Highlights
► We measured EC50 and CC50 of the five well-known or potential antiviral agents. ► The antiviral effect against of 6-Azauridine was demonstrated. ► We tested the combination effects of the pairs of antiviral agents. ► Enhanced antiviral effect of Ad-porcine IFN-α with Ribavirin was demonstrated. ► Enhanced antiviral effect of Ad-porcine IFN-α and Ad-siRNA was demonstrated.
Abbreviations: CPE, cytopathic effect; CC50, cytotoxic concentration required to reduce cell viability by 50%; EC50, effective concentration required to reduce virus-induced cytopathogenicity by 50%; FMD, foot-and-mouth disease; FMDV, foot-and-mouth disease virus; Guanidine-HCl, Guanidine hydrochloride; IFN, interferon; IP, intraperitoneal; SD, standard deviation; siRNA, small interference RNA; shRNA, short hairpin RNA; RNAi, RNA interference; RT-PCR, reverse transcriptase polymerase chain reaction; TCID50, 50% tissue culture infective dose; DPC, days post challenge; P.I., post infection; LD50, 50% lethal dose
Keywords: Foot-and-mouth disease virus, Antiviral agent, Interferon, Small interfernce RNA, Ribavirin, Combination
Abstract
Foot-and-mouth disease (FMD) is an economically significant animal disease because of the speed of its transmission. The current FMD vaccine provides no protection until 7 days after the vaccination, which reduces its effectiveness in the case of an outbreak. Therefore, to find an alternative method of applying antiviral agents for rapid and enhanced inhibition of the FMD virus (FMDV), we compared the antiviral effects of promising antiviral agents and attempted to apply them in combination. First, we measured and compared the 50% effective concentration (EC50) to the mean inhibition effects of FMDV, and the 50% cytotoxic concentration (CC50) to the mean cytotoxicity of antiviral agents such as ribavirin, guanidine-hydrochloride (guanidine-HCl), 6-azauridine, and recombinant adenovirus expressing three small interference RNAs (Ad-siRNA) or porcine interferon-α (Ad-porcine IFN-α) in swine kidney cells (IBRS-2). The selectivity indices of ribavirin (35.2) and 6-azauridine (34.6) were higher than that of guanidine-HCl (26.9). The selectivity indices of Ad-siRNA or Ad-porcine IFN-α were 7 × 103 or 7 × 104 based on the adenoviral titer. Next, we tested the combined effects of the FMDV inhibition agents. Enhanced inhibition effects were observed in the IBRS-2 cells and in suckling mice from the combination of Ad-porcine IFN-α and Ad-siRNA or ribavirin. The combined application of these recombinant adenoviruses and ribavirin may enhance their inhibitory effect on FMDV and overcome FMDV resistance against antiviral agents.
1. Introduction
Foot-and-mouth disease (FMD) is a highly contagious disease that affects cloven-hoofed animals such as cattle, swine, and sheep (Pereira, 1981). The ability of the FMD virus (FMDV) to spread rapidly in susceptible animals has led to FMD being considered a disease serious enough to be listed by the World Organization for Animal Health (OIE). FMDV belongs to the genus Aphthovirus in the family Picornaviridae (Bachrach, 1968). The virus genome is an 8.5-kb, positive-sense single-stranded RNA containing one open reading frame. There are 7 serotypes: A, O, Asia1, C, SAT1, SAT2, and SAT3; moreover, numerous subtypes have evolved within each serotype (Knowles and Samuel, 2003; Domingo et al., 2003). The use of current FMD vaccines to induce early protection is limited because protection cannot take effect until 7 days after the vaccination (Golde et al., 2005). Therefore, an alternative method of applying antiviral agents is required to reduce the spread of FMDV in outbreak situations. Guanidine-hydrochloride (guanidine-HCl), ribavirin, interferon (IFN), and small interference RNA (siRNA) have been proposed as promising antiviral agents with different mechanisms. However, their inhibition effects on FMDV have not been evaluated although they have different antiviral efficiency. Furthermore, treatment with individual antiviral agents does not lead to perfect inhibition because of limitations related to viral resistance.
Ribavirin and guanidine-HCl are known as chemical compounds that inhibit FMDV replication in cells (Airaksinen et al., 2003, Nettleton et al., 1982). Guanidine-HCl inhibits the replication of picornaviruses such as FMDV and poliovirus. Ribavirin has antiviral effects against a broad range of viruses, including FMDV, by inhibition of RNA-capping activity or viral polymerase, and by increasing mutation frequency (Graci and Cameron, 2006). A broad-spectrum anti-metabolite, 6-azauridine inhibits virus replication (Rada and Dragun, 1977). It has been reported that 6-azauridine effectively inhibits RNA viruses such as Chikungunya virus, Semliki Forest virus, and human coronavirus at low concentration (Briolant et al., 2004, Pyrc et al., 2006). However, it has not been reported whether 6-azauridine effectively inhibits FMDV. Recombinant adenovirus expressing porcine IFN-α induces high levels of biologically active IFN in cell supernatants and has antiviral effects against FMDV in cells, and the protection lasts for 3–5 days in swine inoculated with the adenovirus (Chinsangaram et al., 2003). RNA interference (RNAi), which causes gene silencing in a sequence-specific manner, is mediated by short hairpin RNA. Recently, researchers reported the inhibition of FMDV replication using plasmids or adenovirus expressing RNAi (Chen et al., 2006, Chen et al., 2004, Kahana et al., 2004). We have reported significant antiviral effects against FMDV by adenovirus expressing multiple siRNA targeting non-structural protein genes (Kim et al., 2010). The multiple siRNA enhanced antiviral efficiency and had antiviral effects against 7 FMDV serotypes.
However, FMDV possesses resistance mechanisms against antiviral agents, such as amino acid substitutions against mutagens, mutation against siRNA target sequence, and type I IFN suppression (Belsham and Normann, 2008, de la Torre et al., 1987, Pariente et al., 2003, Pfister and Wimmer, 1999, Pusch et al., 2003). Our group anticipated that the combination treatment of antiviral agents with different mechanisms might be more advantageous in overcoming their individual limitations. Moreover, FMD researchers have reported that combination treatment such as type I and II IFN, or guanidine-HCl and ribavirin enhanced the antiviral effect (Moraes et al., 2007, Perales et al., 2009).
Here, we examined and compared the inhibition effect and cytotoxicity of the following well-known or potential antiviral agents: guanidine-HCl, ribavirin, 6-azauridine, and recombinant adenovirus expressing porcine IFN-α (Ad-porcine IFN-α) or multiple siRNA (Ad-siRNA 2B-3C1-3C2 in a previous study (Kim et al., 2010), Ad-siRNA in this study). Furthermore, we tested the combined effects of four pairs of mixed antiviral agents selected from the following five agents: guanidine-HCl, ribavirin, 6-azauridine, Ad-porcine IFN-α, and Ad-siRNA. This is the first time the enhanced antiviral effect of the combination of Ad-porcine IFN-α and Ad-siRNA or Ad-porcine IFN-α and ribavirin were demonstrated.
2. Materials and methods
2.1. Cells, animals, viruses and virus titration
Human embryonic kidney cells, including those that contain human adenovirus type 5 E1 DNA (293A cells), and swine kidney cells (IBRS-2) were propagated in Dulbecco’s modified Eagle’s medium (D-MEM) supplemented with 10% fetal bovine serum (FBS; pH 7.4) and antibiotics at 37 °C with 5% CO2. CD-1 (ICR) suckling mice, 6 days old and weighing 4–5 g, were purchased from Orient Co., Ltd. (Republic of Korea). The animals were treated in accordance with the ethical guidelines of the Animal Welfare Committee of the Animal, Plant, and Fisheries Quarantine and Inspection Agency (QIA). FMDVs from strain O/SKR/2002 (GenBank accession numbers AY312589 and AY312588) were used for the viral challenges. Virus titers for FMDV and adenovirus were determined in IBRS-2 and 293A cells, respectively. The 50% tissue culture infective dose (TCID50) was calculated using the formula of Reed and Muench (Reed and Muench, 1938).
2.2. Antivirals: chemical compounds and recombinant adenoviruses
We purchased the commercial chemical compounds ribavirin (Sigma R9644, USA), guanidine-HCl (Thermo Scientific 24115, USA), and 6-azauridine (Sigma Aldrich A1882, USA), and produced the recombinant adenoviruses (Ad-siRNA and Ad-porcine IFN-α) in our laboratory. Ribavirin and guanidine-HCl have been reported for application in cases of FMDV (Black and Brown, 1969, de la Torre et al., 1987), while 6-azauridine has not. We reported previously that recombinant adenovirus expressing three different siRNA sequences targeting the non-structural protein regions 2B and 3C (Ad-siRNA) significantly inhibits FMDV replication (Kim et al., 2010). To produce Ad-porcine IFN-α, the porcine IFN-α1 gene (GenBank accession number NM_214393) was synthesized by Bioneer Corp. (Republic of Korea) and cloned into a pENTR™/SD-TOPO cloning vector (Invitrogen, USA). Recombinant human adenovirus was produced according to the manufacturer’s instructions. Briefly, we performed a recombination reaction using a pENTR™/SD-TOPO construct (Invitrogen), pAd/CMV/V5-DEST™ Gateway Vector (Invitrogen), and Gateway LR Clonase™ Enzyme Mix (Invitrogen), and 293A cells were transfected with the adenovirus constructs digested with PacI using Lipofectamine 2000 (Invitrogen). When approximately 80% cytopathic effect (CPE) was observed, the adenovirus was harvested. The harvested virus was purified using a ViraBind Adenovirus Miniprep Kit (Cell Biolabs, Inc., USA). Adenovirus stock of 108–109 TCID50/mL was used for the experiment.
2.3. FMDV inhibition assay and cytotoxicity assay
The inhibition effects and cytotoxicity were measured in IBRS-2 cells to compare the effectiveness of the antiviral agents against FMDV. IBRS-2 cells of 3.5 × 104 per well were plated on 96-well plates. On the following day, the plated cells were 90% confluent at the time of infection. Cells were infected with 100 TCID50 FMDV and treated with recombinant adenoviruses and serially diluted chemical compounds 1 h post-FMDV infection. The cells were incubated at 37 °C for 72 h until the maximum CPE of FMDV was reached. The level of viability of the infected cells was determined by an MTS assay using CellTiter 96 AQueous One Solution Proliferation Assay (Promega, USA). The antiviral activity was expressed as the 50% effective concentration (EC50), defined as the concentration required to reduce virus-induced cytopathogenicity by 50% of the control value. A similar procedure was used for the cytotoxicity assay, but the cells were treated with recombinant adenoviruses and chemical compounds without FMDV infection. Cytotoxicity was determined by an MTS assay using CellTiter 96 AQueous One Solution Proliferation Assay (Promega). Cytotoxicity was expressed as the 50% cytotoxic concentration (CC50), defined as the concentration required to reduce cell viability by 50% of the control value.
2.4. Antiviral reagent inoculation, viral challenge, and analysis in IBRS-2 cells
To investigate the antiviral effects of the antiviral agents and their combination in vitro, IBRS-2 cells were inoculated on 96-well plates. Cells were inoculated with recombinant adenovirus expressing porcine IFN-α or siRNA, ribavirin, guanidine-HCl, 6-azauridine, or combinations of two antiviral agents 24 h before FMDV infection for the experiments illustrated in Fig. 1, Fig. 2 . Ad-siRNA, Ad-porcine IFN-α, and ribavirin treatments were carried out 2, 6, and 24 h before FMDV infection, as depicted in Fig. 3 . In the experiment illustrated in Fig. 4 , Ad-porcine IFN-α and ribavirin treatments were carried out 24 or 6 h before FMDV infection for the simultaneous treatment experiment. Ad-porcine IFN-α and ribavirin were added at 24 and 6 h, respectively, before FMDV infection for the sequential treatment experiment. The adenovirus or chemical compound was removed and cells were washed twice with D-MEM, and IBRS-2 cells were immediately infected with 1000 or 3000 TCID50 FMDV. After 1-h absorption, the inocula were removed and 100 μL culture medium containing 2% FBS was added in the experiments depicted in Fig. 3, Fig. 4. The inocula were not removed in the experiments depicted in Fig. 1, Fig. 2. The cells were incubated at 37 °C for 48 h. The supernatant was collected at 48 h post-infection (p.i.) and analyzed with replication analysis. Quantitative real-time RT-PCR was carried out to assay the effects of the antiviral agents on FMDV RNA replication. Viral RNA was extracted by the MagNa Pure LC 96 System (Roche, Switzerland). Real-time RT-PCR was conducted as described previously (Kim et al., 2010). A t-test was performed for statistical analysis using Graph Pad InStat (Ver. 3.05).
Fig. 1.
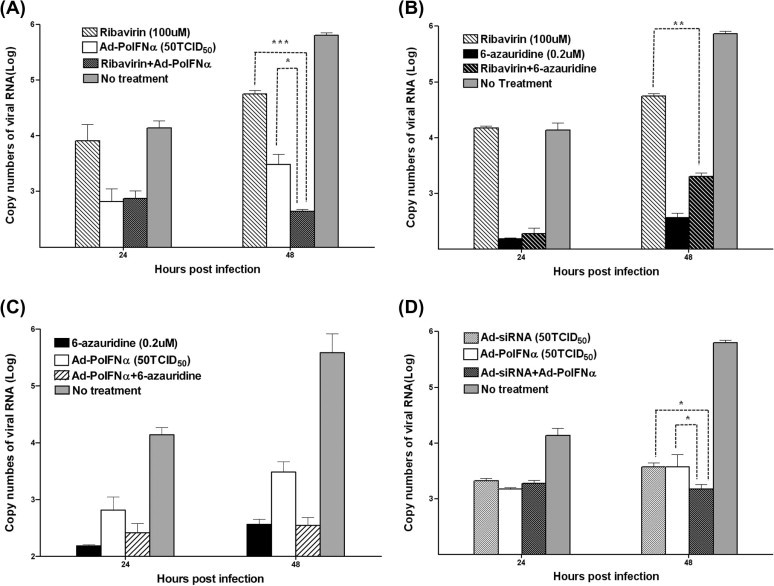
Effects of combined antiviral agents on inhibition of FMDV replication IBRS-2 cells were inoculated with 50 TCID50 adenovirus expressing porcine IFN-α (Ad-porcine IFN-α or Ad-PoIFNα) or multiple siRNAs (Ad-siRNA), ribavirin (100 μM), and 6-azauridine (0.2 μM). Ribavirin and Ad-porcine IFN-α (A), ribavirin and 6-azauridine (B), 6-azauridine and Ad-porcine IFN-α (C), and Ad-siRNA and Ad-porcine IFN-α (D) were administered to the cells to observe the combination effect. After 24 h, cells were infected with 1000 TCID50 FMDV (O/SKR/2002) after the inoculum was removed. The supernatants were analyzed using RNA extraction and real-time RT-PCR at 24 and 48 h post-FMDV infection. A t-test was performed for statistical analysis (∗P < 0.05 and ∗∗∗P < 0.0005). Error bars indicate standard deviations (SD).
Fig. 2.
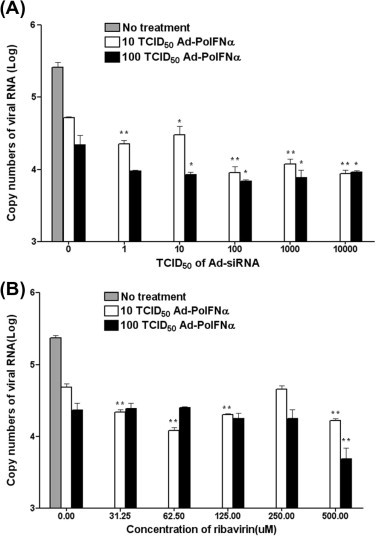
Combination effects of Ad-porcine IFN-α (Ad-PoIFNα) and various amounts of Ad-siRNA or ribavirin. IBRS-2 cells were inoculated with 10 or 100 TCID50 Ad-porcine IFN-α and various titers (or concentrations) of Ad-siRNA (A) and ribavirin (B). After 24 h, cells were infected with 3000 TCID50 FMDV (O/SKR/2002) after the inoculum was removed. The supernatants were analyzed using RNA extraction and real-time RT-PCR at 48 h post-FMDV infection. A t-test was performed for statistical analysis between the single antiviral agent treatment with Ad-porcine IFN-α and others (∗P < 0.05 and ∗∗P < 0.005). Error bars indicate SD.
Fig. 3.
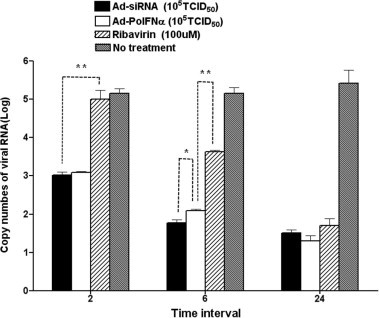
Antiviral effect of Ad-porcine IFN-α, Ad-siRNA, and ribavirin at various times after treatment IBRS-2 cells were inoculated with 105 TCID50 Ad-porcine IFN-α and Ad-siRNA and 100 μM ribavirin at 2, 6, and 24 h before FMDV infection. Cells were infected with 1000 TCID50 FMDV (O/SKR/2002) after the inoculum was removed. After 1-h adsorption, the cells were rinsed with D-MEM, and culture medium containing 2% fetal bovine serum (FBS) was added. The supernatants were collected 48 h post-FMDV infection and analyzed using RNA extraction and real-time RT-PCR. A t-test was performed for statistical analysis (∗P < 0.05 and ∗∗P < 0.005). Error bars indicate SD.
Fig. 4.
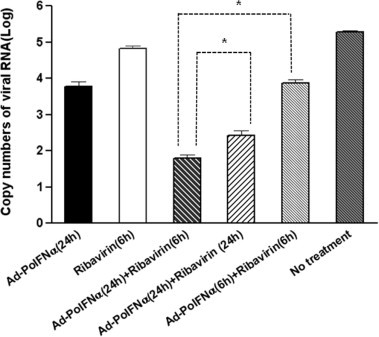
Inhibition effects of simultaneous and sequential treatment of Ad-porcine IFN-α and ribavirin IBRS-2 cells were inoculated with 105 TCID50 Ad-porcine IFN-α or 100 μM ribavirin at 6 or 24 h before FMDV infection. Cells were simultaneously or sequentially inoculated with Ad-porcine IFN-α and ribavirin at 24 and 6 h before FMDV infection. Cells were infected with 1000 TCID50 FMDV (O/SKR/2002) after the inoculum was removed. After 1-h adsorption, the cells were rinsed with D-MEM, and culture medium containing 2% FBS was added. The supernatants were collected 48 h post-FMDV infection and analyzed using RNA extraction and real-time RT-PCR. A t-test was performed for statistical analysis (∗P < 0.05). Error bars indicate SD.
2.5. Antiviral reagent injection, viral challenge, and monitoring in suckling mice
We used 6-day-old suckling mice to investigate the antiviral effects in vivo in this study. The FMDV dose was determined through 10-fold serial dilutions of the virus. The 50% lethal dose (LD50) of FMDV used was estimated by the Reed-Muench method (Reed and Muench, 1938). Suckling mice were inoculated by intraperitoneal (IP) injection with 0.01 mg ribavirin and 2 × 107 TCID50 adenovirus. Combination group 2, treated with Ad-porcine IFN-α and Ad-siRNA, was injected with 2 × 107 TCID50 of each adenovirus. After 18 h, combination group 1, treated with ribavirin and Ad-porcine IFN-α (sequential treatment) was injected with 0.01 mg ribavirin. After 24 h of adenovirus or ribavirin injections, the suckling mice were challenged with 125 LD50 or 250 LD50 FMDV (O/SKR/2002) in a 0.05-mL IP injection. The animals were monitored for 7 days. A log rank test was performed for statistical analysis using Graph Pad InStat (Ver. 3.05).
3. Results
3.1. Selectivity of agents as FMDV inhibitors
The inhibition effect of the antiviral agents was determined by the inhibition of FMDV-induced CPE, and the cytotoxicity of the antiviral agents was determined by the viability of treated IBRS-2 cells. When the selectivity indices for effective antiviral agents were calculated as the ratio of CC50 to EC50, all agents demonstrated selectivity indices >20 against FMDV (Table 1 ).
Table 1.
The effect of antiviral agents on FMDV.
| Agent | CC50a | EC50b | Selectivity index (CC50/EC50) |
|---|---|---|---|
| Ad-Porcine IFN-α | 10.1 ± 3.8 × 104 TCID50 | 1.4 ± 0.5 × 100 TCID50 | 7.1 × 104 |
| Ad-siRNA 2B-3C1-3C2 | 18 ± 7.1 × 103 TCID50 | 2.5 ± 1.4 × 100 TCID50 | 7.0 × 103 |
| Ribavirin | 960.9 ± 11 μM | 27.3 ± 5 μM | 35.2 |
| Guanidine HCl | 147 ± 13 μM | 5.46 ± 1 μM | 26.9 |
| 6-Azauridine | 11.12 ± 2 μM | 0.321 ± 0.0 13 μM | 34.6 |
Data are mean ± standard deviations (SD) from three independent experiments.
Cytotoxic concentration required to reduce cell viability by 50% of the control value.
Effective concentration required to reduce virus-induced cytopathogenicity by 50% of the control value.
Regarding the chemical compounds, ribavirin and 6-azauridine had selectivity indices of 35.2 and 34.6, respectively. These were higher than that of guanidine-HCl, which had a selectivity index of 26.9. The selectivity index of 6-azauridine, whose effect against FMDV had been unknown up to this point, was similar to that of ribavirin. Ribavirin proved to be the most selective FMDV inhibitor, and guanidine-HCl had the lowest selectivity index of the chemical compounds examined in this study.
The selectivity indices of adenoviruses expressing siRNA or porcine IFN-α were 7 × 103 or 7 × 104 based on the adenoviral titer. In the IBRS-2 cells, the selectivity index of porcine IFN-α was 10-fold higher than that of siRNA.
3.2. Enhanced antiviral effects by combination of antiviral agents
To determine if a combination of antiviral agents had a greater antiviral effect than that of an individual agent, FMDV replication in IBRS-2 cells was analyzed after combination and individual treatments (Fig. 1). All the antiviral agents exhibited statistically significant inhibition of FMDV replication compared to the control (P < 0.05, t-test). The antiviral effect of combined treatment using ribavirin (100 μM) and Ad-porcine IFN-α (50 TCID50) was more enhanced than that of the treatment using ribavirin (P = 0.0003, t-test) or Ad-porcine IFN-α (P = 0.02, t-test) alone at 48 h p.i. (Fig. 1A). Additionally, the antiviral effect of combination treatment using Ad-siRNA (50 TCID50) and Ad-porcine IFN-α (50 TCID50) was more enhanced than that of the treatment using Ad-siRNA (P = 0.027, t-test) or Ad-porcine IFN-α (P = 0.043, t-test) alone at 48 h p.i. (Fig. 1D).
However, the inhibition effect due to combination treatment using ribavirin (100 μM) and 6-azauridine (0.2 μM) was lower than that due to 6-azauridine treatment alone, although the inhibition effect of combination treatment was higher than that using ribavirin alone (P = 0.002, t-test) (Fig. 1B). The antiviral effect of the combination of Ad-porcine IFN-α and 6-azauridine (0.2 μM) was similar to that of sole 6-azauridine treatment (Fig. 1C). Ribavirin or Ad-porcine IFN-α was not helpful to the anti-FMDV effect of 6-azauridine.
We also examined the effect of 10 or 100 TCID50 Ad-porcine IFN-α combined with various amounts (or titers) of ribavirin and Ad-siRNA on FMDV replication (Fig. 2). The combinations of 10 or 100 TCID50 Ad-porcine IFN-α with the titers of Ad-siRNA improved the antiviral effects more than the individual Ad-porcine IFN-α treatment did (P < 0.05 or 0.005, Fig. 2A). The inhibition effects were proportional to the Ad-siRNA titers in the combination featuring 10 TCID50 Ad-porcine IFN-α. In particular, the combination of 10 TCID50 Ad-porcine IFN-α with 1 TCID50 Ad-siRNA enhanced the inhibition effects on viral copy number by approximately 2-fold as compared to that by the treatment with 10 TCID50 Ad-porcine IFN-α alone. The combination of Ad-porcine IFN-α with a titer greater than 100 TCID50 Ad-siRNA enhanced the inhibition effects on viral copy number by approximately 5-fold compared to the treatment with Ad-porcine IFN-α alone. The combinations of 10:100, 100:10, and 100:100 TCID50 Ad-porcine IFN-α and Ad-siRNA exhibited similar antiviral effects.
The combinations of 10 TCID50 Ad-porcine IFN-α with most ribavirin concentrations, except 250 μM ribavirin, improved the antiviral effects more than the treatment with 10 TCID50 Ad-porcine IFN-α did (P < 0.005, Fig. 2B). The combination of Ad-porcine IFN-α with 62.5 μM or 500 μM ribavirin enhanced the inhibition effects on viral copy number by approximately 4-fold compared to treatment with Ad-porcine IFN-α alone. Moreover, the combination of Ad-porcine IFN-α with 31.25 μM or 125 μM ribavirin enhanced the inhibition effects on viral copy number by approximately 2.3-fold as compared to that by treatment with Ad-porcine IFN-α alone. However, the combination of 100 TCID50 Ad-porcine IFN-α with most concentrations of ribavirin, except 500 μM ribavirin, did not improve the antiviral effect compared to that of treatment with 100 TCID50 Ad- porcine IFN-α (P > 0.05).
3.3. Comparison of activation time for antiviral effect: ribavirin, Ad-porcine IFN-α, and Ad-siRNA
To investigate how quickly the antiviral effects were induced, we compared the inhibition effects of Ad-siRNA, Ad-porcine IFN-α, and ribavirin administered at various intervals before FMDV infection (2, 6, and 24 h) (Fig. 3). At 2 h before FMDV infection, Ad-siRNA and Ad-porcine IFN-α decreased FMDV copy numbers in the adenovirus treatment group by log 3. However, there was a statistically significant difference in the antiviral effects between ribavirin and the adenoviruses because ribavirin did not have significant antiviral effects (P = 0.0021). Ad-siRNA was more effective than Ad-porcine IFN-α in the initial stage of treatment 6 h before FMDV infection (P = 0.0121). Moreover, the antiviral effects of the adenoviruses were superior to that of ribavirin, though the antiviral effect of ribavirin was improved as compared to that at 2 h before infection. At 24 h before FMDV infection, the Ad-siRNA, Ad-porcine IFN-α, and ribavirin treatments demonstrated considerable antiviral effects with similar levels of statistical significance (P > 0.05). Ad-siRNA and Ad-porcine IFN-α took effect more quickly than ribavirin, though the inhibition effect of the agents depended on the incubation period up until 24 h.
3.4. Comparison of antiviral effect in simultaneous or sequential treatment with ribavirin and Ad-porcine IFN-α
We compared the antiviral effect of simultaneous treatment, administering a mixture of Ad-porcine IFN-α and ribavirin, to that of sequential treatment, in which Ad-porcine IFN-α treatment was followed by ribavirin treatment, because there was a possibility that ribavirin might have antiviral effects on recombinant adenoviruses (Lenaerts and Naesens, 2006). As shown in Fig. 4, the groups that received the combination treatment using ribavirin and Ad-porcine IFN-α exhibited significantly inhibited FMDV replication. However, sequential treatment using Ad-porcine IFN-α and ribavirin at 24 and 6 h, respectively, before FMDV infection was more effective than simultaneous treatment with Ad-porcine IFN-α and ribavirin at 24 h (P < 0.05) or 6 h (P < 0.05) before FMDV infection.
3.5. Combination of antiviral agents: Ad-porcine IFN-α and Ad-siRNA (or ribavirin) and the antiviral effects on suckling mice
To test the anti-FMDV effect of adenovirus-mediated siRNAs, porcine IFN-α, ribavirin, and their combinations in vivo, we injected antiviral agents into suckling mice and then challenged them with 125 LD50 or 250 LD50 FMDV O/SKR/2002 (Fig. 5 ). Adenovirus expressing 3 siRNAs (Ad-siRNA) or porcine IFN-α (Ad-porcine IFN-α), and ribavirin inhibited the viral replication of FMDV in suckling mice (P < 0.005, log rank test). The combination treatment group, treated with 2 × 107 TCID50 Ad-porcine IFN-α and 0.01 mg ribavirin, and the Ad-porcine IFN-α group had a 90% survival rate at 7 days post-challenge (dpc) by 125 LD50 FMDV (Fig. 5A). Of the Ad-siRNA and Ad-porcine IFN-α combination group and the Ad-siRNA group, 2 × 107 TCID50 maintained 100% survival up to 7 dpc by 125 LD50 FMDV (Fig. 5B). In addition, the Ad-porcine IFN-α group maintained a 90% survival rate.
Fig. 5.
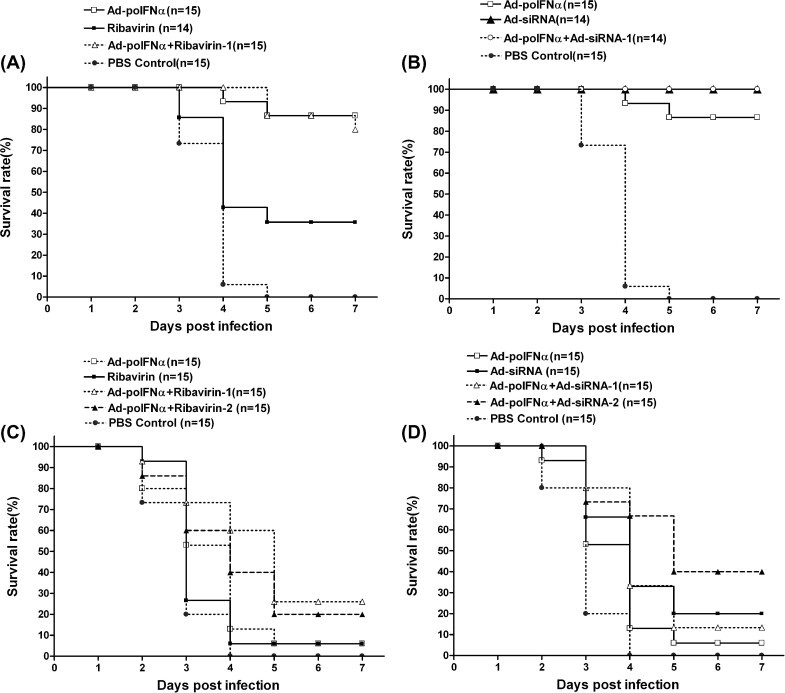
Enhanced survival rate after FMDV challenge by single or combination with Ad-porcine IFN-α, Ad-siRNA, and ribavirin in suckling mice Suckling mice received intraperitoneal injections of 2 × 107 TCID50 Ad-porcine IFN-α and Ad-siRNA, and 0.01 mg ribavirin at 24 h or 6 h before FMDV infection. Ad-porcine IFN-α was administered 24 h before FMDV infection and ribavirin was administered 6 h before FMDV infection except for combination group 2 (A and C). Combination group 2 received a mixture of Ad-porcine IFN-α and ribavirin 24 h before FMDV infection. Combination group 1 received 1 × 107 TCID50 each of Ad-porcine IFN-α and Ad-siRNA (B and D). Combination group 2 received 2 × 107 TCID50 each of Ad-porcine IFN-α and Ad-siRNA. Suckling mice were challenged with 125 LD50 (A and B) or 250 LD50 (C and D) FMDV (O/SKR/2002). Animals were monitored for 7 days.
The combination of Ad-porcine IFN-α with ribavirin enhanced the survival rate of the mice as shown in Fig. 5C. Combination group 1 (sequential treatment) maintained a survival rate of 26%, and combination group 2 (simultaneous treatment) maintained a survival rate of 20% up to 7 dpc, while the Ad-porcine IFN-α or ribavirin group had a survival rate of 6% or below at 7 dpc. The difference of survival rates between the sequential and simultaneous treatments was clearly demonstrated at 3 and 4 dpc, although the survival rate of the sequential treatment group was constantly higher than that of the simultaneous treatment group during the monitoring period. The combination effect of Ad-porcine IFN-α and Ad-siRNA against 250 LD50 FMDV is demonstrated clearly in Fig. 5D. The combination group 1 administered with 1 × 107 TCID50 Ad-porcine IFN-α and 1 × 107 TCID50 Ad-siRNA had a survival rate of 13% at 7 dpc. The survival rate was higher than that of Ad-porcine IFN-α alone (6%) and lower than that of Ad-siRNA alone (20%). However, the combination group 2 with 2 × 107 TCID50 Ad-porcine IFN-α and 2 × 107 TCID50 Ad-siRNA had a survival rate of 40%, which was higher than that of the groups treated solely with Ad-porcine IFN-α or Ad-siRNA at 7 dpc.
4. Discussion
Several antiviral agents against FMDV such as chemical compounds, IFN, and siRNA have been proposed as an alternative method of FMD prevention (Airaksinen et al., 2003, Nettleton et al., 1982, Chinsangaram et al., 2003, Chen et al., 2006). These antiviral agents have different mechanisms for virus inhibition. Ribavirin is a mutagenic nucleoside analogue that is converted to its triphosphate form by cellular enzymes. Recently, the lethal mutagenesis of FMDV by ribavirin was analyzed on a molecular basis (Ehrenfeld et al., 2010, Perales et al., 2011). The study reported that ribavirin leads to lethal mutagenesis by accelerating the occurrence of AU-rich mutant clouds during the early replication stage, and not by increasing viral mutation rate. Guanidine-HCl blocks the ATPase activity of nonstructural protein 2C, a protein involved in poliovirus replication (Saunders et al., 1985). Type I IFNs, IFN-α and IFN-β, lead to the expression of genes that have antiviral activity (Der et al., 1998). Researchers have reported that treatment with IFN-α inhibited FMDV in cells, and the process is involved with IFN α/β-stimulated genes (ISGs), 2′–5′ oligoadenylate synthetase (OAS) and double-stranded RNA-dependent protein kinase (PKR) (Chinsangaram et al., 2001, Chinsangaram et al., 1999). Gene silencing is caused by siRNA in a sequence-specific manner and leads to the inhibition of virus replication. Our group has reported that adenovirus expressing three different siRNAs targeting non-structural protein genes in the 2B or 3C regions possess enhanced and broad antiviral effects against FMDV (Kim et al., 2010).
However, FMDV possesses resistance mechanisms against antiviral agents. FMDV induces amino acid mutation of the 3D region to decrease sensitivity to ribavirin, and the FMDV leader sequence negatively regulates type I IFN (Agudo et al., 2010, de Los Santos et al., 2006, Ferrer-Orta et al., 2010, Sierra et al., 2007, Wang et al., 2011). Amino acid substitutions at non-structural protein 2C have been associated with resistance to guanidine-HCl in FMDV (Belsham and Normann, 2008, Pfister and Wimmer, 1999, Saunders et al., 1985). Additionally, siRNA loses its effect due to escape mutants of the virus (Sabariegos et al., 2006). Therefore, we anticipated that combination treatment using antiviral agents with different inhibition mechanisms would be more effective at circumventing FMDV resistance. Combination antiviral therapy has become standard for some human disease viruses, for example, the human immunodeficiency virus and human hepatitis C virus (Bhagani, 2011).
For this reason, we compared the antiviral effects of ribavirin, guanidine-HCl, 6-azauridine, Ad-porcine IFN-α, and Ad-siRNA to select the most effective inhibitor (Table 1). In the current study, CC50 and EC50 were expressed using “concentration” as the unit of measurement for the chemical compounds and “titer” as the unit of measurement for the recombinant adenovirus. In particular, the inhibition effect of 6-azauridine against FMDV was discovered for the first time in this study. The EC50 of Ad-porcine IFN-α and Ad-siRNA were measured under the 10 TCID50 titer. However, Ad-siRNA led to higher cytotoxicity than Ad-porcine IFN-α, though it also demonstrated an excellent inhibition effect. As we predicted, Ad-siRNA with 3 siRNAs under U6 promoters induced mRNA expression of IFN-β, double-stranded RNA-dependent PKR, and 2′–5′ OAS in IBRS-2 cells, in addition to RNA interference (Kim et al., 2010).
Next, we tested the combination effects of the antiviral agents with different inhibition mechanisms (Fig. 1). The combination of ribavirin and 6-azauridine caused an adverse effect. We suggest that ribavirin and 6-azauridine, nucleoside analogs, create mutual metabolic interference. Moreover, a recent report demonstrated that the combination of ribavirin with a nucleoside polymerase inhibitor resulted in an antagonistic effect (Thibaut et al., 2011). The combination of Ad-porcine IFN-α with 6-azauridine did not demonstrate as much enhanced antiviral effect as the individual Ad-porcine IFN-α or 6-azauridine treatments did. The reason the Ad-porcine IFN-α and 6-azauridine combination did not have an additive inhibition effect is not clear. However, we suggest that 6-azauridine might have an adverse effect on adenoviral infection or IFN expression.
To our knowledge, this is the first report demonstrating that the combination of ribavirin and porcine IFN-α has an enhanced inhibition effect against FMDV. Furthermore, the combination of siRNA with type I IFN was applied to inhibit FMDV. Previous studies have reported that the induction of type I IFN enhances the antiviral effect of siRNA in human hepatitis B virus (Han et al., 2011). Additionally, we have suggested that adenovirus expressing 3 siRNAs under U6 promoters activate the genes related with type I IFN and enhance antiviral effects (Kim et al., 2010). Therefore, we suggest in the current study that siRNA and type I IFN do not interfere with their respective effects, and that their combination may be a potential antiviral strategy.
The combination of 100 TCID50 Ad-porcine IFN-α and ribavirin, except at high concentration (500 μM ribavirin), did not lead to an enhanced antiviral effect, whereas the single adenovirus treatment did (Fig. 2B). The reason might be the combination of the high-dose recombinant adenovirus and ribavirin. The combination of ribavirin and human IFN-α protein is a common treatment method for human hepatitis C (Bhagani, 2011). Therefore, we suggest that ribavirin might interfere with the expression of porcine IFN-α in the recombinant adenovirus, or a part of ribavirin might inhibit the adenovirus. A previous study reported that ribavirin had antiviral effects on adenoviruses (Lenaerts and Naesens, 2006). However, the mechanism of ribavirin against adenovirus and its antiviral effect on recombinant adenovirus is not clear because several mechanisms have been proposed to explain the antiviral activity. Due to this possibility, we compared the simultaneous inoculation of Ad-porcine IFN-α and ribavirin, and sequential inoculation, where ribavirin treatment followed adsorption of Ad-porcine IFN-α (Fig. 4). The sequential inoculation of Ad-porcine IFN-α and ribavirin at 24 and 6 h, respectively, before FMDV infection was more effective than their simultaneous inoculation. The result was consistent with the results of Perales et al. (Perales et al., 2009). Sequential treatment with ribavirin and guanidine-HCl was more effective than the simultaneous combination treatment. Therefore, we carried out the sequential or simultaneous injection of Ad-porcine IFN-α and ribavirin in suckling mice to expand the examination to animals (Fig. 5A and 5C).
The effects of the antiviral agents and their combination were also demonstrated in the animal experiment (Fig. 5). We demonstrated that the mixture of Ad-porcine IFN-α and Ad-siRNA or ribavirin had a positive effect on FMDV inhibition, and that the effect of 1 antiviral did not interfere with that of the other in vivo. The survival rate of Ad-siRNA was higher than that of Ad-porcine IFN-α in the same titer in 125 LD50 and 250 LD50, although the selectivity index of Ad-porcine IFN-α in IBRS-2 cells was 10-fold higher than that of Ad-siRNA (Table 1). One possible reason is that the inhibition effect of multiple siRNA was higher than that of porcine IFN-α. Another possible reason is that the inhibition effect of Ad-porcine IFN-α was enhanced by the stimulation of IFN pathway-related genes in porcine kidney cells (Chinsangaram et al., 2003, Chinsangaram et al., 1999), and that the gene stimulation might not have taken place because the suckling mice model lacks IFN responses (Ryman et al., 2000). The survival rate of combination group 1, treated with Ad-porcine IFN-α and Ad-siRNA, was lower than that treated with Ad-siRNA alone, while that of combination group 2, treated with a mixture of single-dose Ad-porcine IFN-α and Ad-siRNA, was higher than that of single-dose Ad-siRNA. A possible explanation for this result could be that the antiviral effect is proportional to adenovirus titer, and the survival rate of the Ad-siRNA-treated group was higher than that of the group treated with Ad-porcine IFN-α in the same titer.
We anticipated that combination treatment using Ad-porcine IFN-α, Ad-siRNA, or ribavirin would have advantages for FMDV control. The first of these advantages is that the combination treatment could lead to more rapid and effective inhibition of FMDV because antiviral agents have different activation times. We investigated how quickly the antiviral effects of Ad-porcine IFN-α, Ad-siRNA, or ribavirin were induced in IBRS-2 cells (Fig. 3). We expected Ad-siRNA and Ad-porcine IFN-α, especially Ad-siRNA, to have greater advantages than ribavirin, and that the addition of Ad-siRNA and Ad-porcine IFN-α could induce rapid inhibition when applied to an emergency situation. Second, the combination treatment could maintain the inhibition effect for a longer period because the combination of antiviral agents could be advantageous to circumventing FMDV resistance and the antiviral agents might have varying half-lifes in animals. Furthermore, Ad-porcine IFN-α stimulates the genes related to antiviral effects as time progresses after the treatment, and has a direct effect by the porcine IFN-α protein (Chinsangaram et al., 2003). Third, we suggest that their combination can reduce the quantities of single agents used to inhibit FMDV. Therefore, we propose that the combination of Ad-porcine IFN-α and ribavirin or Ad-porcine IFN-α and Ad-siRNA could be effective when used as an emergency or combination agent with vaccines in order to prevent the spread of FMDV. It could be a very promising method if the agent could start to take effect within 24 h and could maintain the antiviral effect until the formation of immunity by vaccination. Further study in a natural host would provide conclusive evidence.
In conclusion, we compared promising antiviral agents and effective combinations of these agents for the inhibition of FMDV in vitro and in vivo. The data reported in this study may be used to develop more effective and long-lasting inhibition of FMDV. We suggest that the combinations proposed in this study: 1) Ad-siRNA with Ad-porcine IFN-α, or 2) Ribavirin with Ad-porcine IFN-α, may be advantageous in inhibiting FMDV because they have different mechanisms and activation times. Further study is necessary to test their enhanced antiviral effect in natural hosts such as swine, cattle, or goats, and against more FMDV strains. A combination ratio and injection dose could be determined in a natural host.
Acknowledgement
This work was supported by the Animal, Plant and Fisheries Quarantine and Inspection Agency (QIA), Republic of Korea.
References
- Agudo R., Ferrer-Orta C., Arias A., de la Higuera I., Perales C., Perez-Luque R., Verdaguer N., Domingo E. A multi-step process of viral adaptation to a mutagenic nucleoside analogue by modulation of transition types leads to extinction-escape. PLoS Pathog. 2010;6:e1001072. doi: 10.1371/journal.ppat.1001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen A., Pariente N., Menendez-Arias L., Domingo E. Curing of foot-and-mouth disease virus from persistently infected cells by ribavirin involves enhanced mutagenesis. Virology. 2003;311:339–349. doi: 10.1016/s0042-6822(03)00144-2. [DOI] [PubMed] [Google Scholar]
- Bachrach H.L. Foot-and-mouth disease. Annu. Rev. Microbiol. 1968;22:201–244. doi: 10.1146/annurev.mi.22.100168.001221. [DOI] [PubMed] [Google Scholar]
- Belsham G.J., Normann P. Dynamics of picornavirus RNA replication within infected cells. J. Gen. Virol. 2008;89:485–493. doi: 10.1099/vir.0.83385-0. [DOI] [PubMed] [Google Scholar]
- Bhagani S. Current treatment for chronic hepatitis C virus/HIV-infected individuals: the role of pegylated interferon-alpha and ribavirin. Curr. Opin. HIV AIDS. 2011;6:483–490. doi: 10.1097/COH.0b013e32834bd257. [DOI] [PubMed] [Google Scholar]
- Black D.N., Brown F. Effect of actinomycin D and guanidine on the formation of a ribonucleic acid polymerase induced by foot-and mouth-disease virus and on the replication of virus and viral ribonucleic acid. Biochem. J. 1969;112:317–323. doi: 10.1042/bj1120317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briolant S., Garin D., Scaramozzino N., Jouan A., Crance J.M. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination. Antiviral Res. 2004;61:111–117. doi: 10.1016/j.antiviral.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Chen W., Liu M., Jiao Y., Yan W., Wei X., Chen J., Fei L., Liu Y., Zuo X., Yang F., Lu Y., Zheng Z. Adenovirus-mediated RNA interference against foot-and-mouth disease virus infection both in vitro and in vivo. J. Virol. 2006;80:3559–3566. doi: 10.1128/JVI.80.7.3559-3566.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Yan W., Du Q., Fei L., Liu M., Ni Z., Sheng Z., Zheng Z. RNA interference targeting VP1 inhibits foot-and-mouth disease virus replication in BHK-21 cells and suckling mice. J. Virol. 2004;78:6900–6907. doi: 10.1128/JVI.78.13.6900-6907.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsangaram J., Koster M., Grubman M.J. Inhibition of L-deleted foot-and-mouth disease virus replication by alpha/beta interferon involves double-stranded RNA-dependent protein kinase. J. Virol. 2001;75:5498–5503. doi: 10.1128/JVI.75.12.5498-5503.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsangaram J., Moraes M.P., Koster M., Grubman M.J. Novel viral disease control strategy: adenovirus expressing alpha interferon rapidly protects swine from foot-and-mouth disease. J. Virol. 2003;77:1621–1625. doi: 10.1128/JVI.77.2.1621-1625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsangaram J., Piccone M.E., Grubman M.J. Ability of foot-and-mouth disease virus to form plaques in cell culture is associated with suppression of alpha/beta interferon. J. Virol. 1999;73:9891–9898. doi: 10.1128/jvi.73.12.9891-9898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J.C., Alarcon B., Martinez-Salas E., Carrasco L., Domingo E. Ribavirin cures cells of a persistent infection with foot-and-mouth disease virus in vitro. J. Virol. 1987;61:233–235. doi: 10.1128/jvi.61.1.233-235.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Los Santos T., de Avila Botton S., Weiblen R., Grubman M.J. The leader proteinase of foot-and-mouth disease virus inhibits the induction of beta interferon mRNA and blocks the host innate immune response. J. Virol. 2006;80:1906–1914. doi: 10.1128/JVI.80.4.1906-1914.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der S.D., Zhou A., Williams B.R., Silverman R.H. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E., Escarmis C., Baranowski E., Ruiz-Jarabo C.M., Carrillo E., Nunez J.I., Sobrino F. Evolution of foot-and-mouth disease virus. Virus Res. 2003;91:47–63. doi: 10.1016/s0168-1702(02)00259-9. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Domingo E., Roos R.P., editors. Mutation, Quasispecies and Lethal Mutagenesis, the Picornaviruses. ASM Press; Washington, DC: 2010. (pp. 1970–211) [Google Scholar]
- Ferrer-Orta C., Sierra M., Agudo R., de la Higuera I., Arias A., Perez-Luque R., Escarmis C., Domingo E., Verdaguer N. Structure of foot-and-mouth disease virus mutant polymerases with reduced sensitivity to ribavirin. J. Virol. 2010;84:6188–6199. doi: 10.1128/JVI.02420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde W.T., Pacheco J.M., Duque H., Doel T., Penfold B., Ferman G.S., Gregg D.R., Rodriguez L.L. Vaccination against foot-and-mouth disease virus confers complete clinical protection in 7 days and partial protection in 4 days: Use in emergency outbreak response. Vaccine. 2005;23:5775–5782. doi: 10.1016/j.vaccine.2005.07.043. [DOI] [PubMed] [Google Scholar]
- Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q., Zhang C., Zhang J., Tian Z. Involvement of activation of PKR in HBx-siRNA-mediated innate immune effects on HBV inhibition. PLoS One. 2011;6:e27931. doi: 10.1371/journal.pone.0027931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana R., Kuznetzova L., Rogel A., Shemesh M., Hai D., Yadin H., Stram Y. Inhibition of foot-and-mouth disease virus replication by small interfering RNA. J. Gen. Virol. 2004;85:3213–3217. doi: 10.1099/vir.0.80133-0. [DOI] [PubMed] [Google Scholar]
- Kim S.M., Lee K.N., Lee S.J., Ko Y.J., Lee H.S., Kweon C.H., Kim H.S., Park J.H. Multiple shRNAs driven by U6 and CMV promoter enhances efficiency of antiviral effects against foot-and-mouth disease virus. Antiviral Res. 2010;87:307–317. doi: 10.1016/j.antiviral.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Knowles N.J., Samuel A.R. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003;91:65–80. doi: 10.1016/s0168-1702(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Lenaerts L., Naesens L. Antiviral therapy for adenovirus infections. Antiviral Res. 2006;71:172–180. doi: 10.1016/j.antiviral.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Moraes M.P., de Los Santos T., Koster M., Turecek T., Wang H., Andreyev V.G., Grubman M.J. Enhanced antiviral activity against foot-and-mouth disease virus by a combination of type I and II porcine interferons. J. Virol. 2007;81:7124–7135. doi: 10.1128/JVI.02775-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton P.F., Davies M.J., Rweyemamu M.M. Guanidine and heat sensitivity of foot-and-mouth disease virus (FMDV) strains. J. Hyg. (London) 1982;89:129–138. doi: 10.1017/s0022172400070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente N., Airaksinen A., Domingo E. Mutagenesis versus inhibition in the efficiency of extinction of foot-and-mouth disease virus. J. Virol. 2003;77:7131–7138. doi: 10.1128/JVI.77.12.7131-7138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales C., Agudo R., Tejero H., Manrubia S.C., Domingo E. Potential benefits of sequential inhibitor-mutagen treatments of RNA virus infections. PLoS Pathog. 2009;5:e1000658. doi: 10.1371/journal.ppat.1000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales C., Henry M., Domingo E., Wain-Hobson S., Vartanian J.P. Lethal mutagenesis of foot-and-mouth disease virus involves shifts in sequence space. J. Virol. 2011;85:12227–12240. doi: 10.1128/JVI.00716-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H.G. Academic Press; New York, NY: 1981. Foot-and-mouth disease animals. pp. 333–363. [Google Scholar]
- Pfister T., Wimmer E. Characterization of the nucleoside triphosphatase activity of poliovirus protein 2C reveals a mechanism by which guanidine inhibits poliovirus replication. J. Biol. Chem. 1999;274:6992–7001. doi: 10.1074/jbc.274.11.6992. [DOI] [PubMed] [Google Scholar]
- Pusch O., Boden D., Silbermann R., Lee F., Tucker L., Ramratnam B. Nucleotide sequence homology requirements of HIV-1-specific short hairpin RNA. Nucleic Acids Res. 2003;31:6444–6449. doi: 10.1093/nar/gkg876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K., Bosch B.J., Berkhout B., Jebbink M.F., Dijkman R., Rottier P., van der Hoek L. Inhibition of human coronavirus NL63 infection at early stages of the replication cycle. Antimicrob. Agents Chemother. 2006;50:2000–2008. doi: 10.1128/AAC.01598-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B., Dragun M. Antiviral action and selectivity of 6-azauridine. Ann. NY Acad. Sci. 1977;284:410–417. doi: 10.1111/j.1749-6632.1977.tb21977.x. [DOI] [PubMed] [Google Scholar]
- Reed L.I., Muench H. A simple method of estimating fifty percent end points. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Ryman K.D., Klimstra W.B., Nguyen K.B., Biron C.A., Johnston R.E. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 2000;74:3366–3378. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabariegos R., Gimenez-Barcons M., Tapia N., Clotet B., Martinez M.A. Sequence homology required by human immunodeficiency virus type 1 to escape from short interfering RNAs. J. Virol. 2006;80:571–577. doi: 10.1128/JVI.80.2.571-577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K., King A.M., McCahon D., Newman J.W., Slade W.R., Forss S. Recombination and oligonucleotide analysis of guanidine-resistant foot-and-mouth disease virus mutants. J. Virol. 1985;56:921–929. doi: 10.1128/jvi.56.3.921-929.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M., Airaksinen A., Gonzalez-Lopez C., Agudo R., Arias A., Domingo E. Foot-and-mouth disease virus mutant with decreased sensitivity to ribavirin: implications for error catastrophe. J. Virol. 2007;81:2012–2024. doi: 10.1128/JVI.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut H.J., Leyssen P., Puerstinger G., Muigg A., Neyts J., De Palma A.M. Towards the design of combination therapy for the treatment of enterovirus infections. Antiviral Res. 2011;90:213–217. doi: 10.1016/j.antiviral.2011.03.187. [DOI] [PubMed] [Google Scholar]
- Wang D., Fang L., Li P., Sun L., Fan J., Zhang Q., Luo R., Liu X., Li K., Chen H., Chen Z., Xiao S. The leader proteinase of foot-and-mouth disease virus negatively regulates the type I interferon pathway by acting as a viral deubiquitinase. J. Virol. 2011;85:3758–3766. doi: 10.1128/JVI.02589-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


