-SN-infected Vero-E6 cells, their culture supernatants and Ad-SN-treated tissues, as visualized by both commercial rabbit anti-spike IgG (Abgent) and sera from convalescent SARS patients, whereas no SN band was found in Ad-LacZ-infected cells or tissues (Fig. 2B).
3.3. Immunization and detection of SARS-CoV-specific antibodies
ELISA analysis showed SARS-CoV-specific antibody responses were detected in rats subjected to three i.n. or s.c. immunizations with Ad-SN, whereas no specific immune responses were found in animals treated with Ad-LacZ or PBS (Fig. 3A). SARS-CoV-specific IgGs were detected in Ad-SN-treated rats 2 weeks after the first immunization, with levels gradually increasing to maximum IgG titers of 1:29 and 1:29.1 in the i.n. and s.c. groups, respectively, by 2 weeks after the last treatment.
Fig. 3.
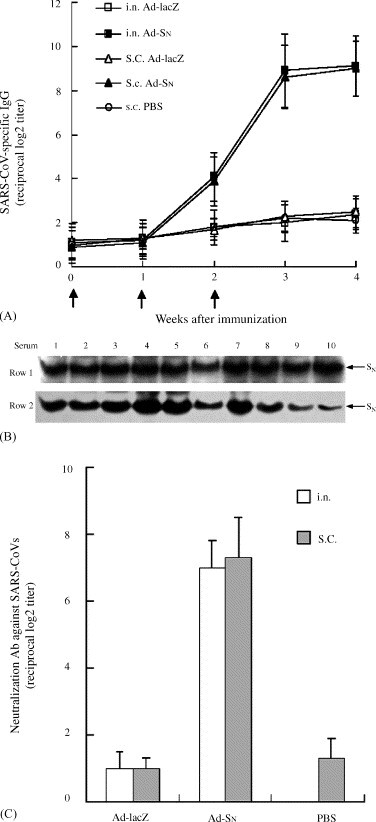
Ad-SN induced rats to produce high titers of antibodies (A, B) and neutralization activity (C). (A) ELISA assay for SARS-CoV-specific serum IgG in the rats immunized subcutaneously (s.c.) or intranasally (i.n.) with Ad-SN. Arrows (↑) indicate time points of immunization. (B) Western blot analysis of SN-specific antibodies in sera of immunized rats. Western blotting was performed with the Phototope-HRP Western Blot Detection System (New England BioLabs). The culture supernatants of Vero-E6 cells infected with Ad-SN (separated by 10% SDS-PAGE) were used as antigen, and sera from rats immunized with Ad-SN subcutaneously (Row 1) or intranasally (Row 2) were used as the primary antibodies. Bands indicate the existence of SN-specific antibodies in the sera of immunized rats. (C) Analysis of neutralizing activities of sera from the rats immunized subcutaneously or intranasally with Ad-SN.
The antibody response specific for SN protein in Ad-SN-vaccinated rats was tested by Western blot analysis. Our results showed that all of the sera from Ad-SN-immunized rats (i.n. and s.c.) showed visible Ag-Ab reactions with SN protein expressed in Ad-SN-infected Vero-E6 cells (Fig. 3B), while sera from Ad-LacZ-treated or PBS-treated rats did not (data not shown).
3.4. Neutralizing activity assay
For detecting the neutralizing activity of sera from immunized rats against SARS-CoV, Vero-E6 cells were challenged with SARS-CoV (BJ01 isolate). As expected, the sera of rats immunized with Ad-SN protected Vero-E6 cells against SARS-CoV (BJ01 stain) challenge in vitro, with neutralization titers up to 1:27 and 1:27.3 in the i.n. and s.c. groups, respectively, while the sera from control animals did not display any neutralization activity (Fig. 3C).
3.5. Histopathology
To evaluate the safety of Ad-SN vaccine candidate, histopathological examination was performed on tissue sections from immunizated rats. There was not any evident pathological change in either Ad-SN-immunized or Ad-LacZ-treated rat tissues (data not shown).
4. Discussion
Various strategies have been explored in the search for an effective vaccine against SARS-CoV, including the use of inactivated whole virus particles, attenuated live virus, purified viral structural proteins and gene vaccines (Tang et al., 2004, Xiong et al., 2004, Choy et al., 2004, Zhang et al., 2004, Zhou et al., 2004, Yang et al., 2004b, Bukreyev et al., 2004, Bisht et al., 2004, Gao et al., 2003). Recently, the gene vaccines, which express SARS-CoV structural proteins and induce host immunity against SARS-CoV, have become increasingly popular.
In this study, we constructed a replication-incompetent recombinant adenovirus containing nucleotides −45 to 1469 of Spike gene of SARS-CoV, named Ad-SN, which encodes a truncated S1 subunit of SARS-CoV S protein. Sequencing analysis revealed that the cloned SN fragment had three nucleotides difference compared with SARS-CoV BJ01 isolate, resulting in two amino-acid alterations in the predicted SN protein (GenBank accession no. AY862402). Computational prediction of antigenic peptides (Kolaskar and Tongaonkar, 1990, Singh and Raghava, 2001) [http://immunax.dfci.harvard.edu/Tools/antigenic.html and http://www.imtech.res.in/raghava/propred/] demonstrated that these variations did not influence the antigenicity of the recombinant protein. The secretory ∼60 kDa truncated SN protein, expressed in Vero-E6 cells and in rats infected by Ad-SN, was recognized by rabbit anti-spike IgG, sera from convalescent SARS patients and Ad-SN-immunized rats (Figs. 2B and 3B). This indicates that the truncated SN fragment is a potential antigen capable of inducing specific antibody responses in rats, rabbits and humans.
Previous studies have reported that several antigenic epitopes on the spike protein are recognized by sera from convalescent SARS patients and are capable of inducing neutralizing antibodies in humans (Hua et al., 2004, Guo et al., 2004). Spike protein or its S1 subunit have been reported that can induce efficient immunity against SARS-CoV and protect from the attack of SARS-CoV in animal models (Yang et al., 2004b, Bukreyev et al., 2004, Gao et al., 2003). In this study, we demonstrated that a truncated S1 subunit of spike protein (490 N-terminal amino-acid residues) could induce a strong humoral immune response against SARS-CoV in rats (Fig. 3A and B), and the sera from Ad-SN-immunized rats could effectively protect Vero-E6 cells against SARS-CoV (BJ01 isolate) infection in vitro (Fig. 3C). No evident pathological changes (e.g., inflammatory response) were observed in various tissues (including lung, liver, colon, heart, brain, spleen, kidney, etc.) of Ad-SN-immunized animals (data not shown), contrast to the finding of Weingartl et al. that immunization with modified vaccinia virus expressing the SARS-CoV spike (S) protein exhibited strong inflammatory responses in liver tissue in ferrets (Weingartl et al., 2004). This difference may be due to the difference of experimental animals, protocols and Spike gene fragments that vectors carried. Our present data cannot conclusively demonstrate whether truncated S1 gene vaccine will be a safer vaccine candidate. However, it can be presumed that truncated S1 gene carries less risk for spontaneous recombination with wild type virus to generate new virus types. Considering these favorites, truncated S1 subunit gene vaccine could be a safer vaccine candidate than the full-length Spike gene and may act as a pharmacologically active vaccine.
Replication-incompetent adenovirus vectors are widely used as foreign gene carriers for vaccines (Xiang and Ertl, 1999, Casimiro et al., 2003, Wu et al., 2003). Previous work has shown that immunization via the respiratory tract (i.e. intranasal) could induce protective mucosal immunity (Sakaue et al., 2003, Bukreyev et al., 2004, Xiang and Ertl, 1999). As respiratory epithelial cells are the natural hosts for adenovirus, recombinant adenovirus Ad-SN immunized through the respiratory tract should induce mucosal immunity as a first line of defense against viral attack. This is particularly important for SARS, which mainly spread through the respiratory tract. In this study, it was demonstrated that SARS-CoV-specific antibodies and neutralizing activities could be induced by intranasal immunization at the same serum level as that elicted by subcutaneous immunization (Fig. 3A and C). This indicates that recombinant adenovirus encoding truncated S1 subunit is a good candidate for development as a mucosal SARS-CoV vaccine.
Clinical observations in SARS patients imply that SARS-CoV seemed to elicit effective humoral immunity but inhibit cellular immunity, especially CD8+ memory T lymphocytes over time (Huang et al., 2005), so developing a vaccine to induce both humoral and cellular immune responses may be required to prevent from SARS-CoV infection (Tang et al., 2003, Li et al., 2003a). It is necessary to make clear whether Ad-SN can elicit T-cell immunity in rats. Comprehensive examinations of the associated immunological parameters will be further needed to evaluate the benefits of the various immunization strategies. In addition, the rat model may not faithfully replicate the immunological characteristics of humans. It will be necessary to test potential SARS vaccine candidates for their immunogenicity, safety and efficacy in other animal models, such as rabbit, primates, raccoons, dog and human.
Acknowledgement
We thank Dr. Wang Jian at the Chinese Academy of Sciences for providing SARS-CoV cDNA. This work was supported by grants from the National Basic Research Program of China (No. 2003CB514107, 2004CB518801), Ministry of Education, Guangdong Provincial Science Council and Guangzhou City Science Council (No. 2003Z3-E0451).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2005.02.009.
Appendix B. Supplementary data
References
- Berger A., Drosten Ch., Doerr H.W., Sturmer M., Preiser W. Severe acute respiratory syndrome (SARS)—paradigm of an emerging viral infection. J. Clin. Virol. 2004;29:13–22. doi: 10.1016/j.jcv.2003.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R., Subbarao K., Moss B. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz U.J., Bukreyev A., Yang L., Lamirande E.W., Murphy B.R., Subbarao K., Collins P.L. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukreyev A., Lamirande E.W., Buchholz U.J., Vogel L.N., Elkins W.R., St Claire M., Murphy B.R., Subbarao K., Collins P.L. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363:2122–2127. doi: 10.1016/S0140-6736(04)16501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro D.R., Chen L., Fu T.M., Evans R.K., Caulfield M.J., Davies M.E., Tang A., Chen M., Huang L., Harris V., Freed D.C., Wilson K.A., Dubey S., Zhu D.M., Nawrocki D., Mach H., Troutman R., Isopi L., Williams D., Hurni W., Xu Z., Smith J.G., Wang S., Liu X., Guan L., Long R., Trigona W., Heidecker G.J., Perry H.C., Persaud N., Toner T.J., Su Q., Liang X., Youil R., Chastain M., Bett A.J., Volkin D.B., Emini E.A., Shiver J.W. Comparartive immunogenicity in Rhesus monkey of DNA plasmid, recombinant Vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 2003;77:6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy W.Y., Lin S.G., Chan P.K., Tam J.S., Lo Y.M., Chu I.M., Tsai S.N., Zhong M.Q., Fung K.P., Waye M.M., Tsui S.K., Ng K.O., Shan Z.X., Yang M., Wu Y.L., Lin Z.Y., Ngai S.M. Synthetic peptide studies on the severe acute respiratory syndrome (SARS) coronavirus spike glycoprotein: perspective for SARS vaccine development. Clin. Chem. 2004;50:1036–1042. doi: 10.1373/clinchem.2003.029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa I., Gebauer F., Bullido M.J., Sune C., Baay M.F., Zwaagstra K.A., Posthumus W.P., Lenstra J.A., Enjuanes L. Localization of antigenic sites of the E2 glycoprotein of transmissible gastroenteritis coronavirus. J. Gen. Virol. 1990;71:271–279. doi: 10.1099/0022-1317-71-2-271. [DOI] [PubMed] [Google Scholar]
- Delmas B., Rasschaert D., Godet M., Gelfi J., Laude H. Four major antigenic sites of the coronavirus transmissible gastroenteritis virus are located on the amino-terminal half of spike glycoprotein S. J. Gen. Virol. 1990;71:1313–1323. doi: 10.1099/0022-1317-71-6-1313. [DOI] [PubMed] [Google Scholar]
- Eykholt R.L., Mitchell M.D., Marvin K.W. Accelerated titering of adenoviruses. Biotechniques. 2000;28:871–873. [Google Scholar]
- Fulginiti V.A., Eller J.J., Downie A.W., Kempe C.H. Altered reactivity to measles virus. A typical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967;202:1075–1080. doi: 10.1001/jama.202.12.1075. [DOI] [PubMed] [Google Scholar]
- Gao W., Tamin A., Soloff A., D’Aiuto L., Nwanegbo E., Robbins P.D., Bellini W.J., Barratt-Boyes S., Gambotto A. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F.L., Smiley J., Russell W.C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Guo J.P., Petric M., Campbell W., McGeer P.L. SARS corona virus peptides recognized by antibodies in the sera of convalescent cases. Virology. 2004;324:251–256. doi: 10.1016/j.virol.2004.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Geier M., Marzi A., Krumbiegel M., Peipp M., Fey G.H., Gramberg T., Pohlmann S. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem. Biophys. Res. Commun. 2004;319:1216–1221. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua R., Zhou Y., Wang Y., Hua Y., Tong G. Identication of two antigenic epitopes on SARS-CoV spike protein. Biochem. Biophys. Res. Commun. 2004;319:929–935. doi: 10.1016/j.bbrc.2004.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.L., Huang J., Duan Z.H., Wei J., Min J., Luo X.H., Li J.G., Tan W.P., Wu L.Z., Liu R.Y., Li Y., Shao J., Huang B.J., Zeng Y.X., Huang W. Th2 predominance and CD8+ memory T cell depletion in patients with severe acute respiratory syndrome. Microbes Infect. 2005;7:427–436. doi: 10.1016/j.micinf.2004.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingallinella P., Bianchi E., Finotto M., Cantoni G., Eckert D.M., Supekar V.M., Bruckmann C., Carfi A., Pessi A. Structural characterization of the fusion-active complex of severe acute respiratory syndrome (SARS) coronavirus. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8709–8714. doi: 10.1073/pnas.0402753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaskar A.S., Tongaonkar P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276:172–174. doi: 10.1016/0014-5793(90)80535-q. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Li G., Chen X., Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N. Engl. J. Med. 2003;349:508–509. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis N., Evelegh C., Graham F.L. Cloning and sequencing of the cellular-viral junctions from the human adenovirus type 5 transformed 293 cell line. Virology. 1997;233:423–429. doi: 10.1006/viro.1997.8597. [DOI] [PubMed] [Google Scholar]
- Marshall E., Enserink M. Medicine. Caution urged on SARS vaccine. Science. 2004;303:944–946. doi: 10.1126/science.303.5660.944. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H., Kay M.A. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum. Gene Ther. 1998;9:2577–2583. doi: 10.1089/hum.1998.9.17-2577. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H., Kay M.A. A simple method for constructing E1- and E1/E4-deleted recombinant adenoviral vectors. Hum. Gene Ther. 1999;10:2013–2017. doi: 10.1089/10430349950017374. [DOI] [PubMed] [Google Scholar]
- Polack F.P., Auwaerter P.G., Lee S.H., Nousari H.C., Valsamakis A., Leiferman K.M., Diwan A., Adams R.J., Griffin D.E. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat. Med. 1999;5:629–634. doi: 10.1038/9473. [DOI] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Sakaue G., Hiroi T., Nakagawa Y., Someya K., Iwatani K., Sawa Y., Takahashi H., Honda M., Kunisawa J., Kiyono H. HIV mucosal vaccine: nasal immunization with gp160-encapsulated hemagglutinating virus of Japan-liposome induces antigen-specific CTLs and neutralizing antibody responses. J. Immunol. 2003;170:495–502. doi: 10.4049/jimmunol.170.1.495. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf P., Ashraf H. Severe acute respiratory syndrome spreads worldwide. Lancet. 2003:361–1017. doi: 10.1016/S0140-6736(03)12843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Raghava G.P.S. Propred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236–1237. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- Song C.S., Lee Y.J., Lee C.W., Sung H.W., Kim J.H., Mo I.P., Izumiya Y., Jang H.K., Mikami T. Induction of protective immunity in chickens vaccinated with infectious bronchitis virus S1 glycoprotein expressed by a recombinant baculovirus. J. Gen. Virol. 1998;79:719–723. doi: 10.1099/0022-1317-79-4-719. [DOI] [PubMed] [Google Scholar]
- Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K., Moore M.J., Tallarico A.S., Olurinde M., Choe H., Anderson L.J., Bellini W.J., Farzan M., Marasco W.A. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Zhu Q., Qin E., Yu M., Ding Z., Shi H., Cheng X., Wang C., Chang G., Zhu Q., Fang F., Chang H., Li S., Zhang X., Chen X., Yu J., Wang J., Chen Z. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol. 2004;23:391–394. doi: 10.1089/104454904323145272. [DOI] [PubMed] [Google Scholar]
- Tang X., Yin C., Zhang F., Fu Y., Chen W., Chen Y., Wang J., Jia W., Xu A. Measurement of subgroups of peripheral blood T lymphocytes in patients with severe acute respiratory syndrome and its clinical significance. Chin. Med. J. 2003;116:827–830. [PubMed] [Google Scholar]
- Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J., Smith G., Jones S., Proulx R., Deschambault Y., Grudeski E., Andonov A., He R., Li Y., Copps J., Grolla A., Dick D., Berry J., Ganske S., Manning L., Cao J. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 2004;78:12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Moraes M.P., Grubman M.J. Recombinant adenovirus co-expressing capsid proteins of two serotypes of foot-and-mouth disease virus (FMDV): in vitro characterization and induction of neutralizing antibodies against FMDV in swine. Virus Res. 2003;93:211–219. doi: 10.1016/s0168-1702(03)00116-3. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Ertl H.C.J. Induction of mucosal immunity with a replication-defective adenoviral recombinant. Vaccine. 1999;17:2003–2008. doi: 10.1016/s0264-410x(98)00449-6. [DOI] [PubMed] [Google Scholar]
- Xiong S., Wang Y.F., Zhang M.Y., Liu X.J., Zhang C.H., Liu S.S., Qian C.W., Li J.X., Lu J.H., Wan Z.Y., Zheng H.Y., Yan X.G., Meng M.J., Fan J.L. Immunogenicity of SARS inactivated vaccine in BALB/c mice. Immunol. Lett. 2004;95:139–143. doi: 10.1016/j.imlet.2004.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang G., Li J., Nie Y., Shi X., Lian G., Wang W., Yin X., Zhao Y., Qu X., Ding M., Deng H. Identification of an antigenic determinant on the S2 domain of the severe acute respiratory syndrome coronavirus spike glycoprotein capable of inducing neutralizing antibodies. J. Virol. 2004;78:6938–6945. doi: 10.1128/JVI.78.13.6938-6945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Wang H., Luo D., Rowe T., Wang Z., Hogan R.J., Qiu S., Bunzel R.J., Huang G., Mishra V., Voss T.G., Kimberly R., Luo M. An exposed domain in the severe acute respiratory syndrome coronavirus spike protein induces neutralizing antibodies. J. Virol. 2004;78:7217–7226. doi: 10.1128/JVI.78.13.7217-7226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


