Abstract
To identify management practices associated with an increased within-herd prevalence of Cryptosporidium parvum shedding on dairy farms in southern Ontario, fecal samples were taken from 1089 calves aged 7–28 days, from 119 herds. Information on management practices was obtained by administering a questionnaire compiled using a modified Delphi technique. Data were analyzed using univariable and multivariable negative binomial regression. Overall, 30% of the calves in the study were shedding C. parvum oocysts, with at least one positive calf detected in 77% of herds. Within-herd prevalence ranged from 0 to 80%. Predictors significantly associated with an increased prevalence of shedding in multivariable modelling were the use of calf scour prophylaxis in cows (risk ratio [RR] 1.70, P < 0.01) and calves (RR 1.38, P = 0.02) and the feeding of milk replacer in the first week of life (RR 1.40, P = 0.02). In contrast, the presence of concrete flooring in calf housing areas (RR 0.59, P < 0.01) and the use of soap or detergent when washing calf feeding utensils (RR 0.61, P < 0.01) appeared to be protective.
Keywords: Cryptosporidium parvum, Dairy calves, Risk factors, Ontario
1. Introduction
Cryptosporidium parvum infection, and the diarrhea it causes, produces losses to the dairy industry in terms of increased labour and veterinary costs associated with calf morbidity and, occasionally, mortality (de Graaf et al., 1999). On dairy farms, disease associated with C. parvum infection occurs primarily in young calves. Infection spreads from animal to animal by the fecal–oral route, and large numbers of infective and resistant oocysts may be shed in the feces of infected calves (Nydam et al., 2001). In addition, C. parvum is a zoonosis, and cattle have been implicated as a potential source of human infection via contamination of drinking water sources by oocysts shed in the feces of infected animals (Sischo et al., 2000).
The results of several studies have revealed that C. parvum is very common among dairy calves. Sischo et al. (2000) found that 15% of 198 dairy calves under 3 weeks of age on 11 farms in the northeastern United States were shedding Cryptosporidium oocysts, with oocysts detected on 10 of the 11 farms. In a larger study, 59% of 7369 pre-weaned dairy calves on 1103 farms in 28 states shed Cryptosporidium oocysts, with a prevalence of 48% among 1607 calves aged 1–3 weeks old (Garber et al., 1994). In Canada, C. parvum oocysts were detected in the feces of 59% of 386 calves under 6 months old on 16 of 20 British Columbia dairies (Olson et al., 1997), while in Québec, infected calves were found on 89% of 505 dairy farms (Ruest et al., 1998). In 2002, the within-herd prevalence of C. parvum shedding by calves 7–21 days of age on 51 southwestern Ontario dairies ranged from 0 to 70% (Trotz-Williams et al., 2005a). In 2003, a within-herd prevalence of 35% to 100% was found in a sample of calves under 1 month old on 11 dairy herds in the same region (Trotz-Williams et al., submitted for publication). Most chemotherapeutic agents and disinfectants are ineffective against this parasite (Korich et al., 1990, Weir et al., 2002). Prevention of infection is, therefore, the most viable option for reducing the incidence and impact of infection in dairy calves.
Various studies have investigated the association between farm management practices and the occurrence of C. parvum on dairy farms. Potential risk factors for shedding identified by such studies have included large herd size, use of multi-cow maternity facilities, and a long calving season (Garber et al., 1994, Atwill et al., 1999). However, factors associated with the prevalence of C. parvum infection and disease on farms may vary depending on geographical region. A calf-level study carried out on 11 dairies in southwestern Ontario identified the type of facilities (individual vs. multi-cow calving pens) in which calves were born as a factor significantly associated with the risk of C. parvum shedding. In that study, most of the variation in the risk of shedding occurred between herds, as opposed to within herds (intra-class correlation coefficient = 0.4; Trotz-Williams et al., submitted for publication). Similarly, in 51 herds in southwestern Ontario from which calves were sampled in 2002, shedding of C. parvum showed apparent clustering within herds as opposed to within herds (ICC 0.2; Trotz-Williams et al., 2005a). Although these herds, for the most part, had previous or current problems with diarrhea or cryptosporidiosis, the large herd-to-herd variation indicated that an investigation of herd-level risk factors for shedding, rather than calf-level parameters, would be more likely to generate information that would effectively control C. parvum on farms. Therefore, the study reported here was carried out with the objective of identifying factors associated with the shedding of C. parvum oocysts at the herd level in southern Ontario.
2. Materials and methods
2.1. Estimation of sample size
In planning the study, the assumption was made that the mean within-herd prevalence of C. parvum shedding in exposed herds was 0.3, with a standard deviation of 0.2, and that, in non-exposed herds, the prevalence was 0.2 with a standard deviation of 0.1. Using these assumptions with the <sampsi> command in Stata 8.0 (Stata Corporation, College Station, Texas) generated an estimated sample size of 40 farms per group for a power of 80% and a confidence level of 95% when analysing the effect of one exposure variable. This number was adjusted to 53 farms per group to allow for the investigation of 8 covariates (Dohoo et al., 2003a).
2.2. Farms
A convenience sample of herds was obtained with the assistance of veterinary practitioners in southern Ontario. Practitioners were asked to encourage their dairy producer clients to participate. Criteria for participation were: anticipated availability of calves for sampling during summer and fall 2004 (the time of the study), location of the farms (no more than 5 hours’ drive from the Ontario Veterinary College (OVC), University of Guelph), and willingness to complete a short questionnaire on farm management practices. In an attempt to optimize the external validity of the results of the work, veterinarians were asked to enrol farms representing as wide a range of farm management practices as possible. In addition, in order to further ensure that such farms would be included, as well as to encourage the recruitment of farms with a wide range of C. parvum within-farm prevalence, they were requested to recruit one farm without neonatal calf diarrhea problems (‘non-diarrheic’) for each farm recruited that was experiencing a high frequency of calf diarrhea (‘diarrheic’). Definitions of ‘diarrheic’ and ‘non-diarrheic’ farms were subject to the interpretation of the veterinarians. All herds recruited were included in the study.
Male (bull) and female (heifer) calves 7–28 days of age were sampled on the farms enrolled in the study. Each farm was visited up to a maximum of four times between May and October 2004, in order to sample as many calves as were available during the course of the study, to a maximum of 15 calves per farm. This maximum number of calves sampled per farm was chosen on the basis of practical limitations to the number of samples that could be processed, and also to ensure a reasonable ability to detect infected farms if C. parvum was present, based on previous estimates of prevalence of >30%.On farms with up to 15 calves 7–28 days of age available for sampling, all eligible calves were included. However, if more than 15 eligible animals were available, specimens were taken from a systematic sample of 15 calves of various ages within the desired age range.
2.3. Sampling
A single fecal specimen was collected per rectum from each calf in a clean, labelled vial. Specimens were transported to the OVC in a cooler and stored at 4 °C for a maximum of 5 days until examined for C. parvum oocysts.
On each farm, blood samples were drawn by jugular venepuncture from up to 5 calves under 7 days old. Blood was collected in 7 ml sterile vacutainers (Becton Dickinson, Mississauga, Ontario, Canada) without anti-coagulant, and was transported to the OVC for separation of serum and assessment of total serum protein by refractometry (Calloway et al., 2002).
2.4. Testing of fecal samples
Fecal specimens were examined for the presence of C. parvum oocysts by a sucrose wet mount method previously described. The performance of this test has been assessed using 199 fecal specimens from diarrheic and non-diarrheic calves 7–28 days old. In that work, in which polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) of the Cryptosporidium oocyst wall protein gene served as a gold standard, the sensitivity and specificity of this test were 88.6% and 93.8%, respectively (Trotz-Williams et al., 2005b).
2.5. Questionnaires
Questionnaires were prepared based on hypothesized predictors selected using a modified Delphi technique (Duffield, 1988). Initially, 50 dairy practitioners enrolled in a continuing education course at the OVC were asked to participate in this process. Practitioners were asked to rank a list of farm and calf management practices to reflect the perceived importance of each factor in causing, directly or indirectly, Cryptosporidium infection and diarrhea among dairy calves. Participants were also encouraged to list any additional factors or practices considered to be important risk factors. The results were used to identify those factors ranked as most important by the group. The same group of veterinary practitioners was then asked to rank each factor in this second list to reflect its perceived importance. Management factors and practices from this second list that were considered by the group to be most important were used to construct survey questions for participating producers. The resulting questionnaire included questions on the management of cows and their calves in the perinatal period, colostrum feeding practices, feeding and housing of pre-weaned calves, cleaning of feeding utensils, and cleaning of maternity and calf housing areas. Following pre-testing on five farms, this questionnaire was administered on each farm by one of two research technicians in a standardized manner. Technicians were blind to the status of herds at the time of administration of the questionnaire. A copy of the questionnaire is available from the authors in English.
2.6. Statistical analysis
Data entry and checking were performed using EpiData 3.02 (EpiData Association, Odense, Denmark) and data were exported to Stata 8.0 (Stata Corporation, College Station, Texas) for screening and analysis.
Categorical variables for which one level was represented by more than 90% of the farms (29 variables) were excluded from the analysis. Variables representing responses to questions that appeared to have been subject to misinterpretation (3 variables) were also excluded.
Negative binomial modelling with robust standard errors was used for univariable and multivariable analysis using the <nbreg> command in Stata 8.0, with the number of positive fecal samples on a farm as the outcome and the total number of fecal samples from the farm as the exposure. The assumption of linearity was assessed for each continuous predictor by visual examination of smoothed scatterplots of the outcome against the continuous variable (Dohoo et al., 2003b), and by tabulating the mean within-farm prevalence of C. parvum shedding by categories of the predictor, achieved using the <lintrend> command in Stata 8.0. Using either quartiles or cut-points that reflected the change in risk, as most appropriate, non-linear continuous predictors were transformed into categorical variables for unconditional and multivariable analysis. Because of the large number of predictors being screened, a liberal significance level of 25% in the univariable models was used as the criterion for inclusion of predictors in the multivariable models. Before model-building, groups of variables were checked for collinearity by examining covariance matrices for subsets of the variables. Where variables were found to be collinear, only the variable most significantly associated with the dependent variable in unconditional analysis was included in the model-building process. Because of the large number of variables that were significant at the 25% level of significance, variables were further screened by constructing 2 preliminary multivariable models before final model-construction. This was done by constructing one multivariable model including management variables concerning the prenatal and perinatal period (a ‘perinatal’ model), and another including those relevant to the pre-weaning period after removal of the calf from the calving area (a ‘pre-weaning’ model). Variables remaining in each of the final models from this process were then combined for construction of a single final model.
For each multivariable model, predictors with significance levels lower than 5% (P < 0.05) were selected for inclusion by a manual backward elimination approach (Dohoo et al., 2003c). Significance levels were evaluated for continuous and dichotomous predictors using Wald's test. The likelihood ratio test was used to assess the significance of categorical variables. During model-building, confounding was assessed by evaluating the effect of adding predictors to, and removing predictors from, the model. Predictors that, when added or removed, caused a change of more than 25% in the model coefficients, were considered confounders and were kept in the model regardless of their level of significance. Variables significant in the final model were checked for interaction by the inclusion of biologically appropriate two-way interaction terms. Statistically significant interaction terms (P < 0.05) were considered to be evidence of interaction, and were retained in the model.
Evaluation of model fit for the final model was achieved by constructing generalized linear models using the <glm> command in Stata 8.0 with the log link and ‘nbinomial’ family specifications, and including the variables present in the final negative binomial models constructed during multivariable analysis. Residuals, leverage, and values for Cook's distance were examined to assess general model fit, outliers, and observations with undue leverage or influence on the model.
3. Results
3.1. Prevalence of C. parvum
In total, fecal samples were collected from 1089 calves on 119 dairy farms. Of these, 324 (30%) tested positive for C. parvum by microscopy. Within-herd prevalence ranged from 0 to 80% (Fig. 1 ). At least one calf was found to be shedding C. parvum oocysts on 92 (77%) of 119 farms. No C. parvum was detected on the remaining 27 farms. The number of calves under 1 month old present per farm over the sampling period ranged from 1 to 140, and the number of fecal samples collected per farm ranged from 1 to 13, with a mean of 9 samples and a median of 10. The number of milking cows per farm ranged from 17 to 950, with a mean of 95 cows and a median of 70.
Fig. 1.
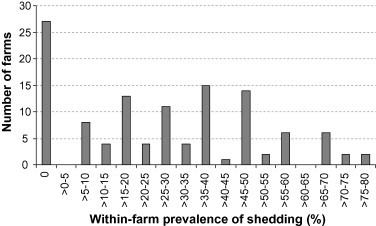
Distribution of within-herd prevalence of Cryptosporidium parvum shedding in calves 7–28 days if age on 119 dairy farms in southern Ontario.
3.2. Passive maternal antibody transfer
The number of calves per farm from which blood samples were drawn ranged from 0 (7 farms) to 5, with a median of 4. The average within-farm proportion of calves with a serum total protein level of less than 5.2 g/dL (Calloway et al., 2002) was 0.32. The median serum total protein level within farms ranged from 4.2 to 7.0 g/dL, with an average of 5.4 g/dL.
3.3. Univariable (unconditional) analysis
For univariable analysis, data from the largest farm in the dataset were excluded, as this farm appeared to have undue influence on the outcome, with large residuals and values for Cook's distance. In total, 47 variables were screened, of which 19 were significant at the 25% level and 6 were significant at the 5% level (Table 1 ).
Table 1.
Results of univariable analysis using negative binomial regression of each variable on the dependent variable (within-farm prevalence of C. parvum shedding)
| Variable | Risk ratio | 95% Confidence interval | P |
|---|---|---|---|
| Farm characteristics | |||
| Number of calves at time of sampling | |||
| 1–3 | 0 | 0.001 (group) | |
| 4–6 | 2.1 | 1.4–3.1 | |
| >6 | 2.3 | 1.5–3.5 | |
| Number of milking cows | |||
| ≤50 | 0.0 | 0.097 (group) | |
| >50–70 | 1.1 | 0.7–1.8 | |
| >70–101 | 1.5 | 1.0–2.4 | |
| >101 | 1.6 | 1.1–2.5 | |
| Perinatal management | |||
| Percentage of calves fed colostrum by esophageal tube | |||
| <10% | 0 | 0.24 (group) | |
| 10–4% | 0.5 | 0.3–1.0 | |
| 15–9% | 0.9 | 0.8–1.5 | |
| 30–100% | 1.0 | 0.7–1.5 | |
| Scraping of calving pen floor during cleaning | |||
| No | 0 | ||
| Yes | 0.8 | 0.5–1.1 | 0.181 |
| Separation of calves from dams as soon as possible after birth | |||
| No | 0 | ||
| Yes | 1.5 | 1.1–2.2 | 0.011 |
| Use of calf scour prophylaxisa on cows before calving | |||
| No | 0 | ||
| Yes | 1.5 | 1.1–2.1 | 0.009 |
| Management of pre-weaned calves | |||
| Concrete flooring in calf housing area | |||
| No | 0 | ||
| Yes | 0.6 | 0.5–0.8 | 0.002 |
| Earth flooring in calf housing area | |||
| No | 0 | ||
| Yes | 1.4 | 0.9–2.1 | 0.099 |
| Feeding of milk replacer in first week of life | |||
| No | 0.0 | ||
| Yes | 1.3 | 1.0–1.8 | 0.089 |
| Feeding of starter in first week of life | |||
| No | 0.0 | ||
| Yes | 1.2 | 0.9–1.7 | 0.207 |
| Gravel flooring in calf housing area | |||
| No | 0.0 | ||
| Yes | 1.4 | 1.0–1.9 | 0.061 |
| Medications given to newborn calves | |||
| No | 0.0 | ||
| Yes | 1.3 | 1.0–1.8 | 0.074 |
| Sweeping of floor (earth-floored calf housing areas) | |||
| No | 0.0 | ||
| Yes | 0.5 | 0.2–1.2 | 0.142 |
| Type of coccidiostat fed in calf starter | |||
| None | 0.0 | ||
| Decoquinate | 2.5 | 1.0–6.3 | 0.046 |
| Monensin | 3.3 | 1.3–8.6 | (group) |
| Other | 3.1 | 1.1–8.3 | |
| Use of First Defenseb Immucell, Portland, ME) for newborn calves | |||
| No | 0.0 | ||
| Yes | 1.4 | 1.0–1.9 | 0.040 |
| Use of injectable vitamin E and selenium for newborn calves | |||
| No | 0.0 | ||
| Yes | 1.3 | 0.9–1.8 | 0.128 |
| Vaccination of newborn calves against any pathogens | |||
| No | 0.0 | ||
| Yes | 1.3 | 0.9–1.8 | 0.213 |
| Washing of feeding utensils with disinfectant | |||
| No | 0.0 | ||
| Yes | 1.2 | 0.9–1.7 | 0.167 |
| Washing of feeding utensils with soap/detergent | |||
| No | 0.0 | ||
| Yes | 0.7 | 0.5–0.9 | 0.015 |
Data were collected from dairy calves 7–28 days old on 119 farms in southern Ontario. Variables significant at the 25% level (P < 0.25) are shown. A full list of all variables tested for significance is available from the authors.
Ecostar® (Novartis Animal Health, Mississauga, Ontario): bovine rotavirus and coronavirus killed virus vaccine with Escherichia coli bacterin; or ScourGuard (Pfizer Animal Health, Orangeville, Ontario): vaccine containing inactivated bovine rotavirus and coronavirus, a K99 Escherichia coli bacterin, and Clostridium perfringens type C toxoid.
First Defense® (Immucell, Portland, Maine): bovine coronavirus-E. coli antibody, bovine origin.
3.4. Multivariable (conditional) analysis
To fully utilise the available data, the largest farm in the dataset, which had been removed for unconditional analysis, was included in the dataset for multivariable modelling. The decision was made to include the farm on the condition that there was no evidence of undue influence in the final model. A categorical variable representing the number of milking cows as an indicator of herd size was included in each model, but was only significant at the 5% level in the ‘perinatal’ preliminary model and was eliminated during the construction of the final model.
No evidence of confounding or interaction was found during the model-building process. The final model that resulted from this procedure is summarized in Table 2 .
Table 2.
Results of multivariable negative binomial modelling of within-farm prevalence of Cryptosporidium parvum shedding on management factors significant at the 25% level in unconditional analysis
| Predictor | Risk ratio | 95% CI | Robust S.E. | P |
|---|---|---|---|---|
| Use of calf scour prophylaxis in pregnant cowsa | 1.70 | 1.30, 2.22 | 0.23 | <0.01 |
| Concrete flooring in calf housing area | 0.59 | 0.45, 0.76 | 0.08 | <0.01 |
| Feeding of milk replacer in first week of life | 1.40 | 1.06, 1.85 | 0.20 | 0.02 |
| Use of calf scour prophylaxis in calvesb | 1.38 | 1.06, 1.81 | 0.19 | 0.02 |
| Use of soap or detergent when washing feeding utensils | 0.61 | 0.46, 0.82 | 0.09 | <0.01 |
Data were collected from dairy calves 7–28 days old on 119 farms in southern Ontario. P-value of likelihood ratio test of alpha = 0 was 0.02 (<0.05).
Ecostar® (Novartis Animal Health, Mississauga, Ontario): bovine rotavirus and coronavirus killed virus vaccine with Escherichia coli bacterin; or ScourGuard (Pfizer Animal Health, Orangeville, Ontario): vaccine containing inactivated bovine rotavirus and coronavirus, a K99 Escherichia coli bacterin, and Clostridium perfringens type C toxoid.
First Defense® (Immucell, Portland, Maine): bovine coronavirus-E. coli antibody, bovine origin.
A scatter-plot of Pearson residuals against predicted mean number of positive fecal samples showed no evidence of outliers. Similarly, a plot of leverage against predicted mean number of positive fecal samples showed no evidence of any observations with unusual leverage in the model. However, one farm, which was not the largest farm, appeared to have undue influence on the model from a plot of Cook's distance against the predicted mean number of positive fecal samples. On this farm, 4 positive samples were collected out of a total of 8 (within-farm prevalence 50%), while the predicted number of positive samples was 1.2. Most of the factors predicted by the model to increase the risk of C. parvum on a farm were absent from this farm (no calf scour prophylaxis was used in the cows or calves, there was no concrete flooring in the calf housing area, and no soap or detergent was used to wash feeding utensils). However, calves were fed milk replacer in the first week of life. Removal of this farm's data from the dataset and re-running the model without them produced little change in the coefficients or P-values in the model. Therefore, data from this farm were retained in the model summarized in Table 2.
4. Discussion
In this study, 30% of 1089 calves aged 7–28 days and sampled once were shedding C. parvum oocysts at the time of sampling. This is within the range of previous figures reported from other regions of Canada. A study conducted in 2002, in which single samples were taken from 500 calves 7–21 days old in the same region, revealed a prevalence of 41% (Trotz-Williams et al., 2005a); however, in that work, one of the major criteria for inclusion of farms was a current or recent history of neonatal calf scours. As a higher proportion of farms in that study had a history of calf scours, it would be expected that the prevalence of shedding would be higher than reported here. Herd-level prevalence of C. parvum shedding among the 119 farms in this study was 77.3%. Despite the fact that farms included in this study represented herds with and without recent or current calf diarrhea problems, this figure was very similar to the 76% herd-level prevalence reported from the previous study in southwestern Ontario (Trotz-Williams et al., submitted for publication).
The outcome variable investigated in this study (within-farm prevalence of C. parvum shedding) was not normally distributed (Fig. 1) and could not be easily transformed into a variable that was approximately normally distributed. For this reason, and because the data did not fit the Poisson distribution, it was decided to use negative binomial regression to analyze the data. Furthermore, diagnostics performed on the resulting model indicated that, of all the options considered (linear regression, Poisson regression and negative binomial regression), this form of model fit the data best.
Variables associated with an increased prevalence of C. parvum shedding included several management factors implicated as risk factors by studies conducted elsewhere. For example, Castro-Hermida et al. (2002) reported that concrete flooring in calf housing areas resulted in a lower prevalence of shedding on farms in Spain. Likewise, in Mexico, Maldonado-Camargo et al. (1998) found that the feeding of starter grain to newborn dairy calves was associated with increased shedding. On the other hand, some practices that were reported as protective by other researchers appeared to be associated here with an increased prevalence of shedding; feeding milk replacer to dairy calves was associated with a decreased risk of shedding in New York State (Mohammed et al., 1999), while in our study, the practice was associated with an increased prevalence of C. parvum. In addition, several factors reported as being significantly associated with C. parvum shedding in other publications, such as use of multi-cow versus individual calving facilities, contact between pre-weaned calves, and cleaning of calf housing areas (Garber et al., 1994, Sischo et al., 2000, Castro-Hermida et al., 2002), showed no significant association with within-farm prevalence of C. parvum in our work.
The association between the use of calf scour prophylaxis in pregnant cows and an increased prevalence of C. parvum shedding was highly significant, both in univariable analysis and in the multivariable model. Controlling for other management factors included in the final model (Table 2), the prevalence was 70% higher on farms using this type of prophylaxis than on farms where no prophylaxis was used in the cows. A plausible explanation for this finding could be that the use of such measures on farms may have been in response to a high incidence of calf scours in those herds. As prophylactic products marketed for calf scours have no known impact on the risk of cryptosporidiosis, the use of these products on farms affected by C. parvum in calves would be ineffective against scouring caused by Cryptosporidium infection. This explanation may also apply to the significant association between the use of calf scour prophylaxis in calves and increased prevalence of C. parvum shedding, with the prevalence of positive calves being 38% higher on farms using the products.
The prevalence of C. parvum shedding was 41% lower on farms where concrete flooring was present in the calf housing areas. Due to the large number of farms using hutches, the most common other types of flooring material were gravel, sand and earth. The presence of concrete flooring is likely to facilitate more thorough cleaning than is possible when other types of flooring (sand, gravel or earth) are used (Castro-Hermida et al., 2002). On the other hand, this variable may also be an indicator of higher quality housing in general, and of better calf management. Similarly, the finding that the prevalence, or the risk of a calf shedding C. parvum, was 39% lower on farms using soap or detergent when washing feeding utensils, may be related to better overall management on the farms. Alternatively, this finding may be a direct result of cleaner, and therefore less contaminated, feeding utensils.
In contrast to results published by Mohammed et al. (1999), the feeding of milk replacer to calves in the first week of life was associated with an increased prevalence of C. parvum shedding; the prevalence of shedding was 40% higher on farms feeding milk replacer to young calves than on farms where no milk replacer was fed to calves of this age. Although the variable representing herd size was dropped due to non-significance in the construction of the multivariable model, there was a moderate correlation between the size of farms and the feeding of milk replacer to calves in the first week of life (Pearson's correlation coefficient 0.23), and the risk of shedding was significantly higher in larger herds. This may partially explain the observed positive association between the feeding of milk replacer and the risk of shedding.
The findings of this work are useful in identifying dairy management practices that are potentially important predictors of the prevalence of shedding in dairy calves under 1 month of age in Ontario. This information may be beneficial to veterinary practitioners and dairy producers involved in the prevention and control of C. parvum infection and disease in calves. However, because of the large number of variables investigated in this study and the fact that this is the first published study of this kind conducted in Ontario, these results provide only an indication of which practices may in reality be of importance. Repeating the study with a smaller number of predictor variables would be of value in confirming the results reported here. Moreover, in this work, sampling was carried out during the time of year when C. parvum is thought to be most prevalent in dairy calves in Ontario. Factors identified here as important predictors of oocyst shedding may, therefore, be of importance only in this season. Future herd-level studies might provide useful information on seasonal differences by including data collection in the winter as well as in summer months.
5. Conclusion
C. parvum shedding is common among dairy calves under 1 month of age in Ontario, Canada: 30% of 1089 calves tested in this study were shedding oocysts, and oocysts were detected in the feces of calves on 77.3% of 119 farms. Predictors found to be associated with increased within-farm prevalence of shedding were the use of calf scour prophylaxis in cows and calves, and feeding of milk replacer to young calves. Presence of concrete flooring in calf housing areas and use of soap or detergent when washing calf feeding utensils appeared to be protective management practices, as both were associated with reduced prevalence of shedding. It would appear that calf feeding and housing practices that facilitate good hygiene may be important in reducing the prevalence of C. parvum shedding in young dairy calves.
Acknowledgements
The authors acknowledge Grazyna Adamska-Jarecka for laboratory diagnostic support, and appreciate the assistance of the dairy practitioners and producers involved in recruitment of herds and data collection. Jen Wilstra and Beth Sockovie coordinated the collection and processing of samples. This work was funded by the Ontario Ministry of Agriculture and Food, Dairy Farmers of Canada, Dairy Farmers of Ontario, and the National Sciences and Engineering Research Council of Canada.
References
- Atwill E.R., Johnson E.M., Pereira M.C. Association of herd composition, stocking rate, and duration of calving season with fecal shedding of C. parvum oocysts in beef herds. J. Am. Vet. Med. Assoc. 1999;215:1833–1838. [PubMed] [Google Scholar]
- Calloway C.D., Tyler J.W., Tessman R.K., Hostetler D., Holle J. Comparison of refractometers and test endpoints in the measurement of serum protein concentration to assess passive transfer status in calves. J. Am. Vet. Med. Assoc. 2002;221:1605–1608. doi: 10.2460/javma.2002.221.1605. [DOI] [PubMed] [Google Scholar]
- Castro-Hermida J.A., González-Losada Y.A., Ares-Mazás E. Prevalence of and risk factors involved in the spread of neonatal bovine cryptosporidiosis in Galicia (N W Spain) Vet. Parasitol. 2002;106:1–10. doi: 10.1016/S0304-4017(02)00036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf D.C., Vanopdenbosch E., Ortega-Mora L.M., Abassi H., Peeters J.E. A review of the importance of cryptosporidiosis in farm animals. Int. J. Parasitol. 1999;29:1269–1287. doi: 10.1016/S0020-7519(99)00076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohoo I., Martin W., Stryhn H. Veterinary Epidemiologic Research. AVC Inc.; Prince Edward Island, Canada: 2003. Sampling. pp. 43–44. [Google Scholar]
- Dohoo I., Martin W., Stryhn H. Veterinary Epidemiologic Research. AVC Inc.; Prince Edward Island, Canada: 2003. Logistic regression. pp. 335–372. [Google Scholar]
- Dohoo I., Martin W., Stryhn H. Veterinary Epidemiologic Research. AVC Inc.; Prince Edward Island, Canada: 2003. Model-building strategies. pp. 317–334. [Google Scholar]
- Duffield C. The Delphi technique. Aust. J. Adv. Nurs. 1988;6:41–45. [PubMed] [Google Scholar]
- Garber L.P., Salman M.D., Hurd H.S., Keefe T., Schlater J.L. Potential risk factors for Cryptosporidium infection in dairy calves. J. Am. Vet. Med. Assoc. 1994;205:86–91. [PubMed] [Google Scholar]
- Korich D.G., Mead J.R., Madore M.S., Sinclair N.A., Sterling C.R. Effects of ozone, chlorine dioxide, chlorine and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 1990;56:1423–1428. doi: 10.1128/aem.56.5.1423-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Camargo S., Atwill E.R., Saltijeral-Oaxaca J.A., Herrera-Alonso L.C. Prevalence and risk factors for shedding of Cryptosporidium parvum in Holstein Freisian dairy calves in central México. Prev. Vet. Med. 1998;36:95–107. doi: 10.1016/s0167-5877(98)00084-1. [DOI] [PubMed] [Google Scholar]
- Mohammed H.O., Wade S.E., Schaaf S. Risk factors associated with Cryptosporidium parvum infection in southeastern New York State. Vet. Parasitol. 1999;83:1–13. doi: 10.1016/s0304-4017(99)00032-1. [DOI] [PubMed] [Google Scholar]
- Nydam D.V., Wade S.E., Schaaf S.L., Mohammed H.O. Number of Cryptosporidium parvum oocysts or Giardia spp cysts shed by dairy calves after natural infection. Am. J. Vet. Res. 2001;62:1612–1615. doi: 10.2460/ajvr.2001.62.1612. [DOI] [PubMed] [Google Scholar]
- Olson M.E., Guselle N.J., O’Handley R.M. Giardia and Cryptosporidium in dairy calves in British Columbia. Can. Vet. J. 1997;38:703–706. [PMC free article] [PubMed] [Google Scholar]
- Ruest N., Faubert G.M., Couture Y. Prevalence and geographical distribution of Giardia spp. and Cryptosporidium spp. in dairy farms in Quebec. Can. Vet. J. 1998;39:697–700. [PMC free article] [PubMed] [Google Scholar]
- Sischo W.M., Atwill E.R., Lanyon L.E., George J. Cryptosporidia on dairy farms and the role these farms may have in contaminating surface water supplies in the northeastern United States. Prev. Vet. Med. 2000;43:253–267. doi: 10.1016/s0167-5877(99)00107-5. [DOI] [PubMed] [Google Scholar]
- Trotz-Williams L.A., Jarvie B.D., Martin S.W., Leslie K.E., Peregrine A.S. Prevalence of Cryptosporidium parvum infection in southwestern Ontario and its association with diarrhea in neonatal dairy calves. Can. Vet. J. 2005;46:349–351. [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams L.A., Martin S.W., Martin D. Multiattribute evaluation of two simple tests for the detection of Cryptosporidium parvum in cal faeces. Vet. Parasitol. 2005;134:15–23. doi: 10.1016/j.vetpar.2005.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams, L.A., Martin, S.W., Leslie, K.E., Duffield, T., Nydam, D.V., Peregrine, A.S., 2006. Calf-level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Prev. Vet. Med., submitted for publication. [DOI] [PMC free article] [PubMed]
- Weir S.C., Pokorny N.J., Carreno R.A., Trevors J.T., Lee H. Efficacy of common laboratory disinfectants on the infectivity of Cryptosporidium parvum oocysts in cell culture. Appl. Environ. Microbiol. 2002;68:2576–2579. doi: 10.1128/AEM.68.5.2576-2579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


